Abstract
This systematic review and meta-analysis aimed to synthesize the evidence on the adverse events (AEs) of coronavirus disease 2019 (COVID-19) vaccinations in Saudi Arabia. A computerized search in MEDLINE via PubMed and OVID, Scopus, CENTRAL, and Web of Science was conducted using relevant keywords. The NIH tools were used for the quality assessment. A total of 14 studies (16 reports) were included. The pooled analysis showed that the incidence of AEs post-COVID-19 vaccination was 40.4% (95% CI:6.4% to 87%). Compared to the AstraZeneca vaccine, the Pfizer-BioNTech vaccine was associated with a lower risk ratio (RR) of wheezing (RR = 0.04), fever (RR = 0.32), chills (RR = 0.41), headache (RR = 0.47), dizziness (RR = 0.49), and joint pain (RR = 0.51). The Pfizer-BioNTech vaccine was associated with significantly higher RR of general allergic reactions (RR = 1.62), dyspnea (RR = 1.68), upper respiratory tract symptoms (RR = 1.71), and lymphadenopathy (RR = 8.32). The current evidence suggests that the incidence of AEs following COVID-19 vaccines is 40%; however, most of these AEs were mild and for a short time. The overall number of participants with AEs was higher in the Pfizer group compared to the AstraZeneca group; however, the AstraZeneca vaccine was associated with a higher RR of several AEs.
Keywords: COVID-19, vaccine, adverse events, AstraZeneca, Pfizer-BioNTech
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease that has resulted in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. COVID-19 has a lower fatality rate than the Middle East respiratory syndrome (MERS) but is spreading much faster [2,3]. To reduce the spread of SARS-CoV-2 and related deaths, several COVID-19 vaccines have been authorized for use in humans. These COVID-19 vaccines are based on several pharmacological methods: inactivated SARS-CoV-2 genes, an adenovirus vector, a protein subunit, and messenger RNA (mRNA) [4].
The mRNA-based vaccination (tozinameran or BNT162b2), publicly known as the Pfizer-BioNTech vaccine, emerged in 2020 during the COVID-19 pandemic. The mRNA vaccines act by directing human cells to generate spike protein, which is contained in SARS-CoV-2. The generated spike will protect the body against SARS-CoV-2 infection by stimulating the immune system [5,6]. The government of Saudi Arabia provides free vaccinations and online registration to all citizens and residents. The Ministry of Health has opened over 500 immunization clinics around the country to improve accessibility [7]. In December 2020, the Saudi Food and Drug Authority (SFDA) approved the tozinameran/BNT162b2 vaccine (Pfizer vaccine) [8]. On the other hand, mutated adenoviruses are used in a vector approach to prompt human cells to produce spike protein and the immune response to stimulate particular T cells for preventing COVID-19.
In February 2021, the SFDA gave the green light to the distribution of ChAdOx1, a vector vaccine developed by a British-Swedish company with the help of Oxford University, known as the AstraZeneca vaccine [8,9]. In contrast to the mRNA and vector vaccines, the inactivated SARS-CoV-2 vaccine was generated by inactivating a specific strain of SARS-CoV-2 using -propiolactone, a chemical that may bind to virus genes and inhibit replication while leaving viral proteins intact. Inactivated viruses may be administered to boost the immune system without producing illness [10]. Recently, the SFDA has approved the use and distribution of Janssen (Johnson & Johnson) and Moderna vaccines; however, they have not yet authorized the use of any inactivated SARS-CoV-2 vaccine. Up to date, more than 66.7 million doses of vaccination have been received by the Saudi population, with 25.1 million individuals vaccinated, meaning 72% of the Saudi population.
Recently, active surveillance of the safety of COVID-19 vaccines (CoVaST) has been initiated; however, no results have been published yet [11]. There are a variety of published publications on the effects of COVID-19 vaccinations on human health, and each vaccine has its unique profile of risks and benefits. There was some inconsistency in these findings based on the specific vaccination and population studied. Due to differences in ethnic background and genetics, such heterogeneity is likely [12,13,14]. Symptoms or signs of upper respiratory inflammation, tachycardia, headache, shivering, myalgia, weakness throughout the body, and pain at the injection site were all documented as adverse drug reactions (ADRs) of the vaccinations [15,16]. This systematic review and meta-analysis aimed to synthesize the evidence on the adverse events (AEs) of COVID-19 vaccinations in Saudi Arabia.
2. Methods
We have followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and Cochrane handbook for systematic reviews of interventions in reporting this study [17,18].
2.1. Eligibility Criteria
The studies were included based on the following criteria:
Population: studies that were conducted on the Saudi population who received the COVID-19 vaccine.
Exposure: studies that included data about the Pfizer-BioNTech vaccine and/or the AstraZeneca vaccine.
Outcomes: studies documenting the AEs of one of the aforementioned vaccines or both.
Study design: observational studies (case-control, cohort, and cross-sectional).
We excluded case reports, conference abstracts, and non-English studies.
2.2. Information Sources and Search Strategy
On April 20, 2022, we searched the following databases: MEDLINE via PubMed and OVID, Scopus, CENTRAL, and Web of Science, using the relevant keywords, such as (“Ad26COVS1”[Mesh] OR “COVID-19 Vaccines”[Mesh] OR “ChAdOx1 nCoV-19”[Mesh] OR “2019-nCoV Vaccine mRNA-1273”[Mesh] OR “BNT162 Vaccine”[Mesh]) AND (“Saudi Arabia”[Mesh] OR Kingdom of Saudi Arabia) to identify the relevant citations. Supplementary File S1 shows the detailed search term for each database. These databases were searched from inception to the date of search. Moreover, the reference lists of all included citations were searched. The retrieved citations were imported to EndNote X9 software, and duplicates were removed.
2.3. Selection Process
Using Microsoft Excel software, a screening sheet was created. the study ID, publication year, title, abstract, keywords, DOI, and URL were all included. The selection process was undertaken using a two-step screening technique by three independent reviewers (M.R.A, A.Y.A, and S.F.A). Step one was screening the title and abstract of all studies found via the literature search to determine which studies might proceed to step two (Full-text screening), where reviewers would read and assess whether each research met eligibility criteria. Any disagreement between the reviewers was solved by the judgment of the study supervisor (A.A).
2.4. Data Items and Collection Process
Four independent reviewers extracted the following data from the included studies to an offline pre-prepared Excel sheet: demographic data of the included participants (age, gender, and residency), study characteristics (studies groups, study duration, total sample size, country, and main findings), and outcomes (incidence rate of adverse events following COVID-19 vaccination).
2.5. Risk of Bias and Quality Assessment
Using the National Institutes of Health (NIH) quality assessment tool for observational cohort, case-control, and cross-sectional studies, two authors (S.M.A and N.I.A) independently evaluated the risk of bias and the quality of each included article. Reviewers can critically evaluate the internal validity of research using this tool. The tool consists of 14 questions; studies that scored <7 were deemed as “poor”, from 7–9 were deemed as “fair”, and >9 were deemed as “Good”. A third author (A.A) resolved disagreements when the authors disagreed on a rating. A detailed list of these questions can be found in Supplementary File S2.
2.6. Data Synthesis
The prevalence of adverse events was calculated using the random-effects model with a 95% CI. Using the I2 statistic, we calculated the percentage of heterogeneity and inconsistency between studies, with values of 25%, 50%, and 75% deemed low, moderate, and high, respectively. The random-effect model was employed if the heterogeneity was considerable and I2 > 50%; otherwise, the fixed-effect model was utilized. The comprehensive meta-analysis was used for all statistical analyses (CMA; USA: version 3.3.070). To resolve heterogeneity, a sensitivity analysis was performed by removing one study in each scenario, known as a sequential sensitivity analysis. Furthermore, a subgroup analysis was performed to minimize the risk of inconsistency. To assess the difference between Pfizer-BioNTech and AstraZeneca in terms of AEs, we used the Review Manager 5.4 software to calculate the risk ratio (RR) between both groups using the Mantel–Haenszel model. Publication bias was assessed based on the criteria of Egger’s test, and a funnel plot was generated for the forest plots that included 10 studies or more.
3. Results
3.1. Study Selection
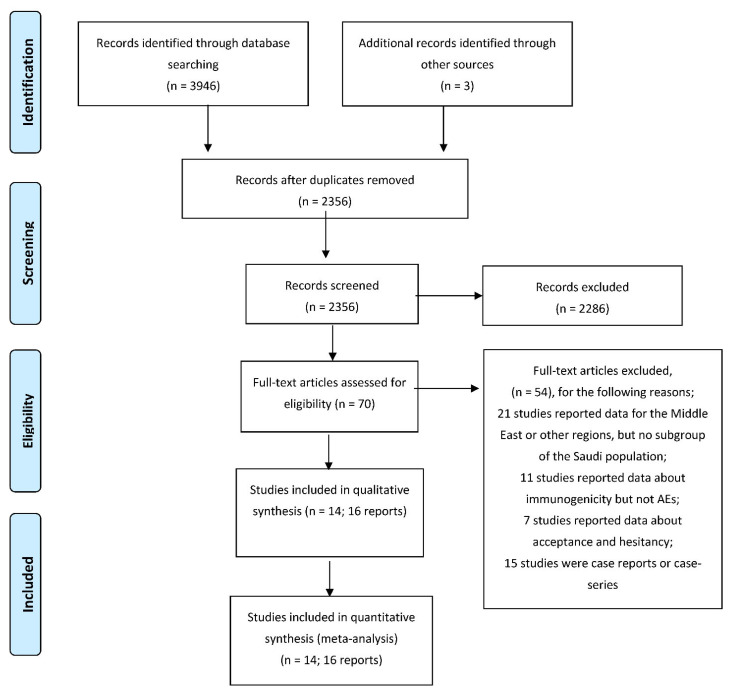
Based on our literature search, we found a total of 3949 relevant citations. After removing duplication, 2356 articles underwent title/abstract screening. Then, 2286 studies were deemed ineligible to our criteria. The full-text screening was performed on 70 articles, and only 14 studies (16 reports) were included in the qualitative (systematic review) and quantitative synthesis (meta-analysis) [7,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Figure 1 shows the PRISMA flow diagram of included studies.
Figure 1.
PRISMA flow diagram.
3.2. Characteristics of Included Studies and Participants
All the included studies were cross-sectional except for one retrospective cohort study. Eight studies collected their data using an online survey, two used a telephone-based survey, two used traditional self-reported questionnaires, one used an interview questionnaire, and one used hospital databases for ADRs. Seven studies compared the Pfizer-BioNTech vaccine and AstraZeneca; five reported data for only Pfizer-BioNTech; and two reported data for only AstraZeneca. Both adults and children were included. More than half of the participants (52.17%) are males across the included studies. Alghamdi A. et al. conducted a study of 528 participants and published three reports; the first report compared males and females [27], the second report compared participants older or younger than 50 years [28], and the third report compared healthcare workers and non-healthcare workers [29]. Table 1 summarizes the baseline characteristics of included studies and participants.
Table 1.
Summary of Included studies.
| ID | Study Design | Method of Data Collection | Study Duration | Sample Size | Vaccines | Age (Years) ** |
Inclusion Criteria | Male |
|---|---|---|---|---|---|---|---|---|
| Jan et al., 2022 | cross-sectional | Questionnaire | May 10 to 20, 2021 | 147 | Pfizer and AstraZeneca | 32.2 (19–49) | All participants with Sickle Cell Disease who received Pfizer or AstraZeneca vaccines | 79 (53.7%) |
| Almohaya et al., 2021 | retrospective cohort | Hospital Databases | February 1st to March 31st, 2021 | 71221 | Pfizer | 32 (16–109) | Participants experienced AEs following the Pfizer vaccine | 39884 (56) |
| Alfaleh et al., 2022 | cross-sectional | Questionnaire | December 2020 to March 2021 * | 4432572 | Pfizer and AstraZeneca | 16 to ≥65 | Individuals had at least one dose of any batch of any of the Pfizer or AstraZeneca vaccines | NA |
| Mohammed et al., 2021 | cross-sectional | Online survey | February to March 2021 | 386 | Pfizer | 18 to ≥65 | Any individual who took the Pfizer vaccine in Saudi Arabia | 177 (45.9) |
| Mahallawi et al., 2021 | cross-sectional | Online survey | February 1st to June 30th, 2021 * | 365 | Pfizer and AstraZeneca | 45.1 ± 14.7 | Participants who received both doses of either the Pfizer or AstraZeneca COVID-19 vaccine | 306 (83.8) |
| EL-Shitany et al.,2021 | cross-sectional | Online survey | January 10 to 21, 2021 | 455 | Pfizer | 16 to ≥65 | Individuals who received at least one dose of the Pfizer COVID-19 vaccine. | 163 (35.8%) |
| Almohaya et al., 2021b | cross-sectional | Online survey | June 1 to 8, 2021 | 3639 | Pfizer | 18 to ≥65 | A resident of the KSA at the time of enrollment and have received at least one dose of the Pfizer vaccine | 1337 (36.7) |
| Alhazmi et al., 2021 | cross-sectional | Online survey | April 7 to April 28, 2021 | 515 | Pfizer and AstraZeneca | 26 ± 9 | Any individual took Pfizer or AstraZeneca vaccines | 221 (43%) |
| Alghamdi N. et al., 2021 | cross-sectional | Questionnaire | - | 4170 | Pfizer and AstraZeneca | 16 to ≥65 | Any individual took Pfizer or AstraZeneca vaccines in Saudi Arabia | 1296 (31.08) |
| Alghamdi A. et al., 2021 | cross-sectional | Telephone-based survey | February 28 to March 12, 2021 | 528 | AstraZeneca | 16 to ≥65 | Individuals who received the AstraZeneca COVID-19 vaccine | 263 (49.81) |
| Alghamdi A. et al., 2021b | cross-sectional | Telephone-based survey | February 28 to March 12, 2021 | 528 | AstraZeneca | 16 to ≥65 | Individuals who received the AstraZeneca COVID-19 vaccine | 263 (49.81) |
| Alghamdi A. et al., 2021c | cross-sectional | Telephone-based survey | February 28 to March 12, 2021 | 528 | AstraZeneca | 16 to ≥65 | Individuals who received the AstraZeneca COVID-19 vaccine | 263 (49.81) |
| Alamer et al., 2021 | cross-sectional | Online survey | 1st August to 24th August of 2021 | 965 | Pfizer | 16 ± 2 | Children received single or double doses of the Pfizer vaccine | 460 (48) |
| Bahrani et al., 2021 | cross-sectional | Telephone-based survey | April to May 2021 | 1592 | AstraZeneca | 37.4 ± 9.6 | Individuals who received the first dose of the AstraZeneca vaccine | 1290 (81) |
| Ahsan et al., 2021 | cross-sectional | Online survey | March 30 to May 13, 2021) | 397 | Pfizer and AstraZeneca | 34.43 ± 6.73 | Individuals who received at least one dose of either the Pfizer or AstraZeneca vaccine | 209 (52.6) |
| Adam et al., 2021 | cross-sectional | Online survey | March to May 2021 | 330 | Pfizer and AstraZeneca | 18 to ≥65 | Participants who received either one or two doses of the AstraZeneca or Pfizer vaccine | 216 (65.5) |
* The duration of vaccine administration. ** Age presented as mean ± SD, median (IQR), or range.
3.3. Quality of the Included Studies
Based on the NIH quality assessment tool for observational studies, six were deemed as “Good,” and eight were deemed as “Fair.” Supplementary File S2 shows the detailed quality assessment based on the NIH tool.
3.4. Meta-Analysis
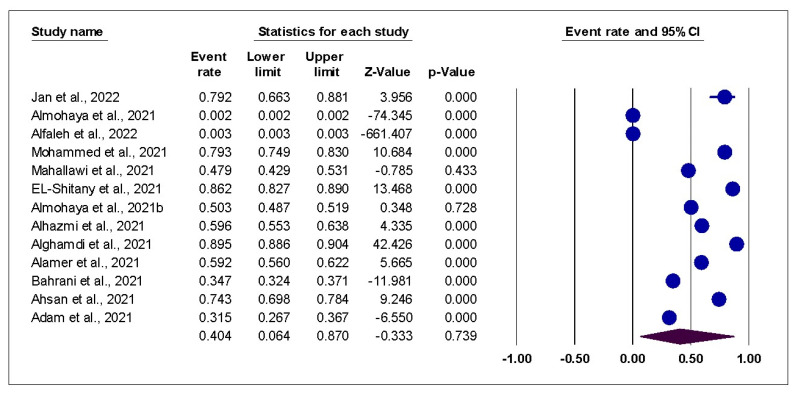
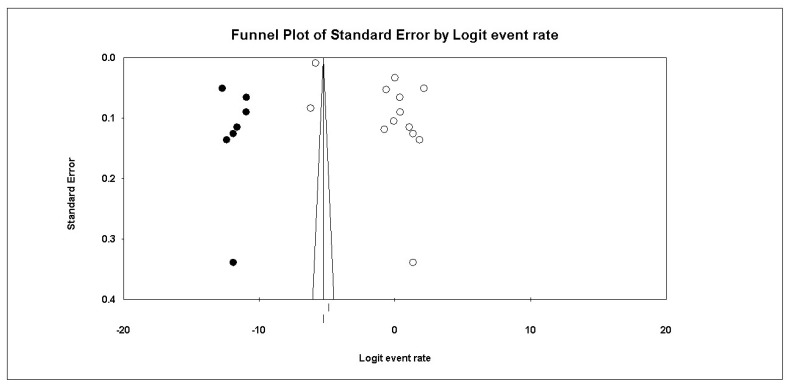
Overall AEs: The pooled analysis of 13 studies showed that the incidence of AEs post-COVID-19 vaccination was 40.4% (95% CI:6.4% to 87%). The pooled data were heterogenous (I2 = 99%; p < 0.001), Figure 2 Sensitivity analysis could not solve the heterogeneity. Subgroup analysis showed that the incidence of AEs post-COVID-19 vaccination in studies that used online surveys was 62.5% (95% CI:52.1–72.0%), traditional questionnaires 13.6% (95% CI:0–99.7%), and other methods 13.7% (95% CI:0.2–92.8%). Based on the type of vaccine, the incidence of AEs post-COVID-19 vaccination in both vaccines was 43.7% (95% CI:1.9–96.9%), Pfizer-BioNTech vaccine 37.0% (95% CI:5.1–86.4%), AstraZeneca vaccine 34.7% (95% CI:32.4–37.1%). The funnel plot and Egger’s test showed a significant risk of publication bias (p = 0.002) which was solved by trimming three studies, resulting in a much smaller effect size of 26.1% (95% CI:5.0–70.2%), as shown in Figure 3.
Figure 2.
Forest Plot of overall AEs; shows the pooled analysis of overall AEs among vaccinated individuals.
Figure 3.
Funnel plot of standard error by the logit event rate.
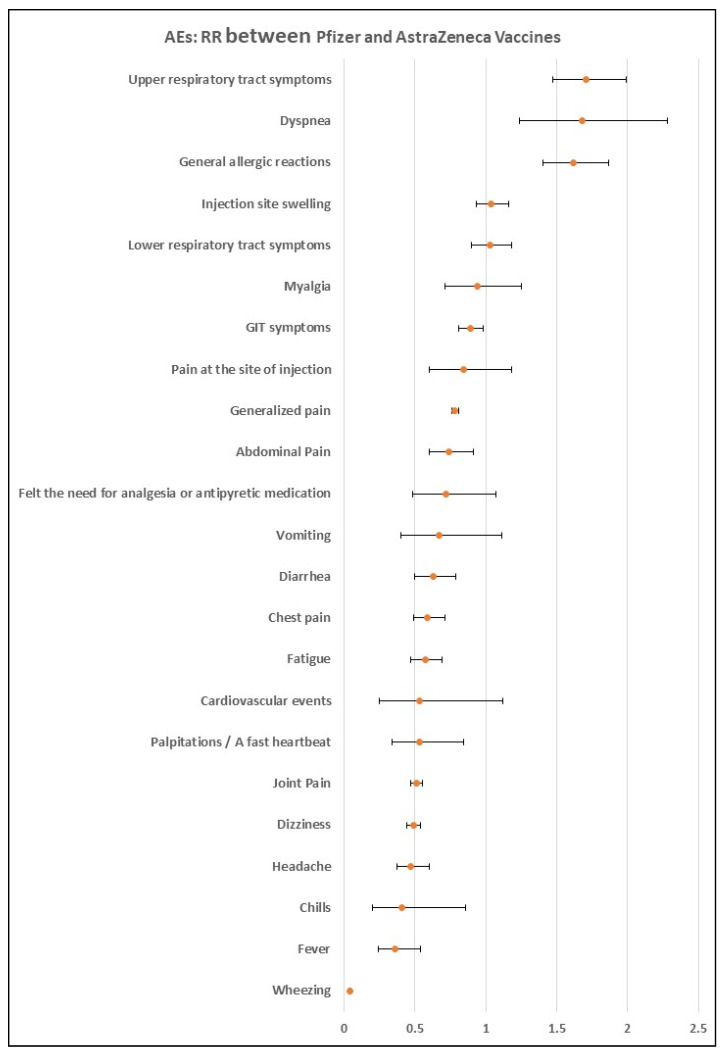
Comparison between Pfizer-BioNTech and AstraZeneca vaccines: The pooled analysis showed that the Pfizer-BioNTech vaccine was associated with lower RR of several AEs, including fever (RR = 0.32, 95% CI: 0.30–0.35; I2 = 0%, p = 0.64), chills (RR = 0.41, 95% CI: 0.20–0.86; I2 = 99%; p < 0.001), headache (RR = 0.47, 95% CI: 0.37–0.60; I2 = 93%, p < 0.001), dizziness (RR = 0.49, 95% CI: 0.44–0.54; I2: 0%, p = 0.73), palpitations (RR = 0.53, 95% CI: 0.34–0.84; I2 = 83%; p = 0.02), fatigue (RR = 0.57, 95% CI: 0.47–0.69; I2 = 58%, p = 0.12), and diarrhea (RR = 0.63, 95% CI: 0.50–0.79; I2 = 0%, p = 0.52). On the other hand, the Pfizer-BioNTech vaccine was associated with significantly higher RR compared to the AstraZeneca vaccine in the following AEs: general allergic reactions (RR = 1.62, 95% CI: 1.40–1.87; I2 = NA), dyspnea (RR = 1.68, 95% CI: 1.24–2.28; I2 = 0%; p = 0.51), upper respiratory tract symptoms (RR = 1.71, 95% CI: 1.47–1.99; I2 = NA), and lymphadenopathy (RR = 8.32, 95% CI: 6.16–11.22; I2 = NA), as shown in Figure 4.
Figure 4.
Forest plot of AEs in Pfizer group compared with AstraZeneca group.
Most common AEs: Table 2 showed that the most common AEs in participants who received the Pfizer-BioNTech vaccine were pain at the site of injection 59.3% (95% CI:44.3–73.0%), myalgia 35.3% (95% CI:28.3–43.0%), fatigue 28.3% (95% CI:20.3–38.0%), joint pain 27.3% (95% CI:25.3–28.0%), and headache 25.3% (95% CI:22.3–29.0%). On the other hand, the most common AEs in participants who received the AstraZeneca vaccine were myalgia 70.7% (95% CI:67.0–80.8%), fatigue 60.8% (95% CI:31.0–80.8%), fever 57.7% (95% CI:42.0–80.8%), joint pain 55.5% (95% CI:53.0–80.8%), and pain at the site of injection 54.8% (95% CI:26.0–80.8%), as shown in Table S1.
Table 2.
Most common local and systemic AEs in both vaccines.
| AEs | Pfizer | AstraZeneca |
|---|---|---|
| Myalgia | 35.3% (28.3–43.0%) | 70.7% (67.0–80.8%) |
| Fatigue | 28.3% (20.3–38.0%) | 60.8% (31.0–80.8%) |
| Fever | 18.3% (14.3–23.0%) | 57.7% (42.0–80.8%) |
| Joint Pain | 27.3% (25.3–28.0%) | 55.5% (53.0–80.8%) |
| Pain at the site of injection | 59.3% (44.3–73.0%) | 54.8% (26.0–80.8%) |
| Headache | 25.3% (22.3–29.0%) | 45.5% (36.0–80.8%) |
| Chills | 10.3% (6.3–14.0%) | 40.6% (21.0–80.8%) |
| Generalized pain | 7.3% (0.3–41.0%) | 26.9% (01.0–80.8%) |
| Injection site swelling | 12.3% (7.3–19.0%) | 25.2% (23.0–80.8%) |
| Dizziness | 20.3% (11.3–33.0%) | 24.6% (04.0–80.8%) |
| Vomiting | 4.3% (1.3–12.0%) | 18.4% (14.9–22.6%) |
| Chest pain | 8.3% (5.3–13.0%) | 13.1% (11.0–80.8%) |
| GIT symptoms | 8.3% (1.3–28.0%) | 10.1% (06.0–80.8%) |
| Diarrhea | 5.3% (3.3–06.0%) | 9.6% (8.2–11.1%) |
| Abdominal Pain | 7.3% (6.3–7.0%) | 09.1% (08.0–80.8%) |
| Numbness | 6.3% (3.3–11.0%) | 8.5% (6.4–11.2%) |
| Nausea | 7.3% (3.3–14.0%) | 07.2% (02.0–80.8%) |
| Palpitations/A fast heartbeat | 6.3% (5.3–7.0%) | 4.9% (0.7–26.1%) |
| Dyspnea | 6.3% (4.3–11.0%) | 3.7% (2.9–4.6%) |
| Sore throat | 1.3% (0.3–15.0%) | 0.9% (0–16.9%) |
| Lymphadenopathy | 2.3% (1.3–6.0%) | 0.6% (0.5–0.8%) |
| Hospitalization due to side effects | 8.3% (6.3–11.0%) | 0.0% (0–8%) |
| High Blood pressure | 1.3% (0.3–13.0%) | NA |
The severity of AEs: Eight studies reported data on the severity of AEs [7,19,21,24,25,26,30,32]. Among the studies that reported data on both Pfizer and AstraZeneca vaccines [7,25,32], 12.06% had serious AEs, and 71.88% had non-serious AEs (RR = 0.17, 95% CI: 0.09–0.34; p < 0.0001). Regarding the participants who received the Pfizer vaccine only [19,21,24,26,30], 17.91% had serious AEs, and 78.73% had non-serious AEs (RR = 0.20, 95% CI: 0.14–0.30; p < 0.00001). One study reported data on participants who received the AstraZeneca vaccine [26]; 2.49% of them had serious AEs, and 76.35% had non-serious AEs (RR = 0.03, 95% CI: 0.02 –0.04; p < 0.00001), as shown in Table 3.
Table 3.
Severity of AEs.
| Vaccines | Number of Studies | Serious vs. Non-Serious (%) | RR (95% CI) | Heterogeneity |
|---|---|---|---|---|
| AstraZeneca and Pfizer | 3 | 12.07 vs. 71.88 | (RR = 0.17, 95% CI: 0.09–0.34; p < 0.0001) | I2 = 89%, p < 0.001 |
| Pfizer | 5 | 17.91 vs. 78.73 | (RR = 0.20, 95% CI: 0.14–0.30; p < 0.00001) | I2 = 98%, p < 0.001 |
| AstraZeneca | 1 | 2.49 vs. 76.35 | (RR = 0.03, 95% CI: 0.02–0.04; p < 0.00001) | - |
Dose-dependent AEs: Five studies reported data regarding the AEs experienced after the first dose and second dose of the Pfizer vaccine [21,23,24,26,30]. The pooled analysis showed that the first dose of the Pfizer vaccine was associated with significantly lower risks of chills (RR = 0.41, 95% CI: 0.36–0.48; p < 0.0001), fever (RR = 0.42, 95% CI: 0.31–0.55; p < 0.00001), headache (RR = 0.74, 95% CI: 0.66–0.83; p < 0.00001), joint pain (RR = 0.39, 95% CI: 0.24–0.61; p < 0.0001), lymph node swelling (RR = 0.48, 95% CI: 0.34–0.67; p < 0.0001), nausea and vomiting (RR = 0.72, 95% CI: 0.60–0.86; p = 0.0004), nerve inflammation symptoms (RR = 0.68, 95% CI: 0.55–0.84; p = 0.0004), tiredness (RR = 0.58, 95% CI: 0.40–0.84; p = 0.004), flu-like symptoms (RR = 0.36, 95% CI: 0.30–0.43; p < 0.0001), and body ache (RR = 0.60, 95% CI: 0.55–0.66; p < 0.0001), as shown in Table 4.
Table 4.
Dose-dependent AEs.
| AEs | Number of Studies | RR (95% CI) | Heterogeneity |
|---|---|---|---|
| Bad rash all over the body | 2 | (RR = 0.88, 95% CI: 0.36–2.14; p = 0.78) | I2 = 11%; p = 0.29 |
| Chest pain | 2 | (RR = 0.90, 95% CI: 0.71–1.14; p = 0.38) | I2 = 0%; p = 0.37 |
| Chills | 4 | (RR = 0.41, 95% CI: 0.36–0.48; p < 0.0001) | I2 = 0%; p = 0.45 |
| Diarrhea | 2 | (RR = 1.25, 95% CI: 0.48–3.29; p = 0.65) | I2 = 32%; p = 0.22 |
| Difficulty of Breathing | 3 | (RR = 0.97, 95% CI: 0.75–1.26; p = 0.82) | I2 = 23%; p = 0.27 |
| Dizziness and giddiness | 2 | (RR = 0.87, 95% CI: 0.75–1.01; p = 0.07) | I2 = 0%; p = 0.83 |
| Elevated blood pressure | 2 | (RR = 0.89, 95% CI: 0.38–2.08; p = 0.78) | I2 = 11%; p = 0.29 |
| Fatigue | 4 | (RR = 0.73, 95% CI: 0.51–1.04; p = 0.08) | I2 = 93%; p < 0.0001 |
| Fever | 5 | (RR = 0.42, 95% CI: 0.31–0.55; p < 0.00001) | I2 = 88%; p < 0.0001 |
| Headache | 5 | (RR = 0.74, 95% CI: 0.66–0.83; p < 0.00001) | I2 = 48%; p = 0.11 |
| Hypersensitivity Symptoms | 2 | (RR = 0.79, 95% CI: 0.45–1.40; p = 0.42) | I2 = 66%; p = 0.09 |
| Injection site swelling and redness | 3 | (RR = 1.08, 95% CI: 0.94–1.25; p = 0.26) | I2 = 45%; p = 0.16 |
| Joint pain | 2 | (RR = 0.39, 95% CI: 0.24–0.61; p < 0.0001) | I2 = 50%; p = 0.13 |
| Lips swelling | 2 | (RR = 1.24, 95% CI: 0.70–2.18; p = 0.46) | I2 = 0%; p = 0.59 |
| Lymph node swelling | 4 | (RR = 0.48, 95% CI: 0.34–0.67; p < 0.0001) | I2 = 29%; p = 0.24 |
| Nausea and vomiting | 3 | (RR = 0.72, 95% CI: 0.60–0.86; p = 0.0004) | I2 = 0%; p = 0.48 |
| Nerve Inflammation Symptoms | 2 | (RR = 0.68, 95% CI: 0.55–0.84; p = 0.0004) | I2 = 43%; p = 0.19 |
| Pain at the site of the injection | 5 | (RR = 1.02, 95% CI: 0.93–1.12; p = 0.67) | I2 = 83%; p = 0.0001 |
| Sore throat | 2 | (RR = 1.00, 95% CI: 0.81–1.24; p = 0.98) | I2 = 0%; p = 0.53 |
| Tiredness | 2 | (RR = 0.58, 95% CI: 0.40–0.84; p = 0.004) | I2 = 45%; p = 0.18 |
| Flu-like symptoms | 2 | (RR = 0.36, 95% CI: 0.30–0.43; p < 0.0001) | I2 = 0%; p = 0.84 |
| Body ache | 2 | (RR = 0.60, 95% CI: 0.55–0.66; p < 0.0001) | I2 = 74%; p = 0.05 |
The interval between COVID-19 vaccination and AEs onset: Alfaleh et al. [20] reported that the median time of AEs onset post-Pfizer vaccine was significantly longer than AstraZeneca vaccine [1.96 (0.1–94.1) vs. 1.64 (0.1–33.4) days; p = 0.001). Similarly, Mohammed et al. [21] found that the median duration of AEs onset after the Pfizer vaccine was 2 days with an IQR of 11 days. They also showed that most events occurred within 24 h (72.6%), and 23.3% occurred between 24–48 h. Alhazmi et al. [25] reported that 84% of the participants experienced AEs within 24 h, 15% within 48 h, and 1% within 72 h, regardless of the type of vaccine. Likewise, Alamer et al. [30] showed that 72% of the participants who received the Pfizer vaccine experienced AEs within 24 h, 20% within 48 h, and 8% within 72 h. Ahsan et al. [32] mentioned that among participants who received either AstraZeneca or Pfizer vaccines, 87.1% experienced AEs within 24 h and 12.9% between 24–48 h. When comparing males vs. females, Alghamdi et al. [27] demonstrated that males were associated with delayed onset of AEs (15 ± 14 h vs. 12.4 ± 10.5 h; p = 0.037). They also compared participants older and younger than 50 years, and their findings showed that 65.4% of the participants younger than 50 experienced AEs within 24 h, compared to 63% in the elderly group [28]. When they compared healthcare workers vs. the general population, they found that the onset of AEs was significantly delayed in the healthcare workers group (16 ± 15.4 vs 12.2 ± 10.2 h; p = 0.002) [29].
4. Discussion
In this systematic review and meta-analysis, our findings showed a high rate of overall AEs among the included studies. This rate was very high in the online surveys compared to the traditional self-reported or telephone-based questionnaires, which is expected as online surveys are associated with a higher response rate [34]. The overall AEs associated with the Pfizer-BioNTech vaccine were slightly higher than the AstraZeneca vaccine. However, the Pfizer-BioNTech vaccine was associated with lower RR of several AEs, including wheezing, fever, chills, headache, dizziness, joint pain, palpitations, fatigue, chest pain, diarrhea, abdominal pain, generalized pain, and GIT symptoms. On the other hand, the Pfizer-BioNTech vaccine was associated with significantly higher RR than the AstraZeneca vaccine in the following AEs: general allergic reactions, dyspnea, upper respiratory tract symptoms, and lymphadenopathy. This is consistent with earlier research showing that Pfizer-BioNTech vaccines had fewer adverse effects than Oxford-AstraZeneca and other companies [35,36]. On the other hand, Klugar et al. reported that the prevalence of local AEs was higher in mRNA-based vaccines compared to viral vector-based vaccines (78.3% vs. 70.4%); however, the difference was not statistically significant (p = 0.064). In terms of systemic AEs, the prevalence was significantly higher in the viral vector-based vaccine (87.2% vs. 61%; p < 0.001) [37]. Previously published findings of phase III clinical studies and vaccine fact sheets consistently reported post-vaccination effects for individuals who got the second dosage [23,38,39,40,41]. Menni et al. recently conducted a prospective observational study and revealed results consistent with previous studies [42]. Similar to our findings, a recent systematic review and meta-analysis showed that headache, fatigue, and fever were the most reported systemic AEs. Most local AEs recorded were pain, swelling, and redness at the injection site [43].
Local and systemic AEs are examples of post-immunization complications [44]. The best vaccines could protect against a targeted virus without causing undesired side effects. However, in real life, all vaccines have the risk of inducing some AEs that may or may not be related to the immunization itself. In most cases, the cause of adverse responses after vaccination is unclear and may be attributable to the vaccine’s adjuvant, stabilizer, or preservative [43,45]. The majority of COVID-19 vaccine-related adverse effects are mild, consistent with the experience of earlier vaccinations. There may be a correlation between IgE-mediated responses and the vaccine’s active antigen, leading to local adverse reactions [46].
For most currently used and formerly used vaccinations, the incidence of vaccine-induced anaphylactic shock is around one in a million doses administered [47]. Vaccine-induced anaphylaxis was almost 10 times more common with Pfizer-BioNTech and Moderna than earlier vaccinations [45]. Unfortunately, the cause of this negative response is yet to be determined. Some inactive components or results of the vaccine manufacturing process, such as lipid or the polyethylene glycol (PEG)-lipid component of the vaccine, may increase susceptibility to non-IgE-related mast-cell activation or complement activation in some individuals [45]. When delivered in vivo, mRNA vaccines are protected from degradation via a lipid-based nanoparticle carrier technology. PEG 2000 lipid conjugate, which provides a hydrophilic layer, significantly stabilizes this carrier system and extends the half-life [47,48]. The vaccines developed by Pfizer-BioNTech and Moderna are the first mRNA vaccines to be authorized. Accordingly, the mechanism of allergic responses to mRNA vaccines remains unknown. The mechanism of allergic reactions to mRNA vaccines was unknown until the Pfizer-BioNTech vaccine, and the Moderna vaccine was granted Emergency Use Authorization (EUA).
Recent systematic reviews and meta-analyses have been published that assess the effectiveness and safety of COVID-19 vaccinations in light of the existing evidence. The most prevalent AEs were fatigue and headache, which is consistent with our findings [49]. Other meta-analyses reported that myalgia, fever, fatigue, and headache were the most common systemic AEs following injection, whereas swelling, erythema, and pain at the injection site were the most prevalent local AEs [50,51,52]. The number of participants who had adverse events and reactions after receiving an injection of an RNA-based vaccination has been found to be greater than with other vaccine types [50]. Serious AEs after immunization against COVID-19 were not documented in any investigations. Notably, the results of this investigation and the existing literature imply that AEs may be moderate and transient owing to the initiation of active immune responses. Since immunization has been shown to be very effective in lowering the likelihood of hospitalization, intensive care unit admission, mechanical ventilation, and mortality, it is recommended that it be continued and the occurrence of these adverse events be anticipated [48,50,51,52]. Current recommendations from the US CDC and the Saudi Ministry of Health (MOH) for the medical management of post-vaccination AEs emphasize the use of symptomatic therapy.
This is the first systematic review and meta-analysis of AEs associated with COVID-19 vaccinations in Saudi Arabia. This study covered more than 13% of the Saudi population and around 20% of all vaccinated individuals in Saudi Arabia. However, our study has some limitations, including the high heterogeneity, which could not be solved by sensitivity or subgroup analysis. Moreover, the observed publication bias is another limitation. Due to the scarcity of data, we could not compare the vaccinated and non-vaccinated and early and late AEs.
In conclusion, the current evidence suggests that the incidence of AEs following COVID-19 vaccines is 40%; however, most of these AEs were mild and lasted for a short time. The most common systemic AEs were myalgia, fatigue, headache, dizziness, fever, and vomiting, whereas the most common local AEs were pain and swelling at the site of injection and joint pain. The overall number of participants with AEs was higher in the Pfizer group compared to the AstraZeneca group; however, the AstraZeneca vaccine was associated with a higher RR of several AEs. Since immunization may be anticipated to be very effective in lowering the likelihood of hospitalization, intensive care unit admission, mechanical ventilation, and mortality, it is recommended that it be continued and the occurrence of these adverse events be anticipated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines10122089/s1, File S1: Used Keywords for search term, File S2: NIH tool of quality assessment, Table S1: AEs among all vaccinated patients, Pfizer vaccine, and AstraZeneca vaccine.
Author Contributions
Conceptualization, A.A.; methodology, A.A. and A.K.A. (Ahoud Khashman Almutairi); software, A.H.A. and Z.S.A.; validation, A.K.A. (Ahoud Khashman Almutairi), A.H.A. and Z.S.A.; formal analysis, A.H.A. and M.R.A.; resources, M.R.A. and A.K.A. (Amjad Khalid Alsaran); data curation, A.K.A. (Amjad Khalid Alsaran); writing—original draft preparation, A.K.A. (Ahoud Khashman Almutairi), A.A., Z.S.A. and A.K.A. (Amjad Khalid Alsaran); writing—review and editing, M.R.A., Z.S.A., A.H.A. and A.A.; visualization, A.K.A. (Amjad Khalid Alsaran) and M.R.A.; supervision, A.A.; project administration, A.A. and A.K.A. (Ahoud Khashman Almutairi). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented within the study text or its Supplementary Files.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kandeel M., Yamamoto M., Tani H., Kobayashi A., Gohda J., Kawaguchi Y., Park B.K., Kwon H.-J., Inoue J.-I., Alkattan A. Discovery of New Fusion Inhibitor Peptides against SARS-CoV-2 by Targeting the Spike S2 Subunit. Biomol. Ther. 2021;29:282–289. doi: 10.4062/biomolther.2020.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelgawad H.A.H., Sayed A., Munir M., Elberry M.H., Sayed I.M., Kamal M.A., Negida A., Ebada M.A., Bahbah E.I. Clinical Review of COVID-19; Pathogenesis, Diagnosis, and Management. Curr. Pharm. Des. 2021;27:4232–4244. doi: 10.2174/1381612826666201222162509. [DOI] [PubMed] [Google Scholar]
- 3.Kim B.M. The Role of Saikosaponins in Therapeutic Strategies for Age-Related Diseases. Oxid. Med. Cell. Longev. 2018;2018:1–10. doi: 10.1155/2018/8275256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Federico M. The Conundrum of Current Anti-SARS-CoV-2 Vaccines. Cytokine Growth Factor Rev. 2021;60:46–51. doi: 10.1016/j.cytogfr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. An MRNA Vaccine against SARS-CoV-2 — Preliminary Report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khehra N., Padda I., Jaferi U., Atwal H., Narain S., Parmar M.S. Tozinameran (BNT162b2) Vaccine: The Journey from Preclinical Research to Clinical Trials and Authorization. Aaps Pharmscitech. 2021;22:172. doi: 10.1208/s12249-021-02058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jan H., Waheeb A., AlAhwal H., Almohammadi A., Al-Marzouki A., Barefah A., Bahashawan S., Radhwi O. COVID-19 Vaccine Perception and Hesitancy Among Patients With Sickle Cell Disease in the Western Region of Saudi Arabia. Cureus. 2022;14:1–9. doi: 10.7759/cureus.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assiri A., Al-Tawfiq J.A., Alkhalifa M., Al Duhailan H., Al Qahtani S., Dawas R.A., El Seoudi A.A., Alomran N., Omar O.A., Alotaibi N., et al. Launching COVID-19 Vaccination in Saudi Arabia: Lessons Learned, and the Way Forward. Travel Med. Infect. Dis. 2021;43:102119. doi: 10.1016/j.tmaid.2021.102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Single-Dose Administration and the Influence of the Timing of the Booster Dose on Immunogenicity and Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine: A Pooled Analysis of Four Randomised Trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirzaei R., Mohammadzadeh R., Mahdavi F., Badrzadeh F., Kazemi S., Ebrahimi M., Soltani F., Kazemi S., Jeda A.S., Darvishmotevalli M., et al. Overview of the Current Promising Approaches for the Development of an Effective Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine. Int. Immunopharmacol. 2020;88:106928. doi: 10.1016/j.intimp.2020.106928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riad A., Schünemann H., Attia S., Peričić T., Žuljević M., Jürisson M., Kalda R., Lang K., Morankar S., Yesuf E., et al. COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects. Int. J. Env. Res. Public Health. 2021;18:7859. doi: 10.3390/ijerph18157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen L.H., Joshi A.D., Drew D.A., Merino J., Ma W., Lo C.-H., Kwon S., Wang K., Graham M.S., Polidori L., et al. Racial and Ethnic Differences in COVID-19 Vaccine Hesitancy and Uptake. MedRxiv. 2021 doi: 10.1101/2021.02.25.21252402. [DOI] [Google Scholar]
- 13.AbdelGhaffar M.M., Omran D., Elgebaly A., Bahbah E.I., Afify S., AlSoda M., El-Shiekh M., ElSayed E.S., Shaaban S.S., AbdelHafez S., et al. Prediction of Mortality in Hospitalized Egyptian Patients with Coronavirus Disease-2019: A Multicenter Retrospective Study. PloS ONE. 2022;17:e0262348. doi: 10.1371/journal.pone.0262348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omran D., Al Soda M., Bahbah E., Esmat G., Shousha H., Elgebaly A., Abdel Ghaffar M., Alsheikh M., El Sayed E., Afify S., et al. Predictors of Severity and Development of Critical Illness of Egyptian COVID-19 Patients: A Multicenter Study. PloS ONE. 2021;16:e0256203. doi: 10.1371/journal.pone.0256203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riad A., Pokorná A., Attia S., Klugarová J., Koščík M., Klugar M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021;10:1428. doi: 10.3390/jcm10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur S.P., Gupta V. COVID-19 Vaccine: A Comprehensive Status Report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;10:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., Thomas J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almohaya A.M., Alsubie H., Alqarni B., Alzayad B., Alghar A., Alshahrani K., Barry M. Acute Unsolicited Adverse Events Following BNT162b2 Vaccine in Saudi Arabia, a Real-World Data. Vaccine. 2022;40:477–482. doi: 10.1016/j.vaccine.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfaleh A., Alkattan A., Radwan N., Elzohri M., Alzaher A., Ibrahim M. Adverse Drug Reactions from Two COVID—19 Vaccines Reported in Saudi Arabia. Drugs. Perspect. 2022;38:84–92. doi: 10.1007/s40267-022-00893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammed R.A., Garout R.M., Wahid S., Ayub F., Firas ZinAlddin L.M., Sultan I. A Survey on the Side Effects of Pfizer/BioNTech COVID-19 Vaccine Among Vaccinated Adults in Saudi Arabia. Cureus. 2021;13 doi: 10.7759/cureus.19222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahallawi W.H., Mumena W.A. Reactogenicity and Immunogenicity of the P Fi Zer and AstraZeneca COVID-19 Vaccines. Front. Immunol. 2021;9:5169. doi: 10.3389/fimmu.2021.794642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Shitany N.A., Harakeh S., Badr-Eldin S.M., Bagher A.M., Eid B., Almukadi H., Alghamdi B.S., Alahmadi A.A., Hassan N.A., Sindi N., et al. Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine Among Saudi Residents: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2021;14:1389–1401. doi: 10.2147/IJGM.S310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almohaya A.M., Qari F., Zubaidi G.A., Alnajim N., Moustafa K., Alshabi M.M., Alsubaie F.M., Almutairi I., Alwazna Q., Al-tawfiq J.A., et al. Early Solicited Adverse Events Following the BNT162b2 MRNA Vaccination, a Population Survey from Saudi Arabia. Prev. Med. Rep. 2021;24:101595. doi: 10.1016/j.pmedr.2021.101595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alhazmi A., Alamer E., Daws D., Hakami M., Darraj M., Abdelwahab S., Maghfuri A., Algaissi A. Evaluation of Side Effects Associated with COVID-19 Vaccines in Saudi Arabia. Vaccines. 2021;9:674. doi: 10.3390/vaccines9060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alghamdi A.N., Alotaibi M.I., Alqahtani A.S., Al Aboud D., Abdel-Moneim A.S. BNT162b2 and ChAdOx1 SARS-CoV-2 Post-Vaccination Side-Effects Among Saudi Vaccinees. Front. Med. 2021;8:1796. doi: 10.3389/fmed.2021.760047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alghamdi A., Ibrahim A., Almutairi R., Joseph M., Alghamdi G., Alhamza A. A Cross-Sectional Survey of Side Effects after COVID-19 Vaccination in Saudi Arabia: Male versus Female Outcomes. J. Adv. Pharm. Educ. Res. 2021;11:51–56. doi: 10.51847/bCwca2qGfP. [DOI] [Google Scholar]
- 28.Alghamdi A., Ibrahim A., Alraey M., Alkazemi A., Alghamdi I., Alwarafi G. Side Effects Following COVID-19 Vaccination: A Cross-Sectional Survey with Age-Related Outcomes in Saudi Arabia. J. Adv. Pharm. Educ. Res. 2021;11:119–125. doi: 10.51847/1UfXSzTR6M. [DOI] [Google Scholar]
- 29.Alghamdi A.A., Alkazemi A., Alissa A., Alghamdi I., Alwarafi G., Waggas H.A. Adverse Events Following AstraZeneca COVID-19 Vaccine in Saudi Arabia: A Cross-Sectional Study among Healthcare and Nonhealthcare Workers. Intervirology. 2022;65:104–109. doi: 10.1159/000519456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alamer E., Alhazmi A., Qasir N.A., Alamer R., Areeshi H., Gohal G., Qadri M., Hashem A.M., Algaissi A. Side Effects of COVID-19 Pfizer-BioNTech MRNA Vaccine in Children Aged 12–18 Years in Saudi Arabia. Vaccines. 2021;9:1297. doi: 10.3390/vaccines9111297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al Bahrani S., Albarrak A., Alghamdi O.A., Alghamdi M.A., Hakami F.H., Al Abaadi A.K., Alkhrashi S.A., Alghamdi M.Y., Almershad M.M., Alenazi M.M., et al. Safety and Reactogenicity of the ChAdOx1 (AZD1222) COVID-19 Vaccine in Saudi Arabia. Int. J. Infect. Dis. 2021;110:359–362. doi: 10.1016/j.ijid.2021.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahsan W., Syed N.K., Alsraeya A.A., Alhazmi H.A., Najmi A., Al Bratty M., Javed S., Makeen H.A., Meraya A.M., Albarraq A.A., et al. Post-Vaccination Survey for Monitoring the Side Effects Associated with COVID-19 Vaccines among Healthcare Professionals of Jazan Province, Saudi Arabia. Saudi Med. J. 2021;42:1341–1352. doi: 10.15537/smj.2021.42.12.20210576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adam M., Gameraddin M., Alelyani M., Alshahrani M.Y., Gareeballah A., Ahmad I., Azzawi A., Komit B., Musa A. Evaluation of Post-Vaccination Symptoms of Two Common COVID-19 Vaccines Used in Abha, Aseer Region, Kingdom of Saudi Arabia. Patient Prefer. Adherence. 2021;15:1963–1970. doi: 10.2147/PPA.S330689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hohwü L., Lyshol H., Gissler M., Jonsson S.H., Petzold M., Obel C. Web-Based versus Traditional Paper Questionnaires: A Mixed-Mode Survey with a Nordic Perspective. J. Med. Internet Res. 2013;15:e173. doi: 10.2196/jmir.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beattie M., Murphy D.J., Atherton I., Lauder W. Instruments to Measure Patient Experience of Healthcare Quality in Hospitals: A Systematic Review. Syst. Rev. 2015;4:97. doi: 10.1186/s13643-015-0089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrzejczak-Grządko S., Czudy Z., Donderska M. Side Effects after COVID-19 Vaccinations among Residents of Poland. Eur. Rev. Med. Pharm. Sci. 2021;25:4418–4421. doi: 10.26355/eurrev_202106_26153. [DOI] [PubMed] [Google Scholar]
- 37.Klugar M., Riad A., Mekhemar M., Conrad J., Buchbender M., Howaldt H.-P., Attia S. Side Effects of MRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology. 2021;10:752. doi: 10.3390/biology10080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapin-Bardales J., Gee J., Myers T. Reactogenicity Following Receipt of MRNA-Based COVID-19 Vaccines. JAMA. 2021;325:2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 40.Kadali R.A.K., Janagama R., Peruru S., Malayala S. V Side Effects of BNT162b2 MRNA COVID-19 Vaccine: A Randomized, Cross-Sectional Study with Detailed Self-Reported Symptoms from Healthcare Workers. Int. J. Infect. Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 Vaccines: Comparison of Biological, Pharmacological Characteristics and Adverse Effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharm. Sci. 2021;25:1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 42.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., et al. Vaccine Side-Effects and SARS-CoV-2 Infection after Vaccination in Users of the COVID Symptom Study App in the UK: A Prospective Observational Study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kouhpayeh H., Ansari H. Adverse Events Following COVID-19 Vaccination: A Systematic Review and Meta-Analysis. Int. Immunopharmacol. 2022;109:108906. doi: 10.1016/j.intimp.2022.108906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rostam-Abadi Y., Gholami J., Noroozi A., Ansari M., Baheshmat S., Hamzehzadeh M., Ghadirzadeh M.R., Vahdani B., Ekhtiari H., Mojtabai R., et al. Public Health Risks Associated with Methadone in Iran: A Systematic Review and Meta-Analysis. Int. J. Drug Policy. 2022;100:103529. doi: 10.1016/j.drugpo.2021.103529. [DOI] [PubMed] [Google Scholar]
- 45.Castells M.C., Phillips E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N. Engl. J. Med. 2021;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone C.A., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., Hemler J.A., Phillips E.J. Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized. J. Allergy Clin. Immunol. Pr. 2019;7:1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone C.A., Rukasin C.R.F., Beachkofsky T.M., Phillips E.J. Immune-mediated Adverse Reactions to Vaccines. Br. J. Clin. Pharm. 2019;85:2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. MRNA Vaccine Delivery Using Lipid Nanoparticles. Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haas J.W., Bender F.L., Ballou S., Kelley J.M., Wilhelm M., Miller F.G., Rief W., Kaptchuk T.J. Frequency of Adverse Events in the Placebo Arms of COVID-19 Vaccine Trials. Jama Netw. Open. 2022;5:e2143955. doi: 10.1001/jamanetworkopen.2021.43955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pormohammad A., Zarei M., Ghorbani S., Mohammadi M., Razizadeh M.H., Turner D.L., Turner R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines. 2021;9:467. doi: 10.3390/vaccines9050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q., Qin C., Liu M., Liu J. Effectiveness and Safety of SARS-CoV-2 Vaccine in Real-World Studies: A Systematic Review and Meta-Analysis. Infect. Dis. Poverty. 2021;10:132. doi: 10.1186/s40249-021-00915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J., Cai Y., Chen Y., Williams A.P., Gao Y., Zeng J. Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials. Vaccines. 2021;9:939. doi: 10.3390/vaccines9080939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are presented within the study text or its Supplementary Files.