Abstract
The development of Chlamydia pneumoniae-specific cell-mediated immunity was studied during a primary C. pneumoniae infection. The immune response was detected as positive lymphocyte proliferation and secretion of interferon gamma. C. pneumoniae-induced activation of both CD4+ and CD8+ T cells was detected in the early phase of infection, but activation of only CD4+ T cells was detected in the later stage.
Chlamydia pneumoniae is an obligate intracellular bacterium that can infect a number of cell types, including monocytes and macrophages (1, 7, 10, 13). Activation of cell-mediated immune (CMI) responses (3, 17) is supposedly important for protective immunity. Contributory factors participating in the outcome or eradication of the primary C. pneumoniae infection have not been investigated in humans.
In this work we followed the development of humoral immunity and CMI in cases of primary C. pneumoniae infection over a period of 4 months. We measured C. pneumoniae-specific antibodies and the development of CMI responses, including the activation of CD4 and CD8 T-cell subsets and the secretion of selected proinflammatory (tumor necrosis factor alpha [TNF-α] and interferon gamma [IFN-γ]) and anti-inflammatory (interleukin-10 [IL-10]) cytokines, which according to experimental infections play a role in the final fate of the host defence against Chlamydia (14, 15, 21).
The series included 291 male patients (age range, 18 to 20 years) who had consulted a doctor because of respiratory tract symptoms (range of duration, 2 to 14 days) and acute fever. The diagnosis of a primary C. pneumoniae infection was based on the detection of specific Immunoglobulin M (IgM) antibodies either at admission or 2 weeks later. The positive diagnosis was used as an inclusion criterion for enrollment in the tests for CMI responses. For that purpose, citrated blood samples were drawn soon after the diagnosis with follow-up specimens taken at weeks 8 and 16 after the symptoms appeared.
C. pneumoniae-specific serum antibodies were measured by the microimmunofluorescence (MIF) method (18). The CMI responses were measured by determining the proliferative response of isolated peripheral blood lymphocytes (PBL) as described previously (17) using C. pneumoniae Kajaani 6 (1 μg/ml) and C. trachomatis L2 (1 μg/ml) whole elementary body (EB) antigens.
Expression of the HLA-DR molecule was analyzed as an activation marker on CD4+ and CD8+ T lymphocytes by double immunofluorescence assay using fluorescein isothiocyanate-conjugated anti-CD4 and CD8 and R-phycoerytherin-conjugated anti-HLA-DR (Caltag Laboratories, San Francisco, Calif.) after in vitro stimulation of 1.5 × 106 PBL with infectious C. pneumoniae EB (1.5 × 105 and 1.5 × 104 inclusion-forming units [IFU]) for 5 days. The cytokine (IFN-γ, TNF-α, and IL-10) analyses were performed from the culture supernatants using commercially available enzyme-linked immunosorbent assay kits (Pelikine Compact; Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands).
The Mann-Whitney U test and Wilcoxon paired t test were performed using SPSS software (SPSS, Inc., Chicago, Ill.).
A primary C. pneumoniae infection was diagnosed by the presence of IgM antibodies in 16 of the 291 patients (5.5%), and 9 of these 16 patients were within reach for the follow-up study of CMI responses. The IgM response to C. pneumoniae continued to increase after admission (geometric mean titer [GMT] of 31.8) and reached its maximum value (GMT of 160.0) 2 to 3 weeks after diagnosis. The C. pneumoniae-specific IgG antibodies were positive (IgG ≥ 32) 8 weeks after the diagnosis in eight of the nine patients (GMT of 50.8) and at 16 weeks in all 9 patients (GMT of 36.8). A positive C. pneumoniae-specific IgA response (IgA ≥ 16) was found in only three of the nine patients (33%) at some point in the follow-up (Table 1), and C. trachomatis-specific IgG antibodies were detected in none of them.
TABLE 1.
Follow-up analysis of C. pneumoniae-specific antibodies and simultaneously analyzed lymphocyte proliferative responses (SI) in nine patients with a primary C. pneumoniae infection
| Patient no. | Time (wk) | Antibody titer
|
LP test (SI) | Clinical diagnosis | ||
|---|---|---|---|---|---|---|
| IgM | IgG | IgA | ||||
| 1 | 3 | 1,280 | 8 | 8 | 101.2 | Bronchitis |
| 8 | 1,280 | 32 | 8 | 57.4 | ||
| 2 | 3 | 640 | 32 | 64 | 46.7 | Pneumonia |
| 8 | 640 | 64 | 16 | 48 | ||
| 16 | 20 | 64 | 8 | 46.3 | ||
| 3 | 3 | 160 | 128 | 8 | 116 | Pneumonia |
| 8 | 40 | 64 | 8 | 9 | ||
| 16 | 160 | 32 | 8 | 2 | ||
| 4 | 3 | 10 | 32 | 8 | 7.3 | Bronchitis |
| 8 | <10 | 32 | 8 | 28.4 | ||
| 16 | <10 | 32 | 8 | 22.1 | ||
| 5 | 3 | 160 | 8 | 8 | 9.2 | Bronchitis |
| 16 | 5 | 8 | 8 | 1.5 | ||
| 6 | 3 | 40 | 8 | 16 | 161.6 | Acute infection |
| 8 | <10 | 64 | 16 | 114.4 | ||
| 7 | 3 | 1,280 | 128 | 8 | 108.8 | Acute infection |
| 8 | 160 | 256 | 8 | 18.5 | ||
| 16 | <10 | 128 | 8 | 24.3 | ||
| 8 | 3 | 320 | 8 | 8 | 159.9 | Bronchopneumonia |
| 8 | 10 | 128 | 8 | 11.7 | ||
| 9 | 3 | 10 | 32 | 8 | 86.1 | Pharyngitis |
| 8 | 10 | 32 | 8 | 44.6 | ||
The PBL proliferative response to C. pneumoniae was positive (stimulation index [SI] ≥ 3) in all nine patients at 3 weeks after the disease symptoms appeared (Table 1; median SI, 86.1; range, 3.1 to 162.0). CMI responses were also studied in five control patients who did not have detectable antibodies to C. pneumoniae, and the median PBL response in these cases was 2.3 (range, 1.0 to 7.1). The PBL responses to C. pneumoniae antigen started to decline after the active phase of the infection but remained clearly positive up to 16 weeks (24.3; range, 3.5 to 97.3) in seven patients. As shown in Table 1, the PBL responses were low (SI < 3) at week 16 in two patients, one of whom no longer had detectable antibodies to C. pneumoniae.
The PBL responses to C. trachomatis did not change and were significantly lower than those to C. pneumoniae at every time point (median responses of 1.6, 7.9, and 2.7 at weeks 3, 8, and 16, respectively; P < 0.05).
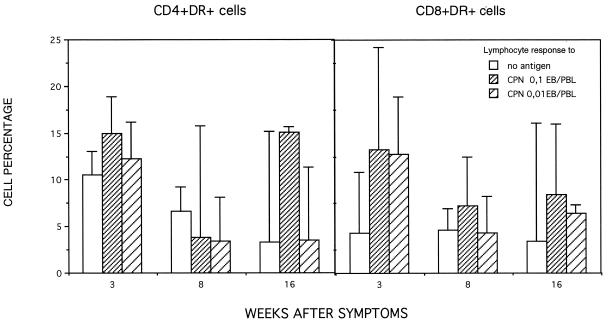
The PBL activation induced by the infectious C. pneumoniae (0.1 and 0.01 EB/PBL) was detected in vitro as an increased number of CD8+ DR+ lymphocytes in comparison to nonstimulated lymphocytes in the specimens taken at 3 weeks (P < 0.01) but less clearly in the later specimens (Fig. 1). The expression of HLA-DR antigen on the nonstimulated CD4+ lymphocytes tended to be high at week 3 relative to the other time points and did not differ statistically significantly from the stimulated level at any time point.
FIG. 1.
Median percentages of C. pneumoniae-induced expression of the HLA-DR molecule on CD4 and CD8-positive T lymphocytes at 3, 8, and 16 weeks after the appearance of respiratory tract symptoms.
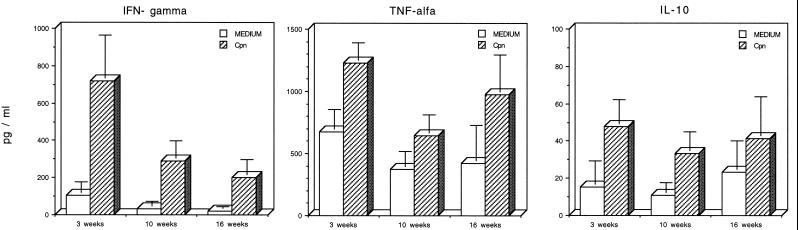
C. pneumoniae-induced cytokine secretion (IFN-γ, IL-10, and TNF-α) was analyzed in the 5-day culture supernatants. The strong PBL proliferative response at week 3 was also reflected in increased production of IFN-γ in response to C. pneumoniae EB (0.1 IFU/PBL) compared to the nonstimulated secretion (Fig. 2). Thereafter, the C. pneumoniae-induced IFN-γ secretion tended to decline, but the difference between the nonstimulated and stimulated PBL remained significant over the whole period. Secretion of TNF-α and IL-10 was clearly detectable even in the culture supernatants of nonstimulated PBL at all the time points, but stimulation with C. pneumoniae enhanced the secretion significantly (Fig. 2).
FIG. 2.
C. pneumoniae-induced (0.1 or 0.01 EB/PBL) secretion of IFN-γ, TNF-α, and IL-10 in lymphocyte cultures at 3, 8, and 16 weeks after the appearance of respiratory tract symptoms. The background secretion of cytokines was measured in cultures in the absence of antigen.
According to our results, C. pneumoniae-specific CMI responses during acute C. pneumoniae infection appeared early after the disease symptoms and simultaneously with the humoral response. The strength of the proliferation did not correlate with the antibody responses, however, which is in accordance with immune responses during C. trachomatis infection (4, 12). Although the PBL responses differed widely in the patients, in spite of antimicrobial therapy, these responses appeared to be strong during the active stage of the infection compared to corresponding results for C. pneumoniae seronegative subjects included in this and an earlier series of ours (17) and with subjects having IgA antibodies to C. pneumoniae (9). Strong PBL reactivity to C. trachomatis antigens has been associated with spontaneous clearance of Chlamydia infection (2), suggesting a protective role for CMI in the development of trachoma.
The fact that the C. pneumoniae-induced PBL reactivity was significantly stronger than that to C. trachomatis antigen shows that CMI reactivity to C. pneumoniae is species specific during a primary infection (17), as was the case in healthy C. pneumoniae-immune responders (9). On the other hand, C. pneumoniae-specific CMI responses in patients with coronary heart disease have been shown to be predominantly induced by antigenic structures that are common among chlamydial species (8). It is difficult to know whether immune responses to chlamydial antigens are linked to the immunopathological mechanism that operates in C. pneumoniae-associated chronic diseases (19); in any case, the PBL cross-reactivity to chlamydial species that was typically found in male patients with coronary heart disease but not in female ones (8) is not a general phenomenon related to the male sex because all of the patients with species-specific PBL reactivity in this series were men.
According to our results, C. pneumoniae-induced T-cell activation seemed to be linked with CD8+ cells during the active stage of infection, since the difference between nonstimulated and C. pneumoniae-stimulated cells expressing the HLA-DR molecule was larger in CD8 than in CD4 cells. Alternatively, the nonsignificant difference in the activated CD4+ T cells between nonstimulated and stimulated cells early in the acute infection suggests that the CD4+ cells were activated in vivo and are thereby important for recovery from the infection.
IFN-γ has proved to be crucial in terms of the eradication of infection and providing immunity to experimental Chlamydia (14, 15). On the other hand, susceptibility and impaired host defence against Chlamydia has been shown to be a consequence of a cytokine production switch to prominence of IL-10 (20), an anti-inflammatory cytokine that attenuates CMI reactions that are needed for host defence against intracellular pathogens (5, 6). According to our results, IFN-γ production was clearly detectable after PBL stimulation with infectious C. pneumoniae EBs in all of the patients studied. IL-10 secretion was simultaneously detected as well, but never in the absence of IFN-γ secretion by the same cells, which argues against the immune response being immunosuppressive in these subjects. Although it is not possible to draw conclusions about the physiological significance of the cytokine concentrations, the presence of IL-10 in the Chlamydia-stimulated cell cultures may reflect a possibility that overproduction of IL-10 at some stages may inhibit the immune response and IFN-γ secretion upon C. pneumoniae stimulation.
In conclusion, we have shown that a primary C. pneumoniae infection induces the development of a positive PBL proliferative response to C. pneumoniae but not to C. trachomatis and also the secretion of IFN-γ in all subjects. C. pneumoniae-induced lymphocyte activation involves CD8+ T cells in the early phase of infection but CD4+ T cells in the later stage. However, in vivo activation of CD4+ cells during the acute disease may relate to their role in recovery from infection. No significant differences were observed in the immune responses among the subjects, although a larger population would be needed to determine whether any immunopathological markers of susceptibility to chronic C. pneumoniae infection (11, 16, 19) can be seen at the acute stage of infection or only with time or after repeated infections.
Acknowledgments
This work was supported by grants from the Academy of Finland and the Jalmari and Rauha Ahokas Foundation.
REFERENCES
- 1.Airenne S, Surcel H M, Alakarppa H, Laitinen K, Paavonen J, Saikku P, Laurila A. Chlamydia pneumoniae infection in human monocytes. Infect Immun. 1999;67:1445–1449. doi: 10.1128/iai.67.3.1445-1449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey R L, Holland M J, Whittle H C, Mabey D C. Subjects recovering from human ocular chlamydial infection have enhanced lymphoproliferative responses to chlamydial antigens compared with those of persistently diseased controls. Infect Immun. 1995;63:389–392. doi: 10.1128/iai.63.2.389-392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun J, Laitko S, Treharne J, Eggens U, Wu P, Distler A, Sieper J. Chlamydia pneumoniae—a new causative agent of reactive arthritis and undifferentiated oligoarthritis. Ann Rheum Dis. 1994;53:100–105. doi: 10.1136/ard.53.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunham R C, Martin D H, Kuo C C, Wang S P, Stevens C E, Hubbard T, Holmes K K. Cellular immune response during uncomplicated genital infection with Chlamydia trachomatis in humans. Infect Immun. 1981;34:98–104. doi: 10.1128/iai.34.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curfs J H, Meis J F, Hoogkamp-Korstanje J A. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daugelat S, Kaufmann S H. Role of Th1 and Th2 cells in bacterial infections. Chem Immunol. 1996;63:66–97. [PubMed] [Google Scholar]
- 7.Godzik K L, O'Brien E R, Wang S K, Kuo C C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halme S, Syrjala H, Bloigu A, Saikku P, Leinonen M, Airaksinen J, Surcel H M. Lymphocyte responses to Chlamydia antigens in patients with coronary heart disease. Eur Heart J. 1997;18:1095–1101. doi: 10.1093/oxfordjournals.eurheartj.a015403. [DOI] [PubMed] [Google Scholar]
- 9.Halme S, von Hertzen L, Bloigu A, Kaprio J, Koskenvuo M, Leinonen M, Saikku P, Surcel H M. Chlamydia pneumoniae-specific cell-mediated and humoral immunity in healthy people. Scand J Immunol. 1998;47:517–520. doi: 10.1046/j.1365-3083.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaukoranta-Tolvanen S-S E, Teppo A-M, Laitinen K, Saikku P, Linnavuori K, Leinonen M. Growth of Chlamydia pneumoniae in cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb Pathog. 1996;21:215–221. doi: 10.1006/mpat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 11.Kuo C C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabey D C W, Holland M J, Viswalingam N D, Goh B T, Estreich S. Lymphocyte proliferation responses to chlamydial antigens in human chlamydial eye infections. Clin Exp Immunol. 1991;86:37–42. doi: 10.1111/j.1365-2249.1991.tb05770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moazed T C, Kuo C C, Grayston J T, Campbell L A. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J Infect Dis. 1998;177:1322–1325. doi: 10.1086/515280. [DOI] [PubMed] [Google Scholar]
- 14.Rank R G. Models of immunity. In: Stephens R S, editor. Chlamydia: intracellular biology, pathogenesis and immunity. Washington, D.C.: American Society for Microbiology; 1999. pp. 239–295. [Google Scholar]
- 15.Rottenberg M E, Gigliotti Rothfuchs A C, Gigliotti D, Svanholm C, Bandholtz L, Wigzell H. Role of innate and adaptive immunity in the outcome of primary infection with Chlamydia pneumoniae, as analyzed in genetically modified mice. J Immunol. 1999;162:2829–2836. [PubMed] [Google Scholar]
- 16.Saikku P, Leinonen M, Mattila K, Ekman M-R, Nieminen M S, Mäkelä P H, Huttunen J K, Valtonen V. Serological evidence of association of novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–984. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 17.Surcel H M, Syrjala H, Leinonen M, Saikku P, Herva E. Cell-mediated immunity to Chlamydia pneumoniae measured as lymphocyte blast transformation in vitro. Infect Immun. 1993;61:2196–2199. doi: 10.1128/iai.61.5.2196-2199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S-P, Grayston J T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970;70:367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]
- 19.Ward M E. Mechanisms of Chlamydia-induced disease. In: Stephens R S, editor. Chlamydia: intracellular biology, pathogenesis and immunity. Washington, D.C.: American Society for Microbiology; 1999. pp. 171–209. [Google Scholar]
- 20.Yang X, Gartner J, Zhu L, Wang S, Brunham R C. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J Immunol. 1999;162:1010–1017. [PubMed] [Google Scholar]
- 21.Yang X, HayGlass K T, Brunham R C. Genetically determined differences in IL-10 and IFN-γ responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–4344. [PubMed] [Google Scholar]