Abstract
The Collaborative Pediatric Critical Care Resesarch Network (CPCCRN) was established by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) in May 2005 to develop an infrastructure for collaborative clinical trials and meaningful descriptive studies in pediatric critical care. This article describes the history of CPCCRN, discusses its financial and organizational structure, illustrates how funds were efficiently used to carry out studies, describes CPCCRN public use datasets and future directions, concluding with the development of the PeRsonalizEd Immunomodulation in PediatriC SepsIS-InducEd MODS (PRECISE) study.
Keywords: research networks, randomize clinical trials, pediatric critical care, children, methodology
In May 2005, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established the Collaborative Pediatric Critical Care Research Network (CPCCRN) with six clinical centers and an independent data coordinating center (DCC). CPCCRN was renewed in 2009 with seven clinical centers (two new) and in 2014 with seven clinical centers (three new). During the first three cycles, CPCCRN investigators successfully competed for nine R01 awards to leverage existing network protocol funds and increase the productivity of CPCCRN, implementing 36 studies resulting in 119 peer reviewed publications. Anticipated competitive renewal in 2019 was delayed with a one year level funding supplement, and in 2020 the current CPCCRN was funded with 12 clinical centers, 12 ancillary sites, and the DCC.
As the Principal Investigator of the DCC since inception of the network, I have had the opportunity to assist the highly talented investigators and research coordinators as they conceived and implemented our studies. In this Special Article for Pediatric Critical Care Medicine, I offer my personal observations about the history and evolution of CPCCRN to its present status, concluding with the development of the PeRsonalizEd Immunomodulation in PediatriC SepsIS-InducEd MODS study (PRECISE).
Organization of the Network
CPCCRN has been funded with individual grants or cooperative agreements to the clinical centers and the DCC; in the first three cycles, funds to support CPCCRN studies were set aside before specific projects were developed and implemented. The network Steering Committee was composed of investigators funded via NIH peer review, and was tasked with responsibility to identify project priorities, develop and conduct the specific studies with scientific input from a NICHD Project Scientist, and efficiently disseminate study results. NICHD appointed an independent chair for the Steering Committee (Douglas F. Willson, M.D. and Allan Doctor, M.D. in the first two cycles, and subsequently Daniel Notterman, M.D.). While the network initially organized subcommittees for various purposes (ethics, research protection, protocol development, capitation financing, etc.), the Steering Committee was so small during the first three cycles that its members served on nearly every subcommittee; thus, the Steering Committee decided to directly conduct all aspects of network business. In the current cycle, there are 12 clinical centers and 12 ancillary sites, totaling 24 investigators on the Steering Committee, and subcommittees have been established and are functioning as would be expected. The current CPCCRN organizational chart is shown in Figure 1.
Figure 1.
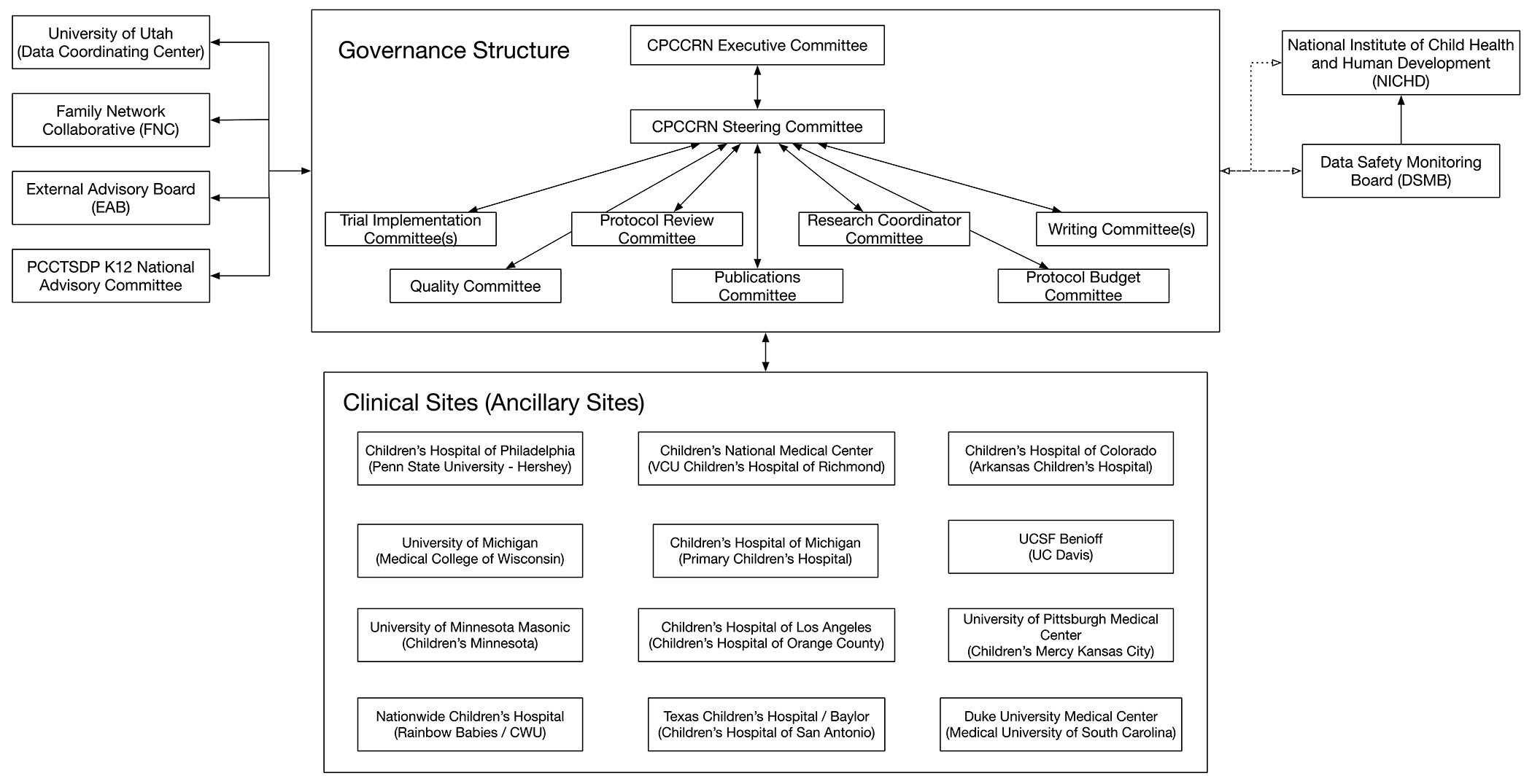
CPCCRN Organizational Chart
In addition to internal subcommittees, CPCCRN interacts with its Family Network Collaborative (FNC) [1], enabling families who have experienced PICU interactions to provide input into the scientific agenda, as well as to provide advice on approaching parents, describing projects, and writing consent documents. The network also formally interacts with the Pediatric Critical Care and Trauma Scientist Development Program (PCCTSDP) [2], with the goal of presenting CPCCRN research to young Scholars in the program, and inviting Scholars to attend CPCCRN meetings to get feedback about their own research.
In the 2020 competitive renewal, NICHD required a single PL1 application to be submitted, including the DCC, clinical and ancillary sites, and one or two large clinical trials for implementation. The mechanism of funding was changed from a cooperative agreement to a grant mechanism, and direct scientific input from NICHD was discontinued. The main purpose of this reorganization was to assure study section review prior to NICHD funding of specific CPCCRN trials, rather than providing generic funds for yet-to-be-developed delayed-onset studies. The PL1 budget was then disaggregated to award individual RL1 grants to the clinical centers, while the DCC remained on the PL1, which includes protocol funds to conduct PRECISE.
Development and Implementation of Studies
During the first six months of CPCCRN, the Steering Committee was required to meet six times in Washington, D.C., with the task of quickly developing study concepts that could be rapidly implemented. Brainstorming was used to identify initial ideas for projects, and the network used a structured approach to subsequent development, iterating from concept proposal to mini-protocol to full protocol, with regular Steering Committee presentations and approvals [3]. Figure 2 shows the current process, which includes the additional steps of NIH grant submission, peer review, and NIH funding decisions. This development process remains in place so that investigators, whether or not they are currently funded by CPCCRN grants, can leverage the network infrastructure to conduct additional studies unrelated to the PRECISE trial that was included in our PL1 application. These additional studies will require competitive submission for NIH funding via usual mechanisms.
Figure 2.
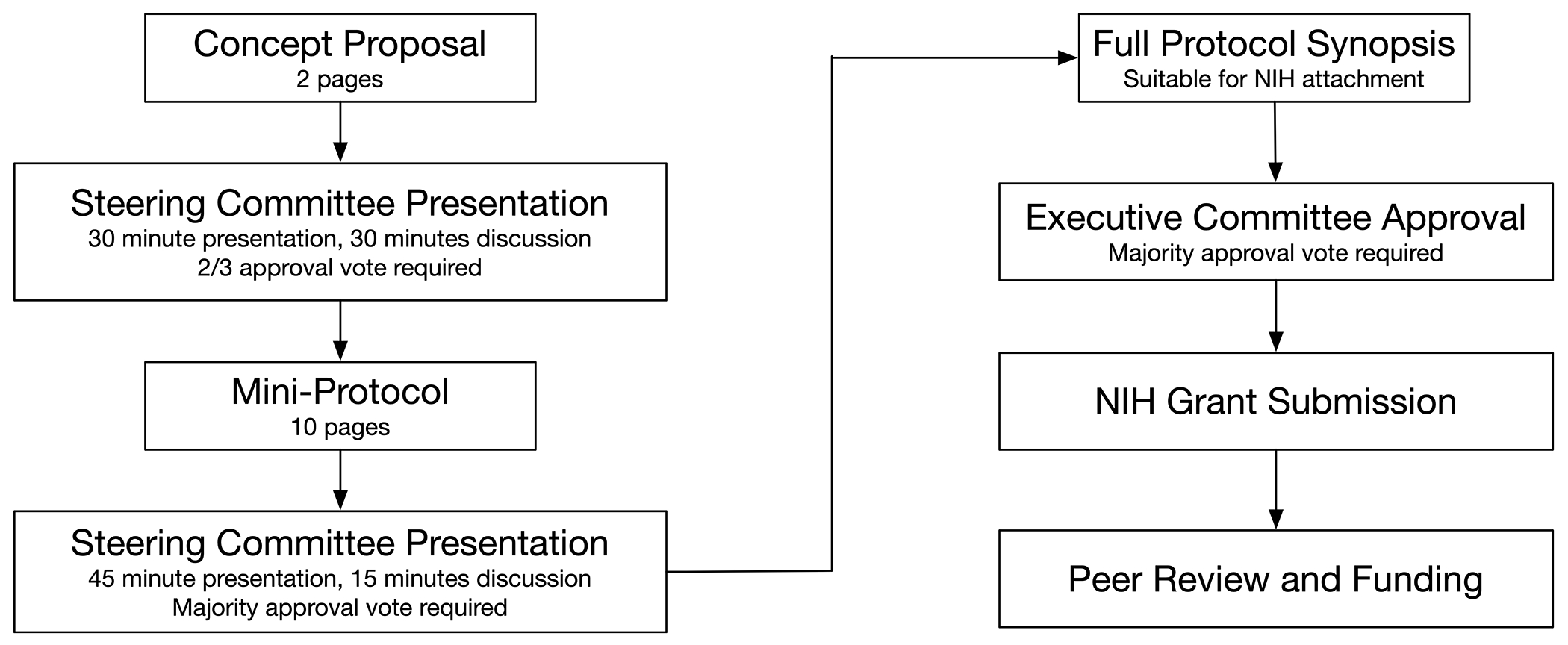
CPCCRN Protocol Development Process
As a nascent network, the investigators and NICHD Project Scientist recognized that lack of productivity could negatively impact future funding of the network. Thus, we placed early emphasis on studies that could be immediately implemented. Figure 3 shows the CPCCRN projects that were implemented through 2019. Many of these projects were very focused and were completed rapidly. While these shorter projects were being implemented by our research coordinators, planning proceeded in parallel for more complex studies such as the CRISIS Prevention Trial [4, 5] and the THAPCA trials [6, 7].
Figure 3.
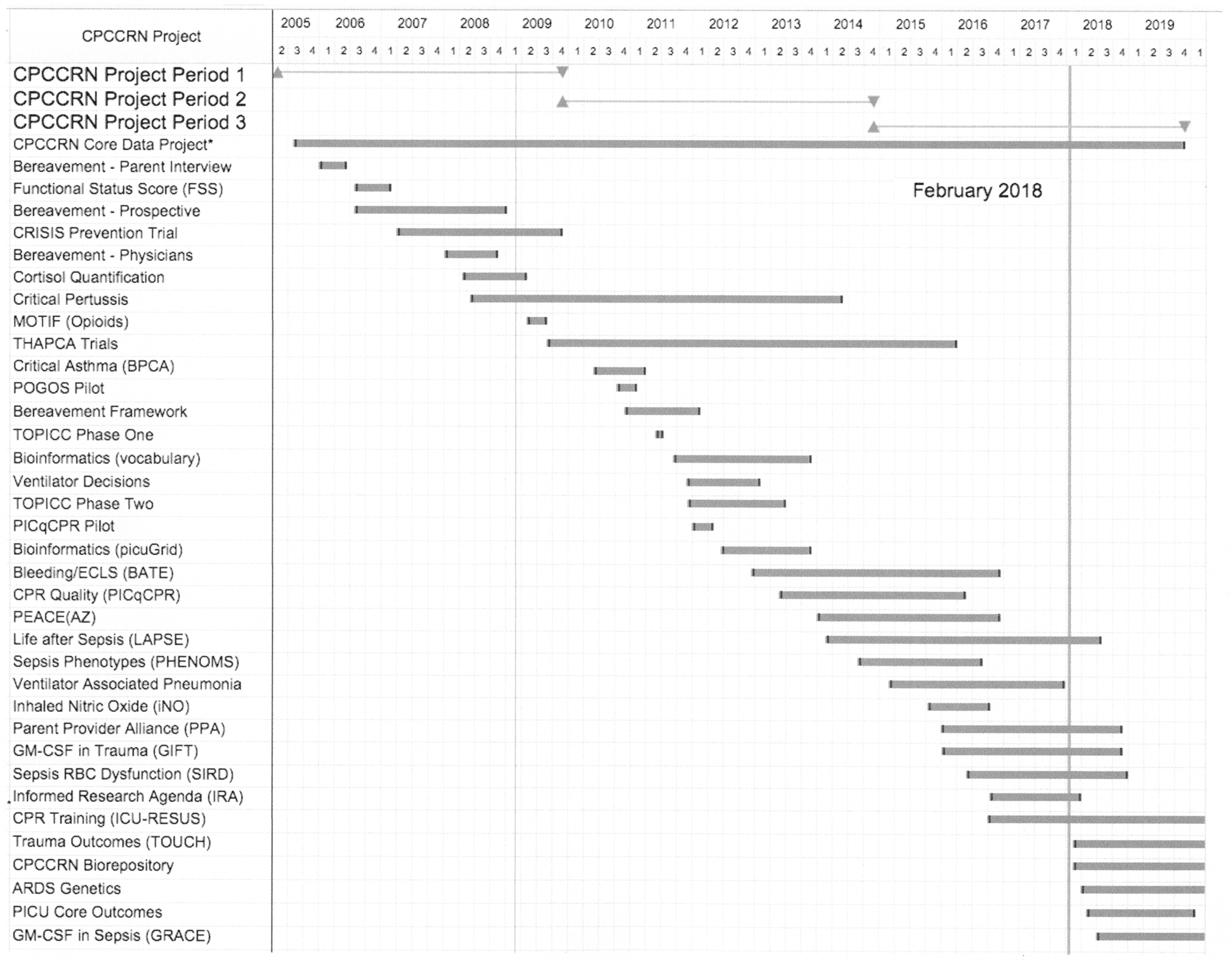
Timeline of CPCCRN Project Implementation 2005 - 2019
There are several themes of research during this period, including parent bereavement following the death of a child in the pediatric intensive care unit (PICU), definitions of meaningful outcomes following critical illnesses, technologies such as ventilators, nitric oxide, and ECMO, cardiac arrest and cardiopulmonary resuscitation, and finally, sepsis and sepsis-induced MODS. The evolution of sepsis-related studies is discussed later in this article when I discuss the development of the current PRECISE study.
Impact of Competitive Funding Mechanism
CPCCRN is a competitively funded research network, which is quite different from research networks such as the UK Paediatric Critical Care Society Study Group (UK PCCS-SG), the Canadian Critical Care Trials Group (CCCTG) which includes the pediatric interest group, and the Pediatric Acute Lung Injury & Sepsis Investigators (PALISI). These networks are freely open to participation at regular meetings, providing input for new projects and enabling voluntary coalitions of investigators to develop proposals for external funding, as well as for institutions to decide whether to enroll subjects in specific projects. This flexibility is advantageous because investigator participation in the network is not dependent on competitive renewal. While individual project grant proposals may or not be funded, investigators are able to maintain continuous voluntary participation in these networks. Complex studies and trials require successful grant funding, and site participation in those studies is not dependent on governance changes inside the underlying networks.
CPCCRN (and other traditionally funded NIH networks) are not freely open to meeting participation by all interested investigators. While non-CPCCRN investigators have been, and remain, welcome to request the opportunity to be invited to attend Steering Committee meetings to present their own research concepts for potential implementation by CPCCRN, the Steering Committee is entirely composed by investigators who remain funded as part of the network. Two CPCCRN clinical sites were replaced at the end of the first cycle, and three sites were replaced at the end of the second cycle. Competitive replacement causes significant disruption in four ways. First, network productivity may lag toward the end of a cycle, as investigators prepare their competitive applications to remain in the network. Second, replaced sites that are actively enrolling in existing studies lose their infrastructure, hobbling subsequent study enrollment. Third, integrating new sites into existing studies is difficult because the new investigators did not help develop existing studies. Fourth, the scientific agenda of the Steering Committee changes when investigators are replaced by new investigators, potentially wasting efforts that may have already gone into planning new projects.
The CPCCRN structure has advantages as well. First, every site is required to participate in projects, so the available patient population is well defined. Second, the scientific agenda becomes relatively stable, enabling sequential planning of network projects. Third, the network has a single DCC for all projects, facilitating the protocol development process and reducing training costs for data management and network procedures. Fourth, of course, is that funds have been specifically set aside to cover the cost of the network research projects. This assures rapid implementation after a protocol has been developed.
The most important advantage of the CPCCRN structure may be that clinical centers were able to be trained to handle laboratory samples in a sophisticated manner. This was accomplished with delayed onset projects during the first three cycles, which would have had unlikely success in a formal study section review. The CPCCRN sites have extensive experience with immune phenotyping from earlier projects (PHENOMS, GRACE, and GIFT), satisfying the study section that we had the infrastructure and ability to carry out the rapid immune phenotyping required for the PRECISE study.
CPCCRN Research Datasets
The NIH has required resource sharing for all research grants and cooperative agreements exceeding $500,000 annual costs for many years, but methods of provision of public use research datasets has been relatively undefined. Since its inception, CPCCRN investigators have been committed to making their research datasets available to other investigators.
The DCC website (https://www.cpccrn.org) provides information about obtaining these datasets, and NICHD established its Data and Specimen Hub (DASH) several years ago. CPCCRN has 14 datasets available on DASH (https://dash.nichd.nih.gov), more than any other NICHD research network, in addition to the THAPCA datasets that are hosted on the National Heart, Lung and Blood Institute (NHLBI) Biologic Specimen and Data Repository (BioLINCC). These datasets are freely available to qualified investigators, including fellows and young faculty, and are greatly underutilized. Collaborations between networks can be enhanced as illustrated by ECMO studies being initiated by CPCCRN and PALISI investigators after analyses of the public use dataset from the Bleeding and Thrombosis During ECMO (BATE) [8] study.
Interactions between Research Networks
The Therapeutic Hypothermia after Pediatric Cardiac Arrest (THAPCA) trials were initially conceived by Frank Moler as an investigator in the Pediatric Emergency Care Applied Research Network (PECARN), and PECARN collaborated with CPCCRN in our application to NHLBI; having the same DCC was considered an important asset by NHLBI program staff. Subsequently, investigators from the UK PCCS-SG were added to the THAPCA project [6,7] and collaborated with a meta-analysis of two independent hypothermia trials [9]. The current on-going P-ICECAP trial (NCT05376267) includes sites and investigators from PALISI, CPCCRN, as well as the Canadian and UK trials groups. These trials have limited eligible patients, and collaborations and cooperation between networks are critical to adequate subject accrual. PALISI and CPCCRN investigators are collaborating on future ECMO projects, as previously mentioned. More recently, the PICU Core Outcomes project, initiated by Erika Fink and Aline Maddux, has led to two publications listing both networks as corporate authors in the byline [10, 11].
It is desirable to enhance interaction between pediatric critical care investigators and networks by formalizing leadership liaisons and integrating regular scientific research meetings. Pediatric critical care is a small specialty with limited numbers of patients who can participate in research. We should aspire to make the PICU a living research laboratory, making research available to many more critically ill children [12].
Development of the PRECISE Study
During the second and third cycles, CPCCRN demonstrated that there were distinct phenotypes of children with MODS [13–15]. These phenotypes included children with immunosupression as well as children with significant hyperinflammation. In the third cycle, two additional CPCCRN projects demonstrated that GM-CSF could successfully reduce immunosuppression after trauma (GIFT) and sepsis (GRACE). In these studies, clinical site staff conducted LPS stimulation and TNF-α production was measured within 24 hours at the Immune Surveillance Laboratory at The Abigail Wexner Research Institute at Nationwide Children’s Hospital (under the direction of Mark Hall), and immunosuppression was defined as failure to generate a significant TNF-α response. These studies did not evaluate whether GM-CSF would impact the ultimate outcome of the patient. The unique ability of CPCCRN sites and the Immune Surveillance Laboratory to be able to measure TNF-α production within 24 hours led to the PRECISE study, Personalized Immunomodulation in Pediatric Sepsis-Induced MODS.
In the PRECISE study, children with sepsis-induced MODS will be immunophenotyped into four distinct groups, as shown in Figure 4. The personalized results will be used to enroll eligible patients into treatment trials or observational cohorts. As shown in red font, the treatment trials will evaluate GM-CSF for immunosuppression (GRACE-2 trial), and Anakinra for hyperinflammation (TRIPS trial). Children who are not eligible for either treatment trial will be observed, and all children in the overall study will have longitudinal immunophenotyping and biomarker measurements. Thus, the overall PRECISE study integrates two treatment trials (GM-CSF for Reversal of Immunoparalysis in Pediatric Sepsis-induced MODS [GRACE-2] and Targeted Reversal of Inflammation in Pediatric Sepsis-induced MODS [TRIPS]).
Figure 4.
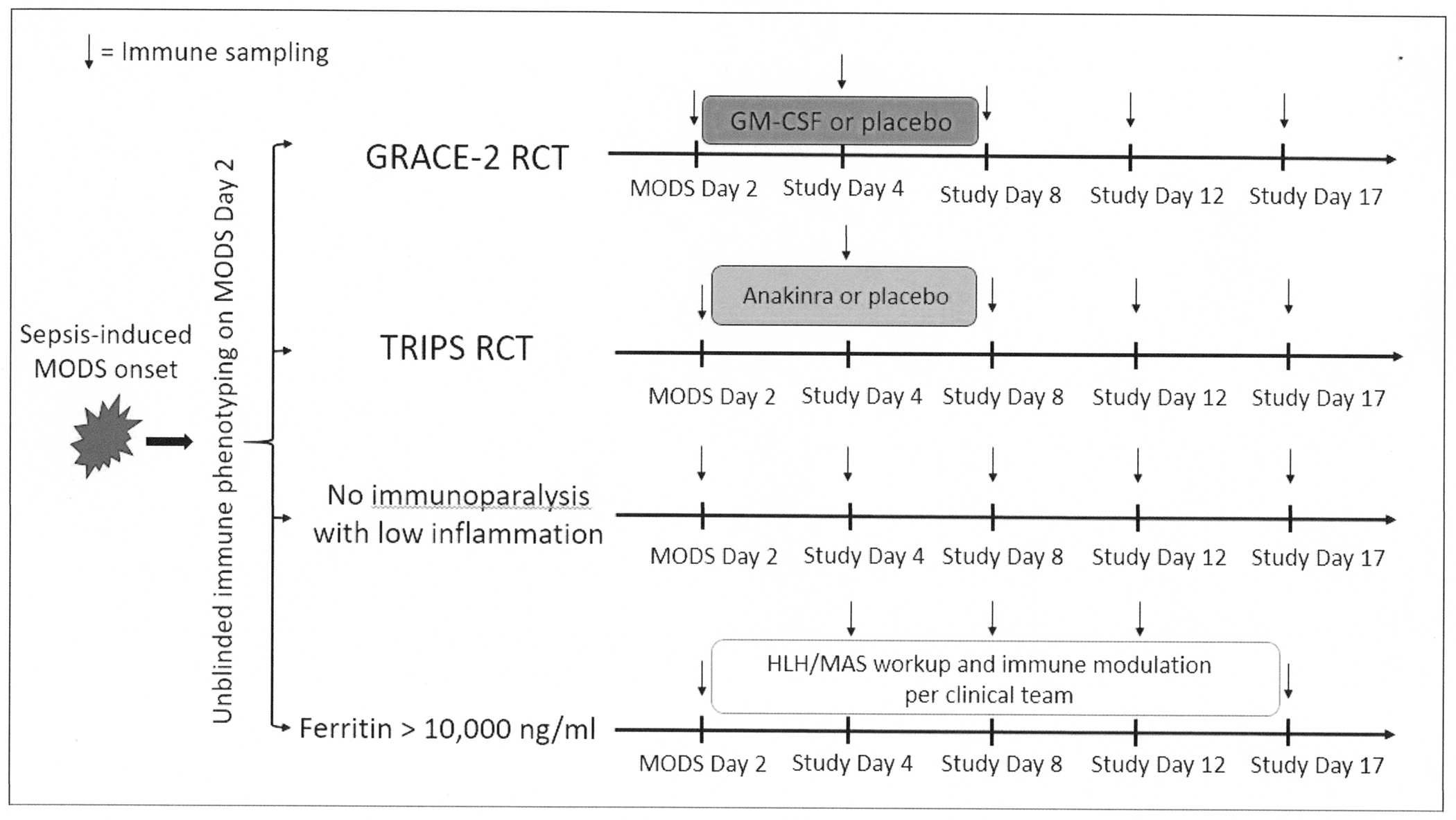
Laboratory immune phenotyping to identify subject study eligibility
Future Directions
Pediatric critical care research has matured significantly in the two decades since PALISI was organized in 2002, and multiple research networks have progressed from observational studies to complex randomized controlled trials. The CPCCRN investigators are eager to collaborate with other investigators and networks to further our universal goal of improving the outcomes for children suffering from critical illness. Investigators from any institution may contact CPCCRN via the DCC website contacts or via the NICHD program officer to express interest in presenting a study concept. During the first three cycles, CPCCRN invited over 40 non-CPCCRN investigators to present their concepts, and several of these collaborations led to RO1 funded projects that were completed by CPCCRN, with the original investigator as the study PI.
We face a shortage of future investigators in pediatric critical care, and we are eager to have young faculty and fellows attend CPCCRN meetings to present their own ideas or research activities, enabling them to receive feedback from the Steering Committee, and also allowing these young physician scientists to see some of the mechanics involved in multicenter research networks. This is not restricted to current CPCCRN institutions, and mentors may contact CPCCRN via the DCC or NICHD to identify opportunities for their trainees to take advantage of the CPCCRN infrastructure.
Acknowledgments
The accomplishments of CPCCRN are entirely the result of the hard work at each enrolling site by research coordinators and investigators, as well as the hard work by project managers, clinical data managers, biostatisticians, and administrative staff at the DCC. NICHD staff (Carol Nicholson, Tammara Jenkins, Robert Tamburro, Valerie Maholmes) also contributed heavily to CPCCRN successes. The sites that have participated in CPCCRN are listed below with the names of the investigators at each site.
Arkansas Children’s Hospital: K.S. Anand, Parthak Prodhan, Ronald Sanders, Olivia Irby, Peter Mourani; Children’s Hospital of Los Angeles: Christopher J.L. Newth, Robinder Khemani, Anoopindar Bhalla; Children’s Hospital of Philadelphia: Robert Berg, Athena Zuppa, Robert Sutton, Julie Fitzgerald, Scott Weiss; Children’s Hospital Orange County: Adam Schwarz; Children’s Wisconsin: Nathan Thompson, Prakad Rajapreyar; Children’s Minnesota: Alberto Orioles; Children’s Hospital Colorado: Peter Mourani, Todd Carpenter, Aline Maddux; Children’s Hospital of Michigan: Kathleen Meert, Sabrina Heidemann, Andrew Prout; Children’s Hospital of Pittsburgh: Joe Carcillo, Shekhar Venkataraman, Michael Bell, Ericka Fink; Children’s Hospital of Richmond/ VCU: Nikki Ferguson, Kim Tabor; Children’s Hospital of San Antonio: Mohammed Salameh, Kirstin Henley, Niveditha Balakumar; Children’s Mercy Kansas City: Yong Yun Han, Paul Bauer; Children’s National Hospital: Murray Pollack, Michael Bell, John Berger, Angela Wratney, David Wessel, Randall Burd, Bao Nguyen Puente; Duke University: Christoph Hornik, Alexandre Rotta, Chi Hornik; Hershey Medical Center: Neal Thomas, Robert Kavanagh; Medical University of South Carolina: John Costello, Elizabeth Mack, Elizabeth Zivick (Emrath), Austin Biggs, Elizabeth MackDiaz; Nationwide Children’s Hospital: Mark Hall, Andrew Yates; Phoenix Children’s Hospital: Murray Pollack, Heidi Dalton; Primary Children’s Hospital: Jill Sweney; Rainbow Babies and Children’s Hospital: Steve Shein, Katherine Slain, Kenneth Remy; Seattle Children’s Hospital: Jerry J. Zimmerman, Thomas Brogan, David Jardine; Texas Children’s Hospital: Ayse Arikan, Trung Nguyen; UC Davis Children’s Hospital: Moonjoo Han, Michelle Lim; UC Los Angeles: Rick Harrison, Anil Sapru; UC San Francisco: Patrick McQuillen, Anil Sapru, Natalie Cvijanovich, Matt Zinter; University of Michigan: Thomas Shanley, Frank Moler, Michael Quasney, Mary Dahmer, Erin Carlton; University of Minnesota Masonic: Marie Steiner, Gwenyth Fischer, Janet Hume, Julia Heneghan; University of Utah: J. Michael Dean, Richard Holubkov, Larry Cook, Charlie Casper, Katherine Sward, Ronald Reeder, John VanBuren, Kevin Watt
Grant Support:
CPCCRN investigators and sites have been continuously supported by grants and cooperative agreements from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD) since 2005. Dr. Dean is currently funded by PL1-HD105462. The views expressed in this paper are solely those of the author and do not necessarily represent the official views of NICHD, NIH, or DHHS..
Footnotes
Conflicts of Interest: No conflicts of interest to declare.
References
- [1].Tamburro R, Pawluszka A, Amey D, et al. The Family Network Collaborative: engaging families in pediatric critical care research. Pediatr Res. 2022. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Keenan HT, Albertine KH, Upperman JS, et al. The Pediatric Critical Care Trauma Scientist Development: Building a Community of Scientists for the Fields of Pediatric Critical Care and Trauma Surgery. Pediatr Crit Care Med. 2020. 07;21(7):672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Willson DF, Dean JM, Newth C, et al. Collaborative Pediatric Critical Care Research Network (CPCCRN). Pediatr Crit Care Med. 2006. Jul;7(4):301–7. [DOI] [PubMed] [Google Scholar]
- [4].Carcillo J, Holubkov R, Dean JM, et al. Rationale and Design of the Pediatric Critical Illness Stress-Induced Immune Suppression (CRISIS) Prevention Trial. JPEN J Parenter Enteral Nutr. 2009;33(4):368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carcillo JA, Dean JM, Holubkov R, et al. The Randomized Comparative Pediatric Critical Illness Stress-Induced Immune Suppression (CRISIS) Prevention Trial. Pediatr Crit Care Med. 2012. Mar;13(2):165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moler FW, Silverstein FS, Holubkov R, al. Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children. N Engl J Med. 2017. 01;376(4):318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015. May;372(20):1898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dalton HJ, Reeder R, Garcia-Filion P, et al. Factors Associated with Bleeding and Thrombosis in Children Receiving Extracorporeal Membrane Oxygenation. Am J Respir Crit Care Med. 2017. 09;196(6):762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Scholefield BR, Silverstein FS, Telford R, et al. Therapeutic hypothermia after paediatric cardiac arrest: Pooled randomized controlled trials. Resuscitation. 2018. 12;133:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fink EL, Maddux AB, Pinto N, et al. A Core Outcome Set for Pediatric Critical Care. Crit Care Med. 2020. 12;48(12):1819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fink EL, Jarvis JM, Maddux AB, et al. Development of a core outcome set for pediatric critical care outcomes research. Contemp Clin Trials. 2020. 04;91:105968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zimmerman JJ, Anand KJS, Meert KL, et al. Research as a Standard of Care in the PICU. Pediatr Crit Care Med. 2016. Jan;17(1):e13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carcillo JA, Berg RA, Wessel D, et al. A Multicenter Network Assessment of Three Inflammation Phenotypes in Pediatric Sepsis-Induced Multiple Organ Failure. Pediatr Crit Care Med. 2019. 12;20(12):1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Carcillo JA, Halstead ES, Hall MW, et al. Three Hypothetical Inflammation Pathobiology Phenotypes and Pediatric Sepsis-Induced Multiple Organ Failure Outcome. Pediatr Crit Care Med. 2017. 06;18(6):513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Carcillo JA, Sward K, Halstead ES, , et al. A Systemic Inflammation Mortality Risk Assessment Contingency Table for Severe Sepsis. Pediatr Crit Care Med. 2017. 02;18(2):143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


