Abstract
Objective
To report a case of a healthy, live birth resulting from a “chaotic” embryo (at least 6 chromosomal aneuploidies) after preimplantation genetic testing for aneuploidy (PGT-A).
Design
Case report.
Setting
University-affiliated fertility clinic.
Patient(s)
A same-sex couple with infertility due to failed donor intrauterine insemination and past implantation failure with in vitro fertilization (IVF)/intracytoplasmic sperm injection using donor sperm.
Intervention(s)
Frozen single embryo transfer of a “chaotic” embryo after genetic counseling and informed consent.
Main Outcome Measure(s)
Live birth of a healthy infant.
Result(s)
Controlled ovarian hyperstimulation and transvaginal oocyte retrieval in a 35-year-old female yielded 10 mature oocytes that underwent intracytoplasmic sperm injection with anonymous donor sperm and in vitro culture for 6 days. A single embryo underwent trophectoderm (TE) biopsy at the blastocyst stage and was cryopreserved. PGT-A revealed a “chaotic” test result. After genetic counseling and proper informed consent, a frozen single embryo transfer of this “chaotic” embryo resulted in a successful pregnancy and live birth of a healthy male infant.
Conclusion(s)
The reproductive potential of embryos with a “chaotic” TE biopsy result is unknown, but herein, we report a healthy, live birth from a “chaotic” embryo. We recommend that patients and providers faced with disposition decisions regarding “chaotic” embryos seek genetic counseling, consider rebiopsy, or consider transfer with informed consent.
Key Words: PGT-A, chaotic Embryo, aneuploidy, TE Biopsy, IVF
Preimplantation genetic testing for aneuploidy (PGT-A) is a widely used process in in vitro fertilization (IVF) that screens for chromosomal abnormalities in embryos before transfer to increase the likelihood of a successful pregnancy. In 2019, 43.8% of the embryo transfers involved at least one embryo that had undergone PGT, which therefore equates to nearly half of all transfers performed in the United States (1). Possible PGT-A results include: euploid, aneuploid, low-level mosaic, high-level mosaic, chaotic, noninformative, and no DNA detected. Ideally, an embryo with a chromosomally normal, euploid trophectoderm (TE) biopsy result is selected for transfer. However, if no embryos with euploid TE biopsy results are available for transfer, embryos with a mosaic TE biopsy result (those with a mix of euploid and aneuploid cells) are sometimes considered, provided that proper genetic counseling and informed consent are obtained. Embryos with aneuploid TE biopsy results are typically excluded from selection for transfer. Reference laboratories define a chaotic TE biopsy result as having 6 or more genetic abnormalities identified via PGT-A, and a chaotic result is further classified as aneuploid. Therefore, the conventional practice is to exclude “chaotic” embryos from transfer.
Several case reports and a recent cohort study have demonstrated that embryos with aneuploid TE biopsy results are capable of implanting and producing healthy, live births (2, 3, 4). However, the reproductive potential of embryos with chaotic TE biopsy results is currently unknown. Interestingly, a recent preliminary retrospective cohort study conducted in Spain by the reference laboratory Igenomix demonstrated that 40.5% of TE biopsies that were initially designated as “chaotic” were called "euploid" on rebiopsy (n = 37) (5). Additional unpublished data collected in the United States by Igenomix suggest that approximately 38% of TE biopsies that are initially found to be “chaotic” in their laboratory are called “euploid” on rebiopsy (6). These findings challenge the conventional practice of refraining from using embryos categorized as “chaotic” after PGT-A. To further support a re-evaluation of current practices regarding the disposition of “chaotic” embryos, here we present a case in which the transfer of an embryo with a chaotic TE biopsy result produced a pregnancy that resulted in a healthy, live birth.
Case report
A female same-sex couple with primary infertility because of a need for donor sperm elected to pursue IVF/intracytoplasmic sperm injection with PGT-A after several failed donor intrauterine inseminations. The 35-year-old egg source underwent controlled ovarian hyperstimulation with injectable gonadotropins followed by transvaginal oocyte retrieval. Ten mature oocytes were obtained and subjected to intracytoplasmic sperm injection with anonymous donor sperm. Eight oocytes fertilized normally and were cultured in vitro. Six blastocysts developed from this cohort of embryos, but 5 of these blastocysts were of poor quality. A single blastocyst underwent TE biopsy for PGT-A on day 6 of embryo culture and was subsequently cryopreserved. Next-generation sequencing revealed a genetically “chaotic” biopsy result, with a total of 6 aneuploidies, and predicted a male sex (Fig. 1). The couple was informed of the aneuploid PGT-A result and advised not to transfer this embryo, per conventional practice at our center. However, they strongly desired intrauterine transfer of the “chaotic” embryo, and they underwent genetic counseling and provided special informed consent to proceed. Frozen embryo transfer (FET) of this “chaotic” embryo resulted in a singleton intrauterine pregnancy with sustained fetal cardiac activity. First-trimester maternal cell-free DNA screening returned a low-risk result, as it was negative for aneuploidy in chromosomes 13, 18, and 21, and it again predicted the sex of the fetus to be male. Second-trimester amniocentesis confirmed a fetus with a 46XY karyotype. The outcome of the FET of a single “chaotic” embryo was a full-term live birth of a healthy, phenotypically male infant. The patient and partner agreed to share their unique family-building journey in a deidentified case report.
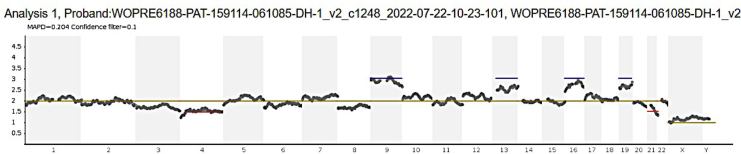
FIGURE 1.
Raw PGT-A data.
Next-generation sequencing of the trophectoderm biopsy submitted to a reference laboratory for preimplantation genetic testing for aneuploidy revealed a chaotic test result (≥ 6 chromosomal abnormalities) and male sex (XY). The 6 chromosomal abnormalities included 4 trisomies (+9, +13, +16, +19), designated by a blue line, and 2 mosaic monosomies (-4, -21), designated by a red line.
Discussion
This case challenges the assumption that embryos identified as “chaotic” through TE biopsy should be immediately excluded from transfer or discarded entirely. There are several reasons that re-evaluation of “chaotic” embryos may be warranted, stemming from the common misinterpretation of PGT-A results, emerging data regarding the rebiopsy of “chaotic” embryos, and the potential inaccuracy of traditional PGT-A results. As technological advancements in reproductive medicine have progressed, PGT has become a widely used tool in IVF. However, PGT-A is often being utilized incorrectly as a diagnostic test rather than its intended use as a screening test. The goal of PGT-A is to minimize the number of embryo transfers required to produce a healthy, live birth by determining which embryos are the most viable and have the highest likelihood of successful implantation. However, this does not mean that embryos with results considered less viable should be excluded from potential use, which is how the test is often currently implemented. Although studies of embryos with mosaicism and aneuploidy identified through PGT-A do suggest a lower success rate after FET (4, 7), PGT-A is not intended to explicitly identify any specific genetic disorders that would warrant an automatic exclusion of embryos from use. This differs from preimplantation genetic testing for monogenic disorders (PGT-M), which is a diagnostic test that identifies specific genetic abnormalities found in an embryo that may result in disease if used. This fundamental misinterpretation of the 2 testing modalities may have contributed to the current practice of excluding embryos that display aneuploidy in TE biopsies from transfer, especially those that are “chaotic,” because they have a greater number of chromosomal abnormalities identified and may be considered “least viable.”
As previously mentioned, emerging data from 2 different pilot studies currently support the re-evaluation of embryos initially called “chaotic” at the time of TE biopsy for PGT-A. One is a retrospective cohort study from the reference laboratory Igenomix, which involved reinvestigation of embryos that were previously identified as “chaotic” by PGT-A (5). In this study, they analyzed results from 381 TE re-biopsies from a total of 39,310 embryos created during PGT-A cycles that occurred at multiple IVF centers between January 2018 and January 2021(5). The average maternal ages (SD) at the time of oocyte retrieval were 38.5 (3.1), 37.4 (4.3), and 37.2 (3.4) for TE biopsies that were initially called “chaotic,” “no DNA detected,” and “noninformative,” respectively (5). Importantly, their results demonstrated that 40.5% of TE biopsies that were initially designated as "chaotic" were called "euploid" on rebiopsy, 46% of TE biopsies that were originally called "no DNA detected " were "euploid" at rebiopsy, and 71.2% of "noninformative" TE biopsies were called "euploid" on rebiopsy.(5) A similar study performed in the United States by Igenomix showed that 38% of embryos that were initially categorized as “chaotic” were called “euploid” on rebiopsy (6). Although the sample sizes in these studies were small, if these findings prove to be consistent with future studies utilizing larger sample sizes, this would suggest that over one-third of all routinely unused or discarded “chaotic” (or ostensibly chaotic) embryos are potentially viable, euploid embryos. In fact, Igenomix recently released a statement via e-mail suggesting that although “chaotic” TE biopsy results do pass quality control parameters in their laboratory and do demonstrate unambiguous aneuploidies, their pilot studies suggest that the predictive value of “chaotic” TE biopsy results may be reduced (8). They also encouraged IVF centers to consider rebiopsy of “chaotic” embryos if blastocyst quality permits (8). As such, these preliminary findings and the clinical statement from Igenomix warrant careful consideration, as the ramifications could lead to a drastic shift in our current view of embryos with chaotic TE biopsy results.
Given the uncertainty this case report lends to the decision not to use “chaotic” embryos for transfer, one must also consider the accuracy of traditional PGT-A results. Although TE biopsy is often used to predict embryo ploidy through PGT-A, the results may not be entirely reliable, as the biopsy is technically analyzing only a small portion of embryonic cells destined to become placental tissue rather than fetal tissue (9, 10, 11). In fact, there are confirmed cases of confined placental mosaicism and studies demonstrating occasional discordance between aneuploid TE biopsy results and genetic sequencing results of the whole embryo, suggesting that using trophectoderm alone to predict whole embryo ploidy may not always be ideal (10, 11, 12, 13). Furthermore, we currently lack a full understanding of the embryo’s ability for repair and self-correction of genetic abnormalities as it develops into a fetus, and it is quite possible that mosaic embryos, “chaotic” embryos, or even aneuploid embryos, in general, are capable of an ample degree of self-repair that allows for viability (10, 14). As mentioned before, there are numerous instances of healthy live births after the transfer of embryos deemed to be mosaic or aneuploid via traditional PGT-A (2, 3, 4, 7). These cases demonstrate that misidentification of embryonic ploidy can certainly occur after a single TE biopsy. They further suggest that the traditional dichotomous view of biopsied embryos as being either “normal/euploid” or “abnormal/aneuploid” based on genetic testing of a few trophectoderm cells may be overly simplified and sometimes even inaccurate, especially given our knowledge that approximately 30% of embryos display some degree of mosaicism at the blastocyst stage in vitro (10, 11).
Noninvasive PGT-A (niPGT-A) bypasses this potential complication by analyzing cell-free DNA originating from the entire embryo found in spent embryo culture media rather than just DNA from a portion of the embryo’s trophectoderm (11). Although the potential for maternal DNA contamination from cumulus cells does exist, a recent study asserted that niPGT-A has the potential to be more accurate than TE biopsy (traditional PGT-A) at predicting embryo ploidy (11). Another potential benefit of niPGT-A is that it may be a better embryonic screening and transfer selection tool because its report provides a euploidy score, which ranks embryos’ fitness for transfer rather than excluding them from transfer completely by definitively “diagnosing” them as aneuploid. Additionally, niPGT-A poses less risk of physical damage to the embryo than a traditional TE biopsy, which can be quite invasive, and it should prove to be less labor-intensive for busy embryology laboratories. Whereas it remains to be determined if the niPGT-A is definitively superior to the TE biopsy, in light of this case report and the Igenomix pilot data regarding “chaotic” embryos, one could argue that the euploidy score may be a more useful way to analyze embryonic viability than the traditional dichotomous view of euploid versus aneuploid.
Finally, we acknowledge our limitation of being unable to prove that the healthy male infant in our case report is, in fact, derived from the transferred “chaotic” male embryo. Unfortunately, the reference laboratory that provided our chaotic PGT-A result was unable to perform DNA fingerprinting of the resulting infant and match it to that of the “chaotic” embryo owing to the legal inability to store the TE biopsy for a clinically appropriate time frame. Thus, the parents decided to forego genetic testing of their infant after birth. However, we are confident that this healthy infant did result from the transferred “chaotic” embryo. Spontaneous conception around the time of FET in a monogamous same-sex couple is highly unlikely. First-trimester ultrasound is known to be the most accurate modality for dating a pregnancy (15), and this couple’s initial viability scan revealed a crown-rump length that was expected for gestational age, based on the embryo transfer date. Furthermore, the sex of the embryo, fetus, and infant was consistent, which also instills confidence that the male infant in our case report did, in fact, result from FET of a “chaotic” male embryo.
Conclusion
Embryos found to be “chaotic” (having 6 or more genetic abnormalities) through traditional PGT-A are conventionally excluded from transfer and may even be immediately discarded because they are considered to be aneuploid. This report describes a case where the transfer of an embryo with a genetically “chaotic” TE biopsy result led to a successful pregnancy and a healthy live birth. In addition, 2 preliminary studies by the reference laboratory Igenomix have shown that when embryos initially identified as “chaotic” through Next-generation sequencing were rebiopsied, approximately 38 - 40.5% were found to be euploid(5, 6). In conclusion, patients and clinicians should be aware that embryos determined to be “chaotic” through PGT-A have the potential to become healthy infants, and patients who receive “chaotic” TE biopsy results should consider rebiopsy and/or potential transfer of these embryos after obtaining genetic counseling and proper informed consent.
Acknowledgments
We would like to thank our patient, DH, for allowing us to share her story with the scientific community, Deb Werzinger, P.A., for gathering antenatal test results from outside facilities, Dr. Alyssa Snider and Dr. Reyfik Kayali at Igenomix for their consultation and genetic insight, and the IVF laboratory team at Strong Fertility Center for their tireless work and dedication to compassionate care of the infertile patient.
Footnotes
J.L. has nothing to disclose. W.V. has nothing to disclose. E.L.S. has nothing to disclose.
References
- 1.CDC Asisted Reproductive Technology (ART)Data. https://nccd.cdc.gov/drh_art/rdPage.aspx?rdReport=DRH_ART.ClinicInfo&ClinicId=9999&ShowNational=1 Accessed.
- 2.Gleicher N., Vidali A., Braverman J., Kushnir V.A., Albertini D.F., Barad D.H. Further evidence against use of PGS in poor prognosis patients: report of normal births after transfer of embryos reported as aneuploid. Fertil Steril. 2015;104:e59. [Google Scholar]
- 3.Darilek S., Nassef S., Huguenard S. Pregnancy outcome following transfer of an aneuploid embryo. Am J Obstet Gynecol. 2018;218:S170. [Google Scholar]
- 4.Barad D.H., Albertini D.F., Molinari E., Gleicher N. IVF outcomes of embryos with abnormal PGT-A biopsy previously refused transfer: a prospective cohort study. Hum Reprod. 2022;37:1194–1206. doi: 10.1093/humrep/deac063. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigo L, Campus Galindo I, Polo Picasso A, Castello Salom D, Lopez de Carvajal LM, Simon Valles C. Embryo rebiopsy as a rescue tool in PGT-A Cycles. 2021 ASEBIR Conference. Toledo, Spain.
- 6.Unpublished data from Igenomix (Accepted as abstract at ASRM 2022) Personal Communication; 2022. [Google Scholar]
- 7.Zhang Y.X., Chen J.J., Nabu S., Yeung Q.S., Tan Y.L., Suksalak W., et al. The pregnancy outcome of mosaic embryo transfer: a prospective multicenter study and meta-analysis. Genes (Basel) 2020;11:973. doi: 10.3390/genes11090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snider A., Kayali R. Clinical interpretation and management of chaotic embryos. Email received, Aug 12, 2022. 2022 Aug 12 https://view.content.igenomix.com/?qs=6a767ac09a1f4f724924c360f7ff95a2378a37f8c9567f0c8ec3248bbb5634898b9d1b8d6ac8f98830e02681c9d63b2be43de48d3ac9449fa50208c0a236d3cba2eca50f41403391670e64461c8ecc26 [Google Scholar]
- 9.Gleicher N., Vidali A., Braverman J., Kushnur V.A., Barad D.F., Hudson C., et al. Accuracy of preimplantation genetic screening (PGS) is compromised by degree of mosaicism of human embryos. Reprod Biol and Endocrinol. 2016;14:54. doi: 10.1186/s12958-016-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esfandiari N., Bunnell M.E., Casper R.F. Human embryo mosaicism: did we drop the ball on chromosomal testing? J Assist Reprod Genet. 2016;33:1439–1444. doi: 10.1007/s10815-016-0797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L., Bogale B., Tang Y., Lu S., Xie X.S., Racowsky C. Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy. Proc Natl Acad Sci U S A. 2019;116:14105–14112. doi: 10.1073/pnas.1907472116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalousek D.K., Dill F.J. Chromosomal mosaicism confined to the placenta in human conception. Science. 1983;221:665–667. doi: 10.1126/science.6867735. [DOI] [PubMed] [Google Scholar]
- 13.Victor A.R., Griffin D.K., Brake A.J., Tyndall J.C., Murphy A.E., LepkowskiLT, et al. Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Hum Reprod. 2019;34:181–192. doi: 10.1093/humrep/dey327. [DOI] [PubMed] [Google Scholar]
- 14.Barzrgar M., Gourabi H., Valojerdi M.R., Yazdi P.E., Beharvand H. Self-correction of chromosomal abnormalities in human preimplantation embryos and embryonic stem cells. Stem Cells Dev. 2013;22:2449–2456. doi: 10.1089/scd.2013.0053. [DOI] [PubMed] [Google Scholar]
- 15.Salomon L.J., Alfirevic Z., Da Silva C.F., Deter R.L., Figueras F., Ghi T., et al. ISUOG practice guidelines: ultrasound assessment of fetal biometry and growth. Ultrasound Obstet Gynecol. 2019;53:715–723. doi: 10.1002/uog.20272. [DOI] [PubMed] [Google Scholar]