Summary
Infection initiates sepsis, but the clinical disease arises through the innate immune response of the host. A rapidly evolving understanding of the biology of that response has not been paralleled by the development of successful new treatment. The COVID-19 pandemic has begun to change this revealing the promise of distinct therapeutic approaches and the feasibility of new approaches to evaluate them. We review the history of mediator-targeted therapy for sepsis and explore the conceptual, biological, technological, and organizational challenges that must be addressed to enable the development of effective treatments for a leading cause of global morbidity and mortality.
Keywords: Sepsis, Innate immunity, Biomarkers, Clinical trials
Introduction
The word “sepsis” has its medical origins in the teachings of Hippocrates almost two and one half millennia in the past. Its contemporary use reflects a still evolving understanding of the complexities of the interactions between multicellular organisms and the microbial world.
To Hippocrates, sepsis was a process through which living organisms died. It was associated with putrefaction, disease, and a bad smell, and contrasted with the process of pepsis, exemplified in the digestion of food or the fermentation of grapes to produce wine.1 The word ‘sepsis’ appears sparingly in medical writings before the twentieth century, but when it does, it denotes rot, putrefaction, and decomposition, and generally in the context of a local process, such as a suppurating wound. With the recognition that these processes arose through the activity of living microorganisms, the word sepsis became synonymous with severe infection. The 1972 version of Stedman's Medical Dictionary defined sepsis as “the presence of pus-forming organisms in the bloodstream”.2
Over the last half century, multiple lines of research have shown that clinical sepsis arises indirectly through the response of the host to infection, rather than as a direct consequence of the action of the microorganism on host cells. This insight has created new therapeutic opportunities, though these come with challenges that have as yet to be met.3 Our viewpoint provides a perspective on these challenges in four broad areas– biological, conceptual, operational, and organizational.
The biologic challenge
Host-microbial interactions are enormously complex. The microbial world is diverse, abundant, and essential to the normal growth and development of eukaryotes. Disruptions in normal lost–microbial interactions – most obviously invasive infection - are common and have been a powerful force driving our evolution.
Infection describes the invasion of normally sterile host tissues by microorganisms. Sepsis, in contrast, is the systemic process that arises through the host response to infection, and results in physiologic organ system dysfunction.4,5 Differentiating these two pathologic mechanisms provided a biologic rationale for treating life-threatening infection by targeting both the invading microorganism and the deleterious consequences of the host response.6
Healthy multicellular organisms, including humans, exist in a largely symbiotic relationship with the microbial world. A complex microbial flora colonises healthy epithelial surfaces, comprising the organism's microbiome, and plays a vital role in homeostasis supporting immunity, metabolism, angiogenesis, and even neurologic function.7 This flora varies from person to person, and can be modified by influences, such as disease, diet, and antibiotic therapy.8 Infection develops when viable microorganisms, either components of the endogenous flora or pathogens in the external environment, breach these epithelial surfaces, and gain access to the interior milieu of the host. Low level transient microbial invasion of the host is likely a common phenomenon, and is thought to play a role in training the gut epithelial immune system.9
Larger inocula can evoke a host immune response of variable nature and severity. Conserved molecular patterns in bacteria, fungi, and viruses are recognized by germline-encoded receptors. Toll-like receptors (TLRs), of which there are ten in humans,10 are the best studied of these. TLR-ligand binding can have dramatic consequences: injection of a single dose of endotoxin into a healthy human volunteer results in the altered transcription of thousands of genes, the majority of which are downregulated.11 Cellular metabolism is fundamentally altered, and the transcribed gene products, in turn, lead to the further release of mediator molecules, activation of the coagulation cascade, and to the stimulation of innate and adaptive immunity, replicating the core features of clinical sepsis.12 Endotoxin challenge has been used extensively as a model for sepsis, and much of what is known about the host response comes from these studies, although murine endotoxemia is a poor model for the complexities of human sepsis. The lethality of endotoxin challenge in the mouse can be attenuated by manipulating any of more than 200 different molecular species,13 none of which has shown unequivocal efficacy in human sepsis.
Host-microbial interactions are further complicated by the variability in both microbial species and host cellular, molecular, and epigenetic regulatory mechanisms. The host microbiome is disrupted. Genetic variability emerging in response to prior infectious disease exposure introduces further biologic heterogeneity. Finally, the treatment context – co-morbidities in the host, exogenous iatrogenic interventions, the capacity of the health care system to respond, and extant views on whom to treat and when – adds to the multidimensional heterogeneity that characterizes the contemporary syndrome of human sepsis. Efforts to modify this response of the host have been consistently disappointing.
Corticosteroids
The role of corticosteroids for patients with sepsis has been an active area of research and controversy, however consistent themes are emerging, largely as a consequence of insights during the COVID-19 pandemic. Meta-analyses suggest that low dose corticosteroids may be beneficial, being associated with lower in-hospital mortality, faster resolution of shock, and reduced organ dysfunction.14 There seems to be heterogeneity in the treatment effect, with the possibility of harm in septic patients with an immunocompetent phenotype (defined on the basis of a transcriptomic signature) who are treated with vasopressors and hydrocortisone.15 Corticosteroids offer a survival benefit in patients with severe COVID-1916 and possibly in non-COVID ARDS.17
Anti-endotoxin strategies
Multiple approaches have been evaluated to disrupt the initial interaction between endotoxin and innate immune cells. Early suggestions of efficacy18 were not replicated in subsequent trials, and currently there are no approved pharmacologic interventions targeting endotoxemia. Heterogeneity of treatment effect is also apparent in trials of anti-endotoxin treatments. Increased mortality has been demonstrated among patients with Gram positive infections has been seen in several trials of anti-endotoxin therapies.19,20 Moreover, the physiologic criteria used to recruit patients to sepsis trials are poor predictors of endotoxemia,21 and endotoxemia is often present in other acute conditions including multiple trauma22 and following cardiopulmonary bypass.23
Tumour Necrosis Factor (TNF) as a therapeutic target
The first endogenous mediator associated with sepsis,24 TNF has also been one of the best studied. Pooled data from 17 trials of anti-TNF strategies showed a modest reduction in mortality, however anti-TNF therapies are not currently used in the treatment of sepsis. Several recurring themes are evident. TNF levels in sepsis ranging from seven to more than 57,000 pg/m in one trial. Anti-TNF therapies have become standards of care for a variety of autoimmune inflammatory diseases including arthritis and inflammatory bowel disease.25
Other pro-inflammatory cytokines
Interleukin-1 has in vivo inflammatory effects similar to those of TNF.26 Its cellular release is accompanied by the synthesis and release of an endogenous antagonist, the interleukin-1 receptor antagonist (IL-1ra). Pooled data from three trials evaluating IL-1ra show a modest survival benefit with therapy.13 Secondary analysis of these trials suggested that a subgroup of patients with features of Macrophage Activation Syndrome were most likely to benefit.27 IL-1ra has also been studied in patients with COVID-19. Although there is no compelling evidence of benefit, it may confer a survival benefit for patients with a hyperinflammatory state, reflected in elevated plasma levels of soluble urokinase plasminogen activator receptor (SUPAR).28 Similar to anti-TNF therapies, anakinra has found a role in the treatment of autoimmune conditions such as rheumatoid arthritis29 and Familial Mediterranean Fever.
Monoclonal antibodies directed against the IL-6 receptor – tocilizumab and sarilumab – have not been evaluated for the treatment of sepsis, but improve survival in patients with severe COVID-19,30,31 and are used to treat rheumatoid arthritis.32
Anticoagulant strategies
Induction of the coagulation cascade typically accompanies the activation of an inflammatory response. Recombinant activated protein C33 was briefly licensed for clinical use in sepsis, however a subsequent trial failed to replicate the findings of the original study,34 and the drug was removed from the market. Other recombinant anticoagulant proteins including tissue factor pathway inhibitor,35 antithrombin36, and soluble thrombomodulin37 similarly failed to provide robust evidence of a survival benefit in clinical trials.
Anticoagulant approaches may benefit patients with COVID-19, based on work showing benefit for heparin in patients with moderately severe disease38; ASA or P2Y12 inhibitors may also reduce mortality risk for patients with severe COVID-19.39
Targeting individual inflammatory mediators has not proven efficacious in sepsis. Whether this primarily reflects patient heterogeneity or biologic redundancy is unclear. If the latter proves to be the case, inhibitors of signal transduction pathways that alter the expression of multiple genes may offer a more rational approach. Jak2 inhibitors have proven effective in COVID-1940,41 as well as in rheumatoid arthritis.42
The conceptual challenge
Despite multiple efforts to define sepsis, there has been a persistent gulf between the concept of sepsis, and the clinical reality of the disorder that clinicians manage. Although sepsis was thought to be indicative of bloodborne infection, it became apparent that bacteraemia was not an invariant, nor even a common finding in patients with clinical sepsis,43 nor were the clinical manifestations of sepsis present in all patients who were bacteraemic.44 Moreover, it was only when supportive care became possible that the clinical features we associate with sepsis – shock in the face of a hyperdynamic circulation,45 acute respiratory distress,46 and organ dysfunction47 –were described: in the absence of effective support of failing organs, the consequence of sepsis was a rapid death.
The contemporary definition of sepsis is acute organ dysfunction arising in association with infection.5 However, organ dysfunction can also be seen in ARDS, trauma, intoxication, heart failure, pancreatitis, autoimmune disease exacerbations and a variety of other acute disorders. An emphasis on infection as the cause of sepsis excludes a substantial number of critically ill patients from recruitment to clinical trials of mediator-directed therapies, and should these be found effective, from an entire class of therapeutic agents. Conversely a focus on sepsis as a specific disorder has blurred the reality that the syndrome is biologically heterogeneous.
In an effort to identify simple pragmatic enrolment criteria, investigators designing a clinical trial of methylprednisolone for septic shock proposed a set of clinical criteria that they termed “sepsis syndrome”.48 Grounded entirely in clinical opinion and dating from a time before the identification of cytokine mediators or the pattern recognition receptors that evoke their expression, the criteria nonetheless embodied a sense that death from severe infection was associated with systemic inflammation and acute organ dysfunction. Variants of these criteria have served as the entry criteria for all subsequent sepsis trials, without evidence that they delineate a discrete biologic process, and with overwhelming evidence that they do not identify patients who might benefit from specific biologic therapies.
So, what is the route forward? Efforts to redefine sepsis seem doomed to failure for reasons grounded in both biology and psychology. The biologic challenge is that the host response responsible for the clinical syndrome of sepsis is activated by viruses, bacterial products such as endotoxin, and non-microbial products released from injured and dying cells.49 The psychologic challenge arises from the recognition that the construct of word sepsis is ingrained in clinical thinking, and change is difficult.
Yet abandonment of concepts, such as sepsis and ARDS as useful descriptors of disease is precisely what is needed if we are to be able to identify a role for treatments that target the host response. An alternate model would focus on a diverse population of patients with acute organ dysfunction and limit trial recruitment to those patients with a derangement in the specific biologic process targeted – a treatable biologic trait.
The operational challenge
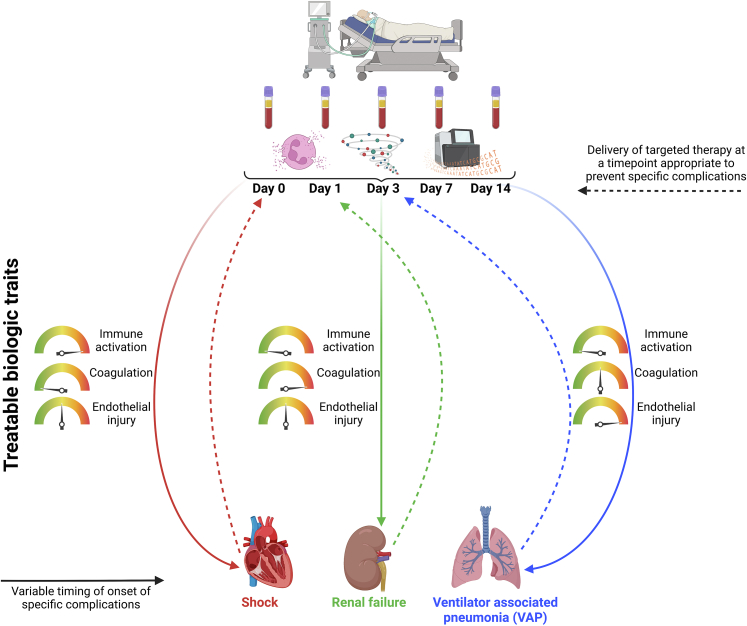
The biologic and conceptual complexities of sepsis create multiple challenges in translating biologic insight into effective treatments. The number of biologic targets, their interdependency, and their potentially beneficial contributions to host defences all complicate the challenge of identifying which patients are most likely to benefit from a particular therapeutic strategy. An attractive approach is to measure a panel of analytes or biomarkers - circulating proteins, lipids, and/or RNA transcripts – that identify modifiable biologic pathways50 (Fig. 1). This approach has transformed the adjuvant therapy of cancer, guiding treatment decisions based on specific biomarkers expressed by the malignant cells.51
Fig. 1.
Quantification of altered immune cell function, circulating proteins, and/or differential expression of transcribed genes at discrete time points after identification of critical illness could identify treatable biologic traits implicated in clinical outcome of interest (e.g.: shock, renal failure, or ventilator-associated pneumonia (VAP)). These complications are associated with different kinetics in biological derangements. For example, shock on day 0, may be best characterised by biological responses at the time of ICU admission (e.g.: excessive immune and endothelial activation). In contrast, development of VAP on week 2 of ICU admission, may be better predicted by analysing biological response kinetics (e.g.: presence of increasing immune tolerance throughout ICU admission, persistent coagulopathy and endothelial injury over time). By identifying time points relative to presentation with sepsis at which biological pathways are most dysregulated in relation to developing shock, renal failure, or VAP, it may be possible to provide therapies to avert these complications. The sequence of these complications varies among critically ill patients, and this graphic simplifies this concept.
A biomarker is a measurable biologic trait that indicates the presence of a clinical abnormality.52 Biomarkers may be prognostic (capable of predicting an increased risk of a particular outcome) or predictive (capable of predicting a response to a particular intervention) or both. A potentially useful biomarker of sepsis is not simply one that correlates with the presence of the syndrome, but rather one that in a heterogeneous population of patients, identifies a subpopulation that is more likely to benefit from a particular treatment approach. Cultures represent one such biomarker: in identifying a specific infecting organism, they guide the selection of the antibiotic that is likely to be most effective. C reactive protein and procalcitonin have shown some utility in diagnosing community-acquired pneumonia in an outpatient setting53 and endotoxemia can be diagnosed by assay of whole blood endotoxin levels.21
More than 200 unique putative biomarkers of sepsis have been proposed,54 yet none of these is used to guide therapies that target the host response. Sepsis is a dynamic process that evolves over time, driven by multiple factors, including treatment or the lack thereof and the inadvertent consequences of that treatment. Co-morbidities and genetic predisposition can be identified in advance of clinical illness. A recent prospective study of patients undergoing elective surgery55 demonstrated the feasibility of early pre-symptomatic detection of infection-related complications up to three days before clinical recognition. But most commonly, it is the clinical presentation that establishes the risk, and so prevention is not possible.
There are many different methods to detect biological responses, each with inherent advantages and challenges (Table 1). Measuring RNA transcripts provides insight into which genes have been expressed or repressed; protein level quantification indicates whether the RNA message has been translated into protein. Characterization of lipids and metabolites provides insight into the dynamic processes active at a cellular level. Cellular assays potentially integrate these approaches to provide insight into how cells have reacted to a threat. These methods may provide complementary, synergistic, or even contradictory conclusions, and a more systematic evaluation of their performance characteristics is warranted.
Table 1.
Diagnostic modalities for targeting sepsis therapies.
| Method | Advantage | Challenges |
|---|---|---|
| RNA | ||
| qPCR | Targeted quantification of key genes, fast, easy interpretation | Limited to a few genes |
| Microarray | High throughput analysis of larger groups of genes (1000s) | Time consuming assay and data analysis |
| RNA sequencing | Comprehensive analysis of very large groups of gene transcripts (10,000s) | Time consuming assay and data analysis |
| Proteins | ||
| Lateral flow test | Fast (minutes), simple | Limited to a single protein, not quantifiable |
| ELISA | Many commercial options for large number of proteins, no interactions between reagents | Time consuming assay |
| Multiplex platforms | Smaller sample volumes, faster data acquisition vs. ELISA, can simultaneously study proteins from many different pathways | Many platforms, many reagent manufacturers, optimisation of sample dilutions, complex analysis |
| O-link | High throughput, high sensitivity | Relative concentration values |
| Lipids & metabolites | ||
| Mass spectrometry | Sensitive and specific detection of metabolites | Expensive, time consuming, complex analyses |
| ELISA | Easy to perform | Single lipids, challenges with sample preparation due to lipid half-lives |
| Cells | ||
| Flow cytometry | Unaltered imaging of cells, ability to study function and phenotype of specific circulating cells | Time consuming, complex protocols and data analysis |
| Functional responses | Ability to study cell function outside the human body | Time consuming Not standardised (antigens, stimulation duration, read-out) |
Linking a biologic with a therapy that can modify its trajectory poses a methodologic challenge. The most reliable means of demonstrating causality is through randomization. Random assignment of study participants provides the greatest likelihood that the groups will be balanced with respect to known and unknown confounders. However, a traditional randomized trial evaluating both a therapy, and the capacity of a biomarker to identify patients more likely to benefit from it poses additional challenges as it is testing two separate and contingent hypotheses. The MONARCS trial of an anti-TNF antibody, for example, tested the hypothesis that therapy was efficacious in patients with sepsis and an elevated IL-6 level.56 The results were equivocal, and in the cohort with elevated IL-6 levels, the differential treatment effect was more pronounced at a higher threshold level than originally hypothesized. The conundrum of testing a marker or stratification scheme that can enhance a differential treatment effect, in the absence of knowing whether such an effect might exist, or how large it might be, has bedevilled recent sepsis clinical trials.57,58
Recent innovations in trial design have provided mechanisms to overcome some of the limitations associated with conventional trial designs. The platform trial, for example, studies patients with a disease, and so can study multiple different interventions simultaneously and sequentially, abandoning interventions that show no evidence of efficacy, and adding new ones as recruitment advances.59 This design provided expedited evidence of the efficacy of corticosteroids60,61 and interleukin-6 blockade62 in patients with severe COVID-19. Platform trials can also assess the effects of differing drug doses and biomarker enrichment and have been used effectively in early phase clinical research to identify therapies with the greatest probability for success in phase III trials. The iSPY2 trial (https://www.ispytrials.org/) has been one such approach to the evaluation of adjuvant treatments for locally advanced breast cancer.63 The efficiency of the design can be further enhanced using Bayesian statistical approaches that enable the trial to learn from accruing data, allowing interventions to graduate, or be dropped as they reach a priori criteria for superiority, inferiority, or harm, and during the trial. The use of response adaptive randomisation enables randomisation proportions to be adjusted to preferentially randomize patients to interventions that are showing the most promise.
An umbrella trial evaluates multiple therapies directed at a single target in a single disease, whereas a basket trial studies a single therapy across multiple diseases or disease subtypes.64 All three designs – platform, umbrella, and basket – are guided by a single master protocol. These designs have been most widely used in oncology,65 although they are well-suited for the challenges faced in sepsis.
An alternate approach that may facilitate the identification of effective therapies is that of Mendelian randomization, a promising strategy in a disease that is heavily impacted by genetic factors.66 Mendelian randomization uses baseline genetic variability in gene expression to estimate the contribution of that gene to an observed outcome that is a plausible consequence of the gene of interest.67 Since genetic variability was present at birth, and preceded the subsequent expression of the gene, the analysis is conceptually analogous to stratification based on the gene product of interest. The technique requires knowledge of which specific single nucleotide polymorphisms (SNPs) are expressed, but as publicly available databases of genome-wide association studies (GWAS) become more prevalent, the approach may identify therapeutic targets with differential treatment responsiveness.
Nonetheless, while prospective RCTs to optimize the targeting of immunomodulatory therapies are challenging, completed trials can be an invaluable source of information. There have been numerous examples of the ability of markers to predict differential treatment responsiveness in retrospective analyses of RCTs,15,27,68,69 although these require prospective confirmation. Incorporating sample collection routinely into RCTs, and creating agreements for data sharing is of fundamental importance to the validation of biomarkers that can predict treatment responsiveness70 and can aid secondary analyses to identify subgroups that are differentially responsive to treatment71 and thus point to heterogeneity in treatment effect.72 However, statistical methods for the identification of heterogeneity of treatment effect vary73 and need to be standardized.
Finally, even in the absence of randomization, advanced analytical techniques statistical and machine learning techniques for very large data sets, can identify subpopulations having differential prognoses and differential treatment responsiveness. Prominent among these are the hyper- and hypoinflammatory subphenotypes of ARDS described by Calfee and her colleagues,71 the SRS1 and SRS2 phenotypes described by Davenport and colleagues,74 the MARS 1-4 subphenotypes described by Scicluna,75 and the α, β, γ, and δ clinical phenotypes reported by Seymour et al.76
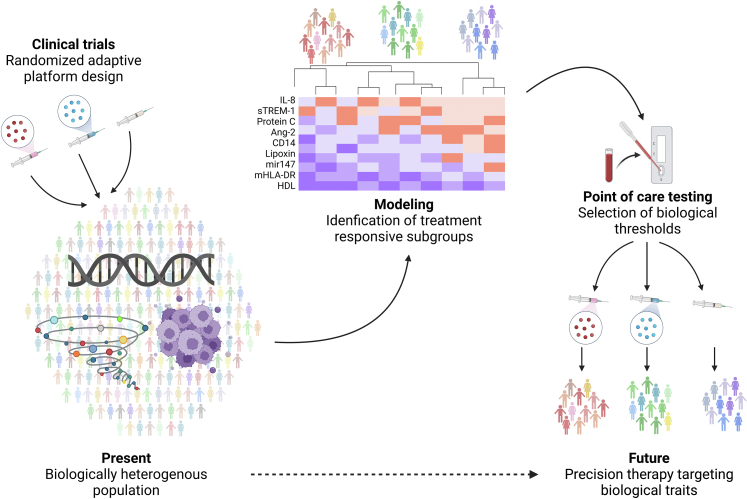
Integration of biomarker-based patient selection and novel clinical trial designs provides a potential roadmap for resolving underlying biologic heterogeneity, and so enabling more precise targeting of therapies to those patients most likely to benefit (Fig. 2).
Fig. 2.
The interplay between novel trial design (e.g.: randomized adaptive platform trials) and quantification of biomarkers implicated in the causal pathway of critical illness syndromes to identify treatment responsive subgroups of patients most likely to experience benefit from therapies targeted at biological mechanisms of illness. Incorporating biological sample collection into trial design enables prospective and retrospective analysis of biological factors, including: cellular functional studies, plasma quantification of proteins, lipids, and metabolites, and RNA sequencing. Machine learning algorithms can be used to incorporate data from biological studies to model clinically-relevant outcomes and identify subgroups of patients most likely to respond to specific therapies. Designing point-of-care tests that can subsequently rapidly and reliably identify biological correlates of clinically-relevant outcomes could then be used in real-time to randomize patients in future precision-guided clinical trials to minimize the heterogeneity in treatment effect (HTE).
The organizational challenge
The biggest challenges facing sepsis research are organizational. The clinical research agenda in sepsis has been driven largely by commercial pharmaceutical companies, motivated by the perception of an unmet clinical need and a correspondingly large commercial market.77 Commercial research is inherently competitive, guided by regulatory dictates, and because of the nature of patent law, driven by a desire to obtain results rapidly. What has been missing has been a complementary academic initiative to understand the epidemiology of sepsis, and to create the staging and stratification models that can frame future work. Insights from other disciplines are instructive.
Our understanding of the epidemiology of cardiovascular diseases owes an enormous debt to the Framingham study, launched in 1948 as a population-based initiative top understand the risk factors for cardiovascular diseases. Driven in large part by the recent death from hypertension and intracranial haemorrhage of President Franklin Delano Roosevelt, the program sought to identify modifiable risk factors for cardiovascular disease.78 Since its inception, the program has generated more than 3000 scientific publications, identified cardinal risk factors such as hypertension and obesity, and, as the study recruits third generation participants, has discovered genetic risk factors that can become druggable targets.79
The Union for International Cancer Control (UICC) was established in 1933 at a meeting in Spain, with the goal of promoting international collaboration in the study and management of cancer.80 An early initiative of the UICC was the development of a staging system that was applicable across multiple histologic types of cancer. The TNM (tumour, nodes, metastasis) system, developed by Pierre Denoix, created a model in which the prognosis of a variety of cancers could be stratified on the basis of tumour spread.81 The model proved effective not only in predicting survival, but also in delineating high risk subgroups that might benefit from adjunctive therapies. The creation of the TNM system has enabled effective multimodal therapy for cancer, and opened the door to treatments that target common biologic pathways shared by histologically distinct cancers.82
Comparable initiatives are needed in acute critical illness, and are beginning to emerge, accelerated by the COVID-19 pandemic. The International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) has created an open access online database to aggregate clinical data on patients with COVID-19.83 To date, more than 700,000 patients from 57 countries have been included. A tiered data collection process enables the contribution of basic epidemiologic data, but also more detailed longitudinal data where such collection is possible.84 Large scale collaboration in understanding the genetics of COVID-19, spearheaded by the GenOMICC consortium85 and the COVID-19 Host Genetics Initiative86 has resulted in the identification of potential therapeutic targets. International platform trials such as the Randomized Embedded Multifactorial Adaptive Platform trial in Community-Acquired Pneumonia (REMAP-CAP) or the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial have facilitated global collaboration in COVID-19 clinical trials. RECOVERY has recruited more than 48,000 hospitalized patients while REMAP-CAP has recruited more than 10,000 patients with severe COVID-19. Together these trials have shown that interventions that target the host response, including IL-6 receptor antagonists,62 corticosteroids,60 Janus kinase inhibitors,87 and even heparin38 and anti-platelet agents39 can improve outcomes for patients with COVID-19. International networks of research networks such as the International Forum for Acute Care Trialists (InFACT) have sought to create a framework for a massive effort to better understand the clinical biology of acute illness,88 and professional societies have embraced the need for a new approach.70
Conclusions
Grand scale collaborative efforts are needed to identify distinct treatable biologic traits within the complex syndromes that characterize critical illness. The COVID-19 pandemic has demonstrated the feasibility of surmounting this challenge. These collaborations have enabled the pooling of genetic data from tens of thousands of patients,86 and the rapid recruitment of tens of thousands of patients to clinical trials.30,89 They also emphasize the enormous challenge transitioning from current research models to more sophisticated ones, grounded in an understanding of the dominant causative underlying biologic processes and the identification of those patients most likely to experience the greatest benefit.
Contributors
JCM conceived the paper, wrote most of the first draft, and edited the final version. AL wrote parts of the initial draft, contributed to the revision, and prepared the figures. Both authors have seen and approved the final version.
Data sharing statement
Not applicable.
Declaration of interests
JCM reports receiving research funding from the Canadian Institutes of Health Research, a salary stipend as Associate Editor of Critical Care Medicine, and fees from AM Pharma as chair of a DSMB and Adrenomed as a consultant. He is chair of the International Forum for Acute Care Trialists. AL reports consulting fees with Janssen Research and Development.
Acknowledgements
No funding was received.
References
- 1.Majno G. The ancient riddle of (Sepsis) J Infect Dis. 1991;163:937–945. doi: 10.1093/infdis/163.5.937. [DOI] [PubMed] [Google Scholar]
- 2.Stedman's Medical Dictionary. 22nd ed. Williams & Wilkins C.; Baltimore: 1972. [Google Scholar]
- 3.Leligdowicz A., Harhay M.O., Calfee C.S. Immune modulation in sepsis, ARDS, and Covid-19 — the road traveled and the road ahead. NEJM EEvidence. 2022;1(11) doi: 10.1056/EVIDra2200118. EVIDra2200118. [DOI] [PubMed] [Google Scholar]
- 4.Bone R.C., Balk R.A., Cerra F.B., et al. ACCP/SCCM CONSENSUS CONFERENCE. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 5.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall J.C., Sweeney D. Microbial infection and the septic response in critical surgical illness. Sepsis, not infection, determines outcome. Arch Surg. 1990;125:17–23. doi: 10.1001/archsurg.1990.01410130019002. [DOI] [PubMed] [Google Scholar]
- 7.Lynch S.V., Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt T.S.B., Raes J., Bork P. The human gut microbiome: from association to modulation. Cell. 2018;172(6):1198–1215. doi: 10.1016/j.cell.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 9.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391–412. doi: 10.1016/j.intimp.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvano S.E., Xiao W., Richards D.R., et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437(7061):1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 12.Taveira Da Silva A.M., Kaulach H.C., Chuidian F.S., Lambert D.R., Stuffredini A.F., Danner R.L. Brief report: shock and multiple organ dysfunction after self administration of salmonella endotoxin. N Engl J Med. 1993;328:1457–1460. doi: 10.1056/NEJM199305203282005. [DOI] [PubMed] [Google Scholar]
- 13.Marshall J.C. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20(4):195–203. doi: 10.1016/j.molmed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Fang F., Zhang Y., Tang J., et al. Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta-analysis. JAMA Intern Med. 2019;179(2):213–223. doi: 10.1001/jamainternmed.2018.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antcliffe D.B., Burnham K.L., Al-Beidh F., et al. Transcriptomic signatures in sepsis and a differential response to steroids. From the VANISH randomized trial. Am J Respir Crit Care Med. 2019;199(8):980–986. doi: 10.1164/rccm.201807-1419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J.A.C., Murthy S., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhuri D., Sasaki K., Karkar A., et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47(5):521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziegler E.J., Fisher C.J., Jr., Sprung C.L., et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 19.Opal S.M., Laterre P.F., Francois B., et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309(11):1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 20.Bone R.C., Balk R.A., Fein A.M., et al. A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, controlled trial. Crit Care Med. 1995;23:994–1006. doi: 10.1097/00003246-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Marshall J.C., Foster D., Vincent J.-L., et al. Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J Infect Dis. 2004;190(3):527–534. doi: 10.1086/422254. [DOI] [PubMed] [Google Scholar]
- 22.Charbonney E., Tsang J.Y., Li Y., et al. Endotoxemia following multiple trauma: risk factors and prognostic implications. Crit Care Med. 2016;44(2):335–341. doi: 10.1097/CCM.0000000000001404. [DOI] [PubMed] [Google Scholar]
- 23.Klein D.J., Briet F., Nisenbaum R., Romaschin A.D., Mazer C.D. Endotoxemia related to cardiopulmonary bypass is associated with increased risk of infection after cardiac surgery: a prospective observational study. Crit Care. 2011;15(1):R69. doi: 10.1186/cc10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beutler B., Milsark I.W., Cerami A.C. Passive immunization against cachectin tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 25.Palladino M.A., Bahjat F.R., Theodorakis E.A., Moldawer L.L. Anti-TNF-alpha therapies: the next generation. Nat Rev Drug Discov. 2003;2(9):736–746. doi: 10.1038/nrd1175. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello C.A., Wolff S.M. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 27.Shakoory B., Carcillo J.A., Chatham W.W., et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44(2):275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyriazopoulou E., Poulakou G., Milionis H., et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27(10):1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikfar S., Saiyarsarai P., Tigabu B.M., Abdollahi M. Efficacy and safety of interleukin-1 antagonists in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol Int. 2018;38(8):1363–1383. doi: 10.1007/s00296-018-4041-1. [DOI] [PubMed] [Google Scholar]
- 30.Gordon A.C., Mouncey P.R., Al-Beidh F., et al. REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Shankar-Hari M., Vale C.L., et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326(6):499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka T., Narazaki M., Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(8) doi: 10.1101/cshperspect.a028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernard G.R., Vincent J.-L., Laterre P.F., et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 34.Ranieri V.M., Thompson B.T., Barie P.S., et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 35.Abraham E., Reinhart K., Opal S., et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290(2):238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 36.Warren B.L., Eid A., Singer P., et al. High-dose antithrombin III in severe sepsis: a randomized, controlled trial. JAMA. 2001;286:1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 37.Vincent J.L., Francois B., Zabolotskikh I., et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. JAMA. 2019;321(20):1993–2002. doi: 10.1001/jama.2019.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.REMAP-CAP Writing Committee for the REMAP-CAP Investigators. Bradbury C.A., Lawler P.R., et al. Effect of antiplatelet therapy on survival and organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1247–1259. doi: 10.1001/jama.2022.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Group R.C. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet. 2022;400(10349):359–368. doi: 10.1016/S0140-6736(22)01109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guimaraes P.O., Quirk D., Furtado R.H., et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385(5):406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka Y., Luo Y., O'Shea J.J., Nakayamada S. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18(3):133–145. doi: 10.1038/s41584-021-00726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sibbald W.J. Sepsis--the Wayne State University Symposium--part III. Bacteremia and endotoxemia: a discussion of their roles in the pathophysiology of gram-negative sepsis. Heart Lung. 1976;5(5):765–771. [PubMed] [Google Scholar]
- 44.Meakins J.L., Wicklund B., Forse R.A., Mclean A.P.H. The surgical intensive care unit: current concepts in infection. Surg Clin North Am. 1980;60:117–132. doi: 10.1016/s0039-6109(16)42038-4. [DOI] [PubMed] [Google Scholar]
- 45.Maclean L.D., Mulligan W.G., Mclean A.P.H., Duff J.H. Patterns of septic shock in man - a detailed study of 56 patients. Ann Surg. 1967;166:543–562. doi: 10.1097/00000658-196710000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashbaugh D.G., Bigelow D.B., Petty T.L., Levine B.E. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 47.Baue A.E. Multiple, progressive, or sequential systems failure. A syndrome of the 1970s. Arch Surg. 1975;110:779–781. doi: 10.1001/archsurg.1975.01360130011001. [DOI] [PubMed] [Google Scholar]
- 48.Bone R.C., Fisher C.J., Clemmer T.P., et al. Sepsis syndrome: a valid clinical entity. Crit Care Med. 1989;17:389–393. [PubMed] [Google Scholar]
- 49.Medzhitov R., Janeway C.A., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 50.Schuurman A.R., Reijnders T.D.Y., Kullberg R.F.J., Butler J.M., van der Poll T., Wiersinga W.J. Sepsis: deriving biological meaning and clinical applications from high-dimensional data. Intensive Care Med Exp. 2021;9(1):27. doi: 10.1186/s40635-021-00383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cercek A., Lumish M., Sinopoli J., et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386(25):2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marshall J.C., Reinhart K., Forum I.S. Biomarkers of sepsis. Crit Care Med. 2009;37(7):2290–2298. doi: 10.1097/CCM.0b013e3181a02afc. [DOI] [PubMed] [Google Scholar]
- 53.Ebell M.H., Bentivegna M., Cai X., Hulme C., Kearney M. Accuracy of biomarkers for the diagnosis of adult community-acquired pneumonia: a meta-analysis. Acad Emerg Med. 2020;27(3):195–206. doi: 10.1111/acem.13889. [DOI] [PubMed] [Google Scholar]
- 54.Pierrakos C., Velissaris D., Bisdorff M., Marshall J.C., Vincent J.L. Biomarkers of sepsis: time for a reappraisal. Crit Care. 2020;24(1):287. doi: 10.1186/s13054-020-02993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukaszewski R.A., Jones H.E., Gersuk V.H., et al. Presymptomatic diagnosis of postoperative infection and sepsis using gene expression signatures. Intensive Care Med. 2022;48(9):1133–1143. doi: 10.1007/s00134-022-06769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panacek E.A., Marshall J.C., Albertson T.E., et al. Efficacy and safety of the monoclonal anti-TNF antibody F(ab')2 fragment in patients with severe sepsis stratified by IL-6 level. Crit Care Med. 2004;32(11):2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 57.Dellinger R.P., Bagshaw S.M., Antonelli M., et al. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA. 2018;320(14):1455–1463. doi: 10.1001/jama.2018.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levi M., Vincent J.L., Tanaka K., et al. Effect of a recombinant human soluble thrombomodulin on baseline coagulation biomarker levels and mortality outcome in patients with sepsis-associated coagulopathy. Crit Care Med. 2020;48(8):1140–1147. doi: 10.1097/CCM.0000000000004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berry S.M., Connor J.T., Lewis R.J. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA. 2015;313(16):1619–1620. doi: 10.1001/jama.2015.2316. [DOI] [PubMed] [Google Scholar]
- 60.Group R.C., Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angus D.C., Derde L., Al-Beidh F., et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Investigators R.-C., Gordon A.C., Mouncey P.R., et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J.W., Liu M.C., Yee D., et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11–22. doi: 10.1056/NEJMoa1513750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woodcock J., LaVange L.M. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 65.Park J.J.H., Siden E., Zoratti M.J., et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. 2019;20(1):572. doi: 10.1186/s13063-019-3664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sorenson T.I., Nielsen G.G., Andersen P.K., Teasdale P.W. Genetic and environmental influences on premature death in adult adoptees. N Engl J Med. 1988;318(12):727–732. doi: 10.1056/NEJM198803243181202. [DOI] [PubMed] [Google Scholar]
- 67.Emdin C.A., Khera A.V., Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 68.Marshall J.C., Albertson T.E., Barchuk W., van Meter L., Group ftAS. Effect of anti-TNF antibody on organ-specific components of MOD score. Am J Resp Crit Care Med. 2001;163(5):A820. [Google Scholar]
- 69.Calfee C.S., Delucchi K.L., Sinha P., et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah F.A., Meyer N.J., Angus D.C., et al. A research agenda for precision medicine in sepsis and acute respiratory distress syndrome: an official American thoracic society research statement. Am J Respir Crit Care Med. 2021;204(8):891–901. doi: 10.1164/rccm.202108-1908ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calfee C.S., Delucchi K., Parsons P.E., et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwashyna T.J., Burke J.F., Sussman J.B., Prescott H.C., Hayward R.A., Angus D.C. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192(9):1045–1051. doi: 10.1164/rccm.201411-2125CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kent D.M., Steyerberg E., van Klaveren D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ. 2018;363:k4245. doi: 10.1136/bmj.k4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davenport E.E., Burnham K.L., Radhakrishnan J., et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med. 2016;4(4):259–271. doi: 10.1016/S2213-2600(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scicluna B.P., van Vught L.A., Zwinderman A.H., et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir Med. 2017;5(10):816–826. doi: 10.1016/S2213-2600(17)30294-1. [DOI] [PubMed] [Google Scholar]
- 76.Seymour C.W., Kennedy J.N., Wang S., et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Angus D.C., Linde-Zwirble W.T., Carcillo J., Lidicker J., Clermont G., Pinsky M.R. Incidence, cost, and outcome of severe sepsis in the United States. Crit Care Med. 2001; Jul;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Mahmood S.S., Levy D., Vasan R.S., Wang T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andersson C., Johnson A.D., Benjamin E.J., Levy D., Vasan R.S. 70-year legacy of the Framingham heart study. Nat Rev Cardiol. 2019;16(11):687–698. doi: 10.1038/s41569-019-0202-5. [DOI] [PubMed] [Google Scholar]
- 80.Gospodarowicz M., Benedet L., Hutter R.V., Flemimg I., Henson D.E., Sobin L.H. History and international developments in cancer staging. Cancer Prev Cont. 1998;2(6):262–268. [PubMed] [Google Scholar]
- 81.Brierley J., National Cancer Institute of Canada Committee on Cancer S The evolving TNM cancer staging system: an essential component of cancer care. CMAJ. 2006;174(2):155–156. doi: 10.1503/cmaj.045113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.The L. 20 years of precision medicine in oncology. Lancet. 2021;397(10287):1781. doi: 10.1016/S0140-6736(21)01099-0. [DOI] [PubMed] [Google Scholar]
- 83.Group I.C.C. The value of open-source clinical science in pandemic response: lessons from ISARIC. Lancet Infect Dis. 2021;21(12):1623–1624. doi: 10.1016/S1473-3099(21)00565-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dunning J.W., Merson L., Rohde G.G., et al. Open source clinical science for emerging infections. Lancet Infect Dis. 2014;14(1):8–9. doi: 10.1016/S1473-3099(13)70327-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pairo-Castineira E., Clohisey S., Klaric L., et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591(7848):92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 86.COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021;600(7889):472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maslove D.M., Tang B., Shankar-Hari M., et al. Redefining critical illness. Nat Med. 2022;28(6):1141–1148. doi: 10.1038/s41591-022-01843-x. [DOI] [PubMed] [Google Scholar]
- 89.Horby P., Lim W.S., Emberson J.R., et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]