Abstract
Lymph node (LN) cells from C3H/HeJ mice (Lyme disease susceptible) infected for 1 week with Borrelia burgdorferi strain JD1 produced higher levels of gamma interferon (IFN-γ) when stimulated in vitro with B. burgdorferi spirochetes than equivalent cells from B. burgdorferi-infected C57BL/6J mice (disease resistant). The interleukin-10 (IL-10) levels were comparable in the two strains, whereas the IL-4 levels were below detection limits. B. burgdorferi-stimulated LN cells from C57BL/6J mice produced significantly higher levels of IFN-γ in the presence of neutralizing anti-IL-10 antibody than cells cultured with B. burgdorferi alone. No effect of IL-10 neutralization on IFN-γ production by LN cells from C3H/HeJ mice was observed. Neutralizing antibody to IFN-γ had no effect on the production of IL-10 by LN cells from C57BL/6J mice. A slight decrease in IL-10 production was detected in culture supernatants of equivalent cells from C3H/HeJ mice. The differential effect of IL-10 on IFN-γ production in C57BL/6J and C3H/HeJ mice suggests that IL-10 is probably involved in the regulation of IFN-γ production by LN cells during infection and may be at the root of the differential susceptibility to Lyme arthritis in these two strains of mice.
Lyme disease, caused by the spirochete Borrelia burgdorferi, is the most frequently reported tick-borne infection in the United States (19). Spirochetal persistence in tissues has been associated with severe pathology (4) in both acute and chronic inflammatory conditions (20). Studies conducted to date have revealed that B. burgdorferi antigens can induce in a variety of cell types the release of proinflammatory cytokines that may contribute to tissue inflammation (8, 15, 17). Additionally, B. burgdorferi antigens also are known to induce the anti-inflammatory cytokine interleukin-10 (IL-10) in mice as well as in both humans and nonhuman primates (3, 8, 9, 16).
The mouse model of Lyme disease is very useful to study Lyme arthritis, in particular the role of cytokines in the pathogenesis of this form of the disease. Severe arthritis, manifested as joint swelling, has been correlated with a Th1 (gamma interferon [IFN-γ]) polarization of the immune response in C3H/HeN mice, whereas mild arthritis, seen in BALB/c and C57BL/6 mice, was thought to be due to a Th2 (IL-4) polarization and cytokine pattern (12, 13). Another study, however, demonstrated that both C3H/HeN and BALB/c mice exhibited severe arthritis early after infection with B. burgdorferi. A rapid resolution of Lyme arthritis in B. burgdorferi-infected BALB/c mice was correlated with the production of IL-4 in draining lymph nodes (LN) (11). More recently, it was shown that IL-10 is a key mediator in innate immune responses induced by B. burgdorferi in mice (3). This study elegantly showed that IL-10 modulates B. burgdorferi-induced inflammation in vivo, since C57BL/6J mice deficient in IL-10 developed more severe arthritis than wild-type C57BL/6J mice (3).
The present study was designed to further understand the role of the Th1 cytokine IFN-γ, the Th2 cytokine IL-4, and the Th2-associated cytokine IL-10 as induced by B. burgdorferi early during the course of infection. Two strains of mouse, C57BL/6J (disease resistant) and C3H/HeJ (disease susceptible), were utilized. We set out to determine the relationship between the levels of IFN-γ, IL-10, and IL-4 at week 1 postinoculation (p.i.) and the extent of cross-regulation between these cytokines. We hypothesized that IFN-γ and IL-10 are key cytokines in Lyme disease pathogenesis and that their balance may explain Lyme disease outcome in C57BL/6J and C3H/HeJ mice.
Four- to six-week-old C3H/HeJ and C57BL/6J female mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Mice (four or five per group) were subcutaneously inoculated in the cervical region with live low-passage B. burgdorferi (4 × 106 spirochetes) of the JD1 strain. Spirochetes were cultivated as described previously (8).
Regional axillary and brachial LN were removed from mice at 1 week p.i. Cells (2 × 106/ml) obtained from these organs were stimulated in vitro with B. burgdorferi JD1 freeze-thawed organisms (1 × 107/ml). The control supernatant was generated by culturing cells in medium alone consisting of 45% RPMI 1640, 45% Iscove's modified Dulbecco's medium (Gibco-BRL, Life Technologies, Grand Island, N.Y.), 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), 1 mM HEPES (Gibco), 0.5 mM sodium pyruvate (Sigma Chemical Co., St. Louis, Mo.), 2 mM l-glutamine, 0.05 mM mercaptoethanol (Sigma), and 1 μg of gentamicin sulfate (Gibco) per ml. Cultures were incubated at 37°C in a humidified atmosphere (5% CO2). Supernatants were harvested at 24 h (concanavalin A [ConA]) or 48 h (B. burgdorferi) and stored at −70°C until they were used.
Freshly isolated LN cells (2 × 106/ml) were obtained from mice at 1 week p.i. and were stimulated in vitro with B. burgdorferi (1 × 107, organisms/ml) in the presence (25 μg/ml) or absence of neutralizing monoclonal antibodies (MAb) to IL-10 (clone JES5-2A5) or IFN-γ (clone R4-6A2) (Pharmingen, San Diego, Calif.). Supernatants were harvested after 48 h and stored at −70°C until they were used.
A sandwich enzyme-linked immunosorbent assay (ELISA) was employed to detect IFN-γ, IL-10, and IL-4 in culture supernatants of LN cells, using cytokine-specific antibody pairs from Pharmingen (6, 7). Secreted IFN-γ was quantified with purified rat anti-mouse IFN-γ MAb (clone R4-6A2) as capture antibody and biotin-conjugated rat anti-mouse IFN-γ MAb (clone XMG1.2) as detection antibody. The production of IL-10 was quantified with purified rat anti-mouse IL-10 MAb (clone JES5-2A5) as capture antibody and biotin-conjugated rat anti-mouse IL-10 MAb (clone SXC-1) as detection antibody. The release of IL-4 was quantified with purified rat anti-mouse IL-4 MAb (clone 11B11) as capture antibody and biotin-conjugated rat anti-mouse IL-4 MAb (clone BVD6-24G2) as detection antibody. The detection levels were 15 pg/ml for IL-10, 20 pg/ml for IFN-γ, and 25 pg/ml for IL-4. Student's t test was used for statistical analysis of the data. Data were considered significantly different at P values of <0.05.
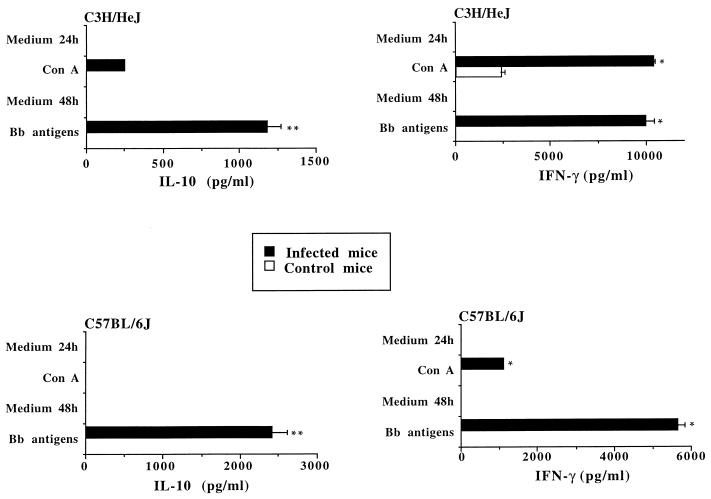
To assess early IFN-γ, IL-10, and IL-4 production, LN cells were obtained from C3H/HeJ and C57BL/6J mice at week 1 p.i. and stimulated with B. burgdorferi. LN cells from C3H/HeJ and C57BL/6J mice produced both IFN-γ and IL-10 when restimulated in vitro with B. burgdorferi. The IFN-γ level was at all times significantly higher (P < 0.006) in C3H/HeJ than in C57BL/6J mice (Fig. 1 and 2a). In contrast, in some experiments the level of IL-10 production by LN cells from C3H/HeJ mice was lower (P < 0.04) (Fig. 1) and in others higher (Fig. 2b) than that produced by equivalent cells from C57BL/6J mice. No IL-4 production was detected in supernatants of LN cells, from either C3H/HeJ or C57BL/6J mice, that were stimulated with either ConA or B. burgdorferi.
FIG. 1.
In vitro production of IFN-γ and IL-10 by LN cells from C3H/HeJ and C57BL/6J mice after 1 week of infection with the JD1 strain of B. burgdorferi (Bb). LN cells from naive mice were used as a control. Cells (2 × 106/ml) were stimulated in vitro with freeze-thawed JD1 spirochetes (1 × 107/ml) or ConA (10 μg/ml). The control supernatant was generated by culturing cells in medium alone. Supernatants were harvested at 24 h (ConA) or 48 h (B. burgdorferi). A sandwich ELISA was performed for cytokine detection. Student's t test was used for statistical analysis. ∗ and ∗∗, P < 0.006 and P < 0.04, respectively. These experiments were conducted in triplicate and repeated four times, with similar results.
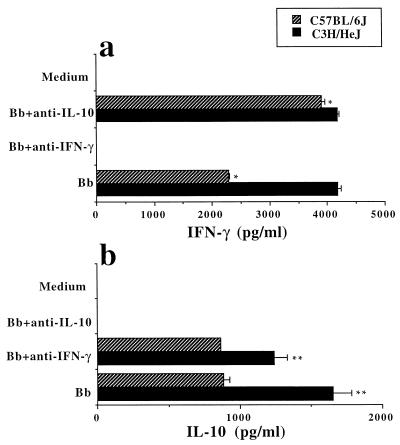
FIG. 2.
Differential effect of in vitro neutralization of IFN-γ and IL-10 on their production by draining LN cells from C3H/HeJ and C57BL/6J mice 1 week p.i. Freshly isolated LN cells (2 × 106/ml) were stimulated in vitro with freeze-thawed B. burgdorferi (Bb) JD1 spirochetes (1 × 107/ml). Neutralizing MAb anti-IL-10 or anti-IFN-γ (25 μg/ml) was added in some of the wells. The control supernatant was generated by culturing cells in medium alone. Supernatants were collected 48 h later and a sandwich ELISA was performed for cytokine detection. Student's t test was used for statistical analysis. ∗ and ∗∗, P < 0.00003 and P < 0.0015, respectively. All samples were run in quadruplicate. Data are representative of four separate experiments.
To study the effect of in vitro neutralization of IL-10 and IFN-γ on the production of these cytokines, draining LN cells obtained 1 week after the inoculation of C3H/HeJ and C57BL/6J mice with B. burgdorferi were stimulated in vitro with B. burgdorferi in the presence of neutralizing anti-murine IL-10 or IFN-γ antibody. Culture supernatants of B. burgdorferi-stimulated LN cells from C57BL/6J mice contained, in the presence of anti-IL-10 antibody, higher levels of IFN-γ than supernatants from cells stimulated with B. burgdorferi alone (P < 0.00003) (Fig. 2a). In contrast, no effect on IFN-γ production was observed in C3H/HeJ mouse LN cells that were cultivated in the presence of neutralizing anti-IL-10 antibodies (Fig. 2a). In some experiments, the level of IFN-γ increased up to 10-fold in LN cells from C57BL/6J mice and 1.2-fold in cells from C3H/HeJ mice (data not shown). Neutralizing antibody to IFN-γ had no effect on the production of IL-10 by B. burgdorferi-induced LN cells from C57BL/6J mice, since IL-10 production was similar in LN cells stimulated with B. burgdorferi antigens alone (Fig. 2b). A slight but significant decrease (P < 0.0015) in IL-10 production was detected in culture supernatants of LN cells from B. burgdorferi-infected C3H/HeJ mice when the cells were stimulated in the presence of neutralizing antibody to IFN-γ (Fig. 2b).
The present study shows that production of IFN-γ and IL-10 but not of IL-4 is specifically induced in the regional LN of mice early during the infection process. Stimulation with B. burgdorferi induced a significantly higher level of IFN-γ in the supernatants of LN cells from C3H/HeJ mice than that observed in cells of C57BL/6J mice. IL-10 production by B. burgdorferi-induced LN cells was variable in both mouse strains. In vitro cytokine neutralization results show that regulation of B. burgdorferi-induced IFN-γ production in LN cells from C57BL/6J (disease-resistant) mice is dependent on the IL-10 level in the cell culture supernatants. Surprisingly, neutralization of endogenous IL-10 appeared to have no effect on IFN-γ production in B. burgdorferi-stimulated LN cells of C3H/HeJ (disease-susceptible) mice.
Although there have been a number of reports describing the effect of IL-4 on the control of Lyme arthritis in mice (11–13, 21), the role of IL-4 in Lyme disease pathogenesis remains unclear. Our findings show that IL-4 is not specifically induced in the regional LN of either C57BL/6J or C3H/HeJ mice early during infection with B. burgdorferi. This suggests a lack of correlation between IL-4 production and Lyme arthritis development. In this regard our data agree with the conclusions drawn by Brown and Reiner (1) from their work with IL-4-deficient mice. These authors noted that IL-4 may play a role in arthritis resolution but not in its development. However, our assessment of IL-4 at 4 weeks p.i. also showed that the production of this cytokine by B. burgdorferi-induced LN cells was below detection levels but that the production of both IFN-γ and IL-10 was still detectable; this further indicates that the latter two cytokines, not IL-4, may play a role in later stages of Lyme disease in mice (data not shown). Recently, Harjacek and coworkers (10) reported that 4% of patients with either Lyme arthritis or juvenile rheumatoid arthritis expressed IL-10 transcript in the synovium, whereas none expressed IL-4 mRNA. However, recent studies have shown that IL-4 modulates B. burgdorferi-induced carditis in vivo, since BALB/c mice deficient in IL-4 developed more severe carditis than wild-type BALB/c mice (18). Further studies are needed to better understand the role played by IL-4 in Lyme disease pathogenesis.
The difference in the ability of IL-10 to regulate IFN-γ production in C3H/HeJ and C57BL/6J mice could be due to differences in the kinetics of production of both cytokines in these mouse strains. It is possible that in C3H/HeJ mice IFN-γ is produced prior to IL-10. Hence, functional inhibition of endogenously produced IL-10 would have no effect on IFN-γ production. If, in contrast, C57BL/6J mouse lymphocytes produce IFN-γ after IL-10 is produced, the inhibition of IL-10 function could affect production of IFN-γ. Alternatively, it is possible that the IL-10 receptors expressed on lymphocytes from C3H/HeJ mice have a higher activation threshold than IL-10 receptors of C57BL/6J mouse cells. Differences in numbers of receptors in the two mouse strains also may account for the observed phenomenon. The decrease in IL-10 production that we observed when LN cells from B. burgdorferi-infected C3H/HeJ mice were cultivated in the presence of neutralizing antibody to IFN-γ is intriguing and suggests that more complex cross-regulatory effects may be at play. This is also suggested by the finding that IFN-γ is not absolutely required for arthritis susceptibility in mice (1).
The contribution of IFN-γ to the initiation and maintenance of inflammatory reactions is well documented. In fact, IFN-γ cooperates with tumor necrosis factor alpha (TNF-α) to enhance expression of cell surface adhesion molecules which have been shown to expand and amplify the overall inflammatory response. Collaboration between IFN-γ and TNF-α leads to an increase in the number of monocytes in the inflammatory site (5). IL-10, in contrast, downregulates the production of TNF-α (2, 14). The balance between IFN-γ and IL-10 production by lymphocytes from each of these mouse strains may help to explain the severity of arthritis in the C3H/HeJ mouse strain compared with that in the C57BL/6J strain. Moreover, as was recently reported, the fact that bone marrow macrophages from C57BL/6J mice produce more IL-10 when stimulated with B. burgdorferi lipoproteins than do macrophages from C3H/HeJ mice indicates that early in the infection process the innate immune response, as mediated by skin macrophages, may further contribute, via IL-10, to the diminished disease severity in C57BL/6J mice (3). Studies are currently being conducted to better understand the effects of these cytokines on B- and T-cell phenotypes and on the pathogenesis of Lyme arthritis.
Acknowledgments
We thank Christie Trew for her excellent secretarial help.
This work was supported by grant U50/CCU606604 from the Centers for Disease Control and Prevention and grant RR00164 from the National Center for Research Resources, National Institutes of Health.
REFERENCES
- 1.Brown C R, Reiner S L. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect Immun. 1999;67:3329–3333. doi: 10.1128/iai.67.7.3329-3333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown E L, Rivas J M, Ulrich S E, Young C R, Norris S J, Kripke M L. Modulation of immunity to Borrelia burgdorferi by ultraviolet irradiation: differential effect on Th-1 and Th-2 immune responses. Eur J Immunol. 1995;25:3017–3022. doi: 10.1002/eji.1830251105. [DOI] [PubMed] [Google Scholar]
- 3.Brown J P, Zachary J F, Teuscher C, Weis J J, Wooten R M. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyle P K. Lyme disease. In: Manning S, editor. Pathogenesis of Lyme disease. St. Louis, Mo: Mosby Year Book; 1993. pp. 179–183. [Google Scholar]
- 5.Farrar M A, Schreiber R D. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 6.Ganapamo F, Rutti B, Brossard M. In vitro production of interleukin-4 and interferon-γ by lymph node cells from BALB/c mice infested with nymphal Ixodes ricinus ticks. Immunology. 1995;85:120–124. [PMC free article] [PubMed] [Google Scholar]
- 7.Ganapamo F, Rutti B, Brossard M. Immunosuppression and cytokine production in mice infested with Ixodes ricinus ticks: a possible role of laminin and interleukin-10 on the in vitro responsiveness of lymphocytes to mitogens. Immunology. 1996;87:259–263. doi: 10.1046/j.1365-2567.1996.450512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giambartolomei G H, Dennis V A, Philipp M T. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect Immun. 1998;66:2691–2697. doi: 10.1128/iai.66.6.2691-2697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giambartolomei G H, Dennis V A, Lasater B L, Philipp M T. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harjacek M, Diaz-Cano S, Alman B A, Coburn J, Ruthazer R, Wolfe H, Steere A C. Prominent expression of mRNA for proinflammatory cytokines in synovium in patients with juvenile rheumatoid arthritis or chronic Lyme arthritis. J Rheumatol. 2000;27:497–503. [PubMed] [Google Scholar]
- 11.Kang I, Barthold S W, Persing D H, Bockenstedt L K. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keane-Myers A, Maliszewski C R, Finkelman F D, Nickell S P. Recombinant IL-4 treatment augments resistance to Borrelia burgdorferi infections in both normal susceptible and antibody-deficient susceptible mice. J Immunol. 1996;156:2488–2494. [PubMed] [Google Scholar]
- 13.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore K W, O'Garra A, DeWaal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 15.Norgard M V, Arndt L L, Akins D R, Curetty L L, Harrich D A, Radolf J D. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-κ B. Infect Immun. 1996;64:3845–3852. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pohl-Koppe A, Balashov K E, Steere A C, Logigian E L, Hafler D A. Identification of a T cell subset capable of both IFN-γ and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J Immunol. 1998;160:1804–1810. [PubMed] [Google Scholar]
- 17.Radolf J D, Arndt L L, Akins D R, Curetty L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 18.Satoskar A R, Elizondo J, Monteforte G M, Stamm L M, Bluethmann H, Katavolos P, Telford S R., 3rd Interleukin-4-deficient BALB/c mice develop an enhanced Th-1-like response but control cardiac inflammation following Borrelia burgdorferi infection. FEMS Microbiol Lett. 2000;183:319–325. doi: 10.1111/j.1574-6968.2000.tb08978.x. [DOI] [PubMed] [Google Scholar]
- 19.Steere A C. Lyme disease. N Engl J Med. 1989;21:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeidner N, Mbow M L, Dolan M, Massung R, Baca E, Piesman J. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun. 1997;65:3100–3106. doi: 10.1128/iai.65.8.3100-3106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]