Abstract
The genus Onosma belongs to the Boraginaceae family and contains over 230 species. The present review sheds light on the ethnopharmacology, phytoconstituents, bioactivity, and toxicology of the Onosma species from previous investigations. Furthermore, the paper also highlights the unresolved issues for the future investigations. The review included previous studies of the genus Onosma available from Google Scholar and Baidu Scholar, Science Direct, SciFinder, Wiley Online Library, and Web of Science. Until now, more than 200 chemical compounds have been detected from the genus Onosma, including naphthoquinone (33), flavonoids (30), hydrocarbon (23), phenolic (22), ester (17), alkaloids (20), aromatics (12), carboxylic acid (11), fatty acids (9), terpenoids (10), while the most important ones are rosmarinic, ferulic, protocatechuic, chlorogenic, caffeic, p-coumaric acids, and apigenin. The Onosma species are reported as traditional medicine for wound healing, heart disease, and kidney disorders, while the pharmacological investigations revealed that the extracts and the phytochemicals of Onosma species have different therapeutic properties including antioxidant, enzyme inhibitory, antitumor, hepatoprotective, antiviral, anti-inflammatory, and antimicrobial actions. The summarized knowledge in this review provides valuable ideas for the current and future drug discovery and a motivation for further investigation on the genus Onosma.
Keywords: Onosma, ethnobotany, phytochemistry, data mining, pharmacological activity, toxicology
1. Introduction
The genus Onosma comprises more than 230 species across the globe. The Asian continent has the highest share in terms of Onosma species existence [1], most of which are represented in Turkey by 88 species [2] followed by countries such as Iran and China by 58 [3] and 29 species [4], respectively. Iraqi Kurdistan represents 32 species of the genus Onosma based on the latest botanical studies [5]. However, recent investigations have revealed seven new species of Onosma in Asian countries, particularly Iran [6]. Continuous exploration on the ethnobotanical and plant taxonomy studies led to the discovery of several new Onosma species across our continent [7,8]. Some Onosma species have been well studied pharmacologically than others, and the most common ones are shown in Figure 1.
Figure 1.
Representative example of the most-studied species of the genus Onosma in terms of pharmacology actions.
The ethnobotanical and in vitro studies have revealed that most of this Onosma species has many medicinal capabilities such as sedatives [9], antioxidant [10], anti-inflammatory [11], gastric disorders [11], antithrombotic [12], wound healing [13], Alzheimer [14], enzyme inhibitory [15], anti-tumor [16], anti-viral [17], antifungal [18], and COVID-19 curatives [19] (Figure 2).
Figure 2.
Chemical profile and pharmacological activities of the Onosma species ((A) O. mutabilis, (B): O. Alborosea). 1: Kingdom, 2: Phylum, 3: Class, 4: Order, 5: Family, 6: Subfamily, 7: Genus.
The phytochemical studies on the genus Onosma have reported several chemical compounds as their main active ingredients, including naphthaquinones (5,8-dihydroxy-2-(4-methylpent-3-enyl) naphthalene-1,4-dione) [16], Phenolics (ferulic acid, vanillic acid), flavonoids (apigenin, luteolin) [10,20], alkannin, and shikonines (deoxyshikonin, isobutyrylshikonin, α-methylbutyrylshikonin, acetylshikonin) [21,22].
The past decades have showed numerous new records, phytochemical, and pharmacological studies of the new Onosma species, and the published two reviews were found lacking integrity as they contained incoherent data with skipping of some biological activities of the genus Onosma [23,24]. Therefore, in order to provide theoretical reference for further research and to comprehensively understand the medicinal applications of this genus, this article systematically reviewed traditional uses, chemical constituents, pharmacological activities, and clinical applications of the Onosma species based on the published literature.
2. Methodology
The authors independently extracted systematic literature data search from seven electronic databases: Google Scholar, PubMed, Science Direct, Sci-Finder, Wiley Online Library, Web of Science, and Baidu Scholar. The scientific name “Onosma” was searched to cover all relevant information from April 1800–2022, including folkloric uses, phytochemical contents, and pharmacological potentials (antimicrobial, anti-inflammatory, anticancer, antioxidant, enzyme inhibitory, and antidiabetic) of the Onosma species are presented in this review. More than 1000 articles were detected with keyword Onosma, about 132 articles were found with keyword Onosma phytochemical, 187 articles were found with keyword Onosma pharmacology, biological activity, medicinal uses, pharmacology, and toxicology. Out of these, 125 articles were published detailing the isolation and properties of different phytochemical contents of the Onosma genus, and a total of 95 papers were selected based on the quality, specificity, and the procedure of the investigation of Onosma extracts and its isolated compounds.
3. Regional (Folkloric) Name
The folkloric names of most Onosma species in most Middle east countries is Gaozaban, an Urdu word. It was first referred to O. bracteatum Wall, and among Arabic populations it is known as “Lisan-al-Thawr” or “Saqil ul-Hammam”. Furthermore, its English popular name is “Vipers Bugloss”, while, in Hindi language, the Onosma species known as “Ratanjot” as first referred to O. echioides L. [25,26].
4. Regional Distribution
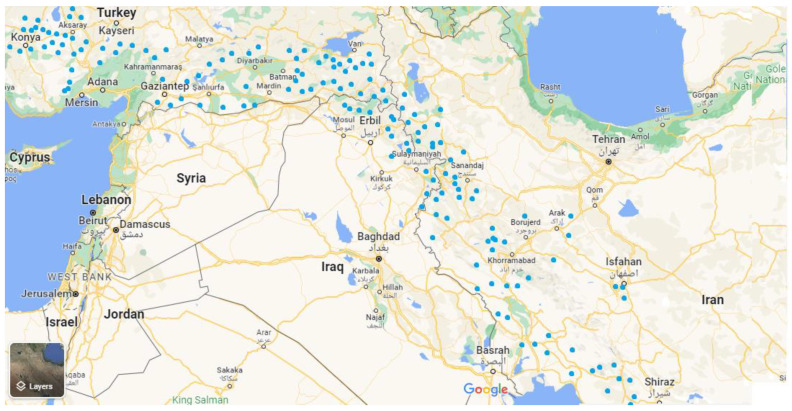
Distribution map of some Onosma species collected from different regions of Iran, Iraq, and Turkey is shown in Figure 3. The point inputs to the models developed in this study were collected from their habitats of Iran such as Fars, Lorestan, Khuzestan, Kermanshah, Hamedan, Markazi, Ilam, Kohgiluyeh, Kerman, and Boyer-Ahmad provinces, and their habitats of Iraq include mainly some areas of Kurdistan, Sulaimani, Hawraman, Rwanduz, and Amedia districts. Meanwhile, their habitats of Turkey includes Sirt, Hakary, Anatolia, and Van [27,28,29].
Figure 3.
Regional distribution of some Onosma species in Iran, Iraq, and Turkey [4,27,29].
5. Onosma Taxonomy
Onosma species belongs to the Boraginaceae family. The Boraginaceae family contains more than 100 genera and over 200 species, which are classified into five subfamilies: Boraginoideae, Cordioideae, Ehretioideae, Hydrophylloideae, and Lennooideae [30,31] (Table 1).
Table 1.
Onosma taxonomy according to Global Biodiversity Information Facility [1].
| Kingdom | Plantea |
|---|---|
| Phylum | Tracheophyta |
| Class | Angiosperms |
| Order | Boraginales |
| Family | Boraginaceae |
| Subfamily | Boraginoideae |
| Genus | Onosma L. |
6. Traditional Use
The folkloric use of many Onosma species as medicinal plants for different health problems by local ethnic groups in several countries such as Iraq, Turkey, Iran, China, and India roots back to hundreds of years ago. Almost all plant parts, such as leaves, roots, underground parts, flowers, and the whole plant of this genus species are reported to have a broad range of therapeutic potentials (Table 2) [32,33]. Species such as Onosma alborosea have traditionally been utilized by Iraqi Kurdistan populations as a remedy for sedative, heart diseases, and kidney disorders through ingesting its aerial part extracts prepared by aqueous extraction methods [33]. The aerial parts of Onosma orientalis has been macerated with hot water for treating sedatives by Kurdish nations living in Iraqi Kurdistan [33]. Furthermore, the O. armeniacum K. has been used as Turkish folkloric medicine for healing wounds, peptic ulcers, burns, dyspnea, hoarseness, hemorrhoids, and abdominal pains through methods of cooking and filtration of its roots with butter [34]. The extracts (oil and aqueous extracts) of O. argentatum and O. chlorotricum has been traditionally utilized in Turkey and Iran (Lorestan province) for the treatment of wounds and cutaneous injures [35,36]. Furthermore, the root extracts of O. hispidum Wall. have been used traditionally by the Iranian nation as curatives for headache, wounds, insect stings, bits, and inflammatory diseases, while its flowers have been ingested for cardiovascular problems [37]. Moreover, same species has been used as a dye and as a substitute for alkanet [38]. The O. bracteatum Wall. extracts have been reported as traditional herbal medicine as a tonic agent for improving the body’s immune system with enhancing regulation of urine output [39]. The O. bracteatum Wall. also has been used as remedy for asthma, respiratory problems, tonic, alterative, demulcent, diuretic, spasmolytic, rheumatoid arthritis, diuretic, and antileprotic in India, Nepal, Kashmir, and northwestern Himalayas countries [40,41]. The root extracts of O. sericeum have been traditionally used in cream preparations for skin injuries and burn scar treatments in Adıyaman, Turkey [13].
The O. microcarpum has traditional medicine record for the healing of wounds and burn scars by rural residents of Il’yca district, Erzurum, Turkey [36,42]. The leaf aqueous extracts of O. echioides DC. are prepared for children suffering from constipation and metabolic disorders. Meanwhile, its flowers are reported as a cordial and as a stimulant for orthopedic and cardiac problems [43]. The dried roots of O. paniculata have a traditional medicinal record in Chinese herbal medicine for curing several human diseases including tumors [44]. The O. aucheriana is another species with traditional medicinal usage for itchiness, leucoderma, bronchitis, abdominal pain, strangury, fever, wounds, burns, and urinary calculi. Meanwhile, its flowers have been highlighted as stimulants and cardio-tonics, and its leaf extracts have been ingested as laxatives, purgatives, and as wound curatives [45]. Out of more than 230 species of Onosma, only 12 species were reported in traditional medicines as herbal medicine until now. This could be due to the large geographical distribution of the Onosma species and lack of scientific interest in the past, but this number is expected to increase in upcoming years as the researchers extensively search and investigate for other Onosma species after discovering some interesting phytochemical and pharmacological potentials of this genus in recent years.
Table 2.
The traditional use of Onosma medicinal plants.
| Species | Traditional Name | Country of Habitat |
Medicinal Parts | Medicinal Use |
|---|---|---|---|---|
| O. alborosea | Safeen mountain, Shaqlawa district, Iraqi Kurdistan | Aerial parts | Sedative, heart diseases, kidney disorders [33] | |
| O. orientalis | Safeen mountain, Shaqlawa district, Iraqi Kurdistan | Aerial parts | Sedative [33] | |
| O. armeniacum | Turkey, Anatolia | Leaves | healing wound, peptic ulcers, burns, dyspnea, hoarseness, hemorrhoids, and abdominal pains [34] | |
| O. argentatum, | Turkey | roots | Wound healing [35] | |
| O. chlorotricum | Iran, Lorestan | roots | Wound healing [36] | |
| O. hispidum | Iran (Korrassan) | roots | headache, wounds, insect stings and bits, inflammatory diseases, while its flowers are used for cardiovascular problems [37] and as a dye and a substitute for alkanet [38] | |
| O. bracteatum Wall | Gaozaban, Sedge | India, Nepal, Kashmir, and in the northwestern Himalayas | Roots, flowers | asthma, respiratory problems, tonic, alterative, demulcent, diuretic, spasmolytic, rheumatoid arthritis, diuretic, and antileprotic [40,41]. |
| O. sericeum | Turkey, Adıyaman | roots | As curatives for cutaneous wounds and burns [13] | |
| O. microcarpum | Turkey, Il’yca district, Erzurum province | Roots and leaves | Wound healing [36] | |
| O. echioides | Turkey | Leaves and flowers | Laxatives for children and as a cordial, stimulant for orthopedic and cardiac problems [46] | |
| O. paniculata | China | roots | Anticancer [44] | |
| O. aucheriana | Turkey | Roots, leaves, flowers | itchiness, leucoderma, bronchitis, abdominal pain, strangury, fever, wounds, burns, and urinary calculi. Stimulants and cardio-tonics. Laxative, purgative, and as wound remedy [45] |
The traditional names, country, ingested parts, and medicinal purposes of the genus Onosma are listed in Table 2.
7. Chemical Profile of Onosma Species
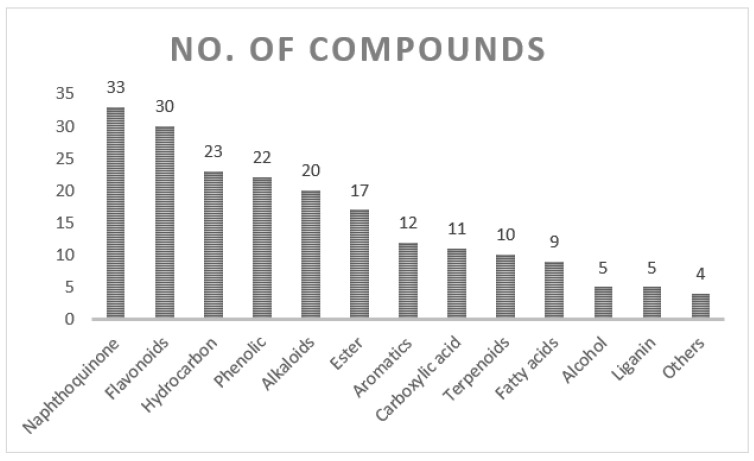
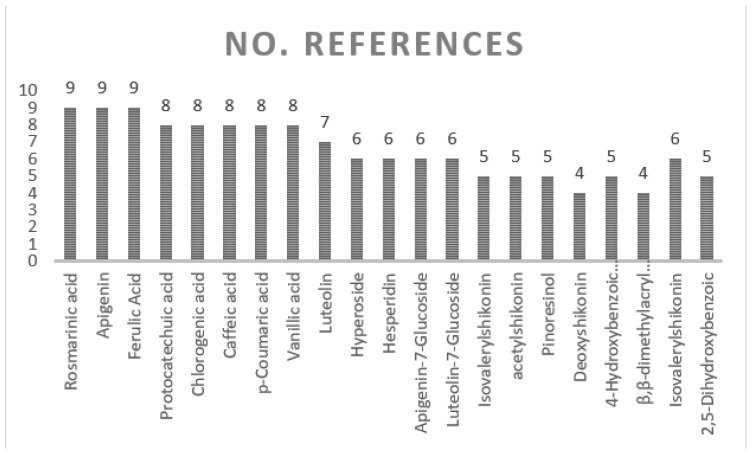
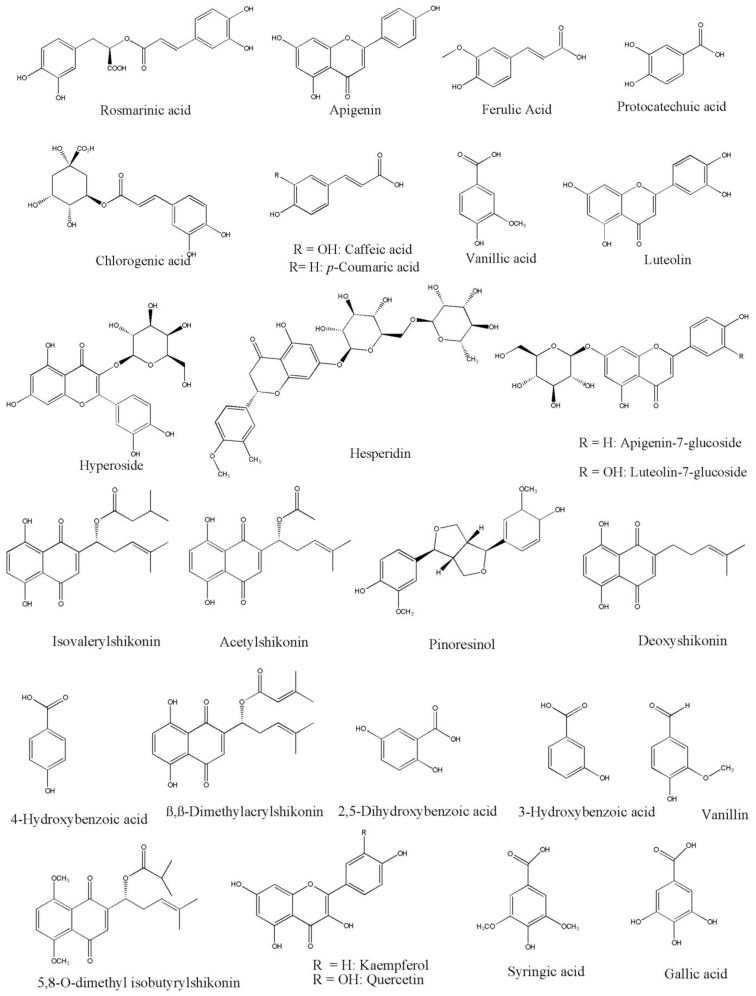
The current systematic review of the phytochemical contents of Onosma species presents major identified organic classes such as naphthoquinone (33), flavonoids (30), hydrocarbon (23), phenolic (22), ester (17), alkaloids (20), terpenoids (10), carboxylic acid (11), fatty acids (9), aromatics (12), and liganin (5) compounds as shown in Figure 4. In addition, miscellaneous chemicals such as 24,25-Dihydroxycholecalciferol, 5-hydroxymethyl-furoic acid, and uplandicine also enrich the diversity of the phytochemistry in Onosma plants. Segregation of phytochemical contents in different classes is challenging and not always a clear and easy task. According to the current search, a total of 198 compounds are detected in the Onosma species as detailed in this review (Table 3), and this will open up new future study opportunities to explore pharmacological potentials of those phytochemicals. Most common Onosma compounds reported were rosmarinic acid, apigenin, ferulic acid, protocatechuic acid, chlorogenic acid, caffeic acid, p-coumaric acid, vanillic acid, luteolin, hyperoside, hesperidin, apigenin-7-Glucoside, luteolin-7, glucoside, isovalerylshikonin, acetylshikonin, pinoresinol, deoxyshikonin, 4, hydroxybenzoic acid, β,β-dimethylacryl, isovalerylshikonin, 2,5-Dihydroxybenzoic, and 3-Hydroxybenzoic acid as presented in Figure 5.
Figure 4.
Organic class contents of Onosma species based on reported compounds.
Table 3.
Names and sources of compounds isolated from genus Onosma.
| No. | Chemical Names | Organic Class | Plant Species | Distribution in Plant | Reference | |
|---|---|---|---|---|---|---|
| 1 | Hyperoside | Flavonoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. trapezuntea, O. rigidum, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,47,48,49,50,51] | |
| 2 | Hesperidin | Flavonoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,48,49,51] | |
| 3 | Vanillic acid | Aromatics | O. isaurica, O. bracteosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. bracteatum, O. inexspectata, O. armenum, O. hispidum, O. mollis | Aerial parts | [10,14,30,39,47,49,51,52] | |
| 4 | Pinoresinol | Phenolics | O. isaurica, O. bracteosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. mollis, O. sericea, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,49,51] | |
| 5 | Apigenin-7-glucoside | Flaconoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,48,49,51] | |
| 6 | Apigenin | Flavonoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. gigantea, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. hispida, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,48,49,51,53,54] | |
| 7 | Ferulic acid | Phenolics | O. isaurica, O. bracteosa, O. sericea, O. lycaonica, O. papillosa. O. aucheriana, O. gigantea, O. pulchra O. frutescens, O. inexspectata, O. armenum, O. hispidum, O. mollis | Aerial parts | [10,14,15,47,48,49,51,52,53,55] | |
| 8 | Luteolin-7-glucoside | Flavonoid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. mollis, O. inexspectata, O. armenum | Aerial parts | [10,14,30,47,48,49,51] | |
| 9 | Luteolin | Flavonoid | O. isaurica, O. bracteosa, O. stenoloba, O. lycaonica, O. papillosa. O. gigantea, O. pulchra, O. frutescens, O. aucheriana, O. inexspectata, O. armenum, O. sericea, O. mollis | Aerial parts | [10,14,15,47,48,49,51,53] | |
| 10 | Rosmarinic acid | Aromatic | O. isaurica, O. inexspectata, O. armenum, O. bracteosa, O. lycaonica, O. papillosa, O. ambigens, O. aucheriana, O. gigantea, O. pulchra, O. frutescens, O. sericea, O. bracteatum, O. trapezuntea, O. rigidum, O. inexspectata, O. armenum, O. mutabilis, O. mollis | Aerial parts | [10,14,16,30,39,45,47,48,50,51,53] | |
| 11 | 3-Hydroxybenzoic acid | Carboxylic acid | O. isaurica, O. bracteosa, O. pulchra, O. aucheriana, O. sericea, O. inexspectata, O. armenum | Aerial parts | [10,14,47,49,51] | |
| 12 | Protocatechuic acid | Carboxylic acid | O. isaurica, O. bracteosa, O. gigantea, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. ambigens, O. bracteatum, O. mollis, O. inexspectata, O. armenum | Aerial parts | [14,30,39,45,47,49,51,53] | |
| 13 | Chlorogenic acid | Quinic acids | O. isaurica, O. bracteosa, O. ambigens, O. aucheriana, O. gigantea, O. pulchra, O. frutescens, O. sericea, O. trapezuntea, O. rigidum, O. mollis, O. inexspectata, O. armenum | Aerial parts | [14,30,45,47,49,50,51,53] | |
| 14 | Gentisic acid | Carboxylic acid | O. isaurica, O. bracteosa, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. lycaonica, O. papillosa, O. mollis | Aerial parts | [14,47,48,49] | |
| 15 | Caffeic acid | Carboxylic acid | O. isaurica, O. bracteosa, O. lycaonica, O. papillosa. O. aucheriana, O. gigantea, O. pulchra, O. bracteatum, O. inexspectata, O. armenum | Aerial parts | [10,14,39,45,47,48,51,53] | |
| 16 | p-Coumaric acid | Aromatics | O. isaurica, O. bracteosa, O. aucheriana, O. gigantea, O. pulchra, O. frutescens, O. sericea, O. lycaonica, O. papillosa, O. ambigens, O. inexspectata, O. armenum | Aerial parts | [10,14,30,45,46,47,48,49,51,53] | |
| 17 | Salvianic acid A | Phenolics | O. stenoloba, O. sericea | Aerial parts | [15] | |
| 18 | Verbascoside | Phenolics | O. sericea, O. aucheriana, | Aerial parts | [15,49] | |
| 19 | Rosmarinic acid-O-hexoside |
Aromatics | O. sericea, O. stenoloba | Aerial parts | [15] | |
| 20 | Apigenin-O-hexoside | Flavonoid | O. sericea, O. stenoloba | Aerial parts | [15] | |
| 21 | Methyl caffeate | Phenolics | O. sericea, O. stenoloba | Aerial parts | [15] | |
| 22 | Apigenin-O-rhamnosylhexoside | Phenolics | O. sericea, O. stenoloba | Aerial parts | [15] | |
| 23 | Diosmin | Flavonoid | O. sericea | Aerial parts | [15] | |
| 24 | O-Methylrosmarinic acid isomer | Phenolics | O. sericea | Aerial parts | [15] | |
| 25 | Tricin | Flavonoid | O. sericea | Aerial parts | [15] | |
| 26 | Cirsiliol | Flavonoid | O. sericea | Aerial parts | [15] | |
| 27 | Diosmetin | Flavonoid | O. sericea | Aerial parts | [15,55] | |
| 28 | Stearic acid | Fatty acid | O. sericea | Aerial parts | [15] | |
| 29 | Intermedine | Ester | O. stenoloba, O. alborosea, O. arenaria | Aerial parts, roots | [15,56] | |
| 30 | Lycopsamine | Alkaloid | O. stenoloba | Aerial parts | [15] | |
| 31 | Caffeoylshikimic acid isomer | Phenolics | O. stenoloba | Aerial parts | [15] | |
| 32 | Heliosupine | Pyrrolizidine Alkaloids | O. stenoloba | Aerial parts | [15] | |
| 33 | Vicenin-2 | Flavonoid | O. stenoloba | Aerial parts | [15] | |
| 34 | Echimidine | Pyrrolizidine Alkaloids | O. stenoloba | Aerial parts | [15] | |
| 35 | Isoferulic acid | Aromatics | O. stenoloba | Aerial parts | [15] | |
| 36 | Rosmarinic acid-di-Ohexoside | Aromatics | O. stenoloba | Aerial parts | [15] | |
| 37 | Quercetin-O-hexoside | Flavonoid | O. stenoloba, O. sericea | Aerial parts | [15] | |
| 38 | Kaempferol-O-hexoside | Ester | O. stenoloba, O. sericea | Aerial parts | [15] | |
| 39 | Isorhamnetin-O-rhamnosylhexoside | Flavonoid | O. stenoloba | Aerial parts | [15] | |
| 40 | Trihydroxyisoflavone | Flavonoid | O. stenoloba | Aerial parts | [15] | |
| 41 | Ursolic acid | O. stenoloba | Aerial parts | [15] | ||
| 42 | 4-Hydroxybenzoic acid | Triterpenoids | O. lycaonica, O. papillosa, O. ambigens, O. pulchra, O. frutescens, O. aucheriana, O. sericea, O. gigantea, O. aucheriana, O. bracteatum | Aerial parts | [14,30,39,48,49] | |
| 43 | Eriodictyol | Carboxylic acid | O. lycaonica, O. papillosa. | Aerial parts | [48] | |
| 44 | Vanillin | Phenolics | O. lycaonica, O. papillosa. O. pulchra, O. frutescens, O.aucherian, O. sericea | Aerial parts | [14,48,49,57] | |
| 45 | (+)-Catechin | Flavonoid | O. lycaonica, O. papillosa O. frutescens | Aerial parts | [48,49,57] | |
| 46 | Homoprotocatechuic acid | Phenolics | O. lycaonica, O. papillosa | Aerial parts | [48,57] | |
| 47 | Acetylshikonin | Naphthoquinones | O. heterophylla | Roots | [58] | |
| 48 | Shikonin derivatives | Naphthoquinones | O. heterophylla | Roots | [58] | |
| 49 | Acetyl shikonin | Naphthoquinones | O. heterophylla | Roots | [58] | |
| 50 | Shikonin derivatives | Naphthoquinones | O. visianii | Roots | [59] | |
| 51 | Shikonin derivatives | Naphthoquinones | O. visianii | Roots | [59] | |
| 52 | Isobutyrylshikonin | Naphthoquinones | O. visianii | Roots | [21,59] | |
| 53 | Isovalerylshikonin | Naphthoquinones | O. visianii, O. paniculata, O. exsertum, O. waltonii, O. paniculatum, O. hookeri, O. confertum, O. echioides, O. heterophylla | Roots | [21,22,59,60,61,62] | |
| 54 | α-methylbutyrylshikonin | Naphthoquinones | O. visianii | Roots | [21,59] | |
| 55 | 5,8-O-dimethyl deoxyshikonin | Naphthoquinones | O. visianii | Roots | [59,63] | |
| 56 | 5,8-O-dimethyl isobutyrylshikonin | Naphthoquinones | O. visianii | Roots | [21,59,61] | |
| 57 | deoxyshikonin | Naphthoquinones | O. visianii, O. paniculata, paniculatum | Roots | [21,59,60,62,63] | |
| 58 | Acetylshikonin | Naphthoquinones | O. visianii, O. confertum, O. echioides, O. setosum, O. paniculata, paniculatum | Roots | [21,59,60,61,64] | |
| 59 | β-Hydroxyisovalerylshikonin | Naphthoquinones | O. paniculata, O. heterophylla | Roots | [63,65] | |
| 60 | β,β-dimethylacrylshikonin | Naphthoquinones | O. paniculata, O. confertum, O. exsertum, O. waltonii, O. paniculatum, hookeri, Onosma hookeri, Onosma zerizaminum | Roots | [62,63,64,65] | |
| 61 | Methylbutyrylshikonin | Naphthoquinones | O. paniculata | Roots | [63] | |
| 62 | Isovalerylshikonin | Naphthoquinones | O. paniculata | Roots | [63] | |
| 63 | Gallic acid | Fatty acid | O. aucheriana, O. pulchra, O. frutescens, O. sericea | Aerial parts | [14,45,49] | |
| 64 | Quercetin | Flavonoid | O. aucheriana, O. pulchra, O. frutescens, O. sericea | Aerial parts | [14,45,49] | |
| 65 | Syringic acid | Fatty acid | O. aucheriana, O. pulchra, O. frutescens, O. sericea | Aerial parts | [14,49] | |
| 66 | Shikonin derivatives | Hydrocarbon | O. mutabilis | Aerial parts | [16] | |
| 67 | Shikonin derivatives | naphthoquinones | O. mutabilis | Aerial parts | [16] | |
| 68 | 3-O-Methyl-d-glucose | Hydrocarbon | O. mutabilis | Aerial parts | [16] | |
| 69 | 24,25-Dihydroxycholecalciferol | Vitamin D | O. mutabilis | Aerial parts | [16] | |
| 70 | β-Sitosterol | Phytosterol | O. mutabilis, O. heterophylla | Aerial parts, roots | [16,65] | |
| 71 | Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite | Phenolics | O. mutabilis | Aerial parts | [16] | |
| 72 | p-Hydroxybenzoic acid | Carboxylic acid | O. gigantea, O. aucheriana, O. bracteatum | Aerial parts | [39,45,53] | |
| 73 | trans-Cinnamic acid | Cinnamic acid | O. gigantea | Aerial parts | [53] | |
| 74 | Kaempferol | Flavonoid | O. gigantea, O. pulchra, O. frutescens, O. aucheriana, O. sericea | Aerial parts | [14,49,53] | |
| 75 | 3,4-Dihydroxyphenylacetic acid | Catechol | O. pulchra | Aerial parts | [14] | |
| 76 | Taxifolin | Flavonoid | O. pulchra, O. frutescens, O. aucheriana, O. sericea | Aerial parts | [14,49] | |
| 77 | Sinapic acid | Aromatics | O. pulchra, O. frutescens, O. aucheriana, O. sericea | Aerial parts | [14,49] | |
| 78 | Eriodictyol | Flavonoid | O. pulchra, O. frutescens, O. aucheriana, O. sericea | Aerial parts | [14,49] | |
| 79 | Shikonin derivatives | Naphthoquinones | O. echioides | Aerial parts | [64] | |
| 80 | Pulmonarioside C | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 81 | 9′-Methoxyl salvianolic acid | Flavonoid | O. bracteatum | Aerial parts | [39] | |
| 82 | 4-O-(E)-p-coumaroyl-l-threonic acid | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 83 | Coumarin | Aromatics | O. bracteatum | Aerial parts | [39] | |
| 84 | Umbelliferone | Aromatics | O. bracteatum | Aerial parts | [39] | |
| 85 | Scopoletin | Aromatics | F | Aerial parts | [39] | |
| 86 | 6,7-Dimethoxycoumarin | Aromatics | O. bracteatum | Aerial parts | [39] | |
| 87 | Esculetin | Aromatics | O. bracteatum | Aerial parts | [39] | |
| 88 | Caffeic acid methyl ester | Ester | O. bracteatum | Aerial parts | [39] | |
| 89 | 1-O-Caffeoyl glycerol | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 90 | Latifolicinin C | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 91 | Oresbiusin A | Phenolics | O. bracteatum | Aerial parts | [39] | |
| 92 | Ethyl 3-(3, 4-dihydroxyphenyl)lactate |
Phenolics | O. bracteatum | Aerial parts | [39] | |
| 93 | 4, 5-Dihydroxy-3-methoxybenzoic acid |
Carboxylic acid | O. bracteatum | Aerial parts | [39] | |
| 94 | 5-Hydroxymethyl-furoic acid | Furoic acid | O. bracteatum | Aerial parts | [39] | |
| 95 | 3,4-Dihydroxybenzyl alcohol | Alcohol | O. bracteatum | Aerial parts | [39] | |
| 96 | Rosmarinic acid methyl ester |
Ester | O. bracteatum | Aerial parts | [39] | |
| 97 | Salviaflaside methyl ester |
Ester | O. bracteatum | Aerial parts | [39] | |
| 98 | 9′-(2,3-Dihydroxypropyl)-rosmarinic acid |
Phenolics | O. bracteatum | Aerial parts | [39] | |
| 99 | p-Coumarinic acid ester of trigonotin |
Ester | O. bracteatum | Aerial parts | [39] | |
| 100 | Echiumin A | Liganin | O. bracteatum | Aerial parts | [39] | |
| 101 | Ternifoliuslignan A | Liganin | O. bracteatum | Aerial parts | [39] | |
| 102 | Ternifoliuslignan D |
Liganin | O. bracteatum | Aerial parts | [39] | |
| 103 | Eritrichin | Liganin | O. bracteatum | Aerial parts | [39] | |
| 104 | Shikonin derivatives | Naphthoquinon | O. bracteatum | Aerial parts | [39] | |
| 105 | Kaempferol 3-O-[α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside] | Flavonoid | O. bracteatum | Aerial parts | [39] | |
| 106 | Kaempferol 3-O-[α-l-rhamno pyranosyl-(1→6)-β-d-glucopyranoside] | Flavonoid | O. bracteatum | Aerial parts | [39] | |
| 107 | Impecylone A | Flavonoid | O. bracteatum | Aerial parts | [39] | |
| 108 | Tigloylshikonin | Naphthoquinon | O. hookeri | Roots | [66] | |
| 109 | Acetyl shikonin | Naphthoquinon | O. hispidum | Roots | [67] | |
| 110 | Alkannan | Naphthoquinon | O. hispidum, O. echioides | Roots | [67] | |
| 111 | Deoxyshikonin | Naphthoquinon | O. hispidum, O. echioides, O. confertum | Roots | [62,67] | |
| 112 | 7-O-acetylechinatine N-oxide | Alkaloid | O. erects | Roots | [68] | |
| 113 | Viridinatine N-oxide stereoisomer | Alkaloid | O. erects | Roots | [68] | |
| 114 | 7-Epi-echimiplatine Noxide | Alkaloid | O. erects | Roots | [68] | |
| 115 | Onosmerectine N-oxide |
Alkaloid | O. erects | Roots | [68] | |
| 116 | Acid 2,3-dimethyl-2,3,4-trihydroxypentanoic acid | Alkaloid | O. erects | Roots | [68] | |
| 117 | Acyloin 4-methyl-2-hydroxypentanon | Alkaloid | O. erects | Roots | [68] | |
| 118 | 2-Methyl-n-butyrylshikonin | Naphthoquinon | O. exsertum, O. waltonii, O. paniculatum, hookeri, O. confertum | Roots | [62] | |
| 119 | β-Acetoxyisovalerylshikonin | Naphthoquinon | O. exsertum, O. waltonii, O. paniculatum, O. hookeri, O. confertum | Roots | [62] | |
| 120 | Isobutylshikonin | Naphthoquinon | O. exsertum, O. waltonii, O. paniculatum, O. hookeri, O. confertum | Roots | [62] | |
| 121 | Alkannin | Naphthoquinon | O. echioides, O. paniculata | Roots | [65] | |
| 122 | Shikonin | Naphthoquinon | O. caucasicum, O. conferitum, O. hookeri, O. livanovii, O. polyphyllum, O. tauricum, O. sericium, O. setosum, O. visianii, O. zerizaminium | Roots | [65] | |
| 123 | β,β-dimethylacrylalkannin | Naphthoquinon | O. heterophylla, O. hookeri, O. paniculata | Roots | [65] | |
| 124 | Heliotridine | Alkaloid | O. heterophyllum | Roots | [58] | |
| 125 | Necine derivative (1-methyl-8(-pyrrolizine) | Alkaloid | O. heterophyllum | Roots | [58] | |
| 126 | Acetylintermedine | Alkaloid | O. alborosea, O. arenaria | Roots | [56,57] | |
| 127 | O7-Acetyllycopsamine | Alkaloid | O. alborosea, O. arenaria | Roots | [56,57] | |
| 128 | 5,6-Dihydro-7,9-dimethoxy 7H- pyrrolizine |
Alkaloid | O. arenaria | Roots | [56] | |
| 129 | 7-Acetylretronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 130 | 9-(Butyryl-2-ene) supinidine | O. arenaria | Roots | [56] | ||
| 131 | 7-Acetyl-9-(2-methylbutyryl) retronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 132 | 7-Acetyl-9-(2,3-dimethylbutyryl) retronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 133 | 7-Acetyl-9-(2-hydroxy-3-methylbutyryl) retronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 134 | 3′-Acetylsupinine | Alkaloid | O. arenaria | Roots | [56] | |
| 135 | 7-Acetyl-9-(2,3-dihydroxybutyryl) retronecine | Alkaloid | O. arenaria | Roots | [56] | |
| 136 | Uplandicine | Pyrrolizines | O. arenaria | Roots | [56] | |
| 137 | Palmitic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 138 | Oleic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 139 | Linolenic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 140 | γ-Linolenic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 141 | Stearidonic acid | Fatty acid | O. irrigans | Fruits | [69] | |
| 142 | monoenoic acids 20:1, 22:1, and 24:1 | Fatty acid | O. irrigans | Fruits | [69] | |
| 143 | Hexahydrofarnesyl acetone | Fatty acid | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 144 | Phytol | Diterpenoid | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 145 | Farnesyl acetone | Diterpenoid | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 146 | Hexadecanal | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 147 | Hexyl hexanoate | Ester | O. isaurica | Aerial parts | [70] | |
| 148 | (E)-2-Decenal | Medium-chain aldehyde Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 149 | 1-Hexadecene | Unsaturated aliphatic Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 150 | Safranal | Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 151 | Heptadecane | Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 152 | Dodecanal | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 153 | E)-2-Undecenal | Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 154 | Tridecanal | Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 155 | (E)-Geranyl acetone | Diterpenoid | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 156 | 1-Isobutyl-4-isopropyl-2,2-dimethyl succinate | Dicarboxylic acid | O. bulbotrichum | Aerial parts | [70] | |
| 157 | Neophytadiene isomer I | Terpenoid | O. isaurica | Aerial parts | [70] | |
| 158 | Tetradecanal | Hydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 159 | (E)-β-Ionone | Sesquiterpenoid | O. bulbotrichum | Aerial parts | [70] | |
| 160 | Neophytadiene | Sesquiterpenoid | O. isaurica | Aerial parts | [70] | |
| 161 | Pentadecanal | Sydrocarbon | O. bulbotrichum | Aerial parts | [70] | |
| 162 | (E)-Nerolidol | Sesquiterpenoid | O. bulbotrichum | Aerial parts | [70] | |
| 163 | Hexadecanal | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 164 | 3,4-Dimethyl-5-pentylidene-2(5H)-furanone | Phenolics | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 165 | 3,4-Dimethyl-5-pentyl-5H-furan-2-one | Phenolics | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 166 | Carvacrol | Monoterpenoid | O. bulbotrichum | Aerial parts | [70] | |
| 167 | Tricosane | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 168 | (2E, 6E)-Farnesol | Sesquiterpenoid | O. bulbotrichum | Aerial parts | [70] | |
| 169 | Tetracosane | Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 170 | Pentacosane | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 171 | Geranyl linalool | Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 172 | Heptacosane | Hydrocarbon | O. bulbotrichum, O. isaurica | Aerial parts | [70] | |
| 173 | Nonacosane | Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 174 | 1-Docosene | Unsaturated aliphatic Hydrocarbon | O. isaurica | Aerial parts | [70] | |
| 175 | isorhamnetin-3-O-rutinoside | Flavonoid | O. stellulata | Aerial parts | [71] | |
| 176 | sinapic acid | Aromatic | O. stellulata | Aerial parts | [71] | |
| 177 | Deoxyshikonin [2-(4-methyl-pent-3-enyl)-5,8-dihydroxynaphthalene-1,4-dione] | Naphthoquinon | O. nigricaule | roots | [72] | |
| 178 | β, β - Dimethylacrylshikonin (5,8-Dihydroxy-2-[1-(β, β -dimethy lacryloyloxy)-4-methyl-3-pentenyl]-1,4-naphthalenedion] |
Naphthoquinon | O. nigricaule | Roots | [72] | |
| 179 | Acetyl shikonin [(+)-Acetic acid 1-(5,8-dihydroxy-1,4- dioxo-1,4-dihydro-naphthalen-2-yl)-4-methyl-pent-3-enyl ester] | Naphthoquinon | O. nigricaule | Roots | [72] | |
| 180 | 2-[(4-methylbenzyl)amino]benzoic acid | Carboxylic acid | O. hispida | Whole plant | [54] | |
| 181 | Methyl 2-[(4-methylbenzyl)amino]benzoate | Flavonoid | O. hispida | Whole plant | [54] | |
| 182 | 6,4′-Dimethoxy-3,5,7-trihydroxyflavone | Flavonoid | O. hispida | Whole plant | [54] | |
| 183 | apigenin 7-O-β-d-glucoside | Flavonoid | O. hispida | Whole plant | [54] | |
| 184 | Paraffins | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 185 | n-Dodecane | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 186 | n-Decatrian | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 187 | Methyl dodecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 188 | Methyl tetradecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 189 | Methyl 4-methyl tetradodecan-9,12 dien-oate | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 190 | Methyl 4-methyl tetradodec-9-ene-oate | Hydrocarbon | O. heterophylla | Roots | [62] | |
| 191 | Methyl 4-methyl hexadec-9-ene-oate | Methyl ester | O. heterophylla | Roots | [62] | |
| 192 | Methyl hexadecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 193 | Ethyl hexadecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 194 | Isopropyl hexadecanoate | Methyl ester | O. heterophylla | Roots | [62] | |
| 195 | Methyl octadeca-9,12,15-triene-oate | Methyl ester | O. heterophylla | Roots | [62] | |
| 196 | Methyl octadeca-9,12-diene-oate | Methyl ester | O. heterophylla | Roots | [62] | |
| 197 | Methyl octadec-9-ene-oate | Methyl ester | O. heterophylla | Roots | [62] | |
| 198 | Methyl octadecanoate | Methyl ester | O. heterophylla | Roots | [65] | |
| 199 | diosmetin-7-O-β-glucoside | Aromatics | O. bourgaei | Aerial parts | [55] | |
| 200 | allantoin | Imidazoles | O. bourgaei | Aerial parts | [55] | |
| 201 | globoidnan A | Liganin | O. bourgaei | Aerial parts | [55] | |
Figure 5.
Most common Onosma compounds based on the repetition in the literature.
The bioactive structures of identified and characterized representative compounds, which are based on the repetition across published studies are shown in Figure 5, in addition to Figure 6.
Figure 6.
Representative of main compounds isolated from Onosma species, repeated in the literature.
8. Toxicity Study of the Onosma Species
8.1. Toxicity In Vivo Experiment
The chloroform and ethanolic extracts of O. aucheranum, O. isauricum O. sericeum, O. tauricum, and O. tauricum were safe in the administered doses from 100 mg/kg to 200 mg/kg based on the assessment of acute toxicity in the carrageenan-induced paw edema experiment as no abnormality in the morbidity nor mortality was recorded after 24 hours post treatment [73]. Furthermore, the 100, 200, 300, and 600 mg/kg of the MeOH of O. mutabilis administration to rats showed no changes in the appearance, behavior, and feed intake of the rats in a 7-day experiment [16]. Moreover, by the tarsal toxicity test, researchers have shown the acaricidal activity of the root extracts of O. visianii experimented against Tetranychus urticae mites in bean plants (P. vulgaris var. Carmen) after 24 h (considered as acute toxicity), which caused significant mortality of T. urticae adults with lethal doses 83.2 and 112.6 μg·cm causing 50% (LD50) and 90% (LD90) inhibition of oviposition, respectively. However, at 5 days (considered as chronic toxicity) from the start of the test, the lethal dose LD50 was more than 30 times lower (2.6 μg·cm−2) as a function of time used in the LD50 calculation [60]. Over the last two decades, several Onosma species have been tested for their toxicity to laboratory animal models. A study on toxicity of the bark extracts of O. echioides roots to Sprague Dawley rats (140 ± 10 g body weight) was performed and reported significant improvement in the body weight, food consumption, water intake, serum glucose, hematology, and biochemistry of rats with no adverse effect at a fixed dose [74].
8.2. Genotoxicity and Mutagenicity
Through the Allium-test, significant genotoxic effect from aqueous extracts of O. stellulata roots and aerial parts were observed in mitosis at meristematic cells of onion. Although the aerial parts showed significant genotoxicity after 4-h treatment (mitotic index was 2, 79%, vs. 9, 18% for control), but the root aqueous extracts had higher genotoxic effects. Genotoxic effects included changes in the structure of chromosomes (conglutination, spirality), and cytotoxic reaction and certain differentiation in the cell cycle, which were found to be in correlation with duration of treatment and solution concentration [75]. A genotoxic study by Allium anaphase–telophase assay reported that the safety of the ethanolic extract of O. aucheriana aerial parts at lower dose (62.5 mg/mL) had no toxic or genotoxic effects, while the higher dose (500 mg/mL) showed significantly the highest genotoxic effect including chromosomal aberrations, cells with multipolarity, cell bridges, and vagrant chromosomes (24.4%), cell fragments, and mitosis entrance [76]. In vivo genotoxic study of methanolic extracts of O. sericea and O. stenoloba at different doses (25, 50, 100, 200, and 400 μg/mL) against EMS-induced DNA damage in the flies and larvae of the wild-type strain of Drosophila melanogaster showed the absence of genotoxic effect of O. sericea and O. stenoloba at concentration 80 mg/mL. Furthermore, significant antigenotoxic effects reported after dual treatment with 80 mg/mL of both plant extracts plus EMS (ethyl methane sulfonate) caused significant decrease in DNA damage (with over 80% reduction) [15]. By using Ames assay, the antimutagenic potential of ethanolic extract of O. bracteatum has been reported against sodium azide and 2-aminofluorene mutagenicity in Salmonella typhimurium in TA100 strain (-S9 mix) as it displayed significant inhibition rate (82.30% at 250 mg/0.1 mL/plate), showing strong modulation of genotoxicity of base-pair substitution mutagen sodium azide when compared to NPD (frameshift mutagen) in TA98 tester strain. The O. bracteatum extracts showed significant antimutagenicity activity for preincubation mode than in co-incubation approach without -S9 in both TA100 and TA98 [77].
9. Pharmacological Activity of the Onosma Species
9.1. Antibacterial Activity
The essential oils isolated from roots of O. sieheana showed appreciable antibacterial activity against gram negative bacteria (Escherichia coli (MIC: 125 μg/mL) and Pseudomonas aeruginosa (MIC: 125 μg/mL) and gram positive bacteria (Staphylococcus aureus (MIC: 125 μg/mL) and Bacillus subtilis (MIC: 250 μg/mL)) [78]. The n-hexane–dichloromethane mixture extracts of O. argentatum roots showed antibacterial activity against Bacillus subtilis, Escherichia coli, and Staphylococcus aureus with MIC values 28, 13, and 32 μg/mL, respectively [18]. The chloroform fraction of O. khyberianum whole plant parts showed significant antibacterial activity against Salmonella typhi, Shigella dysenteriae, and Vibrio cholera inhibition zone 28, 26, 26 mm, respectively. Ethanol fraction of O. khyberianum demonstrated significant antiradical activity against Shigella dysenteriae (21 mm) and Vibrio cholera (20 mm), while the least active fraction of O. khyberianum n-hexane showed activity against Vibrio cholera, S. aureus, and Shigella dysenteriae (inhibition zone: 12, 9, 8 mm, respectively) but completely inactive against Salmonella and E. coli [79]. The crude ethanolic extracts of O. hispidum roots showed significant antibacterial activity against several gram positive and gram negative bacteria (Corynebacterium diphtheria, C. diphtheriticum, Micrococcus lysodiecticus, S. aureus, S. epidermidis, S. saprophyticus, Enterococcus faecalis, E. faecalis 2400, E. faecium, Streptococcus pneumonia, and S. pyogenes) with inhibition zone range between 18–20 mm [52]. The isolated naphtshoquinones (deoxyshikonin, isobutyrylshikonin, α- methylbutyrylshikonin, acetylshikonin, β-hydroxyisovalerylshikonin, 5,8-O-dimethyl isobutyrylshikonin, and 5,8-O-dimethyl deoxyshikonin) from O. visanii roots showed significant antibacterial activity against gram negative bacteria (Citrobacter koseri, Hafnia alvei, maltophilia, Yersinia intermedia, Ps. proteolytica, and Stenotrophomonas) and gram positive bacteria (Bacillus megaterium, Enterococcus faecalis, S. epidermidis, Microbacterium arborescens, and Micrococcus luteus) with MIC50 and MIC90 values between range 4.27–68.27 μg/mL and 4.77–76.20 μg/mL, respectively [61]. The antibacterial activity (MIC values) from methanol extract of aerial parts of O. sericea and O. stenoloba were between 2.5−10 mg/mL. Both Onosma extracts had moderate antibacterial activity only on a few strains, namely A. chroococcum and E. coli with MIC values 2.5 and 5 mg/L, respectively. O. sericea extract exhibited low activity on gram positive strain M. lysodeikticus with MIC 10 mg/mL, while O. stenoloba extract showed notable antibacterial action on E. faecalis and A. tumefaciens with MIC values 5 and 10 mg/mL, respectively [15].
9.2. Antifungal Activity
Antifungal activity of methanolic extracts of O. sericea and O. stenoloba aerial parts against fungal strains Phialophare fastigiata and Fusarium oxysporum has been reported as 2.5 and 5 μg/mLof MIC, respectively. Furthermore, the methanol extracts of O. sericea exhibited moderate activity (MIC range of 2.5−5 μg/mL) on Penicillium canescens FSB 24 and P. cyclopium FSB 23, while O. stenoloba had antifungal activity only against P. cyclopium (MIC 10 μg/mL). Moreover, the same study showed antifungal potentials (MIC 10 μg/mL) of O. sericea against Trichoderma longibrachiatum FSB 13 and Trichoderma harzianum FSB 12. Meanwhile, increased concentration (10 μg/mL) of Onosma extracts showed inactivity against Aspergillus niger FSB 31, Aspergillus glaucus FSB 32, Doratomyces stemonitis FSB 41, Phialophora fastigiata FSB 81, Alternaria alternata FSB 51, and Fusarium oxysporum FSB 91 [15]. The methanol extracts from aerial parts of O. griffithii exhibit antifungal activity against Aspergillus flavus (55%) and Fusarium solani (40%). Meanwhile, the chloroformic extracts showed better antifungal activity against A. flavus (59%) and Fusarium solani (60%) [17]. The antifungal activity of O. kheberianum against three fungal strains, Fusarium oxysporum, Alternaria alternate, and A. flavus were reported as 18, 13, and 7 mm, respectively, for ethanol fractions and 17, 11, and 9 mm, respectively, for chloroform fractions [79]. A previous study also showed a lack of antifungal activity of n-hexane–dichloromethane extracts of O. argentatum roots against Trichophyton tonsurans, Trichophyton interdigitale, Microphyton gypseum, and Candida albicans [18]. The essential oils from O. sieheana Hayek roots showed significant antifungal activity against yeast strains Candida glabrata and C. albicans, and the authors linked this activity with their phytoconstituents, namely Monoterpenes, such as cymene and thymol [80]. The essential oils from O. chlorotricum roots exhibit higher antifungal activity (21 and 19.3 mean of inhibition zones (mm) against C. albicans and C. glaberata, respectively) than that of essential oils from O. microcarpum roots [80]. The O. paniculatum cells showed strong response to fungal elicitors from Aspergillus sp., in an attempt to accelerate shikonin derivative formation and inversely arrest plant cell growth, which resulted in a slight change in shikonin contents [81].
9.3. Antioxidant Activity
Onosma species have been comprehensively studied and researchers have revealed that they are a promising resources of antioxidants using various types of extraction and solvent methods [14,82,83]. The O. ambigens aerial part extracts exhibited notable antioxidant action in the phosphomolybdenum, CUPRAC, FRAP, DPPH, and ABTS assays with values of 1.65, 0.95, 0.52, 1.86, and 1.45 mg/mL, respectively [30]. The antioxidant activity of O. gigantea were significant in phosphomolybdenum (134.31 μmol trolox (TEs)/g air dry matter (adm)), chelating effect (32.97 μmol (EDTAEs)/g adm), on DPPH (32.14 μmol TEs/g adm) and ABTS (58.68 μmol TEs/g adm)), and reducing power (CUPRAC (50.23 μmol TEs/g adm) and FRAP (40.96 μmol TEs/g adm)) assays [53].
The water extract of the aerial part of O. pulchra showed significant antioxidant actions in DPPH, ABTS, CUPRAC, and ferrous ion chelating tests (3.90, 2.55, 2.20, and 1.23 mg/mL, respectively). Meanwhile, the Phosphomolybdenum and FRAP assays showed superiority of MeOH extract (1.98 and 1.02 mg/mL, respectively) [14]. The ethanol extract of aerial parts of O. bracteatum showed significant radical quenching activity in superoxide radical scavenging (EC50: 115.14 μg/mL) and lipid peroxidation (EC50: 199.33 μg/mL) assays [77]. The methanol extracts of O. mutabilis showed higher antioxidant activity than that of water and ethyl acetate fractions, respectively, in which the antioxidant values for methanol extracts were 1.45 ± 0.05, 3.54 ± 0.064, 2.33 ± 0.045, 1.12 ± 0.023, and 1.62 ± 0.079 mg/mL in phosphomolybdenum, DPPH scavenging, ABTS, FRAP, and CUPRAC reducing, respectively [16]. The methanol extract of aerial parts of O. frutescens showed significantly higher antioxidant activity in DPPH (1.14 mg/mL), ABTS (1.04 mg/mL), CUPRAC (0.53 mg/mL), FRAP (0.35 mg/mL), and phosphomolybdenum (1.18 mg/mL) tests than that (1.75,1.50, 0.87, 0.55, 1.97 mg/mL) and (2.18, 1.87, 0.99, 0.63, 1.92 mg/mL) for O. sericea and O. aucheriana, respectively. The ferrous ion chelating assays showed superiority of O. aucheriana (IC50: 2.57 mg/mL) over O. frutescens (4.68 mg/mL) and O. sericea (6.18 mg/mL) [49]. The aqueous extract of O. aucheriana roots showed significant antioxidant activity in radical quenching activity (ABTS, DPPH) with IC50 values as 9.89 and 17.73 μg/mL. Additionally, the same species showed notable lipid peroxidation inhibition, and hydroxyl radical scavenging actions with IC50 values, 23.41 and 31.09 μg/mL, respectively [45]. The methanolic extracts of O. trapezuntea aerial parts showed stronger antioxidant activity (IC50: 3.05 mg/mL in DPPH and 7.19 mg/mL in ABTS) than that (IC50: 2.63 mg/mL in DPPH and 5.23 mg/mL in ABTS) of O. rigidum [50]. The O. argentatum root extracts (0.1% concentration) by n-hexane–dichloromethane mixture (1:1) showed significant 98% antioxidant activity (IC50: 0.0076% w/v) by thiobarbituric acid (TBA) [18]. The methanol extract of aerial parts of O. lycaonica Hub. -Mor. exhibited stronger antioxidant activity in 1,1-diphenyl-2-picrylhydrazyl scavenging activity (2.69 ± 0.10 mg/mL), cupric reducing antioxidant power (1.10 ± 0.01), ferric reducing antioxidant power (0.69 ± 0.01 mg/mL), and ferrous ion chelating activity (2.32 ± 0.16 mg/mL) than that of O. papillosa. However, the O. papillosa showed lower IC50 or EC50 values for phosphomolybdenum (1.90 ± 0.07 mg/mL) when compared to O. lycaonica (2.05 ± 0.07 mg/mL), which could be related to their phytochemical contents as O. lycaonica had higher phenolic contents, with (43.5 ± 1.5 mg (gallic acid equivalent)/g extracts), whereas O. papillosa was higher in flavonoids (32.9 ± 0.3 mg (quercetin equivalent)/g extracts) [48]. The aerial part ethanol extracts of O. hookeri showed the same 2,2-diphenyl-1-picrylhydrazyl (77.77 ± 1.44 μg/mL) scavenging activity as butylated hydroxy toluene (72.70 ± 1.04 μg/mL), but slightly weaker 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (553.56 ± 2.78 μg/mL) scavenging activity and total antioxidant capacity than that of BHT (51.44 ± 1.37 μg/mL), while the ethyl acetate fraction of O. hookeri showed better ABTS scavenger, with IC50 value of 84.83 ± 1.37 μg/mL [66]. The aerial part MeOH extracts of O. sericea significant antioxidant activity in DPPH scavenging (130.23 ± 5.31 mg TE/g extract), ABTS scavenging (235.53 ± 4.62 mg TE/g extract), FRAP (215.65 ± 2.51 mg TE/g extract), CUPRAC (359.63 ± 14.83 mg TE/g extract), total antioxidant capacity (2.46 ± 0.35 mmol TE/g extract), metal chelating activity (24.65 ± 2.21 mgEDTAE/g extract), while O. stenoloba stronger activity with values 53.96 ± 0.78, 95.60 ± 2.30, 76.48 ± 3.26, 142.88 ± 1.49 mg TE/g, 1.16 ± 0.05 mmol TE/g, and 5.51 ± 0.81 mg EDTAE/g in the same essays, respectively [81]. The aerial part extract of O. isauricum exhibited significant antioxidant actions with superiority of its methanol extracts in DPPH (34.75 mg/mL) and CUPRAC (0.643 mg/mL), ferric reducing powers (0.211 mg/mL), ABTS (188.68 mgTE/g extract), superoxide radical scavenging ability (97.50 mgTE/g extract), and total antioxidant ability (86.02 mgAAE/g extract) than that (31.44 mg/mL, 0.471 mg/mL, 0.237 mg/mL, 130.91 mgTE/g, 159.92 mgTE/g, 55.36 mgAAE/g) and (4.69 mg/mL, 0.078 mg/mL, 0.021 mg/mL, 131.94 mgTE/g, 103.23 mgTE/g, 31.17 mgAAE/g extract) for water and ethyl extracts, respectively [83]. The results of antioxidant investigations of O. mollis showed significant radical scavenging actions phosphomolybdenum, DPPH, and ABTS, (2.01, 3.33, 2.30 mg/mL, respectively) while reducing power activity, CUPRAC and FRAP, were found as 1.48 and 0.79 mg/mL, respectively [51].
9.4. Cytotoxicity Activity
For the past decades, several studies have confirmed the traditional usage of the Onosma species as cytotoxic agents, and mammalian cancer cell division was inhibited by its extracts and isolated compounds [45,55,60].
The methanol extract of O. mutabilis aerial parts indicated significant anticancer activity against prostate (DU-145), mammary (MCF-7), and cervical cancer (Hep2c) cells with IC50 values as 35.67 ± 0.15, 28.79 ± 0.23, and 41.83 ± 0.21 μg/mL, respectively [55]. The crude extracts of O. aucheriana showed significant cytotoxicity activity against human rhabdomyosarcoma, human cervix carcinoma Hep2c, and from murine fibroblast (L2OB) cell lines with IC50 values range between 25.54 to 50.57 μg/mL [45]. The isolated compounds acetylshikonin, dimethylacrylshikonin, α-methylbutyrylshikonin, and isovalerylshikonin from the roots of O. paniculata showed appreciable anticancer activity against human CCRF-CEM leukemia, MDA-MB-231 breast cancer, human U251 glioblastoma, HCT 116 colon cancer, and human melanoma (SBcl2, WM35, WM9, WM164) cell lines with IC50 values ranging between 600 nM to 70 μM [60]. The isolated naphtshoquinones α-methylbutyrylshikonin and acetylshikonin compounds from O. visanii roots demonstrated stronger cytotoxic activity against MDAMB-231 cells (IC50: 86.0 μg/mL and 80.2 μg/mL, respectively) than that of 118.9, 204.6, 424.7, 391.6, and 411.5 μg/mL of Deoxyshikonin, β-Hydroxyisovalerylshikonin, Isobutyrylshikonin, 5,8-O-Dimethyl deoxyshikonin, and 5,8-O-Dimethyl isobutyrylshikonin, respectively. Additionally, all compounds except 5,8-O-Dimethyl deoxyshikonin, and 5,8-O-Dimethyl isobutyrylshikonin reduced viability of MDA-MB-231 cells after 48 h of incubation. Furthermore, α-methylbutyrylshikonin demonstrated the higher anticancer activity against HCT116 cells (IC50: 15.2 μg/mL) than that 97.8 μg/mL, 24.6 μg/mL and 30.9 μg/mL of Deoxyshikonin, Acetylshikonin, and β-Hydroxyisovalerylshikonin, respectively [61]. The effect of Onosma bracteatum has been studied against different cancer cell lines and the results showed that various concentrations (0.055, 0.11, 0.22, 0.44, 0.88, 1.7, and 3.52 µg/mL) of O. bracteatum decreased viability of cells in a time- and dose-dependent protocol [84]. Furthermore, the hydrochloric root extracts of O. dichroanthum Boiss. roots have shown significant anticancer actions against gastric cancer cells [11]. Moreover, O. paniculata has shown notable cytotoxicity activity against a number of cancer lines and linked their action with its ability to accelerate apoptosis [60]. The 50 µg/mL ethanolic extract from aerial parts of O. sericeum exhibited significant cytotoxicity activity against the breast cancer cells (MCF-7) with significantly decreased cell viability (28.76 ± 11.31%) [13]. The petroleum ether and aqueous extracts of O. hispidum roots have shown significant anticancer actions against HepG2 liver cancer cell lines [85].
9.5. Enzyme Inhibitory Activity
9.5.1. Antidiabetic Activity
A literature search revealed multiple research works that confirmed the anti-diabetics properties of Onosma species as the in vitro antidiabetic activity of Onosma species was reported based on its inhibitory potentials on α-amylase and glucosidase enzymes. The ethyl acetate extraction of aerial parts of O. gigantea showed higher α-amylase and glucosidase inhibitory activity (15.98 and 1.07 μmol/g) than that (410.50 and 6.75 μmol/g) and (1320.53 and 5.16μmol/g) of methanol and water extracts, respectively [53]. The α-amylase inhibitory activity from MeOH extracts of O. aucheriana and O. sericea were reported higher (2.50 and 2.51 mg/mL, respectively) than that (3.15 mg/mL) of O. frutescens [49]. The ethyl acetate extraction of O. ambigens aerial parts showed stronger α-amylase inhibitory activity (IC50: 2.64 mg/mL) than that (2.98 and 16.34 mg/mL) for methanol and water extracts, respectively [30]. The methanol extracts of O. lycaonica and O. papillosa aerial parts exhibited significant α-amylase inhibitory concentration (IC50: 2.57 and 2.40 mg/mL) and glucosidase inhibition (IC50: 2.60 and 2.61 mg/mL), respectively [48]. The ethyl acetate extract of O. pulchra aerial parts showed higher α-amylase inhibitory activity (2.40 mg/mL) than that (5.47 and 19.23 mg/mL) of methanol and water extracts, respectively [14]. The aerial part extraction of O. rigidum showed higher glucosidase and lower α-amylase enzyme inhibitory activity than that of O. trapezuntea extracts [50]. The MeOH aerial extracts of O. stenoloba exhibited higher α-amylase and lower glucosidase inhibitory activity (0.89 and 43.47 mmol/g) than that (1.26 and 33.38 mmol/g) of O. sericea, respectively [15]. The hydroalcoholic extract of the aerial part of O. Dichroanthum was reported to have anti-diabetic and anti-neuropathy properties based on its ability to down regulation of the MDA and Glutathione levels in homogenized tissues of brain and liver in a rat experiment [86]. The petroleum ether, chloroform, and methanol extracts of O. hispidum wall roots have shown significant anticancer actions with inhibitory percentages reported as 70, 58, and 50%, respectively. Meanwhile, the superiority of petroleum ether extracts has been linked with its higher polyphenolic contents [85].
9.5.2. Alzheimer’s Disease
The protective effect of Onosma species against Alzheimer’s disease was reported depending on its inhibitory activity on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes. The ethyl acetate extraction of aerial parts of O. gigantea showed higher AChE and BChE inhibitory activity (2.76 and 6.87 μmol/g, respectively) than that (31.57 and 1.82 μmol/g, respectively) of methanol extracts [53]. The isolated hispidone and (2S)-5,2-dihydroxy-7,5-dimethoxyflavanone from methanol extractions of whole plant parts of O. hispida showed significant inhibitory activity against AChE (11.6 and 15.7 mg/mL, respectively) and BChE (28.0 and 7.9 mg/mL, respectively) enzymes [38]. The aerial part extracts of O. lycaonica and O. papillosa exhibited significant AChE inhibition activity (IC50:1.32 and increased BChE inhibitory activity (2.31 ± 0.04 and 2.07 ± 0.1 (2.31 ± 0.04 and 2.07 ± 0.08 mg GALAEs/g extracts), respectively [48]. The MeOH extraction of O. rigidum aerial parts showed higher AChE and lower BChE inhibitory activity than that of O. trapezuntea extracts [50]. O. sericea aerial part extracts showed higher inhibitory activity on AChE (3.74 mg/g) and BChE (0.51 mg/g) than that (4.34 and 3.44 mg/g) for O. stenoloba, respectively [15].
9.5.3. Anti-Tyrosinase Activity
Tyrosinase enzymes are well-known for their participation in melanin biosynthesis, and hypersecretion accompanied by accumulation of melanin pigments may lead to hyperpigmentation disorders and photo carcinogenesis [87]. The ethyl acetate partition of aerial parts of O. gigantea showed higher tyrosinase inhibitory activity (0.15 μmol/g) than that (0.49 and 10.48μmol/g) of methanol and water extracts, respectively [53]. The tyrosinase inhibitory activity of methanol extracts of O. aucheriana aerial parts was higher (2.19 mg/mL) than that (2.23 and 2.40 mg/mL) of O. sericea and O. frutescence, respectively [49]. The methanol partition of aerial parts of O. ambigens showed higher tyrosinase inhibitory activity (2.81 mg/mL) than that (3.79 and 4.45 mg/mL) of water and ethyl acetate extracts, respectively [30]. Onosma lycaonica and O. papillosa aerial extracts have been reported as tyrosinase inhibitors with IC50 values 2.20 and 2.05 mg/mL, respectively [48]. The methanol extracts of O. pulchra aerial parts showed higher tyrosinase inhibitory activity (2.47 mg/mL) than that (3.77 and 4.35 mg/mL) of ethyl acetate and water extracts, respectively [14]. The aerial part extracts of O. rigidum and O. trapezuntea showed comparable tyrosinase inhibitory potentials activity [50]. A previous study also reported modest tyrosinase inhibitory activity (136.35 and 135.68 mg/g) for methanol extracts of aerial parts of O. sericea and O. stenoloba, respectively [15]. The ethyl acetate extracts of O. isauricum showed higher tyrosinase inhibitory activity (19.96 mg/g kojic acid equivalents) than that (15.33 and 14.83 mg/g) of methanol and water extracts, respectively [83].
9.5.4. Anti-Lipoxygenases Activity
Lipoxygenases enzymes are known to catalyze oxidation of polyunsaturated fatty acids (linoleic, linolenic, and arachidonic acid) yielding hydroperoxides. Such reactions may be favorable, but also lipoxygenases may interact undesirably. Aromatic compounds are major yields of lipoxygenase reactions that can interfere with food properties, mainly during long-term storage. Lipoxygenase’s impact on unsaturated fatty acids may lead to off-flavor/off-odor formation, leading to food spoilage. Furthermore, lipoxygenase is considered as an important enzyme in stimulation of inflammatory reactions in the human body by playing as a key factor in the biosynthesis of many bio-regulatory compounds such as hydroxyeicosatetraenoic acids (HETEs), leukotrienes, lipoxins, and hepoxylines that were linked to major diseases such as cancer, stroke, and heart and brain diseases [88]. Therefore, searching for natural products that could target this enzyme has become a continuous scientific mission to prevent such diseases. The onosmins A (2-[(4-methylbenzyl)amino]benzoic acid and B (methyl 2-[(4-methylbenzyl)amino]benzoate) compounds isolated from the n-hexane-soluble fraction of ethanol extracts of O. hispida whole plant showed significant lipoxygenase inhibitory activity (IC50: 24.0 and 36.2 μM) [54].
10. Other Biological Activity
10.1. Parasiticidal Activity
The antileishmanial activities of the crude methanol extract of O. griffithii and its fractions were statistically significant (p < 0.05) against the Leishmania promastigotes, Pakistani isolates in comparison with the standard drug called Pentamidine [17].
10.2. Anti-Inflammatory and Analgesic Activity
The chloroform extracts from roots of O. aucheranum, O. isauricum, and O. tauricum showed 28.0%, 34.3%, and 15.6% inhibitory action in p-benzoquinone-induced abdominal constriction experiment, while the ethanol extracts of O. isauricum and O. sericeum demonstrated inhibition action of 24.6% and 27.5%, respectively, in the same test. The chloroform and ethanol extracts of O. isauricum and ethanol extract of O. sericeum also showed significant inhibitory activity, ranging between 12.3–27.3%, 10.5–25.3%, 8.2–22.6%, respectively, in a carrageenan-induced hind paw edema model at 100 mg/kg dose without gastric damage, and the activity was very comparable to indomethacin (32.0–38.4% inhibition) as a standard sample [73]. The chloroform extracts of O. aucheranum and O. isauricum and ethanolic extracts of O. isauricum and O. sericeum exhibited notable antinociceptive activity; 28.0%, 34.3%, 24.6%, and 27.5% inhibition, respectively, against p-benzoquinone-induced abdominal contractions, without induction of any sign of gastric lesion [73]. The methanol extraction of aerial parts of O. bracteatum showed potent analgesic activity by inducing significant increase in the latency period in a dose-dependent manner at different doses at 1, 2, and 3 h (with superiority of 500 mg/kg i.e., 258.9% (p < 0.05) at 3 h) post feeding, respectively, in a tail flick test. Furthermore, the methanol extract of O. bracteatum showed significant analgesic effect at 500 mg/kg body weight dose by inducing 54% inhibition (p < 0.05) in comparison to 45.9% inhibition activity for standard Diclofenac sodium (5 mg/kg body weight) [89].
10.3. Gastric-Ulcerogenic Activity
The chloroform and ethanol extracts from O. aucheranum, O. isauricum, O. sericeum, and O. tauricum roots did not cause any gastric lesions or bleeding in the stomach of mice in a 48-h experiment [71].
10.4. Treatment and Prevention of COVID-19
The Onosma phytochemicals, deoxyshikonin, 3-hydroxy-isovaleryl shikonin, propionyl shikonin, and acetyl shikonin showed significant binding affinities for the Mpro enzyme based on the molecular docking studies using two distinct approaches, in which a SiteMap module of Maestro was used to detect the possible ligand binding sites for the Mpro enzyme. Docking simulations and molecular mechanics suggest that shikonin derivatives might be effective anti-SARS-CoV-2 compounds [19].
11. Conclusions
Application of natural products and their metabolites as chemically diverse starting building blocks has been a major driving force in drug discovery over the last century. However, the use of natural products is not linked only to the modern era, as most folkloric medicines have plant-derived extracts. Moreover, the technological advancement and new technical development for isolation and identification of the natural bioactive compounds in herbs have motivated scientists to investigate and use them as nutrients and nutraceuticals, as well as curatives.
The genus Onosma, known to be widespread worldwide, has a history of medicinal uses against different diseases in the folk medicine system of several civilizations. In this review, the authors rediscover the genus Onosma by detailing the important isolated and identified chemical compounds and extracts, including naphthoquinone (33), flavonoids (30), hydrocarbon (23), phenolic (22), ester (17), alkaloids (20), terpenoids (10), carboxylic acid (11), fatty acids (9), aromatics (12), and liganin (5). The Onosma phytoconstituents that are considered as potential leads amenable for drug development were reported as rosmarinic acid, apigenin, ferulic acid, protocatechuic acid, chlorogenic acid, caffeic acid, p-coumaric acid, vanillic acid.
Several biological activities were reported from Onosma compounds and extracts, including, Genotoxicity and Mutagenicity, antifungal, antibacterial, antioxidant, anticancer, antidiabetic, anti-Alzheimer, anti-tyrosinase, anti-lipoxygenases, parasiticidal, anti-inflammatory, and gastric-ulcerogenic activities. Finally, despite the fact that rosmarinic acid is reported as the most detectable compound in the Onosma species, it was not found in other species such as O. echioides, O. hookeri, O. heterophylla, and O. erecta, requiring further investigation for more confirmation by profiling many other species for comparison.
Author Contributions
Conceptualization, A.A.J.; methodology, A.A.J. and F.O.A.; software, K.F.A.; validation, Y.G., R.R.H. and A.O.H.; formal analysis, A.A.J. and F.O.A.; writing—original draft preparation, A.A.J.; writing—review and editing, E.Q.R. and M.I.S.; review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Binzet R., Kandemir I., Orcan N. Palynological classification of Onosma L. (Boraginaceae) species from east Mediterranean region in Turkey. Acta Bot. Croat. 2010;69:259–274. [Google Scholar]
- 2.Güzel Ö., Duman S., Yılmaz S., Pirhan A.F., Bedir E. Screening of Onosma Species for Cytotoxic Activity. Proceedings. 2017;1:1048. [Google Scholar]
- 3.Rechinger K.H., editor. Flora Iranica, Akademische Druck-u. Verlagsanstalt; Graz, Austria: 1967. Reidl Onosma L. [Google Scholar]
- 4.Onosma R.H. In: Flora of Turkey and the East Aegean Islands. Devis P.H., editor. Vol. 6. Edinburgh University Press; Edinburgh, UK: 1978. pp. 326–376. [Google Scholar]
- 5.Ahmad S.A. Onosma hawramanensis (Boraginaceae), a New Species from Kurdistan, Iraq. Harvard Pap. Bot. 2014;19:201–202. doi: 10.3100/hpib.v19iss2.2014.n6. [DOI] [Google Scholar]
- 6.Mehrabian A.R., Mozaffarian V. Seven new species of Onosma L. (Boraginaceae) with emphasis on their habitats in Iran. Taiwania. 2018;63:366–388. doi: 10.6165/tai.2018.63.366. [DOI] [Google Scholar]
- 7.Cecchi L., Coppi A., Selvi F. Onosma juliae (boraginaceae), a new species from Southern Turkey, with remarks on the systematics of Onosma in the irano-turanian region. Phytotaxa. 2016;288:201–213. doi: 10.11646/phytotaxa.288.3.1. [DOI] [Google Scholar]
- 8.Attar F., Amini Rad M., Mirtadzadini M. Onosma Alburzensis (Boraginaceae), a New Species From Central Alburz Mountains, North Iran. Iran. J. Bot. 2021;27:78–83. doi: 10.22092/ijb.2021.353306.1311. [DOI] [Google Scholar]
- 9.Shilov S.V., Ustenova G.O., Kiyekbayeva L.N., Korotetskiy I.S., Kudashkina N.V., Zubenko N.V., Parenova R.A., Jumagaziyeva A.B., Iskakbayeva Z.A., Kenesheva S.T. Component Composition and Biological Activity of Various Extracts of Onosma gmelinii (Boraginaceae) Int. J. Biomater. 2022;2022:4427804. doi: 10.1155/2022/4427804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarikurkcu C., Tlili N. Onosma inexspectata and Onosma armenum as Novel Sources of Phytochemicals with Determination by High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS/MS) with Evaluation of the Antioxidant and Enzyme Inhibitory Capacities. Anal Lett. 2022;55:1068–1079. doi: 10.1080/00032719.2021.1983583. [DOI] [Google Scholar]
- 11.Hashemi M.M., Marjani M., Poursharifi N., Marjani A. Effects of Onosma dichroanthum Boiss. root extract on AGS human gastric cancer cell-line. J. Basic Clin. Physiol. Pharmacol. 2021 doi: 10.1515/jbcpp-2020-0323. [DOI] [PubMed] [Google Scholar]
- 12.Kundaković T., Stanojković T., Juranić Z., Kovačević N. Cytotoxicity in vitro of naphthazarin derivatives from Onosma arenaria. Phyther. Res. 2006;20:602–604. doi: 10.1002/ptr.1899. [DOI] [PubMed] [Google Scholar]
- 13.Doğan Çalhan S., Gündoğan M. Evaluation of changes in the biological activity of Onosma sericeum Willd (Boraginaceae) based on collection time and extraction solvent, and determination of its mineral and trace element composition. J. Turkish Chem. Soc. Sect. A Chem. 2019;6:355–364. doi: 10.18596/jotcsa.585036. [DOI] [Google Scholar]
- 14.Sarikurkcu C., Sahinler S.S., Ceylan O., Tepe B. Onosma pulchra: Phytochemical composition, antioxidant, skin-whitening and anti-diabetic activity. Ind. Crops Prod. 2020;154:112632. doi: 10.1016/j.indcrop.2020.112632. [DOI] [Google Scholar]
- 15.Katanić Stanković J.S., Ceylan R., Zengin G., Matić S., Jurić T., Diuzheva A., Jeko J., Cziáky Z., Aktumsek A. Multiple biological activities of two Onosma species (O. sericea and O. stenoloba) and HPLC-MS/MS characterization of their phytochemical composition. Ind. Crops Prod. 2020;144:112053. doi: 10.1016/j.indcrop.2019.112053. [DOI] [Google Scholar]
- 16.Jabbar A.A. Onosma mutabilis: Phytochemical composition, antioxidant, cytotoxicity, and acute oral toxicity. Food Sci. Nutr. 2021;9:5755–5764. doi: 10.1002/fsn3.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad B., Ali N., Bashir S., Choudhary M.I., Azam S., Khan I. Parasiticidal, antifungal and antibacterial activities of Onosma griffithii Vatke. Afr. J. Biotechnol. 2009;8:5084–5087. [Google Scholar]
- 18.Özgen U., Houghton P.J., Ogundipe Y., Coşkun M. Antioxidant and antimicrobial activities of Onosma argentatum and Rubia peregrina. Fitoterapia. 2003;74:682–685. doi: 10.1016/S0367-326X(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 19.Kilinç N. Molecular Mechanisms of Possible Action of Naphthoquinones from Onosma in the Treatment and Prevention of COVID-19. Cauc. J. Sci. 2021;8:173–185. doi: 10.48138/cjo.1037727. [DOI] [Google Scholar]
- 20.Mehrabian A., Sheidai M., Noormohammadi Z., Mehrabian A. Palynological diversity in the genus Onosma L. (Boraginaceae) of Iran Shahid Beheshti University, GC, Faculty of Biological Sciences, Tehran, Iran. Ann. Biol. Res. 2012;3:3885–3893. [Google Scholar]
- 21.Vukic M.D., Vukovic N.L., Obradovic A.D., Popovic S.L., Zaric M.M., Djurdjevic P.M., Markovic S.D., Baskic D.D. Naphthoquinone rich Onosma visianii Clem (Boraginaceae) root extracts induce apoptosis and cell cycle arrest in HCT-116 and MDA-MB-231 cancer cell lines. Nat. Prod. Res. 2018;32:2712–2716. doi: 10.1080/14786419.2017.1374271. [DOI] [PubMed] [Google Scholar]
- 22.Sagratini G., Cristalli G., Giardinà D., Gioventù G., Maggi F., Ricciutelli M., Vittori S. Alkannin/shikonin mixture from roots of Onosma echioides (L.) L.: Extraction method study and quantification. J. Sep. Sci. 2008;31:945–952. doi: 10.1002/jssc.200700408. [DOI] [PubMed] [Google Scholar]
- 23.Kumar N., Kumar R., Kishore K. Onosma L.: A review of phytochemistry and ethnopharmacology. Pharmacogn. Rev. 2013;7:140–151. doi: 10.4103/0973-7847.120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajapara A.M., Mamta B. Shah the Genus Onosma L.: A Comprehensive Review. EPRA Int. J. Res. Dev. 2021;7838:219–227. doi: 10.36713/epra9023. [DOI] [Google Scholar]
- 25.Joshi M.C. Hand Book of Indian Medicinal Plants. Scientific Reports; Jodhpor, India: 2019. [Google Scholar]
- 26.Khan M.N., Tariq M., Akhtar J., Khan M.A. Study of a Controversial Unani Drug Gaozaban—A review. World J. Pharm. Res. 2018;7:213–223. doi: 10.20959/wjpr20186-11451. [DOI] [Google Scholar]
- 27.Khajoei Nasab F., Mehrabian A., Mostafavi H. Mapping the current and future distributions of Onosma species endemic to Iran. J. Arid Land. 2020;12:1031–1045. doi: 10.1007/s40333-020-0080-z. [DOI] [Google Scholar]
- 28.Moradi Zeinab H., MEHRABIAN A., Naghizadeh S., MOSTAFAVI H., Khajoi Nasab F. Distribution patterns, diversity and conservation priorities of Onosma L. (Boraginaceae Juss.) in some sections of the northwestern geomorphologic unit of Iran. Environ. Sci. 2019;17:73–94. [Google Scholar]
- 29.Youssef S. Endemic Plant Species of Iraq: From Floristic Diversity to Critical Analysis Review. J. Duhok Univ. 2020;23:90–105. doi: 10.26682/ajuod.2020.23.2.12. [DOI] [Google Scholar]
- 30.Sarikurkcu C., Sahinler S.S., Ceylan O., Tepe B. Onosma ambigens: Phytochemical composition, antioxidant and enzyme inhibitory activity. Ind. Crops Prod. 2020;154:112651. doi: 10.1016/j.indcrop.2020.112651. [DOI] [Google Scholar]
- 31.Luebert F., Cecchi L., Frohlich M.W., Gottschling M., Guilliams C.M., Hasenstab-Lehman K.E., Hilger H.H., Miller J.S., Mittelbach M., Nazaire M., et al. Familial classification of the Boraginales. Taxon. 2016;65:502–522. doi: 10.12705/653.5. [DOI] [Google Scholar]
- 32.Mehrabian A.R., Sheidai M., Mozaffarian V. Micromorphology of leaf trichomes in Onosma (Boraginaceae) and their systematic relevance in Iran. Phytol. Balc. 2014;20:33–48. [Google Scholar]
- 33.Abdullah F.O., Hussain F.H.S., Sardar A.S., Vita-Finzi P., Vidari G. Phytochemistry and Ethnopharmacology of Medicinal Plants Used on Safeen Mountain in the Kurdistan Region of Iraq. Nat. Prod. Commun. 2016;11:1923–1927. doi: 10.1177/1934578X1601101236. [DOI] [PubMed] [Google Scholar]
- 34.Cadirci E., Suleyman H., Aksoy H., Halici Z., Ozgen U., Koc A., Ozturk N. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem. Biol. Interact. 2007;170:40–48. doi: 10.1016/j.cbi.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 35.Reidl H. Additional notes on Cwoiswa-Species (Boraginaceae) from Turkey. Linzer Biol. Beitr. 1987;19:461–465. [Google Scholar]
- 36.Özgen U., Coşkun M., Kazaz C., Seçen H. Naphthoquinones from the roots of Onosma argentatum Hub.-Mor. (Boraginaceae) Turkish J. Chem. 2004;28:451–454. [Google Scholar]
- 37.Ghahremaninejad F., Joharchi M., Vitek E. New plant records for Khorassan province, Iran. Ann. Naturhist. Mus. Wien B. 2005;106:255–293. [Google Scholar]
- 38.Ahmad I., Anis I., Malik A., Nawaz S.A., Choudhary M.I. Cholinesterase Inhibitory Constituents from Onosma hispida. Chem. Pharm. Bull. 2003;51:412–414. doi: 10.1248/cpb.51.412. [DOI] [PubMed] [Google Scholar]
- 39.Sun B., Jiang H., Wang Z.-N., Luo H.-Z., Jia A.-Q. Phytochemical constituents of Onosma bracteatum Wall. Phytochem. Lett. 2021;45:1–5. doi: 10.1016/j.phytol.2021.07.001. [DOI] [Google Scholar]
- 40.Badruddeen , Fareed S., Siddiqui H.H., Haque S.E., Khalid M., Akhtar J. Psychoimmunomodulatory effects of Onosma bracteatum wall. (Gaozaban) on stress model in sprague dawley rats. J. Clin. Diagn. Res. 2012;6:1356–1360. [Google Scholar]
- 41.Ved D., Sureshchandra S.T., Barve V., Srinivas V., Sangeetha S., Ravikumar K. Envis Newsletter. Envis Newsl. Med. Plants Envis Cent Med. Plants. 2016;1:49–116. [Google Scholar]
- 42.Binzet R., Akçin Ö.E. The anatomical properties of two Onosma L. (Boraginaceae) species from Turkey. J. Med. Plants Res. 2012;6:3288–3294. [Google Scholar]
- 43.Kandemir A., Hedge I.C. An Anomalous New Ferulago (Apiaceae) from Eastern Turkey. Willdenowia. 2007;37:273–276. doi: 10.3372/wi.37.37115. [DOI] [Google Scholar]
- 44.Rinner B., Kretschmer N., Knausz H., Mayer A., Boechzelt H., Hao X.-J., Heubl G., Efferth T., Schaider H., Bauer R. A petrol ether extract of the roots of Onosma paniculatum induces cell death in a caspase dependent manner. J. Ethnopharmacol. 2010;129:182–188. doi: 10.1016/j.jep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Mašković P.Z., Diamanto L.D., Vujic J.M., Cvetanović A.D., Radojković M.M., Gadžurić S.B., Zengin G. Onosma aucheriana: A source of biologically active molecules for novel food ingredients and pharmaceuticals. J. Funct. Foods. 2015;19:479–486. doi: 10.1016/j.jff.2015.09.054. [DOI] [Google Scholar]
- 46.Kandemir A., Türkmen Z. The flora of Üzümlü-Sakaltutan (Erzincan-Gümüv’hane) Turk. J. Bot. 2008;32:265–304. [Google Scholar]
- 47.Saravanakumar K., Sarikurkcu C., Sarikurkcu R.T., Wang M.-H. A comparative study on the phenolic composition, antioxidant and enzyme inhibition activities of two endemic Onosma species. Ind. Crops Prod. 2019;142:111878. doi: 10.1016/j.indcrop.2019.111878. [DOI] [Google Scholar]
- 48.Saravanakumar K., Sarikurkcu C., Sahinler S.S., Sarikurkcu R.B., Wang M.-H. Phytochemical Composition, Antioxidant, and Enzyme Inhibition Activities of Methanolic Extracts of Two Endemic Onosma Species. Plants. 2021;10:1373. doi: 10.3390/plants10071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarikurkcu C., Sahinler S.S., Tepe B. Onosma aucheriana, O. frutescens, and O. sericea: Phytochemical profiling and biological activity. Ind. Crops Prod. 2020;154:112633. doi: 10.1016/j.indcrop.2020.112633. [DOI] [Google Scholar]
- 50.Kirkan B., Sarikurkcu C., Zengin G. Bioactive constituents, antioxidant effects and enzyme inhibitory properties of two Onosma species (Onosma trapezuntea and O. rigidum) S. Afr. J. Bot. 2021;145:142–148. doi: 10.1016/j.sajb.2021.09.036. [DOI] [Google Scholar]
- 51.Sihoglu Tepe A. Determination of the Chemical Composition, Antioxidant, and Enzyme Inhibitory Activity of Onosma mollis DC. J. Chem. 2021;2021:5405365. doi: 10.1155/2021/5405365. [DOI] [Google Scholar]
- 52.Naz S., Ahmad S., Ajaz Rasool S., Asad Sayeed S., Siddiqi R. Antibacterial activity directed isolation of compounds from Onosma hispidum. Microbiol. Res. 2006;161:43–48. doi: 10.1016/j.micres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Sarikurkcu C., Kirkan B., Ozer M.S., Ceylan O., Atilgan N., Cengiz M., Tepe B. Chemical characterization and biological activity of Onosma gigantea extracts. Ind. Crops Prod. 2018;115:323–329. doi: 10.1016/j.indcrop.2018.02.040. [DOI] [Google Scholar]
- 54.Ahmad I., Nawaz S.A., Afza N., Malik A., Fatima I., Khan S.B., Ahmad M., Choudhary M.I. Isolation of onosmins A and B, lipoxygenase inhibitors from Onosma hispida. Chem. Pharm. Bull. 2005;53:907–910. doi: 10.1248/cpb.53.907. [DOI] [PubMed] [Google Scholar]
- 55.Erenler R., Yildiz I., Aydin A., Genc N. Antiproliferative and cytotoxic effects of bioactive compounds isolated from Onosma bourgaei. Med. Oncol. 2022;39:116. doi: 10.1007/s12032-022-01705-z. [DOI] [PubMed] [Google Scholar]
- 56.El-Shazly A.M., Abdel-Ghani A.E., Wink M. Pyrrolizidine alkaloids from Onosma arenaria (Boraginaceae) Biochem. Syst. Ecol. 2003;31:477–485. doi: 10.1016/S0305-1978(02)00177-1. [DOI] [Google Scholar]
- 57.Rder E., Wiedenfeld H., Krger R., Teppner H. Pyrrolizidinalkaloide dreier Onosma-Sippen (Boraginaceae-Lithospermeae) Phyton (B Aires) 1993;33:41–49. [Google Scholar]
- 58.Mellidis A.S., Papageorgiou V.P. Naphthazarins from Onosma heterophylla. J. Nat. Prod. 1987;50:618–619. doi: 10.1021/np50052a005. [DOI] [Google Scholar]
- 59.Sut S., Pavela R., Kolarčik V., Cappellacci L., Petrelli R., Maggi F., Dall’Acqua S., Benelli G. Identification of Onosma visianii Roots Extract and Purified Shikonin Derivatives as Potential Acaricidal Agents against Tetranychus urticae. Molecules. 2017;22:1002. doi: 10.3390/molecules22061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vukic M.D., Vukovic N.L., Djelic G.T., Popovic S.L., Zaric M.M., Baskic D.D., Krstic G.B., Tesevic V.V., Kacaniova M.M. Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem. EXCLI J. 2017;16:73–88. doi: 10.17179/excli2016-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu Y., Jiang Z., Leung K.S.-Y., Zhao Z. Simultaneous determination of naphthoquinone derivatives in Boraginaceous herbs by high-performance liquid chromatography. Anal. Chim. Acta. 2006;577:26–31. doi: 10.1016/j.aca.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 62.Mellidis A.S., Papageorgiou V.P. Lipids from roots of Onosma heterophylla. Phytochemistry. 1987;26:842–843. doi: 10.1016/S0031-9422(00)84800-1. [DOI] [Google Scholar]
- 63.Kretschmer N., Rinner B., Deutsch A.J.A., Lohberger B., Knausz H., Kunert O., Blunder M., Boechzelt H., Schaider H., Bauer R. Naphthoquinones from Onosma paniculata induce cell-cycle arrest and apoptosis in melanoma Cells. J. Nat. Prod. 2012;75:865–869. doi: 10.1021/np2006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou W., Jiang H.D.G.L., Peng Y., Li S.S. Comparative study on enantiomeric excess of main akannin/shikonin derivatives isolated from the roots of three endemic Boraginaceae plants in China. Biomed. Chromatogr. 2011;25:1067–1075. doi: 10.1002/bmc.1570. [DOI] [PubMed] [Google Scholar]
- 65.Finefield J.M., Sherman D.H., Kreitman M., Williams R.M. Enantiomeric natural products: Occurrence and biogenesis. Angew. Chem. Int. Ed. Engl. 2012;51:4802–4836. doi: 10.1002/anie.201107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Q., Er-bu A., Liang X., Luan S., He C., Yin L., Yin Z., Zou Y., Li L., Song X. Determination of the main naphthoquinones in Onosma hookeri Clarke. var. longiforum Duthie and its optimization of the ultrasound-assisted extraction using response surface methodology. J. Food Sci. 2021;86:357–365. doi: 10.1111/1750-3841.15460. [DOI] [PubMed] [Google Scholar]
- 67.Khatoon S., Mehrotra S. Naphthaquinones from some Boraginaceous Taxa-A Chemical Review.pdf. Nat. Prod. Sci. 1996;2:75–85. [Google Scholar]
- 68.Damianakos H., Sotiroudis G., Chinou I. Pyrrolizidine alkaloids from Onosma erecta. J. Nat. Prod. 2013;76:1829–1835. doi: 10.1021/np300785g. [DOI] [PubMed] [Google Scholar]
- 69.Yuldasheva N.K., Ul’chenko N.T., Glushenkova A.I. Lipids from fruits of Solenanthus turkestanicus and Onosma irrigans. Chem. Nat. Compd. 2013;49:599–602. doi: 10.1007/s10600-013-0688-8. [DOI] [Google Scholar]
- 70.Yıldız G., Köse Y.B., Kürkçüoğlu M. Essential oil composition of Onosma isaurica boiss. & heldr. and Onosma bulbotrichum dc. from tokat, Turkey. Nat. Volatiles Essent. Oils. 2020;7:30–33. [Google Scholar]
- 71.Kostić A.Ž., Mačukanović-Jocić M.P., Milinčić D.D., Petrović J.D., Gašić U.M., Gligorijević N.N., Jarić S.V., Soković M.D., Tešić Ž.L., Pešić M.B. Hieracium waldsteinii (Asteraceae) and Onosma stellulata (Boraginaceae) as a Source of Antioxidant and Antimicrobial Agents. Chem. Biodivers. 2022;19:e202200069. doi: 10.1002/cbdv.202200069. [DOI] [PubMed] [Google Scholar]
- 72.Ozgen U., Miloglu F.D., Bulut G. Quantitative determination of shikonin derivatives with UV-Vis spectrophotometric methods in the roots of Onosma nigricaule. Rev. Anal. Chem. 2011;30:59–63. doi: 10.1515/revac.2011.014. [DOI] [Google Scholar]
- 73.Tosun A., Akkol E.K., Bahadir O., Yeşilada E. Evaluation of anti-inflammatory and antinociceptive activities of some Onosma L. species growing in Turkey. J. Ethnopharmacol. 2008;120:378–381. doi: 10.1016/j.jep.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Shoaib A., Siddiqui H.H., Badruddeen , Dixit R.K. Evaluation of Noxious Consequence of Bark Extract of Onosma echioides Linn Root: Hematology, Biochemistry, and Histopathological Findings. J. Diet. Suppl. 2020;17:110–119. doi: 10.1080/19390211.2018.1484406. [DOI] [PubMed] [Google Scholar]
- 75.Redvzić A., Redvzić S., Sejdic N. Genotoxic Effects of Aquatic Extract Of Endemic Plant Onosma Stellulata Waldst. & Kit. (Boraginaceae) Afr. J. Tradit. Complement. Altern. Med. 2009;6:346–347. [Google Scholar]
- 76.Maskovic P.Z., Mira A.C., Pavlovic M., Vujosevic M.R., Blagojevic J.V., Djuric M., Moracanin S.V., Djukic D.A. A study on the ethanolic extract of Onosma aucheriana biological and toxicological evaluation. Rev. Chim. 2016;67:2511–2518. [Google Scholar]
- 77.Kumar A., Kaur V., Pandit K., Tuli H.S., Sak K., Jain S.K., Kaur S. Antioxidant Phytoconstituents from Onosma bracteata Wall. (Boraginaceae) Ameliorate the CCl4 Induced Hepatic Damage: In Vivo Study in Male Wistar Rats. Front. Pharmacol. 2020;11:21. doi: 10.3389/fphar.2020.01301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Binzet R., Binzet G., Gumus I., Turunc E., Solmaz U., Keskin E., Dogen A., Arslan H. Chemical Composition and Antimicrobial Activity of Essential Oil and Various Extracts of Onosma sieheana Hayek Roots. J. Essent. Oil Bear. Plants. 2019;22:94–104. doi: 10.1080/0972060X.2019.1600431. [DOI] [Google Scholar]
- 79.Ahmad S., Khan M.A., Ayaz S., Ahamd I. Antibacterial and antifungal studies of the crude extract and solvent fractions of Onosma khyberianum. Pharmacologica. 2013;4:525–528. [Google Scholar]
- 80.Dousti B., Nabipor F. Antibacterial and antifungal activity of Onosma essential oils compared to four common antibioticsMON ANTIBIOTICS; Proceedings of the 21st International Congress of Microbiology of Iran; Tehran, Iran. 18 August 2020; MEDISM21_019. [Google Scholar]
- 81.Ning W., Zhao Q., Xia Z. Effects of Fungal Elicitor on Shikonin Derivatives Formation in Onosma paniculatum Cell Cultures. Chin. Sci. Abstr. Ser. B. 1995;14:33. [Google Scholar]
- 82.Menghani E., Sudhanshu R.N., Mittal S. Free radical scavenging capacity and antioxidant activity of Onosma bracteatum. Int. J. Pharm. Res. Dev. 2011;4:16–20. [Google Scholar]
- 83.Zengin G., Mahomoodally M.F., Picot-Allain C.M.N., Cakmak Y.S., Uysal S., Aktumsek A. In vitro tyrosinase inhibitory and antioxidant potential of Consolida orientalis, Onosma isauricum and Spartium junceum from Turkey. S. Afr. J. Bot. 2019;120:119–123. doi: 10.1016/j.sajb.2018.01.010. [DOI] [Google Scholar]
- 84.Albaqami J., Myles E.L., Tiriveedhi V., Boadi W., Driggins S.N. The Effect of Onosma bracteatum in cancer cells. MOJ Bioequivalence Bioavailab. 2018;5:321–325. doi: 10.15406/mojbb.2018.05.00122. [DOI] [Google Scholar]
- 85.Asghar M., Islam M., Saeed H., Imtiaz F., Saleem B., Saleem Z., Qamar S., Iqtedar M. Investigations on Onosma Hispidum wall root extracts for in-vitro antidiabetic, proliferative and cytotoxic effects. J. Anim. Plant Sci. 2018;28:1339–1347. [Google Scholar]
- 86.Nadri M., Dehpour A.A., Yaghubi S., Fathi H., Ataee R. Effects of the Anti-diabetic and Anti-neuropathy Effects of Onosma Dichroanthum in an Experimental Model of Diabetes by Streptozocin in Mice. Iran J. Endocrinol. Metab. 2017;19:161–169. [Google Scholar]
- 87.Chen W.-C., Tseng T.-S., Hsiao N.-W., Lin Y.-L., Wen Z.-H., Tsai C.-C., Lee Y.-C., Lin H.-H., Tsai K.-C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015;5:7995. doi: 10.1038/srep07995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lončarić M., Strelec I., Moslavac T., Šubarić D., Pavić V., Molnar M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules. 2021;11:152. doi: 10.3390/biom11020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Imran H., Rahman A., Sohail T., Taqvi S.I.H., Yaqeen Z. Onosma bracteatum wall: A Potent analgesic agent. Bangladesh J. Med. Sci. 2018;17:36–41. doi: 10.3329/bjms.v17i1.35276. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.