Abstract
Aging is associated with an increasing burden of morbidity, especially for cardiovascular diseases (CVDs). General cardiovascular risk factors, ischemic heart diseases, heart failure, arrhythmias, and cardiomyopathies present a significant prevalence in older people, and are characterized by peculiar clinical manifestations that have distinct features compared with the same conditions in a younger population. Remarkably, the aging heart phenotype in both healthy individuals and patients with CVD reflects modifications at the cellular level. An improvement in the knowledge of the physiological and pathological molecular mechanisms underlying cardiac aging could improve clinical management of older patients and offer new therapeutic targets.
Keywords: aging, elderly, geriatric cardiology, ischemic cardiomyopathy, heart failure, cardiomyopathies, arrhythmias, molecular mechanisms, oxidative stress, inflammation, microRNAs, telomeres
1. Introduction
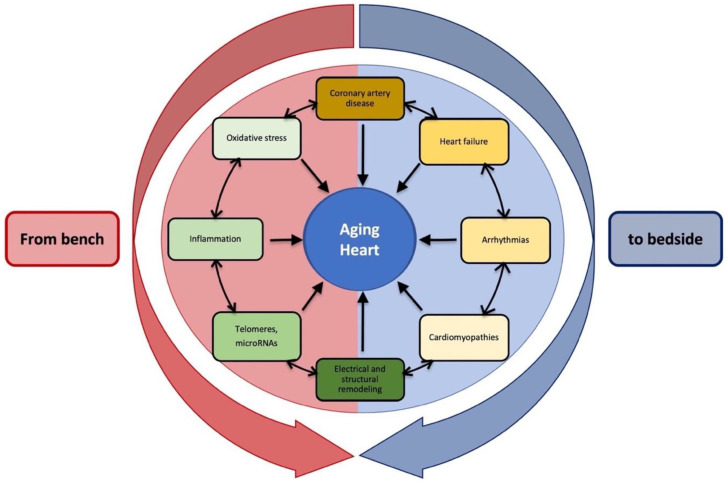
Globally, cardiovascular diseases (CVDs) represent the leading cause of mortality and disability among the growing population of older adults [1]. Problems in the field of “geriatric cardiology” are made more complex by the presence of many comorbidities, multiple treatment regimens, frailty, cognitive impairments, and reduced functional capacity, as well as changes in the social environment [2]. Although the burden of most CVDs, such as hypertension, coronary artery disease, and arrhythmias, rises with age, earlier-onset cardiovascular conditions, such as myocarditis and cardiomyopathies, are often investigated later, resulting in more severe conditions in older patients. All those factors should be taken into consideration when making a differential diagnosis [3] (Figure 1). In addition, clinical guidelines often lack specific recommendations for individuals aged ≥ 75 years, as shown in randomized clinical trials [4]. Age-associated alterations in heart structure and function are linked to changes in signaling pathways and gene expression of the cardiac transcriptome, both in senescence and disease [5]. A better knowledge of intracellular modifications and molecular mechanisms could improve therapeutic strategies for cardiac protection [6,7]. This review aims to provide insight into CVDs in the aging population from both clinical and molecular points of view (see Figure 1).
Figure 1.
Aging heart: clinical and molecular features of cardiac conditions in older patients. Any of these items could contribute to cardiac aging individually or in combination.
2. Definition of the Aging Heart
Biological aging is the gradual deterioration of functional characteristics in living organisms. According to the Framingham Heart Study and the Baltimore Longitudinal Study on Aging (BLSA), aging causes an increase in the prevalence of left ventricular (LV) hypertrophy, a decline in diastolic function, and a decline in exercise capacity despite relatively preserved systolic function at rest, as well as an increase in the prevalence of atrial fibrillation in healthy individuals without concomitant cardiovascular diseases [8]. The characteristics of murine cardiac aging closely resemble those of human cardiac aging [8,9] Echocardiography on a mouse lifespan cohort revealed that the left ventricular mass index (LVMI) and left atrial dimension grew considerably with age. In addition, diastolic function, as evaluated by tissue Doppler, decreased with age; however, systolic function only decreased slightly when the older mice were compared with the young adults. The MPI also deteriorated with age, mirroring the age-related reductions in systolic and diastolic performance [9,10]. Furthermore, the relatively short lifetime and the availability of genetically engineered mice are the benefits of using a mouse model in the investigation of the molecular causes of heart aging [9]. Despite possessing comparable cardiac aging characteristics as humans, laboratory mice do not develop increased blood pressure or unfavorable blood glucose and lipid profiles [9,11,12], allowing the intrinsic cardiac alterations of aging to be explored without the extra problems of cardiovascular risk factors such as hypertension and diabetes [9].
3. Cardiovascular Risk Factors
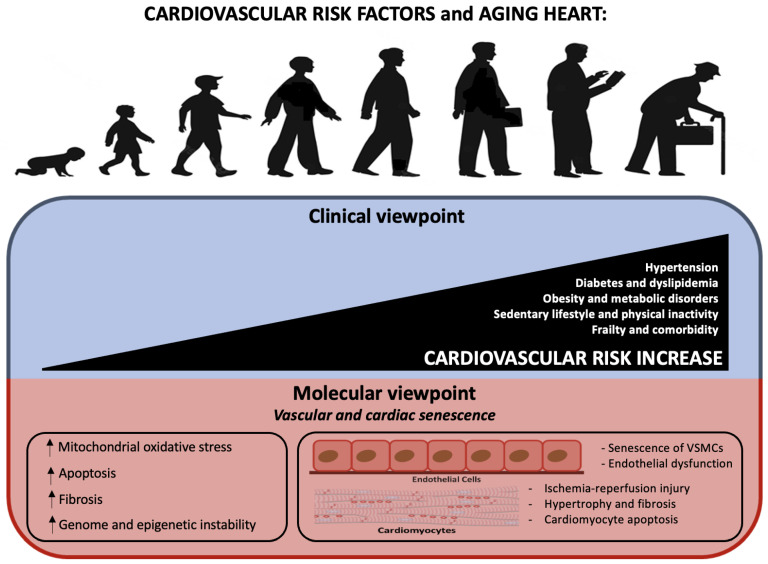
One of the most important risk factors for cardiovascular disease (CVD) is age. Indeed, by 2030, almost 20% of the world population will be over the age of 65, which will result in a major rise in CVD prevalence. This emphasizes the significance of comprehending the processes underlying the aging process and its relationship to cardiovascular disease phenotypes (see Figure 2).
Figure 2.
Aging heart and cardiovascular risk: from clinical to molecular viewpoint. The upper figure shows the correlation between age and CV risk, which is explained by an increase in the number of pathologies such as diabetes and dyslipidemia. Underneath, the same events are explained from a molecular point of view.
Age as an independent risk factor
Age, which is linked to an increased chance of developing a variety of new cardiac risk factors, such as obesity and diabetes, plays a vital role in the deterioration of cardiovascular functionality, resulting in an increased risk of cardiovascular disease (CVD) in older adults [13,14,15]. Furthermore, the prevalence of most types of CVDs is considerably higher among older adults compared with the general population [16]. Throughout an individual’s lifespan, there is an incremental acquisition of several CVD risk factors with age. Nevertheless, age remains an independent risk factor when these risk variables are included in a multivariable regression model [13].
MicroRNAs
microRNAs (miRNAs) are involved in the aging process and help to regulate many mechanisms underlying cardiac changes in the elderly [17]. Aging is specifically associated with an increased expression of miR-34a, which is caused by an upregulation of p53 signaling. Indeed, the miR-34 family induces apoptosis, which emphasizes the central role of miR-34a in the mechanisms underlying aging [18]. Moreover, in aged cells, a reduced amount of miR-146a is found. MiR-146a reduces oxidative stress by downregulating the expression of NOX4, which is the major catalytic subunit of NADPH oxidase [19]. Some miRNAs, including the senescence-associated miR-17-92 cluster, have been shown to inhibit apoptosis [20]. Finally, the expression of miR-17, which is reduced by hypoxia, causes a downregulation of Casp9 and apoptotic protease-activating factor 1 (Apaf-1) [21].
P66shc
It is well known that aging can affect several molecular mechanisms, leading to hypertension and dyslipidemia. Moreover, metabolic disorders, including obesity, diabetes, and insulin resistance, are linked with premature features of vascular and cardiac senescence, pointing out the strong association between aging, metabolism, and cardiovascular disease [22]. This connection might be explained by several factors, such as p66Shc. This enzyme leads to the production of reactive oxygen species (ROS) through the oxidation of cytochrome C and, consequently, to the activation of apoptotic mechanisms [23].
mTOR pathway: AMPK
Growing evidence supports the notion that AMPK plays a crucial role in the regulation of effectors involved in metabolic mechanisms, longevity, and cardiovascular homeostasis [22]. AMPK controls the mTOR pathway through the phosphorylation of the TSC1/2 complex and modulates IGF-1 signaling via the extracellular signal-regulated kinase (Erk) cascade [24]. The fact that the pharmacological triggering of AMPK causes the senescence of vascular smooth muscle cells [25] and the improvement of ROS-driven endothelial dysfunction [26] is particularly important. Moreover, metformin, which is used in diabetes treatment, has been shown to prevent ischemia-reperfusion injury and adverse remodeling of the left ventricle [27]. Considering all the above, AMPK might be considered a therapeutic target to prevent the aging process.
NAD-dependent proteins: SIRT1
Another factor that should be considered is the SIRT1 gene, which is an NAD-dependent protein that protects the heart from senescence, ischemia-reperfusion injury, hypertrophy, and cardiomyocyte apoptosis [28]. In addition, pharmacological activation of SIRT1 by resveratrol causes many benefits, including a decrease in fibrotic collagen deposition, which in turn leads to an improvement of the ejection fraction and fractional shortening [29]. A study showed that SIRT1 improves endothelial function and prevents macrophage foam cell formation and calcification of vascular smooth muscle [30]. SIRT1 can also deacetylate LKB1 and, consequently, activate AMPK, thereby ensuring endothelial integrity thanks to eNOS activity and autophagy [31]. Therefore, impairment of the SIRT1-LKB1-AMPK pathway causes an energy imbalance, cellular stress, and activation of apoptosis mechanisms, which can subsequently lead to vascular aging [32].
NF-κB
NF-κB represents a crucial intermediary between age-induced myocardial inflammation and fibrosis, and its suppression decreases remodeling and cardiac hypertrophy [33]. Activator protein-1 (AP-1) transcription factor JunD is deeply implicated in age-related disease due to its ability to regulate oxidative stress levels; its importance in the vascular context is supported by the observation that its overexpression can rescue endothelial dysfunction in aged mice [34]. Likewise, JunD expression is reduced in peripheral blood monocytes isolated from aged individuals [22]. It is well known that mTOR takes part in the connection between aging and cardiovascular diseases through the stimulation of oxidative stress and inflammatory responses [35]. Aging is associated with an increase of inflammatory adhesion molecules, including ICA-1 and VCAM-1, which contributes to the initiation and progression of atherosclerosis through enhanced monocyte-endothelial cell interactions [36]. Immunosenescence affects the health and survival of elderly individuals. In particular, senescent T cells can produce a large number of proinflammatory cytokines and cytotoxic mediators, which suggests that they may play a role in cardiovascular disease, including hypertension, atherosclerosis, and myocardial infarction.
4. Ischemic Cardiomyopathy
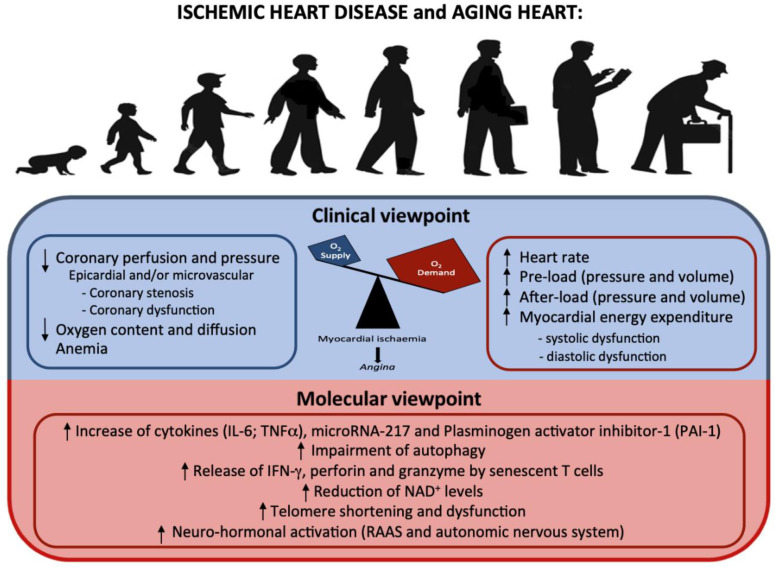
The molecular mechanisms underlying vascular aging are still partially unknown, but the importance of endothelial dysfunction in the context of atherosclerosis and CVD development is clear (see Table 1 and Figure 3).
Figure 3.
Aging heart and ischemic heart disease: from clinical to molecular viewpoint. An increase in O2 demand in the context of a diminished O2 supply leads to myocardial ischemia. The pathophysiological factors that cause these alterations are listed in the upper figure. In the lower figure, the molecular modifications secondary to the increased cellular stress are listed.
Inflammatory markers and cardiovascular risk
In the last years, inflammatory markers have emerged as strong independent risk indicators for cardiovascular disease; however, their specificity and predictivity may differ in older people [37]. Interleukin-6 (IL-6) was shown to be a stronger predictor of incident coronary disease [38], stroke, and cardiovascular mortality than C-reactive protein (CRP) [39]. Tumor necrosis factor-α (TNF-α) is another marker of CVD in older patients, but not of stroke [38]. Fibrinogen was not associated with increased CVD risk in people aged ≥ 70 years [40]. One of the key factors in this context is microRNA-217, which accelerates atherosclerosis and coronary lesion development and triggers impaired left ventricular function. On the other hand, microRNA-217 inhibition improves vascular contractile function and reduces atherosclerotic development, which is suggestive of a role as a biomarker of cardiovascular aging in humans [41]. For example, plasminogen activator inhibitor-1 (PAI-1) could promote age-associated thrombosis and atherosclerosis [42]. In addition, dysregulated activation of the renin-angiotensin-aldosterone system (RAAS) accelerates the atherosclerotic process [43]. Age-related impairment of autophagy, which may lead to endothelial dysfunction, arterial stiffness, and vascular pathologies, including atherosclerosis and calcification, is strongly associated with vascular aging [44].
T cells and cardiovascular risk
It has been suggested that senescent T cells are directly involved in the pathophysiology of atherosclerosis and acute coronary syndrome through the release of several factors, such as IFN-γ, that induce macrophages activation and, consequently, the release of metallo-proteinases that degrade the extracellular matrix [45,46]. These lymphocytes also discharge a great amount of perforin and granzyme, resulting in direct lysis of endothelial and vascular smooth cells [47]. During the process of aging and related ischemic conditions, NAD+ levels decrease and lead to nuclear and mitochondrial dysfunctions that result in age-related diseases. It has been demonstrated that restoring NAD+ using intermediates, including nicotinamide mononucleotide and nicotinamide riboside, may be a good approach for recovering from ischemic injury and age-associated defects [48].
Telomere shortening and cardiovascular risk
Progressive telomere shortening and dysfunction are responsible for physiological and pathological aging, including cardiovascular diseases [49,50]. Telomere ablation, as well as length-independent telomere damage, possibly due to oxidative stress, is responsible for age-related cardiac dysfunction [51]. Massive oxidative stress, as seen in cardiac ischemia-reperfusion injury, has been shown to induce telomere damage, with rescue by the clearance of senescent cells [52]. Telomeres also play a role in vascular pathobiology [53]. Circulating leukocytes and atherosclerotic plaque-associated vascular smooth cells have shorter telomeres than age-matched controls [54,55]. Therapeutic strategies for the selective elimination of senescent cells may improve cardiac function in older patients.
Table 1.
Molecular mechanisms and intracellular modifications underlying ischemic cardiomyopathy (ICM).
| Molecular Mechanisms and Intracellular Modifications Underlying Ischemic Cardiomyopathy | Studies |
|---|---|
| - Increase of cytokines (IL-6; TNFα), microRNA, plasminogen activator inhibitor-1 (PAI-1) | [38,41,42] |
| - Impairment of autophagy | [44] |
| - The release of IFN-γ, perforin and granzyme by senescent T cells | [45,46,47] |
| - Reduction of NAD+ levels | [48] |
| - Telomere shortening and dysfunction | [49,50] |
5. Heart Failure
Heart failure (HF) is a clinical syndrome with a prevalence that increases considerably with age. This disease might be considered the result of the interaction between cardiovascular aging and specific risk factors, comorbidities, and disease modifiers [56] (see Table 2 and Figure 4). The aging process relates to various alterations in the vascular system [57] and myocardium, such as elastin fiber degradation and an increase in collagen quantity, that may predispose an individual to HF. Smooth muscle cells also tend to grow and accumulate. These tissue and cellular changes could result in vascular stiffening and an increased afterload for the left ventricle. The aging process also affects the vascular endothelial cells and their capacity to produce NO and other vital peptides. All these alterations contribute to myocardial interstitial fibrosis, calcium deposition, and amyloid accumulations [8]. Similarly, cardiac valves also suffer through the aging process, thereby exacerbating cardiac stress and HF vulnerability [58]. In this context, the role of activin type II receptor (ActRII) ligands, including FSTL3, which is an endogenous inhibitor of ActRII ligands that increases with aging and HF severity in humans [59], might be crucial. It has been proven that systemic ACTRII inhibition improves systolic function in murine age-related HF models [59]. There are several similarities between the pathophysiology of frailty and HF, including many inflammatory markers such as IL-6, CRP, and TNF-α [60]. Remarkably, several microRNAs regulate aging [61], and there is a large overlap between these age-related microRNAs and the microRNAs involved in both HF and inflammation in Toll-like receptor (TLR) signaling [62,63]. Biomarkers involved in extracellular matrix organization, inflammation, and tumor cell regulation were up-regulated in older patients with heart failure with reduced ejection fraction (HFrEF), with a strong association between aging and WAP four-disulfide core domain protein 2 (WFDC2), while pathways associated with tumor proliferation were down-regulated [64]. On the other hand, heart failure with preserved ejection fraction (HfpEF) is very common among older people with other conditions. HfpEF is characterized by chronic, low-grade, systemic inflammation, with the inflammatory milieu differing according to the specific comorbidities present [65]. From a molecular point of view, levels of TNF and its receptors (TNFR1 and TNFR2), interleukin (IL)-6 and IL-8, high-sensitivity C-reactive protein (hs-CRP), pentraxin-3, and the chemokine (C-C motif) ligand 2 (CCL2) are all often raised in individuals with HfpEF. Chronic, low-grade, systemic inflammation may harm cardiac structure and function. Experimental results indicate that increased pro-inflammatory cytokine production increases oxidative stress, drives fibroblast differentiation into collagen-secreting myofibroblasts, and induces extracellular matrix degradation, resulting in increased myocardial stiffness and coronary microvascular dysfunction (CMD). Local inflammation also decreases the availability of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP), leading to hypophosphorylation of the large sarcomeric protein titin, which increases cardiac stiffness and affects diastolic function. Oxidative stress may also be involved in the development of metabolic heart disease, which suggests that inflammation and cardiac dysfunction could be linked in a bidirectional manner [65]. Understanding the different mechanisms underlying HF in the elderly may help to identify potential therapeutic targets.
Table 2.
Molecular mechanisms and intracellular modifications underlying heart failure (HF).
| Molecular Mechanisms and Intracellular Modifications Underlying Heart Failure | Studies |
|---|---|
| - Degeneration of elastin fibers and increase in collagen | [57] |
| - Clustering and hypertrophy of smooth muscle cells | [57] |
| - Endothelial dysfunction, which affects the production of NO and other peptides | [57] |
| - Myocardial interstitial fibrosis, calcium deposition, and amyloid accumulations | [8] |
| - Upregulation of the activin/ActRII pathway and TLR signaling | [59,62,63] |
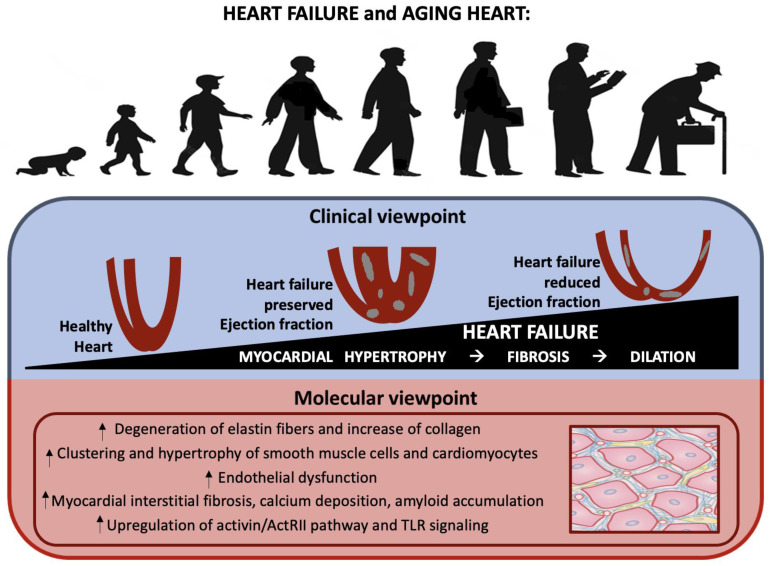
Figure 4.
Aging heart and heart failure: from clinical to molecular viewpoint. Age-related myocardial hypertrophy due to multiple mechanisms (lower figure) results in fibrosis and subsequent cardiac dilation (upper figure).
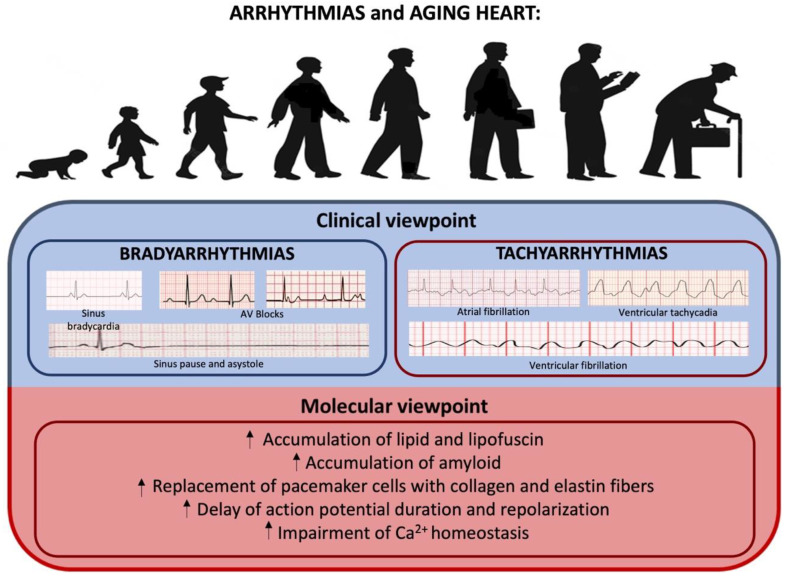
6. Arrhythmias
Aging is associated with an increased prevalence of cardiac arrhythmias, which contribute to higher morbidity and mortality in the elderly [66,67,68,69] (see Table 3 and Figure 5). The incidence of cardiac dysrhythmias, both bradyarrhythmia and tachyarrhythmia, increases with advancing age [70,71,72], with more than 80% of pacemaker implantations in the US needed to relieve symptoms caused by bradycardia and/or chronotropic incompetency from sinus node dysfunction or His-Purkinje disease [70,73,74]. Among tachyarrhythmias, AF is the most common arrhythmia encountered in clinical practice, with a 100-fold higher prevalence in octogenarians (8–10%) compared to those younger than 55 years [68,70,75,76,77,78]. Alterations in the mechanical and electrical cardiac system, as well as energetics and metabolism associated with the aging process, which is exacerbated by comorbidities or use of medications, increase predisposition to cardiac arrhythmias [69,79,80,81]. Deposition of amyloid, lipid, and lipofuscin around the atrial pacemaker tissue contributes to bradyarrhythmia in the aging heart [69,70,74,82,83]. In addition, pacemaker cells within the sinoatrial node and AV conduction fibers are progressively replaced with an extracellular matrix composed of collagen and elastin fibers [84], with up to a 10% reduction of the number of pacemaker cells up in individuals 75 years of age or older compared to young adults [85]. Signaling via β-adrenergic receptors also lowers with age, contributing to a diminished heart rate response and heart rate variability and a resultant reduction in aerobic work capacity in the elderly [84,86,87,88,89]. Furthermore, aging-induced degenerative changes to the cardiac skeleton affect areas close to the AV node, His-Purkinje tissue, and bundle branches, thereby delaying conduction and predisposing elderly patients to arrhythmias [90,91]. An increased prevalence of first-degree AV block, mostly secondary to the pathological fibrosis of the conduction system, has been observed in older people. Although largely considered benign, a prolonged PR interval has been associated with increased AF [92,93]. These modifications also increase the prevalence of fascicular and bifascicular block, which are associated with a high risk of subsequent advanced AV block, syncope, and even sudden cardiac death (SCD), especially in the presence of alternating bundle branch block, type 2 or advanced second-degree AV block, or transient third-degree AV block [92,94]. Another study pointed out that since mitochondria are the primary producer of ROS, this organelle could be considered a potential target for free radical damage. Indeed, a general decrease in mitochondrial-encoded gene expression, which is related to mitochondrial genomic DNA deletions [95,96] and mitochondrial loss, followed by reduced mitochondrial function has been observed with age [96,97,98]. Changes in mRNA abundance associated with aging have recently been examined by gene expression arrays [96]. Bodyak et al. [99] found reduced mRNA levels of several transcription factors (e.g., Nkx2.5, GATA-4, JunB) in ventricular cardiomyocytes that might be implicated in aging. However, Lee et al. [100] showed that only 10% of the transcripts in the whole mouse heart demonstrated significant changes in abundance with aging [99], meaning that many age-associated changes in transcript abundance may instead be associated with non-cardiomyocytes, strain differences, or altered transcript abundances associated with isolation procedures. It is therefore critical to consider biological diversity when performing studies of aging [96].
Figure 5.
Aging heart and arrhythmias: from clinical to molecular viewpoint. The aging process may be one of the main promoting factors for bradyarrhythmias and tachyarrhythmias.
It has been demonstrated that electrical and structural remodeling with action potential duration prolongation and connexin remodeling increases the refractoriness of cardiac tissue and slows conduction [74,101,102,103]. Action potential duration and repolarization are delayed in the senescent heart [74,104,105], in part due to the downregulation of K+ currents, including Ca2+-activated IK+, transient outward (Ito), and ATP-sensitive K+ channels, and in part due to a delay in Ca2+ current inactivation (ICaL) [105,106,107,108]. This delay, along with an increase in sodium-Ca2+ exchanger activity, enhances the tendency for Ca2+-overload-mediated triggered activity and re-entrant arrhythmias [68,78,109,110,111,112,113,114,115]. A decrease in sarcoplasmic reticulum Ca2+-ATPase expression [78,116,117] and post-translational modifications that affect the function of the sarcoplasmic reticulum Ca2+-ATPase, phospholamban, and the sarcoplasmic reticulum Ca2+-release channel (ryanodine receptor 2) further alter Ca2+ homeostasis and the aging heart’s susceptibility to arrhythmias [78,118,119,120,121,122,123]. The effects of age-related changes on cardiac microstructure, including the sarcolemma, cytoskeleton, intercellular gap junctions, cellular geometry, and interstitium, as well as mitochondria [78,124,125], are not well defined and require further studies.
Table 3.
Molecular mechanisms and intracellular modifications underlying arrhythmias.
| Molecular Mechanisms and Intracellular Modifications Underlying Arrhythmias | Studies |
|---|---|
| - Accumulation of amyloid, lipid, and lipofuscin, which leads to bradyarrhythmia | [69,70,74,82,83] |
| - Replacement of pacemaker cells with collagen and elastin fibers | [84] |
| - Delay of action potential duration and repolarization | [74,104,105] |
| - Impairment of Ca2+ homeostasis | [78,118,119,120,121,122,123] |
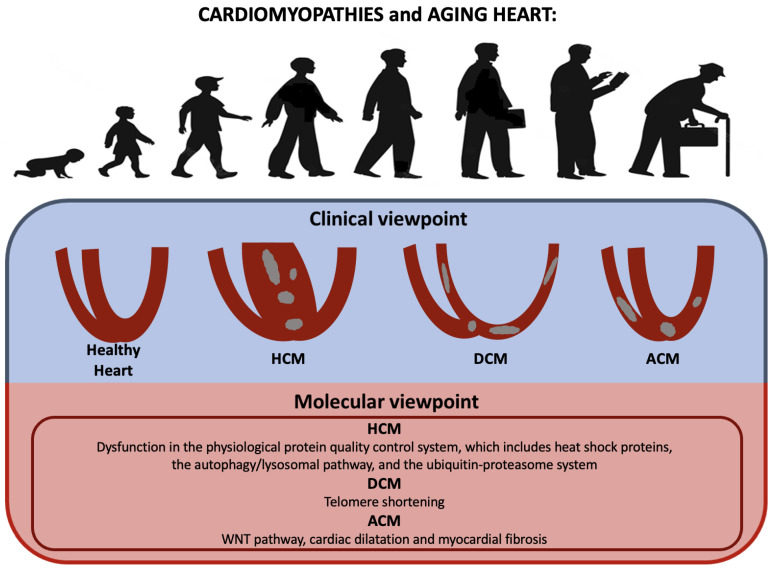
7. Cardiomyopathies
Age-related changes in the heart’s pathophysiology, such as vascular, cellular, and interstitial molecular changes, could result in left ventricular hypertrophy, a general deterioration in organ function, and stress-related cardiovascular illness [126] (see Table 4 and Figure 6). While younger patients tend to be more impacted by DCM and HCM, elderly patients appear to be less afflicted, with only 10% of affected patients being above the age of 65. Restrictive cardiomyopathies are rare in the elderly, while severe and concentric hypertrophy are more commonly associated with hypertrophic cardiomyopathy (HCM) [127]. HCM patients of more advanced age are being increasingly recognized due to greater awareness of this disease and increased use of advanced cardiac imaging in clinical practice [128,129,130]. Exposure to several stressors may cause aggregation of proteins, impair cell viability, and cause pathological conditions, including age-related vascular diseases. To reduce this risk, the cell initiates a mechanism involving molecular chaperones to maintain protein homeostasis. Small heat shock proteins (HSPs) as molecular chaperones prevent aggregation or misfolding of proteins and enable their correct refolding under stress [106,131,132,133]. Among them αB-crystallin (CryaB) [134,135] binds to intermediate filaments and sarcomeric myofibrils, preventing their aggregation during stress [136,137,138]. In terms of function, CryaB phosphorylation has been reported to decrease the ability of this protein to act as a molecular chaperone and to provide protection from oxidative stress [71]. A dysfunction of CryaB could cause various forms of muscular disorder, including restrictive, hypertrophic, and dilated cardiomyopathies, heart failure, and skeletal muscle weakness. The phosphorylation status of CryaB is in dynamic equilibrium under physiological conditions and is usually increased under stress and during aging, although changes in the heart remain unknown [106]. In HCM pathophysiology, disturbances in the physiological protein quality control system (PQS), which is formed by heat shock proteins (HSPs), autophagy/lysosomal, and the ubiquitin-proteasome system (UPS), have been reported. Under conditions of oxidative stress, ROS are found to suppress autophagy, which leads to the accumulation of ubiquitinated proteins and subsequently to cardiac fibrosis and hypertrophy [128]. Furthermore, as reported in recent studies using mice, the knock-out of autophagy-associated genes results in the development of age-related cardiomyopathies. This suggests that continuous constitutive autophagy may play a crucial role in maintaining cardiac structure and function [139]. Furthermore, suppression of the WNT pathway could attenuate age-dependent expression of cardiac dilatation and dysfunction, myocardial fibrosis, and apoptosis in a mouse model of ACM [69,140]. The prognosis of elderly DCM patients has significantly improved over the past 20 years, thanks to advances in pharmacologic treatments and earlier diagnosis [141]. In many patients with HCM, age represents a negative risk marker for sudden death, although they are still more likely to die of non-cardiac competing morbidities [128]. Genetic HCM and DCM are characterized by shorter telomeres in cardiomyocytes [142], with a correlation between hypertrophic phenotype severity and leukocyte telomere length [143]. Myosins and myosin-encoded microRNA networks may explain phenotype differences and could represent putative therapeutic targets in HCM patients [144].
Table 4.
Molecular mechanisms and intracellular modifications underlying cardiomyopathies.
| Molecular Mechanisms and Intracellular Modifications Underlying Cardiomyopathies | Studies |
|---|---|
| - Dysfunction in the physiological protein quality control system, which includes heat shock proteins, the autophagy/lysosomal pathway, and the ubiquitin-proteasome system (HCM) | [128] |
| - WNT pathway, due to its correlation with cardiac dilatation and myocardial fibrosis (ACM) | [69,140] |
| - Telomere shortening (HCM and DCM) | [142] |
Figure 6.
Aging heart and cardiomyopathies: from clinical to molecular viewpoint. Primary cardiomyopathies may be diagnosed even in older people and show specific age-related features.
Finally, older patients with a previously healed myocarditis or a subtle chronic active inflammation may suffer from post-inflammatory DCM, which may cause HF and/or ventricular arrhythmias [145].
LMNA-associated cardiomyopathy may be underdiagnosed in older patients with DCM, atrio-ventricular conduction disorder, AF, and ventricular arrhythmias [146].
Amyloidosis, especially wild-type transthyretin (TTR) amyloidosis, is underdiagnosed in older people, for whom “red flags” may be misread [147] and prognosis may be affected by diagnostic delays, despite available treatments [148]. The aggregation of misfolded TTR monomers and deposition of extracellular fibrils is favored by age-related oxidative modifications [149].
In conclusion, the aging of the healthy heart is a complex process characterized by mild cardiomyocyte hypertrophy, increased cellular senescence, and cell replacement within the extracellular matrix; these changes eventually result in a loss of both contractile function and endogenous protection from irreversible injury [106,136]. However, primary genetic and acquired cardiomyopathies should still be considered among differential diagnoses.
8. Clinical Management of CVD in Older People
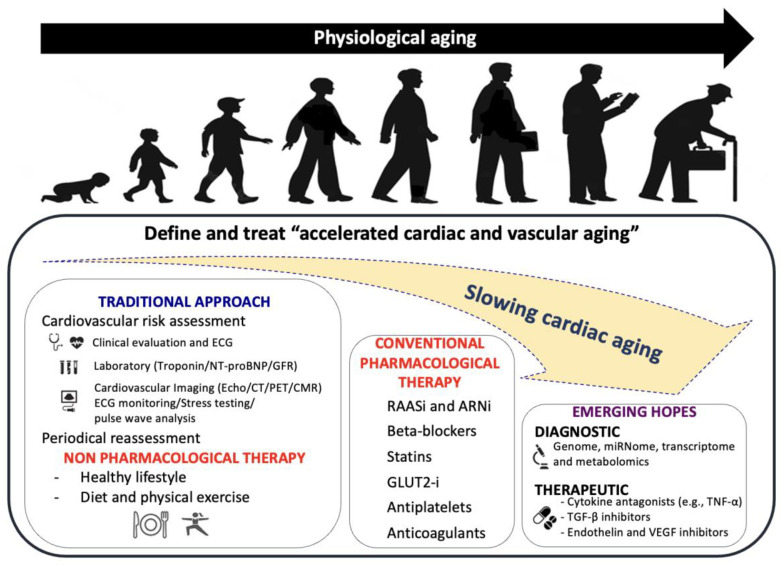
Aging, as well as cardiovascular aging, is a natural and inescapable process; nevertheless, detecting people with accelerated aging is difficult. It is critical to identify patients with accelerated cardiovascular aging and define the mechanisms behind this process to develop preventative interventions targeted at reducing the process of accelerated cardiac and vascular aging. Firstly, it is essential to evaluate and treat each cardiovascular risk factor. This is because the acceleration of cardiovascular system aging primarily depends on the harmful role of both traditional and emerging cardiovascular risk factors, such as familial history, arterial hypertension, dyslipidemia, diabetes, obesity, smoking, and unhealthy lifestyle [150]. Secondly, it is necessary to perform cardiological evaluations to identify cardiac organ damage (hypertrophy, dilation, systolic and/or diastolic dysfunction) and vascular organ damage (atherosclerosis or vascular stiffness) using a multiparametric diagnostic approach [151] (Figure 7). Similarly, non-drug therapies, such as changes in diet and activity, represent the cornerstone of anti-aging medicine. Moreover, identifying the optimal medical therapy for specific cardiac diseases represents the first step toward slowing down accelerated cardiovascular aging [152]. Finally, novel diagnostic (genome, miRNome, transcriptome and metabolomics) and therapeutic tools, such as cytokine antagonists, TGF-β inhibitors or endothelin and VEGF inhibitors [153], are also emerging as potential methods to slow down accelerated cardiovascular aging (Figure 7).
Figure 7.
Clinical management of CVD in older patients. From traditional approaches, such as cardiovascular risk assessment and conventional pharmacological therapies, we are moving towards a new era of diagnostic and therapeutic opportunities, as shown in the figure. Detection and treatment of accelerated aging patients might significantly improve outcomes and delay the aging process.
Older adults, especially those aged ≥ 75 years and with multiple disabilities, are underrepresented in most cardiovascular clinical trials, resulting in knowledge gaps related to cardiovascular care for this population [4]. In addition, there is great heterogeneity and biological diversity in this population, which are independent of age [152]. Adopting a patient-centered-approach, which considers individual comorbidities, life expectancy, cognitive function, frailty, and patient preferences, is critical for establishing the optimal management strategy [152]. According to recent guidelines, reducing blood pressure to a cut-off of <140/90 mmHg is recommended for older adults suffering from hypertension, and a further lowering to 130 mmHg should be considered in individuals aged ≥ 70 years [150]. Aspirin in primary prevention did not demonstrate a reduction in CVD, and increased major bleeding risk in individuals aged ≥ 70 years [154]. A statin-based therapy for primary prevention in older adults aged ≥ 70 years who have a high 10-year CVD risk, as estimated by the SCORE2-OP algorithm, has been proposed [150,155]. Recently, a polypill containing aspirin, ramipril, and atorvastatin was proven to be effective in secondary prevention in adults aged ≥ 75 years [156]. Aging is a risk factor for both ischemic and bleeding events, but the need for antithrombotic therapies is increased in older people, mainly due to atrial fibrillation (AF). New oral anticoagulants (NOACs) have shown better efficacy and safety than warfarin with regards to reducing stroke, all-cause mortality, and intracranial hemorrhage, even in patients aged ≥ 75 years [157]. However, due to competing mortality risks, other studies failed to confirm the net clinical benefit of anticoagulation for AF in older patients [158]. Invasive procedures, such as revascularization and transcatheter valve interventions, have been proven to reduce major cardiovascular adverse events (MACE) and mortality, without additional major bleeding risk, in patients aged ≥ 75 years [159,160,161,162,163,164]. Palliative care and treatment discontinuation, based on the evaluation of life quality, symptom burden, and disease acceptance, are often neglected in CVD [165]. However, patients with end-stage heart failure (HF) could benefit from this kind of intervention [166].
9. Molecular Therapies
mTOR pathway inhibitors
A recent study by Infante et al. [167] showed that the use of the mTOR inhibitor everolimus in kidney transplant recipients dramatically reduced CVD risk by reducing levels of inflammaging markers, namely serum pentraxin-3 and p21ink, and improving mitochondrial function/biogenesis in PBMC, resulting in more efficient oxidative phosphorylation, antioxidant capacity, and glutathione peroxidase activity [167]. Further supporting these antioxidant and anti-inflammatory effects of rapamycin, pathway analysis revealed an upregulation of free radical scavenging genes and a downregulation of NF-κB signaling genes after rapamycin treatment in adult stem cells [168]. A possible effect of berberine, a Chinese herbal medicine, on both the mTOR pathway and AMPK has been reported [169]. A novel possible therapy to modulate the mTOR, AMPK, and sirtuin pathways comes from the CALERIE trial, which suggests that a short-term calorie restriction (CR) of 25% is effective at delaying age-related phenotypes and improving CVD risk factors in adults [170,171,172].
While age-related increases in superoxide production are associated with increased expression and activity of NADPH oxidase [96], CR appears to constrain this source of ROS, with the expression of NOX4 and the p67 subunit of NOX2, as well as the activity of NADPH oxidase, being reduced in old mice after CR [172]. Pharmacologically, AMPK activity can be increased directly after treatment with aminoimidazole carboxamide ribonucleotide (AICAR, an adenosine analog) and indirectly after metformin treatment. It has been shown that long-term metformin treatment can increase an individual’s life span [173]. Direct AMPK activation by AICAR has also been shown to increase tissue antioxidant defenses, including increasing skeletal muscle expression of MnSOD [174]. Moreover, several studies have shown that activating AMPK can lower inflammatory cytokines, and that this is linked to muted NF-κB signaling in a range of tissues, including endothelial cells [172,175].
SIRT1 stimulation
Genetic models provide direct evidence for a protective role of SIRT1. In particular, it has been shown that cardiac-specific SIRT1 overexpression leads to cardiac protection against ROS and delays age-related cardiac phenotypes [172,175]. One such small molecule activator of SIRT1, SRT1720, has recently been shown to increase life span [172,174] and improve metabolic function in aged mice [176]. Furthermore, treatment with SRT1720 seemed to reverse age-associated NF-κB activation and reduced arterial cytokine expression in old mice [177], consistent with the effects of CR on arterial inflammation [172]. Resveratrol, a plant polyphenol, exhibits antiaging, antitumor, and vascular protection effects by enhancing the binding of SIRT1 and LKB1 and subsequent SIRT1 activation. In this way, resveratrol, through LKB1-dependent SIRT1 activation could increase mitochondrial biogenesis and respiration [178].
Telomere-related therapies
Telomeres are repetitive DNA sequences located at the extremities of chromosomes [179]. Telomeres get shorter as we age in most of our tissues, contributing to the organ and tissue failure we see as we age [179]. Telomerase is a reverse transcriptase that adds new telomeric repeats to short telomeres and prevents them from triggering apoptosis or cellular senescence [179,180,181]. Healthy lifespan is also positively correlated with longer telomeres in humans, as not smoking and not being obese at the age of 71 were shown to be the most significant factors associated with survival in men aged 85 years or older [180,182]. Patients suffering from age-related diseases and premature aging syndromes display shorter telomeres compared to healthy individuals [183]. A vast number of studies have shown how genetically engineered mice with an overexpression of telomerase had dramatically increased lifespans [179]. While telomerase gene transfer therapy provides an attractive method for cardiovascular restoration and deserves future investigations, many studies seem to agree that a combination of exercise, healthy diet, low everyday stress, and anti-inflammatory agent intake may be beneficial in promoting human longevity by modulating the telomere system and slowing down the effects of many chronic disorders [180,181].
MicroRNAs inhibition
MiR-217 is a biomarker of vascular aging and cardiovascular risk, as it regulates an endothelial signaling hub and downregulates a network of eNOS, including VEGF, which results in diminished eNOS expression [184]. A recent study by De Yebénes et al. [184] found out that the inhibition of endogenous vascular miR-217 in apoE−/− mice improved vascular contractility and diminished atherosclerosis, highlighting the therapeutic potential of miR-217 inhibitors.
10. Conclusions
Cardiovascular disorders of the aging heart are a difficult pre-clinical and clinical problem. The complexity of cardiac problems in elderly people is not explained by metabolic remodeling, loss of proteostasis, DNA instability and telomere shortening alone, but also by epigenetic transcriptome modifications by microRNAs. Pre-clinical and clinical research demonstrates that dietary restriction with adequate intake of specific nutrients, as well as regular exercise, stress management, and smoking cessation, are effective ways to prevent or delay the accumulation of molecular damage that results in tissue degeneration and cardiometabolic dysfunction. With the growing impact of aging, it is essential to reassess CV research, including the increased use of real-world studies to measure long-term effects. Clinical decision-making should integrate molecular and genetic indicators, pointing to personalized therapy. Remarkably, the identification of new molecular targets, as well as improved clinical characterization of older patients, may enhance knowledge and therapy of the aging heart. Furthermore, the use of pharmacological treatments and other interventions should be based on both the patient’s quality of life and preferences, with the appropriate strategy being defined by a multidisciplinary team that includes the individual patient in the decision-making process.
Abbreviations
ActRII = activin type II receptor; ACM = arrhythmogenic cardiomyopathy; AF = atrial fibrillation; AP-1 = activator protein-1; cGMP = cyclic guanosine monophosphate; CMD = coronary microvascular dysfunction; CRP = C-reactive protein; CryaB = αB-crystallin; CVD = cardiovascular disease; DCM = dilated cardiomyopathy; Erk = extracellular signal-regulated kinase; HCM = hypertrophic cardiomyopathy; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HSPs = heat shock proteins; IL = interleukin; LVMI = left ventricular mass index; MACE = major cardiovascular adverse events; NO = nitric oxide; NOACs = new oral anticoagulants; PAI-1 = plasminogen activator inhibitor-1; RAAS = renin-angiotensin-aldosterone system; ROS = reactive oxygen species; SCD = sudden cardiac death; TLR = Toll-like receptor; TNF-α = tumor necrosis factor-α; TTR = transthyretin; UPS = ubiquitin-proteasome system; WFDC2 = WAP four-disulfide core domain protein 2.
Author Contributions
Conceptualization, G.P. (Giovanni Peretto) and D.L.; methodology, G.P. (Giovanni Peretto); software, D.L.; validation, D.L., A.V. and G.P. (Giovanni Peretto); formal analysis, D.L.; investigation, A.V., G.S. and G.P. (Gianluca Pili); resources, D.L.; data curation, D.L.; writing—original draft preparation, A.V., G.S. and G.P. (Gianluca Pili); writing—review and editing, A.V. and G.P. (Giovanni Peretto); visualization, G.P. (Giovanni Peretto); supervision, G.P. (Giovanni Peretto).; project administration, G.P. (Giovanni Peretto). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. [(accessed on 30 September 2022)];Circulation. 2022 145:e153–e639. doi: 10.1161/CIR.0000000000001052. Available online: https://pubmed.ncbi.nlm.nih.gov/35078371/ [DOI] [PubMed] [Google Scholar]
- 2.Goyal P., Kwak M.J., al Malouf C., Kumar M., Rohant N., Damluji A.A., Denfeld Q.E., Bircher K.K., Krishnaswami A., Alexander K.P., et al. Geriatric Cardiology: Coming of Age. JACC Adv. 2022;1:100070. doi: 10.1016/j.jacadv.2022.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. [(accessed on 30 September 2022)];J. Am. Coll. Cardiol. 2020 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. Available online: https://pubmed.ncbi.nlm.nih.gov/33309175/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rich M.W., Chyun D.A., Skolnick A.H., Alexander K.P., Forman D.E., Kitzman D.W., Maurer M.S., Mcclurken J.B., Resnick B.M., Shen W.K., et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. [(accessed on 30 September 2022)];Circulation. 2016 133:2103–2122. doi: 10.1161/CIR.0000000000000380. Available online: https://pubmed.ncbi.nlm.nih.gov/27067230/ [DOI] [PubMed] [Google Scholar]
- 5.Anisimov S.V., Boheler K.R. Aging-associated changes in cardiac gene expression: Large scale transcriptome analysis. Adv. Gerontol. 2003;11:67–75. [PubMed] [Google Scholar]
- 6.Cannatr A., Camparini L., Sinagra G., Giacca M., Loffredo F.S. Pathways for salvage and protection of the heart under stress: Novel routes for cardiac rejuvenation. [(accessed on 1 October 2022)];Cardiovasc. Res. 2016 111:142–153. doi: 10.1093/cvr/cvw106. Available online: https://pubmed.ncbi.nlm.nih.gov/27371745/ [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Meana M., Bou-Teen D., Ferdinandy P., Gyongyosi M., Pesce M., Perrino C., Schulz R., Sluijter J.P.G., Tocchetti C.G., Thum T., et al. Cardiomyocyte ageing and cardioprotection: Consensus document from the ESC working groups cell biology of the heart and myocardial function. [(accessed on 1 October 2022)];Cardiovasc. Res. 2020 116:1835–1849. doi: 10.1093/cvr/cvaa132. Available online: https://pubmed.ncbi.nlm.nih.gov/32384145/ [DOI] [PubMed] [Google Scholar]
- 8.Lakatta E.G., Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.CIR.0000048893.62841.F7. [DOI] [PubMed] [Google Scholar]
- 9.Chiao Y.A., Rabinovitch P.S. The aging heart. Cold Spring Harb. Perspect Med. 2015;5:a025148. doi: 10.1101/cshperspect.a025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barger J.L., Kayo T., Vann J.M., Arias E.B., Wang J., Hacker T.A., Wang Y., Raederstorff D., Morrow J.D., Leeuwenburgh C., et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. doi: 10.1371/annotation/c54ef754-1962-4125-bf19-76d3ec6f19e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai D.F., Santana L.F., Vermulst M., Tomazela D.M., Emond M.J., MacCoss M.J., Gollahon K., Martin G.M., Loeb L.A., Ladiges W.C., et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng F., Plati A.R., Potier M., Schulman Y., Berho M., Banerjee A., Leclercq B., Zisman A., Striker L.J., Striker G.E., et al. Resistance to glomerulosclerosis in B6 mice disappears after menopause. Am. J. Pathol. 2003;162:1339–1348. doi: 10.1016/S0002-9440(10)63929-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhingra R., Vasan R.S. Age as a Risk Factor. Med. Clin. N. Am. 2012;96:87–91. doi: 10.1016/j.mcna.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 16.Rodgers J.L., Jones J., Bolleddu S.I., Vanthenapalli S., Rodgers L.E., Shah K., Karia K., Panguluri S.K. Cardiovascular risks associated with gender and aging. J. Cardiovasc. Dev. Dis. 2019;6:19. doi: 10.3390/jcdd6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verjans R., van Bilsen M., Schroen B. MiRNA Deregulation in Cardiac Aging and Associated Disorders. [(accessed on 30 November 2022)];Int Rev Cell Mol Biol. 2017 334:207–263. doi: 10.1016/bs.ircmb.2017.03.004. Available online: https://pubmed.ncbi.nlm.nih.gov/28838539/ [DOI] [PubMed] [Google Scholar]
- 18.Seeger T., Boon R.A. MicroRNAs in cardiovascular ageing. [(accessed on 30 November 2022)];J. Physiol. 2016 594:2085–2094. doi: 10.1113/JP270557. Available online: https://pubmed.ncbi.nlm.nih.gov/26040259/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasa-Nicotera M., Chen H., Tucci P., Yang A.L., Saintigny G., Menghini R., Mahè C., Agostini M., Knight R.A., Melino G., et al. miR-146a is modulated in human endothelial cell with aging. [(accessed on 30 November 2022)];Atherosclerosis. 2011 217:326–330. doi: 10.1016/j.atherosclerosis.2011.03.034. Available online: https://pubmed.ncbi.nlm.nih.gov/21511256/ [DOI] [PubMed] [Google Scholar]
- 20.Yan H.L., Xue G., Mei Q., Wang Y.Z., Ding F.X., Liu M.F., Lu M.H., Tang Y., Yu H.Y., Sun S.H., et al. Repression of the miR-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis. [(accessed on 1 December 2022)];EMBO J. 2009 28:2719–2732. doi: 10.1038/emboj.2009.214. Available online: https://pubmed.ncbi.nlm.nih.gov/19696742/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song S., Seo H.H., Lee S.Y., Lee C.Y., Lee J., Yoo K.J., Yoon C., Choi E., Hwang K.C., Lee S. MicroRNA-17-mediated down-regulation of apoptotic protease activating factor 1 attenuates apoptosome formation and subsequent apoptosis of cardiomyocytes. [(accessed on 1 December 2022)];Biochem. Biophys. Res. Commun. 2015 465:299–304. doi: 10.1016/j.bbrc.2015.08.028. Available online: https://pubmed.ncbi.nlm.nih.gov/26265044/ [DOI] [PubMed] [Google Scholar]
- 22.Costantino S., Paneni F., Cosentino F. Ageing, metabolism and cardiovascular disease. [(accessed on 27 September 2022)];J. Physiol. 2016 594:2061–2073. doi: 10.1113/JP270538. Available online: https://pubmed.ncbi.nlm.nih.gov/26391109/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pelliccia G., Luzi L., Minucci S., Marcaccio M., et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. [(accessed on 27 September 2022)];Cell. 2005 122:221–233. doi: 10.1016/j.cell.2005.05.011. Available online: https://pubmed.ncbi.nlm.nih.gov/16051147/ [DOI] [PubMed] [Google Scholar]
- 24.Salminen A., Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. [(accessed on 29 September 2022)];Ageing Res. Rev. 2012 11:230–241. doi: 10.1016/j.arr.2011.12.005. Available online: https://pubmed.ncbi.nlm.nih.gov/22186033/ [DOI] [PubMed] [Google Scholar]
- 25.Sung J.Y., Woo C.H., Kang Y.J., Lee K.Y., Choi H.C. AMPK induces vascular smooth muscle cell senescence via LKB1 dependent pathway. [(accessed on 29 September 2022)];Biochem. Biophys. Res. Commun. 2011 413:143–148. doi: 10.1016/j.bbrc.2011.08.071. Available online: https://pubmed.ncbi.nlm.nih.gov/21872575/ [DOI] [PubMed] [Google Scholar]
- 26.Lesniewski L.A., Zigler M.C., Durrant J.R., Donato A.J., Seals D.R. Sustained activation of AMPK ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. [(accessed on 29 September 2022)];Mech. Ageing Dev. 2012 133:368–371. doi: 10.1016/j.mad.2012.03.011. Available online: https://pubmed.ncbi.nlm.nih.gov/22484146/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Messaoudi S., Rongen G.A., de Boer R.A., Riksen N.P. The cardioprotective effects of metformin. [(accessed on 29 September 2022)];Curr. Opin. Lipidol. 2011 22:445–453. doi: 10.1097/MOL.0b013e32834ae1a7. Available online: https://pubmed.ncbi.nlm.nih.gov/21897229/ [DOI] [PubMed] [Google Scholar]
- 28.Alcendor R.R., Gao S., Zhai P., Zablocki D., Holle E., Yu X., Tian B., Wagner T., Vatner S.F., Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. [(accessed on 29 September 2022)];Circ. Res. 2007 100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. Available online: https://pubmed.ncbi.nlm.nih.gov/17446436/ [DOI] [PubMed] [Google Scholar]
- 29.Sin T.K., Yu A.P., Yung B.Y., Yip S.P., Chan L.W., Wong C.S., Ying M., Rudd J.A., Siu P.M. Modulating effect of SIRT1 activation induced by resveratrol on Foxo1-associated apoptotic signalling in senescent heart. [(accessed on 29 September 2022)];J. Physiol. 2014 592:2535–2548. doi: 10.1113/jphysiol.2014.271387. Available online: https://pubmed.ncbi.nlm.nih.gov/24639483/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein S., Matter C.M. Protective roles of SIRT1 in atherosclerosis. [(accessed on 29 September 2022)];Cell Cycle. 2011 10:640–647. doi: 10.4161/cc.10.4.14863. Available online: https://pubmed.ncbi.nlm.nih.gov/21293192/ [DOI] [PubMed] [Google Scholar]
- 31.Mattagajasingh I., Kim C.S., Naqvi A., Yamamori T., Hoffman T.A., Jung S.B., DeRicco J., Kasuno K., Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. [(accessed on 29 September 2022)];Proc. Natl. Acad. Sci. USA. 2007 104:14855–14860. doi: 10.1073/pnas.0704329104. Available online: https://pubmed.ncbi.nlm.nih.gov/17785417/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillarisetti S. A review of Sirt1 and Sirt1 modulators in cardiovascular and metabolic diseases. [(accessed on 29 September 2022)];Recent Pat. Cardiovasc. Drug Discov. 2008 3:156–164. doi: 10.2174/157489008786263989. Available online: https://pubmed.ncbi.nlm.nih.gov/18991791/ [DOI] [PubMed] [Google Scholar]
- 33.Tas S., Vervoordeldonk M., Tak P. Gene therapy targeting nuclear factor-kappaB: Towards clinical application in inflammatory diseases and cancer. [(accessed on 30 September 2022)];Curr. Gene Ther. 2009 9:160–170. doi: 10.2174/156652309788488569. Available online: https://pubmed.ncbi.nlm.nih.gov/19519361/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paneni F., Osto E., Costantino S., Mateescu B., Briand S., Coppolino G., Perna E., Mocharla P., Akhmedov A., Kubant R., et al. Deletion of the activated protein-1 transcription factor JunD induces oxidative stress and accelerates age-related endothelial dysfunction. [(accessed on 1 October 2022)];Circulation. 2013 127:1229–1240. doi: 10.1161/CIRCULATIONAHA.112.000826. Available online: https://pubmed.ncbi.nlm.nih.gov/23410942/ [DOI] [PubMed] [Google Scholar]
- 35.Yang Z., Ming X.F. mTOR signalling: The molecular interface connecting metabolic stress, aging and cardiovascular diseases. [(accessed on 1 October 2022)];Obes. Rev. 2012 13((Suppl. 2)):58–68. doi: 10.1111/j.1467-789X.2012.01038.x. Available online: https://pubmed.ncbi.nlm.nih.gov/23107260/ [DOI] [PubMed] [Google Scholar]
- 36.Ungvari Z., Kaley G., de Cabo R., Sonntag W.E., Csiszar A. Mechanisms of vascular aging: New perspectives. [(accessed on 1 October 2022)];J. Gerontol. A Biol. Sci. Med. Sci. 2010 65:1028–1041. doi: 10.1093/gerona/glq113. Available online: https://pubmed.ncbi.nlm.nih.gov/20576649/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kritchevsky S.B., Cesari M., Pahor M. Inflammatory markers and cardiovascular health in older adults. [(accessed on 3 October 2022)];Cardiovasc. Res. 2005 66:265–275. doi: 10.1016/j.cardiores.2004.12.026. Available online: https://academic.oup.com/cardiovascres/article/66/2/265/270123. [DOI] [PubMed] [Google Scholar]
- 38.Cesari M., Penninx B.W.J.H., Newman A.B., Kritchevsky S.B., Nicklas B.J., Sutton-Tyrrell K., Rubin S.M., Ding J., Simonsick E.M., Harris T.B., et al. Inflammatory markers and onset of cardiovascular events: Results from the Health ABC study. [(accessed on 1 October 2022)];Circulation. 2003 108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. Available online: https://pubmed.ncbi.nlm.nih.gov/14568895/ [DOI] [PubMed] [Google Scholar]
- 39.Harris T.B., Ferrucci L., Tracy R.P., Corti M.C., Wacholder S., Ettinger W.H., Heimovitz H., Cohen H.J., Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. [(accessed on 1 October 2022)];Am. J. Med. 1999 106:506–512. doi: 10.1016/S0002-9343(99)00066-2. Available online: https://pubmed.ncbi.nlm.nih.gov/10335721/ [DOI] [PubMed] [Google Scholar]
- 40.Kannel W.B., Wolf P.A., Castelli W.P., D’agostino R.B. Fibrinogen and Risk of Cardiovascular Disease: The Framingham Study. [(accessed on 3 October 2022)];JAMA. 1987 258:1183–1186. doi: 10.1001/jama.1987.03400090067035. Available online: https://jamanetwork.com/journals/jama/fullarticle/367933. [DOI] [PubMed] [Google Scholar]
- 41.de Yébenes V.G., Briones A.M., Martos-Folgado I., Mur S.M., Oller J., Bilal F., González-Amor M., Méndez-Barbero N., Silla-Castro J.C., Were F., et al. Aging-Associated miR-217 Aggravates Atherosclerosis and Promotes Cardiovascular Dysfunction. [(accessed on 1 October 2022)];Arter. Thromb. Vasc. Biol. 2020 40:2408–2424. doi: 10.1161/ATVBAHA.120.314333. Available online: https://pubmed.ncbi.nlm.nih.gov/32847388/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto K., Takeshita K., Kojima T., Takamatsu J., Saito H. Aging and plasminogen activator inhibitor-1 (PAI-1) regulation: Implication in the pathogenesis of thrombotic disorders in the elderly. [(accessed on 1 October 2022)];Cardiovasc. Res. 2005 66:276–285. doi: 10.1016/j.cardiores.2004.11.013. Available online: https://academic.oup.com/cardiovascres/article/66/2/276/270209. [DOI] [PubMed] [Google Scholar]
- 43.Durante A., Peretto G., Laricchia A., Ancona F., Spartera M., Mangieri A., Cianflone D. Role of the renin-angiotensin-aldosterone system in the pathogenesis of atherosclerosis. [(accessed on 6 October 2022)];Curr. Pharm. Des. 2012 18:981–1004. doi: 10.2174/138161212799436467. Available online: https://pubmed.ncbi.nlm.nih.gov/22283771/ [DOI] [PubMed] [Google Scholar]
- 44.Larocca T.J., Henson G.D., Thorburn A., Sindler A.L., Pierce G.L., Seals D.R. Translational evidence that impaired autophagy contributes to arterial ageing. [(accessed on 1 October 2022)];J. Physiol. 2012 590:3305–3316. doi: 10.1113/jphysiol.2012.229690. Available online: https://pubmed.ncbi.nlm.nih.gov/22570377/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu H.T., Park S., Shin E.C., Lee W.W. T cell senescence and cardiovascular diseases. [(accessed on 1 October 2022)];Clin. Exp. Med. 2016 16:257–263. doi: 10.1007/s10238-015-0376-z. Available online: https://pubmed.ncbi.nlm.nih.gov/26188489/ [DOI] [PubMed] [Google Scholar]
- 46.Alfaro Leon M.L., Zuckerman S.H. Gamma interferon: A central mediator in atherosclerosis. [(accessed on 1 October 2022)];Inflamm. Res. 2005 54:395–411. doi: 10.1007/s00011-005-1377-2. Available online: https://pubmed.ncbi.nlm.nih.gov/16283107/ [DOI] [PubMed] [Google Scholar]
- 47.Johnson J.L. Matrix metalloproteinases: Influence on smooth muscle cells and atherosclerotic plaque stability. [(accessed on 1 October 2022)];Expert Rev. Cardiovasc. Ther. 2007 5:265–282. doi: 10.1586/14779072.5.2.265. Available online: https://pubmed.ncbi.nlm.nih.gov/17338671/ [DOI] [PubMed] [Google Scholar]
- 48.Hosseini L., Vafaee M.S., Mahmoudi J., Badalzadeh R. Nicotinamide adenine dinucleotide emerges as a therapeutic target in aging and ischemic conditions. [(accessed on 1 October 2022)];Biogerontology. 2019 20:381–395. doi: 10.1007/s10522-019-09805-6. Available online: https://pubmed.ncbi.nlm.nih.gov/30838484/ [DOI] [PubMed] [Google Scholar]
- 49.Rossiello F., Jurk D., Passos J.F., d’Adda di Fagagna F. Telomere dysfunction in ageing and age-related diseases. [(accessed on 3 October 2022)];Nat. Cell Biol. 2022 24:135–147. doi: 10.1038/s41556-022-00842-x. Available online: https://www.nature.com/articles/s41556-022-00842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boniewska-Bernacka E., Pańczyszyn A., Klinger M. Telomeres and telomerase in risk assessment of cardiovascular diseases. [(accessed on 3 October 2022)];Exp. Cell Res. 2020 397:112361. doi: 10.1016/j.yexcr.2020.112361. Available online: https://pubmed.ncbi.nlm.nih.gov/33171154/ [DOI] [PubMed] [Google Scholar]
- 51.Anderson R., Lagnado A., Maggiorani D., Walaszczyk A., Dookun E., Chapman J., Birch J., Salmonowicz H., Ogrodnik M., Jurk D., et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019;38:e100492. doi: 10.15252/embj.2018100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dookun E., Walaszczyk A., Redgrave R., Palmowski P., Tual-Chalot S., Suwana A., Chapman J., Jirkovsky E., Donastorg-Sosa L., Gill E., et al. Clearance of senescent cells during cardiac ischemia-reperfusion injury improves recovery. [(accessed on 3 October 2022)];Aging Cell. 2020 19:e13249. doi: 10.1111/acel.13249. Available online: https://pubmed.ncbi.nlm.nih.gov/32996233/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edo M.D., Andrés V. Aging, telomeres, and atherosclerosis. [(accessed on 3 October 2022)];Cardiovasc. Res. 2005 66:213–221. doi: 10.1016/j.cardiores.2004.09.007. Available online: https://pubmed.ncbi.nlm.nih.gov/15820190/ [DOI] [PubMed] [Google Scholar]
- 54.Samani N.J., Boultby R., Butler R., Thompson J.R., Goodall A.H. Telomere shortening in atherosclerosis. [(accessed on 3 October 2022)];Lancet. 2001 358:472–473. doi: 10.1016/S0140-6736(01)05633-1. Available online: https://pubmed.ncbi.nlm.nih.gov/11513915/ [DOI] [PubMed] [Google Scholar]
- 55.Matthews C., Gorenne I., Scott S., Figg N., Kirkpatrick P., Ritchie A., Goddard M., Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. [(accessed on 3 October 2022)];Circ. Res. 2006 99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. Available online: https://pubmed.ncbi.nlm.nih.gov/16794190/ [DOI] [PubMed] [Google Scholar]
- 56.Triposkiadis F., Xanthopoulos A., Parissis J., Butler J., Farmakis D. Pathogenesis of chronic heart failure: Cardiovascular aging, risk factors, comorbidities, and disease modifiers. [(accessed on 3 October 2022)];Heart Fail. Rev. 2022 27:337–344. doi: 10.1007/s10741-020-09987-z. Available online: https://pubmed.ncbi.nlm.nih.gov/32524327/ [DOI] [PubMed] [Google Scholar]
- 57.Lakatta E.G., Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A ‘set up’ for vascular disease. [(accessed on 3 October 2022)];Circulation. 2003 107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58. Available online: https://pubmed.ncbi.nlm.nih.gov/12515756/ [DOI] [PubMed] [Google Scholar]
- 58.Forman D.E., Ahmed A., Fleg J.L. Heart failure in very old adults. [(accessed on 3 October 2022)];Curr. Heart Fail. Rep. 2013 10:387–400. doi: 10.1007/s11897-013-0163-7. Available online: https://pubmed.ncbi.nlm.nih.gov/24091808/ [DOI] [PubMed] [Google Scholar]
- 59.Roh J.D., Hobson R., Chaudhari V., Quintero P., Yeri A., Benson M., Xiao C., Zlotoff D., Bezzerides V., Houstis N., et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci. Transl. Med. 2019;11:eaau8680. doi: 10.1126/scitranslmed.aau8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uchmanowicz I., Łoboz-Rudnicka M., Szeląg P., Jankowska-Polańska B., Łoboz-Grudzień K. Frailty in heart failure. [(accessed on 3 October 2022)];Curr. Heart Fail. Rep. 2014 11:266–273. doi: 10.1007/s11897-014-0198-4. Available online: https://pubmed.ncbi.nlm.nih.gov/24733407/ [DOI] [PubMed] [Google Scholar]
- 61.Chen L.H., Chiou G.Y., Chen Y.W., Li H.Y., Chiou S.H. MicroRNA and aging: A novel modulator in regulating the aging network. [(accessed on 3 October 2022)];Ageing Res. Rev. 2010 9((Suppl. 1)):S59–S66. doi: 10.1016/j.arr.2010.08.002. Available online: https://pubmed.ncbi.nlm.nih.gov/20708718/ [DOI] [PubMed] [Google Scholar]
- 62.Schroen B., Heymans S. Small but smart—microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing. [(accessed on 3 October 2022)];Cardiovasc. Res. 2012 93:605–613. doi: 10.1093/cvr/cvr268. Available online: https://academic.oup.com/cardiovascres/article/93/4/605/433375. [DOI] [PubMed] [Google Scholar]
- 63.O’Neill L.A., Sheedy F.J., McCoy C.E. MicroRNAs: The fine-tuners of Toll-like receptor signalling. [(accessed on 3 October 2022)];Nat. Rev. Immunol. 2011 11:163–175. doi: 10.1038/nri2957. Available online: https://pubmed.ncbi.nlm.nih.gov/21331081/ [DOI] [PubMed] [Google Scholar]
- 64.Ferreira J.P., Ouwerkerk W., Santema B.T., van Veldhuisen D.J., Lang C.C., Ng L.L., Anker S.D., Dickstein K., Metra M., Cleland J.G.F., et al. Differences in biomarkers and molecular pathways according to age for patients with HFrEF. [(accessed on 3 October 2022)];Cardiovasc. Res. 2021 117:2228–2236. doi: 10.1093/cvr/cvaa279. Available online: https://academic.oup.com/cardiovascres/article/117/10/2228/5917017. [DOI] [PubMed] [Google Scholar]
- 65.Pugliese N.R., Pellicori P., Filidei F., De Biase N., Maffia P., Guzik T.J., Masi S., Taddei S., Cleland J.G. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: Implications for future interventions. Cardiovasc. Res. 2022:cvac133. doi: 10.1093/cvr/cvac133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chugh S.S., Jui J., Gunson K., Stecker E.C., John B.T., Thompson B., Ilias N., Vickers C., Dogra V., Daya M., et al. Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large U. S. community. J. Am. Coll. Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 67.Piccini J.P., Hammill B.G., Sinner M.F., Jensen P.N., Hernandez A.F., Heckbert S.R., Benjamin E.J., Curtis L.H. Incidence and prevalence of atrial fibrillation and associated mortality among medicare beneficiaries: 1993–2007. Circ. Cardiovasc. Qual. Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., et al. AHA Statistical Update Heart Disease and Stroke Statistics-2012 Update A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strait J.B., Lakatta E.G. Aging-Associated Cardiovascular Changes and Their Relationship to Heart Failure. Heart Fail. Clin. 2012;8:143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuster V., Rydén L.E., Cannom D.S., Crijns H.J., Curtis A.B., Ellenbogen K.A., Halperin J.L., Kay G.N., Le-Huezey J.Y., Lowe J.E., et al. 2011 ACCF/AHA/HRS Focused Updates Incorporated Into the ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation. Circulation. 2011;123:1161–1167. doi: 10.1161/CIR.0b013e318214876d. [DOI] [Google Scholar]
- 71.ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) J. Am. Coll. Cardiol. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Gregoratos G., Abrams J., Epstein A.E., Freedman R.A., Hayes D.L., Hlatky M.A., Kerber R.E., Naccarelli G.V., Schoenfeld M.H., Silka M.J., et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines/North American Society for Pacing and Electrophysiology Committee to Update the 1998 Pacemaker Guidelines. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) Circulation. 2002;106:2145–2161. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 73.Rosenqvist M., Obel I.W.P. Atrial Pacing and the Risk for AV Block: Is There a Time for Change in Attitude? Pacing Clin. Electrophysiol. 1989;12:97–101. doi: 10.1111/pace.1989.12.p1.97. [DOI] [PubMed] [Google Scholar]
- 74.Chow G.V., Marine J.E., Fleg J.L. Epidemiology of arrhythmias and conduction disorders in older adults. Clin Geriatr. Med. 2012;28:539–553. doi: 10.1016/j.cger.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mozaffarian D., Furberg C.D., Psaty B.M., Siscovick D. Physical Activity and Incidence of Atrial Fibrillation in Older Adults. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim M.H., Johnston S.S., Chu B.C., Dalal M.R., Schulman K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ. Cardiovasc. Qual. Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 77.Miyasaka Y., Barnes M.E., Gersh B.J., Cha S.S., Bailey K.R., Abhayaratna W.P., Seward J.B., Tsang T.S.M. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 78.Mirza M., Strunets A., Shen W.K., Jahangir A. Mechanisms of Arrhythmias and Conduction Disorders in Older Adults. Clin. Geriatr. Med. 2012;28:555–573. doi: 10.1016/j.cger.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kistler P.M., Sanders P., Fynn S.P., Stevenson I.H., Spence S.J., Vohra J.K., Sparks P.B., Kalman J.M. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J. Am. Coll. Cardiol. 2004;44:109–116. doi: 10.1016/j.jacc.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 80.Preston C.C., Oberlin A.S., Holmuhamedov E.L., Gupta A., Sagar S., Syed R.H.K., Siddiqui S.A., Raghavakaimal S., Terzic A., Jahangir A. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech. Ageing Dev. 2008;129:304–312. doi: 10.1016/j.mad.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roberts-Thomson K.C., Kistler P.M., Sanders P., Morton J.B., Haqqani H.M., Stevenson I., Vohra J.K., Sparks P.B., Kalman J.M. Fractionated atrial electrograms during sinus rhythm: Relationship to age, voltage, and conduction velocity. Heart Rhythm. 2009;6:587–591. doi: 10.1016/j.hrthm.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 82.Olivetti G., Melissari M., Capasso J.M., Anversa P. Cardiomyopathy of the aging human heart. Myocyte Loss React. Cell. Hypertrophy. Circ. Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 83.Rahlf G. Das Herz des älteren Menschen. Pathologie: Makroskopische und lichtmikroskopische Befunde am Herzen [The heart in the elderly. Pathology: Macroscopic and light microscopy findings in the heart] Z. Kardiol. 1985;74((Suppl. 7)):9–16. (In German) [PubMed] [Google Scholar]
- 84.Lakatta E.G. Cardiovascular regulatory mechanisms in advanced age. Physiol. Rev. 1993;73:413–465. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 85.Tellez J.O., Maczewski M., Yanni J., Sutyagin P., Mackiewicz U., Atkinson A., Inada S., Beresewicz A., Billeter R., Dobrzynski H., et al. Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlying sino-atrial node pacemaking. Exp. Physiol. 2011;96:1163–1178. doi: 10.1113/expphysiol.2011.057752. [DOI] [PubMed] [Google Scholar]
- 86.Dun W., Boyden P.A. Aged atria: Electrical remodeling conducive to atrial fibrillation. J. Interv. Card. Electrophysiol. 2009;25:9–18. doi: 10.1007/s10840-008-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fleg J.L., O’Connor F., Gerstenblith G., Becker L.C., Clulow J., Schulman S.P., Lakatta E.G. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J. Appl. Physiol. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 88.Epstein A.E., DiMarco J.P., Ellenbogen K.A., Estes N.A.M., Freedman R.A., Gettes L.S., Gillinov A.M., Gregoratos G., Hammill S.C., Hayes D.L., et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 89.Tresch D.D. Evaluation and management of cardiac arrhythmias in the elderly. Med. Clin. N. Am. 2001;85:527–550. doi: 10.1016/S0025-7125(05)70325-4. [DOI] [PubMed] [Google Scholar]
- 90.Lie J.T., Hammond P.I. Pathology of the Senescent Heart: Anatomic Observations on 237 Autopsy Studies of Patients 90 to 105 Years Old. Mayo Clin. Proc. 1988;63:552–564. doi: 10.1016/S0025-6196(12)64885-X. [DOI] [PubMed] [Google Scholar]
- 91.Cheitlin M.D. Cardiovascular Physiology—Changes With Aging. Am. J. Geriatr. Cardiol. 2003;12:9–13. doi: 10.1111/j.1076-7460.2003.01751.x. [DOI] [PubMed] [Google Scholar]
- 92.Curtis A.B., Karki R., Hattoum A., Sharma U.C. Arrhythmias in Patients ≥80 Years of Age: Pathophysiology, Management, and Outcomes. J. Am. Coll. Cardiol. 2018;71:2041–2057. doi: 10.1016/j.jacc.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mymin D., Mathewson F.A.L., Tate R.B., Manfreda J. The Natural History of Primary First-Degree Atrioventricular Heart Block. N. Engl. J. Med. 1986;315:1183–1187. doi: 10.1056/NEJM198611063151902. [DOI] [PubMed] [Google Scholar]
- 94.Fisch G.R., Zipes D.P., Fisch C. Bundle branch block and sudden death. Prog. Cardiovasc. Dis. 1980;23:187–224. doi: 10.1016/0033-0620(80)90021-3. [DOI] [PubMed] [Google Scholar]
- 95.Wallace D.C. A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp. 2001;235:247–266. doi: 10.1002/0470868694.ch20. [DOI] [PubMed] [Google Scholar]
- 96.Volkova M., Garg R., Dick S., Boheler K.R. Aging-associated changes in cardiac gene expression. Cardiovasc. Res. 2005;66:194–204. doi: 10.1016/j.cardiores.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 97.Lesnefsky E.J., Moghaddas S., Tandler B., Kerner J., Hoppel C.L. Mitochondrial dysfunction in cardiac disease: Ischemia—Reperfusion, aging, and heart failure. J. Mol. Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 98.Ames B.N. Delay in mitochondrial decay of aging. Ann. N. Y. Acad. Sci. 2004;1019:406–411. doi: 10.1196/annals.1297.073. [DOI] [PubMed] [Google Scholar]
- 99.Bodyak N., Kang P.M., Hiromura M., Sulijoadikusumo I., Horikoshi N., Khrapko K., Usheva A. Gene expression profiling of the aging mouse cardiac myocytes. Nucleic Acids Res. 2002;30:3788–3794. doi: 10.1093/nar/gkf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee C.K., Allison D.B., Brand J., Weindruch R., Prolla T.A. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc. Natl. Acad. Sci. USA. 2002;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang C., Ding W., Li L., Zhao D. Differences in the aging-associated trends of the monophasic action potential duration and effective refractory period of the right and left atria of the rat. Circ. J. 2006;70:352–357. doi: 10.1253/circj.70.352. [DOI] [PubMed] [Google Scholar]
- 102.Liu X.K., Jahangir A., Terzic A., Gersh B.J., Hammill S.C., Shen W.K. Age- and sex-related atrial electrophysiologic and structural changes. Am. J. Cardiol. 2004;94:373–375. doi: 10.1016/j.amjcard.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 103.Spach M.S., Heidlage J.F., Dolber P.C., Barr R.C. Mechanism of origin of conduction disturbances in aging human atrial bundles: Experimental and model study. Heart Rhythm. 2007;4:175–185. doi: 10.1016/j.hrthm.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Josephson I.R., Guia A., Stern M.D., Lakatta E.G. Alterations in properties of L-type Ca channels in aging rat heart. J. Mol. Cell Cardiol. 2002;34:297–308. doi: 10.1006/jmcc.2001.1512. [DOI] [PubMed] [Google Scholar]
- 105.Lakatta E.G., Maltsev V.A., Vinogradova T.M. A Coupled SYSTEM of Intracellular Ca2+ Clocks and Surface Membrane Voltage Clocks Controls the Timekeeping Mechanism of the Heart’s Pacemaker. Circ. Res. 2010;106:659–673. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muraleva N.A., Devyatkin V.A., Kolosova N.G. Phosphorylation of αB-crystallin in the myocardium: Analysis of relations with aging and cardiomyopathy. Exp. Gerontol. 2017;95:26–33. doi: 10.1016/j.exger.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 107.Walker K.E., Lakatta E.G., Houser S.R. Age associated changes in membrane currents in rat ventricular myocytes. Cardiovasc. Res. 1993;27:1968–1977. doi: 10.1093/cvr/27.11.1968. [DOI] [PubMed] [Google Scholar]
- 108.Janczewski A.M., Spurgeon H.A., Lakatta E.G. Action potential prolongation in cardiac myocytes of old rats is an adaptation to sustain youthful intracellular Ca2+ regulation. J. Mol. Cell Cardiol. 2002;34:641–648. doi: 10.1006/jmcc.2002.2004. [DOI] [PubMed] [Google Scholar]
- 109.Zhou Y.Y., Lakatta E.G., Xiao R.P. Age-Associated Alterations in Calcium Current and its Modulation in Cardiac Myocytes. Drugs Aging. 1998;13:2. doi: 10.2165/00002512-199813020-00007. [DOI] [PubMed] [Google Scholar]
- 110.Morita N., Lee J.H., Bapat A., Fishbein M.C., Mandel W.J., Chen P.S., Weiss J.N., Karagueuzian H.S. Glycolytic inhibition causes spontaneous ventricular fibrillation in aged hearts. Am. J. Physiol. Heart Circ. Physiol. 2011;301:180–191. doi: 10.1152/ajpheart.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiao R.P., Zhu W., Zheng M., Cao C., Zhang Y., Lakatta E.G., Han Q. Subtype-specific α1- and β-adrenoceptor signaling in the heart. Trends Pharm. Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 112.Jahangir A., Terzic A. KATP channel therapeutics at the bedside. J. Mol. Cell Cardiol. 2005;39:99–112. doi: 10.1016/j.yjmcc.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toro L., Marijic J., Nishimaru K., Tanaka Y., Song M., Stefani E. Aging, ion channel expression, and vascular function. Vasc. Pharmacol. 2002;38:73–80. doi: 10.1016/S0306-3623(02)00128-3. [DOI] [PubMed] [Google Scholar]
- 114.Hamilton S., Terentyev D. Altered Intracellular Calcium Homeostasis and Arrhythmogenesis in the Aged Heart. Int. J. Mol. Sci. 2019;20:2386. doi: 10.3390/ijms20102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rich M.W., Shen W.K. Cardiac rhythm disorders in older adults. Clin. Geriatr. Med. 2012;28:xiii–xiv. doi: 10.1016/j.cger.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 116.Taffet G.E., Tate C.A. CaATPase content is lower in cardiac sarcoplasmic reticulum isolated from old rats. Am. J. Physiol. Circ. Physiol. 1993;264:H1609–H1614. doi: 10.1152/ajpheart.1993.264.5.H1609. [DOI] [PubMed] [Google Scholar]
- 117.Lompre A.M., Lambert F., Lakatta E.G., Schwartz K. Expression of sarcoplasmic reticulum Ca(2+)-ATPase and calsequestrin genes in rat heart during ontogenic development and aging. Circ. Res. 1991;69:1380–1388. doi: 10.1161/01.RES.69.5.1380. [DOI] [PubMed] [Google Scholar]
- 118.Kawamura S., Takahashi M., Ishihara T., Uchino F. Incidence and distribution of isolated atrial amyloid: Histologic and immunohistochemical studies of 100 aging hearts. Pathol. Int. 1995;45:335–342. doi: 10.1111/j.1440-1827.1995.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 119.Anyukhovsky E.P., Sosunov E.A., Chandra P., Rosen T.S., Boyden P.A., Danilo P., Rosen M.R. Age-associated changes in electrophysiologic remodeling: A potential contributor to initiation of atrial fibrillation. Cardiovasc. Res. 2005;66:353–363. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 120.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 121.Xu A., Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 1998;275:H2087–H2094. doi: 10.1152/ajpheart.1998.275.6.H2087. [DOI] [PubMed] [Google Scholar]
- 122.Koban M. Expressional analysis of the cardiac Na–Ca exchanger in rat development and senescence. Cardiovasc. Res. 1998;37:405–423. doi: 10.1016/S0008-6363(97)00276-9. [DOI] [PubMed] [Google Scholar]
- 123.Tsutsui K., Monfredi O.J., Sirenko-Tagirova S.G., Maltseva L.A., Bychkov R., Kim M.S., Ziman B.D., Tarasov K.V., Tarasova Y.S., Zhang J., et al. A coupled-clock system drives the automaticity of human sinoatrial nodal pacemaker cells. Sci. Signal. 2018;11:eaap7608. doi: 10.1126/scisignal.aap7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jahangir A., Ozcan C., Holmuhamedov E.L., Terzic A. Increased calcium vulnerability of senescent cardiac mitochondria: Protective role for a mitochondrial potassium channel opener. Mech. Ageing Dev. 2001;122:1073–1086. doi: 10.1016/S0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 125.Jahangir A., Sagar S., Terzic A. Aging and cardioprotection. J. Appl. Physiol. 2007;103:2120–2128. doi: 10.1152/japplphysiol.00647.2007. [DOI] [PubMed] [Google Scholar]
- 126.Nakou E.S., Parthenakis F.I., Kallergis E.M., Marketou M.E., Nakos K.S., Vardas P.E. Healthy aging and myocardium: A complicated process with various effects in cardiac structure and physiology. Int. J. Cardiol. 2016;209:167–175. doi: 10.1016/j.ijcard.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 127.Shah P.M., Abelmann W.H., Gersh B.J. Cardiomyopathies in the elderly. J. Am. Coll Cardiol. 1987;10:77A–79A. doi: 10.1016/S0735-1097(87)80455-2. [DOI] [PubMed] [Google Scholar]
- 128.Alashi A., Smedira N.G., Popovic Z.B., Fava A., Thamilarasan M., Kapadia S.R., Wierup P., Lever H.M., Desai M.Y. Characteristics and Outcomes of Elderly Patients With Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2021;10:e018527. doi: 10.1161/JAHA.120.018527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Knickelbine T., Lesser J.R., Haas T.S., Brandenburg E.R., Gleason-Han B.K., Flygenring B., Longe T.F., Schwartz R.S., Maron B.J. Identification of Unexpected Nonatherosclerotic Cardiovascular Disease With Coronary CT Angiography. JACC Cardiovasc. Imaging. 2009;2:1085–1092. doi: 10.1016/j.jcmg.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 130.Maron M.S., Maron B.J., Harrigan C., Buros J., Gibson C.M., Olivotto I., Biller L., Lesser J.R., Udelson J.E., Manning W.J., et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J. Am. Coll Cardiol. 2009;54:220–228. doi: 10.1016/j.jacc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 131.Olcum M., Cheedipudi S.M., Rouhi L., Fan S., Jeong H.H., Zhao Z., Gurha P., Marian A.J. The WNT/β-catenin pathway regulates expression of the genes involved in cell cycle progression and mitochondrial oxidative phosphorylation in the postmitotic cardiac myocytes. J. Cardiovasc. Aging. 2022;2:15. doi: 10.20517/jca.2021.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Söti C., Csermely P. Molecular chaperones and the aging process. Biogerontology. 2000;1:225–233. doi: 10.1023/A:1010082129022. [DOI] [PubMed] [Google Scholar]
- 133.Bakthisaran R., Tangirala R., Rao C.M. Small heat shock proteins: Role in cellular functions and pathology. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 134.Clark J.I. Functional sequences in human alphaB crystallin. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2016;1860:240–245. doi: 10.1016/j.bbagen.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hassoun R., Budde H., Zhazykbayeva S., Herwig M., Sieme M., Delalat S., Mostafi N., Gömöri K., Tangos M., Jarkas M., et al. Stress activated signalling impaired protein quality control pathways in human hypertrophic cardiomyopathy. Int. J. Cardiol. 2021;344:160–169. doi: 10.1016/j.ijcard.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 136.Triposkiadis F., Xanthopoulos A., Butler J. Cardiovascular Aging and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019;74:804–813. doi: 10.1016/j.jacc.2019.06.053. [DOI] [PubMed] [Google Scholar]
- 137.Bullard B., Ferguson C., Minajeva A., Leake M.C., Gautel M., Labeit D., Ding L., Labeit S., Horwitz J., Leonard K.R., et al. Association of the Chaperone αB-crystallin with Titin in Heart Muscle. J. Biol. Chem. 2004;279:7917–7924. doi: 10.1074/jbc.M307473200. [DOI] [PubMed] [Google Scholar]
- 138.Braun N., Zacharias M., Peschek J., Kastenmüller A., Zou J., Hanzlik M., Haslbeck M., Rappsilber J., Buchner J., Weinkauf S., et al. Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach. Proc. Natl. Acad. Sci. USA. 2011;108:20491–20496. doi: 10.1073/pnas.1111014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taneike M., Yamaguchi O., Nakai A., Hikoso S., Takeda T., Mizote I., Oka T., Tamai T., Oyabu J., Murakawa T., et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 140.Cheedipudi S.M., Fan S., Rouhi L., Marian A.J. Pharmacological suppression of the WNT signaling pathway attenuates age-dependent expression of the phenotype in a mouse model of arrhythmogenic cardiomyopathy. J. Cardiovasc. Aging. 2021;1:3. doi: 10.20517/jca.2021.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kubo T., Matsumura Y., Kitaoka H., Okawa M., Hirota T., Hamada T., Hitomi N., Hoshikawa E., Hayato K., Shimizu Y., et al. Improvement in prognosis of dilated cardiomyopathy in the elderly over the past 20 years. J. Cardiol. 2008;52:111–117. doi: 10.1016/j.jjcc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 142.Chang A.C.Y., Chang A.C.H., Kirillova A., Sasagawa K., Su W., Weber G., Lin J., Termglinchan V., Karakikes I., Seeger T., et al. Telomere shortening is a hallmark of genetic cardiomyopathies. [(accessed on 3 October 2022)];Proc. Natl. Acad. Sci. USA. 2018 115:9276–9281. doi: 10.1073/pnas.1714538115. Available online: https://pubmed.ncbi.nlm.nih.gov/30150400/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chatterjee S., de Gonzalo-Calvo D., Derda A.A., Schimmel K., Sonnenschein K., Bavendiek U., Bauersachs J., Bär C., Thum T. Leukocyte telomere length correlates with hypertrophic cardiomyopathy severity. [(accessed on 3 October 2022)];Sci. Rep. 2018 8:11227. doi: 10.1038/s41598-018-29072-8. Available online: https://pubmed.ncbi.nlm.nih.gov/30046139/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Foglieni C., Lombardi M., Lazzeroni D., Zerboni R., Lazzarini E., Bertoli G., Pisano A., Girolami F., Andolfo A., Magagnotti C., et al. Myosins and MyomiR Network in Patients with Obstructive Hypertrophic Cardiomyopathy. [(accessed on 6 October 2022)];Biomedicines. 2022 10:2180. doi: 10.3390/biomedicines10092180. Available online: https://pubmed.ncbi.nlm.nih.gov/36140281/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peretto G., Sala S., Rizzo S., de Luca G., Campochiaro C., Sartorelli S., Benedetti G., Palmisano A., Esposito A., Tresoldi M., et al. Arrhythmias in myocarditis: State of the art. [(accessed on 27 March 2022)];Heart Rhythm. 2019 16:793–801. doi: 10.1016/j.hrthm.2018.11.024. Available online: https://pubmed.ncbi.nlm.nih.gov/30476544/ [DOI] [PubMed] [Google Scholar]
- 146.Peretto G., Sala S., Benedetti S., di Resta C., Gigli L., Ferrari M., Bella P. della. Updated clinical overview on cardiac laminopathies: An electrical and mechanical disease. [(accessed on 6 October 2022)];Nucleus. 2018 9:380–391. doi: 10.1080/19491034.2018.1489195. Available online: https://pubmed.ncbi.nlm.nih.gov/29929425/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Garcia-Pavia P., Rapezzi C., Adler Y., Arad M., Basso C., Brucato A., Burazor I., Caforio A.L.P., Damy T., Eriksson U., et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. [(accessed on 3 October 2022)];Eur. J. Heart Fail. 2021 23:512–526. doi: 10.1002/ejhf.2140. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/ejhf.2140. [DOI] [PubMed] [Google Scholar]
- 148.Porcari A., Fontana M., Gillmore J.D. Transthyretin cardiac amyloidosis. Cardiovasc. Res. 2022:cvac119. doi: 10.1093/cvr/cvac119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao L., Buxbaum J.N., Reixach N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. [(accessed on 3 October 2022)];Biochemistry. 2013 52:1913–1926. doi: 10.1021/bi301313b. Available online: https://pubmed.ncbi.nlm.nih.gov/23414091/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Visseren F.L., Mach F., Smulders Y.M., Carballo D., Koskinas K.C., Bäck M., Benetos A., Biffi A., Boavida J.M., Capodanno D., et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. [(accessed on 30 September 2022)];Eur. Heart J. 2021 42:3227–3337. doi: 10.1093/eurheartj/ehab484. Available online: https://pubmed.ncbi.nlm.nih.gov/34458905/ [DOI] [PubMed] [Google Scholar]
- 151.Forman D.E., de Lemos J.A., Shaw L.J., Reuben D.B., Lyubarova R., Peterson E.D., Spertus J.A., Zieman S., Salive M.E., Rich M.W. Cardiovascular Biomarkers and Imaging in Older Adults: JACC Council Perspectives. [(accessed on 1 October 2022)];J. Am. Coll. Cardiol. 2020 76:1577–1594. doi: 10.1016/j.jacc.2020.07.055. Available online: https://pubmed.ncbi.nlm.nih.gov/32972536/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lettino M., Mascherbauer J., Nordaby M., Ziegler A., Collet J.P., Derumeaux G., Hohnloser S.H., Leclercq C., O’neill D.E., Visseren F., et al. Cardiovascular disease in the elderly: Proceedings of the European Society of Cardiology-Cardiovascular Round Table. [(accessed on 30 September 2022)];Eur. J. Prev. Cardiol. 2022 29:1412–1424. doi: 10.1093/eurjpc/zwac033. Available online: https://pubmed.ncbi.nlm.nih.gov/35167666/ [DOI] [PubMed] [Google Scholar]
- 153.Hadley E.C., Lakatta E.G., Morrison-Bogorad M., Warner H.R., Hodes R.J. The future of aging therapies. [(accessed on 30 September 2022)];Cell. 2005 120:557–567. doi: 10.1016/j.cell.2005.01.030. Available online: http://www.cell.com/article/S0092867405001042/fulltext. [DOI] [PubMed] [Google Scholar]
- 154.McNeil J.J., Wolfe R., Woods R.L., Tonkin A.M., Donnan G.A., Nelson M.R., Reid C.M., Lockery J.E., Kirpach B., Storey E., et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. [(accessed on 30 September 2022)];N. Engl. J. Med. 2018 379:1509–1518. doi: 10.1056/NEJMoa1805819. Available online: https://pubmed.ncbi.nlm.nih.gov/30221597/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Collaboration SO working group and EC risk SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021;42:2455. doi: 10.1093/eurheartj/ehab312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Castellano J.M., Pocock S.J., Bhatt D.L., Quesada A.J., Owen R., Fernandez-Ortiz A., Sanchez P.L., Marin Ortuño F., Vazquez Rodriguez J.M., Domingo-Fernández A., et al. Polypill Strategy in Secondary Cardiovascular Prevention. N. Engl. J. Med. 2022;387:967–977. doi: 10.1056/NEJMoa2208275. [DOI] [PubMed] [Google Scholar]
- 157.Ruff C.T., Giugliano R.P., Braunwald E., Hoffman E.B., Deenadayalu N., Ezekowitz M.D., Camm A.J., Weitz J.I., Lewis B.S., Parkhomenko A., et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. [(accessed on 30 September 2022)];Lancet. 2014 383:955–962. doi: 10.1016/S0140-6736(13)62343-0. Available online: https://pubmed.ncbi.nlm.nih.gov/24315724/ [DOI] [PubMed] [Google Scholar]
- 158.Shah S.J., Singer D.E., Fang M.C., Reynolds K., Go A.S., Eckman M.H. Net Clinical Benefit of Oral Anticoagulation Among Older Adults With Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e006212. doi: 10.1161/CIRCOUTCOMES.119.006212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pfisterer M. Trial of invasive versus medical therapy in elderly patients with chronic symptomatic coronary-artery disease (TIME): A randomised trial. [(accessed on 30 September 2022)];Lancet. 2001 358:951–957. doi: 10.1016/S0140-6736(01)06100-1. Available online: https://pubmed.ncbi.nlm.nih.gov/11583747/ [DOI] [PubMed] [Google Scholar]
- 160.Stone G.W., Lindenfeld J., Abraham W.T., Kar S., Lim D.S., Mishell J.M., Whisenant B., Grayburn P.A., Rinaldi M., Kapadia S.R., et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. [(accessed on 30 September 2022)];N. Engl. J. Med. 2018 379:2307–2318. doi: 10.1056/NEJMoa1806640. Available online: https://pubmed.ncbi.nlm.nih.gov/30280640/ [DOI] [PubMed] [Google Scholar]
- 161.Leon M.B., Smith C.R., Mack M., Miller D.C., Moses J.W., Svensson L.G., Tuzcu E.M., Webb J.G., Fontana G.P., Makkar R.R., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. [(accessed on 30 September 2022)];N. Engl. J. Med. 2010 363:1597–1607. doi: 10.1056/NEJMoa1008232. Available online: https://pubmed.ncbi.nlm.nih.gov/20961243/ [DOI] [PubMed] [Google Scholar]
- 162.Kumar S., McDaniel M., Samady H., Forouzandeh F. Contemporary Revascularization Dilemmas in Older Adults. J. Am. Heart Assoc. 2020;9:1–13. doi: 10.1161/JAHA.119.014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Arisha M.J., Ibrahim D.A., Abouarab A.A., Rahouma M., Kamel M.K., Baudo M., Mehta K., Gaudino M.F.L. Percutaneous coronary intervention in the elderly: Current updates and trends. Vessel Plus. 2018;2:14. doi: 10.20517/2574-1209.2018.29. [DOI] [Google Scholar]
- 164.Bangalore S., Maron D.J., Stone G.W., Hochman J.S. Routine Revascularization Versus Initial Medical Therapy for Stable Ischemic Heart Disease: A Systematic Review and Meta-Analysis of Randomized Trials. Circulation. 2020;143:e809–e810. doi: 10.1161/CIRCULATIONAHA.120.048194. [DOI] [PubMed] [Google Scholar]
- 165.Kilic Y., Smer A., Goldstein N. The Importance of Palliative Care in Cardiology: Differences Between Countries. [(accessed on 30 September 2022)];JACC Case Rep. 2020 2:326–329. doi: 10.1016/j.jaccas.2019.11.069. Available online: https://pubmed.ncbi.nlm.nih.gov/34317235/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rogers J.G., Patel C.B., Mentz R.J., Granger B.B., Steinhauser K.E., Fiuzat M., Adams P.A., Speck A., Johnson K.S., Krishnamoorthy A., et al. Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial. [(accessed on 30 September 2022)];J. Am. Coll. Cardiol. 2017 70:331–341. doi: 10.1016/j.jacc.2017.05.030. Available online: https://pubmed.ncbi.nlm.nih.gov/28705314/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Infante B., Bellanti F., Correale M., Pontrelli P., Franzin R., Leo S., Calvaruso M., Mercuri S., Netti G.S., Ranieri E., et al. mTOR inhibition improves mitochondria function/biogenesis and delays cardiovascular aging in kidney transplant recipients with chronic graft dysfunction. Aging. 2021;13:8026–8039. doi: 10.18632/aging.202863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kofman A.E., McGraw M.R., Payne C.J. Rapamycin increases oxidative stress response gene expression in adult stem cells. Aging. 2012;4:279–289. doi: 10.18632/aging.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao R.Y., Zheng Y., Zhang Y., Jiang L., Li Q., Sun W., Gu W., Cao W., Zhou L., Zheng H.Y.J. Berberine on the Prevention and Management of Cardiometabolic Disease: Clinical Applications and Mechanisms of Action. Am. J. Chin. Med. 2021;49:1645–1666. doi: 10.1142/S0192415X21500762. [DOI] [PubMed] [Google Scholar]
- 170.Most J., Gilmore L.A., Smith S.R., Han H., Ravussin E., Redman L.M. Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am. J. Physiol. Endocrinol. Metab. 2018;314:E396–E405. doi: 10.1152/ajpendo.00261.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Belsky D.W., Huffman K.M., Pieper C.F., Shalev I., Kraus W.E., Anderson R. Change in the rate of biological aging in response to caloric restriction: Calerie Biobank analysis. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2018;73:4–10. doi: 10.1093/gerona/glx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gogulamudi V.R., Cai J., Lesniewski L.A. Reversing Age-Associated Arterial Dysfunction: Insight from Preclinical Models. J. Appl. Physiol. 2018;84148:6–7. doi: 10.1152/japplphysiol.00086.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hattori Y., Suzuki K., Hattori S., Kasai K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 174.Brandauer J., Andersen M.A., Kellezi H., Risis S., Frøsig C., Vienberg S.G., Treebak J.T. AMP-activated protein kinase controls exercise training- and AICAR-induced increases in SIRT3 and MnSOD. Front. Physiol. 2015;6:1–16. doi: 10.3389/fphys.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hattori Y., Nakano Y., Hattori S., Tomizawa A., Inukai K., Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-κB activation in vascular endothelial cells. FEBS Lett. 2008;582:1719–1724. doi: 10.1016/j.febslet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 176.Minor R.K., Baur J.A., Gomes A.P., Ward T.M., Csiszar A., Mercken E.M., Abdelmohsen K., Shin Y.K., Canto C., Scheibye-Knudsen M., et al. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gano L.B., Donato A.J., Pasha H.M., Hearon C.M., Sindler A.L., Seals D.R. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang Y., Lu J., Zhan L., Wang M., Shi R., Yuan X., Gao X., Liu X., Zang J., Liu W., et al. Resveratrol-induced Sirt1 phosphorylation by LKB1 mediates mitochondrial metabolism. J. Biol. Chem. 2021;297:100929. doi: 10.1016/j.jbc.2021.100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Boccardi V., Herbig U. Telomerase gene therapy: A novel approach to combat aging. EMBO Mol. Med. 2012;4:685–687. doi: 10.1002/emmm.201200246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yeh J.K., Lin M.H., Wang C.Y. Telomeres as Therapeutic Targets in Heart Disease. JACC Basic Transl. Sci. 2019;4:855–865. doi: 10.1016/j.jacbts.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Martin-Ruiz C.M., Gussekloo J., van Heemst D., von Zglinicki T., Westendorp R.G.J. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: A population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 182.Franzon K., Byberg L., Sjögren P., Zethelius B., Cederholm T., Kilander L. Predictors of Independent Aging and Survival: A 16-Year Follow-Up Report in Octogenarian Men. J. Am. Geriatr. Soc. 2017;65:1953–1960. doi: 10.1111/jgs.14971. [DOI] [PubMed] [Google Scholar]
- 183.Zhu H., Belcher M., Van Der Harst P. Healthy aging and disease: Role for telomere biology? Clin. Sci. 2011;120:427–440. doi: 10.1042/CS20100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tomás-Loba A., Flores I., Fernández-Marcos P.J., Cayuela M.L., Maraver A., Tejera A., Borrás C., Matheu A., Klatt P., Flores J.M., et al. Telomerase Reverse Transcriptase Delays Aging in Cancer-Resistant Mice. Cell. 2008;135:609–622. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.