Abstract
Reading fluency—the speed and accuracy of reading connected text—is foundational to educational success. The current longitudinal study investigates the neural correlates of fluency development using a connected‐text paradigm with an individualized presentation rate. Twenty‐six children completed a functional MRI task in 1st/2nd grade (time 1) and again 1–2 years later (time 2). There was a longitudinal increase in activation in the ventral occipito‐temporal (vOT) cortex from time 1 to time 2. This increase was also associated with improvements in reading fluency skills and modulated by individual speed demands. These findings highlight the reciprocal relationship of the vOT region with reading proficiency and its importance for supporting the developmental transition to fluent reading. These results have implications for developing effective interventions to target increased automaticity in reading.
Keywords: development, fmri, longitudinal, reading fluency, ventral occipitotemporal cortex
We conducted a longitudinal study to examine the neural correlates of fluency using a connected‐text paradigm with an individualized presentation rate during a critical period of time in children's reading development, as they transition from non‐fluent to fluent reading (1st/2nd to 3rd/4th grades). We show a developmental increase in activation of a key reading region, the ventral occipito‐temporal cortex over the span of 1–2 years. Importantly, increased activation was associated with larger gains in reading fluency and was modulated by task fluency demands.

1. INTRODUCTION
Reading fluency is the foundation for proficient reading and is critical to educational success (Panel [U.S.], 2000). The term fluency refers to the speed and accuracy of decoding connected text (Chard et al., 2002). Despite extensive research into the brain basis of reading, the topic of fluency development has been largely overlooked in the neuroimaging literature. Insights into the neural processes underlying fluency development are important for understanding fluency deficits in children with reading difficulties and for the development of effective interventions targeting these deficits. The current longitudinal study investigates the neural correlates of fluency using a connected‐text paradigm during a period of time in which children transition from non‐fluent to fluent reading.
The goal of successful reading acquisition is to read an unfamiliar text fluently, with great automaticity, and comprehend it. In typical reading development in English‐speakers, children acquire fluency in grades 2 and 3 (roughly 8–9 years old; Chall, 1983). The development of fluency has been conceptualized as the outcome of achieving proficiency in the lower‐level component skills of reading (Kame'enui et al., 2001; Wolf & Katzir‐Cohen, 2001). More specifically, fluency is achieved when processing at the phonological, orthographic, semantic, and morphological levels—and critically, among these levels—becomes automatic. Automaticity has been defined as processing without expending attention or effort (Ehri, 2005). Automaticity arises as a result of robust associations being formed between written words and their linguistic representations (i.e., phonological and semantic) through learning and practice (Ehri, 2005; Hudson et al., 2008). Once these associations are established, word identification of familiar words becomes primarily a memory retrieval process that proceeds quickly and without readers' conscious control, resulting in fluent reading of connected text. This allows for processing words in a fashion that support connecting words together into meaningful strings and for allocating cognitive resources to support processes related to comprehension of text (Perfetti, 1985).
Fluency serves as the foundation for the next stage in reading development—reading to learn—that occurs in later grades (Chall, 1983). When word recognition is not efficient, cognitive resources that are needed to support text integration and comprehension are instead deployed to support word identification (Crain & Shankweiler, 1990; Ozernov‐Palchik et al., 2021; Wolf & Katzir‐Cohen, 2001). Indeed, there is evidence that fluency makes a unique contribution to reading comprehension beyond accuracy (Cutting et al., 2009; Joshi & Aaron, 2000; Silverman et al., 2013; Tilstra et al., 2009) and has important implications for children with reading difficulties, as a fluency deficit may describe some of the most impaired readers, particularly in older grades (Wolf & Bowers, 1999). Thus, fluency is a critical prerequisite for reading comprehension, but the neurocognitive processes underlying the development of fluency remain relatively unknown.
Neuroimaging studies of reading development have demonstrated that foundational reading skills such as mapping phonemes (i.e., speech sounds) to their orthographic representations (i.e., letters) are associated with the structure and function of the temporoparietal brain regions. A shift from early‐reading in English (5–6 years) to emergent reading (7–8 years) and subsequently increasingly fluent reading (8–9 years) has been associated with increased development and recruitment of the occipito‐temporal brain regions (Chyl et al., 2021; Pugh et al., 2001). The increased specialization of the ventral occipito‐temporal cortex (vOT) for print has emerged as an important milestone for the development of word reading (Dehaene et al., 2015). In particular, increased response of the vOT region to words has been associated with better reading proficiency (Ben‐Shachar et al., 2011; Brem et al., 2020; Kubota et al., 2019; Maurer et al., 2011; Olulade et al., 2013; Parviainen, 2006), and has been shown longitudinally in response to reading instruction and intervention (Brem et al., 2010; Fraga González et al., 2015; Rezaie et al., 2011; Shaywitz et al., 2004) and in older as compared with younger readers (Ben‐Shachar et al., 2011; Smith et al., 2018).
As a result of its advantageous structural connections to the phonological, semantic, and memory systems in the brain, the vOT region becomes specialized for automatic word recognition with increased reading experience (Centanni et al., 2018; Dehaene et al., 2010, 2015; Dehaene & Cohen, 2007; Saygin et al., 2016; Stevens et al., 2017; Wang et al., 2020). For example, the connectivity of the vOT in pre‐readers, but not its responsiveness to print, has been shown to predict the functional specificity of the region for words 3 years later (Saygin et al., 2016). In earlier stages of reading development, the vOT emerges as a hub linking visual letter patterns with first phonological and then semantic representations; with increased reading expertise, vOT assists in linking orthographic patterns directly with semantic representations. In fluent readers, this region is thought to process words in a similar way that other proximal regions in the left and right hemispheres process objects such as faces, identifying them wholistically and without exerting conscious effort (Dehaene & Cohen, 2007; Mei et al., 2010).
In support of the wholistic processing of words in the vOT of expert readers, studies have demonstrated reduced activation to words with stronger orthographic familiarity (Borowsky et al., 2007; Borowsky & Besner, 2006; Bruno et al., 2008; Kronbichler et al., 2004, 2007, 2009; van der Mark et al., 2009) suggesting that more familiar words are processed with greater automaticity than less familiar words. Word familiarity could only have an effect on vOT activation if words are processed wholistically, rather than decoded letter‐by‐letter. Furthermore, word length by lexicality interaction effects in vOT have been demonstrated, with length having an effect on vOT activation for non‐words, but not for words. This supports whole‐word processing in vOT for familiar orthographic forms and serial sub‐lexical processing for novel orthographic forms (Schurz et al., 2010).
Despite the overall understanding of the development of the reading brain circuitry and the important role of vOT in automatic word recognition, it remains unknown how reading fluency develops in the brain. Studies investigating the brain correlates of reading have primarily focused on single‐word or letter identification for their functional tasks (Aboud et al., 2018; Ben‐Shachar et al., 2011; Brem et al., 2010; Olulade et al., 2013; Shaywitz et al., 2004). Integrating across words while reading connected text, however, is an important feature of fluency during naturalistic reading (Hagoort, 2013). Several studies compared individuals with reading fluency deficits to typical readers using sentence‐level stimuli and observed differences in activation in left temporoparietal (Meyler et al., 2007; Rimrodt et al., 2009; Schulz et al., 2009), occipito‐temporal, and inferior frontal gyrus areas (Kronbichler et al., 2006). These studies, however, focused on sentence comprehension and were not longitudinal.
Longitudinal designs allow investigators to characterize the neural changes associated with a particular cognitive function in the same individuals. Although a limited number of longitudinal studies have used sentence tasks (Nugiel et al., 2019; Roe et al., 2018), these studies focused on measures of comprehension but not fluency and investigated brain differences in relation to intervention response, rather than to business‐as‐usual development and schooling. Furthermore, these studies held the speed of word processing constant, not accounting for individual differences in the rate of word processing, an important indicator of fluency (Chard et al., 2002). Therefore, no previous neuroimaging studies have used naturalistic sentence‐level stimuli and manipulated individual reading speed to longitudinally investigate the neural substrates of fluency development.
A more ecologically valid approach to neuroimaging of fluency was implemented in several previous studies that measured differences in patterns of activation when reading speed is manipulated within the same individuals and sentence‐level stimuli are used (Benjamin & Gaab, 2012; Christodoulou et al., 2014; Kujala et al., 2007; Langer et al., 2013, 2019). For example, (Langer et al., 2013, 2019) presented sentences at constrained, comfortable, and accelerated speeds determined based on individual reading speed to 8–12‐year‐old children with and without a reading disability. Both groups of children showed an increased response in bilateral vOT with increased fluency demands. Using the same task, another study in adult participants also reported increased activity in the vOT regions with higher speed demands (Benjamin & Gaab, 2012). A key finding from these studies is increased activation in the vOT cortex with increased reading speed; however, the developmental significance and timeline of these findings for emerging fluency remains undetermined.
The current study examined longitudinal changes in brain activation associated with fluent reading during the period in which children typically transition from early to fluent reading. All children underwent functional MRI while performing a reading fluency task (Benjamin & Gaab, 2012; Langer et al., 2013, 2019) in which the speed of text presentation was manipulated at both time points. A critical advantage of this approach for developmental research is controlling for task demands across reading proficiency levels. If text were presented at the same speed to all participants, slower readers (in this case younger readers) may be presented with a more challenging task than faster readers. This may result in increased recruitment of multi‐demand domain‐general brain regions, rather than regions that support reading fluency, the focus of this study. Therefore, comfortable reading speed was determined for each child prior to the scan at both time points, and this speed was used for the in‐scanner task manipulation of two speeds of presentation: comfortable and accelerated.
In order to impose increased speed demands on children's reading, stimuli were presented in an accelerated manner (65% of comfortable speed). Previous findings using this task demonstrated increased activation of the vOT region for accelerated as compared with comfortable conditions (Benjamin & Gaab, 2012; Langer et al., 2013, 2019). This manipulation is closely aligned with how fluency is measured in educational settings in which children read single words or passages as quickly as they can (e.g., Woodcock et al., 2001). The constrained condition, where the speed of presentation is set, was also included to allow to establish a “lowest‐common‐denominator” comparison across participants. Presenting the stimuli at the same speed across participants is consistent with previous neuroimaging studies of reading that kept presentation speed consistent across all participants, allowing for comparison with previous literature.
Based on previous findings of increased engagement of vOT with reading proficiency and with increased speed demands, we hypothesized that we would (1) observe increased engagement of the vOT areas in older children as compared with younger children when comparing comfortable reading speeds; (2) increased engagement of these regions with increased reading speed demands in both age groups; and (3) an association between the increased activation in the vOT regions and improvement in reading fluency performance across the two time points.
2. METHODS
2.1. Participants
Children (N = 26) were retrospectively selected from the Boston Longitudinal Dyslexia study (BOLD) aimed to study the neural trajectory underlying typical and atypical reading development in children with and without a family history of developmental dyslexia (Powers et al., 2016; Raschle et al., 2011, 2012; Yu et al., 2020). Only participants whose fluency neuroimaging task and behavioral data were successfully collected at two time points within a time gap of 1–2 years were included in the current study (N = 31). Five children who performed the in‐scanner fluency task with <70% accuracy were excluded from analyses, resulting in a sample of typical developing children. As a result, 26 children (15 male) were included the final sample for the current study. The mean age was 8.25 years (SD = 9 months; children were in first or second grade) for the first time point and 9.5 years (SD = 14 months; children were in third or fourth grade) for the second time point, with a mean of 14 ± 8 months between the two time points. All children were right‐handed, native English speakers with no history of neurological symptoms, head injuries, visual problems, or hearing loss. The study was approved by the Institutional Review Board at Boston Children's Hospital. Written informed consent was obtained from each participant's accompanying parent, and verbal consent was obtained from each participant. Parental education information is summarized in Table S1.
2.2. Psychometric measurements
All children were examined using a comprehensive battery assessing language, pre‐reading, and reading skills. To avoid redundancy and reduce the number of comparisons, group characterization for the two time points focused on assessments that tested specific reading and reading‐related skills: phonological processing (Comprehensive Test of Phonological Processing, CTOPP, [Wagner et al., 1999]), rapid naming (3‐Set subtest of the RAN/RAS [Wolf & Denckla, 2005]), single‐word reading (Word ID and Word Attack subtests of the Woodcock Reading Mastery Test‐Revised [WRMT‐R; Woodcock, 2011]), Passage Comprehension (WRMT‐R) (Woodcock, 2011), and the Reading Fluency subtest of the Woodcock‐Johnson Test of Achievement Third Edition (WJ‐III) (Woodcock et al., 2001). The performance on these assessments for all participants is summarized in Table 1.
TABLE 1.
Mean (SD) standard/scale scores for the reading and reading related subskills psychometric assessment
| 1st time point | 2nd time point | t | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Word ID | 109.85 (12.2) | 108.81 (10.73) | 0.73 |
| Word attack | 111.92 (15.81) | 106.6 (9.27) | 2.33 |
| Passage comprehension | 108.32 (12.4) | 109.23 (11.67) | −0.33 |
| Reading fluency | 105.48 (15.98) | 105.23 (13) | −0.04 |
| Phonological awareness—elision | 11.04 (3.1) | 11 (2.93) | 0.07 |
| Phonological awareness—blending | 10.65 (2.18) | 10.88 (2.88) | 0.57 |
| Phonological awareness—nonword repetition | 9.81 (2.67) | 10.0 (2.97) | −0.33 |
| Rapid alternating stimulus tests | 106 (19.27) | 104.19 (14.23) | 0.68 |
2.3. MRI acquisition and analysis
2.3.1. Fluency task
This task was previously used and described by Benjamin and Gaab (2012) in adults and by Langer et al. (2013, 2019) in typically developing children and children with reading disabilities. For each trial, sentences comprised of four words were presented at a constrained, comfortable, or accelerated speed. The comfortable reading condition represented reading as it unfolds under naturalistic conditions when people read text at the speed that is comfortable to them. The constrained condition was included to dissociate the demands of speed from accuracy of reading and to ground the paradigm in prior literature that investigated reading using predetermined speed for all participants. The accelerated condition was designed to parallel behavioral studies of reading fluency by imposing speed demands on reading within an individually determined range.
The speed of word presentation in the constrained condition was fixed at 1350 ms for all participants. In contrast, the comfortable reading speed was customized for each participant outside the scanner (described in the subsequent section). The speed of the accelerated condition was 35% faster than the comfortable speed. As such, presentation speeds for comfortable and accelerated conditions varied across subjects and time points, while presentation speed for the constrained condition was the same across participants and time points. Word characteristics, including the age of acquisition, word frequency, familiarity, concreteness, imageability, and the number of phonemes and letters, were controlled using the MRC database (http://websites.psychology.uwa.edu.au/school/MRCDatabase/uwa_mrc.htm).
2.3.2. Determination of comfortable sentence reading speed
Before scanning, children underwent testing to determine their individual reading speeds. They were presented with three passages and asked to read them at a comfortable speed, taking as much time as necessary to complete. To capture their reading time, children pressed a key on a laptop to present each passage and another key when they finished reading the passage.
2.3.3. fMRI task
Before undergoing MRI, children underwent intensive training using a mock MRI scanner (for details, see Raschle et al., 2009, 2012). The fMRI implementation of the fluency task was identical to that used in Langer et al. (2013, 2019) and the child‐adapted version of the experimental fluency design employed in Benjamin and Gaab (2012). The task was presented in two 9‐min‐long runs, which included real word sentence (i.e., task) and letter string sentence (i.e., control) conditions, each presented at constrained, comfortable, and accelerated speeds.
Participants were first presented with a picture cue indicating word presentation speed (turtle‐constrained; cat‐normal; rabbit‐accelerated). Participants were then presented with a sentence one word at a time at one of the speeds (e.g., “The cat ran”), followed by a comprehension question. The comprehension phase included selecting one of three pictures that best describes the presented sentence. Children were instructed to choose the image that best represented the meaning of the sentence. For the control letter task, following the speed indicator picture, strings of “n” letters were presented in place of the words, spaced to appear with a similar structure as sentences, with one different target letter. Children were asked to choose the oddball letter (“f”, “p”, or “x”) that appeared in one of the last two letter strings. This control task was designed to probe lower‐level orthographic skills (e.g., visual attention/visual search) and letter recognition but not rely on high‐level reading skills (e.g., semantic processing). Each of the two runs comprised 42 (21 words and 21 letter string) sentences, with the number of letters matched across conditions and runs. Across the two runs, 14 words and 14 letter string sentences appeared for each reading speed (constrained, comfortable, and accelerated).
Task and control trials were presented using an event‐related design with the order of the two conditions (real word and letter string sentences) and speed pseudorandomized. Each trial began with an image cue indicating the upcoming presentation speed, which appeared on the screen for 500 ms and was followed by a black screen for 200 ms. Then, the words or control stimuli appeared from left to right at constrained, comfortable, or accelerated speed until the complete sentence was displayed. This was followed by a blank screen (200 ms). Subsequently, the comprehension or letter viewing testing phase appeared on the screen for 3000 ms or until the participant indicated their response (with a button press). The location of the correct image/letter was pseudorandomized in each trial. Each trial ended with a fixation cross presented for a variable time for up to 2000 ms. Performance was measured by the percent of trials answered correctly.
2.3.4. Imaging protocol and analysis
MRI scans were acquired on a SIEMENS 3.0 T Trio MR whole‐body scanner. 271 whole‐brain images were acquired in each of the two fMRI runs with a 32‐slice functional echo‐planar acquisition (interleaved ascending) using TR = 2000 ms, TE = 30 ms, FOV = 192 mm (full brain coverage), voxel size = 3 × 3 × 4 mm, and flip angle = 90°.
2.3.5. Preprocessing
The first four images of each run were discarded to account for field effects. Data were then preprocessed and analyzed using FSL 5.9 (http://www.fmrib.ox.ac.uk/fsl), beginning with motion correction (MCFLIRT), slice‐timing correction, brain extraction (BET), linear registration (12 degrees of freedom) to the MNI 152 T1 template (FLIRT), spatial smoothing (4 mm FWHM kernel), and high‐pass filtering (50 s). To deal with the relatively high degree of head motion common in pediatric neuroimaging, we used the ART toolbox (http://cibsr.stanford.edu/tools/human-brainproject/artrepair-software.html) to carefully detect volumes using a translation threshold of 2 mm and a rotation threshold of 0.02 mm. All subjects had two runs in which ≥85% of the constituent volumes were free of artifactual volumes. Subjects not meeting this criterion were excluded from further analyses (N = 7). Motion parameters and artifactual volumes were entered as regressors in the first‐level model.
2.3.6. Analysis
Whole‐brain analysis was performed in three stages. (1) A first‐level model was designed for each participant and each run. Data were prewhitened and regressors were modeled for the speed cues; constrained, comfortable and accelerated fluent sentence reading; constrained, comfortable, and accelerated letter string reading; sentence and control comprehension stimuli; and intertrial fixation. Motion parameters and artifactual volumes were defined as confounding extraneous variables. (2) We used an event‐related design in which the four words or letter strings constituted a single event. Note that unequal numbers of images were acquired for each participant and between the two time points since the individual reading speed varied between participants and between the two time points. FSL, however, can accommodate this variance (for a review, see Beckmann & Smith, 2004; Smith et al., 2004). Additionally, using FSL, low‐level design matrices do not need to be identical to compare the subjects on a higher‐level analysis (Smith et al., 2004). (3) For each time point, the two‐runs of each child's data were combined in fixed‐effects models and then entered into a group‐level random‐effects analysis (FLAME 1). Second‐level statistical maps were generated using a (Gaussianized t‐statistic) threshold of Z = 2.3 and a cluster‐corrected threshold of P < .05 for the within‐group and between‐groups (i.e., time points) contrasts.
The following contrasts were examined (see Table 2)
TABLE 2.
Raw scores (number of correct responses) for the psychometric assessments and in‐scanner accuracy for each time point and t‐score for the time points comparisons
| 1st time point | 2nd time point | t | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Word ID | 58.3 (14.18) | 70.27 (14.18) | 6.55** |
| Word attack | 24.27 (10.3) | 28.77 (7.98) | 4.48** |
| Passage comprehension | 30.54 (10.02) | 39.65 (9.12) | 7.38** |
| Reading fluency | 30.65 (13) | 42.77 (14) | 9.09** |
| Phonological awareness—Elision | 13.85 (4.6) | 15.04 (4.85) | 1.28 |
| Phonological awareness—Blending | 13.54 (3.29) | 14.15 (4.09) | 1.31 |
| Phonological awareness—nonword repetition | 10.19 (2.94) | 11.0 (3.49) | 1.28 |
| Rapid alternating stimulus a 3‐Set | 30.65 (17.44) | 42.77 (9.6) | 3.01** |
| Sentence comfortable accuracy | 95.45 (11.27) | 98.4 (5.28) | 2.1* |
| Sentence fast accuracy | 90.63 (13.08) | 97.44 (5.66) | 2.2* |
| Sentence slow accuracy | 92.51 (10.79) | 97.69 (6.83) | 2.39* |
| Letter comfortable accuracy | 96.50 (7.76) | 98.40 (3.35) | 2.1* |
| Letter fast accuracy | 97.85 (5.26) | 99.04 (3.6) | 2.2* |
| Letter slow accuracy | 96.28 (7.08) | 99.36 (1.81) | 2.39* |
Note: **p < 0.001, *p < 0.05.
Raw scores represent time in second. Lower values represent better performance.
1. Developmental reading effects: Validation of sentence reading activation at time 1 and time 2. To characterize the overall patterns of brain activity during sentence reading and replicate previous results with children (Langer et al., 2013, 2019), we examined sentence reading activation at each speed (constrained, comfortable, accelerated) separately in contrast to fixation (rest condition) at both time 1 and time 2 reading stages. We then compared activation for each sentence speed condition (relative to fixation) between reading stages. To examine the specificity of the developmental effects to connected‐text reading, we repeated the analyses for the letters > fixation contrast at east speed.
2. Sentence reading effects: Comparison between sentence reading and letter string reading at time 1 and time 2 regardless of reading speed. We compared fluent sentence reading to letter string reading to identify brain regions that responded selectively to sentence reading. We computed the contrast sentence reading [all speeds] > letter string reading [all speeds], first for each reading stage separately, and then between the two reading stages (time 2 > time 1).
3. Fluency effects: Comparison among reading speeds at time 1 and time 2 reading stages. We compared activation at the two time points for conditions with higher presentation rate with conditions with lower presentation rate to identify brain regions that responded selectively to the increased demands of more rapid reading (sentence reading [accelerated] > sentence reading [comfortable]; sentence reading [comfortable] > sentence reading [constrained] and sentence reading [accelerated] > sentence reading [constrained]).
2.3.7. Individual differences effects: Region‐of‐interest analysis
Based on previous results using this paradigm (Benjamin & Gaab, 2012; Langer et al., 2013, 2019), a region‐of‐interest (ROI) analysis was performed for the bilateral vOT cortex. First, regions engaged in fluent sentence reading were identified through the contrast of sentence reading [comfortable] > sentence reading [constrained]. Second, ROIs were defined as the intersection between the functional activation and the fusiform region (one per hemisphere) as defined with the Harvard–Oxford anatomical atlas. Finally, subjects' mean contrasts of parameter estimates (COPEs) were then extracted from ROIs for fluent sentence reading under increased speed demands (accelerated > constrained) via featquery. (http://www.FMRIb.ox.ac.uk/fsl/feat5/featquery.html) at each reading stage.
We then used these ROIs to investigate longitudinal brain‐behavior associations. We first calculated the change in activation in the left vOT regions during fluent sentence > rest reading (comfortable > constrained speed) and, as a control, letters > rest by subtracting the contrast maps of the time 1 point from the time 2 point for each participant. Next, we calculated the differences in raw scores between the two time points (time 2–time 1) for the WJ Reading Fluency test (Woodcock et al., 2001; a reading fluency measure). This measure was selected because it requires participants to read sentences as quickly as possible, thereby manipulating speed demands during reading of connected text. It is, therefore, most closely aligned with the neuroimaging task requirements. For exploratory purposes and for establishing the specificity of the association with measures of connected text fluency, but not decoding, we repeated the analyses with the other reading measures (TOWRE Phonemic Decoding and Sight Word Efficiency, RAN 2‐Set, and WJ Word Attack). Differences in time passed between time 1 and time 2 behavioral and MRI data collection points, which varied across subjects, were controlled for in all tests for correlations between the brain and behavioral measures of reading.
2.4. RESULTS
2.4.1. Psychometric assessment
Standardized psychometric test scores did not differ between time 1 and time 2 points (Table 1), according to a paired t‐test, indicating that children retained their relative reading proficiency across time. However, as expected, raw psychometric scores differed between the two time points for all reading tests (Word ID, Word Attack, Passage Comprehension, and Reading Fluency) and the RAN (Table 3), indicating improved reading skills across time. No significant differences between 1st and 2nd time points were observed for phonological awareness as measured using the CTOPP raw scores.
TABLE 3.
Results for the sentences reading (all speeds) > rest for each time point and the time points comparisons
| Time point | Speed rate | Voxels | Z‐MAX | MNI coordinates (mm) | Location (Z‐MAX) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| 2613 | 5.11 | −6 | −28 | −6 | Left thalamus extending to the left fusiform gyrus | ||
| 1st | All speeds | 1009 | 4.88 | 22 | −58 | −10 | Right lingual gyrus |
| 300 | 3.98 | −6 | 0 | 54 | Left cingulate/paracingulate gyrus extending into juxtapositional lobule cortex a | ||
| 252 | 3.83 | −30 | 24 | −2 | Left insular cortex | ||
| Constrained | 2887 | 4.49 | −26 | −60 | −10 | Left temporal occipital fusiform cortex | |
| 1575 | 4.9 | 22 | −58 | −10 | Right lingual gyrus | ||
| 261 | 3.94 | 4 | −54 | −38 | Hippocampus | ||
| Comfortable | 865 | 4.88 | −6 | −28 | −6 | Cingulate gyrus | |
| 551 | 4.75 | 26 | −54 | 6 | Right precuneous cortex | ||
| 287 | 3.95 | −26 | −2 | −2 | Intra calcarine cortex | ||
| 259 | 3.84 | −26 | −46 | −8 | Left lingual gyrus | ||
| Accelerated | 1960 | 4.96 | −8 | −16 | 4 | Left thalamus | |
| 768 | 4.45 | 26 | −54 | 6 | Right precuneous cortex | ||
| 496 | 3.77 | −26 | −74 | −8 | Left occipital fusiform gyrus | ||
| 303 | 4.09 | −30 | 22 | −4 | Left insular cortex | ||
| 292 | 4.21 | 34 | 18 | 6 | Right insular cortex | ||
| 275 | 3.97 | −6 | −2 | 54 | Left cingulate/paracingulate gyrus extending into juxtapositional lobule cortex a | ||
| 2nd | All speeds | 6116 | 4.88 | −28 | −72 | −10 | Left occipital fusiform gyrus |
| 283 | 3.92 | −28 | −76 | 24 | Left lateral occipital cortex, superior division | ||
| Constrained | 5973 | 5.32 | −24 | −52 | −14 | Left temporal occipital fusiform cortex | |
| 551 | 3.96 | 0 | −52 | −40 | Hippocampus | ||
| 352 | 3.77 | −30 | −8 | −4 | Left insular cortex | ||
| Comfortable | 5509 | 4.77 | 34 | −44 | −14 | Right temporal occipital fusiform cortex | |
| 290 | 3.69 | −28 | −78 | 26 | Left lateral occipital cortex, superior division | ||
| Accelerated | 2116 | 4.73 | 6 | −28 | −8 | Right thalamus extending to the fusiform cortex | |
| 1727 | 4.32 | 30 | −58 | −10 | Right temporal occipital fusiform cortex | ||
| 2nd > 1st | All speeds | 1506 | 3.69 | −2 | −96 | −14 | Left occipital pole |
| 1184 | 3.92 | −36 | −72 | −10 | Left occipital fusiform gyrus | ||
| 727 | 4.31 | 4 | −68 | 46 | Right precuneous cortex | ||
| 538 | 3.39 | −18 | −70 | −32 | Hippocampus | ||
| 387 | 3.76 | 18 | −68 | 22 | Right cuneal cortex | ||
| 218 | 3.52 | −12 | −92 | −26 | Left occipital fusiform gyrus | ||
| Constrained | 383 | 4.1 | 2 | −68 | 48 | Right precuneous cortex | |
| Comfortable | 1633 | 4.05 | −36 | −72 | −12 | Left occipital fusiform gyrus | |
| 915 | 3.89 | 24 | −72 | −10 | Right occipital fusiform gyrus | ||
| 570 | 3.51 | −24 | −34 | −40 | Hippocampus | ||
| 518 | 3.96 | 4 | −68 | 46 | Right lingual gyrus | ||
| 286 | 3.32 | 38 | −86 | −14 | Right lateral occipital cortex, inferior division | ||
| 222 | 3.69 | −22 | −86 | −20 | Left occipital fusiform gyrus | ||
| 875 | 3.9 | −30 | −66 | −22 | Left occipital fusiform gyrus | ||
| Accelerated | 791 | 3.57 | 36 | −44 | −24 | Right temporal occipital fusiform cortex | |
| 649 | 4.13 | 2 | −70 | 48 | Right precuneous cortex | ||
| 353 | 3.67 | 24 | −86 | 22 | Right occipital fusiform gyrus | ||
| 207 | 3.96 | 40 | −84 | −14 | Right lateral occipital cortex, inferior division | ||
| 567 | 3.73 | 4 | −68 | 48 | Right precuneous cortex | ||
Formerly supplementary motor cortex.
2.4.2. Determining sentence reading speed
For all participants except one, Comfortable reading speed, as determined before each MR scanning session, was faster than the Constrained reading speed (t[24.23] = 12.82, p < 0.001). The Comfortable speed improved across participants (i.e., reading rate increased (from time 1 [ms/word = 621 ± 288 ms] to time 2 [ms/word = 454 ± 132 ms]; t[25] = 4.03, p < 0.001).
2.4.3. In‐scanner performance
We used a three‐way repeated‐measures with time (time 1 and time 2), reading speed (constrained, comfortable, and accelerated), and condition (sentences, letters) as within‐subject variables to test for differences in reading accuracy. Results indicated a main effect of time on in‐scanner reading accuracy [F (1, 288) = 16.61, p < 0.001] with higher accuracy in time 2, compared with time 1. There was also a main effect of condition [F (1, 288) = 8.36, p = 0.001], with higher accuracy in the letters as compared with sentences condition. Neither the main effect of reading speed [F (2, 288) = 0.41, p = 0.66] nor the interactions among the three variables (p's > 0.09) were significant.
2.5. fMRI results
2.5.1. Developmental reading effects
When compared with rest, sentence reading (all speeds combined) for time 1 and time 2 activated the bilateral ventral occipito‐temporal (vOT) regions, including the lingual gyrus and fusiform gyrus, and the insular cortex (Table 4). A comparison between the two time points revealed increased activation at the time 2 in the left vOT for all three reading speeds (Figure 1).
TABLE 4.
Results for the different sentences reading speed comparisons
| Time point | Contrast | Voxels | p value corrected | Z‐MAX | MNI coordinates (mm) | Location (Z‐MAX) | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| 2nd | Accelerated > Constrained | 732 | 8.94E‐07 | 3.73 | 38 | −50 | −16 | Right temporal occipital fusiform cortex |
| 652 | 3.81E‐06 | 3.87 | −26 | −58 | −12 | Left temporal occipital fusiform cortex | ||
| Comfortable > Constrained | 1140 | 8.54E‐09 | 4.23 | −26 | −58 | −12 | Left temporal occipital fusiform cortex | |
| 976 | 1.19E‐07 | 3.84 | 26 | −60 | −10 | Right temporal occipital fusiform cortex | ||
| 2nd > 1st | Accelerated > Constrained | 275 | 0.00703 | 3.35 | −28 | −56 | −14 | Left temporal occipital fusiform cortex |
| Comfortable > Constrained | 1361 | 1.69E‐10 | 4.55 | −28 | −56 | −14 | Left temporal occipital fusiform cortex | |
| 1069 | 1.06E‐08 | 3.78 | 26 | −60 | −10 | Right temporal occipital fusiform cortex | ||
Note: No significant differences for the speed comparisons were found for the first time point.
FIGURE 1.

Fluent sentence reading (all speeds) > rest for the time 1 point, time 2 point, and the comparison between the two time points. Children show increased BOLD responses in several cortical and subcortical region mainly in occipito‐temporal regions. The level of significance was set at p < 0.05 cluster‐corrected.
2.5.2. Sentence reading effects
The contrast of fluent sentence reading (all speeds) versus letter string reading (all speeds) revealed increased activation in the left and right vOT for both time points (Figure 2). A paired t‐test for the contrast sentence > letters resulted in no significant differences between the two time points. The specificity of the developmental effects for sentences was also investigated using an ROI approach (Figure 3). Mixed effects models with the mean of significant voxels in vOT for the contrasts (a) sentences > rest and (b) letters > rest as dependent variables revealed a significant effect of time in the sentences model (b = 0.116, SE = 0.04, t = 2.8, p = 0.01), but not in the letters model (b = 0.033, SE = 0.05, t = 0.613, p = 0.546). These results suggest that the developmental trajectory of vOT specialization for reading sentences is different from its specialization for processing strings of letters.
FIGURE 2.

Fluent sentence reading (all speeds) > letter reading (all speeds) for time 1 and time 2. Children show increased BOLD responses in bilateral ventral occipito‐temporal (vOT) cortex for the sentence reading task vs. the letter reading task. Time points comparison did not reveal significant differences. The level of significance was set at p < 0.05 cluster‐corrected.
FIGURE 3.

Individual growth lines for all participants representing increased activation from time 1 to time 2 in the left ventral occipito‐temporal (vOT) regions for sentences (a) and letters (b) conditions.
2.5.3. Fluency effects
Results for sentence reading rate contrasts (accelerated > constrained; comfortable > constrained; and accelerated > comfortable) are presented in Table 4 and Figure 4. There were no significant differences in activation in response for the different contrasts for time 1. For time 2, greater activation was shown in the left vOT for accelerated > constrained contrasts and the bilateral vOT for comfortable > constrained contrast (Figure 4). No significant activations were found for the accelerated > comfortable contrast. For the longitudinal comparison, greater activation in time 2 compared with time 1 was shown in the bilateral vOT for the accelerated > constrained contrast and in the left fusiform cortex for the comfortable > constrained contrast. No significant differences were found for differences between time 2 and time 1 across speed contrasts for the letters condition.
FIGURE 4.
Comparisons of different reading speed (comfortable> constrained sentence reading; accelerated > constrained sentences reading) for time 2 and the time points comparisons. Children show increased BOLD responses in bilateral vOT cortex in advanced reading stage and for the time points comparisons. No significant effects were found for time 1. The level of significance was set at p < 0.05 cluster‐corrected.
2.5.4. Region of interest analysis
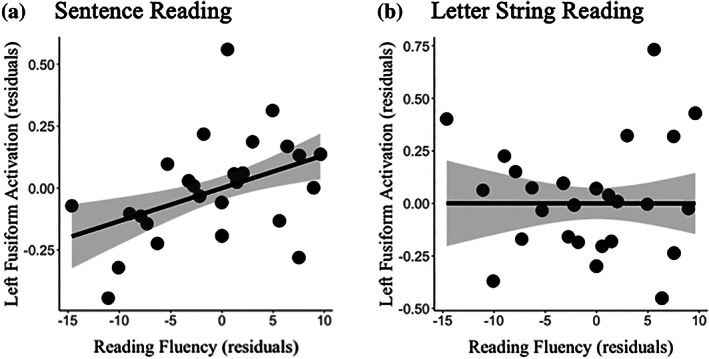
First, we computed the correlation between change in activation for time 2–time 1 for the left vOT cortex for sentences greater than rest (accelerated > constrained condition) and change in reading fluency (WJ‐III reading fluency subtest raw scores for time 2 − raw scores for time 1; Figure 5), while controlling for time passed between the two time points (see Methods for details). A significant positive correlation (r [26] = 0.42, p = 0.033) was observed between change in left vOT activation for sentences and change in reading fluency.
FIGURE 5.

A scatter plot illustrating partial correlation of growth in left vOT cortex activation (ROI) for sentence Reading (a) and letter string Reading (b) conditions with growth in reading fluency (WJ‐III) controlling for the time differences between two measures (r [26] = 0.53, p = 0.007).
Next, to explore whether the association was specific to changes in fluency and not the result of confounding maturational influences on both brain and reading development, we tested the specificity of the association to the sentences contrast, but not letters. Indeed, the association between reading fluency development and increased vOT activation for letters was not significant (r [26] = 0.05, p = 0.82). Finally, to evaluate the specificity of the brain‐behavior association to the domain of connected‐text fluency, we ran exploratory correlations with other behavioral measures administered in the study (Table S1). No other relationships between brain function and reading abilities were significant, but there were small‐to‐medium associations with GORT Fluency (r [24] = 0.32, p = 0.11) and RAN 2‐Set (r [24] = 0.27, p = 0.19).
Finally, to investigate whether the activity of vOT is modulated by the speed of stimulus presentation we tested whether brain activity in the left vOTcortex is related to the individual comfortable speed of presentation. We found that speed of presentation was not correlated with vOT activation for Sentences greater than Rest condition at Time 1 (r [24] = −0.31, p = 0.126) or Time 2 (r [24] = 0, p = 0.98), or with an increase in activation from Time 1 to Time 2 adjusted for age (r [24] = 0.26, p = 0.2). Similarly, there was no significant association with activation for the Letters condition at Time 1 (r [24] = 0.11, p = 0.583) or to Time 2 (r [24] = 0.09, p = 0.668), or to an increase in activation adjusted for age (r [24] = 0.02, p = 0.917). Thus, we did not find evidence that the speed of presentation is related to activation in the vOT cortex, and it is therefore not likely to modulate the association between children's reading fluency and brain activation.
3. DISCUSSION
The current study investigated developmental changes in neural patterns of activation underlying reading fluency. The study is novel in that it uses a longitudinal design and a more ecologically valid task that changes fluency demand by manipulating the presentation speed for each participant according to their individual‐based reading speed. First, we demonstrated increased activation of the bilateral ventral occipito‐temporal regions (vOT) in children during reading at their comfortable speed at time 2 compared with time 1. Second, consistent with studies in same‐age children (Christodoulou et al., 2014; Langer et al., 2013, 2019) and adults (Benjamin & Gaab, 2012), increased engagement of these regions was associated with increased speed demands at time 2, but not in time 1. Finally, increased activation in vOT was associated with a larger growth in reading fluency skills. Taken together, our findings provide critical insights on the association between the development of the vOT and children's transition to fluent reading.
3.1. Reading activation profiles across the two time points
There were differences in activation patterns between time 1 and time 2 points, across all presentation speeds. Specifically, in time 1, children recruited insular, cingulate, and occipito‐temporal regions during sentence reading. In time 2, there was significant recruitment of occipito‐temporal areas only. The results in the older children were strikingly parallel to those obtained in previous studies using the same paradigm of comparable age or older individuals (Benjamin & Gaab, 2012; Langer et al., 2013, 2019). The younger group's patterns of activation, however, were notably distinct (although the direct comparison did not reach significance), with increased recruitment of multi‐demand regions during sentence reading (i.e., insular cortex, cingulate cortex, and precuneus). These regions were shown to support a range of executive control functions (e.g., inhibitory control, attentional selection, conflict resolution, maintenance and manipulation of task sets) for both linguistic and non‐linguistic tasks (Duncan & Owen, 2000; Fedorenko, 2014; Fedorenko et al., 2013; Hugdahl et al., 2015). Previous studies found increased engagement of these systems to support decoding in non‐proficient readers (Ozernov‐Palchik et al., 2021; Roe et al., 2018; Ryherd et al., 2018). These differences in patterns of activation between the two time points support the critical transition proposed around 3rd grade from effortful reading that requires the utilization of considerable cognitive resources, to increasingly automatic word recognition. Such automatic word recognition is akin to the effortless processing of other visual objects such faces (Chall, 1996).
A direct comparison between the two time points revealed that activation in vOT increases longitudinally. Indeed, increased engagement of this region was shown to parallel increased perceptual expertise for processing words in longitudinal studies of early readers (Brem et al., 2010; Dehaene‐Lambertz et al., 2018; Pleisch et al., 2019; Saygin et al., 2016). Specifically, as word processing in vOT becomes increasingly fluent, this region operates to rapidly extract invariant information from the word form, linking this information with the corresponding higher‐level linguistic representations and attentional systems (Chen et al., 2019; Price & Devlin, 2011; Schlaggar & McCandliss, 2007). Although previous studies demonstrated developmental differences in the region, this is the first study to adjust for potential differences in fluency‐related task difficulty in a longitudinal design. This is important because of the sensitivity of vOT to differences in task demands and durations of exposure (Benjamin & Gaab, 2012; Dehaene & Cohen, 2011). By establishing each participant's comfortable reading speed and choosing simple sentence stimuli, we equated task demands and the optimal exposure speed between the two time points, and across individuals. We can therefore confidently interpret our findings as representing increased specialization of the vOT region for fluent reading.
The location of the vOT across contrasts was more medial than its classical coordinates (−43, −54, −12; Cohen & Dehaene, 2004), but it showed consistency in terms of location with other studies that used this task (Benjamin & Gaab, 2012; Langer et al., 2013). The specific coordinates of the region vary across studies and across contrasts in the current study, suggesting sensitivity to differences in task demands. For example, more medial sectors of vOT have previously been shown to exhibit increased sensitivity to lexico‐semantic features (Vinckier et al., 2007) and to unimodal visual, rather than cross‐modal visual and auditory, word processing (Cohen & Dehaene, 2004).
3.2. Early specialization of vOT for sentence reading
The contrast sentence > letter reading (averaged across all speeds) revealed similar patterns of activation in the bilateral vOT in both times 1 and 2. Our results support previous findings of print‐induced activation in the vOT region at the beginning reading stages and its increase with reading experience (Centanni et al., 2017; Dehaene‐Lambertz et al., 2018; Lochy et al., 2016; Saygin et al., 2016). As the region's activation for single letters decreases, its activation for words increases. Specifically, a recent study has documented an inverted U‐shaped pattern of activity of vOT to letter stimuli that peaks in the 1st grade once the alphabetic code has been mastered, but then begins to dip (Fraga González et al., 2015; Fraga‐González et al., 2021). Specialization for words follows a similar trajectory (Maurer et al., 2006). The onset of the vOT activation curve follows the decline in its responses to letters, and with a more extended peak, as word mastery is a longer milestone to reach (Centanni et al., 2017). Since previous developmental studies examined words presented in isolation, it remains unknown whether the course of specialization of vOT to connected text follows a similar trajectory. Our study precludes us from establishing the trajectory of the sentence‐responses beyond our 2nd time point (3rd/4th grades). Longitudinal studies with additional measurement occasions are therefore needed to map out the course of vOT activation under naturalistic reading conditions.
All the vOT effects in the current study were bilaterally distributed. Although the processing of orthographic information in the vOT cortex is considered left‐lateralized (Baker et al., 2007; Cohen & Dehaene, 2004; Glezer & Riesenhuber, 2013; Hirshorn et al., 2016; Woodhead et al., 2011), the extent of the left‐hemispheric dominance depends on multiple factors such participants' age (Centanni et al., 2018; James, 2010), reading proficiency (Maisog et al., 2008; Martin et al., 2015; Richlan et al., 2011), task demands (e.g., semantic demands placed by the task; Seghier & Price, 2011), and type of stimuli (e.g., letters versus words; Fraga‐González et al., 2021). Although single word reading tends to elicit a more left‐lateralized response, increased right‐hemispheric involvement has been demonstrated for sentence reading (Beaucousin et al., 2011). Sentence reading relies on the contextual integration processes in the right hemisphere to construct meaning (for reviews see Bookheimer, 2002; Lindell, 2006).
Moreover, the bilateral distribution of neural responses to words during reading development is consistent with the idea that initial word processing involves the same neural systems as objects and faces in both hemispheres because the feedforward features are similar (Behrmann & Plaut, 2020). As children become increasingly fluent readers, word processing gradually becomes left‐lateralized because of the continuous interactions with the left‐dominant language system. Indeed, bilateral responses to word reading were reported in previous studies of a similar age group as the current sample (age range 7–9 years; [Church et al., 2008; Turkeltaub et al., 2003]), but in studies of older children left‐hemispheric specialization has been reported (age range 8–15 years; [Ben‐Shachar et al., 2011; Maurer et al., 2006, 2011]). Therefore, the bilateral patterns of activation in the current study are consistent with the sensitivity of this region to variations in study design and suggest that left lateralization occurs later in reading development than at the age studied here.
The increased response in the current study of vOT to sentences, as compared with letters, is consistent with the Interactive Account (Price & Devlin, 2011) of vOT specialization. This account applies a predictive coding framework to describe how high‐order language regions (e.g., phonological and semantic regions) generate predictions regarding the identity of words based on the contextual cues and lower‐level visuospatial features. Activation in the vOT region reflects prediction error. i.e., the discrepancy between the predictive and the sensory signals. Predictions are stronger for words, especially when these words are embedded in sentences that provide strong contextual cues to word identification than for letter strings, resulting in more robust error signals and increased activation for words as compared with letters (Price et al., 1996).
This framework has important implications for developmental differences within the vOT. In preliterate children, vOT activation is low because the orthographic inputs fail to trigger the corresponding higher‐level representations; therefore top‐down influences are weak. In early readers, the discrepancy between the top‐down and bottom‐up signals is maximal, resulting in the strongest activation. The prediction errors will decrease with increased reading expertise. The exact timing of the activation peak in the vOT is difficult to ascertain precisely. Various confounding factors could affect activation patterns in the vOT. Transparency of orthography is one such factor (Aro & Wimmer, 2003; Carioti et al., 2021). For example, maximal activity was demonstrated in 2nd grade in children reading in German, which has a more transparent orthography than English (Maurer et al., 2006; van der Mark et al., 2011). Another factor is processing demands imposed by the task. For example, if task demands are not controlled for, some of the vOT signal in younger readers could reflect additional effort. Additional factors include stimulus exposure durations (Schuster et al., 2015) and nature of the task (e.g., lexical decision, ‐overt or covert naming, silent reading, single words) that have varied across different studies and have implications for the generalizability of findings to natural reading (Rayner, 1998; Wehbe et al., 2014; Yarkoni et al., 2008).
It is therefore likely, based on the current findings, that the activation peak in children learning to read English orthography will coincide with the development reading framework that posits that reading mastery is achieved at around 4th grade (Chall, 1983). Thus, increased activation in vOT in more expert readers, as compared with their younger counterparts, is consistent with the interactive specialization framework (Price & Devlin, 2011). This framework sees increased specialization of the region through recurrent connectivity between the linguistic and the vOT components (i.e., higher language and lower sensory levels) through the experience of learning to read.
3.3. Speed demands modulate vOT activation
How does fluency play into the predictive coding framework? We showed that reading speed modulates brain activation only at the time 2 point, after children became more proficient in reading. It is possible that increased speed demands resulted in more prediction errors due to less accurate top‐down predictions. Since the predictive mechanisms are less developed in younger readers, faster presentation rates did not increase error signals in this group. Additionally, children in time 1, but not time 2, activated the cognitive control regions to a greater extent in the accelerated condition. As discussed above, this suggested increased recruitment of the multi‐demand domain‐general regions for executive control in response to higher task demands. Therefore, increased fluency demands increased processing within the vOT for the more proficient readers and increased cognitive control recruitment in the emerging readers. Differences between accelerated and comfortable conditions were not observed at any of the time points. Therefore, increased task demands for the accelerated conditions did not translate into measurable differences in vOT response profile. Only the comparison against individually determined speeds and the constrained speed revealed differences in activation. This suggests that the individually determined speed represented increased speed demands for the comfortable condition as children were attempting to the read the materials at their most efficient pace. Our task, therefore, was sensitive to individual speed differences and represents an ecologically valid neural measure of fluency.
3.4. Alternative explanation for patterns of results
As an alternative to the predictive coding framework, increased activation in the vOT was proposed to reflect the extent of perceptual expertise of the region (McCandliss & Noble, 2003). This idea is consistent with the learning‐curve hypothesis in skill acquisition (Poldrack & Gabrieli, 2001; Pugh et al., 2008), which proposes that initial skill acquisition is accompanied by increased activation in task‐specific cortical areas followed by reduced activation within that area as it becomes specialized. Accordingly, increased response of the region to letters has been demonstrated in newly literate adults (Dehaene et al., 2010), individuals trained on a new orthography (Brem et al., 2010), and in literate as compared with pre‐reading children (Saygin et al., 2016). Consistent with the current study, this framework would predict increased activation in older as compared with younger children to sentences. Inconsistent with the current results, however, increased activation to letter strings (stimuli that children have more expertise in processing) would be expected.
Another possible explanation is that vOT reflects differences in task demands. Such an explanation has previously been shown to account for increased activation in tasks that require processing low‐frequency words, for example, rather than high‐frequency words (Dehaene & Cohen, 2011). This explanation is consistent with the increased activation of the vOT region in faster as compared with slower presentation rates. In the current study, however, children showed a developmental increase in vOT activation from time 1 to time 2, despite reduced task demands as they became more expert readers. Therefore, this account also fails to explain the current findings.
3.5. vOT specialization in relation to fluency skills
The changes in vOT activation across the two time points were related to improvements in reading fluency performance. These findings highlight the reciprocal interaction between reading development and the neural specialization for reading. As children become more proficient readers, vOT activation increases. As vOT becomes more specialized for reading, children become increasingly automatic in their word identification. The majority of the previous studies demonstrating links between reading proficiency and vOT specialization have focused on cross‐sectional comparisons (e.g., Carreiras et al., 2009; Church et al., 2008; Dehaene et al., 2010; Turkeltaub et al., 2003)‐ or lower‐level reading skills (Ben‐Shachar et al., 2011; Maurer et al., 2006, 2011; Skeide et al., 2017). Our findings are important because they demonstrate that variable and individually determined increased fluency demands modulate the specialization of vOT for reading only after word identification proficiency is achieved.
According to Chall's stages of reading, fluency is a bridge that moves students from proficient decoding to the extraction of meaning from connected text (Chall, 1983; Chard et al., 2002). The transition from a focus on accurate word identification to using text to gain new knowledge and ideas through reading increasingly complex texts (reading to learn) is set to occur in 4th grade in English‐readers. Our findings that beyond just proficiency in decoding, rate of decoding is an important contributor to vOT activity, supports the significant role of fluency in reading development. We show that activation of vOT is a sensitive index of speed of processing—and consequently of automaticity in word recognition—that underlies the development of fluency in this critical period of transitioning into reading fluency and proficiency. In accordance with the multi‐componential view of fluency, vOT is an important hub that receives and processes predictive signals from higher‐language brain regions that support semantic, phonological, and attentional processes as well as feed‐forward lower‐level visual signals. Our findings suggest that the automaticity of processing in this hub underlies fluent reading and that the transition to the “reading to learn” stage is a critical time for this region's neural specialization.
The educational implications of individual variability in reading fluency are great. In one study of students who took the NAEP reading assessment in 2002, 40% of the fourth‐grade samples were identified as “non‐fluent” readers (Daane, 2005). Fluency skills account for a significant and unique variance in reading comprehension (Cutting et al., 2009; Joshi & Aaron, 2000; Silverman et al., 2013; Tilstra et al., 2009), and mediates the relationship between word reading and reading comprehension (Kim et al., 2021; Kim & Wagner, 2015). The negative impact of fluency deficits have been shown to extend to many other school subjects (Panel [U.S.], 2000). Indeed, dysfluent reading diverts cognitive resources such as attention and working memory away from comprehension and thereby hinders deep processing of and learning from text (Cain et al., 2004; Perfetti, 1985). Furthermore, reading speed is strongly associated with children's self‐concept regarding their reading skills and affects their motivation for reading (Kasperski et al., 2016).
Our findings can be extended to support the significance of interventions that prioritize fluency‐building strategies. Repeated reading is the most common strategy that explicitly targets reading fluency. In a recent meta‐analysis 90% of fluency intervention studies focused on this strategy (Hudson et al., 2020). These interventions aim to both promote fluency and advance more distal outcomes of reading comprehension. Based on the neurocognitive development model of vOT specialization, repeated reading would strengthen the connections between visual word features and their higher‐level linguistic counterparts, resulting in increased automaticity of processing of orthographic patterns and subsequently greater fluency. Greater fluency would allow the multi‐demand network to be engaged in the cognitively demanding process of assigning meaning, monitoring, inferring, and building coherence while reading connected text.
4. CONCLUSIONS
We examined the development of the neural correlates underlying the development of reading fluency throughout 1–2 years of elementary schooling in a longitudinal design. Our results showed increased ventral occipito‐temporal activation longitudinally and when increasing reading speed demands. Furthermore, increased activation was associated with better fluency development. These findings shed light on the reciprocal importance of the ventral occipito‐temporal cortex for the development of reading fluency. Specifically, the increased engagement of this region in sentence reading (compared with letter strings) and during accelerated reading is modulated by and supports reading proficiency. These findings also provide mechanistic insights for the efficacy of repeated reading strategies to increase reading fluency.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Supporting information
APPENDIX S1 Supporting Information
ACKNOWLEDGMENTS
This study was supported by a grant from the National Institutes of Health–National Institute of Child Health and Human Development (Grants R01HD067312 to NG, F32‐HD100064 to Ola Ozernov‐Palchik). This study was also supported by the Jacobs Foundation and Charles H. Hood Foundation grants to Nadine Gaab, and Harvard Brain Initiative Transitions Program grant to Ted K. Turesky. We thank Laurel Whitfield and Victoria Hue for their editing assistance. We sincerely thank our research testers and participating families.
Ozernov‐Palchik, O. , Sury, D. , Turesky, T. K. , Yu, X. , & Gaab, N. (2023). Longitudinal changes in brain activation underlying reading fluency. Human Brain Mapping, 44(1), 18–34. 10.1002/hbm.26048
Ola Ozernov‐Palchik and Dana Sury have been considered as co‐first authors
Funding information National Institutes of Health, Grant/Award Numbers: F32‐HD100064, R01HD067312; Jacobs Foundation and Charles H. Hood Foundation
DATA AVAILABILITY STATEMENT
The data required to reproduce reported findings will be provided upon request.
REFERENCES
- Aboud, K. S. , Barquero, L. A. , & Cutting, L. E. (2018). Prefrontal mediation of the reading network predicts intervention response in dyslexia. Cortex, 101, 96–106. 10.1016/j.cortex.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro, M. , & Wimmer, H. (2003). Learning to read: English in comparison to six more regular orthographies. Applied PsychoLinguistics, 24(4), 621–635. 10.1017/S0142716403000316 [DOI] [Google Scholar]
- Baker, C. I. , Liu, J. , Wald, L. L. , Kwong, K. K. , Benner, T. , & Kanwisher, N. (2007). Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proceedings of the National Academy of Sciences, 104(21), 9087–9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaucousin, V. , Cassotti, M. , Simon, G. , Pineau, A. , Kostova, M. , Houdé, O. , & Poirel, N. (2011). ERP evidence of a meaningfulness impact on visual global/local processing: When meaning captures attention. Neuropsychologia, 49(5), 1258–1266. [DOI] [PubMed] [Google Scholar]
- Beckmann, C. F. , & Smith, S. M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging, 23(2), 137–152. 10.1109/TMI.2003.822821 [DOI] [PubMed] [Google Scholar]
- Behrmann, M. , & Plaut, D. C. (2020). Hemispheric organization for visual object recognition: A theoretical account and empirical evidence. Perception, 49(4), 373–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin, C. F. A. , & Gaab, N. (2012). What's the story? The tale of reading fluency told at speed. Human Brain Mapping, 33(11), 2572–2585. 10.1002/hbm.21384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Shachar, M. , Dougherty, R. F. , Deutsch, G. K. , & Wandell, B. A. (2011). The development of cortical sensitivity to visual word forms. Journal of Cognitive Neuroscience, 23(9), 2387–2399. 10.1162/jocn.2011.21615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer, S. (2002). Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience, 25(1), 151–188. [DOI] [PubMed] [Google Scholar]
- Borowsky, R. , & Besner, D. (2006). Parallel distributed processing and lexical‐semantic effects in visual word recognition: are a few stages necessary? Psychol Rev. 113(1), 181–95; discussion 196–200. 10.1037/0033-295X.113.1.181. PMID: 16478310. [DOI] [PubMed] [Google Scholar]
- Borowsky, R. , Esopenko, C. , Cummine, J. , & Sarty, G. E. (2007). Neural representations of visual words and objects: A functional MRI study on the modularity of reading and object processing. Brain Topography, 20(2), 89–96. [DOI] [PubMed] [Google Scholar]
- Brem, S. , Bach, S. , Kucian, K. , Kujala, J. V. , Guttorm, T. K. , Martin, E. , Lyytinen, H. , Brandeis, D. , & Richardson, U. (2010). Brain sensitivity to print emerges when children learn letter–speech sound correspondences. Proceedings of the National Academy of Sciences, 107(17), 7939–7944. 10.1073/pnas.0904402107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem, S. , Maurer, U. , Kronbichler, M. , Schurz, M. , Richlan, F. , Blau, V. , Reithler, J. , van der Mark, S. , Schulz, E. , Bucher, K. , Moll, K. , Landerl, K. , Martin, E. , Goebel, R. , Schulte‐Körne, G. , Blomert, L. , Wimmer, H. , & Brandeis, D. (2020). Visual word form processing deficits driven by severity of reading impairments in children with developmental dyslexia. Scientific Reports, 10(1), 18728. 10.1038/s41598-020-75111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, J. L. , Zumberge, A. , Manis, F. R. , Lu, Z.‐L. , & Goldman, J. G. (2008). Sensitivity to orthographic familiarity in the occipito‐temporal region. NeuroImage, 39(4), 1988–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras, M. , Seghier, M. L. , Baquero, S. , Estévez, A. , Lozano, A. , Devlin, J. T. , & Price, C. J. (2009). An anatomical signature for literacy. Nature, 461(7266), 983–986. [DOI] [PubMed] [Google Scholar]
- Cain, K. , Oakhill, J. , & Bryant, P. (2004). Children's reading comprehension ability: Concurrent prediction by working memory, verbal ability, and component skills. Journal of Educational Psychology, 96, 31–42. 10.1037/0022-0663.96.1.31 [DOI] [Google Scholar]
- Carioti, D. , Masia, M. F. , Travellini, S. , & Berlingeri, M. (2021). Orthographic depth and developmental dyslexia: A meta‐analytic study. Annals of Dyslexia, 71(3), 399–438. 10.1007/s11881-021-00226-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni, T. M. , King, L. W. , Eddy, M. D. , Whitfield‐Gabrieli, S. , & Gabrieli, J. D. E. (2017). Development of sensitivity versus specificity for print in the visual word form area. Brain and Language, 170, 62–70. 10.1016/j.bandl.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Centanni, T. M. , Norton, E. S. , Park, A. , Beach, S. D. , Halverson, K. , Ozernov‐Palchik, O. , Gaab, N. , & Gabrieli, J. D. (2018). Early development of letter specialization in left fusiform is associated with better word reading and smaller fusiform face area. Developmental Science, 21(5), e12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chall, J. S. (1983). Stages of reading development. New York: McGraw‐Hill. [Google Scholar]
- Chall, J. S. (1996). American reading achievement: Should we worry? Research in the Teaching of English, 30(3), 303–310. [Google Scholar]
- Chard, D. J. , Vaughn, S. , & Tyler, B.‐J. (2002). A synthesis of research on effective interventions for building Reading fluency with elementary students with learning disabilities. Journal of Learning Disabilities, 35(5), 386–406. 10.1177/00222194020350050101 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Wassermann, D. , Abrams, D. A. , Kochalka, J. , Gallardo‐Diez, G. , & Menon, V. (2019). The visual word form area (VWFA) is part of both language and attention circuitry. Nature Communications, 10(1), 5601. 10.1038/s41467-019-13634-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou, J. A. , del Tufo, S. N. , Lymberis, J. , Saxler, P. K. , Ghosh, S. S. , Triantafyllou, C. , Whitfield‐Gabrieli, S. , & Gabrieli, J. D. E. (2014). Brain bases of reading fluency in typical reading and impaired fluency in dyslexia. PLoS One, 9(7), e100552. 10.1371/journal.pone.0100552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church, J. A. , Coalson, R. S. , Lugar, H. M. , Petersen, S. E. , & Schlaggar, B. L. (2008). A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex, 18(9), 2054–2065. 10.1093/cercor/bhm228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyl, K. , Fraga‐González, G. , Brem, S. , & Jednoróg, K. (2021). Brain dynamics of (a)typical reading development—A review of longitudinal studies. NPJ Science of Learning, 6(1), 4. 10.1038/s41539-020-00081-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, L. , & Dehaene, S. (2004). Specialization within the ventral stream: The case for the visual word form area. NeuroImage, 22(1), 466–476. [DOI] [PubMed] [Google Scholar]
- Crain, S. , & Shankweiler, D. (1990). Explaining failures in spoken language comprehension by children with reading disabilities. In comprehension processes in reading (pp. 539–555). Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Cutting, L. E. , Materek, A. , Cole, C. A. , Levine, T. M. , & Mahone, E. M. (2009). Effects of fluency, oral language, and executive function on reading comprehension performance. Annals of Dyslexia, 59(1), 34–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daane, M. C. , Seghier, M. L. (2005). Nation's report card: Fourth‐grade students reading aloud: NAEP 2002 special study of oral reading. National Center for Education Statistics.
- Dehaene, S. , & Cohen, L. (2007). Cultural recycling of cortical maps. Neuron, 56(2), 384–398. 10.1016/j.neuron.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Dehaene, S. , & Cohen, L. (2011). The unique role of the visual word form area in reading. Trends in Cognitive Sciences, 15(6), 254–262. 10.1016/j.tics.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Dehaene, S. , Cohen, L. , Morais, J. , & Kolinsky, R. (2015). Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nature Reviews Neuroscience, 16(4), 234–244. 10.1038/nrn3924 [DOI] [PubMed] [Google Scholar]
- Dehaene, S. , Pegado, F. , Braga, L. W. , Ventura, P. , Filho, G. N. , Jobert, A. , Dehaene‐Lambertz, G. , Kolinsky, R. , Morais, J. , & Cohen, L. (2010). How learning to read changes the cortical networks for vision and language. Science, 330(6009), 1359–1364. 10.1126/science.1194140 [DOI] [PubMed] [Google Scholar]
- Dehaene‐Lambertz, G. , Monzalvo, K. , & Dehaene, S. (2018). The emergence of the visual word form: Longitudinal evolution of category‐specific ventral visual areas during reading acquisition. PLoS Biology, 16(3), e2004103. 10.1371/journal.pbio.2004103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, J. , & Owen, A. M. (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences, 23(10), 475–483. 10.1016/S0166-2236(00)01633-7 [DOI] [PubMed] [Google Scholar]
- Ehri, L. C. (2005). Development of sight word Reading: Phases and findings. In The science of reading: A handbook (pp. 135–154). Blackwell Publishing. 10.1002/9780470757642.ch8 [DOI] [Google Scholar]
- Fedorenko, E. (2014). The role of domain‐general cognitive control in language comprehension. Frontiers in psychology, 5, 335. 10.3389/fpsyg.2014.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko, E. , Duncan, J. , & Kanwisher, N. (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences, 110(41), 16616–16621. 10.1073/pnas.1315235110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga González, G. , Žarić, G. , Tijms, J. , Bonte, M. , Blomert, L. , & van der Molen, M. W. (2015). A randomized controlled trial on the beneficial effects of training letter‐speech sound integration on reading fluency in children with dyslexia. PLoS One, 10(12), e0143914. 10.1371/journal.pone.0143914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga‐González, G. , Pleisch, G. , di Pietro, S. V. , Neuenschwander, J. , Walitza, S. , Brandeis, D. , Karipidis, I. I. , & Brem, S. (2021). The rise and fall of rapid occipito‐temporal sensitivity to letters: Transient specialization through elementary school. Developmental Cognitive Neuroscience, 49, 100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer, L. S. , & Riesenhuber, M. (2013). Individual variability in location impacts orthographic selectivity in the visual word form area. Journal of Neuroscience, 33(27), 11221–11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort, P. (2013). MUC (memory, unification, control) and beyond. Frontiers in Psychology, 4, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshorn, E. A. , Li, Y. , Ward, M. J. , Richardson, R. M. , Fiez, J. A. , & Ghuman, A. S. (2016). Decoding and disrupting left midfusiform gyrus activity during word reading. Proceedings of the National Academy of Sciences, 113(29), 8162–8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, A. , Koh, P. W. , Moore, K. A. , & Binks‐Cantrell, E. (2020). Fluency interventions for elementary students with reading difficulties: A synthesis of research from 2000–2019. Education Sciences, 10(3), 52. 10.3390/educsci10030052 [DOI] [Google Scholar]
- Hudson, R. F. , Pullen, P. C. , Lane, H. B. , & Torgesen, J. K. (2008). The complex nature of reading fluency: A multidimensional view. Reading & Writing Quarterly, 25(1), 4–32. 10.1080/10573560802491208 [DOI] [Google Scholar]
- Hugdahl, K. , Raichle, M. E. , Mitra, A. , & Specht, K. (2015). On the existence of a generalized non‐specific task‐dependent network. Frontiers in human neuroscience, 9, 430. 10.3389/fnhum.2015.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, K. H. (2010). Sensori‐motor experience leads to changes in visual processing in the developing brain. Developmental Science, 13(2), 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, R. M. , & Aaron, P. (2000). The component model of reading: Simple view of reading made a little more complex. Reading Psychology, 21(2), 85–97. [Google Scholar]
- Kame'enui, E. J. , Simmons, D. C. , Good, R. H. , & Harn, B. A. (2001). The use of fluency‐based measures in early identification and evaluation of intervention efficacy in schools. Dyslexia, fluency, and the brain, (pp. 307–331). [Google Scholar]
- Kasperski, R. , Shany, M. , & Katzir, T. (2016). The role of RAN and reading rate in predicting reading self‐concept. Reading and Writing. Reading and Writing, 29(1), 117–136. [Google Scholar]
- Kim, Y.‐S. G. , Quinn, J. M. , & Petscher, Y. (2021). What is text reading fluency and is it a predictor or an outcome of reading comprehension? A longitudinal investigation. Developmental Psychology, 57(5), 718–732. 10.1037/dev0001167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.‐S. G. , & Wagner, R. K. (2015). Text (Oral) reading fluency as a construct in reading development: An investigation of its mediating role for children from grades 1 to 4. Scientific Studies of Reading, 19(3), 224–242. 10.1080/10888438.2015.1007375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler, M. , Bergmann, J. , Hutzler, F. , Staffen, W. , Mair, A. , Ladurner, G. , & Wimmer, H. (2007). Taxi vs. Taksi: On orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience, 19(10), 1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler, M. , Hutzler, F. , Staffen, W. , Mair, A. , Ladurner, G. , & Wimmer, H. (2006). Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia, 44(10), 1822–1832. 10.1016/j.neuropsychologia.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Kronbichler, M. , Hutzler, F. , Wimmer, H. , Mair, A. , Staffen, W. , & Ladurner, G. (2004). The visual word form area and the frequency with which words are encountered: Evidence from a parametric fMRI study. NeuroImage, 21(3), 946–953. [DOI] [PubMed] [Google Scholar]
- Kronbichler, M. , Klackl, J. , Richlan, F. , Schurz, M. , Staffen, W. , Ladurner, G. , & Wimmer, H. (2009). On the functional neuroanatomy of visual word processing: Effects of case and letter deviance. Journal of Cognitive Neuroscience, 21(2), 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota, E. C. , Joo, S. J. , Huber, E. , & Yeatman, J. D. (2019). Word selectivity in high‐level visual cortex and reading skill. Developmental Cognitive Neuroscience, 36, 100593. 10.1016/j.dcn.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala, J. , Pammer, K. , Cornelissen, P. , Roebroeck, A. , Formisano, E. , & Salmelin, R. (2007). Phase coupling in a cerebro‐cerebellar network at 8–13 Hz during reading. Cerebral Cortex, 17(6), 1476–1485. 10.1093/cercor/bhl059 [DOI] [PubMed] [Google Scholar]
- Langer, N. , Benjamin, C. , Becker, B. L. C. , & Gaab, N. (2019). Comorbidity of reading disabilities and ADHD: Structural and functional brain characteristics. Human Brain Mapping, 40(9), 2677–2698. 10.1002/hbm.24552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer, N. , Benjamin, C. , Minas, J. , & Gaab, N. (2013). The neural correlates of reading fluency deficits in children. Cerebral Cortex, 25(6), 1441–1453. 10.1093/cercor/bht330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell, A. K. (2006). In your right mind: Right hemisphere contributions to language processing and production. Neuropsychology Review, 16(3), 131–148. [DOI] [PubMed] [Google Scholar]
- Lochy, A. , van Reybroeck, M. , & Rossion, B. (2016). Left cortical specialization for visual letter strings predicts rudimentary knowledge of letter‐sound association in preschoolers. Proceedings of the National Academy of Sciences, 113(30), 8544–8549. 10.1073/pnas.1520366113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisog, J. M. , Einbinder, E. R. , Flowers, D. L. , Turkeltaub, P. E. , & Eden, G. F. (2008). A meta‐analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences, 1145(1), 237–259. [DOI] [PubMed] [Google Scholar]
- Martin, A. , Schurz, M. , Kronbichler, M. , & Richlan, F. (2015). Reading in the brain of children and adults: A meta‐analysis of 40 functional magnetic resonance imaging studies. Human Brain Mapping, 36(5), 1963–1981. 10.1002/hbm.22749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer, U. , Brem, S. , Kranz, F. , Bucher, K. , Benz, R. , Halder, P. , Steinhausen, H.‐C. , & Brandeis, D. (2006). Coarse neural tuning for print peaks when children learn to read. NeuroImage, 33(2), 749–758. 10.1016/j.neuroimage.2006.06.025 [DOI] [PubMed] [Google Scholar]
- Maurer, U. , Schulz, E. , Brem, S. , van der Mark, S. , Bucher, K. , Martin, E. , & Brandeis, D. (2011). The development of print tuning in children with dyslexia: Evidence from longitudinal ERP data supported by fMRI. NeuroImage, 57(3), 714–722. 10.1016/j.neuroimage.2010.10.055 [DOI] [PubMed] [Google Scholar]
- McCandliss, B. D. , & Noble, K. G. (2003). The development of reading impairment: A cognitive neuroscience model. Mental Retardation and Developmental Disabilities Research Reviews, 9(3), 196–205. 10.1002/mrdd.10080 [DOI] [PubMed] [Google Scholar]
- Mei, L. , Xue, G. , Chen, C. , Xue, F. , Zhang, M. , & Dong, Q. (2010). The “visual word form area” is involved in successful memory encoding of both words and faces. NeuroImage, 52(1), 371–378. 10.1016/j.neuroimage.2010.03.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyler, A. , Keller, T. A. , Cherkassky, V. L. , Lee, D. , Hoeft, F. , Whitfield‐Gabrieli, S. , Gabrieli, J. D. E. , & Just, M. A. (2007). Brain activation during sentence comprehension among good and poor readers. Cerebral Cortex, 17(12), 2780–2787. 10.1093/cercor/bhm006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugiel, T. , Roe, M. A. , Taylor, W. P. , Cirino, P. T. , Vaughn, S. R. , Fletcher, J. M. , Juranek, J. , & Church, J. A. (2019). Brain activity in struggling readers before intervention relates to future reading gains. Cortex, 111, 286–302. 10.1016/j.cortex.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade, O. A. , Flowers, D. L. , Napoliello, E. M. , & Eden, G. F. (2013). Developmental differences for word processing in the ventral stream. Brain and Language, 125(2), 134–145. 10.1016/j.bandl.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov‐Palchik, O. , Centanni, T. M. , Beach, S. D. , May, S. , Hogan, T. , & Gabrieli, J. D. (2021). Distinct neural substrates of individual differences in components of reading comprehension in adults with or without dyslexia. NeuroImage, 226, 117570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel (U.S.), N. R . (2000). Teaching children to read: An evidence‐based assessment of the scientific research literature on Reading and its implications for Reading instruction: Reports of the subgroups. National Institute of Child Health and Human Development, National Institutes of Health.
- Parviainen, T. (2006). Cortical sequence of word perception in beginning readers. Journal of Neuroscience, 26(22), 6052–6061. 10.1523/JNEUROSCI.0673-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti, C. A. (1985). Reading ability (p. 282). Oxford University Press. [Google Scholar]
- Pleisch, G. , Karipidis, I. I. , Brem, A. , Röthlisberger, M. , Roth, A. , Brandeis, D. , Walitza, S. , & Brem, S. (2019). Simultaneous EEG and fMRI reveals stronger sensitivity to orthographic strings in the left occipito‐temporal cortex of typical versus poor beginning readers. Developmental Cognitive Neuroscience, 40, 100717. 10.1016/j.dcn.2019.100717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack, R. A. , & Gabrieli, J. D. E. (2001). Characterizing the neural mechanisms of skill learning and repetition priming: Evidence from mirror reading. Brain, 124(1), 67–82. 10.1093/brain/124.1.67 [DOI] [PubMed] [Google Scholar]
- Powers, S. J. , Wang, Y. , Beach, S. D. , Sideridis, G. D. , & Gaab, N. (2016). Examining the relationship between home literacy environment and neural correlates of phonological processing in beginning readers with and without a familial risk for dyslexia: An fMRI study. Annals of Dyslexia, 66(3), 337–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. , & Devlin, J. T. (2011). The interactive account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences, 15(6), 246–253. 10.1016/j.tics.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. , Price, C. J. , Wise, R. J. S. , Warburton, E. A. , Moore, C. J. , Howard, D. , Patterson, K. , Frackowiak, R. S. J. , & Friston, K. J. (1996). Hearing and saying: The functional neuro‐anatomy of auditory word processing. Brain, 119(3), 919–931. 10.1093/brain/119.3.919 [DOI] [PubMed] [Google Scholar]
- Pugh, K. R. , Frost, S. J. , Sandak, R. , Landi, N. , Rueckl, J. G. , Constable, R. T. , Seidenberg, M. S. , Fulbright, R. K. , Katz, L. , & Mencl, W. E. (2008). Effects of stimulus difficulty and repetition on printed word identification: An fMRI comparison of nonimpaired and reading‐disabled adolescent cohorts. Journal of Cognitive Neuroscience, 20(7), 1146–1160. 10.1162/jocn.2008.20079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh, K. R. , Mencl, W. E. , Jenner, A. R. , Lee, J. R. , Katz, L. , Frost, S. J. , Shaywitz, S. E. , & Shaywitz, B. A. (2001). Neuroimaging studies of reading development and reading disability. Learning Disabilities Research & Practice, 16(4), 240–249. 10.1111/0938-8982.00024 [DOI] [PubMed] [Google Scholar]
- Raschle, N. M. , Chang, M. , & Gaab, N. (2011). Structural brain alterations associated with dyslexia predate reading onset. NeuroImage, 57(3), 742–749. 10.1016/j.neuroimage.2010.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle, N. M. , Lee, M. , Buechler, R. , Christodoulou, J. A. , Chang, M. , Vakil, M. , Stering, P. L. , & Gaab, N. (2009). Making MR imaging child's play: Pediatric neuroimaging protocol, guidelines and procedure. Journal of Visualized Experiments, 29, 1309. 10.3791/1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle, N. M. , Zuk, J. , & Gaab, N. (2012). Functional characteristics of developmental dyslexia in left‐hemispheric posterior brain regions predate reading onset. Proceedings of the National Academy of Sciences, 109(6), 2156–2161. 10.1073/pnas.1107721109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner, K. (1998). Eye Movements in reading and information processing: 20 years of research. Psychological bulletin, 124(3), 372. [DOI] [PubMed] [Google Scholar]
- Rezaie, R. , Simos, P. G. , Fletcher, J. M. , Cirino, P. T. , Vaughn, S. , & Papanicolaou, A. C. (2011). Engagement of temporal lobe regions predicts response to educational interventions in adolescent struggling readers. Developmental Neuropsychology, 36(7), 869–888. 10.1080/87565641.2011.606404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan, F. , Kronbichler, M. , & Wimmer, H. (2011). Meta‐analyzing brain dysfunctions in dyslexic children and adults. NeuroImage, 56(3), 1735–1742. [DOI] [PubMed] [Google Scholar]
- Rimrodt, S. L. , Clements‐Stephens, A. M. , Pugh, K. R. , Courtney, S. M. , Gaur, P. , Pekar, J. J. , & Cutting, L. E. (2009). Functional MRI of sentence comprehension in children with dyslexia: Beyond word recognition. Cerebral Cortex, 19(2), 402–413. 10.1093/cercor/bhn092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe, M. A. , Martinez, J. E. , Mumford, J. A. , Taylor, W. P. , Cirino, P. T. , Fletcher, J. M. , Juranek, J. , & Church, J. A. (2018). Control engagement during sentence and inhibition fMRI tasks in children with Reading difficulties. Cerebral Cortex, 28(10), 3697–3710. 10.1093/cercor/bhy170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryherd, K. , Jasinska, K. , Van Dyke, J. , Hung, Y.‐H. , Baron, E. , Mencl, W. , Zevin, J. , & Landi, N. (2018). Cortical regions supporting reading comprehension skill for single words and discourse. Brain and Language, 186, 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin, Z. M. , Osher, D. E. , Norton, E. S. , Youssoufian, D. A. , Beach, S. D. , Feather, J. , Gaab, N. , Gabrieli, J. D. E. , & Kanwisher, N. (2016). Connectivity precedes function in the development of the visual word form area. Nature Neuroscience, 19(9), 1250–1255. 10.1038/nn.4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar, B. L. , & McCandliss, B. D. (2007). Development of neural systems for reading. Annual Review of Neuroscience, 30, 475–503. 10.1146/annurev.neuro.28.061604.135645 [DOI] [PubMed] [Google Scholar]
- Schulz, E. , Maurer, U. , van der Mark, S. , Bucher, K. , Brem, S. , Martin, E. , & Brandeis, D. (2009). Reading for meaning in dyslexic and young children distinct neural pathways but common endpoints. Neuropsychologia, 47(12), 2544–2557. 10.1016/j.neuropsychologia.2009.04.028 [DOI] [PubMed] [Google Scholar]
- Schurz, M. , Sturm, D. , Richlan, F. , Kronbichler, M. , Ladurner, G. , & Wimmer, H. (2010). A dual‐route perspective on brain activation in response to visual words: Evidence for a length by lexicality interaction in the visual word form area (VWFA). NeuroImage, 49(3), 2649–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster, S. , Hawelka, S. , Richlan, F. , Ludersdorfer, P. , & Hutzler, F. (2015). Eyes on words: A fixation‐related fMRI study of the left occipito‐temporal cortex during self‐paced silent reading of words and pseudowords. Scientific Reports, 5(1), 12686. 10.1038/srep12686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier, M. L. , & Price, C. J. (2011). Explaining left lateralization for words in the ventral occipitotemporal cortex. Journal of Neuroscience, 31(41), 14745–14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz, B. A. , Shaywitz, S. E. , Blachman, B. A. , Pugh, K. R. , Fulbright, R. K. , Skudlarski, P. , Mencl, W. E. , Constable, R. T. , Holahan, J. M. , Marchione, K. E. , Fletcher, J. M. , Lyon, G. R. , & Gore, J. C. (2004). Development of left occipitotemporal systems for skilled reading in children after a phonologically based intervention. Biological Psychiatry, 55(9), 926–933. 10.1016/j.biopsych.2003.12.019 [DOI] [PubMed] [Google Scholar]
- Silverman, R. D. , Speece, D. L. , Harring, J. R. , & Ritchey, K. D. (2013). Fluency has a role in the simple view of reading. Scientific Studies of Reading, 17(2), 108–133. [Google Scholar]
- Skeide, M. A. , Kumar, U. , Mishra, R. K. , Tripathi, V. N. , Guleria, A. , Singh, J. P. , Eisner, F. , & Huettig, F. (2017). Learning to read alters cortico‐subcortical cross‐talk in the visual system of illiterates. Science Advances, 3(5), e1602612. 10.1126/sciadv.1602612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. J. , Booth, J. R. , & McNorgan, C. (2018). Longitudinal task‐related functional connectivity changes predict reading development. Frontiers in Psychology, 9, 1754. 10.3389/fpsyg.2018.01754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. J. , Johansen‐Berg, H. , Bannister, P. R. , De Luca, M. , Drobnjak, I. , Flitney, D. E. , Niazy, R. K. , Saunders, J. , Vickers, J. , Zhang, Y. , De Stefano, N. , Brady, J. M. , & Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Stevens, W. D. , Kravitz, D. J. , Peng, C. S. , Tessler, M. H. , & Martin, A. (2017). Privileged functional connectivity between the visual word form area and the language system. The Journal of Neuroscience, 37(21), 5288–5297. 10.1523/JNEUROSCI.0138-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilstra, J. , McMaster, K. , Van den Broek, P. , Kendeou, P. , & Rapp, D. (2009). Simple but complex: Components of the simple view of reading across grade levels. Journal of Research in Reading, 32(4), 383–401. [Google Scholar]
- Turkeltaub, P. E. , Gareau, L. , Flowers, D. L. , Zeffiro, T. A. , & Eden, G. F. (2003). Development of neural mechanisms for reading. Nature Neuroscience, 6(7), 767–773. 10.1038/nn1065 [DOI] [PubMed] [Google Scholar]
- Van der Mark, S. , Bucher, K. , Maurer, U. , Schulz, E. , Brem, S. , Buckelmüller, J. , Kronbichler, M. , Loenneker, T. , Klaver, P. , & Martin, E. (2009). Children with dyslexia lack multiple specializations along the visual word‐form (VWF) system. NeuroImage, 47(4), 1940–1949. [DOI] [PubMed] [Google Scholar]
- van der Mark, S. , Klaver, P. , Bucher, K. , Maurer, U. , Schulz, E. , Brem, S. , Martin, E. , & Brandeis, D. (2011). The left occipitotemporal system in reading disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. NeuroImage, 54(3), 2426–2436. 10.1016/j.neuroimage.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Vinckier, F. , Dehaene, S. , Jobert, A. , Dubus, J. P. , Sigman, M. , & Cohen, L. (2007). Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word‐form system. Neuron, 55(1), 143–156. [DOI] [PubMed] [Google Scholar]
- Wagner, R. K. , Torgesen, J. K. , Rashotte, C. A. , & Pearson, N. A. (1999). CTOPP: Comprehensive test of phonological processing. Pro‐ed Austin. [Google Scholar]
- Wang, F. , Karipidis, I. I. , Pleisch, G. , Fraga‐González, G. , & Brem, S. (2020). Development of print‐speech integration in the brain of beginning readers with varying reading skills. Frontiers in Human Neuroscience, 14, 289. 10.3389/fnhum.2020.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehbe, L. , Murphy, B. , Talukdar, P. , Fyshe, A. , Ramdas, A. , & Mitchell, T. (2014). Simultaneously uncovering the patterns of brain regions involved in different story reading subprocesses. PLoS One, 9(11), e112575. 10.1371/journal.pone.0112575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, M. , & Bowers, P. G. (1999). The double‐deficit hypothesis for the developmental dyslexias. Journal of Educational Psychology, 91(3), 415–438. [Google Scholar]
- Wolf, M. , & Denckla, M. B. (2005). RAN/RAS: Rapid automatized naming and rapid alternating stimulus tests. Pro‐ed Austin. [Google Scholar]
- Wolf, M. , & Katzir‐Cohen, T. (2001). Reading fluency and its intervention. Scientific Studies of Reading, 5(3), 211–239. 10.1207/S1532799XSSR0503_2 [DOI] [Google Scholar]
- Woodcock, R. W. (2011). Woodcock reading mastery tests: WRMT‐III. Pearson Assessments. [Google Scholar]
- Woodcock, R. W. , McGrew, K. S. , Mather, N. , & Schrank, F. (2001). Woodcock‐Johnson III NU tests of achievement. Riverside Publishing. [Google Scholar]
- Woodhead, Z. V. J. , Wise, R. J. S. , Sereno, M. , & Leech, R. (2011). Dissociation of sensitivity to spatial frequency in word and face preferential areas of the fusiform gyrus. Cerebral Cortex, 21(10), 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni, T. , Speer, N. K. , Balota, D. A. , McAvoy, M. P. , & Zacks, J. M. (2008). Pictures of a thousand words investigating the neural mechanisms of reading with extremely rapid event‐related fMRI. NeuroImage, 42(2), 973–987. 10.1016/j.neuroimage.2008.04.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Zuk, J. , Perdue, M. V. , Ozernov‐Palchik, O. , Raney, T. , Beach, S. D. , Norton, E. S. , Ou, Y. , Gabrieli, J. D. , & Gaab, N. (2020). Putative protective neural mechanisms in prereaders with a family history of dyslexia who subsequently develop typical reading skills. Human Brain Mapping, 41(10), 2827–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Supporting Information
Data Availability Statement
The data required to reproduce reported findings will be provided upon request.


