Abstract
Cell-mediated immunity is pivotal in host resistance to Blastomyces dermatitidis infection. Immunization of mice with the WI-1 adhesin enhances resistance against experimental pulmonary infection but elicits features of a mixed T-helper-cell immune response. Immune mice acquire delayed-type hypersensitivity (DTH) but also high titers of WI-1-specific immunoglobulin G1 (IgG1) and IgG2b, a result indicative of T-helper-2 cellular immunity. We report that interleukin-12, used as an adjuvant for WI-1 immunization, augments DTH, shifts the balance of the T-helper phenotype toward Th1, and enhances resistance to B. dermatitidis infection.
In many infectious diseases, control or progression of the infection depends on differential expression of CD4+ T-cell subsets and their associated lymphokines. In mice, T-helper-1 (Th1) cells produce gamma interferon (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor β, which are important for the induction of cell-mediated immunity (CMI). Th1 cells are associated with delayed-type hypersensitivity (DTH), macrophage activation, synthesis of complement-fixing immunoglobulin G2a (IgG2a) antibodies, and antibody-dependent, cell-mediated cytotoxicity. In contrast, Th2 cells produce IL-4, IL-5, IL-6, IL-10, and IL-13 and are proficient at providing B-cell help and stimulating production of IgE and non-complement-fixing IgG1 antibodies (7). Whereas many factors influence differentiation of CD4+ T cells, the cytokine microenvironment present during initiation of the immune response has a major influence. IFN-γ and IL-12 are key cytokines for the differentiation of Th1 cells, whereas IL-4 and IL-10 promote Th2-cell development (5, 8, 10).
Protective immunity to infections with endemic fungi, including Blastomyces dermatitidis, is thought to require Th1-dependent CMI responses. In a previous study, we investigated the immunogenicity and protective efficacy of WI-1, a surface protein adhesin of B. dermatitidis, against murine experimental pulmonary blastomycosis (11). WI-1 administration evoked antibody and CMI responses, but immunized mice were only modestly protected against lethal experimental infection. The goal of the present study was to investigate whether IL-12 as an adjuvant could enhance the protective efficacy of WI-1. IL-12 was first described as a vaccine adjuvant in experimental leishmaniasis (1). Soluble leishmania antigen administered to mice led to antigen-specific immune responses with a Th2 phenotype and progressive infection after challenge. The addition of IL-12 converted soluble leishmania antigen from a nonprotective antigen to a protective antigen by enhancing differentiation of CD4+ T cells toward a Th1 subset and cytokine profile needed to foster DTH responses.
Development of DTH is associated with the ability to resist a lethal experimental infection with B. dermatitidis (3, 9). We previously reported that WI-1 immunization evokes DTH responses (11). We wondered whether addition of IL-12 as an adjuvant to WI-1 immunization could increase those DTH responses. Mice were immunized either with 100 μg of WI-1 and complete Freund adjuvant (CFA) as described previously (11) or with WI-1, CFA, and IL-12 (0.5 μg/mouse). Mice receiving IL-12 were boosted intraperitoneally with 0.5 μg of IL-12 at 1, 3, 5, and 7 days after initial immunization. Two weeks after the first immunization, mice were boosted either with antigen and incomplete Freund adjuvant (IFA) or with antigen, IFA, and IL-12 as indicated for the first immunization. This immunization protocol was used for all experiments presented in this study. Two weeks after boosting, DTH responses were assessed by measuring the footpad swelling of immunized and control mice (n = 24 mice per group) as described elsewhere (11). Mice immunized with WI-1 and IL-12 showed significantly greater footpad swelling in response to WI-1 administration (mean ± the standard error of the mean [SEM] of 0.9 ± 0.1 mm) than did mice immunized with WI-1 alone (0.6 ± 0.06 mm) (P = 0.0015) when analyzed statistically using the Wilcoxon rank test for nonparametric data (4). Virtually no footpad swelling was observed in mice immunized with either IL-12 or bovine serum albumin (BSA) alone as a control (0.03 ± 0.01 mm).
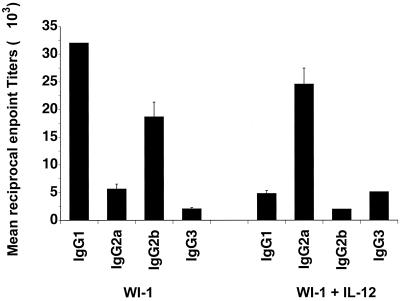
WI-1 immunization evokes humoral immune responses that illustrate a bias toward a Th2 phenotype, based on the subclass distribution of anti-WI-1 IgG antibodies (11). We tested whether recombinant murine IL-12 as an adjuvant together with WI-1 may alter the phenotype of Th cells, as determined by the subclass distribution of WI-1-specific IgG antibodies. Two weeks after immunization, mice (n = 10 per group) were bled and anti-WI-1 IgG subclasses were assessed by enzyme-linked immunosorbent assay as described previously (11). Figure 1 shows that the subclass profile of anti-WI-1 IgG in mice immunized with WI-1 alone was dominated by IgG1 and IgG2b, which is indicative of a Th2 phenotype. The addition of IL-12 as an adjuvant shifted the IgG subclasses toward mainly IgG2a and IgG3, which is indicative of a Th1 phenotype. Mean reciprocal endpoint titers of antibody subclasses were significantly different between the two groups of immunized mice (P = 0.0001).
FIG. 1.
IgG subclass distribution of anti-WI-1 antibodies in mice immunized with WI-1 alone or together with IL-12 as an adjuvant. The bars represent mean reciprocal titers ± the SEM of each antibody subclass. Reciprocal endpoint titers were defined as the maximal dilution of a sample that resulted in a value 2 times greater than the background. Differences in endpoint titers for each subclass were analyzed statistically using the Wilcoxon rank test for nonparametric data (4). P values are for comparison of immunization with WI-1 alone or together with IL-12 adjuvant: IgG1, P = 0.0001; IgG2a, P = 0.0001; IgG2b, P = 0.0001; and IgG3, P = 0.0001. The data shown represent one experiment out of at least three with similar results.
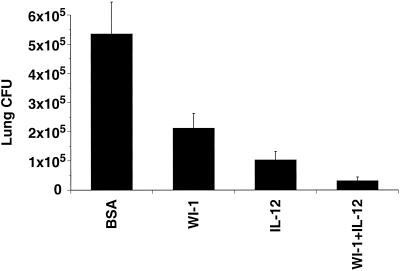
The increase in DTH and shift in the Th phenotype raised the possibility that the use of IL-12 as an adjuvant in WI-1 immunization might also augment resistance against experimental infection. Following immunization, mice (n = 10 per group) were infected intratracheally with a lethal dose of 5 × 10 organisms of B. dermatitidis ATCC 60636. WI-1 used for immunization was purified from ATCC 60636 yeast as described elsewhere (2). Mice immunized with WI-1 alone, with WI-1 in the presence of IL-12, or with IL-12 alone showed a significant reduction in the burden of lung infection at 13 days postinfection compared to mice immunized with the control antigen BSA (P = 0.0211, P = 0.0006, and P = 0.0022, respectively) (Fig. 2). WI-1 immunization in the presence of IL-12 led to a greater reduction of lung infection than did WI-1 immunization alone (P = 0.0017) or IL-12 administration alone (P = 0.0139). These results indicate that IL-12 used as an adjuvant for WI-1 immunization enhances resistance in a murine model of pulmonary blastomycosis, as assessed by reduction in the burden of lung infection. We monitored similar groups of immunized mice (n = 10 per group) for survival after infection. IL-12 as an adjuvant prolonged the survival of WI-1 immune mice significantly (P = 0.0005) (Table 1). WI-1 or IL-12, when used alone for immunization, did not prolong survival after infection (P = 0.6992) and (P = 0.2942), respectively, even though they did reduce the burden of lung infection. In a previous report (11), WI-1 alone did prolong survival modestly, but those mice were infected intranasally. In the present study the mice were infected intratracheally, which may have contributed to the lower protective efficacy of immunization with WI-1 alone. We have found that only ca. 10% of the yeast inoculum reaches the alveoli after intranasal infection (unpublished observations).
FIG. 2.
Burden of lung disease after experimental infection with B. dermatitidis in mice immunized with WI-1 alone or together with IL-12 as an adjuvant. Two weeks after immunization, mice were infected intratracheally with 5 × 10 yeast of ATCC strain 60636. Two weeks postinfection, the mice were sacrificed and analyzed for burden of lung infection, assessed by CFU of yeast on brain heart infusion agar plates. The CFU values are the geometric mean ± the SEM for 10 mice per group. Differences in CFUs were analyzed statistically using the Wilcoxon rank test for nonparametric data (4). The data shown represent one experiment out of at least three with similar results.
TABLE 1.
Survival after experimental B. dermatitidis infection in mice immunized with WI-1 alone or together with IL-12 as an adjuvant
| Groupa (n = 10) | Mean survival time (days) | Pb |
|---|---|---|
| BSA | 27 | 0.0001 |
| WI-1 | 27 | 0.0005 |
| IL-12 alone | 28 | 0.0002 |
| WI-1 plus IL-12 | 32 |
Ten mice per group were immunized with BSA, WI-1, IL-12 alone, or WI-1 plus IL-12. Two weeks after immunization, the mice were infected intratracheally with 5 × 10 yeast of strain ATCC 60636 and monitored for survival. Mice immunized with WI-1 plus IL-12 lived significantly longer than any other group of mice. The data shown represent one experiment out of at least three with similar results. The survival-time data were analyzed by log-rank statistics (Mantel-Haenszel test) (6). Since the number of mice per group is considered to be small, the exact P values were computed using the statistical package StatXact-3 by CYTEL Software Corp. The survival difference between two groups is considered to be significantly different if the two-sided P value is <0.05.
P value is given for the comparison of each respective group versus the group that received WI-1 plus IL-12.
In summary, the use of IL-12 as an adjuvant for WI-1 immunization significantly augmented DTH, shifted the phenotype of Th cells, and enhanced resistance against experimental B. dermatitidis infection. Nevertheless, although the protective efficacy of WI-1 was enhanced, the level of resistance to experimental pulmonary blastomycosis continued to be modest, if not marginal. However, we cannot exclude that another form of WI-1, as in a DNA vaccine, another adjuvant combination, or an increase in the number of boosters might provide a higher level of protection. Alternatively, antigens other than, or in addition to, WI-1 will be needed for a more effective fungal vaccine. Such antigens have recently come under investigation in a recombinant vaccine strain of B. dermatitidis which lacks WI-1 and is correspondingly nonpathogenic (M. Wüthrich, H. I. Filutowicz, and B. S. Klein, submitted for publication).
Acknowledgments
This work was supported by grants from the U.S. Public Health Service (B.S.K.) and from the Swiss National Science Foundation (M. W.). B.S.K. is the recipient of a Research Career Development Award from the National Institutes of Health and is a Burroughs-Wellcome Fund Scholar in Molecular Pathogenic Mycology.
We thank the Genetics Institute, Inc., Cambridge, Mass., for generously providing recombinant murine IL-12. We also thank Lan Zeng of the Department of Biostatistics and Medical Informatics at the University of Wisconsin–Madison for assistance with statistical analyses and Robert Audet and George Cook for help in purifying the secreted WI-1 used in this study.
REFERENCES
- 1.Afonso L C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Audet R, Brandhorst T T, Klein B. Purification in quantity of the secreted form of WI-1: a major adhesin on Blastomyces dermatitidis yeasts. Protein Expr Purif. 1997;11:219–226. doi: 10.1006/prep.1997.0783. [DOI] [PubMed] [Google Scholar]
- 3.Cozad G C, Chang C T. Cell-mediated immunoprotection in blastomycosis. Infect Immun. 1980;28:398–403. doi: 10.1128/iai.28.2.398-403.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher L D, van Belle G. Biostatistics: a methodology for the health sciences. New York, N.Y: John Wiley & Sons; 1993. pp. 611–613. [Google Scholar]
- 5.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 6.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Nat Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 7.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 8.Seder R A, Gazzinelli R, Sher A, Paul W E. Interleukin-12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin-4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer H D, Cozad G C. Role of delayed hypersensitivity in blastomycosis of mice. Infect Immun. 1973;7:329–334. doi: 10.1128/iai.7.3.329-334.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain S L, Weinberg A D, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 11.Wüthrich M, Chang W L, Klein B S. Immunogenicity and protective efficacy of the WI-1 adhesin of Blastomyces dermatitidis. Infect Immun. 1998;66:5443–5449. doi: 10.1128/iai.66.11.5443-5449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]