Abstract
Cesarean scar ectopic pregnancy (CSP) is a rare form of ectopic pregnancy, and treatment of CSP with uterine artery embolization (UAE) is a novel approach. With increasing numbers of cesarean sections being performed annually, the incidence of this condition is likely to increase. The authors became aware of an unusually high number of published studies originating in mainland China regarding this unusual treatment and sought to perform a meta-analysis to provide comprehensive evidence on this novel practice. Methods: We performed a thorough search and included all forms of quality studies on this topic that reported UAE as a part of first-line management of CSP. We included only studies originating in China. Ultimately, 37 studies were included for qualitative and quantitative synthesis of evidence. After screening retrieved records and extracting data from eligible studies, we pooled continuous data as a mean estimate and 95% confidence interval (CI), and dichotomous data as proportion and 95% CI. Results: CSP patients treated with protocols including UAE had a mean time of 30 days for serum β-hCG normalization, 95% CI [26.816, 33.881]. They had a mean estimated intraprocedural blood loss of 4.19 ± 3.76 mL, a mean hospital stay of nine days, 95%CI [7.914, 9.876], and a success rate of 93.4%, 95%CI [0.918, 0.951]. The severe complication rate was 1.2%, 95%CI [0.008, 0.017]. Conclusion: UAE, in combination with other procedures is being used effectively for the treatment of CSP in China. Protocols including UAE have a success rate of approximately 93.4%, and a severe complication rate of approximately 1.2%. This data’s utility is limited by vast differences in the studied protocols and questionable feasibility outside of China.
Keywords: cesarean scar, uterine artery embolization, ectopic pregnancy, extrauterine pregnancy
1. Introduction
Ectopic pregnancy describes pregnancies outside of normal positioning in the uterus, most frequently in the fallopian tube and less frequently in other sites such as the ovaries, abdomen, cesarean scar, and other sites [1]. The incidence of all ectopic pregnancies has increased in recent decades and complicates approximately 2% of all pregnancies, following the increase in the cesarean section rate [1,2].
Cesarean scar pregnancy (CSP) is an ectopic pregnancy located at a previous uterine scar [3]. Its incidence is increasing due to the increased frequency of cesarean sections worldwide [4,5]. It occurs in 1 in 500 pregnancies among women with a previous cesarean delivery and compromises 4% of all ectopic pregnancies [6]. Despite its rarity, CSP can constitute a life-threatening condition [7].
Originally, hysterectomy was considered the only treatment option for CSP [3], however, in recent years, more conservative approaches have been developed. Treatment options now include systemic methotrexate (MTX), uterine artery embolization (UAE), local resection of the ectopic gestational mass, hysteroscopy, and uterine dilation and curettage (D&C) [8,9,10].
Largely used in the treatment of uterine fibroids, uterine artery embolization (UAE) is a widely used procedure generally performed by interventional radiologists under local anesthesia and carried out by catheterization of the uterine arteries through a transfemoral approach. The procedure involves injecting gelatin sponge particles to block the supplying arteries to the uterus, resulting in the cessation of blood supply to the CSP [11]. It may be combined with a dose of MTX given in the intraprocedural period [11,12]. Other authors have reported using polyvinyl alcohol instead of gelatin sponge particles with similar results [13].
UAE may be used alone or combined with local or systemic MTX for treatment of CSP. Moreover, it can be performed before uterine D&C, laparoscopy, hysteroscopy, or local resection [13,14,15,16].
The authors of this study noticed a tremendous increase in published trials that included the use of UAE as a treatment coming out of China. The authors hypothesize that this may likely be due to a decreased regulatory effect on medical care in this country, versus the majority of the rest of the world. As a result, a large body of research on the usage of UAE in CSP from China has surfaced over the last ten years. We aimed to present a global report on the usage of protocols including UAE for the treatment of CSP in China, by conducting this systematic review and meta-analysis.
2. Materials and Methods
We conducted this systematic review and meta-analysis guided by the Cochrane Handbook for Systematic Reviews of Interventions [17], then we reported it using the “preferred reporting items for systematic review and meta-analysis” (PRISMA statement) [18].
2.1. Literature Search
We searched PubMed, Scopus, Web of Science, ClinicalTrials.Gov, MEDLINE, and the Cochrane Central Register of Controlled Trials (CENTRAL) for published studies from inception till April 2021 using the following keywords: “cesarean scar pregnancy,” “ectopic pregnancy”, “extrauterine pregnancy”, “cesarean scar”, “cesarean cicatrization”, and “uterine artery embolization”.
2.2. Eligibility Criteria and Study Selection
We included case series, observational studies, comparative studies, and randomized controlled trials (RCTs) that reported UAE as a part of first-line management of CSP, originating from anywhere within China. Exclusion criteria included: (1) Case reports or review articles, (2) case series describing less than five cases managed by UAE, (3) studies where treatment modality or outcomes were not sufficiently detailed, (4) non-English language studies (5), and in vitro or animal studies. After removing duplicates by Endnote, title, and abstract screening, the full-text screening ensured the studies’ eligibility for inclusion. Moreover, we screened the bibliographies of the included studies manually for other relevant studies. Screening was performed independently by two separate authors, and agreement was reached by consensus between the authors. Per our institute standards, a third researcher was assigned to resolve any disputes but was ultimately never needed. Only three studies that met our criteria were excluded because they were located outside of China.
2.3. Data Extraction
Extracted data included the year of publication, study design, inclusion period, the mean age of participants, gestational age, primary treatment modality, number of cases per group, success rate, causes of treatment failure, rate of severe complications, time for serum β-hCG normalization, length of hospital stay, intraprocedural blood loss, number of cases undergoing hysterectomy or laparotomy, cases with bleeding more than 500 mL or received a blood transfusion, conclusion, and study country of origin. The management was considered successful if there was no major complication and the patient needed no additional treatments. Severe complications included a UAE procedure that required hysterectomy, laparotomy, involved bleeding >500 mL, or necessitated an unexpected blood transfusion.
2.4. Quality Assessment
We used the national institute of health (NIH) tools to assess the quality of cohort and case series studies [19]. For RCTs, we used the Cochrane risk of bias tool described in the Cochrane Handbook for Systematic Reviews of Interventions [17].
2.5. Data Synthesis
Analysis was conducted using Open Meta-Analyst software. We reported dichotomous outcomes as a proportion and a 95% confidence interval (CI) and continuous outcomes as a mean estimate and a 95% CI. When heterogeneity was significant (Chi-square p < 0.1), we employed the random-effects model and then made a sensitivity analysis to solve the heterogeneity.
3. Results
3.1. Literature Search Results
The literature search retrieved 433 records; of them, 109 duplicates were removed. We excluded 232 studies during the title and abstract screening and 57 during full-text screening. In addition to the remaining 35 studies, 2 studies were included through the manual search, and a total of 37 studies were included for qualitative and quantitative synthesis of evidence [11,13,14,15,16,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] (Supplemental Figure S1). Interestingly, only three studies that would have otherwise met our screening criteria were excluded because they originated in countries other than China.
3.2. Characteristic of Included Studies
Included studies are variable in their design, including cohort studies, case series studies, and RCTs with a total of 2655 patients. The most frequent treatment modality in the included studies was UAE combined with D&C or UAE combined with MTX and D&C. Table 1 shows the summary of included studies and the characters of the included patients.
Table 1.
Shows the summary and baseline characteristics of the included studies.
| ID | Year of Publication | Study Design | Inclusion Period | Primary Treatment Modality | Number of Cases | Age | Gestational Age (Days) | Success Rate (%) | Treatment Failure Causes | Severe Complications Rate (%) | Conclusion | Methodological Quality | Country of Origin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cao 2017 [20] | 2017 | Retrospective cohort Study | 2012–2016 | UAE + curettage | 101 | 32.98 (4.96) | – | 93.07% | Treatment failure (n = 7) [underwent curettage again] |
2.97% | Reduced menstrual blood volume can occur in scar pregnancy patients who received uterine artery embolization combined with curettage. | Fair | China |
| Chen 2017 [22] | 2017 | Retrospective cohort Study | 2014–2016 | UAE + curettage | 49 | 33.7 (4.8) | – | 93.90% | Treatment failure (n = 3) [underwent transvaginal hysterotomy] | 4.08% | UAE combined with uterine curettage is less advantageous than transvaginal hysterotomy. | Fair | China |
| Cheng 2020 [23] | 2020 | Retrospective cohort Study | 2010–2015 | UAE + hysteroscopy | 21 | 33.9 (1) | 49 (45.5–65.5) | 100% | – | 0% | Compared with D&C ± UAE, LAOH ± UAE showed a higher success rate for CSP–II patients. | Fair | China |
| UAE + D&C | 61 | 33.5 (0.6) | 52 (42–58) | 82% | Treatment failure (n = 3) [laparoscopic surgery or laparotomy] |
4.90% | |||||||
| Qi 2015 [41] | 2015 | Case series | 2009–2013 | UAE + MTX + D&C | 22 | 31.68 (4.58) | 59.86 (17.67) | 77.30% | Treatment failure (n = 8) additional intra–amniotic MTX injection or systemic MTX + D&C (n = 2), hysterotomy (n = 1). severe vaginal bleeding during curettage (n = 4) [hysterotomy] gelatin sponge separated and embolized the right leg (n = 1) [a second UAE.] |
0% | UAE with or without local MTX infusion might be an effective treatment for CSP. | Good | China |
| UAE + curettage | 28 | 31.68 (4.58) | 54.33 (17.51) | 89.30% | 0% | ||||||||
| Fang 2020 [26] | 2020 | Case series | 2010–2016 | UAE + curettage | 32 | – | 68.05 (23.29) | 43.75% | Treatment failure (n = 18) Massive vaginal bleeding (n = 5) [received blood transfusions and laparoscopy or laparotomy] large gestational sac (n = 13) [underwent surgery] |
27.78% | CSP patients with short gestational age and small gestational sac can be treated with surgery, UAE, and HIFU and achieve safe and effective therapeutic effects. | Good | China |
| Fei 2019 [27] | 2019 | Retrospective cohort Study | 2008–2017 | UAE + MTX | 26 | 31.4 (4.4) | – | 100% | – | 0% | There is no universal agreement on the optimal treatment modality for CSP. | Fair | China |
| Gao 2018 [14] | 2018 | Retrospective cohort study | 2010–2015 | UAE + curettage | 57 | 33.46 (4.47) | 54.25 (11.6) | 86% | Treatment failure (n = 5) [underwent a repeat curettage or intrauterine packing with a water balloon] | 0% | Adding intra–arterial MTX to UAE and curettage significantly promoted postoperative recovery, though success rate and bleeding events were not significantly affected. | Fair | China |
| UAE + MTX +D&C | 36 | 32.18 (5.65) | 55.58 (9.82) | 88.90% | Treatment failure (n = 2) [underwent a repeat curettage and intrauterine packing with a water balloon] | 0% | |||||||
| Guo 2018 [15] | 2018 | Retrospective cohort Study | 2012–2017 | UAE | 51 | 32.21 (5.68) | 54.82 (9.27) | 80.40% | Treatment failure (n = 10) laparotomy hysterectomy (n = 5) LCSPDS operation (n = 3) scar lesion removal by abdominal incision (n = 2) |
9.8% (5/51) | UAE and LCSPDS each have their advantages and disadvantages in treating CSP. Thus, appropriate individualized surgical programs based on specific patient circumstances are needed to avoid indiscriminately performing complete uterine cavity curettage. | Fair | China |
| Hong 2017 [30] | 2017 | Retrospective cohort Study | 2014–2016 | UAE + curettage | 67 | 31.74 (3.69) | – | 88.06% | _ | 0% | UAE combined with suction curettage under hysteroscopy is safe and effective in the management of CSP. | Fair | China |
| Li 2020 [32] | 2020 | Retrospective cohort Study | 2013–2017 | UAE + curettage | 169 | 33.58 (4.88) | – | 96% | Treatment failure (n = 6) repeated curettage (n = 2) resection of gestational tissues (n = 2) hemostatic drugs (n = 2) |
0% | UAE combined with curettage treatment in CSP patients demonstrates a favorable success rate, which can also reduce MBV and proceeding pregnancy rate. | Fair | China |
| Li 2018 [33] | 2018 | Retrospective cohort Study | 2006–2016 | UACE + curettage + MTX | 383 | 32.3 (4.9) | – | 99% | Treatment failure (n = 4) massive blood loss of (n = 1) [systemic methotrexate] residual tissues (n = 3)[underwent hysteroscopic or transabdominal resection] |
0.26% | UACE combined with curettage was found to be an effective fertility–sparing treatment for CSP. Further, the approach did not seem to harm future reproductive ability. | Poor | China |
| Liu 2016 [36] | 2016 | Retrospective cohort Study | 2014–2016 | UAE + MTX + D&C | 42 | 32.43 (4.2) | – | 97.50% | Treatment failure (n = 1) [needed additional treatment.] | 0.00% | The combination of UAE, local MTX injection, and D&C for CSP patients is the optimal treatment strategy. | Fair | China |
| UAE + MTX | 25 | 32.44 (6.16) | – | 76% | Treatment failure (n = 6) [required additional systemic MTX or D&C] | 0% | |||||||
| Liu 2015 [35] | 2015 | Retrospective cohort Study | 2005–2013 | UAE + curettage | 38 | 33.42 (5.29) | 55.42 (14.28) | 100% | – | 0% | UAE combined with curettage appears to be superior to MTX plus curettage for treatment of CSP with high serum b–hCG level. | Fair | China |
| Lou 2020 [37] | 2020 | Retrospective cohort Study | 2013–2015 | UAE + MTX + D&C | 53 | 33 (3.6) | 47 (8.4) | 97.90% | Treatment failure (n = 1) [emergency UAE + Curettage] | 0% | Pretreatment with MTX and UAE prior to curettage is safe and effective for the management of CSP. | Fair | China |
| Ma 2017 [38] | 2017 | Retrospective cohort Study | 2012–2016 | UAE + MTX + D&C | 45 | 33 (6) | – | 91.10% | Treatment failure (n = 4) systemic and local MTX therapy + curettage (n = 1) [supplementary MTX therapy] (n = 2) abdominal CSP mass resection (n = 1) |
0% | All treatments have high success rates and no significant effects on intraoperative bleeding. | Fair | China |
| Ou 2020 [39] | 2020 | Prospective cohort study | 2016–2018 | UAE + curettage | 65 | 34 (4.4) | 52.29 (10.32) | 98.46% | Treatment failure (n = 1) [repeat curettage] | 0% | Suction and curettage alone is a more suitable option than UAE followed by suction and curettage. | Fair | China |
| Qiu 2019 [43] | 2019 | Retrospective cohort Study | 2013–2018 | UAE + curettage | 39 | 32.1 (5.02) | _ | 84.60% | Treatment failure (n = 6) Massive vaginal bleeding (n = 3) [hysteroscopy or iodoform gauze packing.] unsatisfactory decrease in serum β–HCG level (n = 3) [received intramuscular injection of MTX] retained products of conception (n = 3) [underwent hysteroscopy] |
0% | D&C guided by ultrasonography after UAE treatment showed good clinical efficacy. | Fair | China |
| UAE + hysteroscopy | 23 | 32.48 (4.73) | _ | 95.70% | Treatment failure (n = 1) Massive vaginal bleeding received [iodoform gauze packing] |
0% | Hysteroscopy after UAE treatment showed good clinical efficacy. | ||||||
| Wang 2021 [16] | 2021 | Retrospective cohort study | 2017–2019 | UAE+ D&C + Hysteroscopy | 23 | 29.2 (3.6) | _ | 100% | _ | 8.70% | UAE pretreatment method seems to be effective, economical, and with few side effects in the management of CSP. | Fair | China |
| Wang 2019 [50] | 2019 | Retrospective cohort study | 2016–2018 | UAE + MTX + hysteroscopy | 44 | 31.84 (2.47) | _ | 100% | _ | 0% | UAE can effectively reduce intraoperative blood loss but increases the risk of postoperative complications, length of hospital stay, medical costs. | Fair | China |
| Xiao 2018 [48] | 2018 | Retrospective cohort study | 2011–2014 | UACE + curettage + MTX | 102 | 33.1 (4.6) | 51.19 (11.13) | 100% | _ | 0% | UACE combined with D&C is a useful measure for most Type 2 CSP cases in the first trimester. For Type 2 CSP cases in the second trimester, UACE before laparotomy could be a reasonable choice. | Fair | China |
| Xiao 2019 [49] | 2019 | Retrospective case–control study | 2014–2017 | UAE + D&C + hysteroscopy | 35 | 32.67 (6.96) | 52.5 (13.91) | 100% | _ | 0% | combination of UAE and surgery should be selected carefully because of its potential fertility complication. | Fair | China |
| Zhang 2019 [52] | 2019 | Retrospective cohort study | _ | UAE + curettage | 46 | 32.5 (4.7) | 48.7 (9.8) | 100% | _ | 0% | Compared to UAE, lauromacrogol–based sclerotherapy is a safe, effective, and economic approach in the pretreatment for uterine scar pregnancy. | Fair | China |
| Fahg 2009 [25] | 2009 | Prospective cohort study | 2004–2088 | UAE + curettage | 38 | 32.5 (4.8) | 53.35 (7.72) | 100% | _ | 0% | UAE followed by curettage is recommended to medical facilities where UAE is available. | Fair | China |
| Gao 2014 [28] | 2014 | Prospective cohort study | 2009–2012 | UAE + curettage | 93 | 33.4 (4.5) | 49.84 (7.72) | 94.62% | Treatment failure (n = 5) [needed additional interventions] | 0% | UAE combined with D&C within 24 hours was an effective and safe uterine preservation treatment for CSP. | Fair | China |
| Qian 2015 [42] | 2015 | RCT | 2008–2013 | UAE + curettage | 33 | 30.79 (4.29) | 51.33 (7.57) | 100% | _ | 0% | UAE plus curettage was successful in terminating a gestational sac type of CSP. | High | China |
| UAE + D&C + hysteroscopy | 33 | 32 (4.15) | 52 (11.14) | 90.91% | Treatment failure (n = 3) hemorrhage during surgery (n = 1) [Emergency hysterectomy] additional MTX therapy (n = 2) |
3.03% | |||||||
| Wang 2013 [47] | 2013 | Retrospective cohort study | 2007–2012 | UAE + curettage | 128 | 32.28 (4.76) | 48.64 (7.98) | 88.28% | Treatment failure (n = 15) Emergency hysterectomy (n = 5) |
11.72% | For CSP masses with a GA of 8 weeks or more and a diameter of 6 cm or more, the outcome of surgical evacuation after UAE tends to be unsatisfactory. | Fair | China |
| Zhuang 2009 [54] | 2009 | RCT | 2003–2007 | UAE + curettage | 37 | 32.23 (.65) | 51.24 (1.4) | 91.89% | Treatment failure (n = 3) Iodoform meche (n = 1) Readmitted due to bleeding (n = 2) |
0% | UAE followed by suction curettage seems to have more advantages than systemic MTX treatment and maybe a priority option. | Moderate | China |
| Cao 2014 [21] | 2014 | Retrospective cohort study | 2007–2012 | UAE + D&C + hysteroscopy | 52 | 33.3 (4.5) | 49.13 (14.74) | 98.08% | Treatment failure (n = 1) [Resection of the lower uterine segment] |
0% | UAE combined with D&C is a safe and efficient treatment for CSP. | Fair | China |
| Du 2015 [24] | 2015 | Retrospective cohort study | 2006–2012 | UAE + MTX + D&C | 175 | 32.44 (4.6) | 54.05 (14.04) | 96.57% | Treatment failure (n = 6) tamponade with iodoform gauze packs or an inflated balloon catheter (n = 3) emergency local CSP resection via laparotomy (n = 1) Emergency hysterectomy (n = 2) |
3.43% | Increased gestational age increases the risk of bleeding in CSP treated by UAE+MTX+D&C. | Fair | China |
| Huang 2015 [31] | 2015 | Retrospective cohort study | 2009–2014 | UAE + MTX + D&C | 31 | 32.42 (5.94) | 42.12 (6.32) | 100% | _ | 0% | UAE combined with MTX is a safe and efficient treatment of CSP. | Fair | China |
| Li 2011 [11] | 2011 | RCT | 2002–2009 | UAE + MTX + D&C | 31 | 34.15 (5.41) | 70.89 (35.94) | 83.87% | Treatment failure (n = 5) tamponade with iodoform gauze (n = 2) re–embolization (n = 3) |
0% | Arterial chemoembolization with MTX was more effective than systemic MTX treatment for termination of CSP. | Low | China |
| Liang 2010 [34] | 2010 | Retrospective cohort study | 2005–2009 | UAE + MTX + D&C | 42 | 31.3 (3.6) | 5–10.5 weeks | 100% | _ | 0% | The use of UAE for the treatment of CSP is tolerated well and has few complications. | Poor | China |
| Shen 2012 [44] | 2012 | Retrospective cohort study | 2008–2010 | UAE + MTX + D&C | 25 | 32.7 (6) | 55.45 (2.11) | 96.00% | Treatment failure (n = 1) [Hysterectomy] |
4.00% | UAE and MTX appears to be a safe and effective treatment for CSP and causes less morbidity than current approaches. | Fair | China |
| Wu 2012 [13] | 2012 | Retrospective cohort study | 2000–2010 | UAE + MTX + D&C | 16 | 33.09 (4.33) | 48.18 (11.68) | 100% | _ | 0% | UAE combined with intraarterial MTX infusion could be an effective and safe treatment for CSP. | Fair | China |
| Yang 2010 [51] | 2010 | Retrospective cohort study | 2003–2008 | UAE + MTX | 38 | 31.5 (7.25) | 47.73 (11.1) | 31.58% | Treatment failure (n = 26) Re–embolization (n = 2) Additional D&C (n = 24) |
7.89% | UAE combined with local MTX benefits women wishing to preserve fertility and is suitable for use as the primary treatment for CSP. | Fair | China |
| Zhu 2015 [53] | 2015 | Retrospective cohort study | 2014 | UAE + D&C + hysteroscopy | 46 | 31.4 (5.1) | 60.6 (16.4) | 100% | _ | 2.17% | UAE combined with suction curettage under hysteroscopic guidance is safe and effective in treating patients with CSP. | Fair | China |
| He 2014 [29] | 2014 | Retrospective cohort study | 2005–2010 | UAE + MTX + hysteroscopy | 25 | _ | _ | 100% | _ | 0% | Combination of laparoscopy and hysteroscopy is much safer and more effective than uterine curettage as a supplementary measure following the UAE management of CSP. | Fair | China |
| UAE + MTX + D&C | 33 | 78.79% | Treatment failure (n = 7) [underwent multiple curettages] |
6.10% |
SD, standard deviation; MTX, methotrexate; D & C, dilatation and curettage; UAE, uterine artery embolization; CSP, cesarean scar pregnancy; LAOH, laparoscopy–assisted by operative hysteroscopy; LCSPDS, laparoscopic cesarean scar pregnancy debridement surgery; HIFU, high–intensity focused ultrasound; SCEM, selective chemoembolization with methotrexate; GA, gestational age; MBV, menstrual blood volume; UAC, uterine artery chemoembolization.
3.3. Quality Assessment
The quality of most included studies was fair. The final judgments of each study quality are shown in Table 1, and the details of each quality assessment domain are shown in Supplementary Tables S1 and S2.
3.4. Analysis of the Outcomes
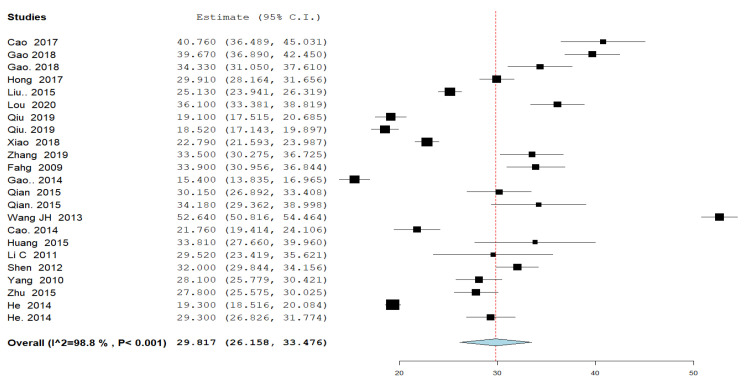
3.4.1. Time for Serum β-Human Chorionic Gonadotropin (β-hCG) Normalization (Figure 1)
The time for serum β-hCG normalization (defined as reaching a level of less than or equal to 5 mIU/mL,) was reported by 20 studies in 23 different study groups. The overall mean time for β-hCG resolution to normal level was 29.817 days; 95% CI [26.158, 33.476], and the analysis was heterogeneous (p < 0.001, I2 = 99%).
Figure 1.
A forest plot of the time for serum β-human chorionic gonadotropin (β-hCG) normalization (defined as reaching a level of less than or equal to 5 mIU/mL,) in days.
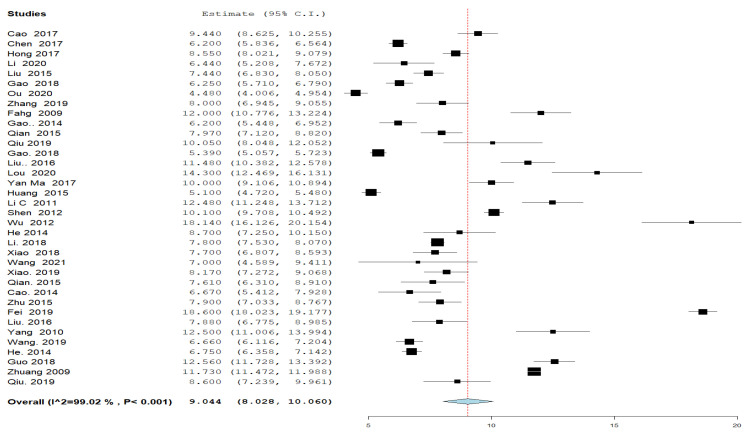
3.4.2. Hospital Stay (Figure 2)
Thirty-six groups in 30 studies reported about the duration of hospital stay following the UAE. The overall mean time of hospitalization was 9.044 days; 95% CI [8.028, 10.060], and the analysis was heterogeneous (p < 0.001, I2 = 99%).
Figure 2.
A forest plot of the duration of postprocedural hospital stay in days.
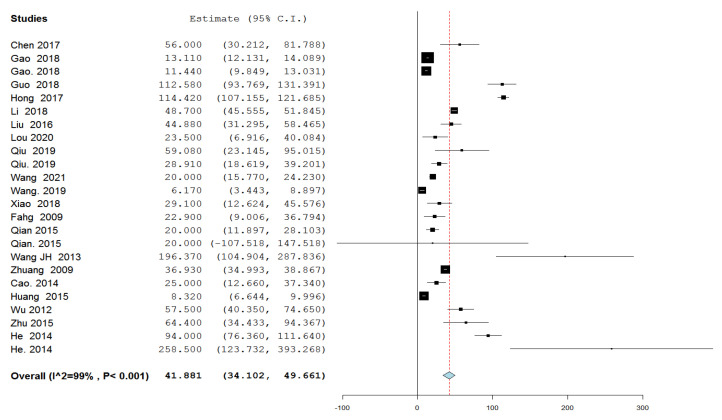
3.4.3. Amount of Intraprocedural Blood Loss (Figure 3)
Twenty studies with 24 variable groups reported the amount of intraprocedural blood loss. The overall effect estimate of the intraprocedural amount of bleeding was 41.881 mL; 95% CI [34.102, 49.661], and the analysis was heterogeneous (p < 0.001, I2 = 99%).
Figure 3.
A forest plot of the amount of intraprocedural blood loss in mL.
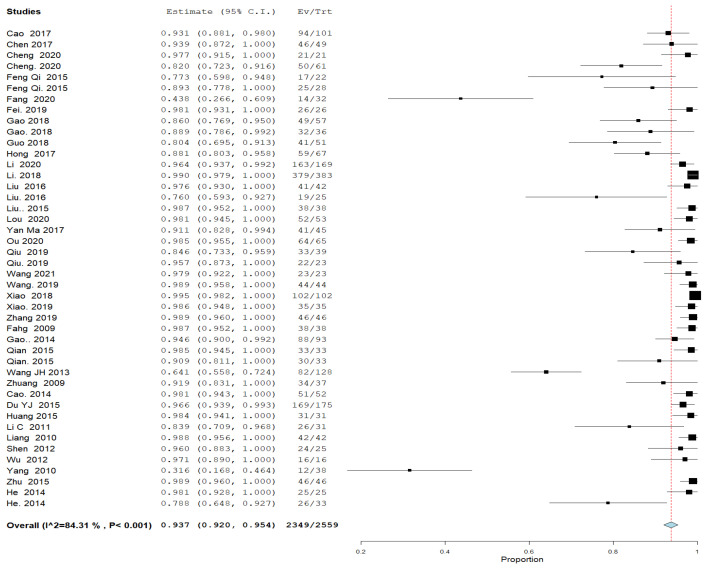
3.4.4. Success Rate (Figure 4)
All included studies reported success rate. The overall UAE success rate was 0.934; 95% CI [0.918, 0.951], and the analysis was heterogeneous (p < 0.001, I2 = 84%).
Figure 4.
A forest plot of UAE success rate.
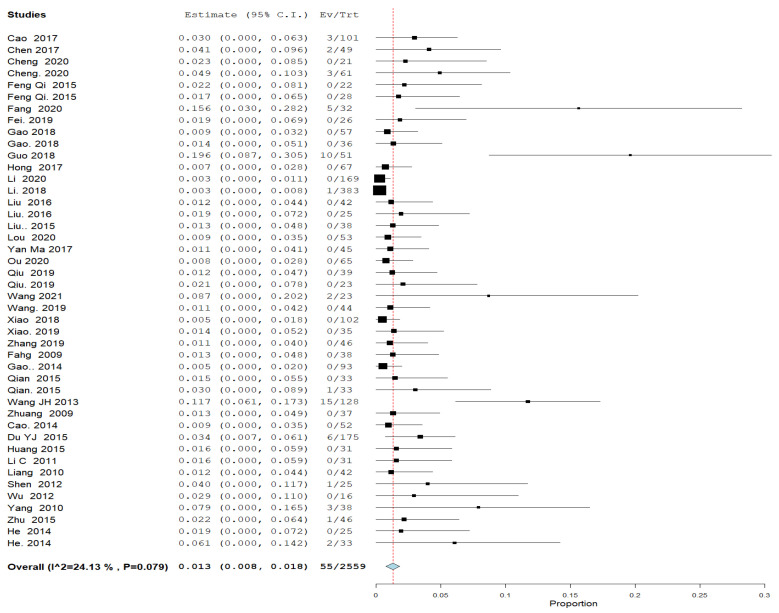
3.4.5. Severe Complication Rate (Figure 5)
All included studies reported a severe complication rate. The overall proportion of severe complication rate was 0.012; 95% CI [0.008, 0.017], and the analysis was homogenous (p = 0.127, I2 = 19%).
Figure 5.
A forest plot of the severe complication rate.
4. Discussion
We analyzed data of 2655 CSP patients treated with UAE as part of first-line management. The results show that UAE was associated with a mean of 30.3 days for β-hCG normalization, a mean hospitalization time of 8.9 days, a mean intraprocedural blood loss of 41.9 mL, a success rate of 93.4%, and a severe complication rate of 1.2%.
Regarding the time for serum β-hCG normalization, it was found to be about 30.3 days. Qiao et al. [55] reported in their meta-analysis comparing adjuvant therapies to D&C, that UAE plus D&C had a shorter β-hCG normalization period than MTX plus D&C, which supports our results and shows that UAE may help decrease the β-hCG normalization time. Other studies have also shown a shorter normalization time after UAE compared with other treatments [28,30,36,51].
The mean amount of blood loss was 41.9 mL in our study. A recent systematic review and meta-analysis of MTX therapy for CSP reported that the mean blood loss was 76.3 mL [56], which strongly indicates that UAE probably helps decrease bleeding. Other studies also reported that the addition of UAE to the treatment protocol led to less bleeding [11,14,54,57,58].
In this study, UAE was associated with a mean post procedural hospital stay of 8.9 days. A recent meta-analysis of MTX for CSP showed an average stay of 11.7 days when MTX was used on an inpatient basis as a solo agent for CSP [56]. Another study found that UAE followed by D&C had significantly less hospital stay than MTX plus D&C [55].
The success rate of UAE in our study was 93.4%, which means that about 2432 patients managed with UAE as a part of their therapy needed no additional follow-up treatments. The success rate for MTX combined treatment was lower and equaling 90.7% [56].
Fifty-five patients (1.2%) managed by UAE reported events of severe complications. Forty-three of them suffered bleeding more than 500 mL or received a blood transfusion, fifteen underwent a hysterectomy, and fourteen had laparotomy. Petersen et al. reported a severe complication rate in different modalities, reaching 3.4% in UAE plus D&C, 1.2% in UAE plus D&C and hysteroscopy, and 2.8% in UAE plus D&C and MTX, and higher proportions for other modalities devoid of UAE [59].
4.1. Limitations
Despite being the first study to group all available research on UAE in China as part of CSP treatment, our study has many limitations. The wide variety of treatment options used in the included studies makes summarizing the results challenging and prohibits any meaningful combination of protocols to show that any one regiment is superior or even notably efficacious. Therefore, our recommendations are somewhat limited as we are combining many different protocols, all of which included UAE as part of the primary treatment of CSP. The small sample size for some studies and heterogeneity in the results are also major limitations. Most studies were of fair rating of quality, so future research is needed with more structured study designs and a larger scale of participants to ensure the effectiveness of UAE in CSP treatment. Moreover, as our analysis looked at this novel technique’s usage only in China, some of our findings may not be applicable to other countries. Notable is the fact that many consider China’s outpatient care system to still be developing, which likely accounts for the very long inpatient stays associated with these studies. This would not be expected in most developed countries with robust outpatient care systems, and further limits the versatility of this data.
4.2. Strengths
This was the first meta-analysis to look at all the available research on the use of UAE for CSP coming from China, and we were able to find a very wide breadth of studies to include. Therefore, although the usefulness of this data in the rest of the world may be limited, we provide researchers and physicians outside of China a first look at a novel practice that is likely very foreign to their own modes of practice. In addition, we used strict adherence to PRISMA guidelines, and were able to solve heterogeneity in most situations. Many gynecologists around the world may not previously have been aware that these novel techniques were being used in China.
5. Conclusions
China is producing a large amount of literature on the novel usage of UAE in the treatment of CSP. Although our study was limited by including many variations in protocols and modalities that were included with UAE in the treatment of CSP, we were able to calculate an overall success rate of approximately 93.4%, and a severe complication rate of approximately 1.2%. Because of conditions unique to the healthcare system in China, this data may have limited utility in application to patient care in other countries. More high-quality trials will be needed to further elucidate which exact treatment combinations and protocols yield the safest and most efficacious results for patients.
Acknowledgments
The Marchand Institute for Minimally Invasive Surgery would like to acknowledge the efforts of all the students, researchers, residents, and fellows at the institute who put their time and effort into these projects without compensation, only for the betterment of women’s health. We firmly assure them that the future of medicine belongs to them.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11247393/s1, Figure S1: PRISMA flow chart summarizing the process of study selection; Table S1: The Risk of Bias for all included Cohort studies (by NIH tool); Table S2: Risk of Bias for all included Case series studies (by NIH tool).
Author Contributions
All authors attest to significant contributions to this work. At a minimum, G.J.M. and A.T.M. were responsible for conception and initial draft; C.C., H.U., A.M. and J.P. were responsible for data analysis, results, and discussion writing; A.A., S.G., C.M., A.M. and M.G. were responsible for data collection and the final draft. Many of the listed authors made other contributions to other parts of this work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This Manuscript has been reviewed by the institutional IRB board at Marchand Institute and was found to be exempt from IRB review. (May 2021). Data used was exempt from consent to participate or publish secondary to the nature of the study being a systematic review, retrospectively looking at previously published data.
Informed Consent Statement
Not applicable to systematic review.
Data Availability Statement
All supporting data is included or referenced in this manuscript. The authors have no additional data used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Commitment to Diversity
The Marchand Institute remains committed to diversity and tolerance in its research, and actively maintains a workplace free of racism and sexism. Greater than half of the authors for this study are female and many represent diverse backgrounds and under-represented ethnic groups.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Della-Giustina D., Denny M. Ectopic pregnancy. Emerg. Med. Clin. N. Am. 2003;21:565–584. doi: 10.1016/S0733-8627(03)00036-1. [DOI] [PubMed] [Google Scholar]
- 2.Mettler L., Sodhi B., Schollmeyer T., Mangeshikar P. Ectopic pregnancy treatment by laparoscopy, a short glimpse. Minim. Invasive Ther. Allied Technol. 2006;15:305–310. doi: 10.1080/13645700600771942. [DOI] [PubMed] [Google Scholar]
- 3.Jurkovic D., Hillaby K., Woelfer B., Lawrence A., Salim R., Elson C.J. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2003;21:220–227. doi: 10.1002/uog.56. [DOI] [PubMed] [Google Scholar]
- 4.Mi J., Liu F. Rate of caesarean section is alarming in China. Lancet (Lond. Engl.) 2014;383:1463–1464. doi: 10.1016/S0140-6736(14)60716-9. [DOI] [PubMed] [Google Scholar]
- 5.Lavender T., Hofmeyr G.J., Neilson J.P., Kingdon C., Gyte G.M.L. Caesarean section for non-medical reasons at term. Cochrane Database Syst. Rev. 2012;2012:CD004660. doi: 10.1002/14651858.CD004660.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Maymon R., Svirsky R., Smorgick N., Mendlovic S., Halperin R., Gilad K., Tobvin Y. Fertility performance and obstetric outcomes among women with previous cesarean scar pregnancy. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2011;30:1179–1184. doi: 10.7863/jum.2011.30.9.1179. [DOI] [PubMed] [Google Scholar]
- 7.Seow K.M., Huang L.W., Lin Y.H., Lin M.Y.-S., Tsai Y.L., Hwang J.L. Cesarean scar pregnancy: Issues in management. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2004;23:247–253. doi: 10.1002/uog.974. [DOI] [PubMed] [Google Scholar]
- 8.Arslan M., Pata O., Dilek T.U.K., Aktas A., Aban M., Dilek S. Treatment of viable cesarean scar ectopic pregnancy with suction curettage. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2005;89:163–166. doi: 10.1016/j.ijgo.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Hois E.L., Hibbeln J.F., Alonzo M.J., Chen M.E., Freimanis M.G. Ectopic pregnancy in a cesarean section scar treated with intramuscular methotrexate and bilateral uterine artery embolization. J. Clin. Ultrasound JCU. 2008;36:123–127. doi: 10.1002/jcu.20374. [DOI] [PubMed] [Google Scholar]
- 10.Rotas M.A., Haberman S., Levgur M. Cesarean scar ectopic pregnancies: Etiology, diagnosis, and management. Obstet. Gynecol. 2006;107:1373–1381. doi: 10.1097/01.AOG.0000218690.24494.ce. [DOI] [PubMed] [Google Scholar]
- 11.Li C., Li C., Feng D., Jia C., Liu B., Zhan X. Transcatheter arterial chemoembolization versus systemic methotrexate for the management of cesarean scar pregnancy. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2011;113:178–182. doi: 10.1016/j.ijgo.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B., Jiang Z.-B., Huang M.-S., Guan S.-H., Zhu K.-S., Qian J.-S., Zhou B., Li M.-A., Shan H. Uterine artery embolization combined with methotrexate in the treatment of cesarean scar pregnancy: Results of a case series and review of the literature. J. Vasc. Interv. Radiol. JVIR. 2012;23:1582–1588. doi: 10.1016/j.jvir.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Wu X., Zhang X., Zhu J., Di W. Caesarean scar pregnancy: Comparative efficacy and safety of treatment by uterine artery chemoembolization and systemic methotrexate injection. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;161:75–79. doi: 10.1016/j.ejogrb.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Gao L., Hou Y.-Y., Sun F., Xia W., Yang Y., Tian T., Chen Q.-F., Li X.-C. A retrospective comparative study evaluating the efficacy of adding intra-arterial methotrexate infusion to uterine artery embolisation followed by curettage for cesarean scar pregnancy. Arch. Gynecol. Obstet. 2018;297:1205–1211. doi: 10.1007/s00404-018-4686-8. [DOI] [PubMed] [Google Scholar]
- 15.Guo J., Yu J., Zhang Q., Song X. Clinical Efficacy and Safety of Uterine Artery Embolization (UAE) versus Laparoscopic Cesarean Scar Pregnancy Debridement Surgery (LCSPDS) in Treatment of Cesarean Scar Pregnancy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018;24:4659–4666. doi: 10.12659/MSM.907404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Zhao R., Qian H., Lv H. Pituitrin local injection versus uterine artery embolization in the management of cesarean scar pregnancy: A retrospective cohort study. J. Obstet. Gynaecol. Res. 2021;47:1711–1718. doi: 10.1111/jog.14720. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P.T. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0. 1. The Cochrane Collaboration. 2008. [(accessed on 3 March 2021)]. Available online: http://www.cochrane-handbook.org.
- 18.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.NIH National Heart, Lung, and Blood Institute Study Quality Assessment Tools: Quality Assessment Tool for Observational Cohort, Cross-Sectional and Case Series Studies. [(accessed on 3 March 2021)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 20.Cao G.-S., Liu R.-Q., Liu Y.-Y., Liu J.-W., Li L.-P., Zhang Q., Cao H.-C., Li T.-X. Menstruation recovery in scar pregnancy patients undergoing UAE and curettage and its influencing factors. Medicine. 2018;97:e9584. doi: 10.1097/MD.0000000000009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao S., Zhu L., Jin L., Gao J., Chen C. Uterine artery embolization in cesarean scar pregnancy: Safe and effective intervention. Chin. Med. J. 2014;127:2322–2326. [PubMed] [Google Scholar]
- 22.Chen H., Zhou J., Wang H., Tan W., Yao M., Wang X. The Treatment of Cesarean Scar Pregnancy with Uterine Artery Embolization and Curettage as Compared to Transvaginal Hysterotomy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;214:44–49. doi: 10.1016/j.ejogrb.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Q., Tian Q., Chang K.-K., Yi X.-F. Comparison of the efficacy and safety of different surgical strategies for patients with type II cesarean scar pregnancy. Reprod. Dev. Med. 2020;4:89–96. doi: 10.4103/2096-2924.288024. [DOI] [Google Scholar]
- 24.Du Y.J., Zhang X.H., Wang L.Q. Risk Factors for Haemorrhage during Suction Curettage after Uterine Artery Embolization for Treating Caesarean Scar Pregnancy: A Case-Control Study. Gynecol. Obstet. Investig. 2015;80:259–264. doi: 10.1159/000381263. [DOI] [PubMed] [Google Scholar]
- 25.Fahg A.-H., Chen Q.-F., Qian Z.-X., Li Q.-Y., Meng Y. Correlation Questions Clinical Discussion of Uterine Artery Embolization in Induced Abortion Patients with Management of Cesarean Scar Pregnancy. J. Reprod. Contracept. 2009;20:153–160. doi: 10.1016/S1001-7844(09)60020-1. [DOI] [Google Scholar]
- 26.Fang S., Zhang P., Zhu Y., Wang F., He L. A Retrospective Analysis of the Treatment of Cesarean Scar Pregnancy by High-Intensity Focused Ultrasound, Uterine Artery Embolization and Surgery. Front. Surg. 2020;7:23. doi: 10.3389/fsurg.2020.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fei H., Jiang X., Li T., Pan Y., Guo H., Xu X., Shu S. Comparison Of Three Different Treatment Methods For Cesarean Scar Pregnancy. Ther. Clin. Risk Manag. 2019;15:1377–1381. doi: 10.2147/TCRM.S220852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao L., Huang Z., Gao J., Mai H., Zhang Y., Wang X. Uterine artery embolization followed by dilation and curettage within 24 hours compared with systemic methotrexate for cesarean scar pregnancy. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2014;127:147–151. doi: 10.1016/j.ijgo.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 29.He Y., Wu X., Zhu Q., Wu X., Feng L., Wu X., Zhao A., Di W. Combined laparoscopy and hysteroscopy vs. uterine curettage in the uterine artery embolization-based management of cesarean scar pregnancy: A retrospective cohort study. BMC Women’s Health. 2014;14:116. doi: 10.1186/1472-6874-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong Y., Guo Q., Pu Y., Lu D., Hu M. Outcome of high-intensity focused ultrasound and uterine artery embolization in the treatment and management of cesarean scar pregnancy: A retrospective study. Medicine. 2017;96:e7687. doi: 10.1097/MD.0000000000007687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y., Li Y., Xi R., Chen Z., Ying D., Li Z., Yang Y. An application of uterine artery chemoembolization in treating cesarean scar pregnancy. Int. J. Clin. Exp. Med. 2015;8:2570–2577. [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Niu H., Li J., Zhang L., Qu Q. Clinical assessment of uterine artery embolization combined with curettage when treating patients with cesarean scar pregnancy: A retrospective study of 169 cases. J. Obstet. Gynaecol. Res. 2020;46:1110–1116. doi: 10.1111/jog.14258. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Lu L., Wang W., Sun J., Zhang X., Huang X. Retrospective study of patients with cesarean scar pregnancies treated by uterine artery chemoembolization and curettage. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2018;143:172–177. doi: 10.1002/ijgo.12636. [DOI] [PubMed] [Google Scholar]
- 34.Liang F., He J. Methotrexate-based bilateral uterine arterial chemoembolization for treatment of cesarean scar pregnancy. Acta Obstet. Et Gynecol. Scand. 2010;89:1592–1594. doi: 10.3109/00016349.2010.512973. [DOI] [PubMed] [Google Scholar]
- 35.Liu G., Wu J., Cao J., Xue Y., Dai C., Xu J., Jia X. Comparison of three treatment strategies for cesarean scar pregnancy. Arch. Gynecol. Obstet. 2017;296:383–389. doi: 10.1007/s00404-017-4426-5. [DOI] [PubMed] [Google Scholar]
- 36.Liu W., Shen L., Wang Q., Wang W., Sun Z. Uterine artery embolization combined with curettage vs. methotrexate plus curettage for cesarean scar pregnancy. Arch. Gynecol. Obstet. 2016;294:71–76. doi: 10.1007/s00404-015-3952-2. [DOI] [PubMed] [Google Scholar]
- 37.Lou T., Gao Y., Feng Y., Lu J., Zhang Z., Bai H. Reproductive outcomes of cesarean scar pregnancies pretreated with methotrexate and uterine artery embolization prior to curettage. Taiwan. J. Obstet. Gynecol. 2020;59:381–386. doi: 10.1016/j.tjog.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Ma Y., Shao M., Shao X. Analysis of risk factors for intraoperative hemorrhage of cesarean scar pregnancy. Medicine. 2017;96:e7327. doi: 10.1097/MD.0000000000007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ou J., Peng P., Li C., Teng L., Liu X. Assessment of the necessity of uterine artery embolization during suction and curettage for caesarean scar pregnancy: A prospective cohort study. BMC Pregnancy Childbirth. 2020;20:378. doi: 10.1186/s12884-020-03062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyra K., Szmygin M., Bérczi V., Tsitskari M., Sojka M., Pietras G. Clinical outcome and analysis of procedural failure during uterine artery chemoembolization as a treatment of caesarean scar pregnancy. Videosurgery Other Miniinvasive Tech. 2021;16:243–248. doi: 10.5114/wiitm.2020.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi F., Zhou W., Wang M.-F., Chai Z.-Y., Zheng L.-Z. Uterine artery embolization with and without local methotrexate infusion for the treatment of cesarean scar pregnancy. Taiwan. J. Obstet. Gynecol. 2015;54:376–380. doi: 10.1016/j.tjog.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Qian Z.-D., Huang L.-L., Zhu X.-M. Curettage or operative hysteroscopy in the treatment of cesarean scar pregnancy. Arch. Gynecol. Obstet. 2015;292:1055–1061. doi: 10.1007/s00404-015-3730-1. [DOI] [PubMed] [Google Scholar]
- 43.Qiu J., Fu Y., Xu J., Huang X., Yao G., Lu W. Analysis on clinical effects of dilation and curettage guided by ultrasonography versus hysteroscopy after uterine artery embolization in the treatment of cesarean scar pregnancy. Ther. Clin. Risk Manag. 2019;15:83–89. doi: 10.2147/TCRM.S184387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen L., Tan A., Zhu H., Guo C., Liu D., Huang W. Bilateral uterine artery chemoembolization with methotrexate for cesarean scar pregnancy. Am. J. Obstet. Gynecol. 2012;207:386.e1–386.e6. doi: 10.1016/j.ajog.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Stępniak A., Paszkowski T., Jargiełło T., Czuczwar P. Effectiveness, complications and reproductive outcome of selective chemoembolization with methotrexate followed by suction curettage for caesarean scar pregnancy—A prospective observational study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;241:56–59. doi: 10.1016/j.ejogrb.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Tumenjargal A., Tokue H., Kishi H., Hirasawa H., Taketomi-Takahashi A., Tsushima Y. Uterine Artery Embolization Combined with Dilation and Curettage for the Treatment of Cesarean Scar Pregnancy: Efficacy and Future Fertility. Cardiovasc. Interv. Radiol. 2018;41:1165–1173. doi: 10.1007/s00270-018-1934-z. [DOI] [PubMed] [Google Scholar]
- 47.Wang J.-H., Qian Z.-D., Zhuang Y.-L., Du Y.-J., Zhu L.-H., Huang L.-L. Risk factors for intraoperative hemorrhage at evacuation of a cesarean scar pregnancy following uterine artery embolization. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2013;123:240–243. doi: 10.1016/j.ijgo.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 48.Xiao F.-Y., Xue X.-H., Lu X. Comparison of Five Treatment Strategies for Cesarean Scar Pregnancy. Reprod. Dev. Med. 2018;2:88–94. doi: 10.4103/2096-2924.242751. [DOI] [Google Scholar]
- 49.Xiao Z., Cheng D., Chen J., Yang J., Xu W., Xie Q. The effects of methotrexate and uterine arterial embolization in patients with cesarean scar pregnancy: A retrospective case-control study. Medicine. 2019;98:e14913. doi: 10.1097/MD.0000000000014913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Luo F., Xia Y., Mei L., Xie L., Liu H. Clinical analysis of 211 cases of cesarean scar pregnancy. Clin. Exp. Obstet. Gynecol. 2019;46:948–952. doi: 10.12891/ceog5013.2019. [DOI] [Google Scholar]
- 51.Yang X.Y., Yu H., Li K.M., Chu Y.X., Zheng A. Uterine artery embolisation combined with local methotrexate for treatment of caesarean scar pregnancy. BJOG Int. J. Obstet. Gynaecol. 2010;117:990–996. doi: 10.1111/j.1471-0528.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S., Zhou T., Li M., Sheng C., Shou J. Dilation and curettage following local sclerotherapy for cesarean scar pregnancy. Int. J. Clin. Exp. Med. 2019;12:730–734. [Google Scholar]
- 53.Zhu X., Deng X., Xiao S., Wan Y., Xue M. A comparison of high-intensity focused ultrasound and uterine artery embolisation for the management of caesarean scar pregnancy. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. North Am. Hyperth. Group. 2016;32:144–150. doi: 10.3109/02656736.2015.1104733. [DOI] [PubMed] [Google Scholar]
- 54.Zhuang Y., Huang L. Uterine artery embolization compared with methotrexate for the management of pregnancy implanted within a cesarean scar. Am. J. Obstet. Gynecol. 2009;201:152.e1–152.e3. doi: 10.1016/j.ajog.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 55.Qiao B., Zhang Z., Li Y. Uterine Artery Embolization Versus Methotrexate for Cesarean Scar Pregnancy in a Chinese Population: A Meta-analysis. J. Minim. Invasive Gynecol. 2016;23:1040–1048. doi: 10.1016/j.jmig.2016.08.819. [DOI] [PubMed] [Google Scholar]
- 56.Salari N., Kazeminia M., Shohaimi S., Nankali A.A.-D., Mohammadi M. Evaluation of treatment of previous cesarean scar pregnancy with methotrexate: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. RBE. 2020;18:108. doi: 10.1186/s12958-020-00666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng Y., Chen S., Li C., Zhang X., Duan H., Sooranna S. Curettage after uterine artery embolization combined with methotrexate treatment for caesarean scar pregnancy. Exp. Ther. Med. 2016;12:1469–1475. doi: 10.3892/etm.2016.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lan W., Hu D., Li Z., Wang L., Yang W., Hu S. Bilateral uterine artery chemoembolization combined with dilation and curettage for treatment of cesarean scar pregnancy: A method for preserving the uterus. J. Obstet. Gynaecol. Res. 2013;39:1153–1158. doi: 10.1111/jog.12051. [DOI] [PubMed] [Google Scholar]
- 59.Birch Petersen K., Hoffmann E., Rifbjerg Larsen C., Svarre Nielsen H. Cesarean scar pregnancy: A systematic review of treatment studies. Fertil. Steril. 2016;105:958–967. doi: 10.1016/j.fertnstert.2015.12.130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data is included or referenced in this manuscript. The authors have no additional data used in this study.