Abstract
Sera from six adults, collected before and after acellular pertussis vaccination, and from a placebo control were examined for the ability to elicit two bactericidal immune defenses, (i) antibody-dependent complement-mediated bacterial lysis and (ii) opsonization and phagocytosis by human neutrophils. The samples were chosen based on low preimmunization titers and strong postimmunization responses to various combinations of vaccine antigens. All but two prevaccination samples demonstrated activity indicative of complement-mediated lysis. Preimmunization activity could have been due to prior infection or childhood immunization. Immunization did not result in improved bactericidal activity for any of the individuals, and in two cases immunization caused a statistically significant decrease in complement-mediated lysis. Similarly, opsonization with the postimmunization sera failed to enhance attachment or phagocytosis of bacteria by neutrophils, and one postimmunization sample with a strong response to filamentous hemagglutinin caused an inhibition of phagocytosis that was statistically significant compared to that observed for the no-serum control. In summary, booster immunization of adults with acellular pertussis vaccines was not found to increase bactericidal activity over preimmunization levels. Identifying ways to promote bactericidal immune responses might improve the efficacy of acellular pertussis vaccines.
The molecular basis for the pathogenicity of Bordetella pertussis is becoming clear. Pertussis is characterized by growth of B. pertussis on the respiratory mucosa, resulting in local damage and production of toxins that cause systemic symptoms. B. pertussis can attach to human cells using several adhesins (15), including filamentous hemagglutinin (FHA), pertactin, BrkA (Bordetella resistance to killing), pili, and tracheal colonization factor, with other potential adhesins appearing in the genome sequence. As a result of the redundancy of adhesins, with the exception of BrkA, which also promotes resistance to complement (25), mutants deficient in production of a single adhesin are often as virulent as the wild-type strain in animal models of disease (7, 11, 25, 26). Only mutants lacking more than one adhesin are reduced in virulence. B. pertussis produces potent toxins (pertussis toxin and adenylate cyclase toxin) that can poison the host immune response, and this may allow it to escape detection even with only a limited number of adhesins. Pertussis toxin is required for long-term persistence of the bacteria, but its absence makes no difference in the first few weeks of infection (7). In contrast, adenylate cyclase toxin is critical for establishment of infection, since mutants lacking adenylate cyclase toxin were unable to survive past the first week of infection (7).
The new acellular pertussis vaccines contain inactivated pertussis toxin and various combinations of the bacterial adhesins (FHA, pertactin, and fimbrial antigens). Antibodies to these antigens should block adherence and neutralize the effects of pertussis toxin. These vaccines are highly effective at preventing the severe manifestations of pertussis but are less effective at preventing bacterial colonization (1, 4, 8, 17, 20). This is consistent with animal experiments which suggest that adhesins not targeted by the vaccine may permit a bit of colonization and that neutralization of pertussis toxin would limit the severity of the disease but would not have an impact on the initial stages of infection.
From a public health point of view there are many advantages to a vaccine that would prevent the organisms from becoming established in the respiratory tract. In this study we have examined the ability of acellular vaccines to promote two immune defenses that could kill the bacteria and prevent colonization: (i) complement mediated lysis and (ii) opsonization and phagocytosis by neutrophils.
Human immune serum.
Human immune serum from an individual with occupational exposure to B. pertussis has been described previously (12, 22–24, 27). This sample has antibodies to B. pertussis lipopolysaccharide (LPS) and several surface-localized protein virulence factors, including FHA (27). Antibodies to adenylate cyclase were not present in this serum sample, as determined by Western blot analysis using purified adenylate cyclase toxin obtained from List Biologicals. Using this technique, we observed antibodies to adenylate cyclase toxin in sera from some, but not all, convalescent individuals (data not shown). Sera from adults participating in an acellular vaccine trial (10) are described in Table 1. Pre- and postvaccination serum samples were selected based on patterns of high and low responses to the four antigens in the acellular vaccines (Table 1). All of the preimmunization samples had low titers of antibodies to the four vaccine antigens, which were pertussis toxin, FHA, pertactin, and fimbriae. The individual receiving a placebo vaccine had low postimmunization titers of antibodies to all of the vaccine antigens. Four samples were chosen because each individual had very high postimmunization titers of antibodies to a single vaccine antigen (the four individuals, designated PT-Hi, FHA-Hi, Prn-Hi, and Fim-Hi, displayed high levels of antibody to pertussis toxin, FHA, pertactin, and fimbriae, respectively, in serum), although a rise in titers of antibodies to other antigens occurred in some cases. One individual (designated 4-Hi) had high postimmunization titers of antibodies to all four antigens, and another individual (designated 3-Hi) had high postimmunization titers of antibodies to pertussis toxin, FHA, and pertactin. All human serum samples were heat-inactivated at 56°C for 30 min to eliminate complement activity.
TABLE 1.
Pre- and post-acellular vaccine titersa of antibodies to vaccine antigens
| Serum sample | Vaccine | Titer of antibody to:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pertussis toxin
|
FHA
|
Pertactin
|
Fimbriae
|
||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| Placebo | None | 5 | 4 | 16 | 17 | 11 | 12 | 35 | 3 |
| 4-Hi | CLL-4F | 4 | 552 | 16 | 656 | 25 | 576 | 261 | 6,752 |
| 3-Hi | Biocine-3P | 17 | 4,264 | 42 | 1,496 | 44 | 3,008 | 32 | 57 |
| Fim-Hi | CLL-4F | 4 | 64 | 3 | 232 | 46 | 84 | 63 | 3,760 |
| Prn-Hi | Biocine-3P | 32 | 77 | 12 | 245 | 31 | 4,304 | 35 | 54 |
| FHA-Hi | Biocine-3P | 4 | 130 | 7 | 1,904 | 3 | 50 | 7 | 10 |
| PT-Hi | Amvax-1 | 36 | 4,288 | 36 | 90 | 40 | 34 | 115 | 109 |
Antibody-dependent complement-mediated bacterial lysis.
Complement is an important defense in the lungs and is also extruded to mucosal surfaces. Intact mucosal surfaces have about 10% as much complement as serum, and the amount increases during infection (16). B. pertussis expressing the surface protein, BrkA, resists killing by complement (5, 6, 27). However, some individuals mount an immune response that can overcome the BrkA defense (27). In one study, sera from adults with different exposure to B. pertussis were characterized for bactericidal activity against a wild-type strain and a BrkA complement-sensitive mutant (27). All of the sera killed the complement-sensitive mutant, suggesting that adults often have preexisting immune responses to B. pertussis, resulting from childhood immunization or from exposure to B. pertussis or cross-reactive organisms. However, only the sera collected from the convalescent and occupationally exposed individuals activated complement to kill the wild-type strain. Individuals receiving a two-component acellular vaccine (pertussis toxoid and FHA) lacked antibodies that promoted complement-mediated lysis (27). Pertussis toxin is secreted and FHA is easily detached from the bacterial surface, making these poor targets for complement fixation and bactericidal killing. Pertactin (an outer membrane protein) and fimbriae remain attached to the bacteria and would be better targets for complement killing. In this study we examined the ability of acellular vaccines containing pertactin and fimbriae as antigens to promote complement killing.
A virulent wild-type B. pertussis strain, BP338, was inoculated onto a Bordet-Gengou agar (BGA) plate and allowed to grow at 37°C for 15 to 20 h. The bacteria were harvested in prewarmed Stainer-Scholte broth lacking supplements (5). The optical density of the culture was determined at 600 nm, and the culture was diluted to a calculated optical density of 0.002. A 10-μl volume (about 4 × 105 bacteria) was added to the well of a U-bottom 96-well microtiter plate, 5 μl of human serum or phosphate-buffered saline (PBS) was added to the cells, and they were shaken briefly (150 rpm) at 37°C. Ten microliters of guinea pig serum (lot no. 116H9412; Sigma) lacking antibodies to B. pertussis (27) was added as a source of complement, and the bacteria were incubated for 1 h at 37°C with shaking at 150 rpm. To stop the reaction, 180 μl of PBS with 10 mM EDTA was added. Serial dilutions in PBS were performed, a 0.1-ml volume was plated on 1-day-old BGA plates, and the number of bacterial colonies was determined.
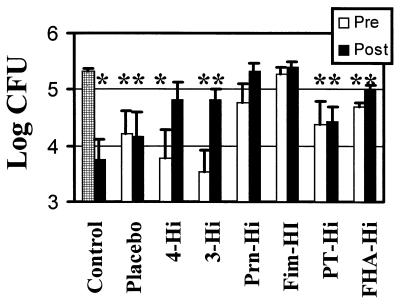
Guinea pig serum as a source of complement was unable to kill B. pertussis (Fig. 1). The value for the control following a 1-h incubation with guinea pig complement alone was 2.1 × 105 CFU, a value nearly identical to that obtained with a PBS control lacking complement (2.0 × 105 CFU). However, when both heat-inactivated immune serum and guinea pig complement were combined, significant bacterial killing occurred (Fig. 1). Addition of human serum from an occupationally exposed individual reduced viability to 5.7 × 103 CFU, corresponding to a 37-fold reduction in viability, and this was a statistically significant change (P < 0.002).
FIG. 1.
Antibody-dependent complement-mediated killing of B. pertussis. Bacterial survival was determined after a 1-h incubation with guinea pig complement. The control samples included a no-serum control (checked bar) and a heat-inactivated human immune serum (27) with known bactericidal activity (closed bar immediately to right of checked bar). The bactericidal activity of preimmunization (Pre) and postimmunization (Post) samples from an adult acellular vaccine trial are plotted. The preimmunization antibody titers were low for all vaccine antigens tested, and the individuals from whom the samples were collected were assigned designations on the basis of the pattern of their postvaccination response to the vaccine antigens pertussis toxin, FHA, pertactin, and fimbriae (Fim). 4-Hi displayed a high response to all of the vaccine antigens, and 3-Hi displayed a high response to pertussis toxin, FHA, and pertactin, as indicated in Table 1. Data were analyzed by the Student t test. ∗, samples with statistically significant activity compared to the no-serum control (P of <0.05 to 0.002).
The pre- and postimmunization sera from the acellular vaccine recipients were also characterized for antibody-dependent, complement-mediated lysis. Addition of the preimmune sera from all but two individuals (Prn-Hi and Fim-Hi) caused a reduction in viability that was statistically significant compared to that observed with the no-serum control (Fig. 1). Following immunization, the serum from 4-Hi had reduced bactericidal activity, and killing was not statistically different from that of the no-serum control. Both 3-Hi (P < 0.03) and FHA-Hi (P < 0.05) had a statistically significant loss of bactericidal activity following vaccination, although the postimmunization serum still possessed bactericidal activity that was statistically significant compared to that of the no-serum control.
Phagocytosis by human neutrophils.
Neutrophils are an important part of the innate immune response, and opsonizing antibodies enable neutrophils to contribute to microbial clearance in the presence of an acquired immune response. The early phases of inflammation due to infectious agents are characterized by an infiltration by leukocytes, especially neutrophils. Neutrophils are recruited in response to inflammatory mediators such as tumor necrosis factor alpha and interleukin-1 or bacterial products, such as LPS or formylated peptides (e.g., formylmethionyl-leucine-phenylalanine) and a recent study confirmed that neutrophil recruitment occurs in human airways in response to such agents (9). Neutrophil infiltration was observed in the lungs of mice following aerosol challenge with B. pertussis (13).
Two virulence factors (FHA and adenylate cyclase toxin) influence phagocytosis of B. pertussis by neutrophils (22–24). In the absence of opsonization, FHA mediates efficient attachment to neutrophils; however, very few bacteria are phagocytosed (23). Adenylate cyclase toxin plays a critical role in blocking phagocytosis. In the absence of opsonization, phagocytosis of the adenylate cyclase toxin mutant was not different from that of the wild-type strain. However, after opsonization the adenylate cyclase toxin mutants but not the wild-type strain were efficiently internalized (23). These results suggest that wild-type B. pertussis uses FHA to attach to neutrophils in a way that does not provoke phagocytosis. Following opsonization, engagement of the Fc receptor should allow the neutrophils to recognize B. pertussis as foreign and initiate phagocytosis (21); however, adenylate cyclase toxin blocks this process. Our previous studies have shown that opsonization with a human immune serum could inhibit both attachment and phagocytosis of wild-type B. pertussis by neutrophils (12, 22–24). We wanted to determine if serum from individuals receiving acellular pertussis vaccines would have a positive or negative impact on the ability of neutrophils to phagocytose B. pertussis.
Neutrophils were purified from human peripheral blood by dextran sedimentation and Ficoll-Paque centrifugation, and phagocytosis assays were performed using opsonized or nonopsonized B. pertussis strain BP338(pCW504), expressing green fluorescent protein as previously described (22, 23). To opsonize the bacteria, 30 μl of human serum was added to approximately 3 × 106 bacteria suspended in 30 μl of HBSA (Hanks' buffer supplemented with 0.25% bovine serum albumin and 2 mM HEPES) and incubated at 37°C for 15 min. Due to the limited amount of serum obtained from the vaccine recipients, phagocytosis studies were not performed in duplicate but were repeated seven to eight times.
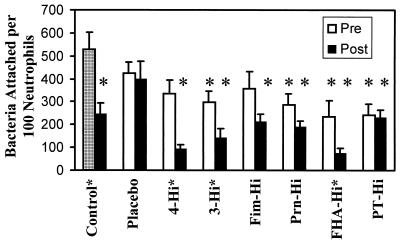
Phagocytosis can be broken down into two steps. First the bacteria must attach to the neutrophil, and then a signal must stimulate the neutrophil to ingest the microorganism. When attachment was examined, several of the samples displayed decreased attachment that was statistically significant compared to the nonopsonized control (Fig. 2). These included the preimmunization samples from 3-Hi, Prn-Hi, FHA-Hi, and PT-Hi and the postimmunization samples from all individuals except the individual receiving the placebo. The attachment of bacteria opsonized with the preimmunization serum was compared to the postimmunization serum, and the responses of 4-Hi (P < 0.002), 3-Hi (P < 0.03), and FHA-Hi (P < 0.05) were significantly different. The presence of low preimmunization titers but high postimmunization titers of antibodies to FHA is the common factor among these paired samples.
FIG. 2.
Effect of opsonization by human serum on bacterial attachment to neutrophils. Bacteria were not opsonized (checked bar) or were opsonized by the addition of either a control serum characterized in previous studies (12, 22–24) or sera from human volunteers participating in an acellular pertussis vaccine trial and incubated with human neutrophils for 1 h. One hundred consecutive neutrophils were examined microscopically for the number of adherent extracellular bacteria. Data were analyzed by the Student t test. Each bar represents the mean (+ standard error of the mean [error bars]) from seven to eight independent experiments. An asterisk above a bar indicates a result that is significantly different from that of the nonopsonized control (P of <0.05 to 0.00001). An asterisk placed after a label indicates a result that is significantly different from that of the preimmunization control (P of <0.05 to 0.002). Pre, preimmunization; Post, postimmunization.
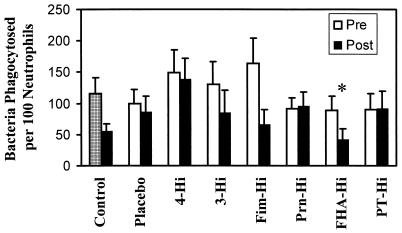
The ability of the serum samples to influence phagocytosis was also examined (Fig. 3). None of the samples from the vaccine study was statistically significantly different from the no-serum control with the exception of the postimmunization sample from FHA-Hi. This individual had a statistically significant (P < 0.04) reduction in phagocytosis compared to the nonopsonized control.
FIG. 3.
Effect of opsonization by human serum on bacterial phagocytosis by neutrophils. Bacteria were not opsonized (checked bar) or were opsonized by addition of human sera as described in the legend to Fig. 2, and phagocytosis was allowed to occur. One hundred consecutive neutrophils were examined for the number of intracellular bacteria. Data were analyzed by the Student t test. Each bar represents the mean (+ standard error of the mean [error bar]) from seven to eight independent experiments. ∗, significantly different from nonopsonized control result (P < 0.04). Pre, preimmunization; Post, postimmunization.
Pertussis vaccines have been used for half a century, but there is no indication that the disease is going away. Immunity to B. pertussis is complicated and could be achieved by different mechanisms. In this study we have examined how immunization with acellular pertussis vaccines influences two bactericidal immune defenses against B. pertussis, antibody-dependent complement fixation and phagocytosis by neutrophils.
The B. pertussis BrkA protein protects the bacteria from killing by complement (5, 6), but some individuals can mount an immune response that overcomes this resistance mechanism (27). The surface-associated proteins, pertactin and fimbriae, should be targets for antibody-mediated complement killing, while pertussis toxin, a secreted protein, and FHA, a loosely surface-associated protein, would be poor targets for antibody-dependent complement-mediated killing. In this study we have found no evidence that immunization with acellular vaccines induced complement-mediated killing, and for two individuals (3-Hi and FHA-Hi), immunization cause a statistically significant loss in complement-mediated killing. The loss of activity following immunization is perplexing; however, in a previous study (27) members of our group observed that most sera can kill a BrkA mutant strain but killing of wild-type B. pertussis was associated with the presence of immunoglobulin G3 antibodies, the most potent at fixing complement. Less-potent antibodies could be blocking the antibodies capable of fixing complement.
The role of neutrophils in immunity to pertussis has received little attention until recently. Following aerosol challenge, a significant neutrophil infiltration was observed in the lungs of naïve mice and mice immunized with the whole-cell pertussis vaccine, but neutrophils were not observed in the lungs mice immunized with an acellular pertussis vaccine (13). The difference between the two vaccine groups could be correlated with the type of immune response. Natural infection or whole-cell pertussis vaccine selectively induced Th1 helper T cells in mice, while the acellular pertussis vaccine selectively induced Th2 helper T cells (3, 14). However, this polarized response was not observed in humans, where acellular vaccines induced a mixed Th1-Th2 response (2, 18, 19). Proinflammatory cytokines produced during a Th1 type of response will activate and recruit neutrophils to the site of infection, particularly in the presence of LPS. B. pertussis sheds LPS, making it likely that neutrophils will be recruited during human disease and could potentially play an important role in clearing the infection.
The ability of serum from acellular vaccine recipients to promote phagocytosis by neutrophils was examined. None of the serum samples increased phagocytosis, and one caused a statistically significant decrease in phagocytosis. Phagocytosis requires attachment of the organisms to the neutrophil and a signaling event to initiate internalization (21). In a previous study members of our group showed that FHA mediates efficient attachment of B. pertussis to human neutrophils, but few organisms are internalized, suggesting that FHA-mediated attachment does not activate signaling pathways needed for internalization (23). Following opsonization, adenylate cyclase toxin mutants but not wild-type organisms are efficiently phagocytosed (23). This suggests that signaling through Fc receptors is blocked by adenylate cyclase toxin.
Most of the sera characterized in this study inhibited bacterial attachment to neutrophils. Opsonization with sera from the three individuals with the highest titers of antibodies to FHA (4-Hi, 3-Hi, and FHA-Hi) resulted in a statistically significant reduction in attachment of the bacteria compared to the preimmunization levels, and in addition, the sera from FHA-Hi caused a statistically significant reduction in phagocytosis. Interestingly, two independent clinical trials were unable correlate levels of antibody to FHA in serum with protection (4, 20). We have shown that addition of neutralizing antibodies to adenylate cyclase toxin can reverse the effects of a serum that by itself inhibited phagocytosis, resulting in efficient uptake (24). Adenylate cyclase toxin is not included in any of the acellular vaccine formulations presently used, and inclusion of this antigen might be beneficial in promoting immunity to pertussis.
Immunity can be achieved in several ways. The acellular vaccines presently in use are highly effective at preventing severe disease but are less effective at preventing bacterial infection (1, 4, 8, 17, 20). Presumably, they achieve protection by blocking bacterial attachment and neutralizing the adverse effects of pertussis toxin. In this small study, we found no evidence that acellular vaccines promoted antibody-dependent killing by complement or enhanced phagocytosis by neutrophils. A larger sample size will be needed to determine if, in general, acellular vaccines are unable to activate these immune responses. However, BrkA has been shown to inhibit killing by complement (5) and adenylate cyclase toxin has been shown to inhibit phagocytosis by neutrophils (23), and it seems unlikely that a vaccine that does not contain these antigens could efficiently promote clearance via these mechanisms. Promoting clearance by these immune defenses could lead to a more efficacious vaccine, particularly if it prevented mild as well as severe disease, and further studies are needed to clarify the roles of neutralizing antibodies to adenylate cyclase toxin and BrkA in immunity to pertussis.
Acknowledgments
This work was supported in part by grants RO1 AI38415 and AI45715 to A.A.W. and NO1 AI25135 to W.A.K. and K.M.E.
REFERENCES
- 1.Ad Hoc Group for the Study of Pertussis Vaccines. Placebo-controlled trial of two acellular pertussis vaccines in Sweden—protective efficacy and adverse events. Lancet. 1988;i:955–960. [PubMed] [Google Scholar]
- 2.Ausiello C M, Urbani F, la Sala A, Lande R, Cassone A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect Immun. 1997;65:2168–2174. doi: 10.1128/iai.65.6.2168-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnard A, Mahon B P, Watkins J, Redhead K, Mills K H G. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology. 1996;87:372–380. doi: 10.1046/j.1365-2567.1996.497560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherry J D, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. 1998;16:1901–1906. doi: 10.1016/s0264-410x(98)00226-6. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez R C, Weiss A A. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect Immun. 1994;62:4727–4738. doi: 10.1128/iai.62.11.4727-4738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez R C, Weiss A A. Serum resistance in bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol Lett. 1998;163:57–63. doi: 10.1111/j.1574-6968.1998.tb13026.x. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin M S M, Weiss A A. Adenylate cyclase toxin is critical for bacterial colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect Immun. 1990;58:3445–3447. doi: 10.1128/iai.58.10.3445-3447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewlett E L. Pertussis: current concepts of pathogenesis and prevention. Pediatr Infect Dis J. 1997;16(Suppl. 4):S78–S84. doi: 10.1097/00006454-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 9.Jagels M A, Daffern P J, Zuraw B L, Hugli T E. Mechanisms and regulation of polymorphonuclear leukocyte and eosinophil adherence to human airway epithelial cells. Am J Respir Cell Mol Biol. 1999;21:418–427. doi: 10.1165/ajrcmb.21.3.3478. [DOI] [PubMed] [Google Scholar]
- 10.Keitel W A, Muenz L R, Decker M D, Englund J A, Mink C M, Blumberg D A, Edwards K M. A randomized clinical trial of acellular pertussis vaccines in healthy adults: dose-response comparisons of 5 vaccines and implications for booster immunization. J Infect Dis. 1999;180:397–403. doi: 10.1086/314869. [DOI] [PubMed] [Google Scholar]
- 11.Khelef N, Bachelet C M, Vargaftig B B, Guiso N. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect Immun. 1994;62:2893–2900. doi: 10.1128/iai.62.7.2893-2900.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenz D H, Weingart C L, Weiss A A. Phagocytosed Bordetella pertussis fail to survive in human neutrophils. Infect Immun. 2000;68:956–959. doi: 10.1128/iai.68.2.956-959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuirk P, Mills K H G. A regulatory role for interleukin 4 in differential inflammatory responses in the lung following infection of mice primed with Th1- or Th2-inducing pertussis vaccines. Infect Immun. 2000;68:1383–1390. doi: 10.1128/iai.68.3.1383-1390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills K H G, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parton R. Review of the biology of Bordetella pertussis. Biologicals. 1999;27:71–76. doi: 10.1006/biol.1999.0182. [DOI] [PubMed] [Google Scholar]
- 16.Persson C G A, Erjefalt I, Alkner U, Baumgarten C, Grief L, Gustafsson B, Luts A, Pipkorn U, Sundler F, Svensson C, Wollmer P. Plasma exudation as a first line respiratory mucosal defence. Clin Exp Allergy. 1991;21:17–24. doi: 10.1111/j.1365-2222.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin S A, Cadoz M. The acellular pertussis vaccine trials: an interpretation. Pediatr Infect Dis J. 1997;16:508–517. doi: 10.1097/00006454-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills K H. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J Infect Dis. 1997;175:1246–1250. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 19.Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Gothefors L, Oymar K, Miller E, Storsaeter J, Mills K H. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storsaeter J, Hallander H O, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16:1907–1916. doi: 10.1016/s0264-410x(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 21.Strzelecka A, Kwiatkowska K, Sobota A. Tyrosine phosphorylation and Fcγ receptor-mediated phagocytosis. FEBS Lett. 1997;400:11–14. doi: 10.1016/s0014-5793(96)01359-2. [DOI] [PubMed] [Google Scholar]
- 22.Weingart C L, Broitman-Maduro G, Dean G, Newman S, Peppler M, Weiss A A. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 1999;67:4264–4267. doi: 10.1128/iai.67.8.4264-4267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weingart C L, Weiss A A. Bordetella pertussis virulence factors affect phagocytosis by human neutrophils. Infect Immun. 2000;68:1735–1739. doi: 10.1128/iai.68.3.1735-1739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weingart C L, Mobberley P S, Hewlett E L, Gray M C, Weiss A A. Neutralizing antibodies to adenylate cyclase toxin promote phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 2000;68:7152–7155. doi: 10.1128/iai.68.12.7152-7155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss A A, Goodwin M S. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989;57:3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss A A, Hewlett E L, Myers G A, Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984;150:219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- 27.Weiss A A, Mobberley P S, Fernandez R C, Mink C A. Characterization of human bactericidal antibodies to Bordetella pertussis. Infect Immun. 1999;67:1424–1431. doi: 10.1128/iai.67.3.1424-1431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]