Abstract
We sequenced a 705-bp fragment of the recA gene from 113 Vibrio cholerae strains and closely related species. One hundred eighty-seven nucleotides were phylogenetically informative, 55 were phylogenetically uninformative, and 463 were invariant. Not unexpectedly, Vibrio parahaemolyticus and Vibrio vulnificus strains formed out-groups; we also identified isolates which resembled V. cholerae biochemically but which did not cluster with V. cholerae. In many instances, V. cholerae serogroup designations did not correlate with phylogeny, as reflected by recA sequence divergence. This observation is consistent with the idea that there is horizontal transfer of O-antigen biosynthesis genes among V. cholerae strains.
Understanding the transfer of genes within and among bacteria requires knowledge of the genetic relatedness of the bacteria. Pandemic strains of Vibrio cholerae have acquired the major virulence factors cholera toxin and toxin-coregulated pilus (TCP) by lysogeny (14, 27). The recent appearance of the O139 epidemic strain of V. cholerae probably occurred via acquisition of a new surface polysaccharide through a horizontal gene transfer event (5, 8, 23). In order to better understand this event and in hopes of predicting future events, we have begun to generate multilocus sequencing (MLS) genotypes of various strains of V. cholerae. MLS has three advantages over multilocus enzyme electrophoresis: (i) MLS detects more variation for each locus (e.g., silent substitutions), (ii) convergence of alleles is less likely, and (iii) MLS data are easily compared across laboratories (16). Sequencing of aldA and the cholera toxin genes, ctxA and ctxB, has proven useful in studying the epidemiology of pandemic strains but is limited to toxigenic isolates (12, 26). Sequencing the asd gene has broader application but has been done for only 24 non-O1 isolates (13). Studies using the pattern of IS1004 insertions and pulsed-field gel electrophoresis (PFGE) have also looked at only limited numbers of non-O1 V. cholerae isolates (5, 6).
In this brief communication, we report our results from sequencing a 705-bp fragment of the recA gene from 107 strains that had initially been designated V. cholerae and 5 strains of other Vibrio species. The locus chosen for study was recA because it has been shown to be useful for estimating phylogeny, in contrast to some other genes (9). Strains are listed in Table 1; strains are from our collection at the University of Maryland and include strains representative of known outbreaks, as well as serogroup type strains from the Smith Vibrio Reference Laboratory collection (22) of non-O1 V. cholerae.
TABLE 1.
Strains useda
| Strain | Serogroup
|
Place where isolated | Yr isolated | Classification or description | Specimen origin | Source and/or reference | |
|---|---|---|---|---|---|---|---|
| Sakazaki | Smith | ||||||
| AS119 | ND | ND | India | 1996 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| 6707 | ND | O15 | Hong Kong | 1958 | Night soil | H. Smith collection | |
| AM25 | O39 | ND | India | 1995 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| 6337 | ND | O352 | H. Smith collection | ||||
| 6313 | ND | O309 | Bangladesh | 1962 | Diarrhea | H. Smith collection | |
| AM107 | O107 | ND | India | 1996 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| 5411 | ND | O42 | Bangladesh | 1961 | Diarrhea | H. Smith collection | |
| ATCC 25873 | O37 | ND | Czechoslovakia | Diarrhea | ATCC 1 | ||
| CS365 | O1 | ND | Brazil | El tor | Diarrhea | C. Salles | |
| N16961 | O1 | O1 | India | El tor Inaba | Diarrhea | CVD 7 | |
| AM2 | O9 | ND | India | 1995 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| S21 | O37 | ND | Sudan | Diarrhea | CVD 2 | ||
| N16117 | O1 | O1 | El tor | Diarrhea | CVD | ||
| AI1837 | O139 | ND | Bangladesh | Bengal | Diarrhea | J. Albert 11 | |
| 981-75 | O65 | ND | India | 1975 | Diarrhea | T. Shimada 21 | |
| CA385 | O1 | O1 | Rough | CVD 21 | |||
| ATCC 25872 | O37 | ND | Czechoslovakia | Diarrhea | ATCC 1 | ||
| 1322-69 | O37 | ND | India | 1969 | T. Shimada 21 | ||
| 322 | O1 | ND | El tor | Diarrhea | CVD | ||
| ATCC 25874 | ND | ND | Czechoslovakia | Diarrhea | ATCC 1 | ||
| MO10 | O139 | ND | India | 1992 | Diarrhea | P. Echeverria | |
| MO45 | O139 | ND | India | 1992 | Diarrhea | T. Shimada | |
| E7946 | O1 | O1 | Bahrain | El tor Ogawa | Diarrhea | CVD | |
| 8585 | ND | O340 | Iraq | 1966 | Feces | H. Smith collection | |
| 3083 | O1 | O1 | El tor Ogawa | Diarrhea | CVD | ||
| N15870 | O1 | O1 | Bangladesh | Diarrhea | CVD | ||
| RV79 | O1 | O1 | Sulawesi | 1937 | El tor Inaba | Diarrhea | CVD |
| 5066 | ND | O24 | Thailand | 1960 | Diarrhea | H. Smith collection | |
| 6355 | ND | O57 | Bangladesh | Diarrhea | H. Smith collection | ||
| 5053 | ND | O56 | Thailand | 1959 | Water | H. Smith collection | |
| 5078 | ND | O37 | Thailand | 1959 | Water | H. Smith collection | |
| 6970 | ND | O312 | Bangladesh | 1966 | Diarrhea | H. Smith collection | |
| 5069 | ND | O48 | Thailand | 1959 | Contact | H. Smith collection | |
| 498-7 | O139 | ND | Thailand | CT+ TCP− | Diarrhea | P. Echeverria | |
| 569B | O1 | O1 | India | 1948 | Classical Inaba | Diarrhea | CVD 7 |
| 5011 | ND | O333 | H. Smith collection | ||||
| 395 | O1 | O1 | India | Classical | Diarrhea | CVD 7 | |
| NIH35A3 | O1 | ND | 1941 | T. Shimada 21 | |||
| 7236 | ND | O361 | H. Smith collection | ||||
| 7261 | ND | O362 | H. Smith collection | ||||
| 5152 | ND | O50 | United States (Maryland) | 1960 | Water | H. Smith collection | |
| C0545 | O5 | ND | India | 1994 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| 8-76 | 77 | ND | India | 1976 | Diarrhea | T. Shimada 21 | |
| 571-88 | O105 | ND | India | 1988 | Diarrhea | T. Shimada 21 | |
| 234-93 | O141 | ND | India | 1993 | Diarrhea | T. Shimada 21 | |
| 1421-77 | O80 | ND | India | 1977 | Diarrhea | T. Shimada 21 | |
| AS67 | O190 | ND | India | 1996 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| AM124 | O11 | ND | India | 1996 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| 5043 | ND | O175 | H. Smith collection | ||||
| 5811 | ND | O102 | H. Smith collection | ||||
| 7977 | ND | O18 | Bangladesh | 1965 | Water | H. Smith collection | |
| 8635 | ND | O321 | H. Smith collection | ||||
| 5714 | ND | O351 | H. Smith collection | ||||
| 7920 | ND | O33 | Japan | 1968 | H. Smith collection | ||
| 9183 | ND | O347 | Guam | 1977 | Unknown | H. Smith collection | |
| 5051 | ND | O94 | Thailand | 1959 | Water | H. Smith collection | |
| 5052 | ND | O38 | Thailand | 1959 | Water | H. Smith collection | |
| C677 | ND | O14 | Thailand | NAG-ST | Diarrhea | P. Echeverria | |
| 9794 | ND | O357 | H. Smith collection | ||||
| AS12-1 | O10 | ND | India | 1995 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| 5180 | ND | O176 | India | 1963 | H. Smith collection | ||
| 6701 | ND | O19 | Hong Kong | 1958 | Water | H. Smith collection | |
| 32-90 | ND | ND | Thailand | 1990 | NAG-ST | Diarrhea | P. Echeverria 3 |
| 5694 | ND | O77 | Bangladesh | 1962 | Water | H. Smith collection | |
| C0845 | O83 | ND | India | 1995 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| 5096 | ND | O16 | Thailand | 1960 | H. Smith collection | ||
| 169-68 | O22 | ND | 1968 | Environment | CVD (Sozhamannan, unpublished) | ||
| 8105 | ND | O358 | H. Smith collection | ||||
| 7995 | ND | O320 | 1968 | Sewer | H. Smith collection | ||
| AS414 | O39 | ND | India | 1997 | Diarrhea | CVD (Sozhamannan, unpublished) | |
| NG288-36 | O139 | ND | Thailand | CT− TCP− | Diarrhea | P. Echeverria | |
| C0560 | O27 | ND | India | 1994 | Diarrhea | CVD (Sozhamannan, unpublished) | |
| 9211 | ND | O348 | United States (Maryland) | 1977 | Water | H. Smith collection | |
| Sakazaki | Smith | ||||||
| 9248 | ND | O349 | United States (Maryland) | 1977 | Water | H. Smith collection | |
| 5029 | ND | O61 | Thailand | 1959 | Water | H. Smith collection | |
| 8691 | ND | O363 | H. Smith collection | ||||
| M556 | O74 | ND | Argentina | 1993 | D. Karaolis 13 | ||
| PS15 | ND | O106 | United States (Maryland) | Sediment | C. Kaysner | ||
| 7449 | ND | O64 | Philippines | 1962 | H. Smith collection | ||
| C0668 | ND | ND | India | 1994 | Diarrhea | CVD (Sozhamannan, unpublished) | |
| C0639 | O11 | ND | India | 1994 | Diarrhea | CVD (Sozhamannan, unpublished) | |
| 984-81 | 89 | ND | India | 1981 | Diarrhea | T. Shimada 21 | |
| 5037 | ND | O44 | Thailand | 1959 | H. Smith collection | ||
| C0603B | O108 | ND | India | 1994 | Diarrhea | G.B. Nair (Sozhamannan, unpublished) | |
| 1074-78 | O1 | O1 | Brazil | 1978 | Nonpathogenic El Tor | Sewer | CVD 15 |
| 6291 | ND | O46 | H. Smith collection | ||||
| CS367 | ND | ND | Brazil | Diarrhea | C. Salles | ||
| 8497 | ND | O343 | United States | 1973 | Septic | H. Smith collection | |
| 5047 | ND | O175 | Thailand | 1959 | H. Smith collection | ||
| 7165 | ND | O201 | Bangladesh | 1966 | Water | H. Smith collection | |
| 5697 | ND | O83 | Bangladesh | 1962 | Water | H. Smith collection | |
| 5064 | ND | O12 | Nanking, People's Republic of China | 1932? | H. Smith collection | ||
| 5103 | ND | O22 | Thailand | 1959 | H. Smith collection | ||
| 7902 | ND | O115 | Bangladesh | 1962 | Diarrhea | H. Smith collection | |
| NG653-36 | O139 | ND | Thailand | CT− TCP− | Diarrhea | P. Echeverria | |
| VO11627 | O139 | ND | Thailand | CT− TCP− | Diarrhea | P. Echeverria | |
| 8536 | ND | O332 | Yugoslavia | Sewer | H. Smith collection | ||
| Arg3 | O139 | ND | NAG-ST | Environment | CVD | ||
| 9009 | ND | O175 | Hungary | 1976 | Environment | H. Smith collection | |
| M554 | O83 | ND | Germany | 1994 | D. Karaolis 13 | ||
| A-5 | O31 | ND | Japan | Shrimp | CVD 2 | ||
| NRT36S | O31 | ND | Japan | NAG-ST | Diarrhea | CVD 18 | |
| MO6-24 | NA | NA | United States | V. vulnificus | Blood | CVD | |
| 5310 | NA | NA | V. vulnificus | H. Smith collection | |||
| 9115 | NA | O345 | Philippines | 1976 | V. parahaemolyticus | NonHD | H. Smith collection |
| AS530 | O45 | NA | India | 1997 | Diarrhea | G.B. Nair | |
| AS555 | NA | NA | India | 1997 | Diarrhea | G.B. Nair | |
| 6358 | NA | O160 | V. mimicus | H. Smith collection | |||
| 6306 | NA | O107 | Bangladesh | 1961 | V. mimicus | Diarrhea | H. Smith collection |
| 61956 | NA | NA | Bangladesh | V. mimicus | Diarrhea | CVD, Kaper | |
| 523-80 | O115 | NA | India | 1980 | V. mimicus | Diarrhea | T. Shimada 21 |
| 8643 | NA | NA | V. mimicus | H. Smith collection | |||
ND, not determined; CT, cholera toxin; ATCC, American Type Culture Collection; CVD, Center for Vaccine Development, University of Maryland, Baltimore; NA, not applicable.
Minipreparations of chromosomal DNA were made from each strain using the Wizard genomic DNA purification kit (Promega). DNAs were diluted to 10 ng/μl, and 1 μl was used for PCR amplification of the recA gene. The base sequence from 813 to 1598 (numbering based on the V. cholerae sequence, GenBank accession no. X71969) of the recA gene was determined in two directions from PCR products using cycle sequencing and an ABI Prism 377 automatic sequencer (Perkin-Elmer). PCR was initiated with the primers rec-1 (GAAACCATTTCGACCGGTTC) and rec-2 (CCGTTATAGCTGTACCAAGCGCCC). These two primers were selected from two regions conserved between V. cholerae and Vibrio anguillarum (accession no. M80525). The 30-μl PCR mixture contained 0.5 U of Taq polymerase, a 10 μM concentration of each primer, 20 mM KCl, 1.5 mM MgCl2, and 10 mM Tris, pH 8.5. The reaction was carried out using one step of 94°C for 4 min, 28 cycles of 94°C for 30 s, 64°C for 45 s, and 72°C for 30 s, and a final step of 72°C for 6 min in a 9600 thermocycler (Perkin-Elmer). The 788-bp amplified product was purified using Wizard PCR purification columns (Promega). The eluted DNA was precipitated with ethanol. Cycle sequencing reactions were initiated with a 0.3 μM concentration of one of the PCR primers using fluorescent-dye-labeled dideoxynucleotides (Big Dye kit; Perkin-Elmer). There were 28 cycles of 94°C for 10 s, 50°C for 5 s, and 60°C for 4 min. The products were separated on 6% denaturing gels under standard conditions in an ABI Prism 377 automatic sequencer.
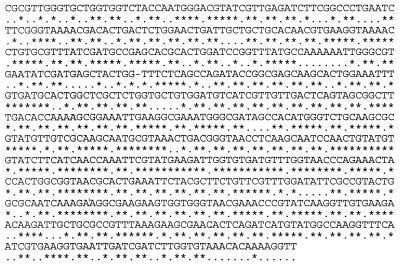
Data were analyzed using Genescan (version 2.0) and Fractura (version 3.0) software (Perkin-Elmer). Sequencing reactions were performed in both directions to maximize the quality of the sequence. After trimming the low-quality sequence on the ends, the remaining 705 bp of high-quality sequence were aligned using CLUSTAL X (24). The aligned sequence is shown in Fig. 1. Most (134 of 243) of the variable bases are in the third, or wobble, position of the codons. The hyphen at bp 201 represents a 1-bp insertion in C0545; 11 bp later this strain has a 1-bp deletion. Phylogenetic trees were calculated using distance matrixes, unweighted pair group method with arithmetic mean (UPGMA), neighbor joining, and bootstrapping methods (PAUP, version 3.1; Sinauer Assoc., Sunderland, Mass.). Each of the methods produced similar results.
FIG. 1.
Partial sequence for the recA gene of the El Tor strain N16961, as aligned with 111 other analyzed sequences. An asterisk below the base indicates that the base is conserved in all 112 strains; a period indicates that the base varies in at least one strain. The hyphen at bp 201 is to accommodate strain C0545, which has a 1-bp insertion at this point and a 1-bp deletion 11 bp later.
All strains were streaked on Luria agar and on thiosulfate-citrate-bile salts-sucrose agar to check for purity and were tested for oxidase and spot indole (Remel, Lenexa, Kans.). The 20 most divergent strains were subjected to additional biochemical tests for Vibrio species (17). These included API 20E (bioMerieux, Hazelwood, Mo.), indole (Remel), and the string test. Additional tube biochemicals were run after the addition of 1% NaCl, including o-nitrophenyl-β-d-galactopyranoside, Moeller's ornithine decarboxylase, lysine decarboxylase, and arginine dihydrolase, and purple broth with glucose, sucrose, or arabinose (Remel). Critical reactions for selected strains are shown in Table 2.
TABLE 2.
Selected biochemical reactions of out-group V. cholerae strains
| Strain | Reaction with:
|
||||||
|---|---|---|---|---|---|---|---|
| Arginine dihydrolyase | Lysine decarboxylase | Ornithine decarboxylase | Arabinose | Sucrose | ONPGa | VP (API 20E)b | |
| 6358 | − | + | + | − | + | + | − |
| 6306 | − | + | + | − | − | + | − |
| 61956 | − | + | + | + | − | + | − |
| 8691 | − | + | + | − | + | + | + |
| AS530 | − | + | + | − | + | + | + |
| AS555 | − | + | + | − | + | + | − |
| 9115 | − | + | + | − | − | − | − |
| NRT-36S | − | + | + | − | + | + | + |
| AM25 | − | + | + | − | + | + | + |
| AS119 | − | + | + | − | + | + | − |
| A-5 | − | + | + | − | + | + | + |
| 6707 | − | + | + | − | + | + | − |
| AM1070 | − | + | + | − | + | + | + |
| 5411 | − | + | + | − | + | + | + |
| 6313 | − | + | − | − | + | + | + |
| 6337 | − | + | + | − | + | + | + |
ONPG, o-nitrophenyl-β-d-galactopyranoside.
VP, Voges-Proskauer.
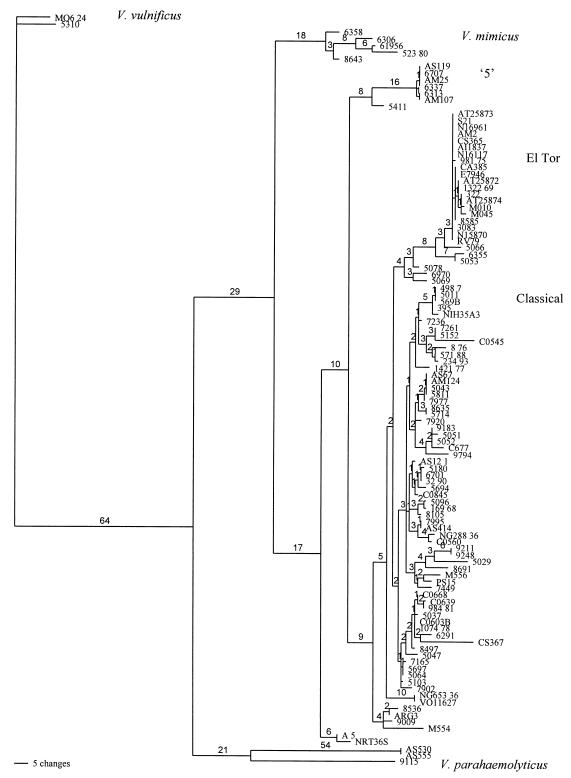
Figure 2 shows the phylogenic tree analysis on 705 bases of sequence from the recA gene of 113 bacterial strains. A total of 187 nucleotides were phylogenetically informative, 55 were phylogenetically uninformative, and 463 were invariant. As expected, the Vibrio vulnificus and Vibrio parahaemolyticus sequences formed out-groups. One strain from the Smith collection (strain 5310), previously designated non-O1 V. cholerae, clustered with V. vulnificus, an identification that was confirmed biochemically. Each of these clusters occurred 100% of the time in 1,000 bootstrap replicates. When the V. vulnificus and V. parahaemolyticus groups were removed, the number of phylogenetically informative sites was reduced to 156.
FIG. 2.
Neighbor-joining tree of selected strains of Vibrio. The numbers on the branches indicate the number of the nucleotide changes; terminal branch lengths were suppressed.
The cluster labeled Vibrio mimicus in Fig. 2 contains four strains that are sucrose negative (characteristic for V. mimicus) and a strain that is biochemically indistinguishable from V. cholerae (strain 8643). The cluster occurred 100% of the time in 1,000 bootstrap replicates. The cluster labeled V. parahaemolyticus in Fig. 2 contains one typical strain (9115) that is arabinose positive and sucrose, lactose, and citrate negative and contains two strains that are biochemically similar to V. cholerae (AS530 and AS555). AS530 and AS555 show considerable distance (100% in 1,000 bootstrap replicates) from the more typical V. parahaemolyticus strain and may be atypical V. parahaemolyticus strains, V. cholerae strains with atypical recA sequences, or a previously uncharacterized species of Vibrio. The cluster labeled ‘5’ in Fig. 2 contains five strains that are typical of V. cholerae, one Smith strain (6313) that is ornithine decarboxylase negative (V. cholerae is 99% positive [17]), and one strain differing from typical V. cholerae in three tests (AM25, which was indole negative and sorbitol and rhamnose positive). It was distinct 95% of the time in 1,000 bootstrap replicates from the rest of the cholera-causing strains. A final cluster containing only NAG-ST-producing Sakazaki serogroup O31 strains (A5 and NRT36S) also diverges from the main V. cholerae cluster (distinct in 97% of 1,000 bootstrap replicates). The presence of biochemically identified V. cholerae in these four clusters suggests that there is a substantial amount of genetic divergence within V. cholerae or that biochemical tests may be more variable within the species than previously recognized.
Our analysis indicates that several Smith-type strains are actually other species of Vibrio, reflecting the improvements in Vibrio taxonomy since the collection was first assembled. More than 90% of the V. cholerae strains diverge from each other by less than 10% of the sequence. Thus, the tree is shallow, and although some clusters are well supported by bootstrap analysis, potentially interesting subdivisions of this species cannot be unambiguously identified.
In keeping with previously reported studies utilizing other molecular typing techniques (4, 19, 25, 26), V. cholerae O1 El Tor isolates tended to cluster together. The cluster was distinct in 73% of 1,000 bootstrap replicates. While O139 Bengal strains generally fell within the El Tor cluster, there were several strains (an environmental strain from Argentina [Arg-3] and three nontoxigenic clinical isolates from Thailand [NG653/36, VO11627, and NG288/36], which were also atypical of El Tor by ribotyping [P. Echeverria, personal communication]) that were not within the El Tor clade. The observation that toxigenic O139 strains group with El Tor O1 strains is consistent with previous studies, suggesting that the Bengal O139 strain arose from a seventh-pandemic strain that had acquired new genes for O-antigen synthesis. The El Tor group did include four toxigenic Sakazaki serogroup O37 strains from Czechoslovakia and Sudan. In ribotyping studies the O37 strains showed slight divergence from both El Tor (seventh-pandemic) and classical (sixth-pandemic) clades (D. K. R. Karaolis, S. Sozhamannan, J. A. Johnson, and J. B. Kaper, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., abstr. B-179, p. 85, 1998). Analysis of genes associated with CTXφ and the Vibrio pathogenicity island (VPI) demonstrated variations unique to the O37 strains, suggesting that acquisition of these phages by strains within this serogroup occurred at a different points in time (Karaolis et al., Abstr. 98th Gen. Meet. Am. Soc. Microbiol.).
Separation of El Tor and classical clades is well supported by ribotyping, multilocus enzyme electrophoresis, PFGE, and sequencing of the asd, ctxA, and ctxB genes (6, 13, 26). However, we found that classical strains 395 and 569B formed a cluster with a Sakazaki O139 strain (498-7) and a Smith serogroup O333 strain (5011). In keeping with our results with V. cholerae O1 El Tor strain 1074-78, prior studies have demonstrated that environmental O1 strains generally do not fall within the sixth- or seventh-pandemic clades (13). Four clinical isolates from India (AM2, AM107, C0639, and C0545) within nonepidemic Sakazaki serogroups (i.e., not O1, O37, or O139), which have been shown in the laboratory to cause severe diarrhea in rabbits (S. Sozhamannan, unpublished data), had divergent recA sequences. One strain (AM2, Sakazaki serogroup O9) was identical to the El Tor strains, suggesting a common strain background. The other three (in Sakazaki serogroups O5, O11, and O144) had recA sequences that diverged widely from those of epidemic strains.
As suggested by the above observations, V. cholerae serogroup designations do not correlate with phylogeny, at least as manifested by recA sequence divergence. There is sufficient recA sequence divergence among strains within Sakazaki serogroups O1, O11, O39, O83, and O139 and Smith serogroup O175 for strains within a single serogroup to appear in different clades. The largest clade (El Tor) contains Sakazaki serogroups O1, O139, O37, and O9 and one strain for which we do not have Sakazaki typing data. The O139 pandemic strain is thought to have arisen by horizontal transfer when the biosynthesis genes for the O side chain of lipopolysaccharide O1 were replaced in an O1 El Tor strain. Most O139 strains are clonal, as indicated by molecular fingerprinting methods, such as ribotyping and PFGE. Several unusual nontoxigenic isolates included in this study fell in other clusters. One of these strains may have served as the source of the O139-specific DNA acquired by the Bengal strain. Our data, showing O139 strains in four distinct clades, raises the possibility that there were many such exchanges. Clustering of the O9 clinical isolate AM2 and the O37 toxigenic strains within the El Tor clade may reflect horizontal transfer of these other O side chain lipo-polysaccharide biosynthesis genes into the O1 El Tor strain background. While there is clearly a need for further analysis of sequence data from other loci, our observation of divergent recA sequences in each of a number of different serogroups (O1, O11, O37, O83, and O139) and the presence of strains from multiple serotypes (O1, O37, O139, and O9) with identical recA sequences supports the hypothesis that there is frequent horizontal transfer of genes associated with O-antigen synthesis among V. cholerae strains.
Nucleotide sequence accession numbers.
Individual sequences were entered into GenBank as accession no. AF301020 through AF301131.
REFERENCES
- 1.Aldova E, Laznickova K, Stepankova E, Lietva J. Isolation of nonagglutinable vibrios from an enteritis outbreak in Czechoslovakia. J Infect Dis. 1968;118:25–31. doi: 10.1093/infdis/118.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Arita M, Takeda T, Honda T, Miwatani T. Purification and characterization of Vibrio cholerae non-O1 heat-stable enterotoxin. Infect Immun. 1986;52:45–49. doi: 10.1128/iai.52.1.45-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagchi K, Echeverria P, Arthur J D, Sethabutr O, Serichantalergs O, Hoge C W. Epidemic of diarrhea caused by Vibrio cholerae non-O1 that produced heat-stable toxin among Khmers in a camp in Thailand. J Clin Microbiol. 1993;31:1315–1317. doi: 10.1128/jcm.31.5.1315-1317.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrán P, Delgado G, Navarro A, Trujillo F, Selander R K, Cravioto A. Genetic diversity and population structure of Vibrio cholerae. J Clin Microbiol. 1999;37:581–590. doi: 10.1128/jcm.37.3.581-590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bik E M, Bunschoten A E, Gouw R D, Mooi F R. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron D N, Khambaty F M, Wachsmuth I K, Tauxe R V, Barrett T J. Molecular characterization of Vibrio cholerae O1 strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1994;32:1685–1690. doi: 10.1128/jcm.32.7.1685-1690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements M L, Levine M M, Young C R, Black R E, Lim Y L, Robins-Browne R M, Craig J P. Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J Infect Dis. 1982;145:465–473. doi: 10.1093/infdis/145.4.465. [DOI] [PubMed] [Google Scholar]
- 8.Comstock L E, Johnson J A, Michalski J M, Morris J G, Jr, Kaper J B. Cloning and sequence of a region encoding surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol. 1996;19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x. [DOI] [PubMed] [Google Scholar]
- 9.Feil E, Zhou J, Maynard Smith J, Spratt B G. A comparison of the nucleotide sequences of the adk and recA genes of pathogenic and commensal Neisseria species: evidence for extensive interspecies recombination within adk. J Mol Evol. 1996;43:631–640. doi: 10.1007/BF02202111. [DOI] [PubMed] [Google Scholar]
- 10.Johnson J A, Salles C A, Panigrahi P, Albert M J, Wright A C, Johnson R J, Morris J G., Jr Vibrio cholerae O139 synonym Bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect Immun. 1994;62:2108–2110. doi: 10.1128/iai.62.5.2108-2110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaper J B, Moseley S L, Falkow S. Molecular characterization of environmental and nontoxigenic strains of Vibrio cholerae. Infect Immun. 1981;32:661–667. doi: 10.1128/iai.32.2.661-667.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaolis D K R, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karaolis D K R, Lan R, Reeves P R. The sixth and seventh cholera pandemics are due to independent clones separately derived from environmental, nontoxigenic, non-O1 Vibrio cholerae. J Bacteriol. 1995;177:3191–3198. doi: 10.1128/jb.177.11.3191-3198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karaolis D K R, Somara S, Maneval D R, Jr, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 15.Levine M M, Black R E, Clements M L, Cisneros L, Saah A, Nalin D R, Gill D M, Craig J P, Young C R, Ristaino P. The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J Infect Dis. 1982;145:296–299. doi: 10.1093/infdis/145.3.296. [DOI] [PubMed] [Google Scholar]
- 16.Maiden M C J, Bygraves J A, Feil E, Morelli G, Russell J E, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant D A, Feavers I M, Achtman M, Spratt B G. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin J C. Vibrio. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: ASM Press; 1995. pp. 465–476. [Google Scholar]
- 18.Morris J G, Jr, Takeda T, Tall B D, Losonsky G A, Bhattacharya S K, Forrest B D, Kay B A, Nishibuchi M. Experimental non-O group 1 Vibrio cholerae gastroenteritis in humans. J Clin Investig. 1990;85:697–705. doi: 10.1172/JCI114494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popovic T, Bopp C, Olsvik Ø, Wachsmuth K. Epidemiologic application of a standardized ribotype scheme for Vibrio cholerae O1. J Clin Microbiol. 1993;31:2474–2482. doi: 10.1128/jcm.31.9.2474-2482.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salles C A, Momen H. Identification of Vibrio cholerae by enzyme electrophoresis. Trans R Soc Trop Med. 1991;85:544–547. doi: 10.1016/0035-9203(91)90251-s. [DOI] [PubMed] [Google Scholar]
- 21.Shimada T, Arakawa E, Itoh K, Okitsu T, Matsushima A, Asai Y, Yamai S, Nakazato T, Nair G B, Albert M J, Takeda T. Extended serotyping scheme for Vibrio cholerae. Curr Microbiol. 1994;28:175–178. [Google Scholar]
- 22.Smith H L., Jr Serotyping of non-cholera vibrios. J Clin Microbiol. 1979;10:85–90. doi: 10.1128/jcm.10.1.85-90.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroeher U H, Parasivam G, Dredge B K, Manning P A. Novel Vibrio cholerae O139 genes involved in lipopolysaccharide biosynthesis. J Bacteriol. 1997;179:2740–2747. doi: 10.1128/jb.179.8.2740-2747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wachsmuth I K, Evins G M, Fields P I, Olsvik O, Popovic T, Bopp C A, Wells J G, Carrillo C, Blake P A. The molecular epidemiology of cholera in Latin America. J Infect Dis. 1993;167:621–626. doi: 10.1093/infdis/167.3.621. [DOI] [PubMed] [Google Scholar]
- 26.Wachsmuth K, Olsvik Ø, Evins G M, Popovic T. Molecular epidemiology of cholera. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C.: ASM Press; 1994. pp. 357–370. [Google Scholar]
- 27.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]