Abstract
Background
Antibiotic-impregnated calcium sulfate has excellent curative efficacy in chronic osteomyelitis. However, its curative efficacy in pediatric hematogenous osteomyelitis has not been sufficiently studied. The purpose of this study was to evaluate the curative effects of antibiotic-impregnated calcium sulfate in the treatment of pediatric hematogenous osteomyelitis.
Methods
Overall, twenty-one pediatric patients with hematogenous osteomyelitis treated at our hospital between 2013 and 2018 were included for assessment. The clinical history, clinical manifestation, infection recurrence rate, sinus leakage, incision leakage, pathological fractures, bone growth and surgical procedures were analyzed.
Results
The infection recurrence rate was 0% (0/21) at a minimum of 31 months (range 31 to 91 months) of follow-up. Postoperative incision leakage was found in one pediatric patient. Osteolysis was found in one pediatric patient. Acceleration of bone growth occurred in one pediatric patient. Retardation of bone growth occurred in one pediatric patient. Genu valgus deformity occurred in one pediatric patient.
Conclusions
Although noninfectious complications occurred, the curative effect of antibiotic-impregnated calcium sulfate in pediatric hematogenous osteomyelitis was satisfactory.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-022-03791-4.
Keywords: Hematogenous osteomyelitis, Pediatric, Calcium sulfate, Local antibiotic
Background
Osteomyelitis is an inflammatory disorder of bone caused by infection leading to necrosis and destruction [1]. The prevalence of osteomyelitis has increased in recent decades with a serious disease burden and socioeconomic impact [2]. Hematogenous osteomyelitis(HO) is a type of osteomyelitis in which bacteria reach bone through hematogenous seeding. The methods for avoiding disease progression are surgical intervention and drainage [3]. Surgical intervention includes decompression, excision of necrotic tissue, sequestrectomy, irrigation and aspiration [4]. Treating the bone loss caused by surgical intervention is a challenge for orthopedic surgeons. Polymethylmethacrylate (PMMA), a nonabsorbable bone filler, was reported to achieve satisfactory curative effects in chronic pediatric osteomyelitis when it was administered in combination with antibiotics into the areas of bone loss caused by surgical intervention [5]. However, there are disadvantages: PMMA is not biodegradable; the material does not allow bone regrowth; and in most cases, an additional surgical procedure is required for removal of the beads and subsequent bone grafting [6–8]. In contrast, calcium sulfate(CS) which can be impregnated with antibiotics, is osteoconductive and does not require a two stage procedure for removal; in addition, CS has been well characterized clinically as a bone void filler [9–12]. Tobramycin, vancomycin and gentamicin are common choices for CS loading [13]. Antibiotic-impregnated CS pellets have been applied in the treatment of pediatric HO and have been reported to yield satisfactory outcomes [14, 15]. As a supplement, the aim of this study was to share our experience with antibiotic-impregnated CS and its efficacy in the treatment of pediatric HO.
Methods
Study design and setting
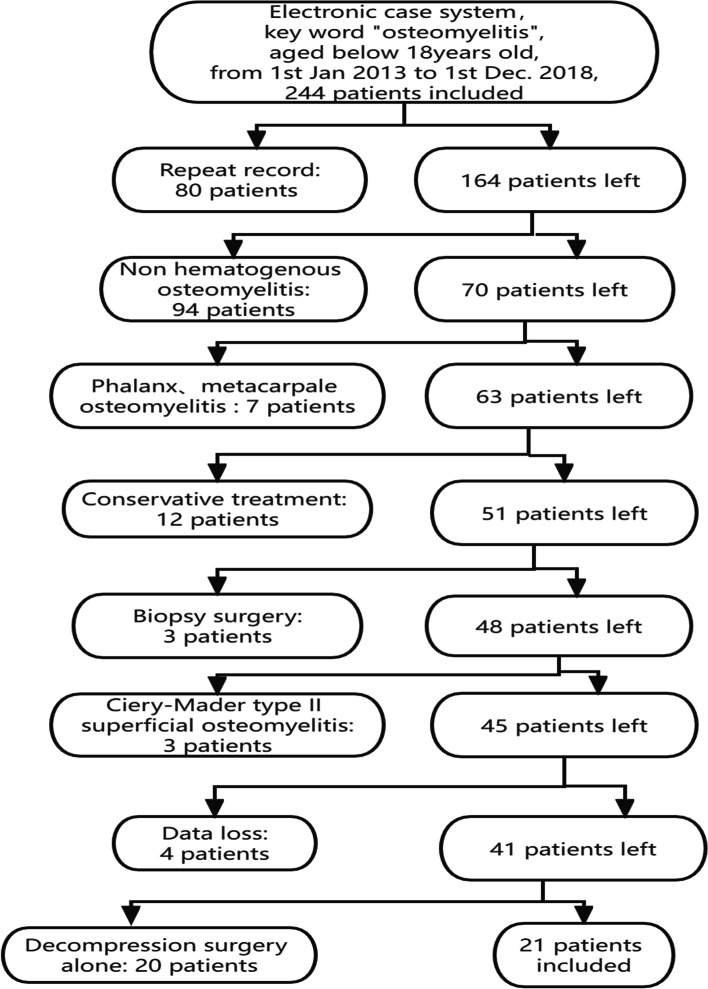
We recruited subjects by searching the electronic medical record system of Southern Medical University Nanfang Hospital using key words (Fig. 1). A case series study of pediatric HO patients treated at our hospital from January 2013 to December 2018 was performed. The main inclusion criteria were as follows: 1) pediatric patients less than 18 years old; 2) patients with HO who underwent surgical intervention and were treated with CS; and 3) patients who remained under follow-up monitoring for at least 24 months. The main exclusion criteria were as follows: 1) patients who suffered from direct spreading osteomyelitis, such as posttraumatic osteomyelitis, or infection after internal fixation; 2) patients who underwent soft tissue surgery and 3) patients who were lost to follow-up. The diagnosis of osteomyelitis was made according to Peltola and Vahvanen's definition [16] or a positive culture. The collected clinical data included demographics, clinical history, serum inflammatory indexes (white blood cell count (WBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level), etiology, imaging data, osteomyelitis site, operation process and follow-up duration.
Fig. 1.
Flow diagram of recruitment for the study
Descriptions of the experiment, treatment and surgery
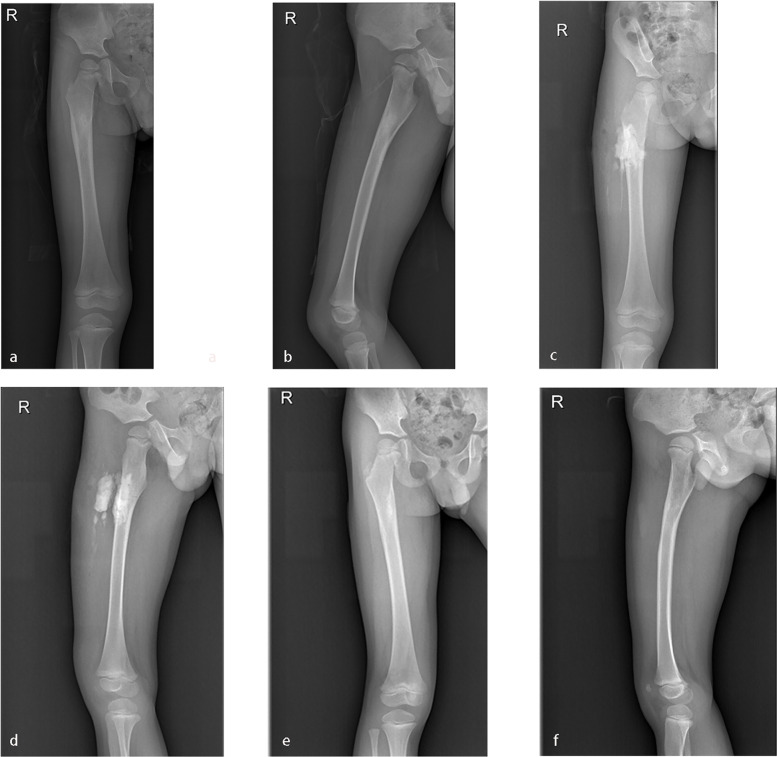
The surgical approach was guided by preoperative imaging and employed the safest, most direct route to debride all foci of infection. Deep soft-tissue fluid collections, abscesses and subperiosteal tissues were removed. In patients with long bone osteomyelitis, a cortical window was created at the epicenter of infection to enable access to the region. Intramedullary abscesses were debrided with a suitable curet, and the adjacent physis was carefully protected. In patients with calcaneus osteomyelitis, eggshell-like decompression technology [17] was used. The inside abscess was debrided with a suitable curet, and the adjacent physis was carefully protected. The lesions and/or abscesses were sent for biopsy and bacterial culture. Then antibiotic-impregnated CS was applied to fill the void and deliver local antibiotics. One gram vancomycin (or 160 mg of gentamicin or both) and 10 mL of CS were mixed thoroughly using the solvent provided by the manufacturer until a smooth paste was formed (approximately 30 s). The paste was allowed to cure undisturbed for at least 15 min after mixing. The volume of CS varied according to the size of the bone defect. The CS formulation (beads or block-shaped or both) was then placed into the defect as well as the superior-inferior medullary cavity (Fig. 2). The periosteum, subcutaneous tissue and skin were sutured in turn. A cast or external fixation was applied if the bone was unstable after decompression. Bone transport technology was applied in cases of extensive bone defects.
Fig. 2.
a-b: A three-year-old boy suffered right proximal femoral HO. c-d: Vancomycin impregnated CS was used to fill the void left by decompression and debridement. e–f: X-ray examination indicated good recovery 12 months postoperatively
Aftercare and Follow-up
All patients were routinely given intravenous antibiotics postoperatively. Cefmetazole 100 mg/kg/day was used in 14 patients. Clindamycin 30 mg/kg/day was used in 2 patients who were allergic to cefmetazole. Vancomycin 40 mg/kg/day was used in 5 patients when the pathogenic bacterium showed resistance to cefmetazole. Intravenous antibiotics were administered for a mean of 8.19 days, followed by the administration of oral antibiotics for approximately two weeks. The patients were released when their symptoms improved. The patients were regularly reviewed by the outpatient department or by telephone interview. Infection recurrence was defined as a worsening clinical symptom, continuously increasing serum inflammatory markers, or development of sinus tract and/or continuous bone tissue destruction on X-ray examination. We considered patients in remission of infection when there was an absence of clinical, laboratory, or radiological signs of infection as evaluated during the last medical visit (in patients with a minimum of two years of follow-up) and in patients in which there was no need for reoperation or administration of an extra course of antibiotic therapy at the same site of infection following the end of therapy.
Results
In all, thirteen males and eight females were included for assessment, and the average age was 10.14 years (range, 3–18 years). The site of onset was the femur in six patients, the tibia in five patients, the calcaneus in three patients, the fibula in three patients, the humerus in two patients, the radius in one patient, and the clavicle in one patient. The average time of onset was 7.74 months (range, 0.25–36 months). The average WBC count was 8.76*109/L (range, 6.03–12.07 *109/L). The average ESR was 44.14 mm/L (range, 26–70 mm/L). The average CRP was 12.35 mg/L (range, 1.03–35.04 mg/L). Thirteen patients were primary surgical pediatric patients. Eight patients had a surgical history at the site of onset (Tables 1 and 2).
Table 1.
Demographics and clinical outcome of primary operation patients
| Patient number | Age/Sex | Bone | Side | Time of onset (months) | History | Symptoms | WBC (103/μl) | ESR (mm/h) | CRP (mg/l) | Etiology/Type of specimen | Comorbidities | Local Antibiotic | Systemic antibiotic/time | Follow up duration (months) | Outcome of treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16/M | Femur | L | 6 | Topical application of traditional chinese medicine | Pain, Swelling, Redness | 15.35 | 40 | 51.8 | Negative Intraoperative specimen | - | Vancomycin | Cefmetazole/21 days | 55 | Cured, Leakage of the incision |
| 2 | 10/M | Calcaneus | R | 12 | - | Pain | 7.25 | 17 | 2.44 | Negative Intraoperative specimen | - | Vancomycin | Clindamycin/5 days | 31 | Cured |
| 3 | 12/M | Tibia | L | 0.25 | - | Pain, Swelling,Redness, Fever | 11.35 | 91 | 190 | Negative Intraoperative and blood specimen | - | Vancomycin | Cefmetazole/10 days | 54 | Cured |
| 4 | 13/M | Femur | L | 2 | Arthrocentesis Culture of articular puncture fluid: Staphylococcus aureus | Pain | 6.63 | 52 | 79.93 | S.S.S.S.aureus Intraoperative specimen | Suppurative arthritis of the hip | Vancomycin | Cefmetazole/ 17 days | 35 | Cured |
| 5 | 3/M | Femur | R | 8 | - | Limping | 13.49 | 20 | 37.5 | S. aureus Intraoperative specimen | - | Vancomycin | Clindamycin/3 days | 88 | Cured |
| 6 | 13/F | Femur | R | 12 | - | Pain, Fever | 8.04 | 40 | 18.66 | S. aureus Intraoperative specimen | - | Vancomycin | Cefmetazole/4 days | 35 | Cured |
| 7 | 5/F | Tibia | R | 1 | Topical application of traditional chinese medicine | Pain, Sinus | 14.36 | 70 | 18.5 | S. aureus Intraoperative specimen | - | Gentamycin | Cefmetazole/9 days | 91 | Cured |
| 8 | 15/M | Clavicle | R | 8 | - | Pain, Swelling | 7.54 | 6 | 1.05 | MRSA Intraoperative and pus puncture specimen | - | Vancomycin | Vancomycin/2 days | 43 | Cured |
| 9 | 18/M | Humerus | L | 36 | - | Pain | 8.37 | 5 | 1.65 | Negative Intraoperative specimen | - | Gentamycin | Cefmetazole/4 days | 42 | Cured |
| 10 | 10/M | Radius | L | 6 | - | Pain | 7.31 | 3 | 0 | Negative Intraoperative specimen | - | Vancomycin | Cefmetazole/6 days | 45 | Cured |
| 11 | 12/F | Fibula | L | 12 | Topical application of traditional chinese medicine | Pain, Sinus | 5.99 | 5 | 0.11 | MRSA Intraoperative and sinus specimen | - | Vancomycin | Vancomycin/5 days | 69 | Cured |
| 12 | 10/F | Fibula | L | 0.33 | Blood culture: MRSA | Pain, Fever | 21.78 | 102 | 48.77 | MRSA Intraoperative and blood specimen | Acute tonsillitis, Septicemia | Vancomycin | Vancomycin/14 days | 59 | Cured, Osteolysis |
| 13 | 10/M | Tibia | R | 1 | - | Pain | 7.54 | 23 | 1.78 | Negative Intraoperative specimen | G6PD Deficiency | Vancomycin | Cefmetazole/7says | 35 | Cured |
Abbreviation: WBC White Blood Cell count, ESR Erythrocyte Sedimentation Rate, CRP C-Reactive Protein, S aureus Staphylococcus aureus, MRSA Methicillin-resistant S.aureus, G6PD Glucose-6-phosphate Dehydrogenase
Table 2.
Demographics and clinical outcome of reoperation patients
| Patient number | Age/Sex | Bone | Side | Time of onset (months) | History | Symptoms | WBC (109/l) | ESR (mm/h) | CRP (mg/l) | Etiology/Type of specimen | Comorbidities | Local Antibiotic | Systemic antibiotic/time | Follow up duration (months) | Outcome of treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6/F | Fibula | L | 5 | Decompression surgery 5 months prior | Pain, Fever, | 8.01 | 44 | 3.66 | S. aureus Intraoperative specimen | - | Vancomycin + Gentamycin | Cefmetazole/6 days | 31 | Cured |
| 2 | 14/M | Humerus | R | 6 | Decompression surgery 6 months prior | Pain,Sinus | 13.64 | 92 | 87.18 | S. aureus Intraoperative specimen | - | Gentamycin | Cefmetazole/8 days | 58 | Cured |
| 3 | 15/M | Femur | L | 3 | Decompression surgery 1 month prior | Pain | 6.75 | 12 | 4.47 | aureus Intraoperative specimen | - | Vancomycin | Cefmetazole/8 days | 37 | Cured |
| 4 | 3/M | Calcaneus | R | 9 | Debridement surgery of soft tissue 9 months prior | Pain,Sinus | 7.72 | 38 | 28.2 | Negative Intraoperative specimen | - | Vancomycin | Cefmetazole/3 days | 36 | Cured |
| 5 | 6/F | Tibia | L | 5 | Multiple debridement surgery 4 months prior | Pain,Sinus | 8.49 | 41 | 4.81 | MRSA Intraoperative specimen | - | Vancomycin | Vancomycin/7 days | 40 |
Cured, Knee valgus deformity |
| 6 | 10/M | Calcaneus | L | 1 | Debridement surgery 7 days prior | Pain,Unclosed wound | 5.84 | 36 | 3.3 | cloaca Intraoperative specimen | - | Vancomycin | Cefmetazole/10 days | 53 | Cured |
| 7 | 5/F | Femur | R | 5 | Decompression surgery 5 months ago, pathological fracture 3 months prior | Pain, Swelling, Pathological fracture | 7.14 | 44 | 8.1 | MRSA Intraoperative specimen | - | Vancomycin | Vancomycin/12 days | 31 | Cured, Retardation of bone growth |
| 8 | 7/F | Tibia | L | 24 | Decompression surgery 12 months prior | Pain | 6.28 | 14 | 0.5 | Negative Intraoperative specimen | - | Vancomycin | Cefmetazole/11 days | 37 | Cured, Acceleration of bone growth |
Abbreviation: WBC White Blood Cell count, ESR Erythrocyte Sedimentation Rate, CRP C-Reactive Protein, S. aureus Staphylococcus aureus, E. cloacae Enterobacter cloacae, MRSA Methicillin-resistant S.aureus, G6PD Glucose-6-phosphate Dehydrogenase
This study included twenty-one patients with HO. The etiology was Staphylococcus aureus (S. aureus) in seven patients, Methicillin-resistant S.aureus (MRSA) in five patients, and Enterobacter cloacae (E. cloacae) in one patient, and the culture was negative in eight patients. The average follow-up was 47.9 months (range, 31–91 months). The infection recurrence rate was 0%(0/21) and infection eradication was achieved in all of the patients (Tables 1 and 2).
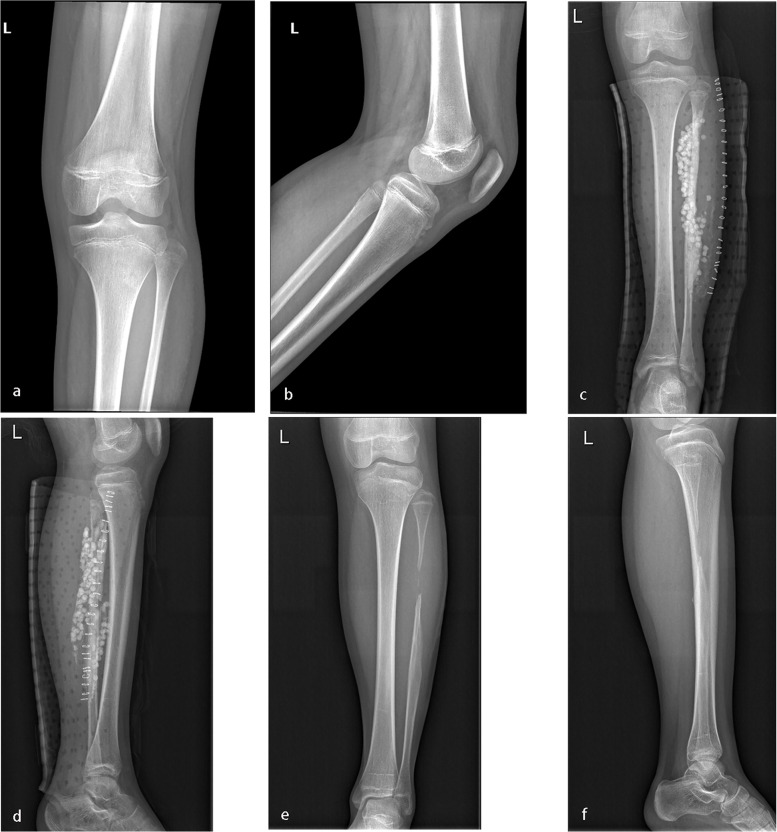
Thirteen patients underwent a primary operation. Among these patients, three had a history of traditional Chinese medicine usage. Of these three patients, two had a clinical manifestation of sinus leakage at the site of onset preoperatively, and one had incision leakage postoperatively (Table 1). The duration of incision leakage was approximately four weeks, at which point the leakage disappeared. One patient with fibular osteomyelitis who underwent the surgical procedures was discovered to have osteolysis at a followed-up visit (Fig. 3). None of the patients had pain or walking or adjacent joint activity limitations. All of the patients had a daily life comparable with that of their normal peers.
Fig. 3.
a-b: A ten-year-old girl suffered left proximal fibular HO. c-d: Vancomycin impregnated CS was used to fill the void left by decompression and debridement. e–f: X-ray examination indicated osteolysis 18 months postoperatively
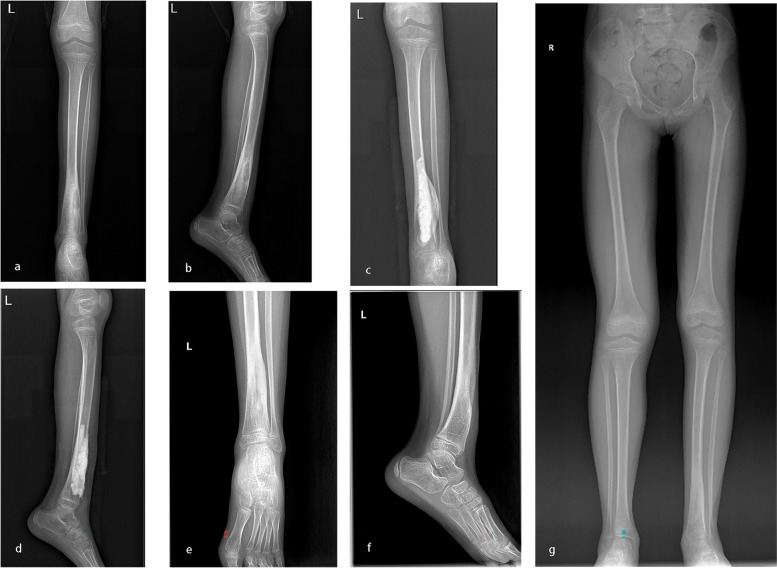
Eight patients had a history of decompression or debridement at the site of onset. Among these patients, five had a serious clinical manifestation: three had sinus leakage, one had an unclosed wound and one had a pathological fracture (Table 2). Moreover, among these patients, three had serious complications: one with tibial osteomyelitis who underwent the surgical procedures experienced acceleration of bone growth as a complication (Fig. 4), one with femoral osteomyelitis who underwent the surgical procedures and bone transport experienced retardation of bone growth (Fig. 5), and one with tibial osteomyelitis who underwent the surgical procedures and bone transport developed genu valgus deformity (Fig. 6). These three patients needed to walk with a crutch to avoid bear load in the first month removing the external fixation. Their myodynamia and motion of adjacent joints were slightly limited in the initial three months after removing the external fixation. After six months removing the external fixation, they had no pain and no walking limitations with appropriate treatment, such as heightened insoles and knee braces. The remaining patients had no pain nor walking nor adjacent joint activity limitations and had a daily life comparable to that of their normal peers.
Fig. 4.
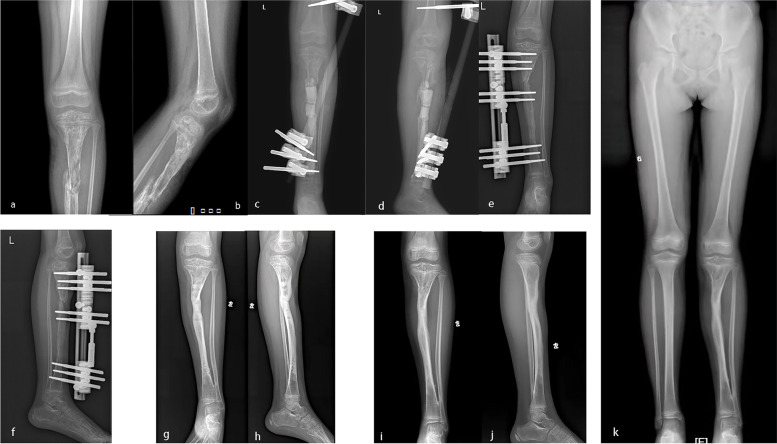
a-b: A seven-year-old girl suffered left distal tibial HO. c-d: Vancomycin impregnated CS was used to fill the void left by decompression and debridement. e–g: X-ray examination indicated acceleration of bone growth 12 months postoperatively
Fig. 5.
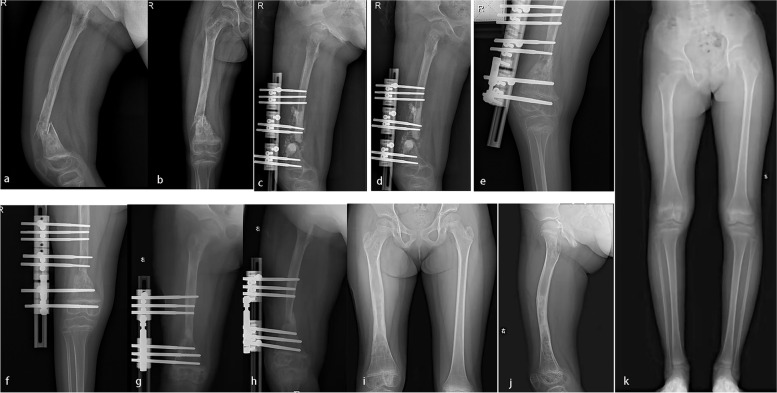
a-b: A five-year-old girl suffered right distal femoral HO and pathological fracture. c-d: Vancomycin impregnated CS was used to fill the void left by decompression and debridement, and the bone transport technique was applied. e–f: Bone transport was finished 3 months postoperatively. g-h: Removing of the distal screw and adjusting the external fixation device. i-k: X-ray examination indicated retardation of bone growth and lower limb discrepancy 19 months postoperatively
Fig. 6.
a-b: A six-year-old girl had suffered left proximal tibia HO. c-d: Vancomycin impregnated CS was placed in the void spaces left by decompression and debridement. The tibia was fixed by a temporary external fixator. e–f: A terminal unilateral external fixator was used for bone transport. Bone transport was finished 9 months postoperatively. g-h: External fixation was removed 18 months postoperatively. i-k: The latest X-ray was taken at 34 months postoperatively. The girl showed genu valgus deformity
Discussion
HO is a type of pediatric musculoskeletal infection. The strategies for avoiding disease progression are surgical intervention and drainage [3]. HO is the most difficult condition to understand in the realm of pediatric musculoskeletal infection and continues to present a significant clinical challenge [18]. Copley et al. [4] presented a surgical approach consisting of bone decompression and curettage of intramedullary abscesses in 2009. However, they did not describe filling of the void left by surgery. Bar-On et al. [5] reported the successful treatment of four patients with chronic pediatric osteomyelitis with intramedullary reaming and antibiotic-impregnated PMMA in 2010. This was the first report of a nonabsorbable local antibiotic release system applied in pediatric osteomyelitis. Antonio Andreacchio et al. [14] reported the successful treatment of twelve pediatric patients with chronic osteomyelitis by debridement and filling with antibiotic-impregnated CS in 2019. This was the first report of an absorbable local antibiotic release system applied in pediatric osteomyelitis. Vikas Ellur et al. [15] reported the successful treatment of thirty-four pediatric patients with chronic osteomyelitis by debridement and filling with antibiotic-impregnated CS in 2021. In our study, we report the successful treatment of twenty-one pediatric patients with HO by decompression, debridement and filling with antibiotic-impregnated CS. All of the patients were infected via the hematologic route. Our study is a supplement to these previous studies and presents a deeper understanding of the application of CS in HO.
Masquelet [19] proposed a two-step operation technique for the treatment of chronic osteomyelitis. The first step is sharp debridement of necrotic tissue, abundant lavage, sequestrectomy, and removal of any hardware and/or foreign bodies, followed by delivery of antibiotic-impregnated PMMA. The second step is an additional surgical procedure required for removal of the beads and subsequent bone grafting. This technique can be used in cases of initial infection and to control established infection before final bone reconstruction. Although the application of antibiotic-impregnated PMMA has shown an excellent curative effect in the eradication of infection, its deficiencies have been obvious. First, PMMA has the potential to serve as a reservoir for recurrent infection if left in the void past the period of effective antibiotic elution due to its nonbiodegradable nature [20, 21]. Second, PMMA does not contribute to bone regrowth and requires an additional surgical procedure to be removed. This second procedure delays the healing process and increases the cost [22]. Additionally, other disadvantages include the increased risk of the development of antibiotic-resistant organisms and decreased immune function of the host with PMMA application [20, 23, 24]. Moreover, donor site complications such as instability, infection and fatigue fractures occur when autogenous iliac bone grafting is applied [22]. Therefore, CS, as an absorbable material, has drawn clinical attention. Antibiotic-impregnated CS has unique advantages. First, it exhibits characteristic steady and gradual resorption, it is osteoconductive, and it does not require an additional surgical procedure to be removed [25–27]. Second, antibiotic-impregnated CS has been found to be osteoinductive, suggesting that it can induce the differentiation of bone marrow mesenchymal stem cells into osteoblasts [28]. Additionally, antibiotic-impregnated CS has been found to induce the formation of a vascularized membrane that can prevent graft resorption and create a favorable microenvironment for vascularization and corticalization, thus promoting bone formation [29]. In our study, the infection was eradicated in all 21 patients and none of them required additional surgery. These results confirmed the curative effect of antibiotic-impregnated CS in eradicating the infection.
Although any water-soluble antibiotic can be incorporated into CS, the ideal antibiotic remains controversial. Vancomycin, gentamicin and tobramycin were the common choices for CS loading. In a vitro experiment, vancomycin and tobramycin impregnated materials had similar germicidal properties and elution efficiency [11]. Furthermore, a systematic review indicated that the choice of tobramycin-loaded CS or vancomycin combined with gentamicin-loaded CS did not affect the eradication rate and the incidence of postoperative complications in chronic osteomyelitis patients [13]. The choice of a local antibiotic depends on the local epidemiological data in patients who have no accurate bacterial data. We chose vancomycin empirically for gram-positive patients or patients who were highly suspected to have S.aureus infection and gentamicin for gram-negative patients. We chose vancomycin and gentamicin together empirically for patients with hard-to-identify bacterial infections. Compared with long-term intravenous antibiotics, local antibiotics have unique advantages. One advantage is the higher and more effective concentrations that can be obtained in the local area of infected bone through local antibiotic applications over a prolonged period of time. Another is the prevention of adverse events related to systemic chemotherapy and the reduced risk of systemic toxicity [30]. Zhang et al. [31] measured the blood vancomycin levels of 24 osteomyelitis patients locally treated with vancomycin-impregnated CS beads (a dose ranging from 1.5 ml to 5 ml at a ratio of 1 g of vancomycin: 5 ml of calcium sulfate). The results showed that the mean blood vancomycin level was still within a safe range for application. P. Wahl et al. [32] found that when 6 g of vancomycin was applied locally, the systemic concentration remained within a safe range, and the local concentration was still below the reported cellular toxicity thresholds. In our study, intravenous and oral antibiotics were administered to patients over the short term. Adverse events related to systemic chemotherapy and systemic toxicity did not occur.
Ping-chung Leung et al. [33] reported that the topical application of traditional Chinese medicine presented satisfactory results in plantar fasciitis, nonundisplaced metatarsal fracture, Tennis elbow and de-Quervain's disease. The authors indicated that pain relief and inflammation control were the advantages of topical traditional Chinese medicine administration. However, the authors did not describe the condition of the skin. In our study, three patients with no history of surgery were treated with topical traditional Chinese medicine by their parents. Among these patients, two developed sinus leakage preoperatively and one of them had incision leakage postoperatively. Aseptic exudation is one of the disadvantages of CS [30, 34]. A commonly reported observation associated with the surgical use of CS is fluid discharge from the wound/surgical site, occurring in 4% to 51% of patients [11, 22, 35]. The reported duration of fluid discharge is variable, ranging from 2 to 24 weeks [35]. In our study, the duration of fluid discharge was approximately four weeks. We hypothesize that the application of topical traditional Chinese medicine may damage the skin and increase the risk of sinus and aseptic exudation caused by CS. How to prevent aseptic exudation after antibiotic-impregnated CS use is an urgent problem to be solved. In view of this, we present some advice from our own experience: 1. prevent the CS from becoming too wet; 2. place the CS in an area rich with soft tissue; and 3. do not use topical traditional Chinese medicine.
Osteolysis is the most frequent complication of total joint arthroplasty and internal fixation [36, 37]. The mechanisms underlying osteolysis mainly include the following: 1. inflammation caused by inflammatory stimuli; 2. inflammation caused by stimulation of innate immune receptors; 3. regulation of debris-induced inflammation; and 4. inflammation-associated bone resorption [37]. The treatment of osteolysis consists of bone grafting using either bone allografts or bone-graft substitutes (such as various osteoconductive or osteoinductive materials) or combinations thereof [38]. CS itself was developed as a bone-graft substitute that can cure osteolysis. However, in our study, 1 patient treated with CS developed osteolysis (Fig. 3). We speculate that there may be two reasons for this outcome: 1. stimulation caused by bone decompression and debridement; and 2. stimulation caused by the vancomycin-impregnated CS.
The disease of patients who had a history of decompression or debridement at the site of onset was more serious than that of patients who had no history of surgery. In our study, more serious clinical manifestations, such as sinus leakage, unclosed wounds, and pathological fractures, occurred in 62.5% (5/8) of these patients. We do not know how decompression or debridement surgery proceeded in these patients at other hospitals. However, we know that antibiotic-impregnated CS was not applied to the void left by decompression or debridement in these patients. We estimated that failure to apply antibiotic-impregnated CS was a contributing factor of the serious clinical manifestations.
Bone growth has been found to be affected by systemic and local hormonal pathways and mechanical loading [39]. In our study, two patients had experienced abnormal bone growth as a serious complication. One patient experienced acceleration of bone growth, and the other experienced retardation of bone growth (Figs. 4 and 5). We believe that the number of surgeries and the stimulation by CS may have resulted in abnormally accelerated or retarded bone growth.
The bone transport technique refers to the production of new bone between vascular bone surfaces created by an osteotomy and separated by gradual distraction [40]. In our study, two patients who had large bone defects left by surgical intervention underwent treatment with this technique (Figs. 5 and 6). These two patients had a history of decompression or debridement at the site of onset without the application of antibiotic-impregnated CS. As a result, one of them developed genu valgus deformity as a complication. Fortunately, these patients had no pain and no limitations in walking or squatting.
Limitations
This study had a number of limitations. First, this study was not a retrospective control study, and comparisons were not performed to confirm the results. Second, this study was a small-sample retrospective study, and only twenty-one patients were included. However, the 100% infection eradication rate and the rarity of complications are information that can supplement the findings of existing studies. We expect large-sample randomized controlled trials to confirm our findings.
Conclusions
Although noninfectious complications occurred, such as incision leakage, osteolysis, and acceleration or retardation of bone growth, the curative effect of antibiotic-impregnated CS in pediatricHO was satisfactory.
Supplementary Information
Acknowledgements
We would like to thank all the people who helped us with the current study.
Authors’ contributions
RT, JW and JL contributed equally to this work. Scientific idea: RT, JW, JL and CQ. Project planning: RT, JW, JL, LH, CZ, GC snd CQ. Date collection: RT, JW, JL, LH, CZ and GC. Manuscript writing: RT, JW, JL, LH, CZ and GC. All authors have read and approved the final manuscript.
Funding
This study was not externally founded.
Availability of data and materials
The data supporting our findings come from Southern Medical University Nanfang Hospital. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Medical Ethics Committee of Nanfang Hospital of Southern Medical University has approved this research. Human participants: All informed consent was obtained from all subjects and/or their legal guardian(s). Information or images that could lead to identification of a study participant: Not applicable. All procedures were conducted according to the Declaration of Helsinki.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rui Tao, Email: doctortaorui@hotmail.com.
Jian-qun Wu, Email: wjq2627@126.com.
Ji-wei Luo, Email: rui04@126.com.
Liang Hong, Email: 13565409@qq.com.
Chun-hao Zhou, Email: 1204723822@qq.com.
Guo-yun Cheng, Email: 648632698@qq.com.
Cheng-he Qin, Email: orthoqin@163.com.
References
- 1.Rao N, Ziran BH, Lipsky BA. Treating Osteomyelitis: Antibiotics and Surgery. Plast Reconstr Surg. 2011;127:177S–187S. doi: 10.1097/PRS.0b013e3182001f0f. [DOI] [PubMed] [Google Scholar]
- 2.Walter N, Baertl S, Alt V, Rupp M. What is the burden of osteomyelitis in Germany? an analysis of inpatient data from 2008 through 2018. BMC Infect Dis. 2021;21(1):550. doi: 10.1186/s12879-021-06274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danielsson LG, Düppe H. Acute hematogenous osteomyelitis of the neck of the femur in children treated with drilling. Acta Orthop Scand. 2002;73(3):311–316. doi: 10.1080/000164702320155310. [DOI] [PubMed] [Google Scholar]
- 4.Copley LAB. Pediatric Musculoskeletal Infection: Trends and Antibiotic Recommendations. J Am Acad Orthop Sur. 2009;17(10):618–626. doi: 10.5435/00124635-200910000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bar-On E, Weigl DM, Bor N, Becker T, Katz K, Mercado E, Livni G. Chronic osteomyelitis in children: treatment by intramedullary reaming and antibiotic-impregnated cement rods. J Pediatr Orthop. 2010;30(5):508–513. doi: 10.1097/BPO.0b013e3181e00e34. [DOI] [PubMed] [Google Scholar]
- 6.Buchholz HW, Elson RA, Heinert K. Antibiotic-loaded acrylic cement: current concepts. Clin Orthop Relat Res. 1984;190:96–108. doi: 10.1097/00003086-198411000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Patzakis MJ, Mazur K, Wilkins J, Sherman R, Holtom P. Septopal beads and autogenous bone grafting for bone defects in patients with chronic osteomyelitis. Clin Orthop Relat Res. 1993;295:112–118. doi: 10.1097/00003086-199310000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Scott CP, Higham PA, Dumbleton JH. Effectiveness of bone cement containing tobramycin. an in vitro susceptibility study of 99 organisms found in infected joint arthroplasty. J Bone Joint Surg Br . 1999;81(3):440–443. doi: 10.1302/0301-620X.81B3.0810440. [DOI] [PubMed] [Google Scholar]
- 9.Beardmore AA, Brooks DE, Wenke JC, Thomas DB. Effectiveness of local antibiotic delivery with an osteoinductive and osteoconductive bone-graft substitute. J Bone Joint Surg Am. 2005;87(1):107–112. doi: 10.2106/JBJS.C.01670. [DOI] [PubMed] [Google Scholar]
- 10.Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg (Hong Kong) 2002;10(1):53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]
- 11.McKee MD, Wild LM, Schemitsch EH, Waddell JP. The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: early results of a prospective trial. J Orthop Trauma. 2002;16(9):622–627. doi: 10.1097/00005131-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Nelson CL, McLaren SG, Skinner RA, Smeltzer MS, Thomas JR, Olsen KM. The treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pellets. J Orthop Res. 2002;20(4):643–647. doi: 10.1016/S0736-0266(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 13.Shi X, Wu Y, Ni H, Li M, Zhang C, Qi B, Wei M, Wang T, Xu Y. Antibiotic-loaded calcium sulfate in clinical treatment of chronic osteomyelitis: a systematic review and meta-analysis. J Orthop Surg Res. 2022;17(1):104. doi: 10.1186/s13018-022-02980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreacchio A, Alberghina F, Paonessa M, Cravino M, De Rosa V, Canavese F. Tobramycin-impregnated calcium sulfate pellets for the treatment of chronic osteomyelitis in children and adolescents. Journal of Pediatric Orthopaedics B. 2019;28(3):189–195. doi: 10.1097/BPB.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 15.Ellur V, Kumar G, Sampath JS. Treatment of chronic hematogenous osteomyelitis in children with antibiotic impregnated calcium sulphate. J Pediatr Orthoped. 2021;41(2):127–131. doi: 10.1097/BPO.0000000000001723. [DOI] [PubMed] [Google Scholar]
- 16.Peltola H, Vahvanen V. A comparative study of osteomyelitis and purulent arthritis with special reference to aetiology and recovery. Infection. 1984;12(2):75–79. doi: 10.1007/BF01641675. [DOI] [PubMed] [Google Scholar]
- 17.Qin C, Zhou C, Ren Y, Cheng G, Zhang H, Fang J, Tao R. Extensive eggshell-like debridement technique plus antibiotic-loaded calcium sulphate for one-stage treatment of chronic calcaneal osteomyelitis. Foot Ankle Surg. 2020;26(6):644–649. doi: 10.1016/j.fas.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Funk SS, Copley LAB. Acute Hematogenous Osteomyelitis in Children. Orthop Clin N Am. 2017;48(2):199–208. doi: 10.1016/j.ocl.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Azi ML, de Almeida Teixeira AA, Cotias RB, Joeris A, Kfuri M. Induced-Membrane Technique in the Management of Posttraumatic Bone Defects. JBJS Essential Surgical Techniques. 2019;9(2):e21–e22. doi: 10.2106/JBJS.ST.18.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowyer GW, Cumberland N. Antibiotic release from impregnated pellets and beads. J Trauma. 1994;36(3):331–335. doi: 10.1097/00005373-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Kanellakopoulou K, Giamarellos-Bourboulis EJ. Carrier systems for the local delivery of antibiotics in bone infections. Drugs. 2000;59(6):1223–1232. doi: 10.2165/00003495-200059060-00003. [DOI] [PubMed] [Google Scholar]
- 22.McKee MD, Li-Bland EA, Wild LM, Schemitsch EH. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion. J Orthop Trauma. 2010;24(8):483–490. doi: 10.1097/BOT.0b013e3181df91d9. [DOI] [PubMed] [Google Scholar]
- 23.Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res. 1992;278:244–252. doi: 10.1097/00003086-199205000-00037. [DOI] [PubMed] [Google Scholar]
- 24.Granchi D, Ciapetti G, Savarino L, Cenni E, Pizzoferrato A, Baldini N, Giunti A. Effects of bone cement extracts on the cell-mediated immune response. Biomaterials. 2002;23(4):1033–1041. doi: 10.1016/S0142-9612(01)00215-0. [DOI] [PubMed] [Google Scholar]
- 25.Borrelli JJ, Prickett WD, Ricci WM. Treatment of nonunions and osseous defects with bone graft and calcium sulfate. Clin Orthop Relat Res. 2003;411:245–254. doi: 10.1097/01.blo.0000069893.31220.6f. [DOI] [PubMed] [Google Scholar]
- 26.Dahners LE, Funderburk CH Gentamicin-loaded plaster of Paris as a treatment of experimental osteomyelitis in rabbits. Clin Orthop Relat Res. 1987;219:278–282. [PubMed] [Google Scholar]
- 27.Urban RM, Turner TM, Hall DJ, Infanger S, Cheema N, Lim TH. Healing of large defects treated with calcium sulfate pellets containing demineralized bone matrix particles. Orthopedics. 2003;26(5 Suppl):s581–s585. doi: 10.3928/0147-7447-20030502-11. [DOI] [PubMed] [Google Scholar]
- 28.Kim YK, Lee JY, Kim SG, Lim SC. Guided bone regeneration using demineralized allogenic bone matrix with calcium sulfate: case series. J Adv Prosthodont. 2013;5(2):167–171. doi: 10.4047/jap.2013.5.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma YF, Jiang N, Zhang X, Qin CH, Wang L, Hu YJ, Lin QR, Yu B, Wang BW. Calcium sulfate induced versus PMMA-induced membrane in a critical-sized femoral defect in a rat model. Sci Rep. 2018;8(1):637. doi: 10.1038/s41598-017-17430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauschmann MA, Wichelhaus TA, Stirnal V, Dingeldein E, Zichner L, Schnettler R, Alt V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26(15):2677–2684. doi: 10.1016/j.biomaterials.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 31.Menon A, Soman R, Rodrigues C, Phadke S, Agashe VM. Careful interpretation of the wound status is needed with use of antibiotic impregnated biodegradable synthetic pure calcium sulfate beads: Series of 39 cases. J Bone Jt Infect. 2018;3(2):87–93. doi: 10.7150/jbji.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lum ZC, Pereira GC. Local bio-absorbable antibiotic delivery in calcium sulfate beads in hip and knee arthroplasty. J Orthop. 2018;15(2):676–678. doi: 10.1016/j.jor.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung P, Ko EC, Siu W, Pang ES, Lau CB. Selected topical agents used in traditional Chinese medicine in the treatment of minor injuries- a review. Front Pharmacol. 2016;7:16. doi: 10.3389/fphar.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helgeson MD, Potter BK, Tucker CJ, Frisch HM, Shawen SB. Antibiotic-impregnated calcium sulfate use in combat-related open fractures. Orthopedics. 2009;32(5):323. doi: 10.3928/01477447-20090501-03. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson JY, Dudareva M, Riley ND, Stubbs D, Atkins BL, McNally MA: The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. Bone Joint J 2014, 96-B(6):829–836. [DOI] [PubMed]
- 36.Joaquim AF, Lee NJ, Lehman RA, Tumialán LM, Riew KD. Osteolysis after cervical disc arthroplasty. EUR SPINE J. 2020;29(11):2723–2733. doi: 10.1007/s00586-020-06578-2. [DOI] [PubMed] [Google Scholar]
- 37.Goodman SB, Gallo J. Periprosthetic osteolysis: mechanisms, prevention and treatment. J Clin Med. 2019;8(12):2091. doi: 10.3390/jcm8122091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheth NP, Rozell JC, Paprosky WG. Evaluation and treatment of patients with acetabular osteolysis after total hip arthroplasty. J Am Acad Orthop Surg. 2019;27(6):e258–e267. doi: 10.5435/JAAOS-D-16-00685. [DOI] [PubMed] [Google Scholar]
- 39.Gkiatas I, Lykissas M, Kostas-Agnantis I, Korompilias A, Batistatou A, Beris A. Factors affecting bone growth. Am J Orthop (Belle Mead NJ) 2015;44(2):61–67. [PubMed] [Google Scholar]
- 40.ARONSON J: Current Concepts Review - Limb-Lengthening, Skeletal Reconstruction, and Bone Transport with the Ilizarov Method. J Bone JT Surg. American volume 1997, 79(8):1243–1258. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting our findings come from Southern Medical University Nanfang Hospital. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.