Abstract
Background
The risk of hypertensive disorders of pregnancy (HDP) varies in women with gestational diabetes mellitus (GDM), depending on the degree of insulin resistance and is also influenced by obesity. The aim of this study was to evaluate clinical features, blood pressure (BP) profiles and inflammatory markers, to identify patients with an elevated risk of developing HDP.
Methods
A total of 146 normotensive pregnant women were studied. We analysed the relationships of BP profiles detected by ambulatory blood pressure monitoring (ABPM) with serum biomarkers and angiogenic factors and their association with the development of HDP.
Results
Fourteen (9.6%) women developed HDP, of which 11 had GDM and 8 had obesity. Women with HDP had higher values of 24-h and daytime systolic/diastolic BP (113/69 vs. 104/64; 115/72 vs. 106/66 mmHg, respectively; p < 0.05). Higher levels of leptin (10.97 ± 0.82 vs. 10.2 ± 1.11; p = 0.018) andmonocyte chemoattractant protein-1 (MCP-1) (5.24 ± 0.60 vs. 4.9 ± 0.55; p = 0.044) and a higher soluble fms-like tyrosine kinase-1/placental growth factor (sFlt-1/PlGF) ratio (4.37 ± 2.2 vs. 2.2 ± 1.43; p = 0.003) were also observed in the HDP patients. Multivariate analysis showed that a higher sFlt-1/PlGF ratio was associated with an increased risk of developing HDP [OR = 2.02; IC 95%: 1.35–3.05]. Furthermore, higher daytime systolic BP [OR = 1.27; IC 95% 1.00–1.26] and prepregnancy body mass index (BMI) [OR = 1.14; IC 95%: 1.01–1.30] significantly increased the risk of developing HDP.
Conclusions
Higher daytime systolic BP values, prepregnancy BMI and the sFlt-1/PlGF ratio are useful for identifying normotensive pregnant women with an increased risk of developing HDP.
Keywords: Ambulatory blood pressure monitoring, Predictor, Hypertensive disorders of pregnancy, Cytokine profile; sFlt-1/PIGF ratio
Background
Gestational diabetes mellitus (GDM) and hypertensive disorders of pregnancy (HDP) are two pathologies that most frequently complicate pregnancies, and imply an increase in maternal and neonatal morbidity [1, 2] and an increased risk of developing cardiovascular disease [1, 3, 4]. Both conditions share risk factors and are related to each other, as HDP (as gestational hypertension and preeclampsia) is 1.5–2 times more common in women with GDM [5, 6], and an increased risk of developing GDM has been described in women with history of preeclampsia in previous pregnancies [7].
Several studies have proposed that both clinical conditions share – at least partly – some pathophysiological mechanisms in which insulin resistance may play a key role [8]. In addition, an increased proinflammatory state associated with HDP [9, 10] and GDM [11, 12] has been described, and in that respect, our group has observed an angiogenic imbalance, characterized by an increased soluble fms-like tyrosine kinase–1/placental growth factor (sFlt-1/PlGF) ratio, as a valid predictor of the development of HDP in women with GDM, as well as its association with obstetric and perinatal complications [13].
Ambulatory blood pressure monitoring (ABPM) provides a larger number of blood pressure (BP) measurements, allows assessment of the circadian rhythm and detects BP alterations that correlate with target organ involvement and cardiovascular morbidity and mortality in the general population [14]. In pregnancy, previous works have supported the use of ABPM to detect subclinical changes in BP patterns (BP changes/alterations that can only be detected by ABPM) in pregnant women who are at higher risk of developing HDP [15–17]. Importantly, we recently published a study reporting that high nocturnal systolic BP levels are related to the development of HDP in pregnant women with GDM and obesity [18]. In addition, these alterations were found to be related to poor obstetric and perinatal outcomes.
While the interaction between endothelial damage, insulin resistance, and the development of HDP has been reported [19–21], the complex relationship between proinflammatory cytokines and subclinical BP alterations in pregnant women with GDM has not been demonstrated. The aim of this study was to evaluate the relationship between inflammatory markers and BP profiles as measured by ABPM, in normotensive women with and without GDM to identify those with an elevated risk of developing HDP.
Methods
A prospective observational study was performed by recruiting normotensive pregnant women who were selected consecutively from the Endocrinologist and Obstetric clinic of the Puerta del Mar University Hospital (Cádiz, Spain). The inclusion criteria were as follows: women with singleton physiological pregnancy and normal BP at the time of enrolement (ambulatory systolic BP ≤ 130 mmHg and diastolic BP ≤ 80 mmHg). The exclusion criteria were as follows: women with chronic hypertension or receiving antihypertension medication; a diagnosis of pregestational diabetes, morbid obesity (defined by body mass index (BMI) ≥ 40 kg/m2), or placental insufficiency, and the presence of concomitant systemic disease or smoking.
Procedure
The study was approved by the Hospital Research Ethics Board of Puerta del Mar Hospital (code number 1507-N-16) following the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants.
At the time of inclusion in the study, maternal clinical data were recorded: family history of hypertension, age, pregestational weight and BMI, presence of obesity (defined as having a prepregnancy BMI ≥ 30 kg/m2), diagnosis of GDM, obstetric history, parity and history of HDP or GDM in a previous pregnancy. Pregnant women at high risk of preeclampsia were treated with 100 mg of aspirin daily from 12 until 37 weeks according to our hospital protocol based on NICE guidelines [22]: presence of any high-risk factors, including: hypertensive disease during a previous pregnancy, chronic kidney disease, autoimmune disease such as systemic lupus erythematosus or antiphospholipid syndrome, type 1 or type 2 diabetes and chronic hypertension, or presence of more than one moderate risk factor, including first pregnancy, age of 40 years or older, pregnancy interval of more than 10 years, BMI > 35 kg/m2 at first visit, family history of preeclampsia, and multi-fetal pregnancy. The diagnosis of GDM was established using a two-step approach according to the criteria of the National Diabetes Data Group [23]: in all pregnant women between 24 and 28 weeks of gestation a screening test was performed with a 50 g glucose test. Women with a positive screening test (1-h blood glucose > 7.8 mmol/L) underwent a confirmatory 3-h, 100 g oral glucose tolerance test (OGTT). GDM was diagnosed with abnormally high values of two of the following thresholds: fasting glucose, 5.8 mmol/L; 1-h, 10.5 mmol/L; 2-h, 9.1 mmol/L; 3-h, 8.0 mmol. All women with GDM received complex dietary counselling at diagnosis, which consisted of a high-protein diet (at least 1.1 g protein/kg per day) with a caloric intake between 25 and 35 kcal/kg of b.w. per day adjusted for pregestational BMI. If glucose targets were not achieved (fasting glucose < 5.3 mmol/L and postprandial glucose at 1 h < 7.8 mmol/L), insulin therapy was initiated.
During the third trimester of pregnancy, pregnant women were scheduled for a first and single visit between 28 and 32 weeks at 08:30–09:30. An interview and physical examination were carried out, fasting blood samples were collected, BP was measured and 24-h ABPM was performed. After delivery, obstetric and perinatal data were retrospectively reviewed.
Blood pressure measurements
The conventional office BP was measured on the nondominant arm with an automated BP monitor (Omron HEM-7200-E (Kyoto, Japan)) in a sitting position. The measurement was performed twice on the same day and before ABPM was undertaken. For the 24-h recordings, the Spacelabs 90,207 monitor (Spacelabs, Redmond,WA, USA) was used. The monitor was programmed to perform the measurements every 20 min during the day and every 30 min at night; daytime hours were set as the period between 6.00 and 22.00 h, and night-time hours were set from 22.00 to 6.00. Having completed a diary of activities for the night-time rest, the actual sleep time was corrected for each patient. ABPM values with at least 66% successful measurements and at least one record per hour were considered valid. The following ABPM circadian patterns were established: dipper pattern (defined by a nocturnal BP reduction between 10 and 20% compared to the daytime period), extreme dipper pattern (nocturnal BP reduction of 20% or more), nondipper pattern (BP decrease of less than 10% in the nocturnal period compared to the daytime period) and riser pattern (mean nocturnal BP increase relative to the daytime period). The last three patterns were consideredpathological circadian patterns.
Laboratory measurements
Specimen blood samples were collected for biochemical analysis (including glucose, HbA1c, uric acid level, HOMA index and lipid profile), following a minimum of 8 h fast. The blood sample was centrifuged (7 min; 3000 rpm) and aliquots were stored at − 80 °C in a freezer until the batch measurement of cytokines. Cytokine levels (including sFlt-1, PlGF, adiponectin, leptin, MCP-1, PAI-1, resistin, NGF, TNFα, HGF, and FGF-2) were measured in maternal plasma using commercial kits following the manufacturer’s instructions (Millipore, Billerica, MA, USA) that uses the xMAP technology (Luminex Corporation, Austin, TX, USA). Levels of cytokines were logarithmically transformed due to large values.
Pregnancy outcomes
After delivery, the following obstetric and perinatal data were retrospectively reviewed: maternal weight gain, gestational age at delivery, route of delivery, delivery complications, birthweight and customized percentile, and the Apgar score of the newborn. Intrauterine growth restriction (IUGR) was defined as a birthweight less than the 5th percentile on a customized pediatric curve; small for gestational age (SGA) was defined as a birthweight below the 10th percentile, and macrosomia was designated as a birthweight above the 95th percentile for gestational age. We considered the presence of HDP, including gestational hypertension (BP > 140/90 mmHg in a woman who was normotensive before the 20th week of gestation and whose BP returned to normal by 12 weeks after delivery) and preeclampsia (defined as the new onset of hypertension after the 20th week of gestation in a previously normotensive woman, who developed proteinuria or end-organ dysfunction [24]).
Statistical analysis
Statistical analyses carried out using the IBM SPSS program (version 24.0 software for MS Windows). The normality of the variables was assessed with the Shapiro–Wilk test. Continuous variables were reported as the mean and standard deviation (SD) and were compared between independent groups using Student’s t –test or the Mann–Whitney U –test for nonparametric variables. Categorical variables were expressed as frequencies and percentages and were compared using the χ2 test or Fisher’s exact test as appropriate. The correlation between two variables was studied with the Pearson test or Spearman’s correlation coefficient. p values less than 0.05 were defined as significant in all two-tailed analyses. Multivariate analysis was performed using nonconditional logistic regression. The stepwise technique was used to select the independent variables introduced into the model based on clinical and statistical criteria of p < 0.05 in a bivariate analysis. The fit of the final model was tested using the Hosmer–Lemeshow test.
Results
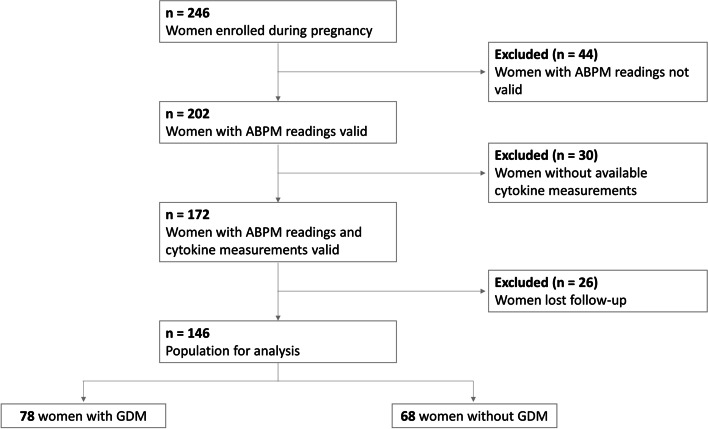
A total of 246 normotensive pregnant women were enrolled. We excluded women with < 66% of valid ABPM readings and nonavailability of cytokine measurements and women who gave birth elsewhere (Fig. 1). Finally, 146 pregnant women with normal BP were included in the analysis, 78 patients diagnosed with GDM and 68 with normal glucose tolerance. Women with GDM were found to be older than non-GDM women (34.3 ± 3.6 vs. 32.8 ± 4.8; p = 0.029) and, as expected, had significantly higher triglycerides (2.3 ± 0.9 vs. 2.0 ± 0.6 mmol/L; p = 0.04), basal glucose (4.9 ± 0.6 vs. 4.6 ± 0.3 mmol/L; p = 0.018) and HbA1c levels (5 ± 0.4 vs. 4.8 ± 0.3%; p = 0.003). The rest of the studied variables showed no statistically significant differences between the groups.
Fig. 1.
Study flowchart. ABPM: ambulatory blood pressure monitoring; GDM: gestational diabetes mellitus
Fourteen patients (9.6%) developed HDP; 10 presented with gestational hypertension, and four developed added preeclampsia. The demographic and clinical characteristics of women who developed some type of HDP and normotensive women are summarized in Table 1. No differences in maternal age, family history of hypertension, obstetric history, parity or gestational age at the time of inclusion were observed. Of the 14 hypertensive pregnant women, eight had obesity (57.16%) and 11 had GDM (78.6%), these included the four patients who developed preeclampsia. Hence, pregnant women with HDP showed significantly greater prepregnancy BMI and higher HbA1c and basal glucose levels, without statistically significant differences in the rest of the laboratory variables measured (Table 1). We also found that office systolic and diastolic BP (measured at 28–32 weeks of pregnancy) were significantly higher in women who subsequently developed HDP, and the rate of aspirin prophylaxis was higher in this group.
Table 1.
Baseline clinical characteristics and laboratory variables in women with and without HDP
| Variable | HDP (n = 14) | Non-HDP (n = 132) | p Value |
|---|---|---|---|
| Clinical characteristics | |||
| Maternal age (y) * | 33.6 ± 4.4 | 33.6 ± 4.3 | 0.9 |
| Family history AHT † | 4 (28.6%) | 58 (43.9%) | 0.2 |
| Parity * | 1.71 ± 1.4 | 1.89 ± 0.9 | 0.5 |
| Previous history of GDM † | 1 (7.1%) | 19 (14.4%) | 0.4 |
| Previous history of preeclampsia † | 1 (7.1%) | 2 (1.5%) | 0.1 |
| Gestational age at enrollement (w) * | 31.2 ± 1.9 | 31.1 ± 1.9 | 0.8 |
| Pregestational BMI (kg/m2) * | 30.1 ± 6.8 | 26.0 ± 5.0 | 0.046 |
| Obesity † | 8 (57.1%) | 28 (21.2%) | 0.006 |
| GDM † | 11 (78.6%) | 67 (50.2%) | 0.047 |
| Insulin treatment † | 5 (45.4%) | 24 (35.8%) | 0.2 |
| Office Systolic BP (mmHg) * | 118.5 ± 15.7 | 109.7 ± 15.4 | 0.043 |
| Office Diastolic BP (mmHg) * | 74.5 ± 9.7 | 65.9 ± 9.0 | 0.001 |
| ASA prophylaxis † | 5 (35.7%) | 14 (10.6%) | 0.008 |
| Laboratory parameters | |||
| Basal glucose (mmol/L) * | 4.9 ± 0.5 | 4.8 ± 0.5 | 0.039 |
| HbA1c (%)* | 5.2 ± 0.5 | 4.8 ± 0.3 | 0.002 |
| HOMA-IR * | 2.9 ± 1.1 | 2.0 ± 1.3 | 0.09 |
| Albumin/creatinine (mg/g) * | 28.3 ± 60.0 | 6.3 ± 6.6 | 0.1 |
| Uric acid (mmol/L) * | 0.24 ± 0.06 | 0.22 ± 0.13 | 0.6 |
| Total-cholesterol (mmol/L) * | 6.52 ± 0.86 | 6.45 ± 1.19 | 0.8 |
| LDL-cholesterol (mmol/L) * | 3.76 ± 0.8 | 3.66 ± 1.22 | 0.7 |
| HDL-cholesterol (mmol/L) * | 1.99 ± 0.42 | 1.92 ± 0.47 | 0.6 |
| Triglycerides (mmol/L) * | 2.2 ± 0.6 | 2.1 ± 0.8 | 0.6 |
* Data expressed as means ± standard deviation; comparisons between different groups were done by the Student’s t– test for parametric variables and the Mann–Whitney U– test for nonparametric variables
† Data expressed as n (%); comparisons among groups were done using the χ2 test, or Fisher’s exact test for nonparametric contrasting
HDP hypertensive disorders of pregnancy, AHT arterial hypertension, BMI body mass index, GDM gestational diabetes mellitus, BP blood pressure, ASA acetylsalicylic acid, HbA1c glycated hemoglobin, LDL low-density lipoprotein, HDL high-density lipoprotein
With regard to obstetric and perinatal outcomes, hypertensive women delivered earlier and the difference in gestational age at delivery was almost significant; however, preterm delivery was significantly more common in this group. On the other hand, birthweight and customized percentile were significantly lower in women who developed HDP than in those who did not, and a higher rate of SGA and IUGR was observed in these patients, as shown in Table 2. Regarding neonatal complications, the rate of hypoglycemia was significantly higher in women who developed HDP, without significant differences in the remaining complications analysed.
Table 2.
Obstetric and perinatal outcomes in women with and without HDP
| Variable | HDP (n = 14) | Non-HDP (n = 132) | p Value |
|---|---|---|---|
| Obstetric and perinatal outcomes | |||
| Weight gain (kg) * | 10.9 ± 5.0 | 8.8 ± 4.2 | 0.1 |
| Gestational age at delivery; (wk) * | 38.4 ± 1.8 | 39.4 ± 1.1 | 0.06 |
| Preterm delivery (< 37 wk)† | 3 (21.4%) | 0 | < 0.001 |
| Instrumental delivery † | 5 (35.7%) | 30 (22.7%) | 0.2 |
| Cesarean section † | 5 (35.7%) | 34 (25.8%) | 0.3 |
| Birthweight (g) * | 2901 ± 667 | 3318 ± 472 | 0.038 |
| Customized percentile * | 28.6 ± 29.8 | 47.1 ± 29.1 | 0.026 |
| Neonatal complications † | |||
| Macrosomia | 0 | 16 (12.1%) | 0.1 |
| SGA | 6 (42.9%) | 16 (12.1%) | 0.008 |
| IUGR | 4 (28.6%) | 7 (5.3%) | 0.012 |
| Hypoglycemia | 3 (21.4%) | 4 (3%) | 0.002 |
| Hyperbilirubinemia | 1 (7.1%) | 6 (4.5%) | 0.6 |
| Congenital malformations | 1 (7.1%) | 1 (0.8) | 0.051 |
| Admission to the neonatal ICU | 1 (7.1%) | 5 (3.8%) | 0.5 |
* Data expressed as means ± standard deviation; comparisons between different groups were done by the Student’s t– test for parametric variables and the Mann–Whitney U– test for nonparametric variables
† Data expressed as n (%); comparisons among groups were done using the χ2 test, or Fisher’s exact test for nonparametric contrasting
SGA small for gestational age, IUGR intrauterine growth restriction
By analysing the relationship between cytokine and angiogenic factor concentrations and the development of HDP, we observed significantly lower maternal plasma PlGF levels in women with HDP than in normotensive women. MCP-1 and leptin levels, as well as the sFlt-1/PlGF ratio, were significantly higher in women who developed HDP (Table 3). No statistically significant differences were found in the rest of the measured markers.
Table 3.
Bivariate analysis of the association between the development of HDP, biomarkers’ levels and ABPM parameters
| Variable | HDP (n = 14) | Non-HDP (n = 132) | p Value |
|---|---|---|---|
| Cytokines and biomarkers levels * | |||
| Adiponectin, (pg/ml) | 10.22 ± 2.54 | 13.08 ± 2.93 | 0.06 |
| Resistin (pg/ml) | 7.43 ± 3.82 | 8.28 ± 3.28 | 0.37 |
| PAI-1 (pg/ml) | 8.04 ± 4.08 | 8.69 ± 3.48 | 0.51 |
| NGF (pg/ml) | 0.62 ± 0.84 | 0.73 ± 0.9 | 0.67 |
| Leptin (pg/ml) | 10.97 ± 0.82 | 10.2 ± 1.11 | 0.018 |
| HGF (pg/ml) | 6.65 ± 1.07 | 7.03 ± 1.23 | 0.41 |
| MCP-1 (pg/ml) | 5.24 ± 0.60 | 4.9 ± 0.55 | 0.044 |
| TNFα (pg/ml) | 0.35 ± 2.02 | 0.59 ± 1.13 | 0.49 |
| FGF-2 (pg/ml) | 3.8 ± 0.62 | 4.02 ± 0.63 | 0.22 |
| sFlt-1 (pg/ml) | 7.56 ± 0.93 | 7.25 ± 0.97 | 0.26 |
| PIGF (pg/ml) | 3.18 ± 1.79 | 5.1 ± 1.12 | 0.002 |
| sFlt-1/PlGF ratio | 4.37 ± 2.2 | 2.2 ± 1.43 | 0.003 |
| BP parameters | |||
| 24 h SBP (mmHg) * | 113.1 ± 14.4 | 104.2 ± 7.9 | 0.04 |
| 24 h DBP (mmHg) * | 69.7 ± 9.2 | 64.1 ± 5.6 | 0.04 |
| Daytime SBP (mmHg) * | 115.7 ± 13.4 | 106.7 ± 8.6 | 0.001 |
| Daytime DBP (mmHg) * | 72.3 ± 8.2 | 66.6 ± 6.0 | 0.002 |
| Nocturnal SBP (mmHg) * | 107.5 ± 17.5 | 98.7 ± 7.8 | 0.08 |
| Nocturnal DBP (mmHg) * | 63.5 ± 12.1 | 58.5 ± 5.6 | 0.15 |
| Pathological circadian pattern † | 9 (64.3%) | 62 (46.9%) | 0.17 |
| Riser pattern † | 2 (14.3%) | 6 (4.5%) | 0.13 |
| Nondipper pattern † | 4 (28.6%) | 44 (33.3%) | 0.72 |
| Extrem dipper pattern | 3 (21.4%) | 12 (9.1%) | 0.15 |
| Dipper pattern † | 5 (35.7%) | 70 (53%) | 0.22 |
* Data expressed as means ± standard deviation; Comparisons between different groups were done by the Student’s t– test for parametric variables and the Mann–Whitney U– test for nonparametric contrasting
† Data expressed as n (%); comparison among groups were done using the Fisher’s exact test
HDP hypertensive disorders of pregnancy, PAI-1 plasminogen activator inhibitor-1, NGF nerve growth factor, HGF hepatocyte growth factor, MCP-1 Monocyte Chemoattractant Protein-1, TNFα tumor necrosis factor alpha, FGF-2 fibroblast growth factor-2, sFlt-1 soluble fms-like tyrosine kinase-1, PlGF placental growth factor, PB blood pressure, SBP systolic blood pressure, DBP diastolic blood pressure
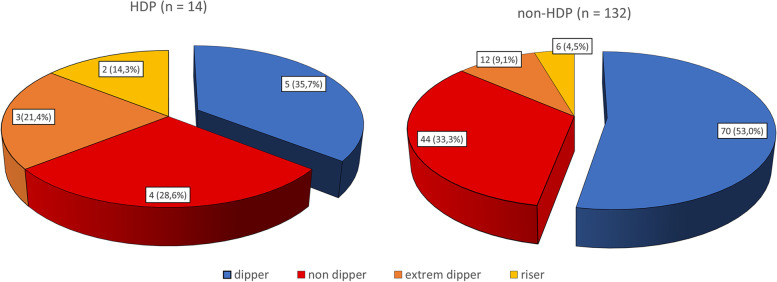
Regarding ABPM parameters, significantly higher 24-hour and daytime systolic and diastolic BP levels were detected by ABPM between 28 and 32 weeks in patients who subsequently developed HDP compared to those who remained normotensive, (Table 3). Furthermore, the rate of pathological circadian patterns (including nondipper, extreme dipper and riser patterns) was higher in hypertensive women (64.3% vs. 46.9%; p = 0.17), but the difference did not reach statistical significance (Fig. 2). Conversely, we found that women with a pathological circadian pattern had significantly higher concentrations of triglycerides (2.3 ± 0.9 vs. 2.0 ± 0.6; p = 0.05) and sFlt-1/PlGF ratio (2.77 ± 1.7 vs. 2.07 ± 1.5; p = 0.01) than those who presented a dipper pattern. In addition, a lower customized percentile was also observed in patients without a dipper pattern relative to those with a dipper pattern (39.3 ± 27.2 vs. 51.1 ± 30.7; p = 0.017). No differences were found in the remainder of variables analysed.
Fig. 2.
Prevalence of circadian patters in women with and without HDP. HDP: hypertensive disorders of pregnancy; extreme dipper pattern: subjects with a > 20% fall in nocturnal blood pressure; dipper pattern: subjects with a 10–20% fall in nocturnal blood pressure; non-dipper pattern: subjects with a 0–10% fall in nocturnal blood pressure; riser pattern: subjects with nocturnal blood pressure higher than diurnal blood pressure
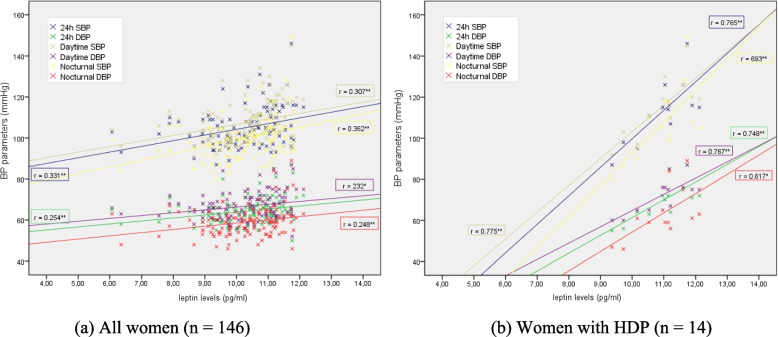
There were moderate positive correlations between the levels of leptin and BP parameters detected by ABPM (Fig. 3a), but this correlation was stronger among the women who developed HDP (Fig. 3b): systolic (r: 0.765; p = 0.002) and diastolic BP (r: 0.748; p = 0.003) over 24 h, systolic (r: 0.775; p = 0.002) and diastolic BP (r: 0.767; p = 0.002) in the daytime period, and systolic (r: 0.693; p = 0.009) and diastolic BP (r: 0.617; p = 0.025) in the nocturnal period. Regarding the remaining cytokines measured, which were low but statistically significant (p < 0.05), positive correlations were found between MCP–1 levels and 24-h and nocturnal systolic BP and 24-h, daytime and nocturnal diastolic BP. In the group of women who had HDP, this correlation was significantly stronger for nocturnal systolic BP (r = 0.576; p = 0.039).
Fig. 3.
Correlation between ABPM and leptin levels in whole population (a); in women with HDP (b). * p < 0.05; ** p < 0.01. HDP: hypertensive disorders of pregnancy; PB: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure
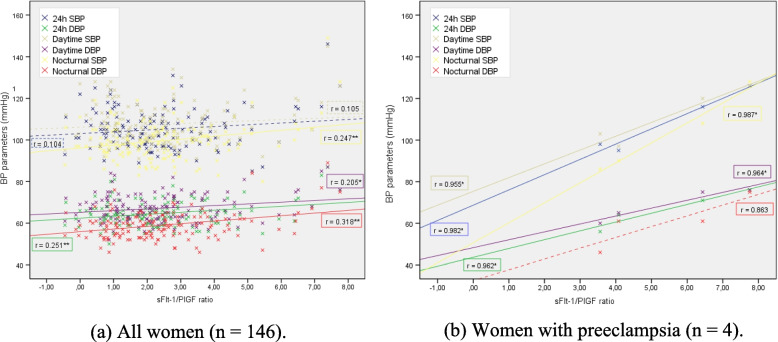
Additionally, moderate negative correlations between PlGF levels and all ABPM parameters: systolic BP (24 h: r = − 0.301; daytime: r = − 0.289; and nocturnal: r = − 0.309; p < 0.001) and diastolic BP (24 h: r = − 0.353; daytime: r = − 0.356; and nocturnal: r = − 0.329; p < 0.001) were observed. Similarly, a low-moderate positive correlation was observed between the sFlt-1/PlGF ratio and the average 24 h diastolic BP (r: 0.251; p = 0.002), diastolic BP in the daytime period (r: 0.205; p = 0.013) and systolic (r: 0.247; p = 0.003) and diastolic BP (r: 0.318; p < 0.001) in the nocturnal period (Fig. 4a). When analysing these correlations separately in women who had preeclampsia (n = 4), stronger associations were found between ABPM parameters and PlGF levels for 24 h systolic BP (r = − 0.967; p = 0.033) and systolic BP in the daytime period (r = 0.973; p = 0.027), as well as the sFlt-1/PlGF ratio (Fig. 4b); however, these associations were not observed in women who presented with isolated gestational hypertension (without preeclampsia).
Fig. 4.
Correlation between ABPM and sFlt-1/PlGF ratio in whole population (a); in women with preeclampsia (b). * p < 0.05; ** p < 0.01. HDP: hypertensive disorders of pregnancy; PB: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; sFlt-1/PlGF ratio: soluble fms-like tyrosine kinase-1 / placental growth factor ratio
Table 4 summarizes the results of the final multivariate logistic regression model; following adjustment for potential confounding factors, sFlt-1/PlGF ratio (OR: 2.02), daytime SBP (OR: 1.27) and prepregnancy BMI (OR: 1.14) were found to be independent risk predictors for the development of HDP.
Table 4.
Multivariate logistic regression for the risk prediction of HDP
| Variable | OR | Z score | p Value | IC 95% |
|---|---|---|---|---|
| Age (y) | 0.95 | - 0.46 | 0.57 | [0.81–1.11] |
| Prepregnancy BMI (kg/m2) | 1.14 | 0.13 | 0.036 | [1.01–1.30] |
| HbA1c (%) | 0.96 | - 0.03 | 0.88 | [0.61–1.51] |
| Daytime SBP (mmHg) | 1.27 | 0.11 | 0.036 | [1.00–1.26] |
| Nocturnal SBP (mmHg) | 0.92 | - 0.07 | 0.21 | [0.82–1.04] |
| sFlt-1/PIGF ratio | 2.02 | 0.70 | 0.001 | [1.35–3.05] |
Multivariate analysis was performed using binary logistic regression models. The Hosmer–Lemeshow statistic (8.28 for 8 degrees of freedom (df); p = 0.41) indicates an adequate fit to the logistic regression model. OR odd ratio, 95% CI 95% confidence interval, BMI body mass index, HbA1c glycated hemoglobin, SBP systolic blood pressure, DBP diastolic blood pressure, sFlt-1/PlGF ratio soluble fms-like tyrosine kinase-1 / placental growth factor ratio
Discussion
Evidence exists that an angiogenic imbalance and increasing inflammatory biomarkers play a crucial role in both GDM and HDP [8, 20], as both entities share several pathophysiological features including endothelial dysfunction, oxidative stress and inflammatory activation. In the data we present here, we evaluated the association between the pro-inflammatory state and endothelial damage by measuring maternal serum inflammatory markers and the subclinical blood pressure alterations detected by ABPM during the third trimester of pregnancy in women with and without GDM.
The incidence of HDP in our study was 9.6%; of which, 78.6% (n = 11) presented with GDM and 57.1% (n = 8) with obesity. As described in the literature, maternal obesity is the most important modifiable risk factor for the development of HDP [25, 26] and GDM [27, 28]. In our cohort, the presence of obesity was similar in women with and without GDM; however, prepregnancy BMI was significantly higher in patients who developed HDP (Table 1). Although it has been previously described that increasing BMI is associated with a progressively increased risk of HDP [29–31], the mechanism by which excess adipose tissue causes the development of HDP in pregnant women remains unclear. Overweight is associated with alterations in lipid concentrations and the activation of inflammatory markers [32], and both of these metabolic abnormalities are characteristic of preeclamptic pregnancies before the onset of clinically evident disease [33]. In our study, we found higher levels of triglycerides in women with subclinical alterations in circadian rhythm, however, we could not establish its relationship in the pathogenesis of HDP, possibly due to the small number of patients developing HDP. Hence, in agreement with previous investigators, HDP may be secondary to an underlying placental insufficiency coupled with chronic oxidative stress from maternal metabolic disorders such as obesity and insulin resistance [30, 34]. In addition, we found that the rate of patients who received low doses of aspirin was higher in the HDP group, which we attributed to the fact that in these pregnant women presented more risk factors for preeclampsia and the indication of ASA prophylaxis.
On the other hand, GDM is also a recognized risk factor for developing HDP [5, 6, 35], although the rate is more variable [36] and could be influenced by glycemic control [37]. We observed higher basal glucose and HbA1c levels in women who subsequently presented with HDP, and in agreement with other studies, these showed a positive correlation between fasting glucose and HDP, even amongst women without GDM [38]. These results are consistent with the previous hypothesis that reported the causal role of insulin resistance in endothelial dysfunction and the development of HDP [8, 34]. Since insulin resistance can be indirectly measured by circulating adipokine markers, we investigated whether pro- and antiangiogenic biomarkers are related to subclinical BP alterations detected by ABPM in normotensive pregnant women who subsequently develop HDP.
It is widely recognized that there is an association between leptin concentration and fat mass, and since higher maternal leptin levels have been described in both pregnant women with obesity and pregnant women with GDM [12, 39] circulating leptin levels have been shown to be involved in the physiology of insulin resistance [40, 41]. Vitoratos et al. [42] found greater leptin levels in women with HDP, in agreement with a recent publication from our group among GDM women who develop HDP [13]. The data we present here demonstrate a low-moderate correlation between leptin levels and BP parameters when ABPM is performed before the development of HDP, and this correlation is even stronger amongt women who subsequently develop gestational hypertension or preeclampsia (Fig. 3).
With respect to other proinflammatory cytokines, a higher maternal concentration of MCP-1 was observed in women who developed HDP, as well as a positive correlation with BP levels, which is consistent with current studies describing that MCP-1 is involved in the endothelial inflammatory process [43], atherosclerosis and cardiovascular damage [44], and has also been described in preeclampsia [45].
In relation to angiogenic factors, evidence supports an angiogenic imbalance, characterized by greater concentrations of sFlt-1, lower PlGF levels and, particularly, a higher sFlt-1/PlGF ratio correlated with the risk of developing preeclampsia in pregnant women [9, 46–48] as well as those with preexisting diabetes [49, 50]. Recently, Nuzzo et al. [19] reported similar findings in women with GDM. Additionally, we have published a study describing that in a cohort of GDM women, the best predictor for HDP was the sFlt-1/PIGF ratio, which is directly correlated with diastolic BP values at delivery [13]. The relationship between BP levels and concentrations of sFlt-1, PlGF and sFlt-1/PlGF ratio has been investigated in women with chronic hypertension [51] and preeclampsia [46, 52, 53], but the association with BP values detected by ABPM has not been evaluated. Although in our cohort of patients the relationship between BP levels detected by ABPM and the sFlt-1/PlGF ratio was statistically significant, the correlation coefficient showed a low direct relationship, so any interpretation should be made with caution. Of note, the majority of studies, such as the SaPPPhirE Study [54], have measured cytokine markers in the development of preeclampsia or preeclampsia over preexisting hypertension, but did not include the development of gestational hypertension. It remains uncertain whether gestational hypertension and preeclampsia are different states of the same disease or two different entities [55]. Among previous studies that have also evaluated angiogenic biomarkers in other HDP, Yang et al. concluded that the sFlt-1/PlGF ratio is a valuable tool for the diagnosis of preeclampsia and severe preeclampsia rather than other types of HDP (including gestational hypertension and chronic hypertension) [56]. A similar conclusion was also reported by Engels et al. [57]. In our research, because of the small number of women who had HDP, we studied preeclampsia and gestational hypertension together, and it is possible that the lower incidence of events could influence the precision of our results. However, when we analysed the relationship between ABPM parameters and the concentration of PlGF and the sFlt-1/PlGF ratio in women who presented preeclampsia (n = 4), we observed a statistically significant stronger correlation, in line with the findings from the abovementioned studies about these angiogenic factors that may be useful in the prediction of preeclampsia compared to gestational hypertension.
Numerous studies consistently substantiate a strong association between an abnormal physiological circadian rhythm (including nondipper [58–60], extreme dipper [61] and riser patterns [62]) and a higher cardiovascular risk in hypertensive patients and in the general population [63]. The nondipper pattern has also been associated with cardiovascular events, left ventricular hypertrophy and a higher rate of clinical conditions, such as obstructive sleep apnea, diabetes or heart failure [64–66]. In pregnancy, several studies have attempted to determine whether ABPM could be a useful tool for predicting early changes in BP circadian rhythm in patients who subsequently develop HDP [17]. In fact, pregnant women with type 1 DM have shown an increase in the frequency of the nondipper pattern in the second trimester of pregnancy to be predictive of the development of HDP [67], and in previous studies of this cohort, our group reported that a predominance of nondipper patterns can be seen in GDM [68]. In our study, we found that the mean 24-h and daytime systolic and diastolic BP values were higher in women who subsequently developed HDP, and in agreement with previous studies [15], these patients had more frequently altered circadian patterns, although the results did not reach statistical significance. This altered circadian pattern was associated with higher levels of triglycerides and a higher sFlt-1/PlGF ratio, which could indicate that an atherogenic profile is related to a subclinical inflammatory status, the elevation of biomarkers and the alterations in BP circadian rhythm, all of which lead to vascular damage with implications for obstetric and perinatal outcomes.
To our knowledge, this is the first study to report the role of ABPM values as a valid predictor of HDP and to further evaluate inflammatory status and endothelial dysfunction using proinflammatory biomarkers and angiogenic factors in pregnant women who develop HDP. In our study, multivariate analysis identified daytime systolic BP and the sFlt-1/PlGF ratio as independent risk factors for the development of HDP. In addition, we found that prepregnancy obesity also increases the risk of developing HDP, and we have previously reported a positive correlation between ABPM parameters and prepregnancy BMI [18]. Therefore, we demonstrate that the use of ABPM along with the sFlt-1/PlGF ratio in women with GDM and obesity may be useful tools to identify those with an increased risk of developing HDP. This, in turn, could be of great value to the health system because a better prediction would entail a greater benefit both for the planning of prenatal care and for the adoption of effective strategies for the reduction of maternal and perinatal complications.
Nevertheless, there are several limitations in our study. First, 40% of participants (100 of 246 women enrolled) were excluded due to missing data which may result in potential selection bias. Second, the reproducibility of ABPM is limited, and there are few studies with which our results may be compared. Third, the small number of patients who developed HDP may have limited the power to detect some differences of smaller size, so our findings require confirmation in larger studies.
Conclusions
In our cohort of normotensive pregnant women, HDP occurred significantly more frequently in women with GDM. BP values on ABPM are directly related to levels of leptin and MCP-1 as well as the sFlt-1/PlGF ratio, and inversely correlated with PlGF levels. We found that higher prepregnancy BMI, higher daytime systolic BP values on ABPM and an elevated sFlt-1/PlGF ratio were associated with an increased risk of developing HDP, and could be useful to identify women at higher risk who may benefit from further interventions to minimize the impact on pregnancy.
Acknowledgements
We appreciate the written assistance of Dr. Deborah Wallace and all the clinicians and laboratory researchers at the Puerta del Mar University Hospital.
Abbreviations
- ABPM
ambulatory blood pressure monitoring
- AHT
arterial hypertension
- ASA
acetysalicylic acid
- BMI
body mass index
- BP
blood pressure
- DBP
diastolic blood pressure
- FGF-2
fibroblast growth factor 2
- GDM
gestational diabetes mellitus
- HbA1c
glycated hemoglobin
- HDL
high-density lipoprotein
- HDP
hypertensive disorders of pregnancy
- HGF
hepatocyte growth factor
- IUGR
intrauterine growth restriction
- LDL
low-density lipoprotein
- LGA
large for gestational age
- MCP-1
monocyte chemoattractant protein-1
- NGF
nerve growth factor
- ns
not statically significant
- PAI-1
plasminogen activator inhibitor-1
- PlGF
placental growth factor
- SBP
systolic blood pressure
- SD
standard deviation
- sFlt-1
soluble fms-like tyrosine kinase-1
- SGA
small for gestational age
- TNF
tumor necrosis factor alpha
Authors’ contributions
C.L-T: Project development. A.L-B and B.S-L: Data collection; A.L-B, B.S-L and C.L-T: Data cleaning and standardization; A.L-B and C.L-T: Manuscript writing and data analysis; M.A-D: Manuscript revision and project development. The authors read and approved the final manuscript.
Funding
This study was funded by the Instituto de Salud Carlos III, grant number PI16/00370 (cofunded by the European Regional Development Fund, ERDF), and by grants from Convocatoria de Subvenciones para la Financiación de la Investigación y la Innovación Biomédica y en Ciencias de la Salud en el Marco de la Iniciativa Territorial Integrada 2014–2020 para la Provincia de Cádiz, Fondos ITI-FEDER, grant number PI-0029-2017.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Board of Puerta del Mar Hospital (1507-N-16) in Cádiz, Spain. Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Almudena Lara-Barea, Email: almlarbar@gmail.com.
Begoña Sánchez-Lechuga, Email: bsanchezle@gmail.com.
Manuel Aguilar-Diosdado, Email: manuelaguilardiosdado@gmail.com.
Cristina López-Tinoco, Email: cristinalopeztinoco@gmail.com.
References
- 1.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 2.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35(4):780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fadl H, Magnuson A, Östlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG An Int J Obstet Gynaecol. 2014;121(12):1530–1536. doi: 10.1111/1471-0528.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burlina S, Dalfrà MG, Chilelli NC, Lapolla A. Gestational diabetes mellitus and future cardiovascular risk: an update. Int J Endocrinol. 2016;2016. 10.1155/2016/2070926. [DOI] [PMC free article] [PubMed]
- 5.Nerenberg KA, Johnson JA, Leung B, Savu A, Ryan EA, Chik CL, et al. Risks of gestational diabetes and preeclampsia over the last decade in a cohort of Alberta women. J Obstet Gynaecol Canada. 2013;35(11):986–994. doi: 10.1016/S1701-2163(15)30786-6. [DOI] [PubMed] [Google Scholar]
- 6.Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. 2003;158(12):1148–1153. doi: 10.1093/aje/kwg273. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Ouh YT, Ahn KH, Hong SC, Oh MJ, Kim HJ, et al. Preeclampsia: a risk factor for gestational diabetes mellitus in subsequent pregnancy. PLoS One. 2017;12(5):1–8. doi: 10.1371/journal.pone.0178150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McElwain CJ, Tuboly E, McCarthy FP, McCarthy CM. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: windows into future Cardiometabolic health? Front Endocrinol. 2020;11:1–19. doi: 10.3389/fendo.2020.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rǎdulescu C, Bacârea A, Huanu A, Gabor R, Dobreanu M. Placental growth factor, soluble fms-like tyrosine kinase 1, soluble Endoglin, IL-6, and IL-16 as biomarkers in preeclampsia. Mediat Inflamm. 2016;2016:1–8. doi: 10.1155/2016/3027363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202(2):161.e1–161.e11. doi: 10.1016/j.ajog.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Abell SK, De Courten B, Boyle JA, Teede HJ. Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. Int J Mol Sci. 2015;16(6):13442–13473. doi: 10.3390/ijms160613442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Tinoco C, Roca M, Fernández-Deudero A, García-Valero A, Bugatto F, Aguilar-Diosdado M, et al. Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus. Cytokine. 2012;58(1):14–19. doi: 10.1016/j.cyto.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Lara-Barea A, Sánchez-Lechuga B, Campos-Caro A, Córdoba-Doña JA, de la Varga-Martínez R, Arroba AI, et al. Angiogenic imbalance and inflammatory biomarkers in the prediction of hypertension as well as obstetric and perinatal complications in women with gestational diabetes mellitus. J Clin Med. 2022;11(6):1514. doi: 10.3390/jcm11061514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorostidi M, Banegas JR, de la Sierra A, Vinyoles E, Segura J, Ruilope LM. Ambulatory blood pressure monitoring in daily clinical practice - the Spanish ABPM registry experience. Eur J Clin Investig. 2016;46(1):92–98. doi: 10.1111/eci.12565. [DOI] [PubMed] [Google Scholar]
- 15.Bhide A, Sankaran S, Moore J, Khalil A, Furneaux E. Ambulatory blood pressure measurements in mid-pregnancy and development of hypertensive pregnancy disorders. Hypertens Pregnancy. 2014;33:159–167. doi: 10.3109/10641955.2013.842585. [DOI] [PubMed] [Google Scholar]
- 16.Saremi AT, Shafiee M-A, Montazeri M, Rashidi N, Montazeri M. Blunted overnight blood pressure dipping in second trimester; a strong predictor of gestational hypertension and preeclampsia. Curr Hypertens Rev. 2018;15(1):70–75. doi: 10.2174/1573402114666180924143801. [DOI] [PubMed] [Google Scholar]
- 17.Salazar MR, Espeche WG, Leiva Sisnieguez CE, Leiva Sisnieguez BC, Balbín E, Stavile RN, et al. Nocturnal hypertension in high-risk mid-pregnancies predict the development of preeclampsia/eclampsia. J Hypertens. 2019;37(1):182–186. doi: 10.1097/HJH.0000000000001848. [DOI] [PubMed] [Google Scholar]
- 18.Lara-Barea A, Sánchez-Lechuga B, Vidal-Suárez Á, Arroba AI, Bugatto F, López-Tinoco C. Blood pressure monitoring and perinatal outcomes in normotensive women with gestational diabetes mellitus. J Clin Med. 2022;11(5):1435. doi: 10.3390/jcm11051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuzzo AM, Giuffrida D, Moretti L, Re P, Grassi G, Menato G, et al. Placental and maternal sFlt1/PlGF expression in gestational diabetes mellitus. Sci Rep. 2021;11(2312). 10.1038/s41598-021-81785-5. [DOI] [PMC free article] [PubMed]
- 20.Karacay Ö, Sepici-Dincel A, Karcaaltincaba D, Sahin D, Yalvaç S, Akyol M, et al. A quantitative evaluation of total antioxidant status and oxidative stress markers in preeclampsia and gestational diabetic patients in 24-36 weeks of gestation. Diabetes Res Clin Pract. 2010;89(3):231–238. doi: 10.1016/j.diabres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 21.De Resende Guimarães MFB, Brandão AHF, De Lima Rezende CA, Cabral ACV, Brum AP, Leite HV, et al. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Arch Gynecol Obstet. 2014;290(3):441–447. doi: 10.1007/s00404-014-3220-x. [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence (NICE) Hypertension in pregnancy: the management ofHypertensive disorders during pregnancy. London: RCOG Press; 2010. [Google Scholar]
- 23.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 24.Leeman L, Dresang LT, Fontaine P. Hypertensive disorders of pregnancy. Am Fam Physician. 2016;93(2):121–127. [PubMed] [Google Scholar]
- 25.Athukorala C, Rumbold AR, Willson KJ, Crowther CA. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth. 2010;10:1–8. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steegers EAP, Von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 27.Sathyapalan T, Mellor D, Atkin SL. Obesity and gestational diabetes. Semin Fetal Neonatal Med. 2010;15(2):89–93. doi: 10.1016/j.siny.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Wei T, Ni W, Zhang A, Zhang J, Xing Y, et al. Incidence and risk factors of gestational diabetes mellitus: a prospective cohort study in Qingdao, China. Front Endocrinol. 2020;11(636):1–9. doi: 10.3389/fendo.2020.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callaway LK, Prins JB, Chang AM, Mcintyre HD. Australian obstetric population. Med J Aust. 2006;184(2):56–59. doi: 10.5694/j.1326-5377.2006.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 30.Bicocca MJ, Mendez-Figueroa H, Chauhan SP, Sibai BM. Maternal obesity and the risk of early-onset and late-onset hypertensive disorders of pregnancy. Obstet Gynecol. 2020;136(1):118–127. doi: 10.1097/AOG.0000000000003901. [DOI] [PubMed] [Google Scholar]
- 31.Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, north American and Australian cohorts. BJOG Int J Obs Gy. 2019;126(8):984–995. doi: 10.1111/1471-0528.15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162(12):1198–1206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- 34.Seely EW, Solomon CG. Insulin resistance and its potential role in pregnancy-induced hypertension. J Clin Endocrinol Metab. 2003;88(6):2393–2398. doi: 10.1210/jc.2003-030241. [DOI] [PubMed] [Google Scholar]
- 35.Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open. 2014;1:e000101. doi: 10.1136/bmjopen-2011-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan SD, Umans JG, Ratner R. Hypertension complicating diabetic pregnancies: pathophysiology, management, and controversies. J Clin Hypertens. 2011;13(4):275–284. doi: 10.1111/j.1751-7176.2011.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yogev Y, Xenakis EMJ, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191(5):1655–1660. doi: 10.1016/j.ajog.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 38.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr UH. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 39.Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, et al. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol. 2005;193(3 SUPPL):979–983. doi: 10.1016/j.ajog.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 40.Gözüküçük M, Gürsoy AY, Destegül E, Taşkin S, Şatıroğlu H. Adiponectin and leptin levels in normal weight women with polycystic ovary syndrome. Horm Mol Biol Clin Invest. 2020;41(4). 10.4103/GMIT.GMIT_30_20. [DOI] [PubMed]
- 41.Xiao W, He J, Shen S, Lu J, Kuang Y, Wei X, et al. Maternal circulating leptin profile during pregnancy and gestational diabetes mellitus. Diabetes Res Clin Pract. 2020:108041. 10.1016/j.diabres.2020.108041. [DOI] [PubMed]
- 42.Vitoratos N, Chrystodoulacos G, Kouskouni E, Salamalekis E, Creatsas G. Alterations of maternal and fetal leptin concentrations in hypertensive disorders of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2001;96(1):59–62. doi: 10.1016/S0301-2115(00)00401-2. [DOI] [PubMed] [Google Scholar]
- 43.Cortez A, Muxfeldt E. Monocyte chemoattractant protein-1 and hypertension: an overview. Hipertens Riesgo Vasc. 2022;39(1):14–23. doi: 10.1016/j.hipert.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Tucci M, Quatraro C, Frassanito MA, Silvestris F. Deregulated expression of monocyte chemoattractant protein-1 (MCP-1) in arterial hypertension: role in endothelial inflammation and atheromasia. J Hypertens. 2006;24(7):1307–1318. doi: 10.1097/01.hjh.0000234111.31239.c3. [DOI] [PubMed] [Google Scholar]
- 45.Ma Y, Ye Y, Zhang J, Ruan CC, Gao PJ. Immune imbalance is associated with the development of preeclampsia. Medicine. 2019;98(14):1–6. doi: 10.1097/MD.0000000000015080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206(1):58.e1–58.e8. doi: 10.1016/j.ajog.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 47.Nikuei P, Rajaei M, Roozbeh N, Mohseni F, Poordarvishi F, Azad M, et al. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth. 2020;20(80):4–9. doi: 10.1186/s12884-020-2744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herraiz I, Simón E, Gómez-Arriaga P, Quezada M, García-Burguillo A, López-Jiménez E, et al. Clinical implementation of the sFlt-1 / PlGF ratio to identify preeclampsia and fetal growth restriction: a prospective cohort study. Pregnancy Hypertens. 2018;13:279–285. doi: 10.1016/j.preghy.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Cohen AL, Wenger JB, James-Todd T, Lamparello BM, Halprin E, Serdy S, et al. The association of circulating angiogenic factors and HbA1c with the risk of preeclampsia in women with preexisting diabetes. Hypertens Pregnancy. 2014;33(1):81–92. doi: 10.3109/10641955.2013.837175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Jenkins AJ, Nankervis AJ, Hanssen KF, Scholz H, Henriksen T, et al. Anti-angiogenic factors and pre-eclampsia in type 1 diabetic women. Diabetologia. 2009;52:160–168. doi: 10.1007/s00125-008-1182-x. [DOI] [PubMed] [Google Scholar]
- 51.Minhas R, Young D, Naseem R, Mueller A, Chinthala S, Lopes J, et al. Association of antepartum blood pressure levels and angiogenic profile among women with chronic hypertension. Pregnancy Hypertens. 2018;14:110–114. doi: 10.1016/j.preghy.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;122(1):33–39. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Molvarec A, Szarka A, Walentin S, Szucs E, Nagy B, Rigó J. Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens Res. 2010;33(9):892–898. doi: 10.1038/hr.2010.92. [DOI] [PubMed] [Google Scholar]
- 54.Agrawal S, Cerdeira AS, Redman C, Vatish M. Meta-analysis and systematic review to assess the role of soluble FMS-like tyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: the SaPPPhirE study. Hypertension. 2018;71(2):306–316. doi: 10.1161/HYPERTENSIONAHA.117.10182. [DOI] [PubMed] [Google Scholar]
- 55.ACOG Clinical management guidelines for obstetrician – gynecologists gestational hypertension and. Obstet Gynecol. 2020;135(202):237–260. [Google Scholar]
- 56.Yang H, Guo F, Guo Q, Wang Y, He P, Zhang H. Clinica Chimica Acta the clinical value of PlGF and the sFlt1 / PlGF ratio in the management of hypertensive pregnancy disorders: a retrospective real-world study in China. Clin Chim Acta. 2022;528:90–97. doi: 10.1016/j.cca.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 57.Engels T, Pape J, Schoofs K, Henrich W, Verlohren S. Automated measurement of sFlt1, PlGF and sFlt1/PlGF ratio in differential diagnosis of hypertensive pregnancy disorders. Hypertens Pregnancy. 2013;32(4):459–473. doi: 10.3109/10641955.2013.827205. [DOI] [PubMed] [Google Scholar]
- 58.Hermida RC, Chayán L, Ayala DE, Mojón A, Fontao MJ, Fernández JR. Relationship between metabolic syndrome, circadian treatment time, and blood pressure non-dipping profile in essential hypertension. Chronobiol Int. 2011;28(6):509–519. doi: 10.3109/07420528.2011.580871. [DOI] [PubMed] [Google Scholar]
- 59.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, et al. Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension. 1994;24(6):793–801. doi: 10.1161/01.HYP.24.6.793. [DOI] [PubMed] [Google Scholar]
- 60.Hermida RC, Smolensky MH, Ayala DE, Portaluppi F. Ambulatory blood pressure monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int. 2015;32(10):1329–1342. doi: 10.3109/07420528.2015.1113804. [DOI] [PubMed] [Google Scholar]
- 61.Amah G, Ouardani R, Pasteur-Rousseau A, Voicu S, Safar ME, Kubis N, et al. Extreme-dipper profile, increased aortic stiffness, and impaired subendocardial viability in hypertension. Am J Hypertens. 2017;30(4):417–426. doi: 10.1093/ajh/hpw209. [DOI] [PubMed] [Google Scholar]
- 62.Nakai K, Fujii H, Watanabe K, Watanabe S, Awata R, Kono K, et al. Riser pattern is a predictor of kidney mortality among patients with chronic kidney disease. Clin Exp Hypertens. 2016;38(5):476–481. doi: 10.3109/10641963.2016.1163368. [DOI] [PubMed] [Google Scholar]
- 63.Hermida RC, Ayala DE, Mojón A, Fernández JR. Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level-the “normotensive non-dipper” paradox. Chronobiol Int. 2013;30(1–2):87–98. doi: 10.3109/07420528.2012.701127. [DOI] [PubMed] [Google Scholar]
- 64.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the ohasama study. J Hypertens. 2002;20(1):2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 65.García-Serrano C, Micol-Bachiller M, Betrán-Biurrun D, Aran-Solé L, Pujol-Salud J. Circadian rhytm of blood pressure and the relation to cardiovascular risk factors. Enferm Nefrol. 2019;22(2):151–158. [Google Scholar]
- 66.Cuspidi C, Giudici V, Negri F, Sala C. Nocturnal nondipping and left ventricular hypertrophy in hypertension: an updated review. Expert Rev Cardiovasc Ther. 2010;8(6):781–792. doi: 10.1586/erc.10.29. [DOI] [PubMed] [Google Scholar]
- 67.Flores L, Levy I, Aguilera E, Martinez S, Gomis R, Esmatjes E. Usefulness of ambulatory blood pressure monitoring in pregnant women with type 1 diabetes. Diabetes Care. 1999;22:1507–1511. doi: 10.2337/diacare.22.9.1507. [DOI] [PubMed] [Google Scholar]
- 68.Sánchez-Lechuga B, Lara-Barea A, Córdoba-Doña JA, Montero Galván A, Abal Cruz A, Aguilar-Diosdado M, et al. Usefulness of blood pressure monitoring in patients with gestational diabetes mellitus. Endocrinol Diabetes y Nutr. 2018;65:394–401. doi: 10.1016/j.endien.2018.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.