Abstract
A susceptible strain of mice infected intravaginally with the mouse pneumonitis biovar of Chlamydia trachomatis became infertile and sustained high rates of hydrosalpinx formation regardless of prior infection with a human serovar. Conversely, susceptible mice infected with human serovars remained fertile unless challenged with a homologous human serovar.
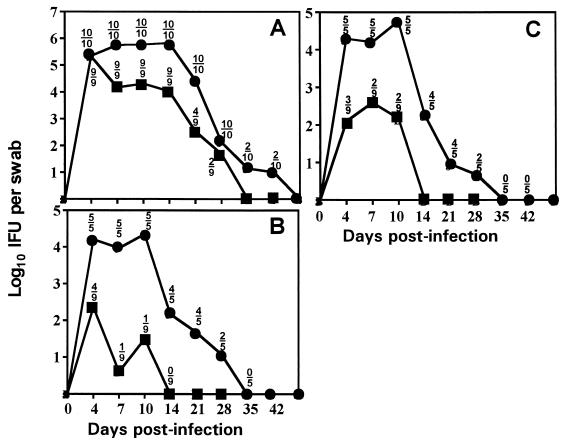
It is a widely held paradigm that protection against infection with Chlamydia trachomatis is conferred by serovar-specific epitopes but that cross-reactive genus-specific epitopes elicit hypersensitizing responses (2, 8, 17). This theory is supported by experimental human conjunctivitis studies (7), previous human vaccine trials (6, 18), nonhuman primate models (10), and in vitro antibody neutralization experiments (1, 2, 17). However, using a common murine model, we have previously found that primary infection with the mouse pneumonitis biovar of C. trachomatis (MoPn) not only yielded protection against homotypic MoPn challenge but also provided significant protection against heterotypic challenge with either human serovar E or L2 (12). In the same study, we found that when the order of challenge was reversed, primary infection with human serovar E resulted in significant protection against a secondary infection with serovar E or serovar L2 and, to a lesser extent, MoPn (Fig. 1). The infections induced antibody and cellular immune responses against all chlamydial strains tested. Thus, it was concluded that broadly cross-reactive epitopes exist that are capable of eliciting at least partial protection against chlamydial infection in this model. Considering the dependence of protective immunity on type 1 immune responses in this model, it is likely that the observed heterotypic protective immunity and the epitopes involved in eliciting the protective response in this study were also type 1 response dependent (12, 13).
FIG. 1.
Primary murine genital infection with C. trachomatis serovar E protects against homotypic or heterotypic challenge (reproduced from reference 12). The course of infection is shown by the solid lines, with each point representing the mean IFU from cervico-vaginal swabs of culture-positive mice at the time points indicated. Above each point is the ratio of the number of culture-positive animals to the number of animals in each group. Symbols: ●, previously uninfected controls; ■, mice previously infected with serovar E. (A) Mice challenged with MoPn (a total of two experiments). (B) Mice challenged with human serovar L2 (one experiment). (C) Mice challenged with human serovar E (one experiment).
Currently, two versions of the murine model of chlamydial genital infection exist, which vary with regard to the chlamydial biovars used. In both models, mouse strains can be categorized as susceptible or resistant to disease based primarily on the disease outcomes of hydrosalpinx formation and infertility. One version uses human serovars of C. trachomatis. The inoculum is administered directly into the upper genital tract following surgical exposure of the tissues (15) or can be instilled intravaginally (4). Infection by direct inoculation into the upper genital tract of susceptible strains of mice with human serovars results in hydrosalpinx formation and infertility, whereas infection by intravaginal inoculation with human serovars normally resolves without these outcomes (4, 16). In contrast, intravaginal infection of susceptible strains of mice with MoPn routinely leads to hydrosalpinx formation and infertility, obviating the need for instillation subsequent to surgical manipulation in order to assess the outcome of disease (5). It has been stated that both infection and disease outcomes are enhanced in both variations of the model if progesterone pretreatment is used (13).
Herein, we extend our previous work related to heterotypic protective immune responses to the subsequent assessment of disease outcomes. Using the mouse model, we sought to assess the hypothesis that hyperresponsiveness to antigens shared among the genus Chlamydia would elicit pathological responses, whereas serovar-unique antigens elicit protective ones. In confirmation of this hypothesis, one should observe enhanced disease and decreased protection from infection subsequent to primary infection and heterotypic secondary challenge compared to results with primary infection and homotypic challenge. Our study design was to assess the pathological outcomes of infertility and hydrosalpinx formation for a susceptible strain of mice subsequent to primary infection with either MoPn or a human serovar (in this case, E) and secondary infection with either MoPn or human serovar A, E, or L2. The strains used should possess a great deal of amino acid sequence homology in the stress response proteins that are implicated in hypersensitizing immune responses but significant variation in the sequences of the epitopes of the implicated protective antigen, the major outer membrane protein (2, 17).
Six- to seven-week-old C3H/HeN female mice were obtained from Harlan-Sprague-Dawley, Indianapolis, Ind., pretreated with progesterone (medroxyprogesterone acetate [Depoprovera; Upjohn]) and inoculated intravaginally with 200 50% infectious doses corresponding to 104 inclusion-forming units (IFU) of MoPn or 106 IFU of human serovars exactly as previously described (12). The infection was assessed by sequential collection of cervical-vaginal swabs at 4, 7, 10, and 14 days postinfection and every 7 days thereafter until cessation of chlamydial shedding. Chlamydial strains were isolated from swabs in HeLa 229 monolayers and enumerated using indirect fluorescence microscopy (3). The primary infection course peaked after 4 to 10 days and resolved in all mice after 28 to 35 days (4, 12) (Fig. 1).
For challenge infections, progesterone pretreatment was administered again and the inoculum was increased by 1 log10. The challenge infections took place at day 56 after the primary infection. Most mice that were first infected with MoPn were solidly immune to heterotypic challenge infection with serovars E and L2. A small number of mice sustained infection subsequent to challenge, but these shed only low numbers of organisms over a shortened course compared to results for the primary infection. Mice that had primary infections with human serovar E and were secondarily challenged with MoPn uniformly became infected, although they shed significantly lower numbers of IFU and sustained a shortened course of infection (12) (Fig. 1A). Last, most mice challenged with homotypic or heterotypic human strains either were solidly immune or sustained an infection course that was blunted in time and magnitude (12) (Fig. 1B and C). In the current experiment, control mice consisted of age-matched and similarly progesterone-treated mice and mice with a primary infection only.
Fifty-six days subsequent to the secondary challenge infections, all mice were assessed for fertility by adapting the method of De La Maza et al. (5). Briefly, experimental or control female mice were placed three to four per cage with a proven breeder male. Baseline weights were recorded initially and at 7, 10, 14, and 18 days later. In addition, at each time interval, abdomens were visually checked and palpated for pregnancy. Obviously pregnant mice were sacrificed at 18 days, and embryos were counted in the left and right uterine horn. If a mouse was not obviously pregnant at the end of 21 days, she was monitored for an additional week in the absence of a male and then introduced to a different male who had successfully mated in the first round of breeding. If at the end of the second round the mouse remained not pregnant, she was considered infertile, sacrificed, and necropsied. Fertility rates were compared between groups of infected mice and the uninfected age-matched progesterone-treated controls. Statistical significance was assessed by the Fisher exact test with comparisons to the controls.
Hydrosalpinx formation has also been used as a means to assess gross upper genital tract pathology in the mouse subsequent to chlamydial infection, and some use it as a surrogate marker for infertility (13). At the time of necropsy, hydrosalpinx formation was assessed by gross macroscopic or microscopic observation using a dissecting microscope with 10× magnification. In most cases, hydrosalpinx formation was readily observable without the aid of a microscope.
As shown in Table 1, primary infection of mice with MoPn uniformly resulted in infertility and a high rate of hydrosalpinx formation regardless of the presence or nature of a secondary challenge infection. Similarly, a secondary MoPn challenge infection elicited a significant loss of fertility (one of nine fertile) and a high rate of hydrosalpinx formation (seven of eight) when the primary infection was with a human serovar, in this case serovar E. When these results are considered in conjunction with our previous observations (12) (Fig. 1A), we conclude that although prior infection with human serovar E provides a significant degree of protection against MoPn challenge infection, it does not provide sufficient protection against the disease outcome of infertility.
TABLE 1.
Gross pathological outcome subsequent to infection
| Primary infection | Secondary infection | No. of fertile mice/ total no. (%) | Mean no. of embryos per mouse (SD) | No. of mice with hydrosalpinx/ total no. (%)a | P valueb |
|---|---|---|---|---|---|
| MoPn | None | 0/25 (0) | 0 | 21/25 (84) | <0.0001 |
| MoPn | Serovar A | 0/9 (0) | 0 | 7/9 (77.7) | <0.0001 |
| MoPn | Serovar E | 0/8 (0) | 0 | 8/8 (100) | <0.0001 |
| MoPn | Serovar L2 | 2/9 (22.2) | 4 | 7/9 (77.7) | 0.0011 |
| Serovar E | None | 14/17 (82.4) | 7.9 (1.6) | 0/3 | NSc |
| Serovar E | MoPn | 1/9 (11.1) | 2 | 7/8 (87.5) | <0.0001 |
| Serovar E | Serovar A | 8/9 (88.9) | 7.3 (2.4) | 1/1 | NS |
| Serovar E | Serovar E | 4/10 (40) | 6.8 (2.2) | 0/6 | 0.02 |
| Serovar E | Serovar L2 | 9/9 (100) | 7.4 (1.6) | NS | |
| Serovar A | None | 4/5 (80) | 9.0 (0.0) | 0/1 | NS |
| Serovar L2 | None | 5/5 (100) | 8.8 (1.3) | NS | |
| None | 10/10 (100) | 7.4 (2.2) |
Includes bilateral and unilateral hydrosalpinx in infertile mice.
Results were compared to those for age-matched, uninfected mice by the Fisher exact test.
NS, not significant; P > 0.05.
The observations recorded in Table 1 also indicate that mice infected with serovar E during the primary infection retained their fertility subsequent to the primary infection and following secondary challenge with serovar A or L2. In contrast, a homotypic secondary challenge resulted in an increased pathological outcome, with 6 of 10 mice challenged with serovar E in the secondary infection becoming infertile. Hence, heterotypic challenge infection with human serovars does not lead to exacerbated disease pathology in this model, but homotypic challenge does. Interestingly, the six mice that were rendered infertile by two serovar E infections did not form hydrosalpinges as a result, thus indicating a possible alternative mechanism for the induction of infertility, one different from the tubal dilation and scarring that occurs with MoPn infection.
In summary, several conclusions can be drawn from our findings. First of all, we confirmed the finding of De La Maza et al. that a single urogenital infection with MoPn routinely results in infertility in a susceptible mouse strain (5). Also, we report that although prior infection with a human serovar may lead to partial protective immunity against challenge infection as measured by reduced chlamydial shedding and a shortened infection course (12) (Fig. 1A), it does not prevent an adverse disease outcome from a challenge with the potentially more virulent MoPn strain. Lastly, while heterotypic challenge with human serovars does not enhance the gross pathological outcomes assessed (infertility and hydrosalpinx formation), homotypic challenge resulted in a significant enhancement of infertility. This is similar to the findings of Rank et al., who have observed increased oviduct pathology following multiple chlamydial infections in guinea pigs (14). These observations also accentuate the need to distinguish between immunological protection from infection and protection from disease when assessing “protective” immune responses in this model.
Our findings indicate that the murine model is not a useful model for studying exacerbation of disease as a result of secondary heterotypic infections. They also imply that either repetitive homotypic antigen challenges with human strains or perhaps biological characteristics unique to MoPn are responsible for the observed disease outcome in this model. With regard to the former implication, it would be interesting to further explore the nature of the infertility outcome that was observed as a result of homotypic serovar E challenge infection. With regard to the latter, this is likely due to the fact that although the exact origin of MoPn is not clear, it is likely a natural pathogen of the mouse and therefore more virulent in this host than the human strains (4, 9). In support of this explanation is the fact that MoPn infection in the mouse routinely results in IFU counts as much as 2 log scales higher than that of human serovars during much of the infection (4, 12) (Fig. 1). These behavioral differences between the murine and human strains of C. trachomatis in the mouse are at least partly due to disparate sensitivities to gamma interferon production, because human serovars are highly sensitive to gamma interferon-dependent mechanisms, whereas MoPn is relatively resistant (3, 11).
Acknowledgments
We thank Michael A. Yanez for his excellent technical assistance on this project.
This work was supported by Public Health Service grant AI37807 (to K.H.R.) and a Midwestern University Summer Research Fellowship (to J.L.D.).
REFERENCES
- 1.Brunham R C. Vaccine design for the prevention of Chlamydia trachomatis infection. In: Orfila J, Byrne G I, Chernesky M A, Grayston J T, Jones R B, Ridgway G L, Saikku P, Schachter J, Stamm W E, Stephens R S, editors. Chlamydial infections: proceedings of the Eighth International Symposium on Human Chlamydial Infections. Bologna, Italy: Societa Editrice Esculapio; 1994. pp. 73–82. [Google Scholar]
- 2.Brunham R C, Peeling R W. Chlamydia trachomatis antigens: role in immunity and pathogenesis. Infect Agents Dis. 1994;3:218–233. [PubMed] [Google Scholar]
- 3.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darville T, Andrews C W, Laffoon K K, Shymasani W, Kishen L R, Rank R G. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De La Maza L M, Pal S, Khamesipour A, Peterson E M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grayston J T, Wang S-P, Yang Y F, Woolridge R L. The effects of trachoma vaccine on the course of experimental infection in blind volunteers. J Exp Med. 1962;115:1009–1022. doi: 10.1084/jem.115.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jawetz E, Rose L, Hanna L, Thygeson P. Experimental inclusion conjunctivitis in man. J Am Med Assoc. 1965;194:150–162. [PubMed] [Google Scholar]
- 8.Morrison R P. Immune responses to Chlamydia are protective and pathogenic. In: Bowie W R, Caldwell H D, Jones R P, Mardh P A, Ridgway G L, Schachter J, Stamm W E, Ward M E, editors. Chlamydial infections: proceedings of the Seventh International Symposium on Human Chlamydial Infections. Cambridge, United Kingdom: Cambridge University Press; 1990. pp. 163–172. [Google Scholar]
- 9.Nigg C, Eaton M D. Isolation from normal mice of a pneumotropic virus which forms elementary bodies. J Exp Med. 1944;79:497–510. doi: 10.1084/jem.79.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patton D L, Sweeney Y T C, Kuo C C. Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in monkeys: a pathogenic mechanism of tubal damage. J Infect Dis. 1994;169:680–683. doi: 10.1093/infdis/169.3.680. [DOI] [PubMed] [Google Scholar]
- 11.Perry L L, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell H D. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 12.Ramsey K H, Cotter T W, Salyer R D, Miranpuri G S, Yanez M A, Poulsen C E, DeWolfe J L, Byrne G I. Prior genital tract infection with a murine or human biovar of Chlamydia trachomatis protects mice against heterotypic challenge infection. Infect Immun. 1999;67:3019–3025. doi: 10.1128/iai.67.6.3019-3025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rank R G. Models of immunity. In: Stephens R S, editor. Chlamydia: intracellular biology, pathogenesis, and immunity. Washington, D.C.: American Society for Microbiology; 1999. pp. 239–295. [Google Scholar]
- 14.Rank R G, Sanders M M, Patton D L. Increased incidence of oviduct pathology in the guinea pig after repeat vaginal inoculation with the chlamydial agent of guinea pig inclusion conjunctivitis. Sex Transm Dis. 1995;22:48–54. doi: 10.1097/00007435-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Tuffrey M, Alexander F, Woods C, Taylor-Robinson D. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil. 1992;95:31–38. doi: 10.1530/jrf.0.0950031. [DOI] [PubMed] [Google Scholar]
- 16.Tuffrey M, Falder P, Taylor-Robinson D. Genital tract infection and disease in nude and immunologically competent mice after inoculation of a human strain of Chlamydia trachomatis. Br J Exp Pathol. 1982;63:539–544. [PMC free article] [PubMed] [Google Scholar]
- 17.Ward M E. The immunobiology and immunopathology of chlamydial infections. APMIS. 1995;103:769–796. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 18.Woolridge R L, Grayston J T, Chang I H Y C Y, Cheng K H. Long-term follow-up of the initial (1959–1960) trachoma vaccine field trial on Taiwan. Am J Ophthalmol. 1967;63:1650–1655. doi: 10.1016/0002-9394(67)94159-1. [DOI] [PubMed] [Google Scholar]