Abstract
The aim of this investigation was to determine the associations of serum irisin and fibroblast growth factor-21 (FGF-21) with the measures of energy homeostasis, training stress and other energy homeostasis hormones in highly trained adolescent rhythmic gymnasts (RG). Thirty-three RG and 20 untrained controls (UC) aged 14–18 years participated in this study. Body composition, resting energy expenditure (REE), peak oxygen consumption, and different energy homeostasis hormones in serum, including irisin, FGF-21, leptin, and resistin, were measured. Irisin and FGF-21 were not significantly different (p > 0.05) between RG and UC groups. In RG, serum irisin was positively associated with REE (r = 0.40; p = 0.021) and leptin (r = 0.60; p = 0.013), while serum FGF-21 was related to body fat mass (r = 0.46; p = 0.007) and leptin (r = 0.45; p = 0.009). Irisin was related to FGF-21, independent of age, body fat, and lean masses (r = 0.36; p = 0.049) in RG. In conclusion, serum irisin concentration was associated with energy expenditure and serum FGF-21 level with energy availability measures in lean adolescent athletes, while no relationships of irisin and FGF-21 with energy status measures were observed in lean nonathletic adolescents.
Keywords: rhythmic gymnasts, irisin, fibroblast growth factor-21, energy homeostasis, training stress
1. Introduction
The regulation of energy homeostasis and high training stress is dependent on several peripheral factors that communicate the status of body energy stores to the brain [1]. These peripheral factors are also synthesized from adipose, muscle, and bone tissues, which may act as endocrine organs [2]. For example, it has been found that specific adipose-derived factors, including circulating leptin and adiponectin concentrations, can be sensitive to changes in training volume and could be used to characterize physical stress conditions in athletes [1]. In elite female rowers, Kurgan et al. [3] investigated such peripheral markers as tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), insulin-like growth factor-1 (IGF-1), and leptin to assess variations in energy homeostasis and training stress over a training year. It appeared that fluctuations in training load (high vs. low) were accompanied by parallel changes in TNF-α and IL-6, while IGF-1 and leptin remained relatively stable over a training season in this population of young female athletes with suitable energy availability [3]. Similarly, adipokines such as circulating leptin, adiponectin, resistin, and visfatin concentrations have been used to characterize energy homeostasis in highly trained adolescent rhythmic gymnasts (RG), who begin to exercise at an early age and often adopt negative energy balance to retain lean physique [4,5,6]. Adiponectin was positively associated with weekly training volume in elite young RG participating in World Championships [7], while leptin levels in highly trained adolescent RG can be as low as in anorectic individuals and chronic athletic activity in the presence of prolonged high energy expenditure state decreases leptin concentrations in growing and maturing RG athletes [8]. The importance of tissue crosstalk in energy homeostasis has been highlighted by studies examining the role of different muscle-derived factors in regulating several adipose tissue adaptations to energy metabolism [2,9,10].
Recently, various myokines have been found to mediate training-induced energy and metabolic processes [9,11], besides the most investigated and well-known myokine-IL-6 [1,12]. These myokines include myostatin [13], follistatin [14], irisin [15], and fibroblast growth factor-21 (FGF-21) [16], which have emerged as potential mediators of training-induced energy metabolism. While myostatin is a negative regulator of muscle mass [2], follistatin is a myostatin-binding peptide that promotes skeletal muscle development and exerts metabolic benefits by improving glucose metabolism [14]. One of the more recently identified myokine, irisin, is primarily secreted by muscle tissue and released into circulation during exercise, resulting in increased energy expenditure and improved glucose metabolism [17]. Irisin levels have been reported to be positively associated with body fat mass (FM) as a surrogate measure of energy availability [18]. However, no differences in serum irisin concentrations were observed between amenorrheic athletes, eumenorrheic athletes and nonathletes aged 14–21 years [19], and between normal weight and overweight young women with a mean age of 18 years [20]. Furthermore, serum irisin concentrations were not related to measures of physical activity and physical fitness in a group of healthy lean women of a wide age range [21]. In addition to irisin, FGF-21 has also emerged as an energy homeostasis hormone that has been implicated in the modulation of energy metabolism in athletes [16,22]. Accordingly, FGF-21 has been proposed as a myokine with metabolic effects on glucose and lipid metabolism that promotes body FM loss [2,23]. It, therefore, appears that irisin and FGF-21 may signal energy status in specific groups of individuals. However, the response of these myokines to chronic exercise training remains to be elucidated in lean adolescent females.
The exact role of circulating irisin and FGF-21 levels in energy homeostasis in female athletes is still not clear. We have previously demonstrated that acute negative energy balance caused by prolonged aerobic exercise elicited the increment in serum irisin and FGF-21 levels and the increase in irisin was related to weekly training volume, while the increase in FGF-21 was associated with exercise energy expenditure in young female rowers with a mean age of 18 years [16]. The present study was undertaken to examine the effect of prolonged athletic activity on serum irisin and FGF-21 concentrations in highly trained adolescent RG athletes. To our best knowledge, whether these myokine levels are related to the measures of energy homeostasis, such as body FM as an index of energy stores, resting energy expenditure (REE), training volume, or other hormones involved in energy homeostasis have not been studied in lean adolescent athletes. We hypothesized that serum irisin and FGF-21 concentrations are higher in highly trained adolescent RG in comparison with nonathletes, and secondly that these circulating myokine levels would be associated with other measures of energy homeostasis in highly trained female athletes with chronically increased energy expenditure state.
2. Materials and Methods
2.1. Participants and Research Design
This study included 53 healthy adolescent females with ages ranging from 14 to 18 years. Participants were divided into rhythmic gymnasts (RG; n = 33) and untrained controls (UC; n = 20). Before entering the study, participants completed medical and training history questionnaires. Athletes were recruited from local training groups and were competing at the international level. Rhythmic gymnasts had trained regularly for the last 10.3 ± 0.9 years with a mean weekly training volume of 17.6 ± 5.3 h/week. The UC group consisted of adolescents, who took part only in compulsory physical education classes and were not involved in any training groups. Information about the age of menarche, changes in the menstrual cycle, past or present diseases, and any kind of medication, vitamin, or mineral supplement, was collected [24]. None of the participants received any medications or had a history of any chronic diseases. No restrictions were placed on dietary intake, and participants consumed their ordinary everyday diet [25]. All UC adolescent females were eumenorrheic, while 22 participants in the RG group were eumenorrheic and 11 were oligomenorrheic or had secondary amenorrhea. Menstruating participants were examined during the follicular phase, where the blood sample was taken between days 7 and 11 from the onset of menstruation [24].
The study design, purpose, and possible risks were explained to the participants and their parents, who gave their written informed consent before entering the study. The study protocol was approved by the Medical Ethics Committee of the University of Tartu, Estonia and was conducted in accordance with the Declaration of Helsinki. Participants underwent an observational cross-sectional examination. Measurements of the current investigation included anthropometry, body composition, energy expenditure, peak oxygen consumption, and blood analyses.
2.2. Measurements
2.2.1. Body Composition
Body height (Martin metal anthropometer, GPM Anthropological Instruments, Zurich, Switzerland) and body mass (medical electronic scale, A&D Instruments Ltd., Abingdon, UK) were measured to the nearest 0.1 cm and 0.05 kg, and body mass index (BMI) was also calculated (kg/m2). Body composition was measured by dual-energy X-ray absorptiometry (DXA) using the DPX-IQ densitometer (Lunar Corporation, Madison, WI; USA) Participants were scanned in light clothing while lying flat on their backs with arms on their sides. Whole body fat percent (body fat %), FM, and lean body mass (LBM) values were obtained. All DXA measurements and results were evaluated by the same examiner. The coefficient of variations (CVs) for the obtained results was less than 2% [25].
2.2.2. Resting Energy Expenditure and Aerobic Performance
Resting energy expenditure (REE) was measured in the morning after an overnight fast. Participants were instructed to avoid any intense physical activity for the 24 h period before REE measurement. After voiding, subjects laid down for 15 min before the measurement of oxygen consumption (VO2) and carbon dioxide (VCO2) production over 30 min. The first 5 min and last 5 min of the measurement were discarded to ensure adequate measurement [26]. A portable open circuit spirometry system (MetaMax 3B, Cortex Biophysic GmbH, Leipzig, Germany) was used, data were stored at 10 s intervals, and the mean of the 20 min was used to calculate REE according to Weir`s equation [27]: Basal metabolic rate (BMR) (kcal/min) = 3.9 [VO2 (l/min)] + 1.1 [VCO2 (l/min)], and REE (kcal/day) = BMR × 1440 min.
Maximal aerobic performance was determined by a stepwise incremental exercise test until volitional exhaustion using an electrically braked bicycle ergometer (Corival V3; Lode, Netherlands) [28]. The initial work rate was 40 W, and the stage increment was 35 W every 3 min until the maximal voluntary exhaustion was reached. The test was designed to elicit maximal power output at approximately 15–18 min for each subject [28]. Pedaling frequency was set to 60–70 rpm. Participants were strongly encouraged to produce the maximal effort. Respiratory gas exchange variables were measured throughout the test using breath-by-breath mode with data being recorded at 10 s intervals. Subjects breathed through a facemask. Oxygen consumption, carbon dioxide output and minute ventilation were continuously measured using a portable open-air spirometry system (MetaMax 3B, Cortex Biophysic GmbH, Leipzig, Germany). The analyzer was calibrated with gases of known concentration before the test according to the manufacturer`s guidelines. All data were calculated by means of computer analysis using standard software (MetaMax-Analysis 3.21, Cortex, Leipzig, Germany). Peak oxygen consumption was measured, and maximal aerobic performance was defined as described previously [28].
2.2.3. Blood Analysis
Venous blood samples were drawn between 8:00 and 9:00 a.m. after an overnight fast from an antecubital vein with the participants sitting in an upright position. Blood serum was separated and frozen at −80 °C for further analyses. Irisin was determined using an enzyme-linked immunosorbent assay (ELISA) kit using a specific Irisin/FDNC5 monoclonal antibody (R&D Systems Inc., Minneapolis, MN, USA) [29]. This assay had intra- and inter-assay CVs of 2.5% and 8.7%, respectively, and the least detection limit was 0.25 ng/mL. Fibroblast growth factor-21 (FGF-21) was assessed by a commercially available ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) with a minimum detectable level of 1.61 pg/mL, and intra-assay CV 3.5% and inter-assay CV 5.2%. Leptin was determined by Evidence® Biochip Technology (Randox Laboratories Ltd., Crumlin, UK) with the intra- and inter-assay CVs of 4.6% and 6.0%. Resistin was also measured by Evidence® Biochip Technology (Randox Laboratories Ltd., Crumlin, UK) with the intra- and inter-assay CVs of 5.2% and 9.1%.
2.3. Statistical Analysis
Data analysis was performed using the SPSS software version 21.0 package for Windows (Chicago, IL, USA). Standard statistical methods were used to calculate means and standard deviations (±SD). Evaluation of data normality was performed with the Kolmogorov-Smirnov method. Data that were not normally distributed were logarithmically transformed prior to analyses to approximate a normal distribution. This was necessary for body FM and serum leptin values. Statistical comparisons between the groups were made using an independent t-test. In addition, effect size (ES, eta squared) thresholds of 0.01, 0.06, and 0.14 were used to identify small, moderate, and large differences, respectively, to define the magnitude of the difference [30]. Pearson correlation coefficients were calculated to assess linear relationships. In addition, partial correlation analyses controlling for age, body FM, and LBM were used to control for confounders [19]. The level of significance was set at p < 0.05
3. Results
The studied RG and UC groups did not differ (ES < 0.05; p > 0.05) in chronological age, body height, body mass, BMI, and REE (Table 1). Age at menarche was higher in RG compared with UC groups, body fat %, FM, and REE/kg were lower, and LBM, training volume, VO2peak/kg, and Wmax/kg higher in RG in comparison with UC (p < 0.05; ES > 0.12). Although mean serum irisin was not significantly (p > 0.05) different between the groups, RG had moderately higher (ES = 0.06) irisin values compared with UC (Table 2). The difference in FGF-21 concentrations between groups was only small in magnitude (ES < 0.05; p > 0.05). In addition, leptin levels were largely (ES = 0.34; p < 0.0001) and resistin concentrations moderately (ES = 0.06; p = 0.077) lower in RG in comparison with UC.
Table 1.
Body composition and energy metabolism values (mean ± SD) in rhythmic gymnasts (RG) and untrained controls (UC).
| Variable | RG (n = 33) | UC (n = 20) | p Value | ES |
|---|---|---|---|---|
| Age (yrs) | 16.0 ± 1.2 | 16.5 ± 1.6 | 0.202 | 0.03 |
| Age at menarche (yrs) | 13.6 ± 1.2 | 12.5 ± 0.7 | <0.0001 | 0.26 |
| Body height (cm) | 166.8 ± 5.3 | 166.8 ± 5.0 | 0.976 | 0.01 |
| Body mass (kg) | 55.7 ± 7.0 | 58.4 ± 7.4 | 0.180 | 0.04 |
| BMI (kg/m2) | 20.0 ± 2.0 | 21.0 ± 2.2 | 0.100 | 0.05 |
| Body fat % | 19.5 ± 5.7 | 30.4 ± 6.2 | <0.0001 | 0.45 |
| Body fat mass (kg) | 11.2 ± 4.3 | 17.8 ± 4.8 | <0.0001 | 0.35 |
| Body lean mass (kg) | 42.2 ± 4.1 | 37.7 ± 3.7 | <0.0001 | 0.25 |
| REE (kcal/day) | 1495 ± 208 | 1520 ± 208 | 0.669 | 0.01 |
| REE/kg (kcal/day/kg LBM) | 33.4 ± 4.8 | 38.6 ± 5.0 | <0.0001 | 0.22 |
| Training volume (h/week) | 17.6 ± 5.3 | 2.1 ± 1.3 | <0.0001 | 0.76 |
| VO2peak/kg (mL/min/kg LBM) | 53.6 ± 7.7 | 48.4 ± 5.6 | 0.012 | 0.12 |
| Wmax/kg (W/kg) | 3.2 ± 0.6 | 2.3 ± 0.4 | <0.0001 | 0.41 |
ES, effect size (eta squared); BMI, body mass index; REE, resting energy expenditure; VO2peak/kg, peak oxygen consumption per kg lean body mass; Wmax/kg, maximal power output per kg body mass.
Table 2.
Energy homeostasis regulating hormone concentrations (mean ± SD) in rhythmic gymnasts (RG) and untrained controls (UC).
| Variable | RG (n = 33) | UC (n = 20) | p Value | ES |
|---|---|---|---|---|
| Irisin (ng/mL) | 272.7 ± 140.0 | 207.3 ± 113.7 | 0.084 | 0.06 |
| FGF-21 (pg/mL) | 169.6 ± 56.4 | 188.1 ± 54.3 | 0.249 | 0.03 |
| Leptin (ng/mL) | 1.2 ± 0.6 | 3.7 ± 2.6 | <0.0001 | 0.34 |
| Resistin (ng/mL) | 4.6 ± 2.0 | 5.7 ± 2.4 | 0.077 | 0.06 |
ES, effect size (eta squared); FGF-21, fibroblast growth factor-21.
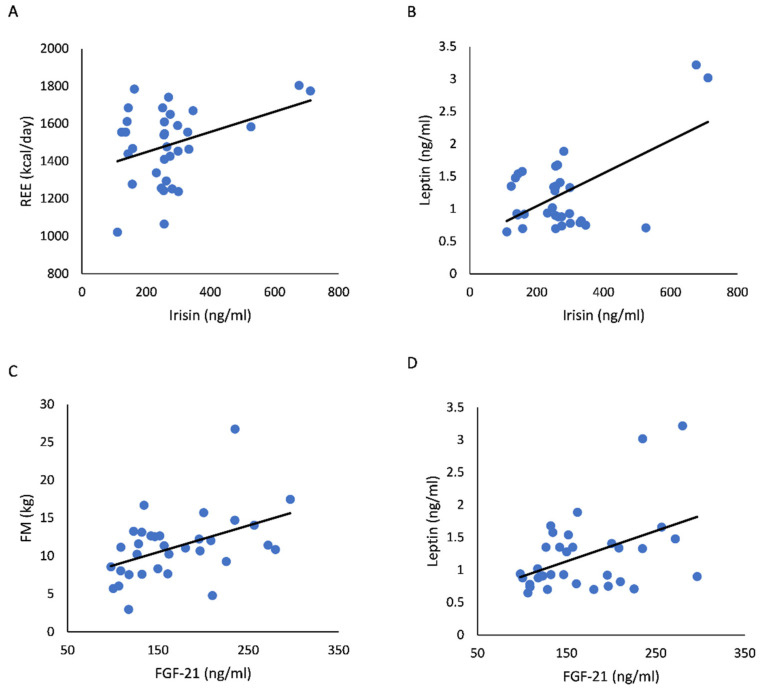
Table 3 presents correlations of irisin and FGF-21 concentrations with energy measures. In the RG group, serum irisin concentration was positively correlated with REE (r = 0.40; p = 0.021) and serum leptin level (r = 0.60; p = 0.013) (Figure 1). In addition, the relationship between irisin and leptin was independent of age, body FM, and LBM (r = 0.57; p = 0.001). In the UC group, serum irisin concentration was positively correlated to resistin levels (r = 0.31; p = 0.036), which remained significant after controlling for age, body FM, and LBM (r = 0.57; p = 0.016). In the RG group, serum FGF-21 concentration was significantly correlated to body FM (r = 0.46; p = 0.007) and serum leptin levels (r = 0.45; p = 0.009) (Figure 1), as opposed to the UC group only to leptin (r = 0.54; p = 0.014). Finally, irisin was related to FGF-21 (r = 0.36; p = 0.012) only in RG, and the association between irisin and FGF-21 was independent of age, body FM and LBM (r = 0.36; p = 0.049).
Table 3.
Relationships of irisin and fibroplast growth factor-21 (FGF-21) with energy measures in rhythmic gymnasts (RG) and untrained controls (UC).
| Variables | Irisin (ng/mL) | FGF-21 (ng/mL) | ||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Fat mass (kg) | ||||
| RG | 0.25 | 0.154 | 0.46 | 0.007 |
| UC | −0.07 | 0.764 | 0.44 | 0.054 |
| Lean mass (kg) | ||||
| RG | 0.29 | 0.101 | 0.28 | 0.144 |
| UC | −0.20 | 0.398 | −0.31 | 0.187 |
| REE (kcal/day) | ||||
| RG | 0.4 | 0.021 | 0.26 | 0.143 |
| UC | −0.05 | 0.838 | 0.26 | 0.27 |
| Training volume (h/week) | ||||
| RG | 0.14 | 0.426 | −0.34 | 0.056 |
| UC | 0.03 | 0.886 | −0.21 | 0.365 |
| VO2peak/kg (mL/min/kg) | ||||
| RG | 0.04 | 0.826 | 0.19 | 0.299 |
| UC | 0.12 | 0.618 | 0.23 | 0.316 |
| Leptin (ng/mL) | ||||
| RG | 0.6 | <0.0001 | 0.45 | 0.009 |
| UC | 0.07 | 0.777 | 0.54 | 0.014 |
| Resistin (ng/mL) | ||||
| RG | 0.31 | 0.076 | 0.04 | 0.808 |
| UC | 0.47 | 0.036 | 0.21 | 0.376 |
REE, resting energy expenditure; VO2peak/kg, peak oxygen consumption per kg lean body mass. Correlations with p < 0.05 are listed in bold.
Figure 1.
Relationships of irisin levels with resting energy expenditure (REE) (A) (r = 0.40; p = 0.021) and leptin (B) (r = 0.60; p < 0.0001), and relationships of fibroblast growth factor-21 (FGF-21) with body fat mass (FM) (C) (r = 0.46; p = 0.007) and leptin (D) (r = 0.45; p = 0.009) in rhythmic gymnasts.
4. Discussion
The present study was undertaken to examine the effect of prolonged athletic activity on energy homeostasis regulating hormones, irisin, and FGF-21 in highly trained adolescent RG. We found that serum irisin and FGF-21 concentrations were not significantly different between RG and UC groups. In addition, serum irisin levels were associated with REE and FGF-21 levels with body FM in RG. In contrast, irisin and FGF-21 were not related to energy expenditure and energy availability measures in lean nonathletic UC. These results demonstrate that circulating irisin and FGF-21 levels may play a role in signaling energy status in a setting of a state of long-term high energy expenditure in adolescent athletes.
It has been suggested that the myokine irisin could play an endocrine control of energy metabolism [22,31]. Circulating irisin levels are elevated in obesity as an excess energy state [32] and reduced in anorexia nervosa as a depleted energy state [33], suggesting that irisin levels may reflect energy stores. Indeed, irisin is known to increase energy expenditure by inducing the browning of subcutaneous white adipocytes, which are metabolically favorable for burning energy through thermogenesis [10,17]. Accordingly, irisin is thought to improve glucose and lipid metabolism in response to exercise training [34,35]. Our data linking irisin levels with REE in highly trained adolescent RG are consistent with the literature indicating that irisin may signal energy availability and promote energy expenditure [34]. Similarly, irisin levels were related to REE in young female runners [22]. No such correlation was found in the UC group in our study. One potential explanation for this could be that the UC subjects were in normal body weight and relatively balanced in terms of energy intake and expenditure. Furthermore, irisin concentrations were also not related to different parameters of energy expenditure in patients with anorexia nervosa [10,36]. It could be speculated that the adolescent RG in our study were in a state of subtle energy deficit, as indicated by their reduced body FM and lower measured REE after correcting for LBM, but not in the state of extreme energy deficit observed in anorexia nervosa. In accordance with young female runners [22], no relationship between irisin with body FM was observed in studied RG, while elevated irisin levels have been reported to be independently associated with obesity risk factors, including body FM in obese adolescents [18]. However, significantly lower (ES = 0.34; p < 0.0001) leptin concentrations in RG were related to irisin levels, and this association was independent of age, body fat, and lean masses (r = 0.57; p = 0.001), demonstrating the muscle-adipose tissue crosstalk in energy homeostasis in adolescent lean females with chronic athletic activity. These seemingly conflicting results demonstrate the specificity of irisin interactions with different markers of energy metabolism in various populations and further studies are needed to clarify the exact role of irisin in energy homeostasis. However, the results of our study and that of Singhal et al. [22] would suggest that irisin concentrations may accentuate the increase in energy expenditure in lean adolescent female athletes, as indicated by the positive associations of circulating irisin levels with measured REE in these individuals.
Studies investigating the effects of chronic athletic activity on circulating irisin levels in adolescent athletes are rare. Earlier studies demonstrated that irisin levels were not significantly different between moderately trained young eumenorrheic runners, amenorrheic runners, and nonathletic controls, although irisin levels were the lowest in amenorrheic runners [19]. Another study found that irisin concentrations were lower in young amenorrheic athletes compared with eumenorrheic athletes and nonathletes [22], while a third study in elite male adolescent tennis players did not observe large variations in irisin concentrations over a competitive tournament season, although it was suggested that irisin may modify overall performance during a long-lasting season [37]. In our study, serum irisin levels were moderately, but not significantly higher (ES = 0.06; p > 0.05) in RG compared with the UC group. However, although VO2peak/kg was higher (ES = 0.12; p = 0.012) in RG compared with UC, no relationship between irisin concentration and maximal aerobic performance was observed in RG, similar to previous studies demonstrating that maximal aerobic performance does not influence circulating irisin concentrations in blood [21,38]. In accordance with our results, training volume did not modify circulating irisin concentrations in adult highly trained athletes [15,39] and basal irisin may not be a good marker of training volume over a training macrocycle [38]. However, as interval training caused moderate and significant increases in serum irisin levels in previously untrained adults [40,41], circulating irisin may be a more sensitive marker of training intensity rather than training volume in adult athletes [16,34] as well as in exercising adolescents [42]. It is known that training in rhythmic gymnastics is quite intensive involving numerous jumping exercises daily [6,8] and our studied RG athletes were tested during the preparatory period with a relatively high training volume. It has also been suggested that irisin may be a marker of muscle damage [43] and can provide anti-inflammatory protection [34]. Although the biological role of irisin as a moderator of energy metabolism in response to acute training load remains to be fully elucidated [13], circulating irisin levels have been reported to increase as a result of an acute training session in young female athletes [16]. According to the results of our study, it appears that moderately higher irisin levels in highly trained adolescent RG did not reflect training stress in a setting of chronic high energy expenditure state, whereas decreased irisin levels in amenorrheic athletes likely represent an adaptive response to reduce training stress and conserve energy [22].
In accordance with irisin levels, serum FGF-21 concentrations are higher in obesity [44], reduced in anorexia nervosa [45] and related to body FM [22,23], suggesting that FGF-21 levels may reflect energy stores. Accordingly, 12 weeks of aerobic exercise training decreased circulating FGF-21 concentrations, body mass and glucose uptake in overweight and obese men [46]. It has been suggested that short-term energy expenditure results in increases in circulating FGF-21 levels [47], while long-term chronic energy expenditure may lead to decreased FGF-21 levels to preserve energy [22,48]. Accordingly, acute training load with adequate duration increased serum FGF-21 concentrations in young female athletes [16]. In our study, serum FGF-21 levels were similar between the RG and UC groups in accordance with the previous studies [19,22]. In addition, we found that serum FGF-21 levels were positively correlated with body FM and leptin concentrations in adolescent RG. These relationships suggest crosstalk between muscle and adipose tissue in energy homeostasis and that FGF-21 could be used as a marker of energy stores in adolescent lean females with chronically increased energy expenditure.
Serum FGF-21 concentrations were positively correlated with irisin levels in the RG group. The secretion of FGF-21 and irisin leads to the white adipose tissue browning, uncoupling protein-1-mediated thermogenesis and energy expenditure [19,22]. The secretion of FGF-21 and irisin is increased by the upregulation of peroxisome proliferator-activated receptor-γ, an exercise-induced transcriptional coactivator that promotes energy metabolism [17,49]. Accordingly, our finding of a positive relationship between irisin and FGF-21 suggests a shared pathway for the regulation of energy metabolism in adolescent athletes with high athletic activity.
This study has some limitations. At first, our cross-sectional design rules out the possibility of identifying causal relationships, particularly from the correlation analysis with some individual outliers. Secondly, a relatively small sample size was used, although the number of individuals in both groups was comparable to previous similar studies with athletes in this area [15,19,31,37]. The main strength of the present study is that, to the best of our knowledge, this is the first study investigating whether specific myokine levels such as irisin and FGF-21 are related to the measures of energy homeostasis in highly trained female adolescent athletes as the participants of this study were international level Estonian rhythmic gymnasts from different sports clubs.
5. Conclusions
Serum irisin and FGF-21 concentrations were not significantly different between lean adolescent athletes and nonathletic control subjects. Irisin was associated with energy expenditure and FGF-21 with energy availability in lean adolescent athletes with a state of heavily increased energy expenditure, while no relationships of irisin and FGF-21 with energy status measures were observed in lean nonathletic adolescents with normal daily energy expenditure levels.
Acknowledgments
We thank all the study participants and staff for their assistance.
Author Contributions
Conceptualization, J.J., L.R. and V.T.; methodology, J.J., A.-L.T. and L.R.; formal analysis, J.J, L.R. and V.T.; investigation, L.R., P.P. and K.M.; writing-original draft preparation, J.J.; writing-review and editing, L.R., A.-L.T., P.P., K.M. and V.T..; project administration, J.J.; funding acquisition, J.J. and V.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of the University of Tartu (ethical approval code number 274/T-3).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on a request from the corresponding author for researchers who meet the criteria for access to confidential data.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Estonian Ministry of Education and Science Institutional Grant PRG 1428.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jürimäe J., Mäestu J., Jürimäe T., Mangus B., von Duvillard S.P. Peripheral signals of energy homeostasis as possible markers of training stress in athletes: A review. Metab. Clin. Exp. 2011;60:335–350. doi: 10.1016/j.metabol.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Kirk B., Feehan J., Lombardi G., Duque G. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 2020;18:388–400. doi: 10.1007/s11914-020-00599-y. [DOI] [PubMed] [Google Scholar]
- 3.Kurgan N., Logan-Sprenger H., Falk B., Klentrou P. Bone and inflammatory responses to training in female rowers over an Olympic year. Med. Sci. Sports Exerc. 2018;50:1810–1817. doi: 10.1249/MSS.0000000000001640. [DOI] [PubMed] [Google Scholar]
- 4.Gruodyte R., Jürimäe J., Cicchella A., Stefanelli C., Pasariello C., Jürimäe T. Adipocytokines and bone mineral density in adolescent female athletes. Acta Paediatr. 2010;99:1879–1884. doi: 10.1111/j.1651-2227.2010.01905.x. [DOI] [PubMed] [Google Scholar]
- 5.Jürimäe J., Tillmann V., Cicchella A., Stefanelli A., Võsoberg K., Tamm A.L., Jürimäe T. Increased sclerostin and preadipocyte factor-1 levels in prepubertal rhythmic gymnasts: Associations with bone mineral density, body composition, and adipocytokine values. Osteoporos. Int. 2016;27:1239–1243. doi: 10.1007/s00198-015-3301-0. [DOI] [PubMed] [Google Scholar]
- 6.Roupas N.D., Mamali I., Armeni A.K., Markantes G.K., Theodoropoulou A., Alexandrides T.K., Leglise M., Markou K.B., Georgopoulos N.A. The influence of intensive physical training on salivary adipokine levels in elite rhythmic gymnasts. Horm. Metab. Res. 2012;44:980–986. doi: 10.1055/s-0032-1321816. [DOI] [PubMed] [Google Scholar]
- 7.Roupas N.D., Maimoun L., Mamali I., Coste O., Tsouka A., Mahadea K.K., Mura T., Philibert P., Gaspari L., Mariano-Goulart D., et al. Saliva adiponectin levels are associated with training intensity but not with bone mass or reproductive function in elite rhythmic gymnasts. Peptides. 2014;51:80–85. doi: 10.1016/j.peptides.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Jürimäe J., Gruodyte-Racience R., Baxter-Jones A.D.G. Effects of gymnastics activities on bone accrual during growth: A systematic review. J. Sports Sci. Med. 2018;17:245–258. [PMC free article] [PubMed] [Google Scholar]
- 9.Domin R., Dadej D., Pytka M., Zybek-Kocik A., Ruchala M., Guzik P. Effect of various exercise regimens on selected exercise-induced cytokines in healthy people. Int. J. Environ. Res. Public Health. 2021;18:1261. doi: 10.3390/ijerph18031261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maimoun L., Mariano-Goulart D., Huguet H., Renard E., Lefebvre P., Picot M.C., Dupuy A.M., Cristol J.P., Courtet P., Boudousq V., et al. In patients with anorexia nervosa, myokine levels are altered but are associated with bone mineral density loss and bone turnover alteration. Endocr. Connect. 2022;11:e210488. doi: 10.1530/EC-21-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbalho S.M., Prado Neto E.V., De Alvares Goulart R., Bechara M.D., Baisi Chagas E.F., Audi M., Guissoni Campos L.M., Landgraf Guiger E., Buchaim R.L., Buchaim D.V., et al. Myokines: A descriptive review. J. Sports Med. Phys. Fit. 2020;60:1583–1590. doi: 10.23736/S0022-4707.20.10884-3. [DOI] [PubMed] [Google Scholar]
- 12.Rämson R., Jürimäe J., Jürimäe T., Mäestu J. The influence of increased training volume on cytokines and ghrelin concentration in college level male rowers. Eur. J. Appl. Physiol. 2008;104:839–846. doi: 10.1007/s00421-008-0839-y. [DOI] [PubMed] [Google Scholar]
- 13.Sliwicka E., Cison T., Pilaczyriska-Szczesniak L., Ziemba A., Straburzynska-Lupa A. Effect of marathon race on selected myokines and sclerostin in middle-aged male amateur runners. Sci. Rep. 2021;11:2813. doi: 10.1038/s41598-021-82288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Z., Tian Y., Valenzuela P.L., Huang C., Zhao J., Hong P. Myokine response to high-intensity interval vs. resistance exercise: An individual approach. Front. Physiol. 2018;9:1735. doi: 10.3389/fphys.2018.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudio A., Rapisarda R., Xourafa A., Zanoli L., Manfre V., Catalano A., Singorelli S.S., Castellino P. Effects of competitive physical activity on serum irisin levels and bone turnover markers. J. Endocrinol. Investig. 2021;44:2235–2241. doi: 10.1007/s40618-021-01529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jürimäe J., Vaiksaar S., Purge P., Tillmann V. Irisin, fibroplast growth factor-21, and follistatin responses to endurance rowing training session in female rowers. Front. Physiol. 2021;12:689696. doi: 10.3389/fphys.2021.689696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J., Rasbach K.A., Boström E.A., Choi J.H., Long J.Z., et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang H.B., Kim H.J., Kang J.H., Park S.I., Park K.H., Lee H.J. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metab. Clin. Exp. 2017;73:100–108. doi: 10.1016/j.metabol.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Lawson E.A., Ackerman K.E., Slattery M., Marengi D.A., Clarker H., Misra M. Oxytocin secretion is related to measures of energy homeostasis in young amenorrheic athletes. J. Clin. Endocrinol. Metab. 2014;99:E881–E885. doi: 10.1210/jc.2013-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez Munoz I.Y., Del Socorro Camarillo Romero E., Correa Padillo T., Guadalupe Santillan Benitez J., Del Socorro Camarillo Romero M., Montenegr Morales L.P., Huitron Bravo G.G., De Jesus Garduno Garcia J. Association of irisin serum concentration and muscle strength in normal-weight and overweight young women. Front. Endocrinol. 2019;10:621. doi: 10.3389/fendo.2019.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biniaminov N., Bandt S., Roth A., Haertel S., Neumann R., Bub A. Irisin, physical activity and fitness status in healthy humans: No association under resting conditions in a cross-sectional study. PLoS ONE. 2018;13:e0189254. doi: 10.1371/journal.pone.0189254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhal V., Lawson E.A., Ackerman K.E., Fazeli P.K., Clarke H., Lee H., Eddy K., Marengi D.A., Derrico N.P., Bouxsein M.L., et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-anthletes and are associated with bone density and strength estimates. PLoS ONE. 2014;9:e100218. doi: 10.1371/journal.pone.0100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalafi M., Alamdari K.A., Symonds M.E., Nobari H., Carlos-Vivas J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: Systematic review and meta-analysis. Hormones. 2021;20:23–33. doi: 10.1007/s42000-020-00245-3. [DOI] [PubMed] [Google Scholar]
- 24.Vaiksaar S., Jürimäe J., Mäestu J., Purge P., Kalytka S., Shakhlina L., Jürimäe T. No effect of menstrual cycle phase on fuel oxidation during exercise in rowers. Eur. J. Appl. Physiol. 2011;111:1027–1034. doi: 10.1007/s00421-010-1730-1. [DOI] [PubMed] [Google Scholar]
- 25.Lätt E., Jürimäe J., Haljuaste K., Cicchella A., Purge P., Jürimäe T. Physical development and swimming performance during biological maturation in young female swimmers. Coll. Antropol. 2009;33:117–122. [PubMed] [Google Scholar]
- 26.Melin A., Tornberg A.B., Skouby S., Moller S.S., Sundgot-Borgen J., Faber J., Sidelmann J.J., Aziz M., Sjödin A. Energy availability and the female athlete triad in elite endurance athletes. Scand. J. Med. Sci. Sports. 2015;25:610–622. doi: 10.1111/sms.12261. [DOI] [PubMed] [Google Scholar]
- 27.Weir J.V.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949;109:4521. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remmel L., Tamme R., Tillmann V., Mäestu E., Purge P., Mengel E., Riso E.M., Jürimäe J. Pubertal physical activity and cardiorespiratory fitness in relation to late adolescent body fatness in boys: A 6-year follow-up study. Int. J. Environ. Res. Public Health. 2021;18:4881. doi: 10.3390/ijerph18094881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jürimäe J., Purge P., Tillmann V. Serum sclerostin and cytokine responses to prolonged sculling exercise in highly-trained male rowers. J. Sports Sci. 2021;39:591–597. doi: 10.1080/02640414.2020.1837428. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins W., Marshall S.W., Batterham A.M., Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009;41:3–13. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 31.Benedini S., Dozio E., Invernizzi P.L., Vianello E., Banfi G., Terruzzi I., Luzi L., Corsi Romanelli M.M. Irisin, a potential link between physical exercise and metabolism—An observational study in differently trained subjects, from elite athletes to sedentary people. J. Diabetes Res. 2017;2017:1039161. doi: 10.1155/2017/1039161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Meneck F., De Souza L.V., Brioschi M.L., Do Carmo Franco M. Emerging evidence for the opposite role of circulating irisin levels and brown adipose tissue activity measured by infrared thermography in anthropometric and metabolic profile during childhood. J. Therm. Biol. 2021;99:103010. doi: 10.1016/j.jtherbio.2021.103010. [DOI] [PubMed] [Google Scholar]
- 33.Stengel A., Hofmann T., Goebel-Stengel M., Elbelt U., Kobelt P., Klapp B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity—Correlation with body mass index. Peptides. 2013;39:125–130. doi: 10.1016/j.peptides.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Fatouros I.G. Is irisin the new player in exercise-induced adaptations or not? A 2017 update. Clin. Chem. Lab. Med. 2018;56:525–548. doi: 10.1515/cclm-2017-0674. [DOI] [PubMed] [Google Scholar]
- 35.Korkmaz A., Venojärvi M., Wasenius N., Manderoos S., Deruisseau K.C., Gidlund E.K., Heinonen O.J., Lindholm H., Aunola S., Eriksson J.G., et al. Plasma irisin is increased following 12 weeks of Nordic walking and associates with glucose homeostasis in overweight/obese men with impaired glucose regulation. Eur. J. Sport Sci. 2019;19:258–266. doi: 10.1080/17461391.2018.1506504. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann T., Elbelt U., Ahnis A., Kobelt P., Rose M., Stengel A. Irisin levels are not affected by physical activity in patients with anorexia nervosa. Front. Endocrinol. 2014;4:202. doi: 10.3389/fendo.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witek K., Zurek P., Zmijewski P., Jaworska J., Lipinska P., Dzedzej-Gmiat A., Antosiewicz J., Ziemann E. Myokines in response to a tournament season among young tennis players. BioMed Res. Int. 2016;2016:1460892. doi: 10.1155/2016/1460892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grzebisz-Zatonska N., Poprzecki S., Pokora I., Mikolajec K., Kaminski T. Effect of seasonal variation during annual cyclist training on somatic function, white blood cell composition, immunological system, selected hormones and their interaction with irisin. J. Clin. Med. 2021;10:3299. doi: 10.3390/jcm10153299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jürimäe J., Purge P. Irisin and inflammatory cytokines in elite male rowers: Adaptation to volume-extended training period. J. Sports Med. Phys. Fit. 2021;61:102–108. doi: 10.23736/S0022-4707.20.11076-4. [DOI] [PubMed] [Google Scholar]
- 40.Dünwald T., Melmer A., Gatterer H., Salzmann K., Ebenbichler C., Burtscher M., Schobersberger W., Grander W. Supervised short-term high-intensity training on plasma irisin concentrations in type 2 diabetic patients. Int. J. Sports Med. 2019;40:158–164. doi: 10.1055/a-0828-8047. [DOI] [PubMed] [Google Scholar]
- 41.Jürimäe J., Purge P., Remmel L., Ereline J., Kums T., Kamandulis S., Brazaitis M., Venckunas T., Pääsuke M. Changes in irisin, inflammatory cytokines and aerobic capacity in response to three weeks of supervised sprint interval training in older men. J. Sports Med. Phys. Fit. 2023;63:162–169. doi: 10.23736/S0022-4707.22.13949-6. [DOI] [PubMed] [Google Scholar]
- 42.Morelli C., Avolio E., Galluccio A., Caparello G., Manes E., Ferraro S., De Rose D., Santoro M., Basone I., Catalano S., et al. Impact of vigorous-intensity physical activity on body composition parameters, lipid profile markers, and irisin levels in adolescents: A cross-sectional study. Nutrients. 2020;12:742. doi: 10.3390/nu12030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaughan R.A., Gannon N.P., Mermier C.M., Conn C.A. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J. Physiol. Biochem. 2015;71:679–689. doi: 10.1007/s13105-015-0433-9. [DOI] [PubMed] [Google Scholar]
- 44.Berti L., Imler M., Zdichavsky M., Meile T., Böhm A., Stefan N., Fritsche A., Beckers J., Köningsrainer A., Häring H.U. Fibroblast growth factor 21 is elevated in metabolically unhealthy obesity and affects lipid deposition, adipogenesis, and adipokine secretion of human abdominal subcutaneous adipocytes. Mol. Metab. 2015;4:519–527. doi: 10.1016/j.molmet.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dostalova I., Kavalkova P., Haluzikova D., Lacinova Z., Mraz M., Papezova H., Haluzik M. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J. Clin. Endocrinol. Metab. 2008;93:3627–3632. doi: 10.1210/jc.2008-0746. [DOI] [PubMed] [Google Scholar]
- 46.Matsui M., Kosaki K., Myoenzono K., Yoshikawa T., Park J., Kuro-O M., Maeda S. Effect of aerobic exercise training on circulating fibroplast growth factor-21 response to glucose challenge in overweight and obese men: A pilot study. Exp. Clin. Endocrinol. Diabetes. 2022;130:723–729. doi: 10.1055/a-1902-3872. [DOI] [PubMed] [Google Scholar]
- 47.Cuevas-Ramos D., Paloma A.V., Meza-Arana C.E., Brito-Cordova G., Gomez-Perez F.J., Mehta R., Oseguera-Moguel J., Aguilar-Salinas C.A. Exercise increases serum Fibroblast Growth Factor 21 (FGF21) levels. PLoS ONE. 2012;7:e38022. doi: 10.1371/journal.pone.0038022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniguchi H., Tanisawa K., Sun X., Kubo T., Higuchi M. Endurance exercise reduces hepatic fat content and serum fibroblast growth factor 21 levels in elderly men. J. Clin. Endocrinol. Metab. 2016;101:191–198. doi: 10.1210/jc.2015-3308. [DOI] [PubMed] [Google Scholar]
- 49.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R., Mohammadi M., Finck B.N., Mangelsdorf D.J., Kliewer S.A., et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on a request from the corresponding author for researchers who meet the criteria for access to confidential data.