Abstract
Background and Objectives: Identification and targeting of membrane proteins in tumor cells is one of the key steps in the development of cancer drugs. The receptor tyrosine kinase-like orphan receptor (ROR) type 1 is a type-I transmembrane protein expressed in various cancer tissues, which is in contrast to its limited expression in normal tissues. These characteristics make ROR1 a candidate target for cancer treatment. This study aimed to identify the prognostic value of ROR1 expression in cancers. Materials and Methods: We conducted a comprehensive systematic search of electronic databases (PubMed) from their inception to September 2021. The included studies assessed the effect of ROR1 on overall survival (OS) and progression-free survival (PFS). Hazard ratios (HR) from collected data were pooled in a meta-analysis using Revman version 5.4 with generic inverse-variance and random effects modeling. Results: A total of fourteen studies were included in the final analysis. ROR1 was associated with worse OS (HR 1.95, 95% confidence interval (CI) 1.50–2.54; p < 0.001) with heterogeneity. The association between poor OS and ROR1 expression was high in endometrial cancer, followed by ovarian cancer, and diffuse large B cell lymphoma. In addition, ROR1 was associated with poor PFS (HR 1.84, 95% CI 1.60–2.10; p < 0.001), but heterogeneity was not statistically significant. In subgroup analysis, high ROR1 expression showed a significantly higher rate of advanced stage or lymph node metastasis. Conclusions: This meta-analysis provides evidence that ROR1 expression is associated with adverse outcome in cancer survival. This result highlights ROR1 as a target for developmental therapeutics in cancers.
Keywords: cancer, meta-analysis, ROR1, receptor tyrosine kinase-like orphan receptor 1
1. Introduction
Patients with cancer have been treated with surgery, chemotherapy, and radiotherapy. In recent decades, individualized approaches targeting gene alterations or membrane proteins on tumor cells have emerged as novel therapies for patients with cancer [1]. Therefore, the challenge of identifying novel biomarkers or surface proteins continues, and new strategies, such as antibody–drug conjugates (ADCs) or adaptive cell therapy, have been introduced into clinical practice [2,3].
Receptor tyrosine kinase-like orphan receptor type 1 (ROR1), a type I orphan receptor tyrosine kinase-like surface protein, is a key protein in embryogenesis and its expression is restricted in normal tissues. The Wnt signaling pathway is a developmental pathway in embryogenesis and regulates cell proliferation and migration during organogenesis through two distinct arms: β-catenin-dependent (canonical) and β-catenin-independent (non-canonical) [4]. It is known that dysregulation of these pathways has been identified in several cancers [5,6]. β-Catenin-dependent Wnt signaling has been associated with breast, colon, gastric, and endometrial cancer development by mutations in β-catenin or adenomatous polyposis coli (APC) [6,7,8]. Additionally, binding of Wnt5a ligand to ROR1 receptor can activate β-catenin-independent Wnt signaling and result in cell proliferation and migration related with carcinogenesis [9,10,11].
However, several studies have shown that ROR1 is highly expressed in neoplastic cells in many different types of cancers, such as leukemia, breast cancer, and ovarian cancer [12,13,14,15,16,17]. It is also associated with the expression of several epithelial–mesenchymal transition markers [16,18], tumor cell proliferation, and relapse [19,20]. Some studies have reported that ROR1 expression is a potential predictive factor for poor survival [21,22]. As such, this surface antigen has been considered as a new target candidate for cancer treatment.
Several preclinical studies evaluating monoclonal antibodies [23,24] or chimeric antigen receptor (CAR)-T cells [25] in leukemia have been conducted. The present meta-analysis assessed whether ROR1 expression has an impact on survival in various types of cancer using published data.
2. Materials and Methods
2.1. Data Sources and Searches
The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [26] and recommendations of the Cochrane Collaboration [27]. We performed electronic searches of MEDLINE (host: PubMed) from 1946 to September 2021 using keywords such as “receptor tyrosine-kinase-like orphan receptor 1” or “ROR1” for intervention; “cancer” or “malignancy” for disease; and “progression”, “survival” or “recurrence” for outcome, and the total number of searched literatures was 243.
2.2. Study Selection
Studies that met the following conditions were included in this meta-analysis: (1) patients had been diagnosed with hematologic or solid cancer; (2) studies with comparative results according to ROR1 expression level; (3) studies reporting a hazard ratio (HR) for progression-free survival (PFS), overall survival (OS), or survival curves, which allowed for the estimation of HR for survivals; and (4) English language publications. Case reports, conference abstracts, or letters were excluded from the study. If a HR with a 95% confidence interval (CI) for survival was not reported directly and could not be calculated, the study was also removed.
2.3. Data Extraction
The following variables were collected for coding the study: publication year, name of first author, journal, country, type of cancer, number of patients included in the analysis, detection methods, agents used for ROR1 expression, cutoff values used for ROR1 intensity, and cancer status (stage, lymph node (LN) metastasis). The primary outcome was OS and the secondary outcome was PFS according to ROR1 expression. In some primary reports, these parameters were extracted directly. In other studies, we estimated them from other reported survival curves using the methods described by Parmar et al. [28]. Additionally, univariable HR and estimated HRs from survival plots were collected using a hierarchal approach.
2.4. Statistical Analysis
The analyses were performed using ReyMan version 5.4 analysis software (Cochrane Collaboration, Copenhagen, Denmark). Extracted HRs were pooled and weighted by generic inverse variance and computed by random effects modeling. Statistical heterogeneity in the results of the studies was assessed by Cochran’s Q and expressed as the I2 index [29]. Subgroup analyses were performed for cancer types, stages, and status of LN metastasis. A funnel plot was produced to assess the possibility of publication bias. Two-sided tests were applied and p-values < 0.05 were considered statistically significant.
3. Results
3.1. Literature Search and Reporting of Information
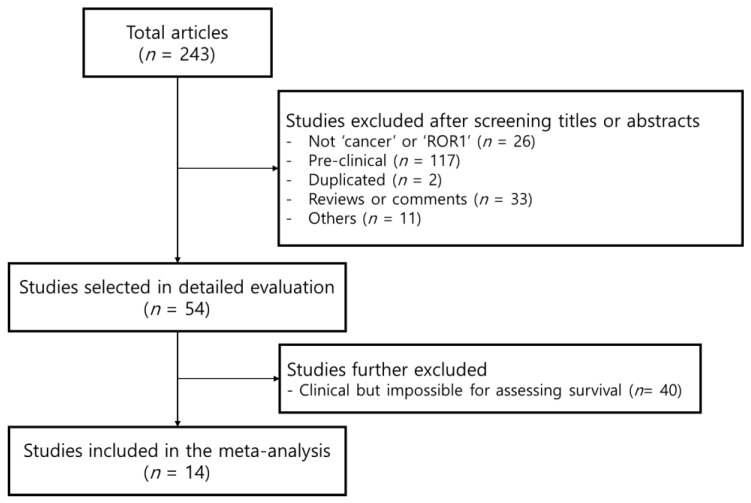
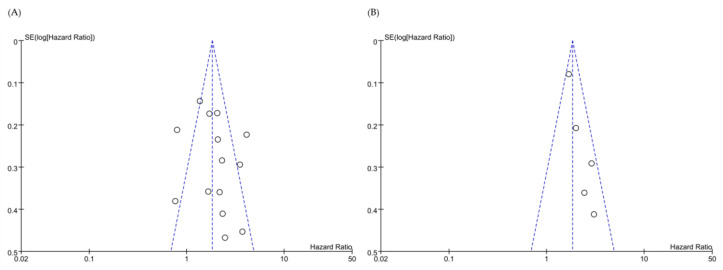
A flowchart of the systematic search process is summarized in Figure 1. A total of 243 retrospective studies were identified in the MEDLINE database (host: PubMed). After review by all the authors, a total of 14 studies, including 4035 subjects, qualified for this study [16,21,22,30,31,32,33,34,35,36,37,38,39,40]. Detailed descriptions of all included studies are outlined in Table 1. Of the 14 studies, three were conducted on leukemia, three on breast cancer, two on ovarian cancer, two on endometrial cancer, two on lung cancer, one on gastric cancer, and one on colorectal cancer. The funnel plot was symmetrical in appearance (Figure 2).
Figure 1.
Flowchart for the receptor tyrosine kinase-like orphan receptor 1 (ROR1) data selection.
Table 1.
Characteristics included for the study of ROR1.
| First Author (Year) [Ref] |
Country | Cancer Type | No. | Detection Method | Agent Used | Cutoff Used | HR for PFS (95% CI) |
HR for OS (95% CI) |
|---|---|---|---|---|---|---|---|---|
| S. Zhang (2012) [16] |
China | Breast cancer | 295 | IHC | Alexa-647-conjugated monoclonal Ab (mAb; 4A5) | Intensity score > 50% | - | 2.30 (1.32–4.01) p = 0.003 |
| H. Zhang (2014) [30] |
China | Ovarian cancer | 100 | IHC | Rabbit polyclonal Ab (1:200, Abcam) | Staining score * ≥ 2 | 2.90 (1.64–5.13) p = 0.01 |
3.54 (1.99–6.29) p = 0.01 |
| S. Zhang (2014) [22] |
U.S.A. | Ovarian cancer | 285 | RT-PCR | N/A | Upper third expression of ROR1 mRNA | 2.0 (1.4–3.0) p = 0.0003 |
1.72 (1.22–2.42) p < 0.05 |
| H. Chang (2015) [31] |
Republic of Korea | Gastric cancer | 424 | IHC | Rabbit polyclonal antibody (1:25; Abcam) | Staining > 50% | - | 0.8 (0.53–1.21) p = 0.189 |
| Chien (2016) [21] |
China | Triple negative breast cancer | 210 | IHC | Rabbit polyclonal antibody (1:100 dilution; proteintech) | Staining score * ≥ 2 | 3.06 (1.36–6.86) p = 0.007 |
2.17 (1.07–4.39) p = 0.031 |
| Cui (2016) [32] |
U.S.A. | CLL | 1568 | IHC | Alexa-647-conjugated monoclonal Ab (mAb; 4A5) | ΔMFI (mean fluorescence intensity) > 32 | 1.69 (1.45–1.98) p < 0.0001 |
2.07 (1.48–2.90) p < 0.0001 |
| Zheng (2016) [33] |
China | Lung cancer | 161 | IHC | Anti-ROR1 (Abcam 135669, 1:20) | Staining score * > 2 | - | 4.11 (2.51–6.38) p < 0.001 |
| Zhou (2017) [35] |
China | Colorectal | 186 | IHC | Polyclonal rabbit Ab (1:20, Abcam) | Staining score * > 2 | - | 2.08 (1.31–3.29) p = 0.002 |
| Li (2017) [34] |
U.S.A. | Triple negative breast cancer | 150 | IHC | N/A | N/A | - | 1.357 (1.024–1.798) p = 0.0336 |
| Henry (2018) [36] |
Australia | Endometrial cancer | 87 | IHC | Anti-ROR1 (Abcam ab135669) | Intensity score = 3 | - | 3.74 (1.54–9.07) p = 0.004 |
| Mao (2019) [37] |
China | DLBCL | 150 | IHC | Primary polyclonal rabbit anti-ROR1 antibody (ab135669, 1:300, Abcam, Cambridge, MA) | Staining score * ≥ 4 | - | 1.67 (0.831–3.370), p = 0.149 |
| Liu (2020) [39] |
Australia | Endometrial cancer | 330 | IHC | Anti-ROR1 (1:50, #564464, BD Bioscience) | Intensity score = 3 | 2.45 (1.21–4.97) p = 0.01 |
2.48 (0.99–6.18) p = 0.05 |
| Giovanna (2020) [40] |
Switzerland | Lung adenocarcinoma | 56 | qRT-PCR | >median of expression | - | 0.769 (0.364–1.62) p = 0.4915 |
|
| Ghaderi (2020) [38] |
Sweden | DLBCL | 33 | IHC | Polyclonal antibody against ROR1 (Proteintech, Manchester, United Kingdom) | >10% (level of unequivocal cytoplasmic and/or membranous staining in the neoplastic B cells) |
- | 2.33 (1.04–5.20) p = 0.039 |
* Staining score = product of staining intensity (0–3) and percentage of ROR1 positive (1–4). ROR1, receptor tyrosine kinase-like orphan receptor 1; HR, hazard ratio; IHC, immunohistochemistry; RT-PCR, reverse transcription-polymerase chain reaction; PFS, progression-free survival; OS, overall survival; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B cell lymphoma.
Figure 2.
Funnel plot for (A) overall survival, and (B) progression-free survival, according to ROR1 expression. Dots(o) represent individual studies.
3.2. Survival
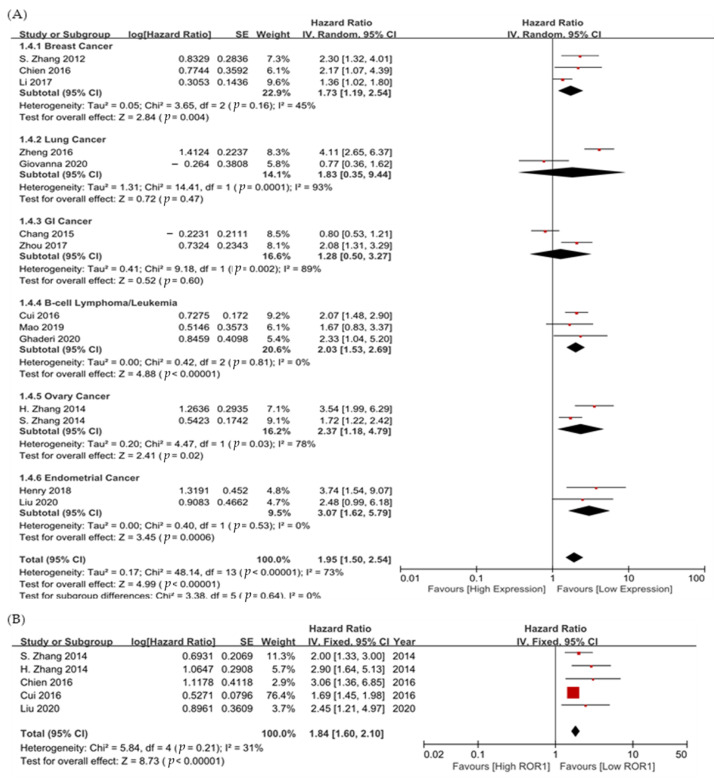
In all 14 studies, ROR1 was significantly associated with poor OS (HR 1.95, 95% CI 1.50–2.54; p < 0.001, Figure 3A). The sample was heterogeneous (χ2 = 48.14, df = 13, Cochran’s Q p < 0.001, I2 = 73%).
Figure 3.
Forest plot showing hazard ratios for (A) overall survival, and (B) progression-free survival, according to ROR1 expression.
For subgroup analysis for each cancer type, endometrial cancer (HR 3.07, 95% CI 1.62–5.79, p = 0.0006), ovarian cancer (HR 2.37, 95% CI 1.18–4.79, p = 0.02), B-cell lymphoma/leukemia (HR 2.03, 95% CI 1.53–2.69, p < 0.0001), and breast cancer (HR 1.73, 95% CI 1.19–2.54, p = 0.004) showed significant results indicating better prognosis of low ROR1 expression.
Data on the association between ROR1 and PFS were reported in five studies, including triple-negative breast cancer, chronic lymphocytic leukemia (CLL), and gynecologic cancers. ROR1 was associated with poor PFS (HR 1.84, 95% CI 1.60–2.10; p < 0.001, Figure 3B). Heterogeneity was not observed (χ2 = 5.84, df = 4, Cochran’s Q p = 0.21, I2 = 31%) because there were few studies which reported PFS. Triple negative breast cancer had significantly worse PFS (HR 3.06, 95% CI 1.36–6.85), followed by ovarian cancer (HR 2.90, 95% CI 1.64–5.13) and endometrial cancer (HR 2.45, 95% CI 1.21–4.97).
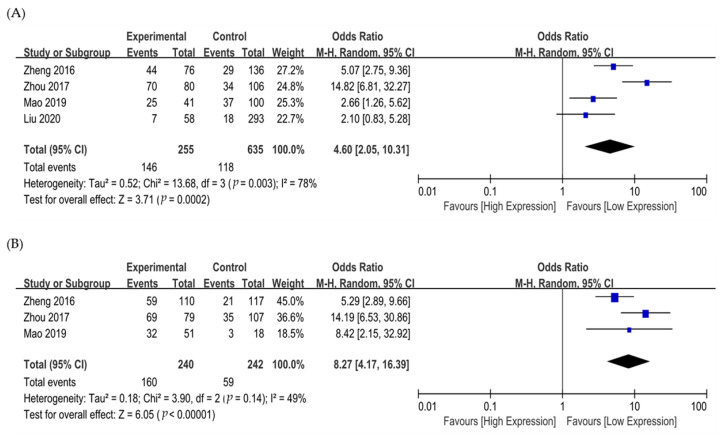
Four studies were included in the analysis of odds ratio (OR) for tumor stage (I/II vs. III/IV) and LN metastasis (Table S1) [33,35,37,39]. There was a significant association between ROR1 expression and advanced stages (OR 4.60, 95% CI 2.05–10.31, p = 0.0002) (Figure 4A), and tumors with high ROR1 expression showed a significantly higher rate of LN metastasis (OR 8.27, 95% CI 4.17–16.39, p < 0.00001) (Figure 4B).
Figure 4.
Forest plot of odds ratio for (A) advanced stages (I/II vs. III/IV) and (B) lymph node metastasis.
4. Discussion
The receptor tyrosine kinase ROR1, a transmembrane glycoprotein, plays an important role in embryogenesis and is overexpressed in many malignant tumors, such as leukemia [12,15], breast cancer [17,18], prostate cancer, and ovarian cancer [22]. Several studies have shown that it plays a critical role in carcinogenesis by activating cell survival signaling events with non-canonical Wnt signaling pathways [41]. Interestingly, ROR1 was shown to be enriched in chemoresistant breast and ovarian cancers. Fultang et al. recently reported that ROR1 is an upstream regulator of the adenosine triphosphate (ATP)-binding cassette (ABC) transporter protein (ABCB1), and promotes its stability in breast cancer [42]. Hanna et al. reported that ROR1-mediated stemness promotes chemoresistance in ovarian cancer [43]. This study revealed that ROR1 expression was significantly associated with poor prognosis in various cancer types. Given the aberrant expression of ROR1 in cancer cells and its important role in cell proliferation, new therapeutic strategies targeting ROR1 have been evaluated in preclinical studies and clinical trials. Among these, monoclonal antibody (mAbs)-based strategies have advanced the most [24,44,45]. The mAbs can bind ligands directly and induce antibody-dependent cellular cytotoxicity or complement–dependent cytotoxicity [46]. Cirmtuzumab, the first humanized mAb targeting ROR1, inhibits ROR1 signaling and stemness signatures in CLL [25]. Clinical trials evaluating the efficacy of its combination with other agents are ongoing: a phase Ib/II trial of cirmtuzumab and Ibrutinib in B cell CLL and mantle cell lymphoma (NCT03088878, NCT03420183), and a phase Ib trial of cirmtuzumab and paclitaxel in breast cancer (NCT02776917, NCT02860676).
Other strategies, such as ADC, bispecific T-cell engager, and CAR T-cells, have also been developed. VLS-101 (a combination of mAb and monomethyl auristatin E) and NBE-002 (a combination of mAb and PNU-159682) are ADCs that have been demonstrated to have anti-cancer effects in preclinical studies [47]. They have also been evaluated in clinical trials (NCT03833180, NCT04504916, NCT04441099). NVG-111, a bispecific antibody targeting ROR1 and CD3, showed anti-tumor effects by T-cell mediated cytotoxicity in preclinical data [48]. ROR1-CAR T-cells are cytotoxic specific to ROR1-expressed tumor cells [49,50]. A phase I trial with ROR1-CART-cells in refractory hematologic malignancies, breast cancer, and lung cancer is ongoing (NCT02706392).
This study has some limitations. First, all included studies were retrospective; therefore, they might have been affected by publication bias and several methodological shortcomings. In addition, there was variation in effect sizes (heterogeneity), scoring systems, and antibodies among the studies. Furthermore, we calculated HR estimates from survival plots in several studies that did not report HRs. This could have resulted in inaccurate estimates.
5. Conclusions
This meta-analysis revealed that ROR1 expression is associated with poor survival in patients with various cancers. Our results suggest that ROR1 expression may be a valuable prognostic biomarker for identifying patients at higher risk of mortality. Considering that ROR1 is a membrane protein and an excellent target for immunotherapy, there is urgent need for new therapeutic agents that target ROR1.
Acknowledgments
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
List of Abbreviation
ADCs, antibody-drug conjugates; ROR, the receptor tyrosine kinase-like orphan receptor; APC, adenomatous polyposis coli; CAR, chimeric antigen receptor; HR, hazard ratio; PFS, progression-free survival; OS, overall survival; CI, confidence interval; LN, lymph node; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B cell lymphoma; OR, odds ratio; ATP, adenosine triphosphate; ABC, ATP-binding cassette; mAb, monoclonal antibody.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina58121867/s1, Table S1: Summary of four studies in analysis for odds ratio related with cancer stage and lymph node metastasis.
Author Contributions
J.H.K. and S.T.P. conceived the original idea. S.Y.P. and J.C. participated in literature searching, data extraction and original draft preparation, and S.T.P. consulted to resolve disagreement. H.S.K. and K.-j.L. carried out statistical analysis and data interpretation. S.Y.J. wrote, and J.-J.L. and N.K. revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by Hallym University Research Fund (HURF-2021-04) and Medical Technology Development Program of the National Research Foundation (NRF), and funded by the Korean government, Ministry of Science and ICT (MSIT) (NRF-2020R1G1A1005483, NRF-2022R1F1A1074597) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare (HR21C0198).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu W., Hu W., Kavanagh J.J. Proteomics in cancer research. Int. J. Gynecol. Cancer. 2002;12:409–423. doi: 10.1136/ijgc-00009577-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y.M., Lian F., Zhou X.F., Chen W.C., Liu H.K., Yao W., Fan W.Z., Li J.P., Chen J., Wang Y. Safety and efficacy of transarterial embolization combined with octreotide LAR on reducing tumor burden for neuroendocrine tumor liver metastasis. Zhonghua Yi Xue Za Zhi. 2019;99:1142–1146. doi: 10.3760/cma.j.issn.0376-2491.2019.15.005. [DOI] [PubMed] [Google Scholar]
- 3.Esparis-Ogando A., Montero J.C., Arribas J., Ocana A., Pandiella A. Targeting the EGF/HER Ligand-Receptor System in Cancer. Curr. Pharm. Des. 2016;22:5887–5898. doi: 10.2174/1381612822666160715132233. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H., Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Bourroul G.M., Fragoso H.J., Gomes J.W., Bourroul V.S., Oshima C.T., Gomes T.S., Saba G.T., Palma R.T., Waisberg J. The destruction complex of beta-catenin in colorectal carcinoma and colonic adenoma. Einstein. 2016;14:135–142. doi: 10.1590/S1679-45082016AO3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin P.J., Sparks A.B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K.W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 7.Clements W.M., Wang J., Sarnaik A., Kim O.J., MacDonald J., Fenoglio-Preiser C., Groden J., Lowy A.M. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503–3506. [PubMed] [Google Scholar]
- 8.Ashihara K., Saito T., Mizumoto H., Nishimura M., Tanaka R., Kudo R. Mutation of beta-catenin gene in endometrial cancer but not in associated hyperplasia. Med. Electron. Microsc. 2002;35:9–15. doi: 10.1007/s007950200001. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda T., Chen L., Endo T., Tang L., Lu D., Castro J.E., Widhopf G.F., 2nd, Rassenti L.Z., Cantwell M.J., Prussak C.E., et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc. Natl. Acad. Sci. USA. 2008;105:3047–3052. doi: 10.1073/pnas.0712148105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho H.Y., Susman M.W., Bikoff J.B., Ryu Y.K., Jonas A.M., Hu L., Kuruvilla R., Greenberg M.E. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc. Natl. Acad. Sci. USA. 2012;109:4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford C.E., Punnia-Moorthy G., Henry C.E., Llamosas E., Nixdorf S., Olivier J., Caduff R., Ward R.L., Heinzelmann-Schwarz V. The non-canonical Wnt ligand, Wnt5a, is upregulated and associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Gynecol. Oncol. 2014;134:338–345. doi: 10.1016/j.ygyno.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Baskar S., Kwong K.Y., Hofer T., Levy J.M., Kennedy M.G., Lee E., Staudt L.M., Wilson W.H., Wiestner A., Rader C. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin. Cancer Res. 2008;14:396–404. doi: 10.1158/1078-0432.CCR-07-1823. [DOI] [PubMed] [Google Scholar]
- 13.Gentile A., Lazzari L., Benvenuti S., Trusolino L., Comoglio P.M. Ror1 is a pseudokinase that is crucial for Met-driven tumorigenesis. Cancer Res. 2011;71:3132–3141. doi: 10.1158/0008-5472.CAN-10-2662. [DOI] [PubMed] [Google Scholar]
- 14.Rabbani H., Ostadkarampour M., Danesh Manesh A.H., Basiri A., Jeddi-Tehrani M., Forouzesh F. Expression of ROR1 in patients with renal cancer--a potential diagnostic marker. Iran Biomed. J. 2010;14:77–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Shabani M., Asgarian-Omran H., Jeddi-Tehrani M., Vossough P., Faranoush M., Sharifian R.A., Toughe G.R., Kordmahin M., Khoshnoodi J., Roohi A., et al. Overexpression of orphan receptor tyrosine kinase Ror1 as a putative tumor-associated antigen in Iranian patients with acute lymphoblastic leukemia. Tumour Biol. 2007;28:318–326. doi: 10.1159/000121405. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S., Chen L., Cui B., Chuang H.Y., Yu J., Wang-Rodriguez J., Tang L., Chen G., Basak G.W., Kipps T.J. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS ONE. 2012;7:e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S., Chen L., Wang-Rodriguez J., Zhang L., Cui B., Frankel W., Wu R., Kipps T.J. The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am. J. Pathol. 2012;181:1903–1910. doi: 10.1016/j.ajpath.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui B., Zhang S., Chen L., Yu J., Widhopf G.F., 2nd, Fecteau J.F., Rassenti L.Z., Kipps T.J. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res. 2013;73:3649–3660. doi: 10.1158/0008-5472.CAN-12-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry C., Llamosas E., Knipprath-Meszaros A., Schoetzau A., Obermann E., Fuenfschilling M., Caduff R., Fink D., Hacker N., Ward R., et al. Targeting the ROR1 and ROR2 receptors in epithelial ovarian cancer inhibits cell migration and invasion. Oncotarget. 2015;6:40310–40326. doi: 10.18632/oncotarget.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Yang H., Chen T., Luo Y., Xu Z., Li Y., Yang J. Silencing of Receptor Tyrosine Kinase ROR1 Inhibits Tumor-Cell Proliferation via PI3K/AKT/mTOR Signaling Pathway in Lung Adenocarcinoma. PLoS ONE. 2015;10:e0127092. doi: 10.1371/journal.pone.0127092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien H.P., Ueng S.H., Chen S.C., Chang Y.S., Lin Y.C., Lo Y.F., Chang H.K., Chuang W.Y., Huang Y.T., Cheung Y.C., et al. Expression of ROR1 has prognostic significance in triple negative breast cancer. Virchows Arch. 2016;468:589–595. doi: 10.1007/s00428-016-1911-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S., Cui B., Lai H., Liu G., Ghia E.M., Widhopf G.F., 2nd, Zhang Z., Wu C.C., Chen L., Wu R., et al. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc. Natl. Acad. Sci. USA. 2014;111:17266–17271. doi: 10.1073/pnas.1419599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassannia H., Amiri M.M., Jadidi-Niaragh F., Hosseini-Ghatar R., Khoshnoodi J., Sharifian R.A., Golsaz-Shirazi F., Jeddi-Tehrani M., Shokri F. Inhibition of tumor growth by mouse ROR1 specific antibody in a syngeneic mouse tumor model. Immunol. Lett. 2018;193:35–41. doi: 10.1016/j.imlet.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Yin Z., Gao M., Chu S., Su Y., Ye C., Wang Y., Pan Z., Wang Z., Zhang H., Tong H., et al. Antitumor activity of a newly developed monoclonal antibody against ROR1 in ovarian cancer cells. Oncotarget. 2017;8:94210–94222. doi: 10.18632/oncotarget.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi M.Y., Widhopf G.F., 2nd, Ghia E.M., Kidwell R.L., Hasan M.K., Yu J., Rassenti L.Z., Chen L., Chen Y., Pittman E., et al. Phase I Trial: Cirmtuzumab Inhibits ROR1 Signaling and Stemness Signatures in Patients with Chronic Lymphocytic Leukemia. Cell Stem Cell. 2018;22:951–959.e953. doi: 10.1016/j.stem.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furlan A.D., Pennick V., Bombardier C., van Tulder M., Editorial Board C.B.R.G. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine. 2009;34:1929–1941. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- 28.Parmar M.K., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H., Qiu J., Ye C., Yang D., Gao L., Su Y., Tang X., Xu N., Zhang D., Xiong L., et al. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci. Rep. 2014;4:5811. doi: 10.1038/srep05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang H., Jung W.Y., Kang Y., Lee H., Kim A., Kim B.H. Expression of ROR1, pAkt, and pCREB in gastric adenocarcinoma. Ann. Diagn. Pathol. 2015;19:330–334. doi: 10.1016/j.anndiagpath.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Cui B., Ghia E.M., Chen L., Rassenti L.Z., DeBoever C., Widhopf G.F., 2nd, Yu J., Neuberg D.S., Wierda W.G., Rai K.R., et al. High-level ROR1 associates with accelerated disease progression in chronic lymphocytic leukemia. Blood. 2016;128:2931–2940. doi: 10.1182/blood-2016-04-712562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y.Z., Ma R., Zhou J.K., Guo C.L., Wang Y.S., Li Z.G., Liu L.X., Peng Y. ROR1 is a novel prognostic biomarker in patients with lung adenocarcinoma. Sci. Rep. 2016;6:36447. doi: 10.1038/srep36447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C., Wang S., Xing Z., Lin A., Liang K., Song J., Hu Q., Yao J., Chen Z., Park P.K., et al. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat. Cell Biol. 2017;19:106–119. doi: 10.1038/ncb3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J.K., Zheng Y.Z., Liu X.S., Gou Q., Ma R., Guo C.L., Croce C.M., Liu L., Peng Y. ROR1 expression as a biomarker for predicting prognosis in patients with colorectal cancer. Oncotarget. 2017;8:32864–32872. doi: 10.18632/oncotarget.15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry C.E., Llamosas E., Daniels B., Coopes A., Tang K., Ford C.E. ROR1 and ROR2 play distinct and opposing roles in endometrial cancer. Gynecol. Oncol. 2018;148:576–584. doi: 10.1016/j.ygyno.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Mao Y., Xu L., Wang J., Zhang L., Hou N., Xu J., Wang L., Yang S., Chen Y., Xiong L., et al. ROR1 associates unfavorable prognosis and promotes lymphoma growth in DLBCL by affecting PI3K/Akt/mTOR signaling pathway. Biofactors. 2019;45:416–426. doi: 10.1002/biof.1498. [DOI] [PubMed] [Google Scholar]
- 38.Ghaderi A., Daneshmanesh A.H., Moshfegh A., Kokhaei P., Vagberg J., Schultz J., Olin T., Harrysson S., Smedby K.E., Drakos E., et al. ROR1 Is Expressed in Diffuse Large B-Cell Lymphoma (DLBCL) and a Small Molecule Inhibitor of ROR1 (KAN0441571C) Induced Apoptosis of Lymphoma Cells. Biomedicines. 2020;8:170. doi: 10.3390/biomedicines8060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu D., Gunther K., Enriquez L.A., Daniels B., O’Mara T.A., Tang K., Spurdle A.B., Ford C.E. ROR1 is upregulated in endometrial cancer and represents a novel therapeutic target. Sci. Rep. 2020;10:13906. doi: 10.1038/s41598-020-70924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiavone G., Epistolio S., Martin V., Molinari F., Barizzi J., Mazzucchelli L., Frattini M., Wannesson L. Functional and clinical significance of ROR1 in lung adenocarcinoma. BMC Cancer. 2020;20:1085. doi: 10.1186/s12885-020-07587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billiard J., Way D.S., Seestaller-Wehr L.M., Moran R.A., Mangine A., Bodine P.V. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol. Endocrinol. 2005;19:90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- 42.Fultang N., Illendula A., Lin J., Pandey M.K., Klase Z., Peethambaran B. ROR1 regulates chemoresistance in Breast Cancer via modulation of drug efflux pump ABCB1. Sci. Rep. 2020;10:1821. doi: 10.1038/s41598-020-58864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karvonen H., Arjama M., Kaleva L., Niininen W., Barker H., Koivisto-Korander R., Tapper J., Pakarinen P., Lassus H., Loukovaara M., et al. Glucocorticoids induce differentiation and chemoresistance in ovarian cancer by promoting ROR1-mediated stemness. Cell Death Dis. 2020;11:790. doi: 10.1038/s41419-020-03009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daneshmanesh A.H., Hojjat-Farsangi M., Khan A.S., Jeddi-Tehrani M., Akhondi M.M., Bayat A.A., Ghods R., Mahmoudi A.R., Hadavi R., Osterborg A., et al. Monoclonal antibodies against ROR1 induce apoptosis of chronic lymphocytic leukemia (CLL) cells. Leukemia. 2012;26:1348–1355. doi: 10.1038/leu.2011.362. [DOI] [PubMed] [Google Scholar]
- 45.Yang J., Baskar S., Kwong K.Y., Kennedy M.G., Wiestner A., Rader C. Therapeutic potential and challenges of targeting receptor tyrosine kinase ROR1 with monoclonal antibodies in B-cell malignancies. PLoS ONE. 2011;6:e21018. doi: 10.1371/journal.pone.0021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson A.L., Dhimolea E., Reichert J.M. Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 2010;9:767–774. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- 47.Vaisitti T., Arruga F., Vitale N., Lee T.T., Ko M., Chadburn A., Braggio E., Di Napoli A., Iannello A., Allan J.N., et al. ROR1 targeting with the antibody-drug conjugate VLS-101 is effective in Richter syndrome patient-derived xenograft mouse models. Blood. 2021;137:3365–3377. doi: 10.1182/blood.2020008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gohil S.H., Paredes-Moscosso S.R., Harrasser M., Vezzalini M., Scarpa A., Morris E., Davidoff A.M., Sorio C., Nathwani A.C., Della Peruta M. An ROR1 bi-specific T-cell engager provides effective targeting and cytotoxicity against a range of solid tumors. Oncoimmunology. 2017;6:e1326437. doi: 10.1080/2162402X.2017.1326437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallstabe L., Gottlich C., Nelke L.C., Kuhnemundt J., Schwarz T., Nerreter T., Einsele H., Walles H., Dandekar G., Nietzer S.L., et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight. 2019;4:e126345. doi: 10.1172/jci.insight.126345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuber T., Monjezi R., Wallstabe L., Kuhnemundt J., Nietzer S.L., Dandekar G., Wockel A., Einsele H., Wischhusen J., Hudecek M. Inhibition of TGF-beta-receptor signaling augments the antitumor function of ROR1-specific CAR T-cells against triple-negative breast cancer. J. Immunother. Cancer. 2020;8:e000676. doi: 10.1136/jitc-2020-000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.