Abstract
Analysis of cancer biomarkers has enormous promise for advancing our molecular understanding of illness and facilitating more precise and timely diagnosis and follow-up care. MicroRNA, exosomes, ctDNA, CTCs, and proteins are only some of the circulating biomarkers that can be detected by liquid biopsy instead of the more intrusive and time-consuming process of doing a tissue biopsy. As the cancer diagnosis bio-markers reveal ultra-low levels in the early stages of the disease, highly sensitive approaches are urgently required. Researchers have taken an interest in a optical biosensor for detecting cancer biomarkers as a potential tool for early disease diagnosis. These techniques have the potential to aid in the development of effective treatments, ultimately leading to a higher rate of patient survival. This review briefly discuss the i) understanding of cancer and biomarkers for early diagonosis purpose ii) Molecular methods and ii) biosensor-based diagnostics. The reseach primary focus on advancement in biosensor design using various concepts ie., Electrochemical, Chemiluminescence and Colorimetric, Surface plasmons (SP), Surface plasmon resonance (SPR), localized surface plasmon resonance (LSPR), Fluorescence, Fiber-based sensors, Terahertz based biosensors, and Surface enhanced Raman spectroscopy (SERS). As a result of the local electric field amplification around plasmonic (usually gold and silver) nanostructures, surface-enhanced Raman spectroscopy (SERS) has emerged as a rapid, selective, and sensitive alternative to conventional laboratory analytical methods, making significant strides in a number of biosensing applications but still under developing stage to be used as diagnostic tool in clinical research.
Keywords: Cancer, biosensors, cancer biomarker, disease diagnosis, nanoparticles, SP, SPR, SERS
INTRODUCTION
In recent decades, cancer has seen the second-greatest reduction in mortality rates among the most persistent diseases. Limitations in available cancer analysis techniques make it impossible to determine an accurate prognosis for the patient. Due to this, it is essential to discover cancer at an early stage for effective treatment. As early detection of cancer is associated with better patient outcomes and longer life expectancy, accurate diagnostic tools must be developed. Cancer accounted for a disproportionately high number of deaths, particularly from prostate, lung, breast, and colon cancers, confirming the disease's status as the world's leading cause of death.[1,2,3] Cancer has many potential origins, some of which are genetic predispositions, others environmental (carcinogenic chemicals or radiation), and still others microbiological (bacterial or viral infections, as in stomach cancer) (e.g., cervical cancer).[4] Cancer is a complex disease with many potential origins, making accurate diagnosis difficult. Tumor-associated antigens have been studied extensively as potential cancer biomarkers. Tumor cells, blood, urine, and other bodily fluids can be tested for biological molecules that are overexpressed in cancer.[5] Biomarkers may exist on the surface of cancer cells or within the cells themselves. In the past, cell lysis was accomplished by centrifugation with magnetic beads and a density gradient in the event of cellular presence.[6,7,8] The colorimetric biosensors are quick, easy to use, portable, and cheap, but the major hurdle in application as diagnotic tool is due to lack of sensitivity and multiplexing capacity. On other hand, the fiber-based biosensor can be easily inserted into the body in places where conventional biosensors have trouble reaching due to barriers. Many methods have been claimed to be effective in boosting the optical signal and detecting low quantities of various biomarkers and cancer cells; they include various nanoparticles (NPs), nanocavities, 2D materials, and biosensor fibre. Due to lack of multiplexing, a phototoxicity, photobleaching, and lengthy assay fluorescence biosensors have trouble in applications. Plasmonic biosensors (includes SPR, LSPR, and SERS) having high specificity, and multiplexing capabilities make them ideal for use with non-clear fluids (like blood and urine). However, THz waves having benefits of providing a much larger measurement area and no need of conventional medical personnel. Therefore, the purpose of this article is to discuss the progress made in building biosensors for disease (cancer) detection and to inquire into the distance yet to be travelled by the optical biosensor before it can be used in clinical practise.
DIAGNOSIS METHODS FOR DISEASE BIOMARKERS
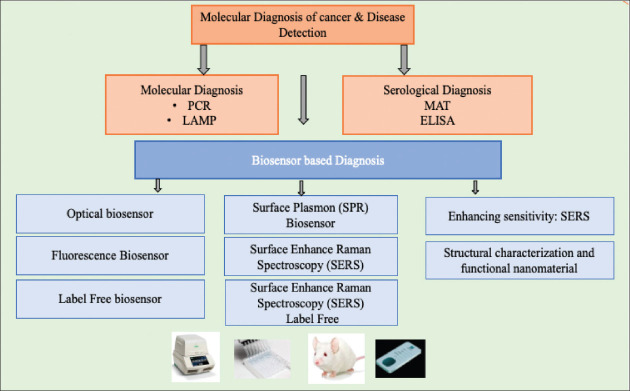
Biosensors disclose new biomarkers useful for evaluating appropriate pharmaceutical therapies, making them invaluable analytical tools for both accurate clinical diagnosis and a deeper understanding of the molecular mechanisms behind the illness.[9] The various diagnosis tool for the detection of disease is shown in Figure 1. Various gene amplication methods such as PCR, RT-PCR, and LAMP popular and used in disease (DNA) detection Advantages of PCR include its high specificity and the sensitivity of its processes, which allow for the detection of genomic material with as few as ten oligomers. To contrast, in order to eliminate false positives caused by contamination, RT-PCR analysis necessitates but expensive equipment, a lengthy analysis, and precise control as limitation in diagnosis. However, LAMP is a gene amplification technique that permits rapid and sensitive DNA detection.[10,11] The quick amplification (can produce 109 copies of DNA/hr), straightforward operation, and low detection threshold of a LAMP are all to its benefit.[12] The microscope agglutination test (MAT), a widely accepted and often used diagnostic method. Despite its status as the standard in serological testing, MAT requires a high level of technical expertise.[14] However, ELISA shows promise because it can be performed at a lesser cost in a wider range of laboratories than the MAT test. In order to diagnose an infection caused by a pathogen, ELISA can be used to measure the amount of immunoglobulin M (IgM) and other biomarkers produced in the body. One of the most promising alternate solutions for the diagnosis or therapeutic monitoring of significant diseases like allergies, celiac disease, diabetes, neurological disorders, and cancer is the use of biosensor devices, which are expected to deliver rapid and reliable biomedical analysis using modest sample quantities and minimal pretreatment.[13]
Figure 1.
Diagnosis tool for detection of cancer and disease
BIOSENSOR BASED DIAGNOSIS
By analysing molecular biomarkers using biosensor platforms, early diagnosis and monitoring of pathological conditions, especially cancer disease, may significantly improve prognosis and survival rates, thereby reducing disease burden and helping social development and expanding access to healthcare for people all over the world. An analytical system with a biological sensing component is called a biosensor. Possible illness biomarkers include a wide range of DNA and bio-molecules that can be detected by a variety of biosensor detection techniques.[15,16,17,18] Cancer biomarkers, which are tumor-specific signature markers expressed on the cell surface, aid in the detection and early diagnosis of the disease. Preliminary evidence suggests that early cancer diagnosis can be achieved by combining these cancer marker signals with various biosensing technologies.[19] Biosensors are devices that can assess the expression levels of disease. Together, the biological detecting molecules as cancer cell receptor protein and the electronic sensor system (including the transducer) in these diagnostic and analytical devices can be utilised to process biological samples (analytes) and collect data of a specified nature. Biocomputers, glucometers, biochips, immunological markers, and DNA, are just some examples of the types of biosensors that can be built dependent on the target analyte. Biosensors can take many forms; examples include resonant mirrors, glucose monitors, biochips, and miniature computers.[20,21] Optical biosensors are being developed as a non-invasive alternative to the conventional methods now utilised for cancer diagnosis. Cancer-related materials such microRNAs (miRNAs), proteins, circulating tumour cells (CTCs), deoxyribonucleic acid (DNA), and exosomes from bodily fluids like blood, urine, saliva, plasma, and serum provide the basis of most optical biosensors.[22] Optical biosensors are classified into two types: fluorescence-based and different other classified biosensors.[23] Overall various classifications fall under biosensor for disease detection which includes; i) Electrochemical, Chemiluminescence and Colorimetric ii) Surface plasmons iii) Surface plasmon resonance iv) localized surface plasmon resonance v) Fluorescence iv) Fiber-based sensors vii) Terahertz based biosensors viii) Surface enhanced Raman spectroscopy.
Electrochemical, Chemiluminescence and Colorimetric
Several areas, including medical diagnosis, food processing quality assurance, and environmental monitoring, have shown great promise for the application of electrochemical biosensors. Electrochemical biosensors inspired by nanotechnology have made it possible to measure parameters of interest in a wide range of matrices or media in real time, without the need of any reagents or labels, without causing any damage to the sample, and without disrupting the native environment. Electrochemical biosensors offer easy production (flexibility and tunability) and testing using “liquid biopsies” while overcoming most of the limitations of present technologies to attain sensing ultra-trace amounts, faster detection.[24] To clarify, electrochemiluminescence (ECL) is a subset of chemiluminescence in which photons are emitted with the aid of electrodes as opposed to purely through chemical reaction. It has been reported that human platelet-derived growth factor-BB can be detected with a pH-colorimetric sensor based on glucose oxidase. Glucose oxidase boosts cancer biomarker identification because it cancels out noise introduced by varying sample types and analysis methods.[25] Colorimetric biosensors utilise chemo-responsive dyes that undergo an easily observable colour change upon interaction with the sensing analyte in order to calculate absorbance or reflectance.
Terahertz based biosensor and Fluorescence biosensor
A portion of the electromagnetic spectrum, terahertz (THz) waves have a frequency range of 1 to 10 THz. Specifically, the design is utilised between 1.5 and 3.0 THz for the diagnosis of breast cancer.[26] The chemical potential of graphene is adjusted between 0.1eV and 0.6eV. Because of graphene's low loss, chemical potential-dependent conductivity that can be adjusted with a bias voltage, and adaptability, it is used in the study of THz-range sensor performance. In response to a rise in chemical potential, the resonant frequency of a substance moves to a higher.[27] Due to these advantages the terahertz based biosensor aim to detect the cancer type as per THz frequency.
The detection limits of biosensors based on fluorescence can be as low as a single molecule, allowing for extremely sensitive analysis.[28] To identify the liver cancer (HepG2) cell lines a fluorescent aptasensor based on the nucleic acid based TLS11a aptamer designed. The advantages of nucleic acid-based aptamer include high affinity and specificity for the target. Aptamer (in hairpin structure), which acts as quencher for fluorescent chemical compound, is developed using two DNA strands. Fluorescent substance is attached to one strand, and a quencher is attached to the other.[29] The study conducted by Zhao et., 2018[30] shows that the fluorescence sensor for CEA detection based on MoS2 nanosheets and a protein aptamer. The good quenching efficiency and the ability to initiate the energy transfer via fluorescence resonance are two of the many benefits of MoS2 nanosheets.
Surface plasmons, Surface plasmon resonance, Localized surface plasmon resonance, Fiber-based sensors, Surface enhanced Raman spectroscopy
This effect occurs when incident light energy is transferred to surface plasmons (SPs) at the “metal-dielectric” contact, resulting in a decrease in the reflected light's intensity. The incident light (wavelength interrogation) or the “angle of incidence” can be used to acquire the reflected light (angular interrogation). There are a couple of optical sensing techniques that rely on resonance, and two of those are “surface plasmon resonance” (SPR) and “localised surface plasmon resonance” (LSPR). SPR demonstrates the “angular or wavelength” change as RI (refractive index) of sensing media changes as a result of biomolecular interactions. In LSPR, the field is limited around the nanoparticles (NPs) and consists of the oscillation of the NPs' free electrons.[31] Yet, because to its robustness, multiplexing, and adaptability, SPR-based sensing has become the most preferred technology for cancer detection in recent years.
“SPR-based biosensor” used for detection of cancer (breast). The two breast genes, 916delTT and 6174delT, are shown to be detectable by a graphene-coated fibre optic SPR biosensor. Similarly, in case of prostate cancer, the “prostate specific antigen” (PSA) detected by utilising “functional monomer of methacrylic acid” based on “micro-contact” imprinting. The micro-contact imprinting method has many benefits, including its inexpensive price, simple fabrication, reusability, and strong binding capability that is on par with that of natural binders. The frequently occuring protein enzymes (Exosomes) could be used as biomarker in various cancer detection due to high enough concentrations in several cancers.[32] The optical fibre biosensor application utilized in detection of breast cancer HER2 protein biomarker. Breast cancer detection performed by utilising the HER2 protein biomarker via Direct functionalization of HER2 ssDNA aptamers on a Au film (50nm).[33] The photonic crystals have vast utilization in optical biosensing. The 1D photonic crystals have a structure made up of alternating thin-layers of materials with dielectric constants (high and low). Due to the fact that, cost of a 1D crystal fabrication is far lower than that of a 2D or 3D photonic crystal design. The 1D photonic crystals applications brightened in the biosensing field in recent years.
The SERS used for detection of lung cancer and breast cancer (in MDA-MB-231 and MCF-7) cell lines. To improve the detection sensitivity, SERS reporters or substrates, including plasmonic nanostructures of Au and Ag, are employed to boost scattering efficiency. For lungs cancer detection, a SERS sensor based on Ag nanorod arrays is created with an attomolar (aM) detection limit. It is stated that miR-141, a microRNA biomarker, can be detected using a SERS (Surface enhanced Raman spectroscopy) sensor comprised of Raman-active Au-NPs and an Au-coated paramagnetic NPs.[34] Similarly, in breast cancer cell lines, the SERS nanoprobe used to detect the uPAR and EGFR receptor expression. Another research on how to differentiate between (MDA-MB-231 and MCF-7) breast cancer cell lines using a SERS nanoprobe. Differential expression of uPAR and EGFR has been observed in two breast cell lines, uPAR is high in “MDA-MB-231” but low in “MCF-7”. Similar levels of EGFR expression can be found in the (MDA-MB-231 and MCF-7) breast cancer cell lines. Using the SERS based biosensor the expression level of two proteins, biomarkers detected.[35]
CONCLUSION
Various diagnostic tools typically identify a single disease biomarker at a time. The inability to detect many targets poses a significant obstacle to cancer detection. In different types of cancer, multiple biomarkers are involved, hence a single detection cannot provide information on the enormous range of cancer types. In addition, simultaneous detection of many biomarkers is essential. However, because DNA is more stable than other molecules, DNA-based biosensors can play a vital role in the clinical use of optical biosensors. The application of DNA-based biosensors and NPs nanoparticles, fibre, and 2D materials is still in its developmental stages, with both positives and cons. Therefore, colorimetric and electrochemiluminescence-based techniques are the most effective and now available in clinics for detecting cancer. For clinical applications, biosensors still require a substantial amount of research and investigation to determine which type are the most promising for cancer diagnosis.
Financial support and sponsorship
Authors would like to acknowledge the UMT for TAPERG/ 2021/UMT/807 grant provided to Gul-e-Saba Chaudhry.
Conflicts of interest
The authors declare no conflict of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Gunawardana CG, Diamandis EP. High throughput proteomic strategies for identifying tumour-associated antigens. Cancer Lett. 2007;249:110–9. doi: 10.1016/j.canlet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Research UK. November 8, 2008, http://info.cancerresearchuk.org/ cancerstats/mortality/
- 4.Soper SA, Brown K, Ellington A, Frazier B, Garcia-Manero G, Gau GV, et al. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosens Bioelectron. 2006;21:1932–42. doi: 10.1016/j.bios.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Lyon: IARC Press; 2004. World Health Organization classification of tumours. pathology and genetics of tumours of the urinary system and male genital organs. [Google Scholar]
- 6.Hu XC, Wang Y, Shi DR, Loo TY, Chow LW. Immunomagnetic tumor cell enrichment is promising in detecting circulating breast cancer cells. Oncology. 2003;64:160–5. doi: 10.1159/000067776. [DOI] [PubMed] [Google Scholar]
- 7.Zieglschmid V, Hollmann C, Gutierrez B, Albert W, Strothoff D, Gross E, et al. Combination of immunomagnetic enrichment with multiplex RT- PCR analysis for the detection of disseminated tumor cells. Anticancer Res. 2005;25:1803–10. [PubMed] [Google Scholar]
- 8.Choesmel V, Pierga JY, Nos C, Vincent-Salomon A, Sigal-Zafrani B, Thiery JP, et al. Enrichment methods to detect bone marrow micrometastases in breast carcinoma patients: clinical relevance. Breast Cancer Res. 2004;6:R556–70. doi: 10.1186/bcr898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellassai N, Spoto G. Biosensors for liquid biopsy: circulating nucleic acids to diagnose and treat cancer. Anal. Bioanal. Chem. 2016;408:7255–7264. doi: 10.1007/s00216-016-9806-3. [DOI] [PubMed] [Google Scholar]
- 10.Güven Gökmen T, Soyal A, Kalayci Y, Önlen C, Köksal F. Comparison of 16s rrna-pcr-rflp, lipl32-PCR and ompl1-PCR methods in the diagnosis of leptospirosis. Rev. Inst. Med. Trop. Sao Paulo. 2016;58(1):2–7. doi: 10.1590/S1678-9946201658064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Notomi T. Nucleic Acids Res. 2000;28(12):63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koizumi N, Nakajima C, Harunari T, Tanikawa T, Tokiwa T, Uchimura E, et al. A new loop-mediated isothermal amplification method for rapid, simple, and sensitive detection of Leptospira spp. in urine. J. Clin. Microbiol. 2012;50(6):2072–74. doi: 10.1128/JCM.00481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, Han R, Su X, Li Y, Xu G, Luo X. Zwitterionic peptide anchored to conducting polymer PEDOT for the development of antifouling and ultrasensitive electrochemical DNA sensor. Biosens. Bioelectron. 2017;92:396–401. doi: 10.1016/j.bios.2016.10.088. [DOI] [PubMed] [Google Scholar]
- 14.Chirathaworn C, Inwattana R, Poovorawan Y, Suwancharoen D. Interpretation of microscopic agglutination test for leptospirosis diagnosis and seroprevalence. Asian Pac. J. Trop. Biomed. 2014;4(1):S162–S164. doi: 10.12980/APJTB.4.2014C580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Zhou J, Du X. Electrochemical biosensors for detection of foodborne pathogens. Micromachines. 2019:10. doi: 10.3390/mi10040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopinath PG, Anitha VR, Mastani SA. Microcantilever based Biosensor for Disease Detection Applications. J. Med. Bioeng. 2015;(4):307–311. doi: 10.12720/jomb.4.4.307-311. [DOI] [Google Scholar]
- 17.Murillo AE, Melo-Máximo L, García-Farrera B, Martínez OS, Melo-Máximo DV, Oliva-Ramírez J, et al. Development of AlN thin films for breast cancer acoustic biosensors. J. Mater. Res. Technol. 2019;(8):350–358. doi: 10.1016/j.jmrt.2018.02.007. [DOI] [Google Scholar]
- 18.Meyer MHF, Stehr M, Bhuju S, Krause HJ, Hartmann M, Miethe P, et al. Magnetic biosensor for the detection of Yersinia pestis. J. Microbiol. Methods. 2007;68(2):218–224. doi: 10.1016/j.mimet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Cheng N, Du D, Wang X, Liu D, Xu W, Luo Y. Recent Advances in Biosensors for Detecting Cancer-Derived Exosomes. Trends Biotechnol. 2019;37:1236–54. doi: 10.1016/j.tibtech.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Naresh V, Lee N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors. 2021;21:1109. doi: 10.3390/s21041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrotra Biosensors and their applications—A review. J. Oral. Biol. Craniofac. Res. 2016;6:153–9. doi: 10.1016/j.jobcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underwood JJ, Quadri RS, Kalva SP, Shah H, Sanjeeviah AR, Beg MS, Sutphin P.D. Radiology. 2020;294(1):5–17. doi: 10.1148/radiol.2019182584. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, White IM, Shopova SI, Zhu H, Suter JD, Sun Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta. 2008;620:8–26. doi: 10.1016/j.aca.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhary M., Arora K. Electrochemical biosensors for early detection of cancer. In: Khan R., Parihar A., Sanghi S. K., editors. Biosensor Based Advanced Cancer Diagnostics: From Lab to Clinics. London, UK: Academic Press; 2022. pp. 123–151. [Google Scholar]
- 25.Miao X, Zhu Z, Jia H, Lu C, Liu X, Mao D, Chen G. Sensor. Actuator. B Chem. 2020;320:128435. [Google Scholar]
- 26.Bulbul AAM, Rahaman H, Biswas S, Hossain MB, Nahid AAl. Sens. Bio-Sensing Res. 2020;30:100388. [Google Scholar]
- 27.Hlali A, Oueslati A, Zairi H. IEEE Sensor. J. 2021;21:9844–51. [Google Scholar]
- 28.Mouffouk F, Rosa da Costa AM, Martins J, Zourob M, Abu-Salah KM, Alrokayan S. A. Development of a highly sensitive bacteria detection assay using fluorescent pH-responsive polymeric micelles. Biosens. Bioelectron. 2011;26(8):3517–3523. doi: 10.1016/j.bios.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Lai Z, Tan J, Wan R, Tan J, Zhang Z, Hu Z, Li J. Oncol. Rep. 2017;37:2688–2694. doi: 10.3892/or.2017.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao L, Cheng M, Liu G, Lu H, Gao Y, Yan X, Liu F, Sun P, Lu G. 2018. Sensor. Actuator. B Chem. 273:185–190. [Google Scholar]
- 31.Hammond JL, Bhalla N, Rafiee SD, Estrela P. 2014. Biosensors. 4(2):172–188. doi: 10.3390/bios4020172. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hlali, A., Oueslati, A., Zairi, H. IEEE Sensor. J. 2021;21(8):9844–9851. [Google Scholar]
- 32.Dilsiz N. Futur. Sci. OA. 2020;6(4):FSO465. doi: 10.2144/fsoa-2019-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loyez M, Lobry M, Hassan EM, DeRosa MC, Caucheteur C, Wattiez R. Talanta. 2021;221:121452. doi: 10.1016/j.talanta.2020.121452. [DOI] [PubMed] [Google Scholar]
- 34.Song CY, Yang YJ, Yang BY, Sun YZ, Zhao YP, Wang LH. Nanoscale. 2016;8(39):17365–17373. doi: 10.1039/c6nr05504d. [DOI] [PubMed] [Google Scholar]
- 35.Li Liao M, Chen Y, Shan B, Li M. J. Mater. Chem. B. 2019;7(5):815–822. doi: 10.1039/c8tb02828a. [DOI] [PubMed] [Google Scholar]