Abstract
Background: Excessive supraventricular ectopic activity (ESVEA) is correlated with the development of atrial fibrillation (AF) and is frequently observed in ischemic stroke patients. This meta-analysis aims to summarize the evidence on the association between ESVEA and the risk of AF and stroke. Methods: PubMed and Embase databases were systematically searched to identify all publications providing relevant data from inception to 23 August 2022. Hazard ratio (HR) and 95% confidence interval (CI) were pooled using fixed-effect or random-effect models. Results: We included 23,272 participants from 20 studies. Pooled results showed that ESVEA was associated with an increased risk of AF in the general population (HR: 2.57; 95% CI 2.16–3.05), increased risk of AF in ischemic stroke patients (HR: 2.91; 95% CI 1.80–4.69), new-onset ischemic stroke (HR: 1.91; 95% CI 1.30–2.79), and all-cause mortality (HR: 1.41; 95% CI 1.24–1.59). Pooled analysis indicated that ESVEA was not associated with recurrent ischemic stroke/transient ischemic attack (TIA) (HR: 1.24; 95% CI 0.91–1.67). Conclusions: ESVEA is associated with AF, new-onset ischemic stroke, and all-cause mortality.
Keywords: stroke, excessive supraventricular ectopic activity, atrial fibrillation, mortality, meta-analysis
1. Introduction
Each year, approximately 795,000 people experience a new or recurrent stroke. Of all strokes, 87% are ischemic strokes [1]. Cryptogenic ischemic strokes (or strokes of unknown cause) are thought to comprise about 25% of all ischemic strokes, and most cryptogenic strokes are thromboembolic [2]. Atrial fibrillation (AF) is the most common cardiac arrhythmia and is an independent risk factor for stroke [3]. Meanwhile, AF is associated with increased in-hospital mortality, not only in ischemic stroke patients [4,5,6,7] but also in patients with cardioembolic stroke [8]. It has also been reported that AF is a predictor of early embolic recurrence in patients with cardioembolic stroke [9] and that early recurrent embolization is the most important predictor of in-hospital mortality [10]. Accumulating studies demonstrated a close relationship between premature atrial contractions (PACs) and AF [11,12,13,14,15,16,17,18,19,20]. Moreover, PACs are common in the general population [21] and patients with ischemic stroke [22]. The term ‘excessive supraventricular ectopic activity’ (ESVEA), with varying definitions, has been used to describe different manifestations of excessive atrial ectopic beats in previous studies, which was defined as >30 PACs per hour and/or runs of ≥20 PACs [12,23], PAC/h > 4 and/or supraventricular runs of >5 beats [24], or >100 PACs per 24 h [13,25]. Therefore, ESVEA has been interpreted as a combination of frequent PACs (the number of PACs/h) and/or frequent atrial tachycardia (the number of continuous PACs in any episode). Recently, more observational studies have indicated associations between ESVEA and AF, stroke, and mortality [11,13,15,26,27,28,29,30].
This meta-analysis aims to summarize the evidence on the associations between ESVEA and AF, stroke, and mortality. Recognizing the risk of stroke after ESVEA is vital for informing early primary and secondary stroke prevention.
2. Materials and Methods
The meta-analysis was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [31]. The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), ID CRD42022353287. As we reviewed only previously published data, local institutional review board or ethics committee approval and subjects′ informed consent were not required.
2.1. Study Search
PubMed and Embase were searched from inception to 23 August 2022 by 2 reviewers (MY and HC). Variations of the following search terms were used: stroke, atrial premature complexes, and atrial fibrillation. The complete search strategy is provided in Supplementary Materials. The reference lists of eligible articles were also scrutinized to find additional data sources.
2.2. Study Selection
Two investigators (MY and HC) independently searched the titles and abstracts for articles relevant to our systematic review. If a decision could not be made based on the information in the title and abstract, then the full text was reviewed. Studies were included if they met the following criteria: (1) reported PAC as a risk factor for AF and/or stroke in participants age ≥ 18 years; (2) prospective or retrospective cohort study; (3) follow-up period ≥ 6 months; (4) PAC was detected using ECG or other cardiac telemetry methods, and PAC burden was greater than the presence of PAC; (5) AF and/or stroke were reported as outcome events; (6) the hazard ratio (HR) and the corresponding 95% confidence interval (CI) were reported. The exclusion criteria were as follows: (1) patients with a known history of AF; (2) patients with a history of catheter ablation, percutaneous coronary intervention, or coronary artery bypass graft; (3) patients with implantable cardiac monitoring. There were no language restrictions.
2.3. Data Extraction and Quality Assessment
The two investigators independently extracted data from the included studies using prepared forms. The extracted information included the name of the first author, year of publication, country, study design, study population, number of subjects, age, sex, the definition of ESVEA, methods of ESVEA detection, the prevalence of ESVEA, outcomes, and number of interesting outcomes. MY and HC assessed the quality of the included studies according to the Newcastle–Ottawa Scale (NOS) [32]. Any disagreements were resolved by a third author (JY).
2.4. Outcomes
The primary outcomes were AF and stroke (including new-onset and recurrent stroke), and the secondary outcome was all-cause mortality.
2.5. Statistical Analysis
We pooled the adjusted effect estimates to investigate the independent relationship between ESVEA and the outcomes of AF, stroke, or mortality. The pooled effect estimates were presented as HRs and 95% CIs using the random-effects model (if the heterogeneity was obvious I2 statistics > 50%); otherwise, the fixed-effects model was adopted. The Egger test was used, and a funnel plot was constructed to evaluate publication bias (p-value < 0.05 was considered significant). Statistical analysis was performed with Stata, version 15.
3. Results
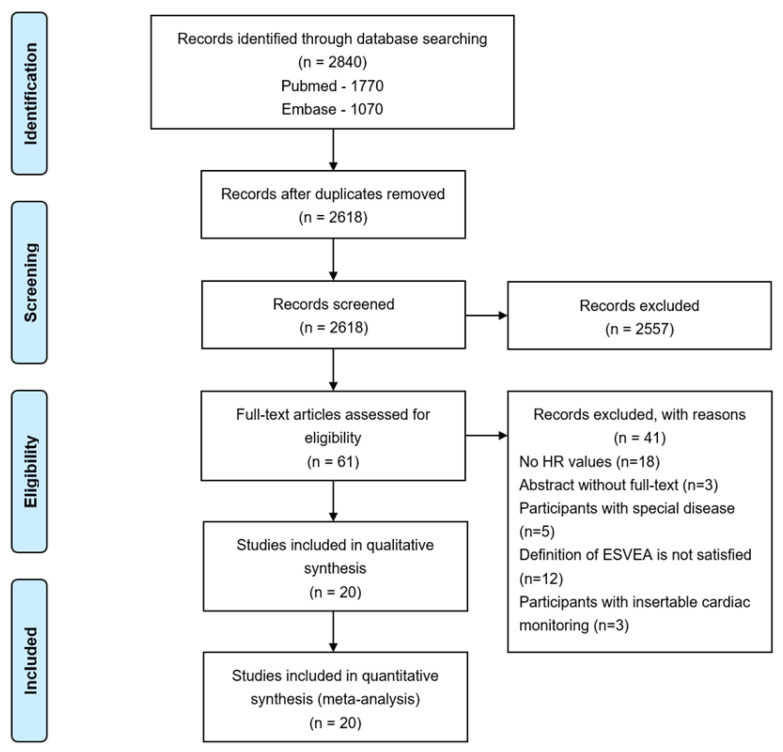
In total, 2840 records were retrieved through database searching, of which 222 were duplicates. After screening titles and abstracts, 61 articles were included for full-text review. Finally, 20 studies that satisfied all eligibility criteria were included in the review. A detailed flowchart of the screening process is presented in Figure 1.
Figure 1.
PRISMA flowchart. Abbreviations: HR—hazard ratio; ESVEA—excessive supraventricular ectopic activity.
3.1. Characteristics of the Included Studies
In total, 20 studies involving 23,272 participants were included, and the characteristics of the included studies are shown in Table 1 and Table 2. Of the 20 studies, 13 were prospective cohort studies [12,13,14,23,33,34,35,36,37,38,39,40,41] and 7 were retrospective cohort studies [15,22,42,43,44,45,46]. The majority of studies were conducted in Europe (n = 13) [12,22,23,34,36,38,42,44,45], followed by Asia (n = 4) [13,33,43,46] and America (n = 3) [14,15,37]. The number of participants at baseline ranged from 68 [33] to 6100 [45]. The studies used different measurement methods to determine ESVEA: 24 h ECG was used in 13 studies [13,14,15,33,34,35,36,38,41,42,43,44,46], 48 h ECG was used in 4 studies [12,22,23,40], routine ECG was used in 1 study [39], 30 s ECG was used in 1 study [45], and polysomnogram-based ECG was used in 1 study [37]. No studies were graded as having low-quality scores (<5 on the NOS) (Table S1).
Table 1.
Characteristics of the included studies.
| Study | Country | Study Design |
Population | Number of Subjects | Age (Means ± SD) |
Male n (%) |
Concomitant Diseases (%) |
Follow-up |
|---|---|---|---|---|---|---|---|---|
| Binici 2010 [12] |
Denmark | Prospective cohort | Without CVD, stroke, or AF | 678 | 64.5 ± 6.8 | 397 (58.6) | Diabetes (11.1) | 6.3 years |
| Chong 2012 [13] |
China | Prospective cohort | Without AF or structural heart disease | 428 | 66.7 ± 10.2 | 187 (43.7) | Hypertension (45.3) Diabetes (17.1) CVD (17.5) |
6.1 years |
| Dewland 2013 [14] | United States | Prospective cohort | Without prevalent AF | 1260 | 71 | 569 (45) | Hypertension (55) Diabetes (15) CVD (20) |
13.0 years |
| Yodogawa 2013 [33] | Japan | Prospective cohort | With AIS, without a history of AF | 68 | 69.9 ± 9.6 | 37 (54.4) | Hypertension (66.2) Diabetes (14.7) |
11 ± 4 months |
| Pinho 2015 [42] |
Portugal | Retrospective cohort | With CIS or TIA | 184 | 55.2 ± 15.1 | 96 (52.2) | Hypertension (56.5) Diabetes (14.7) Dyslipidemia (72.8) CVD (3.3) |
27.5 months |
| Acharya 2015 [15] |
United States | Retrospective cohort | Free of AF | 1357 | 64 | 1262 (93) | Hypertension (66) Diabetes (22.6) CVD (19.7) |
7.5 years |
| Johnson 2015 [34] |
Sweden | Prospective cohort | Free of AF | 383 | 64.6 ± 5.9 | 172 (45) | - | 10.3 years |
| Lin 2015 [43] |
China | Retrospective cohort | Without AF and a PPM | 5371 | 61.8 ± 18.6 | 3222 (60) | Hypertension (35.6) Diabetes (20.2) Dyslipidemia (12.8) CVD (29.4) |
10 ± 1 years |
| Larsen 2015 [23] |
Denmark | Prospective cohort | Without CVD, stroke, or AF | 678 | 64.5 ± 6.8 | 397 (58.6) | Diabetes (11.1) | 14.4 years |
| Vinther 2016 [22] |
Denmark | Retrospective cohort | With IS and without known AF | 565 | - | 313 (55.4) | Hypertension (41.9) Diabetes (10.8) |
4 years |
| Cabrera 2016 [44] |
Spain | Retrospective cohort | Free of AF | 299 | 62.5 ± 17.9 | 160 (53.5) | Hypertension (52.3) Diabetes (17.4) |
39.1 months |
| Marinheiro 2017 [35] | Portugal | Prospective cohort | Without stroke or AF | 362 | - | 204 (56.4) | Hypertension (77.6) Diabetes (25.1) |
7.1 years |
| Vinther 2017 [36] |
Denmark | Prospective cohort | With AIS and without AF | 256 | 73 ± 12.6 | 141 (55) | Hypertension (57) Diabetes (13) Dyslipidemia (28) CVD (13) |
32 months |
| Raman 2017 [37] |
United States | Prospective cohort | Without baseline AF | 2350 | 75.8 ± 5.3 | 2350 (100) | Hypertension (49) Diabetes (13.1) |
8.0 ± 2.6 years |
| Persson 2019 [38] |
Sweden | Prospective cohort | Free of AF | 377 | 65 ± 6 | 170 (45) | - | 17 years |
| Ntaios 2020 [39] |
United Kingdom | Prospective cohort | Embolic Stroke of Undetermined Source | 853 | 67 | 486 (57) | Hypertension (61.9) Diabetes (18.5) CVD (15) |
3.4 years |
| Sejr 2020 [40] |
Denmark | Prospective cohort | with AIS or TIA and without AF | 1453 | 72.8 ± 7.7 | 822 (56.6) | Hypertension (58.6) Diabetes (14.3) |
2.3 ± 1.3 years |
| Hygrell 2021 [45] |
Sweden | Retrospective cohort | Free of AF | 6100 | 76 | 2755 (45) | Hypertension (28) Diabetes (10) |
4.2 years |
| Sasaki 2021 [46] |
Japan | Retrospective cohort | Free of AF | 138 | 72 ± 10 | 108 (52) | Hypertension (62.3) Diabetes (23.9) Dyslipidemia (39.1) |
5 years |
| Vetta 2022 [41] |
Italy | Prospective cohort | With cryptogenic stroke | 112 | 72.2 ± 12.2 | 65 (58) | Hypertension (81) Diabetes (21) CVD (9) |
6 months |
CVD—cardiovascular disease; AF—atrial fibrillation; IS—ischemic stroke; AIS—acute ischemic stroke; CIS—cryptogenic ischemic stroke; TIA—transient ischemic attack; PPM—permanent pacemaker.
Table 2.
Characteristics of ESVEA definition, ESVEA prevalence, and outcomes.
| Study | Definition of ESVEA |
Detection of ESVEA | Prevalence of ESVEA n (%) |
Definition of Outcome |
Numbers of Outcome n (%) |
|---|---|---|---|---|---|
| Binici 2010 [12] |
≥30 SVEC/h or any episode of runs of ≥20 SVEC | 48 h ECG | 99 (14.6) | AF IS All-cause mortality |
22 (5.5) 27 (6.7) 87 (21.4) |
| Chong 2012 [13] |
>100 PACs/24 h | 24 h ECG | 107 (25) | AF IS Death |
60 (14) 41 (9.6) 60 (14) |
| Dewland 2013 [14] | The median PAC count was 2.5 beats/h (IQR, 0.8 to 9.5 beats/h) | 24 h ECG | - | AF All-cause mortality |
343 (27.2) 837 (66.4) |
| Yodogawa 2013 [33] | >100 PACs/24 h | 24 h ECG | - | AF | 17 (25) |
| Pinho 2015 [42] |
>30 APCs/h | 24 h ECG | 17 (9.2) | Recurrent IS/TIA | 22 (12) |
| Acharya 2015 [15] |
≥100 PACs/24 h | 24 h ECG | 486 (35.8) | AF | 155 (11.4) |
| Johnson 2015 [34] |
30 SVE/h and/or any SVT lasting for ≥20 consecutive beats | 24 h ECG | - | AF | 45 (11.7) |
| Lin 2015 [43] |
PAC burden >76 beats per day | 24 h ECG | 2072 (38.6) | All-cause mortality AF |
1209 (22.5) 418 (7.8) |
| Larsen 2015 [23] |
≥30 PACs/h or any episode of runs of ≥20 PACs | 48 h ECG | 99 (14.6) | IS All-cause mortality |
73 (10.8) 259 (38.2) |
| Vinther 2016 [22] |
≥3 PACs lasting less than 30 s during 48 h | 48 h of CICT | 161 (28) | Recurrent IS/TIA All-cause mortality AF |
73 (12.9) 158 (28) 22 (3.9) |
| Cabrera 2016 [44] |
Percentage of PAC (during the 24 h period) ≥0.2% | 24 h ECG | - | AF | 31 (10.4) |
| Marinheiro 2017 [35] |
>97 PACs/h | 24 h ECG | 124 (34.3) | IS All-cause mortality |
54 (14.9) 129 (35.6) |
| 30–97 PACs/h | 114 (31.5) | ||||
| Vinther 2017 [36] |
>14 PACs/h and ≥3 runs of PACs/24 h | 24 h ECG | 31 (12.1) | Recurrent stroke All-cause mortality |
20 (7.8) 34 (13.3) |
| Raman 2017 [37] |
PAC/h of sleep ≥ 21.15 | PSG based ECG | - | AF | 269 (11.4) |
| Persson 2019 [38] |
Top quartile of PACs (≥5.5 per hour) or SVT (≥0.13 per hour) | 24 h ECG | - | AF | 80 (21.2) |
| Ntaios 2020 [39] |
>0–1 SVE per 10 s | 12-lead ECG | 111 (13) | AF Recurrent IS All-cause mortality |
125 (14.7) 103 (12.1) 149 (17.5) |
| >1–2 SVE per 10 s | 57 (6.7) | ||||
| >2 SVE per 10 s | 58 (6.8) | ||||
| Sejr 2020 [40] |
0.5–1.5 PACs/h | 48 h ECG | 359 (24.7) | AF Recurrent IS Recurrent TIA All-cause mortality |
44 (2.9) 78 (5.4) 76 (5.2) 123 (8.5) |
| 1.6–5.9 PACs/h | 365 (25.1) | ||||
| ≥6.0 PACs/h | 362 (24.9) | ||||
| Hygrell 2021 [45] |
the top tenth percentile according to SVECs count | 30 s ECG | 709 (11.6) | AF IS Death |
387 (6.3) 161 (2.6) 354 (5.8) |
| Sasaki 2021 [46] |
PAC burden ≥ 0.4% | 24 h ECG | - | AF | 61 (29.3) |
| Vetta 2022 [41] |
PACs burden ≥ 7 | 24 h ECG | - | AF | 24 (21.4) |
SVEC—supraventricular ectopic complexes; AF—atrial fibrillation; ECG—electrocardiography; PACs—premature atrial complexes; IQR—interquartile range; APCs—atrial premature complexes; IS—ischemic stroke; TIA—transient ischemic attack; SVE—supraventricular extrasystoles; SVT—supraventricular tachycardias; CICT—continuous inpatient cardiac telemetry; SVECs—supraventricular ectopic complexes; PSG—polysomnogram.
3.2. Association between ESVEA and AF
3.2.1. Association between ESVEA and AF in the General Population
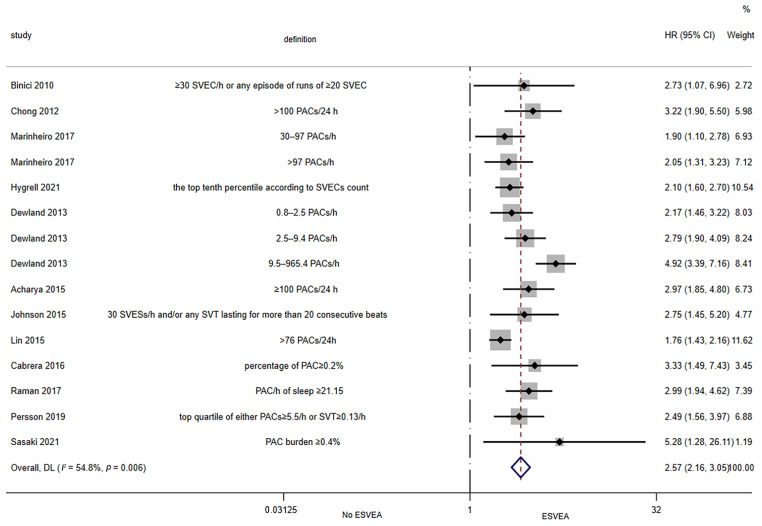
Twelve studies assessed the association between ESVEA and the risk of AF in the general population [12,13,14,15,34,35,37,38,43,44,45,46]. The random-effects pooled adjusted HR was 2.57 (95% CI 2.16–3.05; I2 = 54.8%, p = 0.006), indicating that ESVEA increased the risk of AF in the general population (Figure 2). The results of the Egger’s tests (p = 0.039) and funnel plot (Figure S1) showed publication bias.
Figure 2.
Forest plot of the association between ESVEA and AF in the general population. Abbreviations: SVEC—supraventricular ectopic complexes; PACs—premature atrial complexes; SVESs—supraventricular extrasystoles; SVT—supraventricular tachycardias; ESVEA—excessive supraventricular ectopic activity; HR—hazard ratio; CI—confidence interval. Definition represents the definitions of ESVEA in different studies [12,13,14,15,34,35,37,38,43,44,45,46].
3.2.2. Association between ESVEA and AF in the Ischemic Stroke Patients
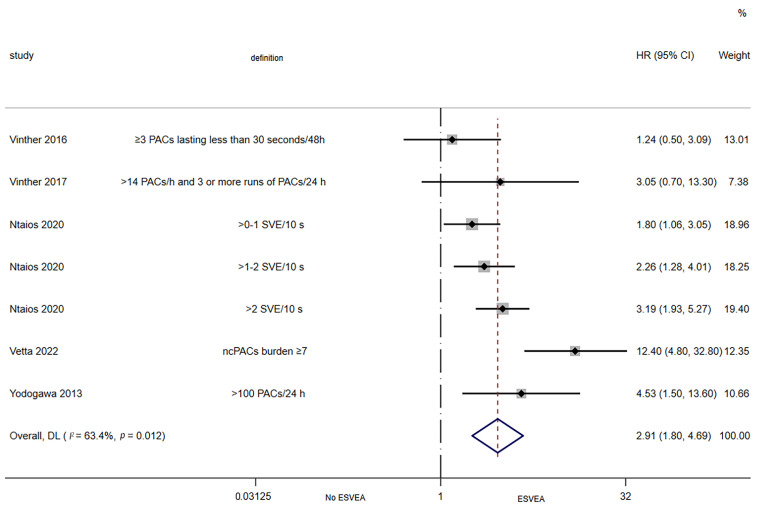
Five studies assessed the association between ESVEA and the risk of AF in ischemic stroke patients [22,33,36,39,41]. The random-effects pooled adjusted HR was 2.91 (95% CI 1.80–4.69; I2 = 63.4%, p = 0.012), indicating that ESVEA increased the risk of AF in ischemic stroke patients (Figure 3).
Figure 3.
Forest plot of the association between ESVEA and AF in the ischemic stroke patients. Abbreviations: PACs—premature atrial complexes; SVE—supraventricular extrasystoles; ncPACs—non-conducted premature atrial complexes; ESVEA—excessive supraventricular ectopic activity; HR—hazard ratio; CI—confidence interval. Definition represents the definitions of ESVEA in different studies [22,33,36,39,41].
3.3. Association between ESVEA and Stroke
3.3.1. Association between ESVEA and Risk of New-Onset Ischemic Stroke
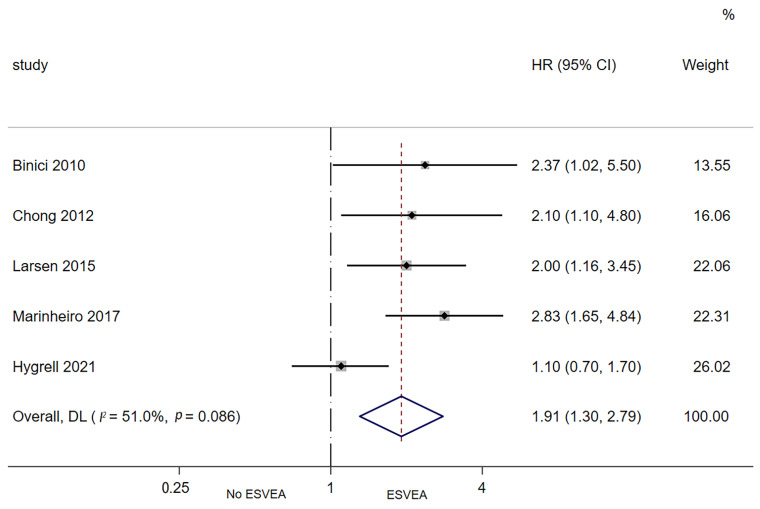
Five studies assessed the association between ESVEA and the risk of new-onset ischemic stroke [12,13,23,35,45]. The random-effects pooled adjusted HR was 1.91 (95% CI 1.30–2.79; I2 = 51%, p = 0.086), indicating that ESVEA increased the risk of new-onset ischemic stroke (Figure 4).
Figure 4.
Forest plot of the association between ESVEA and new-onset ischemic stroke. Abbreviations: ESVEA—excessive supraventricular ectopic activity; HR—hazard ratio; CI—confidence interval [12,13,23,35,45].
3.3.2. Association between ESVEA and Risk of Recurrent Ischemic Stroke/Transient Ischemic Attack (TIA)
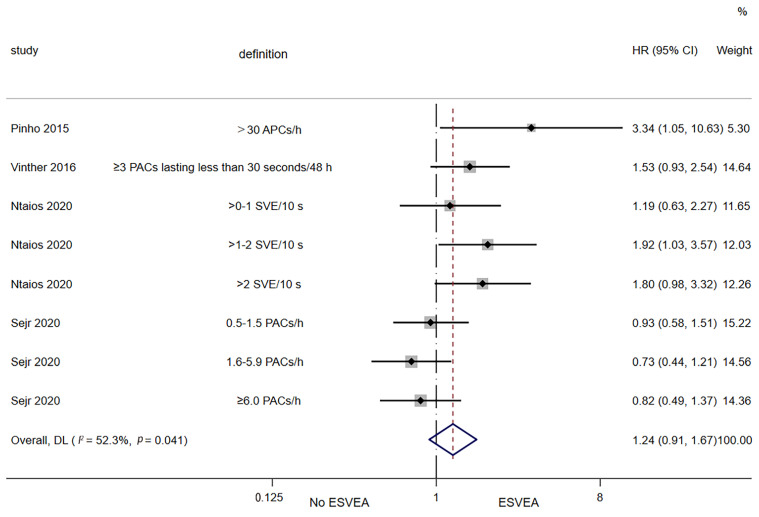
Four studies assessed the association between ESVEA and the risk of recurrent ischemic stroke/TIA [22,39,40,42]. The random-effects pooled adjusted HR was 1.24 (95% CI 0.91–1.67; I2 = 52.3%, p = 0.041), indicating that ESVEA did not increase the risk of recurrent ischemic stroke/TIA (Figure 5).
Figure 5.
Forest plot of ESVEA and the risk of recurrent ischemic stroke/transient ischemic attack. Abbreviations: APCs—atrial premature complexes; PACs—premature atrial complexes; SVE—supraventricular extrasystoles; ESVEA—excessive supraventricular ectopic activity; HR—hazard ratio; CI—confidence interval. Definition represents the definitions of ESVEA in different studies [22,39,40,42].
3.4. Association between ESVEA and All-Cause Mortality
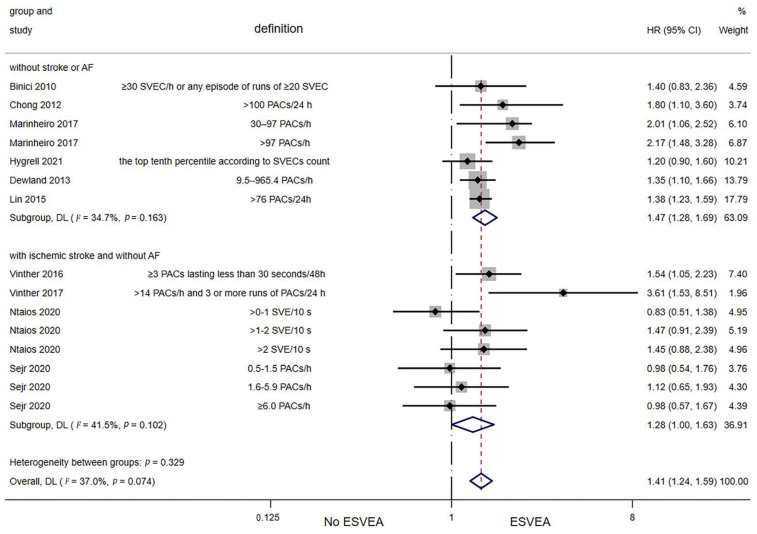
Ten studies assessed the association between ESVEA and the risk of all-cause mortality [12,13,14,22,35,36,39,40,43,45]. The random-effects pooled adjusted HR was 1.41 (95% CI 1.24–1.59; I2 = 37%, p = 0.074), indicating that ESVEA increased the risk of all-cause mortality (Figure 6). The results of the Egger′s test (p = 0.655) and funnel plot (Figure S2) demonstrated a lack of publication bias.
Figure 6.
Forest plot of the association between ESVEA and all-cause mortality. Abbreviations: SVEC—supraventricular ectopic complexes; PACs—premature atrial complexes; SVE—supraventricular extrasystoles; ESVEA—excessive supraventricular ectopic activity; HR—hazard ratio; CI—confidence interval. Definition represents the definitions of ESVEA in different studies [12,13,14,22,35,36,39,40,43,45].
4. Discussion
In this systematic review and meta-analysis, we summarized the relationship between ESVEA and AF, stroke, and death. Pooled data showed that ESVEA had more than doubled the risk of AF in the general population from twelve studies and in ischemic stroke patients from five studies. Pooled data based on five studies showed that ESVEA was correlated with a nearly two-fold increase in the risk of new-onset ischemic stroke. Ten studies indicated that ESVEA increases more than doubled the risk of all-cause mortality.
4.1. Thoughts on Antithrombotic Therapy of ESVEA
Our results indicated that ESVEA increased the risk of AF and new-onset stroke. According to the guidelines, anticoagulation in patients with AF depends on the CHA2DS2-VASc score [47]. However, the current guidelines do not recommend antiplatelet or anticoagulant therapy in patients with ESVEA. Whether non-stroke patients with ESVEA would benefit from antithrombotic therapy is still unclear. Future trials on the primary prevention of stroke using antiplatelet therapy for patients with ESVEA are warranted. Before these trials are carried out, high-quality studies are urgently needed to verify the relationship between ESVEA and the risk of new-onset stroke. In addition, our analysis indicated that ESVEA did not increase the risk of recurrent ischemic stroke/TIA, which may be due to the use of antiplatelets in 62% of patients after their first stroke [48]. Thus, antiplatelet therapy may be an effective secondary prevention treatment for stroke patients with ESVEA, and further studies are needed to prove this hypothesis.
4.2. Definition of ESVEA
At present, the term ‘ESVEA’ has various definitions from different studies, mainly because the cut-off value for the word ‘excessive’ is not clear. For example, Binici et al. [12] had set the cut-off value at the top 10th percentile for both frequency of supraventricular ectopic complexes (SVEC) and length of runs of SVEC, so they defined ESVEA as ≥30 SVEC per hour or any episode of runs of ≥20 SVEC. Weber-Krüger et al. [24] had set the cut-off value at the median of PAC frequency and the longest supraventricular run on 24 h-Holter (SV-run 24 h), so they defined ESVEA as PAC/h >4 and longest SV-run 24 h >5. In the future, larger and more standardized studies are urgently needed to unify the cut-off values and definitions of ESVEA. Thus, we could better diagnose ESVEA among patients, with a view toward early AF or stroke prevention.
4.3. Detection Methods of ESVEA
Furthermore, we found that the methods for detecting ESVEA were different in the included studies, including routine ECG, 24 h ECG, 48 h ECG, and polysomnogram-based ECG. The American Heart Association/American Stroke Association guideline for the prevention of stroke recommends that prolonged rhythm monitoring (≈30 days) is reasonable within six months of the index event for ischemic stroke or transient ischemic attack (TIA) patients with no other apparent cause [47]. Some studies have indicated the use of prolonged, continuous ECG monitoring for the detection of undiagnosed AF in stroke/TIA patients [49,50]. We need large sample studies with appropriate ECG monitoring methods and adequate follow-up duration to verify that ESVEA increases the risk of AF and stroke in the future.
4.4. Limitations
There are several limitations in the present systematic review and meta-analysis. Firstly, the pooled results were highly heterogeneous; we conducted subgroup and sensitivity analyses to find the source of heterogeneity in these studies. When different ECG durations were analyzed as subgroups, the heterogeneity of pooled outcomes between ESVEA and risk of new-onset and recurrent stroke were significantly reduced (Figure S3, Figure S4). Although our results indicated that ESVEA increased the risk of AF in the general population, the Egger′s test and funnel plot showed publication bias. Large samples and high-quality studies are needed to confirm the results in the future. Secondly, all included studies were observational, stroke subtypes and the duration of follow-up were variable, the detection method and definition of ESVEA were different, and the influence of confounders could not be fully excluded. Thirdly, we could not include any randomized controlled trials or large prospective studies in our analysis due to the lack of such studies, which could affect the reliability of our results.
4.5. Future Research
We advocate further investigation of the underlying mechanisms of ESVEA. For example, Bayés syndrome is associated with a high incidence of atrial tachyarrhythmias, which could be the cause of delayed and retrograde activation of the left atrium [51,52]. Bayés syndrome has also been shown to be a predictor of cardioembolic stroke [53,54,55]. Furthermore, high-quality studies are needed to unify the cut-off points of ESVEA so that we can monitor the occurrence of ESVEA in the clinic. Finally, we must conduct clinical trials of ESVEA interventions for the prevention of atrial fibrillation or stroke.
5. Conclusions
In conclusion, ESVEA is associated with an increased risk of incident AF, new-onset ischemic stroke, and all-cause mortality. Larger and more rigorous studies are urgently needed in the future to verify the relationship between ESVEA and ischemic stroke.
Acknowledgments
Not applicable.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcdd9120461/s1, Figure S1: The funnel plot for estimating the association between ESVEA and the risk of AF in the general population, Figure S2: The funnel plot for estimating the association between ESVEA and the risk of all-cause mortality, Figure S3: Forest plot of the association between ESVEA and new-onset ischemic stroke by subgroups, Figure S4: Forest plot of the association between ESVEA and recurrent ischemic stroke/transient ischemic attack (TIA) by subgroups; Table S1: Quality assessment for the included studies.
Author Contributions
Conceptualization, J.Y. and X.W.; writing—original draft preparation, M.Y.; writing—review and editing, M.Y., J.Y., X.W., Y.L., H.C., D.Z., S.T., L.Z. and Z.L.; funding acquisition, J.Y. and Y.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (82171295), Sichuan Science and Technology Program (2021YFS0376), Chengdu Science and Technology Bureau (2020-GH02-00057-HZ), Special Scientific Research Fund of the First Affiliated Hospital of Chengdu Medical University (CYFY2021YB04), and Key project of Chengdu Medical University-Chengdu Seventh People′s Hospital Joint Scientific Research Fund (2021LHJYZD-01).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Hart R.G., Diener H.C., Coutts S.B., Easton J.D., Granger C.B., O’Donnell M.J., Sacco R.L., Connolly S.J. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 3.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Heuschmann P.U., Kolominsky-Rabas P.L., Misselwitz B., Hermanek P., Leffmann C., Janzen R.W., Rother J., Buecker-Nott H.J., Berger K. Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: The German Stroke Registers Study Group. Arch. Intern. Med. 2004;164:1761–1768. doi: 10.1001/archinte.164.16.1761. [DOI] [PubMed] [Google Scholar]
- 5.Kongbunkiat K., Kasemsap N., Travanichakul S., Thepsuthammarat K., Tiamkao S., Sawanyawisuth K. Hospital mortality from atrial fibrillation associated with ischemic stroke: A national data report. Int. J. Neurosci. 2015;125:924–928. doi: 10.3109/00207454.2014.986266. [DOI] [PubMed] [Google Scholar]
- 6.Ong C.T., Wong Y.S., Wu C.S., Su Y.H. Atrial fibrillation is a predictor of in-hospital mortality in ischemic stroke patients. Ther. Clin. Risk Manag. 2016;12:1057–1064. doi: 10.2147/TCRM.S105703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller K., Hobohm L., Wenzel P., Münzel T., Espinola-Klein C., Ostad M.A. Impact of atrial fibrillation/flutter on the in-hospital mortality of ischemic stroke patients. Heart Rhythm. 2020;17:383–390. doi: 10.1016/j.hrthm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Arboix A., García-Eroles L., Massons J.B., Oliveres M., Pujades R., Targa C. Atrial fibrillation and stroke: Clinical presentation of cardioembolic versus atherothrombotic infarction. Int. J. Cardiol. 2000;73:33–42. doi: 10.1016/S0167-5273(99)00214-4. [DOI] [PubMed] [Google Scholar]
- 9.Flach C., Muruet W., Wolfe C.D.A., Bhalla A., Douiri A. Risk and Secondary Prevention of Stroke Recurrence: A Population-Base Cohort Study. Stroke. 2020;51:2435–2444. doi: 10.1161/STROKEAHA.120.028992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arboix A., García-Eroles L., Massons J., Oliveres M. Predictive clinical factors of in-hospital mortality in 231 consecutive patients with cardioembolic cerebral infarction. Cerebrovasc. Dis. 1998;8:8–13. doi: 10.1159/000015809. [DOI] [PubMed] [Google Scholar]
- 11.Wallmann D., Tuller D., Wustmann K., Meier P., Isenegger J., Arnold M., Mattle H.P., Delacretaz E. Frequent atrial premature beats predict paroxysmal atrial fibrillation in stroke patients: An opportunity for a new diagnostic strategy. Stroke. 2007;38:2292–2294. doi: 10.1161/STROKEAHA.107.485110. [DOI] [PubMed] [Google Scholar]
- 12.Binici Z., Intzilakis T., Nielsen O.W., Køber L., Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 13.Chong B.H., Pong V., Lam K.F., Liu S., Zuo M.L., Lau Y.F., Lau C.P., Tse H.F., Siu C.W. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14:942–947. doi: 10.1093/europace/eur389. [DOI] [PubMed] [Google Scholar]
- 14.Dewland T.A., Vittinghoff E., Mandyam M.C., Heckbert S.R., Siscovick D.S., Stein P.K., Psaty B.M., Sotoodehnia N., Gottdiener J.S., Marcus G.M. Atrial ectopy as a predictor of incident atrial fibrillation: A cohort study. Ann. Intern. Med. 2013;159:721–728. doi: 10.7326/0003-4819-159-11-201312030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acharya T., Tringali S., Bhullar M., Nalbandyan M., Ilineni V.K., Carbajal E., Deedwania P. Frequent Atrial Premature Complexes and Their Association WITH Risk of Atrial Fibrillation. Am. J. Cardiol. 2015;116:1852–1857. doi: 10.1016/j.amjcard.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Kochhäuser S., Dechering D.G., Dittrich R., Reinke F., Ritter M.A., Ramtin S., Duning T., Frommeyer G., Eckardt L. Supraventricular premature beats and short atrial runs predict atrial fibrillation in continuously monitored patients with cryptogenic stroke. Stroke. 2014;45:884–886. doi: 10.1161/STROKEAHA.113.003788. [DOI] [PubMed] [Google Scholar]
- 17.Murakoshi N., Xu D., Sairenchi T., Igarashi M., Irie F., Tomizawa T., Tada H., Sekiguchi Y., Yamagishi K., Iso H., et al. Prognostic impact of supraventricular premature complexes in community-based health checkups: The Ibaraki Prefectural Health Study. Eur. Heart J. 2015;36:170–178. doi: 10.1093/eurheartj/ehu407. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen K.T., Vittinghoff E., Dewland T.A., Dukes J.W., Soliman E.Z., Stein P.K., Gottdiener J.S., Alonso A., Chen L.Y., Psaty B.M., et al. Ectopy on a Single 12-Lead ECG, Incident Cardiac Myopathy, and Death in the Community. J. Am. Heart Assoc. 2017;6:e006028. doi: 10.1161/JAHA.117.006028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neal W.T., Kamel H., Judd S.E., Safford M.M., Vaccarino V., Howard V.J., Howard G., Soliman E.Z. Usefulness of Atrial Premature Complexes on Routine Electrocardiogram to Determine the Risk of Atrial Fibrillation (from the REGARDS Study) Am. J. Cardiol. 2017;120:782–785. doi: 10.1016/j.amjcard.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durmaz E., Ikitimur B., Ebren C., Tokdil H., Karaca O.F., Polat F., Ongen Z. The clinical significance of premature atrial contractions: How frequent should they become predictive of new onset atrial fibrillation. Anatol. J. Cardiol. 2019;22:47. doi: 10.1111/anec.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conen D., Adam M., Roche F., Barthelemy J.C., Felber Dietrich D., Imboden M., Künzli N., von Eckardstein A., Regenass S., Hornemann T., et al. Premature atrial contractions in the general population: Frequency and risk factors. Circulation. 2012;126:2302–2308. doi: 10.1161/CIRCULATIONAHA.112.112300. [DOI] [PubMed] [Google Scholar]
- 22.Vinther K.H., Tveskov C., Möller S., Rosen T., Auscher S., Osmanagic A., Egstrup K. Prevalence and Prognostic Significance of Runs of Premature Atrial Complexes in Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016;25:2338–2343. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Larsen B.S., Kumarathurai P., Falkenberg J., Nielsen O.W., Sajadieh A. Excessive Atrial Ectopy and Short Atrial Runs Increase the Risk of Stroke Beyond Incident Atrial Fibrillation. J. Am. Coll. Cardiol. 2015;66:232–241. doi: 10.1016/j.jacc.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Weber-Krüger M., Gröschel K., Mende M., Seegers J., Lahno R., Haase B., Niehaus C.F., Edelmann F., Hasenfuß G., Wachter R., et al. Excessive supraventricular ectopic activity is indicative of paroxysmal atrial fibrillation in patients with cerebral ischemia. PLoS ONE. 2013;8:e67602. doi: 10.1371/journal.pone.0067602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaillard N., Deltour S., Vilotijevic B., Hornych A., Crozier S., Leger A., Frank R., Samson Y. Detection of paroxysmal atrial fibrillation with transtelephonic EKG in TIA or stroke patients. Neurology. 2010;74:1666–1670. doi: 10.1212/WNL.0b013e3181e0427e. [DOI] [PubMed] [Google Scholar]
- 26.Im S.I., Park D.H., Kim B.J., Cho K.I., Kim H.S., Heo J.H. Clinical and electrocardiographic characteristics for prediction of new-onset atrial fibrillation in asymptomatic patients with atrial premature complexes. Int. J. Cardiol. Heart Vasc. 2018;19:70–74. doi: 10.1016/j.ijcha.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cha J.J., Lee K.Y., Chung H., Kim I.S., Choi E.Y., Min P.K., Yoon Y.W., Lee B.K., Hong B.K., Rim S.J., et al. Frequent Premature Atrial Contractions as a Poor Prognostic Factor in Cryptogenic Stroke Patients with Concomitant Non-Sustained Atrial Tachycardia. Yonsei Med. J. 2020;61:965–969. doi: 10.3349/ymj.2020.61.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durmaz E., Ikitimur B., Kilickiran Avci B., Atıcı A., Yurtseven E., Tokdil H., Ebren C., Polat F., Karaca O., Karadag B., et al. The clinical significance of premature atrial contractions: How frequent should they become predictive of new-onset atrial fibrillation. Ann. Noninvasive Electrocardiol. 2020;25:e12718. doi: 10.1111/anec.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neal W.T., Kamel H., Kleindorfer D., Judd S.E., Howard G., Howard V.J., Soliman E.Z. Premature Atrial Contractions on the Screening Electrocardiogram and Risk of Ischemic Stroke: The Reasons for Geographic and Racial Differences in Stroke Study. Neuroepidemiology. 2016;47:53–58. doi: 10.1159/000448619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sajeev J.K., Koshy A.N., Dewey H., Kalman J.M., Rajakariar K., Tan M.C., Street M., Roberts L., Cooke J.C., Wong M., et al. Association between excessive premature atrial complexes and cryptogenic stroke: Results of a case-control study. BMJ Open. 2019;9:e029164. doi: 10.1136/bmjopen-2019-029164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 33.Yodogawa K., Seino Y., Ohara T., Hayashi M., Miyauchi Y., Katoh T., Mizuno K. Prediction of atrial fibrillation after ischemic stroke using P-wave signal averaged electrocardiography. J. Cardiol. 2013;61:49–52. doi: 10.1016/j.jjcc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Johnson L.S., Juhlin T., Juul-Möller S., Hedblad B., Nilsson P.M., Engström G. A prospective study of supraventricular activity and incidence of atrial fibrillation. Heart Rhythm. 2015;12:1898–1904. doi: 10.1016/j.hrthm.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Marinheiro R., Parreira L., Amador P., Sá C., Duarte T., Caria R. Excessive atrial ectopic activity as an independent risk factor for ischemic stroke. Int. J. Cardiol. 2017;249:226–230. doi: 10.1016/j.ijcard.2017.08.054. [DOI] [PubMed] [Google Scholar]
- 36.Vinther K.H., Tveskov C., Möller S., Auscher S., Osmanagic A., Egstrup K. Excessive Premature Atrial Complexes and the Risk of Recurrent Stroke or Death in an Ischemic Stroke Population. J. Stroke Cerebrovasc. Dis. 2017;26:1163–1170. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 37.Raman D., Kaffashi F., Lui L.Y., Sauer W.H., Redline S., Stone P., Cawthon P.M., Stone K.L., Ensrud K.E., Ancoli-Israel S., et al. Polysomnographic Heart Rate Variability Indices and Atrial Ectopy Associated with Incident Atrial Fibrillation Risk in Older Community-dwelling Men. JACC Clin. Electrophysiol. 2017;3:451–460. doi: 10.1016/j.jacep.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persson A.P., Fedorowski A., Hedblad B., Persson M., Juul-Moller S., Engstrom G., Johnson L.S.B. Heart rate and premature atrial contractions at 24hECG independently predict atrial fibrillation in a population-based study. Heart. 2020;106:287–291. doi: 10.1136/heartjnl-2019-315119. [DOI] [PubMed] [Google Scholar]
- 39.Ntaios G., Perlepe K., Lambrou D., Sirimarco G., Strambo D., Eskandari A., Karagkiozi E., Vemmou A., Koroboki E., Manios E., et al. Supraventricular Extrasystoles on Standard 12-lead Electrocardiogram Predict New Incident Atrial Fibrillation after Embolic Stroke of Undetermined Source: The AF-ESUS Study. J. Stroke Cerebrovasc. Dis. 2020;29:104626. doi: 10.1016/j.jstrokecerebrovasdis.2019.104626. [DOI] [PubMed] [Google Scholar]
- 40.Sejr M.H., May O., Damgaard D., Bruun N.H., Nielsen J.C. Burden of Premature Atrial Complexes and Risk of Recurrent Stroke and Death in Patients with Mild to Moderate Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020;29:104490. doi: 10.1016/j.jstrokecerebrovasdis.2019.104490. [DOI] [PubMed] [Google Scholar]
- 41.Vetta G., Parlavecchio A., Caminiti R., Crea P., Magnocavallo M., Della Rocca D.G., Lavalle C., Vetta F., Marano G., Ruggieri C., et al. Non-conducted premature atrial complexes: A new independent predictor of atrial fibrillation in cryptogenic stroke. J. Electrocardiol. 2022;74:46–53. doi: 10.1016/j.jelectrocard.2022.07.071. [DOI] [PubMed] [Google Scholar]
- 42.Pinho J., Braga C.G., Rocha S., Santos A.F., Gomes A., Cabreiro A., Magalhães S., Ferreira C. Atrial ectopic activity in cryptogenic ischemic stroke and TIA: A risk factor for recurrence. J. Stroke Cerebrovasc. Dis. 2015;24:507–510. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 43.Lin C.Y., Lin Y.J., Chen Y.Y., Chang S.L., Lo L.W., Chao T.F., Chung F.P., Hu Y.F., Chong E., Cheng H.M., et al. Prognostic Significance of Premature Atrial Complexes Burden in Prediction of Long-Term Outcome. J. Am. Heart Assoc. 2015;4:e002192. doi: 10.1161/JAHA.115.002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabrera S., Vallès E., Benito B., Alcalde Ó., Jiménez J., Fan R., Martí-Almor J. Simple predictors for new onset atrial fibrillation. Int. J. Cardiol. 2016;221:515–520. doi: 10.1016/j.ijcard.2016.07.077. [DOI] [PubMed] [Google Scholar]
- 45.Hygrell T., Stridh M., Friberg L., Svennberg E. Prognostic Implications of Supraventricular Arrhythmias. Am. J. Cardiol. 2021;151:57–63. doi: 10.1016/j.amjcard.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki K., Nakajima I., Higuma T., Yamada M., Kasagawa A., Togashi D., Harada T., Akashi Y.J. Revisit to the Prognostic Value of Premature Atrial Contraction Burden in 24-h Holter Electrocardiography for Predicting Undiagnosed Atrial Fibrillation-A Propensity Score-Matched Study. Circ. J. 2021;85:1265–1272. doi: 10.1253/circj.CJ-20-1277. [DOI] [PubMed] [Google Scholar]
- 47.Kernan W.N., Ovbiagele B., Black H.R., Bravata D.M., Chimowitz M.I., Ezekowitz M.D., Fang M.C., Fisher M., Furie K.L., Heck D.V., et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 48.Yang M., Cheng H., Wang X., Ouyang M., Shajahan S., Carcel C., Anderson C., Kristoffersen E.S., Lin Y., Sandset E.C., et al. Antithrombotics prescription and adherence among stroke survivors: A systematic review and meta-analysis. Brain Behav. 2022;12:e2752. doi: 10.1002/brb3.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziegler P.D., Glotzer T.V., Daoud E.G., Singer D.E., Ezekowitz M.D., Hoyt R.H., Koehler J.L., Coles J., Jr., Wyse D.G. Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. Am. J. Cardiol. 2012;110:1309–1314. doi: 10.1016/j.amjcard.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Lazzaro M.A., Krishnan K., Prabhakaran S. Detection of atrial fibrillation with concurrent holter monitoring and continuous cardiac telemetry following ischemic stroke and transient ischemic attack. J. Stroke Cerebrovasc. Dis. 2012;21:89–93. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Ariyarajah V., Apiyasawat S., Fernandes J., Kranis M., Spodick D.H. Association of atrial fibrillation in patients with interatrial block over prospectively followed controls with comparable echocardiographic parameters. Am. J. Cardiol. 2007;99:390–392. doi: 10.1016/j.amjcard.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 52.Arboix A., Martí L., Dorison S., Sánchez M.J. Bayés syndrome and acute cardioembolic ischemic stroke. World J. Clin. Cases. 2017;5:93–101. doi: 10.12998/wjcc.v5.i3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes T.M., Worrall B.B. Acute interatrial block is a distinct risk factor for ischemic stroke. Neurology. 2016;87:344–345. doi: 10.1212/WNL.0000000000002905. [DOI] [PubMed] [Google Scholar]
- 54.Bacharova L., Wagner G.S. The time for naming the Interatrial Block Syndrome: Bayes Syndrome. J. Electrocardiol. 2015;48:133–134. doi: 10.1016/j.jelectrocard.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 55.Ariyarajah V., Puri P., Apiyasawat S., Spodick D.H. Interatrial block: A novel risk factor for embolic stroke? Ann. Noninvasive Electrocardiol. 2007;12:15–20. doi: 10.1111/j.1542-474X.2007.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.