Abstract
Several alkaline earth or rare earth binary monosilicides and -germanides possess complex bonding properties, such as polycation formation exceeding the scope of classical electron counting rules. In this study, we present characterization by powder and single-crystal diffraction and thermal analysis of CeGe, one of the few monogermanides crystallizing in the FeB-type structure. Comparative computational investigations for structure types experimentally observed for monogermanides and alternative structures with different structural motifs were performed to gain energetical insights into this family of compounds, underlining the preference for infinite germanium chains over other structural motifs. Formation enthalpy calculations and structural chemical analysis highlight the special position of FeB-type compounds among the monogermanides.
Keywords: intermetallic compounds, crystal chemistry, X-ray diffraction, chemical bonding
1. Introduction
Electron counting rules are simple but powerful schemes allowing for the explanation of electronic properties and chemical bonding or even the prediction of structural arrangements for a surprisingly large number of compounds. Whereas this approach works well for certain families among the inorganics, such as Zintl [1] or cluster compounds [2], the neighboring class of intermetallics lacks general systematics explaining their structure, properties, and bonding interrelation [3,4]. In order to disclose such relationships, combined experimental and theoretical studies, including energy and bonding calculations, have to be systematically performed.
Interestingly, such analyses have often revealed unanticipated bonding features for groups of compounds that formally follow electron counting schemes, e.g., the Zintl–Klemm concept. The latter, in its stricter interpretation, assumes the formation of homopolar two-center, two-electron bonds among p-block elements with the aim to fulfil the octet rule after a formal electron transfer from electropositive metal species. Up to now, several polyanionic fragments with intriguing structural and chemical peculiarities, often exceeding chemists’ expectations and predictions, have been reported. Among them, zig-zag chains are common for binary alkaline earth (AE) or rare earth metal (RE) silicides and germanides. Complex bonding interactions were discovered both for compounds formally fulfilling the octet rule, such as CrB-type CaSi [5], and FeB-type LuGe, wherein the formation of two-bonded germanium chains, (2b)Ge, formally bearing a −2 charge, leads to an ionic formulation comprising excess electrons: Lu3+[(2b)Ge2−] × 1 e− [6].
Recently, such electrons were found to be responsible for the formation of four-atomic Lu4 bonds besides Lu-Ge polar interactions [6]. There are different possibilities of how these interactions can influence the resulting structure, especially the infinite germanium chains as the main structural element. Three supposable scenarios were selected and investigated using electronic structure and energetical calculations and structure chemical analysis to gain a deeper understanding of the origin of bonding interactions and their influence on structural features in FeB-type monogermanides. The chains can show (1) a Peierls distortion resulting in a superstructure formation comprising dumbbells instead of chains at low temperatures [7], (2) a deformation by changing the chain distances dGe-Ge, or (3) a deformation combined with an inclination perpendicular to the running direction of the chains. However, only four rare-earth monogermanides with the same structure type were reported (Table 1). The compound CeGe has been known since the work of Parthé [8], but the structure of the compound (FeB-type) has only been inferred from the indexing of the powder X-ray diffraction pattern. This motivated a reinvestigation of CeGe based on single-crystal diffraction data.
Table 1.
Overview of structure types of known binary monosilicides and –germanides with alkaline and rare earth elements comprising infinite chains (C1—(oP8)FeB, C2—(oS8)CrB, C3—(oS16)LT-LaSi) or isolated polyanions (P—(oI40)SrSi). Availability of full structure refinement data indicated by blue letters [6,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30].
| Mg | Ca | Sr | Ba | Sc | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | C 2 | C 2 | C 2 | C 2 | C 2 | C 1 | C 1 | C 1 | C 1 | C 1 | C 2 | C 1 | C 1 | C 1 | C 1 | C 1 | C 2 | C 2 | C 2 | |
| P | C 3 | C 2 | C 2 | C 2 | ||||||||||||||||
| Ge | C 2 | C 2 | C 2 | C 2 | C 2 | C 1 | C 1 | C 1 | C 2 | C 2 | C 2 | C 2 | C 2 | C 2 | C 2 | C 2 | C 2 | C 1 | ||
| P | C 3 | C 2 |
2. Materials and Methods
Synthesis. Sample handling was performed in an argon-filled glovebox. The synthesis was conducted in an arc furnace under argon atmosphere. To ensure homogenization, each sample was melted two times. Cerium was added in excess (1.8%) to compensate for evaporation loss.
X-ray diffraction. For the analysis, the samples were prepared for powder X-ray diffraction (Stoe Stadi MP (STOE & Cie GmbH, Darmstadt, Germany), Mythen 1k detector, Cu-Kα radiation, λ = 1.54178 Å) in transmission mode. Single crystals were chosen and analyzed with single-crystal X-ray diffraction (Xcalibur (Rigaku Oxford Diffraction, Sevenoaks, Kent, UK), EosS2, CCD plate detector, graphite monochromator, Mo-Kα radiation, λ = 0.71073 Å) at room temperature and at −173 °C. Data integration, structure solution, and refinements were conducted with the programs CrysAlisPro [31], Superflip [32], and JANA2006 [33].
Thermal analysis. Differential scanning calorimetry (DSC) was performed between −90 and 100 °C in a DSC Q2000 device (T.A. Instruments, New Castle, DE, USA) with heating and cooling rates of 10 K min−1 (sample mass 25.568 mg) in an Al pan and lid.
Computational Details. Total energy calculations were performed at a DFT/PBE level of theory using the Quantum Espresso software package (v5.4) [34] for the following compounds: CeGe simulated with the oP8–FeB, oS8–CrB, oS16–LaSi, hP12–Na2O2, and hP8–Li2O2-β structures, YGe simulated as oS8–CrB and hP12–Na2O2, LaGe as oS16–LaSi and hP12–Na2O2, CaAs and KSe as oP8–FeB, oS8–CrB, oS16–LaSi, and hP12–Na2O2. For the sake of conciseness, such structures will just be indicated by their prototypes in the following ways. The recommended projector-augmented wave (PAW) [35] sets, available at the PSlibrary [36], were employed. The semicore states 5s, 5p, and 5d for Ce and La; 4s and 4p for Y; 3s and 3p for K and Ca; and 3d for Ge and Se were included in the valence configuration. Thus, the 4f states of Ce were not included in such configuration, and all calculations were non-spin polarized. The orbital occupancies at the Fermi level were treated with Gaussian smearing of 0.01 Ry. The plane-wave and density cut-off were set to 45 Ry and 450 Ry, respectively. The Brillouin zone was sampled within uniform grids (Monkhorst–Pack [37]) generated with the k-point meshes listed in Table 2.
Table 2.
k-point meshes employed to perform total energy calculations for the selected crystal structures.
| Crystal Structure | k-Mesh |
|---|---|
| oP8–FeB | (6, 14, 10) |
| oS8–CrB | (12, 4, 14) |
| oS16–LaSi | (12, 4, 8) |
| hP12–Na2O2 | (8, 8, 10) |
| hP8–Li2O2-β | (10, 10, 6) |
The convergence threshold for self-consistency was set to 1.0∙10−9 Ry. The electronic density of states (DOS) for CeGe with the FeB, Na2O2, and Li2O2 type of structures were computed with the aid of the LOBSTER software (v4.1.0) [38,39,40,41], which reconstructs electronic structures through the projection of PAW-based wave functions onto atomic-like basis sets.
For this purpose, the PAW wave functions were generated by running an additional single-point calculation slightly reducing the k-mesh: (4, 8, 6) for FeB, (6, 6, 8) for Na2O2, and (8, 8, 4) for Li2O2. The projection was performed with the local pbeVaspFit2015 [39] basis set; Ce 5s, 5p, 5d, 6s, and Ge 3d, 4s, 4p orbitals were selected, leading to excellent absolute charge spilling (<0.9%). DOS curves were plotted utilizing the wxDragon software (v2.1.8) [42].
3. Results and Discussion
3.1. Crystal Structure and Thermal Analysis
CeGe, obtained by the arc-melting of the elements with a 1.8% excess of Ce, is crystallizing in space group Pnma with a = 8.3524(4) Å, b = 4.0852(2) Å, and c = 6.0322(3) Å (Table 3) in accordance with previous powder diffraction studies (Table S1, Figures S1 and S2) [8,43,44]. The structure refinement was performed on single-crystal X-ray diffraction data (Figure S3, acquired at room temperature), resulting in residuals R = 0.0352, wR = 0.0321 (Table 3, Table 4 and Table 5).
Table 3.
X-ray diffraction data for CeGe. Further details on the crystal structure investigations can be obtained from the FachinformationszentrumKarlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (email: crysdata@fiz-karlsruhe.de, http:///www.fiz-karlsruhe.de/request_for_deposited_data.html) on quoting the depository numbers CSD-2213844.
| Composition | CeGe |
| Space group, Pearson symbol | Pnma (No. 62), oP8 |
| Unit cell parameters | |
| a [Å] | 8.3524(4) |
| b [Å] | 4.0852(2) |
| c [Å] | 6.0322(3) |
| V [Å3] | 205.86(2) |
| Formula units Z | 4 |
| Diffractometer | Rigaku Xcalibur 3, CCD detector, graphite monochromator, Mo Kα radiation, λ = 0.71073 Å |
| Reflections collected/independent within F > 4σ(F) | 3250/303 |
| Measurement range | −10 ≤ h ≤ 11, −5 ≤ k ≤ 5, −8 ≤ l ≤ 8 |
| Fourier difference ρmin/ρmax (electrons/Å3) | −6.56/5.58 |
| Residuals and GOF | R = 0.0352, wR = 0.0321, GOF = 1.37 |
Table 4.
Position and displacement parameters for CeGe.
| Atom | Site | a/x | b/y | c/z | U ani |
|---|---|---|---|---|---|
| Ce | 4c | 0.1806(1) | 0.25 | 0.6164(2) | 0.0077(3) |
| Ge | 4c | 0.0372(2) | 0.25 | 0.1334(3) | 0.0104(6) |
Table 5.
Components of the anisotropic displacement tensor U for CeGe.
| Atom | U 11 | U 22 | U 33 | U 12 | U 13 | U 23 |
|---|---|---|---|---|---|---|
| Ce | 0.0073(5) | 0.0084(5) | 0.0073(5) | 0 | 0.0013(4) | 0 |
| Ge | 0.010(1) | 0.009(1) | 0.012(1) | 0 | 0.0003(8) | 0 |
The compound is isotypic to FeB [45]. In CeGe, each cerium atom possesses seven germanium and ten cerium neighbors (Figure S2). Germanium is coordinated by two germanium and seven cerium atoms. The crystal structure of CeGe constitutes columns of triangular prisms along [010], connected by two rectangular faces. Each prism has one shared basal edge and one common atom at the apexes opposite this basal edge with four other prisms. Within these prisms, chains of interconnected germanium atoms run along the [010] direction. The Ge-Ge chains are arranged in sheets along the (011) plane. These sheets are alternately stacked parallel to the [100] direction, in which solely Ce or Ge atoms can be observed.
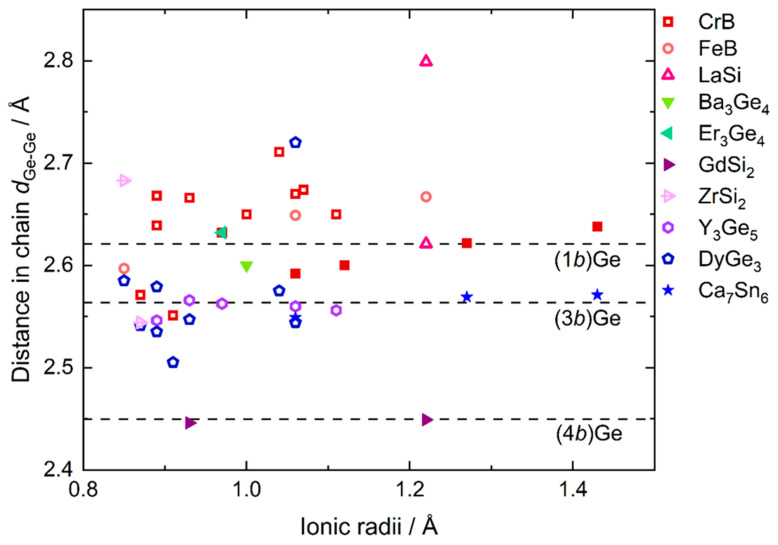
The distances dCe-Ge range between 3.1186(4) and 3.3403(4) Å (Table 6) and dGe-Ge amount to 2.6739(4) Å. In comparison to dGe-Ge in the element (2.450 Å [46]), the distances dGe-Ge are noticeably long (Figure 1), corresponding to a significantly reduced bond order of sij = exp() = 0.68 (d1: dGe-Ge in cF8 Ge [46]; dij: dGe-Ge in CeGe; c = 0.6) [47], calculated as a first estimate using the Pauling formula which approximates bond orders varying exponentially with bond distances.
Table 6.
Selected interatomic distances in CeGe.
| Atom | Distance/Å | Atom | Distance/Å |
|---|---|---|---|
| Ce-2 Ge | 3.1186(4) | Ge-2 Ge | 2.6739(4) |
| Ce-2 Ge | 3.1232(4) | Ge-2 Ce | 3.1186(4) |
| Ce-1 Ge | 3.1495(3) | Ge-2 Ce | 3.1232(4) |
| Ce-1 Ge | 3.3358(4) | Ge-1 Ce | 3.1495(3) |
| Ce-1 Ge | 3.3403(4) | Ge-1 Ce | 3.3358(4) |
| Ce-4 Ce | 3.8222(5) | Ge-1 Ce | 3.3403(4) |
| Ce-2 Ce | 3.9028(5) | ||
| Ce-2 Ce | 4.0852(7) |
Figure 1.
Distances dGe-Ge of selected compounds comprising Ge-chains [6,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] versus ionic radii (for trivalent rare earth and divalent alkaline earth metals, as well as Eu [68]), ordered by their structure types. Filled symbols denote electron-precise compounds, empty symbols compounds with non-electron precise electron balances. Distances for (4b)Ge (cF8-Ge [46]), (3b)Ge (NaGe [69]), and (1b)Ge (Li7Ge2 [70]) serve as references.
Similar one-dimensional chains of germanium occur in compounds crystallizing with different structure types [6,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] (see Table S2), whereas chains in connection with other structural motifs are found both for electron-precise and non-electron-precise phases. When appearing in combination with other structural elements, the distances in Ge–Ge chains are mostly longer than in elemental Ge but relatively similar to distances among (3b)Ge within the electron-precise tetrahedral clusters in NaGe [69] (Figure 1). Compounds with infinite chains as the only structural element are electron-precise for divalent elements such as AE metals and possess, in that case, interatomic distances similar to (1b)Ge dumbbells in Li7Ge2 [70].
Although an electron-precise electron balance could be realized for rare earth monosilicides and -germanides with structures comprising solely (1b)Ge dumbbells in the anionic partial structure, this connectivity scenario is only found for alkaline, AE, and RE metal silicides or germanides for non-electron-precise compositions. In the first possible scenario, a Peierls distortion of the Ge-chains resulting in the formation of a superstructure at low temperatures and, therefore, dimerization in line with an electron-precise electron balance did not occur, as evidenced by the absence of features pointing towards a phase transition during DSC experiments in the range of −90 to 100 °C (Figure S4). Additional single-crystal diffraction experiments at −173 °C confirm a structure comprising Ge-chains with equal Ge–Ge distances.
3.2. Stability and Electronic Structure
Theoretical calculations were performed to explain why monogermanides are an exception among A- (alkali metal), AE-, and RE-tetrel (group 14 elements) compounds [71,72] realizing a structural arrangement in line with the Zintl–Klemm concept and the 8 − N rule. Total energy calculations, including the simulation of CeGe with the structure types FeB, CrB, and LaSi reported for the REGe compounds (for optimized structural data, see Table 7) indicate that there are no relevant energy differences (see Table 7) among them. In contrast, the simulation of CeGe in the structure types Na2O2 and β-Li2O2 experimentally observed, e.g., for CaAs and KSe comprising (1b)As2− and (1b)Se− dumbbells, revealed lower stability compared to the structure types comprising chains (Table 7). These results confirm that the formation of (1b)Ge3− dumbbells is energetically not favored.
Table 7.
Experimental and calculated unit cell parameters and total energy differences (ΔE) for different CeGe polymorphs, simulated to crystallize with the FeB, CrB, LaSi, β-Li2O2, and Na2O2 structure types. The ΔE values are calculated with respect to the oP8–FeB structure, experimentally found to be the most stable one: E(FeB)–E(CrB); E(FeB)–E(LaSi); E(FeB)–E(β-Li2O2); E(FeB)–E(Na2O2).
| Experimental | Calculated | |||||
|---|---|---|---|---|---|---|
| Ge Fragments | (2b)Ge | (2b)Ge | (1b)Ge | |||
| Pearson Symbol Prototype |
oP8 FeB |
oP8 FeB |
oS8 CrB |
oS16 LaSi |
hP8 β-Li2O2 |
hP12 Na2O2 |
| a (Å) | 8.3529(5) | 8.4324 | 4.5573 | 4.5402 | 4.7252 | 8.0172 |
| b (Å) | 4.0878(3) | 4.1185 | 11.275 | 13.757 | 4.7252 | 8.0172 |
| c (Å) | 6.0346(3) | 6.0816 | 4.1198 | 6.7556 | 12.2202 | 6.3456 |
| ΔE (eV/at) | - | 0.000 | 0.000 | 0.002 | −0.101 | −0.068 |
The comparison of the interatomic distances of the optimized CeGe with FeB and Li2O2 structures shows that the distances in the dumbbells (dGe-Ge = 2.79 Å) are larger than those within the zig-zag chains (dGe-Ge = 2.70 Å), suggesting a higher electrostatic repulsion among the (1b)Ge3− species. For CeGe simulated in the Na2O2 structure type, two kinds of Ge dumbbells with dGe1–Ge1 = 2.68 Å and dGe2–Ge2 = 2.53 Å were observed. Such a difference was reported neither for CaAs [73] nor for the Na2O2 [74] prototype. Hence, although the hexagonal symmetry is retained, this observation for CeGe (Na2O2) is probably caused by more complex factors.
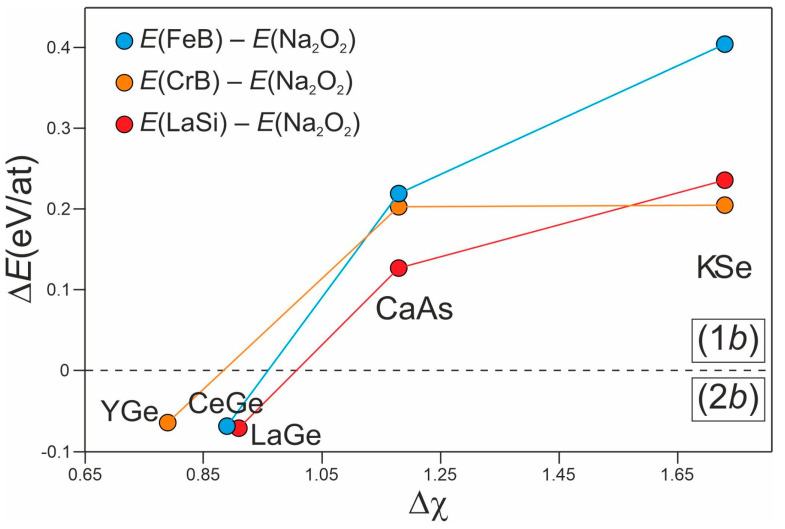
One reason for the preference of (2b)Ge chains could be found in the electronegativity difference among the constituents (Δχ), which is not high enough to allow for an almost complete charge transfer of three valence electrons from Ce to Ge so that only two are formally transferred. The remaining one is probably participating in Ce–Ce interactions. Since the formation of dumbbells is disfavored along the series of the rare-earth monogermanides, YGe and LaGe have been included in this analysis as representative for the CrB and LaSi types, respectively. To prove this assumption, CaAs and KSe total energies have also been calculated in the FeB, CrB, LaSi, and Na2O2 structures and analyzed together with Δχ. For CaAs and KSe, the Na2O2 modification is reported to be the most stable one. Thus, the selected REGe (RE = Y, La, Ce) have also been simulated with the Na2O2 structure, i.e., comprising (1b)Ge dumbbells. Energy differences (ΔE) have been calculated according to the following formulae: E(FeB)–E(Na2O2); E(CrB)–E(Na2O2); E(LaSi)–E(Na2O2). Thus, if ΔE < 0, the FeB/CrB/LaSi type of structure is favored. Otherwise, the Na2O2 one is expected to be most stable. The results of such an analysis are reported in Figure 2. Focusing on the blue circles in Figure 2, which indicate the energy difference between the FeB and Na2O2 structure types, one can see that, at low Δχ value corresponding to the CeGe compounds, a negative ΔE is obtained. The aforementioned equation indicates that CeGe is most stable with the FeB structure; thus, (2b)Ge chains are preferred with respect to (1b)Ge dumbbells. Following the blue line to the right, the Δχ is gradually increased until reaching first CaAs and then KSe. As clearly visible, in both cases ΔE > 0, indicating that these compounds are more stable when simulated in the Na2O2 type than in the FeB one, well in line with experimental results. The same trend is realized for all of the considered REGe phases (red and orange circles in Figure 2), displaying that a gradual increase of Δχ, i.e., of charge transfer from metal species to the p-block elements, stabilizes the dumbbells-containing Na2O2 structure with respect to the other types. The only exception in this trend is represented by KSe, which displays the same E(CrB)–E(Na2O2) of CaAs (orange dots and line in Figure 2). Such anomaly is caused by the crystal structure obtained for KSe after the relaxation procedure that comprises Se dumbbells, even though the input structure was CrB. Nevertheless, the energy difference is still in favor of the Na2O2 structure.
Figure 2.
Energy difference (ΔE) as a function of the Pauling electronegativity difference (Δχ) among the constituents of CeGe, YGe, LaGe, CaAs, and KSe. The dashed black line corresponds to ΔE = 0 and separates the stability region of (2b) polyanions, ΔE < 0, from that of the (1b) ones, ΔE > 0.
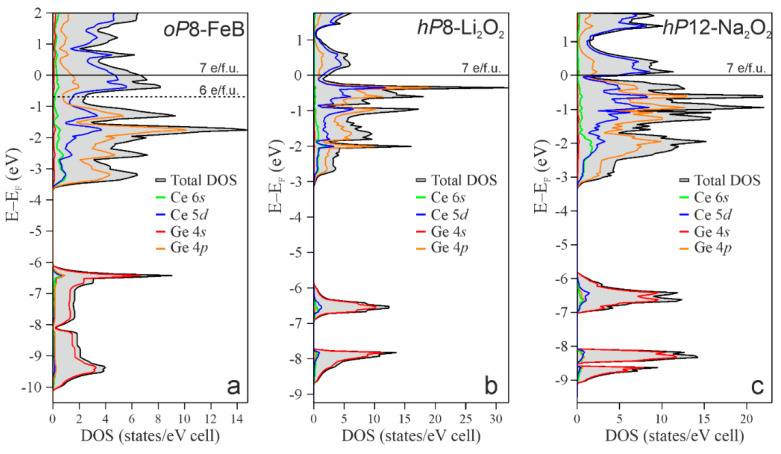
Furthermore, the electronic density of states (DOS) for CeGe in the FeB, Li2O2, and Na2O2 modifications have been calculated (see Figure 3) to give more insight into the chemical reasons behind these experimental and energetical outcomes.
Figure 3.
Total and orbital-projected electronic density of states for CeGe with the oP8-FeB structure (a) and simulated as hP8–Li2O2 (b) and as hP12–Na2O2 (c).
Calculated DOS curves indicate in all cases a metal-like behavior but with a noticeable difference: Li2O2 (Figure 3b) and Na2O2 (Figure 3c) show a pseudogap at the Fermi level, as typically expected for metallic germanides formally accomplishing the Zintl formalism [75,76,77]. Conversely, the pseudogap in the DOS of CeGe (FeB) occurs at approximately −0.6 eV (Figure 3a), so that the Fermi level lies in a region with a high density of states. The DOSs can be conveniently separated into two regions. The first one, located below −5 eV, is essentially dominated by the 4s states of Ge. The second one, in the −4 and −0.6 eV energy range for CeGe (FeB) and between −4 and 0 eV for CeGe (Li2O2 and Na2O2), is mainly contributed by the Ge 4p states that energetically overlap with the 5d of Ce. This finding indicates the incomplete ionization of Ce and the consequent formation of polar covalent interaction between Ce and Ge, analogously to other binary and ternary rare-earth germanides, such as LuGe [6], LuGe3 [65], RE2MGe6 [75,78], RE2Pd3Ge5 [77]. In fact, in the ideal case of a complete ionization, Ce 5d states are expected to be empty, i.e., located above the EF. The CeGe (FeB) DOS has a third region, between about −0.6 eV and EF, in which the contribution of the 5d states of Ce exceeds that of Ge 4p. The integral of the DOS in this region gives 1 e/f.u. (electron per formula unit) (Figure 3a). This situation is very similar to the scenario observed in LuGe [6] wherein the “excess” electrons in the region between the pseudogap and EF were responsible for the formation of Lu4 4-atomic bonds. Similarly, the states in the third DOS region of CeGe could contribute to the formation of cerium–cerium bonding. This kind of interaction does not occur in the Li2O2 and Na2O2 type of structures.
3.3. Crystal-Chemical Insights Based on Bonding Considerations
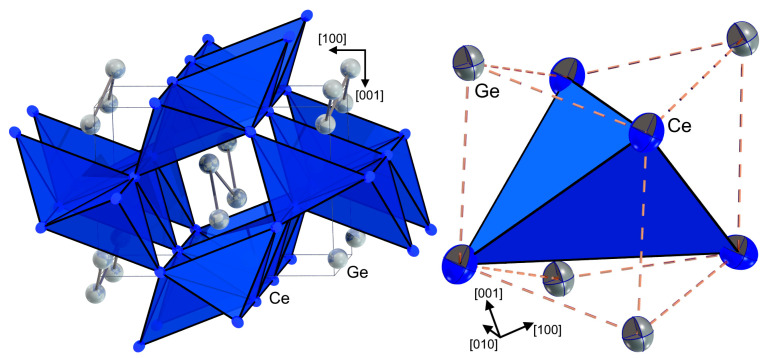
The crystal and the electronic structure analogies observed between CeGe and LuGe allow assuming that the RE–RE interactions are realized within the distorted RE4 tetrahedra evidenced for the Lu-analogue [6]. Considering this chemical feature, the structure can be alternatively described as a network of corner-sharing RE4 tetrahedra with Ge chains running in the channels within the tetrahedra network (Figure 4, left). The displacement ellipsoid of the Ce atoms shows a flattening towards the channels. The displacement of Ge is slightly larger in the [001] direction, and the ellipsoid shows an elongation within the 2-D planes perpendicular to the running direction of the chains (Figure 4) that is directed towards the center of the nearest RE4 tetrahedron. Each tetrahedron is capped by four Ge atoms, resulting in a stella quadrangula (Figure 4, right).
Figure 4.
Crystal structure of CeGe depicted as (left) network of corner-sharing Ce4 tetrahedra and (right) stella quadrangula formed around each Ce4 tetrahedron.
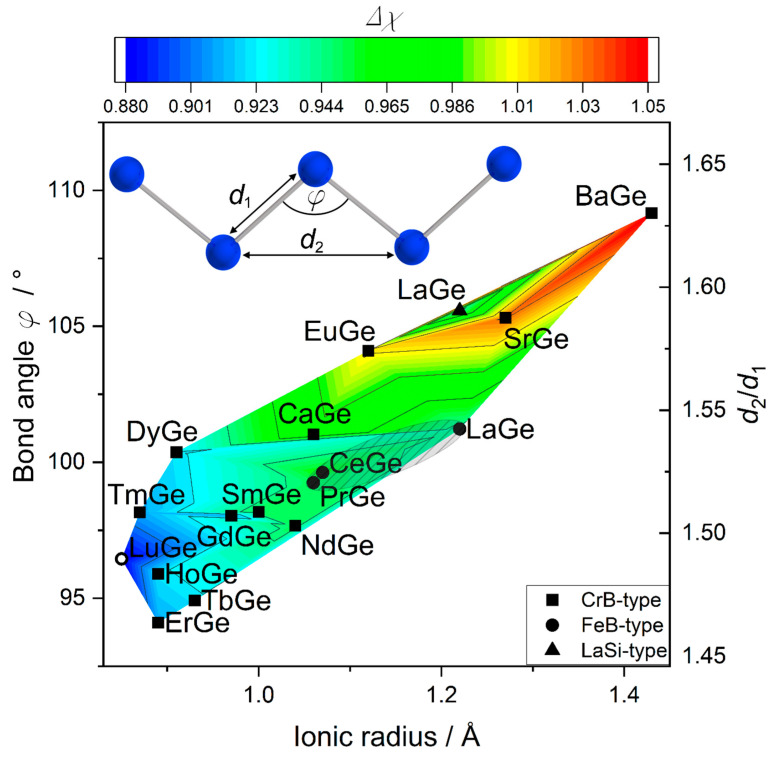
Thus, the RE4 bonding interaction seems to influence the Ge chains significantly. The chains can react by deformation and, therefore, cause a change in the chain distances dGe-Ge and bond angles φ (Figure 5). For a more comprehensive picture of monogermanides, such analysis was extended also to germanides with the CrB type structures. Furthermore, the FeB type structure also offers the possibility of combining a deformation with an inclination perpendicular to the running direction of the chains (in [100] direction).
Figure 5.
Relation between bond angle in the chains φ, ratio of the chain distances d2/d1 and difference in electronegativity (filled symbols—ambient pressure, open symbols—high-pressure synthesis). Striped ellipsoid marks the narrow field of ambient-pressure FeB-type rare-earth germanides.
The next-neighbor distances d1 with dependence on the ionic radii (for trivalent rare earth and divalent alkaline earth metals, as well as Eu [68]) remain almost constant along the series of alkaline earth and rare-earth monogermanides (Figure 1), whereas both the distance d2 after the next neighbor and bond angle increase with ionic radius. Therefore, the bond angles evolve in the same manner as the ratio d2/d1 (Figure 5), denoting that the Ge–Ge chains adjust to the size of the cation via the bond angle (and the related distance after the next d2) instead of the next-neighbor distance d1. Whereas a variety of compounds with a large range of bond angles, ionic radii, and electronegativity differences realize the CrB-type structure, FeB-type rare-earth monogermanides cover only a small range of bond angles and electronegativity differences (Figure 5, striped ellipsoid). Only the high-pressure compound LuGe does not fall into this small field. With the increasing size of the ion, the bond angle increases, underlining the high flexibility of Ge–Ge chains as a function of the cationic dimensions, probably allowing the retention of the RE–Ge or AE–Ge polar interactions that have been already described for some representatives [6,75] and also deduced from DOS calculations for CeGe.
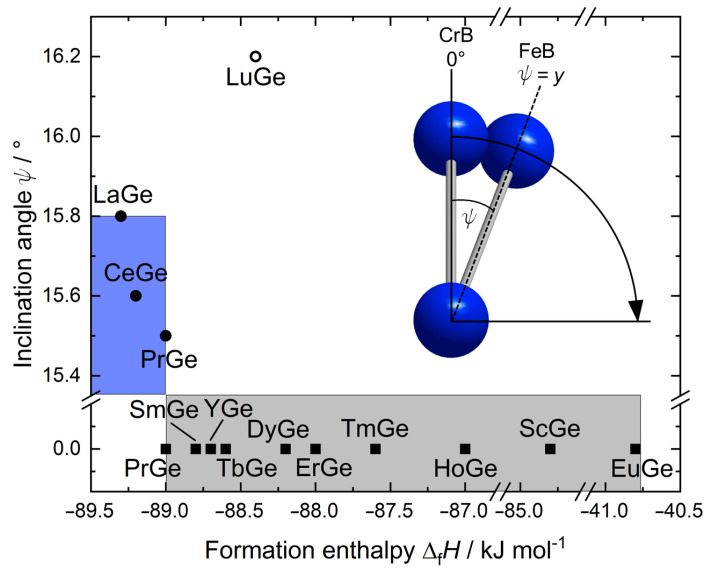
To evaluate the influence of the inclination of the Ge–Ge chains as an additional degree of freedom for FeB-type compounds, the inclination angle (ψ) of the chains (Figure 6) was calculated from experimental data [6,13,20,21,23,24,25,26,27,28,29,30]. The inclination angle seems to mostly depend on the ionic radius (for trivalent rare earth and divalent alkaline earth metals, as well as Eu [68]), a deviation from the vertical (inclination in [100] direction, ψ ≠ 0°) is indeed observed only for the light rare-earth elements. The formation enthalpies ΔfH, calculated by the Miedema method [79], change according to the lanthanide contraction. The plot of the formation enthalpies ΔfH versus the inclination angle revealed that FeB- and CrB-type compounds are distributed in two fields, whereas low energy goes along with higher inclination for FeB-type compounds. For less negative energies, the CrB-type structure is realized; therefore, no inclination of the chains is observed. The dimorphic compound PrGe crystallizing in both the FeB- and the CrB-type structure is energetically located at the transition between both fields. Again, the high-pressure compound LuGe does not fall into those ranges.
Figure 6.
Inclination angle ψ of the chains vs. the enthalpy of formation ΔfH. Squares indicate CrB-type and circles FeB-type compounds. Blue and grey regions indicate fields pertaining to the FeB- and CrB-types, respectively.
4. Conclusions
Despite the power of simple electron counting rules, such as the Zintl–Klemm concept, interesting bonding interactions can hide even within widely “countable” compounds. Total energy and electronic structure calculations evidence the preference for complex structural interactions in contrast to what is expected from this simple scheme. Cation–cation interactions occurring in addition to classical two-center, two-electron (2c-2e) bonds in the anionic partial structure influence the overall structure. The resulting structural adaptions and the special position of FeB-type compounds among other monogermanides were traced by formation enthalpy calculations and structural chemical analysis.
Calculated DFT total energies have been related to the electronegativity difference among the constituents, used in first approximation as a measure of the effective charge transfer from the metal species to the p-block elements, clearly showing the key role of such a parameter in the formation of structures that fulfill, like KSe and CaAs, and do not fulfill, as REGe compounds, the Zintl rules.
For a deeper understanding, similar analyses and further chemical bonding calculations are planned for various chain compounds.
Acknowledgments
We thank Sven Lidin, Serena De Negri and Michael Baitinger for their support and valuable discussions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma15249089/s1, Table S1: Comparison of lattice parameters of CeGe. Figure S1: Powder diffraction pattern of one obtained sample. Figure S2: Crystal structure of CeGe. (left) Unit cell with Ge-chains running along [010] direction. (right) Coordination environment of Ce and Ge, respectively. Figure S3: Diffraction pattern of CeGe with reciprocal lattice reconstructions of (a) (hk0), (b) (h0l), and (c) (0kl) layers. Figure S4: DSC, 1,3-first and second cooling, 2,4-first and second heating. Note the absence of any indication of a phase transformation in the interval measured. Table S2: Binary alkaline earth and rare earth metal germanides comprising Ge-polyanions. Average distances are given for chain motifs. References [6,8,10,12,13,20,21,23,25,26,27,28,29,30,43,44,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,65,66,67] are cited in the supplementary materials.
Author Contributions
Conceptualization, J.-M.H.; methodology, J.-M.H., E.F., C.W. and R.F.; software, J.-M.H. and R.F.; validation, J.-M.H. and R.F.; formal analysis, J.-M.H., E.F., C.W. and R.F.; investigation, J.-M.H. and R.F.; resources, J.-M.H.; data curation, J.-M.H. and R.F.; writing—original draft preparation, J.-M.H. and R.F.; writing—review and editing, J.-M.H. and R.F.; visualization, J.-M.H. and R.F.; supervision, J.-M.H.; project administration, J.-M.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
CCDC 2213844 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
J.-M.H. gratefully acknowledges funding by the Swedish Research Council (Vetenskapsrådet), grant VR Intermet Kemi.2016/339.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nesper R. The Zintl-Klemm Concept—A Historical Survey. Z. Anorg. Allgem. Chem. 2014;640:2639–2648. doi: 10.1002/zaac.201400403. [DOI] [Google Scholar]
- 2.Wade K. The structural significance of the number of skeletal bonding electron-pairs in carboranes, the higher boranes and borane anions, and various transition-metal carbonyl cluster compounds. J. Chem. Soc. D. 1971;15:792–793. doi: 10.1039/c29710000792. [DOI] [Google Scholar]
- 3.Fredrickson D.C., Miller G.J. Intermetallic Chemistry: New Advances in Humanity’s Age-Old Exploration of Metals and Alloys. Acc. Chem. Res. 2018;51:213. doi: 10.1021/acs.accounts.8b00035. [DOI] [PubMed] [Google Scholar]
- 4.Nesper R. Bonding Patterns in Intermetallic Compounds. Angew. Chem. Int. Ed. 1991;30:789–817. doi: 10.1002/anie.199107891. [DOI] [Google Scholar]
- 5.Kurylyshyn I.M., Fässler T.F., Fischer A., Hauf C., Eickerling G., Presnitz M., Scherer W. Probing the Zintl–Klemm Concept: A Combined Experimental and Theoretical Charge Density Study of the Zintl Phase CaSi. Angew. Chem. Int. Ed. 2014;53:3029–3032. doi: 10.1002/anie.201308888. [DOI] [PubMed] [Google Scholar]
- 6.Freccero R., Hübner J.-M., Prots Y., Schnelle W., Schmidt M., Wagner F.R., Schwarz U., Grin Y. “Excess” electrons in LuGe. Angew. Chem. Int. Ed. 2021;60:6457. doi: 10.1002/anie.202014284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peierls R. More Surprises in Theoretical Physics. Princeton University Press; Princeton, NJ, USA: 1991. [Google Scholar]
- 8.Hohnke D., Parthe E. AB compounds with Sc, Y and rare earth metals. II. FeB and CrB type structures of monosilicides and germanides. Acta Crystallogr. 1966;20:572–582. doi: 10.1107/S0365110X66001282. [DOI] [Google Scholar]
- 9.Currao A., Curda J., Nesper R. Kann man Arten von Zintl-Anionen steuern? Variationen ueber das Thema Si2− im System Sr/Mg/Si. Z. Anorg. Allg. Chem. 1996;622:85–94. doi: 10.1002/zaac.19966220113. [DOI] [Google Scholar]
- 10.Eisenmann B., Schaefer H., Turban K. On a new SrSi-modification and the new compound SrGe0.76. Z. Naturforsch. B. 1974;29:464–468. doi: 10.1515/znb-1974-7-803. [DOI] [Google Scholar]
- 11.Schob O., Parthe E. AB compounds with Sc, Y and rare earth metals. I. Scandium and yttrium compounds with Cr B and CsCl structure. Acta Crystallogr. 1965;19:214–224. doi: 10.1107/S0365110X65003134. [DOI] [Google Scholar]
- 12.Mattausch H.J., Oeckler O., Simon A. Eine neue Modifikation von Lanthanmonosilicid-IT-LaSi. Z. Anorg. Allg. Chem. 1999;625:1151–1154. doi: 10.1002/(SICI)1521-3749(199907)625:7<1151::AID-ZAAC1151>3.0.CO;2-4. [DOI] [Google Scholar]
- 13.Duerr I., Bauer B., Roehr C. Lanthan-Triel/Tetrel-ide La (Al, Ga)x (Si, Ge)1−x. Experimentelle und theoretische Studien zur Stabilitaet polarer 1:1-Phasen. Z. Naturforsch. B. 2011;66:1107–1121. doi: 10.1515/znb-2011-1105. [DOI] [Google Scholar]
- 14.Schobinger Papamantellos P., Buschow K.H.J. Incommensurate magnetic structure of Ce Si as observed by neutron diffraction. J. Magn. Magn. Mater. 1994;130:242–246. doi: 10.1016/0304-8853(94)90679-3. [DOI] [Google Scholar]
- 15.Gladyshevskii E.I., Kripyakevich P.I. Monosilicides of rare earth metals and their crystal structures. J. Struct. Chem. 1965;5:789–794. doi: 10.1007/BF00744231. [DOI] [Google Scholar]
- 16.Nagaki D.A., Simon A. Structure of gadolinium monosilicide. Acta Crystallogr. 1990;46:1197–1199. doi: 10.1107/S0108270189011820. [DOI] [Google Scholar]
- 17.Schobinger Papamantellos P., Janssen T., Buschow K.H.J. Thermal variation of incommensurate magnetic phases in TbSi as observed by neutron diffraction. J. Magn. Magn. Mater. 1993;127:115–128. doi: 10.1016/0304-8853(93)90204-F. [DOI] [Google Scholar]
- 18.Roger J., Babizhetskii V., Guizouarn T., Hiebl K., Guerin R., Halet J.F. The ternary RE–Si–B systems (RE = Dy, Ho, Er and Y) at 1270 K: Solid state phase equilibria and magnetic properties of the solid solution REB2−xSix (RE = Dy and Ho) J. Alloys Compd. 2006;417:72–84. doi: 10.1016/j.jallcom.2005.09.012. [DOI] [Google Scholar]
- 19.Schobinger Papamantellos P., Brunelli M., Rodriguez Carvajal J., Buschow K.H.J., Ritter C., Gramm F. Magneto structural transition in the DySi (Cr B)- and micro-structural changes in the (Fe B)-type compounds by XRPD and neutron diffraction. J. Magn. Magn. Mater. 2011;323:903–914. doi: 10.1016/j.jmmm.2010.11.026. [DOI] [Google Scholar]
- 20.Eckerlin B., Meyer H.J., Woelfel E. Die Kristallstruktur von CaSn and CaGe. Z. Anorg. Allg. Chem. 1955;281:322–328. doi: 10.1002/zaac.19552810508. [DOI] [Google Scholar]
- 21.Rieger W., Parthe E. Alkaline earth silicides, germanides and stannides with CrB structure type. Acta Crystallogr. 1967;22:919–922. doi: 10.1107/S0365110X67001793. [DOI] [Google Scholar]
- 22.Mattausch H.J., Simon A. Eine neue Modifikation von Lanthanmonogermanid—IT-LaGe. Z. Naturforsch. B. 2004;59:559–561. doi: 10.1515/znb-2004-0513. [DOI] [Google Scholar]
- 23.Schobinger Papamantellos P., Buschow K.H.J. Ferromagnetism of NdGe and PrGe studied by neutron diffraction and magnetic measurements. J. Less Common Met. 1985;111:125–138. doi: 10.1016/0022-5088(85)90178-X. [DOI] [Google Scholar]
- 24.Gladyshevskii E.I., Uhryn N.S. Monogermanides of the rare-earth metals and their crystal structures. Dop. Akad. Nauk. Ukrain. 1965;10:1326–1329. [Google Scholar]
- 25.Tharp A.G., Smith G.S., Johnson Q. Structures of the rare earth germanides at or near equiatomic proportions. Acta Crystallogr. 1966;20:583–585. doi: 10.1107/S0365110X66001294. [DOI] [Google Scholar]
- 26.Merlo F., Fornasini M.L. CrB-type equiatomic compounds of europium, ytterbium and alkaline-earth metals with Si, Ge, Sn, Pb. J. Less-Common Met. 1967;13:603–610. doi: 10.1016/0022-5088(67)90105-1. [DOI] [Google Scholar]
- 27.Schobinger Papamantellos P., Buschow K.H.J. A neutron diffraction and magnetic study of the first-order phase transition in TbGe1−xSix (0 = x = 0.4) J. Magn. Magn. Mater. 1986;62:15–28. doi: 10.1016/0304-8853(86)90729-8. [DOI] [Google Scholar]
- 28.Buschow K.H.J., Schobinger Papamantellos P., Fischer P. Magnetic structure and properties of equiatomic rare earth germanides. J. Less-Common Met. 1988;139:221–231. doi: 10.1016/0022-5088(88)90003-3. [DOI] [Google Scholar]
- 29.Schobinger Papamantellos P., Buschow K.H.J. Magnetic structure and incommensurate phase transition in HoGe. J. Magn. Magn. Mater. 1984;44:149–157. doi: 10.1016/0304-8853(84)90058-1. [DOI] [Google Scholar]
- 30.Eremenko V.N., Meleshevich K.A., Buyanov Y.I., Martsenyuk P.S. Structure of the alloys and phase diagram of the thulium-germanium system. Powder Metall. Met. Ceram. 1989;28:543–547. doi: 10.1007/BF00794867. [DOI] [Google Scholar]
- 31.Agilent . CrysAlis PRO. Agilent Technologies Ltd.; Yarnton, Oxfordshire, UK: 2014. [Google Scholar]
- 32.Palatinus L., Chapuis G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007;40:786–790. doi: 10.1107/S0021889807029238. [DOI] [Google Scholar]
- 33.Petříček V., Dusek M., Palatinus L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr.—Cryst. Mater. 2014;229:345–352. doi: 10.1515/zkri-2014-1737. [DOI] [Google Scholar]
- 34.Giannozzi P., Baroni S., Bonini N., Calandra M., Car R., Cavazzoni C., Ceresoli D., Chiarotti G.L., Cococcioni M., Dabo I., et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. [(accessed on 5 April 2022)];J. Phys. Condens. Matter. 2009 21:395502. doi: 10.1088/0953-8984/21/39/395502. Available online: https://www.quantum-espresso.org/ [DOI] [PubMed] [Google Scholar]
- 35.Blöchl P.E. Projector Augmented-Wave Method. Phys. Rev. B. 1994;50:17953–17979. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 36.Dal Corso A. Pseudopotentials periodic table: From H to Pu. Comput. Mater. Sci. 2014;95:337. doi: 10.1016/j.commatsci.2014.07.043. [DOI] [Google Scholar]
- 37.Monkhorst H.J., Pack J.D. Special points for Brillouin-zone integrations Special points for Brillouin-zone integrations. Phys. Rev. B. 1976;13:5188. doi: 10.1103/PhysRevB.13.5188. [DOI] [Google Scholar]
- 38.Maintz S., Deringer V.L., Tchougreeff A.L., Dronskowski R. Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J. Comput. Chem. 2013;34:2557–2567. doi: 10.1002/jcc.23424. [DOI] [PubMed] [Google Scholar]
- 39.Maintz S., Deringer V.L., Tchougreeff A.L., Dronskowski R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. [(accessed on 25 October 2021)];J. Comput. Chem. 2016 37:1030–1035. doi: 10.1002/jcc.24300. Available online: http://www.cohp.de/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maintz S., Esser M., Dronskowski R. Efficient Rotation of Local Basis Functions Using Real Spherical Harmonics. Acta Phys. Pol. B. 2016;47:1165–1175. doi: 10.5506/APhysPolB.47.1165. [DOI] [Google Scholar]
- 41.Nelson R., Ertural C., George J., Deringer V.L., Hautier G., Dronskowski R. LOBSTER: Local orbital projections, atomic charges, and chemical-bonding analysis from projector-augmented-wave-based density-functional theory. J. Comput. Chem. 2020;41:1931–1940. doi: 10.1002/jcc.26353. [DOI] [PubMed] [Google Scholar]
- 42.Eck B. wxDragon Program, v-2.1.8. 2019. [(accessed on 10 January 2022)]. Available online: http://wxdragon.de.
- 43.Haschke H., Nowotny H., Benesovsky F. Untersuchungen im System Cer-Silicium-Germanium. Mon. Chem. 1966;97:1452–1458. doi: 10.1007/BF00902596. [DOI] [Google Scholar]
- 44.Das P.K., Kumar N., Kulkarni R., Dhar S.K., Thamizhavel A. Anisotropic magnetic properties and superzone gap formation in CeGe single crystal. J. Phys. Condens. Matter. 2012;24:146003. doi: 10.1088/0953-8984/24/14/146003. [DOI] [PubMed] [Google Scholar]
- 45.Bjurström T., Arnfelt H. Röntgenanalyse des Eisen-Bor-Systems. Z. Phys. Chem. B. 1929;4:469–474. doi: 10.1515/zpch-1929-0434. [DOI] [Google Scholar]
- 46.Straumanis M.E., Aka E.Z. Lattice parameters, coefficients of thermal expansion and atomic weights of purest silicon and germanium. J. Appl. Phys. 1952;23:330–334. doi: 10.1063/1.1702202. [DOI] [Google Scholar]
- 47.Pauling L. Atomic Radii and Interatomic Distances in Metals. J. Am. Chem. Soc. 1947;69:542–553. doi: 10.1021/ja01195a024. [DOI] [Google Scholar]
- 48.Zürcher F., Nesper R. Ba3Ge4: Polymerization of Zintl Anions in the Solid and Bond Stretching Isomerism. Angew. Chem. 1998;37:3314–3318. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3314::AID-ANIE3314>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 49.Tobash P.H., DiFilippo G., Bobev S., Hur N., Thompson J.D., Sarrao J.L. Structure and properties of Gd3Ge4: The orthorhombic RE3Ge4 structures revisited (RE = Y, Tb–Tm) Inorg. Chem. 2007;46:8690–8697. doi: 10.1021/ic7009034. [DOI] [PubMed] [Google Scholar]
- 50.Schobinger Papamantellos P., de Mooij D.B., Buschow K.H.J. Crystallographic and magnetic structure of TbGe2. J. Less Common Met. 1988;144:265–274. doi: 10.1016/0022-5088(88)90140-3. [DOI] [Google Scholar]
- 51.Guloy A.M., Corbett J.D. Syntheses and structures of lanthanum germanide, LaGe2−x, and lanthanum aluminum germanide, LaAlGe: Interrelationships among the α-ThSi2, α-GdSi2, and LaPtSi structure types. Inorg. Chem. 1991;30:4789–4794. doi: 10.1021/ic00025a021. [DOI] [Google Scholar]
- 52.Tobash P.H., Meyers J.J., DiFilippo G., Bobev S., Ronning F., Thompson J.D., Sarrao J.L. Structure and properties of a new family of nearly equiatomic rare-earth metal-tin-germanides RESn1+xGe1−x (RE = Y, Gd–Tm): An unusual example of site preferences between elements from the same group. Chem. Mater. 2008;20:2151–2159. doi: 10.1021/cm7033799. [DOI] [Google Scholar]
- 53.Francois M., Venturini G., Malaman B., Roques B. Nouveaux isotypes de CeNiSi2 dans les systemes R-M-X (R ident to La-Lu, M ident to metaux des groupes 7 A 11 et X ident to Ge, Sn). I compositions et parametres cristallins. J. Less Common Met. 1990;160:197–213. doi: 10.1016/0022-5088(90)90381-S. [DOI] [Google Scholar]
- 54.Bruskov V.A., Bodak O.I., Pecharskii V.K., Gladyshevskii E.I., Muratova L.A. Crystal structure of Y3Ge5 (“YGe1.7”) Kristallografiya. 1983;28:260–263. [Google Scholar]
- 55.Tobash P.H., Lins D., Bobev S., Hur N., Thompson J.D., Sarrao J.L. Vacancy Ordering in SmGe2−x and GdGe2−x (x = 0.33): Structure and Properties of Two Sm3Ge5 Polymorphs and of Gd3Ge5. Inorg. Chem. 2006;45:7286–7294. doi: 10.1021/ic060913f. [DOI] [PubMed] [Google Scholar]
- 56.Schobinger-Papamantellos P., de Mooij D.B., Buschow K.H.J. Crystallographic and magnetic structure of Dy3Ge5 and DyGe1.9. J. Less-Common Met. 1990;163:319–330. doi: 10.1016/0022-5088(90)90598-E. [DOI] [Google Scholar]
- 57.Zaharko O., Schobinger Papamantellos P., Ritter C. Antiferromagnetic ordering in the novel Ho3Ge5 and HoGe1.85 compounds studied by X-ray and neutron diffraction. J. Alloys Compd. 1998;280:4–15. doi: 10.1016/S0925-8388(98)00555-6. [DOI] [Google Scholar]
- 58.Belyavina N.M., Markiv V.Y., Speka M.V. Crystal structure of YGe3, YGe1.9 and a novel Y3Ge4 compound. J. Alloys Compd. 1999;283:162–168. doi: 10.1016/S0925-8388(98)00858-5. [DOI] [Google Scholar]
- 59.Schobinger-Papamantellos P., Andre G., Rodriguez-Carvajal J., de Groot C.H., Buschow K.H.J. The magnetic ordering of the novel compound TbGe3. J. Alloys Compd. 1996;232:165–168. doi: 10.1016/0925-8388(95)01949-9. [DOI] [Google Scholar]
- 60.Schobinger-Papamantellos P., de Mooij D.B., Buschow K.H.J. Crystal structure of the compound DyGe3. J. Alloys Compd. 1992;183:181–186. doi: 10.1016/0925-8388(92)90743-S. [DOI] [Google Scholar]
- 61.Schobinger-Papamantellos P., Rodriguez Carvajal J., Tung L.D., Ritter C., Buschow K.H.J. Competing multiple-q magnetic structures in HoGe3: I. The magnetic phase diagram of HoGe3. J. Phys. Condens. Matter. 2008;20:195201. doi: 10.1088/0953-8984/20/19/195201. [DOI] [Google Scholar]
- 62.Eremenko V.N., Obushenko I.M. Phase Diagram of the Erbium-Germanium System. Sov. Non-Ferr. Met. Res. 1981;9:216–220. [Google Scholar]
- 63.Fukuoka H., Yoshikawa M., Baba K., Yamanaka S. Preparation and Structures of Lanthanoid Germanides, PrGe3.36, NdGe3.25, and TmGe3 with Double Square Ge-Mesh Structures. Bull. Chem. Soc. Jpn. 2010;83:323–327. doi: 10.1246/bcsj.20090310. [DOI] [Google Scholar]
- 64.Harada M., Fukuoka H., Matsumura D., Inumaru K. Structure and Chemical Bonding of Binary Ytterbium Germanides, Yb3Ge5 and YbGe3, Prepared by High-Pressure and High-Temperature Reactions. J. Phys. Chem. C. 2012;116:2153–2158. doi: 10.1021/jp206207c. [DOI] [Google Scholar]
- 65.Hübner J.-M., Bobnar M., Akselrud L., Prots Y., Grin Y., Schwarz U. Lutetium Trigermanide LuGe3: High-Pressure Synthesis, Superconductivity, and Chemical Bonding. Inorg. Chem. 2018;57:10295–10302. doi: 10.1021/acs.inorgchem.8b01510. [DOI] [PubMed] [Google Scholar]
- 66.Palenzona A., Manfrinetti P., Fornasini M.L. The phase diagram of the Ca-Ge system. J. Alloys Compd. 2002;345:144–147. doi: 10.1016/S0925-8388(02)00326-2. [DOI] [Google Scholar]
- 67.Siggelkow L., Hlukhyy V., Fässler T.F. Sr7Ge6, Ba7Ge6 and Ba3Sn2—Three new binary compounds containing dumbbells and four-membered chains of tetrel atoms with considerable Ge–Ge π-bonding character. J. Solid State Chem. 2012;191:76–89. doi: 10.1016/j.jssc.2012.03.008. [DOI] [Google Scholar]
- 68.Emsley J. The Elements. Clarendon Press; Oxford, UK: 1989. [Google Scholar]
- 69.Witte J., von Schnering H.G., Hagenmuller P. Das Verhalten der Alkalimetalle zu Halbmetallen. XI. Die Kristallstruktur von NaSi und NaGe. Z. Anorg. Allgem. Chem. 1964;327:260–273. doi: 10.1002/zaac.19643270319. [DOI] [Google Scholar]
- 70.Hopf V., Mueller W., Schaefer H. Die Struktur der Phase Li7Ge2. Z. Naturforsch. B. 1972;27:1157–1160. doi: 10.1515/znb-1972-1009. [DOI] [Google Scholar]
- 71.Busmann E. Die Kristallstruktur von KGe und isotypen Germaniden und Siliciden. Naturwissenschaften. 1960;47:82. doi: 10.1007/BF00628474. [DOI] [Google Scholar]
- 72.Arguilla M.Q., Cultrara N.D., Scudder M.R., Jiang S., Ross R.D., Goldberger J.E. Optical properties and Raman-active phonon modes of two-dimensional honeycomb Zintl phases. J. Mater. Chem. C. 2017;5:11259–11266. doi: 10.1039/C7TC01907F. [DOI] [Google Scholar]
- 73.Iandelli A., Franceschi E. On the Crystal Structure of the Compounds CaP, SrP, CaAs, SrAs and EuAs. J. Less Common Met. 1973;30:211–216. doi: 10.1016/0022-5088(73)90107-0. [DOI] [Google Scholar]
- 74.Tallman R.L., Margrave J.L., Bailey S.W. The crystal structure of sodium peroxide. J. Am. Chem. Soc. 2002;79:2979–2980. doi: 10.1021/ja01568a087. [DOI] [Google Scholar]
- 75.Freccero R., Solokha P., de Negri S., Saccone A., Grin Y., Wagner F.R. Polar-Covalent Bonding beyond the Zintl Picture in Intermetallic Rare-Earth Germanides. Chem. Eur. J. 2019;25:6600–6612. doi: 10.1002/chem.201900510. [DOI] [PubMed] [Google Scholar]
- 76.Mudring A.V., Corbett J.D. Unusual Electronic and Bonding Properties of the Zintl Phase Ca5Ge3 and Related Compounds. A Theoretical Analysis. J. Am. Chem. Soc. 2004;126:5277–5281. doi: 10.1021/ja030216b. [DOI] [PubMed] [Google Scholar]
- 77.Freccero R., De Negri S., Rogl G., Binder G., Michor H., Rogl P.F., Saccone A., Solokha P. La2Pd3Ge5 and Nd2Pd3Ge5 Compounds: Chemical Bonding and Physical Properties. Inorg. Chem. 2021;60:3345–3354. doi: 10.1021/acs.inorgchem.0c03744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Solokha P., De Negri S., Proserpio D.M., Blatov V.A., Saccone A. Vacancy Ordering as a Driving Factor for Structural Changes in Ternary Germanides: The New R2Zn1−x Ge6 Series of Polar Intermetallics (R = Rare-Earth Metal) Inorg. Chem. 2015;54:2411–2424. doi: 10.1021/ic5030313. [DOI] [PubMed] [Google Scholar]
- 79.Miedema A.R. The electronegativity parameter for transition metals: Heat of formation and charge transfer in alloys. J. Less-Common Met. 1973;32:117–136. doi: 10.1016/0022-5088(73)90078-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CCDC 2213844 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.