Abstract
Infection of polarized MDCK epithelial layers by Salmonella enterica serovar Typhimurium is accompanied by increased tight junction permeability and by contraction of perijunctional actinomyosin. We localized dysfunctional tight junctions in serovar Typhimurium-infected MDCK layers by imaging apical-basolateral intramembrane diffusion of fluorescent lipid and found that loss of the apical-basolateral diffusion barrier (tight junction fence function) was most marked in areas of prominent perijunctional contraction. The protein kinase inhibitor staurosporine prevented perijunctional contraction but did not reverse the effects of serovar Typhimurium on tight junction barrier function. Hence, perijunctional contraction is not required for Salmonella-induced tight junction dysfunction and this epithelial response to infection may be multifactorial.
Salmonella species are a major cause of food poisoning and can induce a broad spectrum of diseases from mild diarrhea to typhoid. In common with other enteropathogens, Salmonella enterica serovar Typhimurium has developed means of breaching the mucosal epithelial barrier by usurping signaling mechanisms within host cells (4). Modulation of epithelial permeability properties is one of the common outcomes of bacterial infection of epithelial layers in vitro, and the in vivo correlates of these effects may induce or amplify diarrhea (20, 36). Several bacterial pathogens have been shown to modulate epithelial tight junctions (zonula occludens), the principal structure limiting paracellular permeability. Frequently bacteria achieve this by producing proteins which engage signaling mechanisms in epithelial cells, modulate the actin cytoskeleton, or degrade tight junction proteins (20, 36). For example, Clostridium difficile toxins A and B and Escherichia coli cytotoxic necrotizing factor 1 induce tight junction dysfunction in epithelial monolayers via the regulation of Rho GTPase activity and disruption of actin microfilaments (12, 17, 21, 22, 27). Similarly, the Vibrio cholerae zonula occludens toxin appears to contribute to diarrhea by disrupting tight junctions secondary to its modulation of protein kinase C and microfilaments (10, 11). The less well-characterized V. cholerae hemagglutinin/protease has recently been shown to disrupt tight junctions, an activity which may be related to proteolytic degradation of the tight junction protein occludin (39). Hemagglutinin/protease thus appears to belong to a diverse group of prokaryotic and eukaryotic proteases which enhance epithelial permeability (1, 28, 35, 38).
Infection of intestinal epithelial layers with either enteropathogenic or enterohemorrhagic E. coli (EPEC or EHEC) also diminishes epithelial barrier function (29, 37). The tight junction-modulating effects of EPEC and EHEC have been proposed to occur via the activity of actinomyosin, secondary to protein kinase activation and possibly the mobilization of intracellular calcium (30, 40), although the role of calcium in EPEC- and EHEC-induced cellular responses has been disputed (2). The bacterial components mediating the tight junction effects of EPEC and EHEC infection remain unknown.
It has also been shown that serovar Typhimurium invasion of intestinal epithelia is accompanied by a loss of epithelial integrity and consequent loss of epithelial function (7, 25). In vitro models of Salmonella infection have revealed modulation of epithelial permeability, as indicated by a reduction in transepithelial electrical resistance (TER) across serovar Typhimurium-infected MDCK and Caco-2 cell monolayers (13, 14). In the case of MDCK cells, it involves rapid changes in both tight junction permeability and transcellular conductance, which occur following serovar Typhimurium invasion and which appear not to involve direct interaction between bacteria and tight junctions (23, 24). Serovar Typhimurium infection induces contraction of the perijunctional actin ring, which follows a time course similar to that of increased paracellular permeability (23). Contraction of the perijunctional actinomyosin ring may physically disrupt tight junctions, and tension generated through actinomyosin has been widely implicated in the modulation of tight junction permeability (6, 26, 31). Although immunocytochemical staining of tight junction and adherens junction proteins failed to detect the breakdown of intercellular junctions following serovar Typhimurium infection (23), it was hypothesized that distortion of the intercellular junctions resulting from contraction of infected cells and consequent stretching of the apical portions of neighboring, uninvaded cells might lead to changes in tight junction permeability in the absence of an overt separation of cells (23). The hypothesis that there is a causal relationship between perijunctional actinomyosin contraction and permeability changes has not been tested. Similarly, although many studies have focused on the mechanisms underlying Salmonella invasion and coincident remodeling of the actin cytoskeleton and the apical membrane to form membrane ruffles (16), the causes of epithelial barrier dysfunction induced by Salmonella have remained largely unexplored.
To address these questions, we sought a method of localizing aberrant tight junctions in Salmonella-infected MDCK monolayers. In addition to their property of limiting paracellular permeability, tight junctions also provide a barrier to the diffusion of lipids and integral membrane proteins between the apical and basolateral compartments of the plasma membrane. A consequence of the barrier properties of tight junctions is that the incubation of polarized epithelial cell layers with fluorescent lipids, such as BODIPY-sphingomyelin (Molecular Probes, Eugene, Oreg.) in the apical chamber results in selective labeling of the outer leaflet of the apical plasma membrane. Consequently, alterations in the ability of tight junctions to limit apical-basolateral intramembrane diffusion can be monitored by the appearance of apically applied fluorescent lipid in the lateral membrane (3).
MDCK II monolayers grown at high density on permeable supports (Anocell; Nunc) as described previously (8) were infected with serovar Typhimurium at a multiplicity of infection (MOI) of 100 for 15 to 60 min in Krebs buffer containing 25 μM bovine serum albumin. They were then incubated with the same buffer containing 25 μM BODIPY FL-sphingomyelin for 10 min on ice by using an adaptation of the labeling technique described by Balda et al. (3). Cells were then washed with cold Krebs buffer containing 25 μM bovine serum albumin, and culture supports were removed and mounted under coverslips in the same buffer. Confocal microscope images in the xy and xz planes were obtained immediately by using a Leica TCS NT confocal system attached to a Leica DM RBE fluorescence microscope and equipped with a Kr-Ar mixed gas laser.
We observed a clear increase in fluorescence throughout the lateral membrane of some cells following serovar Typhimurium infection, which was apparent after 15 min of infection (the earliest time point used) and became more prominent after 60 min of infection (Fig. 1). BODIPY-sphingomyelin was almost exclusively retained in the apical compartment of uninfected cell layers. Loss of the apical-basolateral intramembrane diffusion barrier was most prominent in areas where the cells had contracted (Fig. 1). The infected cell layers exhibited distortions similar to those described previously after cytochemical staining of actin and tight junction proteins (23, 24), with intensely labeled, contracted cells surrounded by a radiating pattern of stretched cells which also exhibited some loss of tight junction function that resulted in accumulation of BODIPY-sphingomyelin (Fig. 1). Monitoring of tight junction barrier function by fluorescence microscopy is likely to be a valuable technique in situations, such as bacterial infection, where changes in tight junction permeability exhibit marked heterogeneity. This technique provides a useful adjunct to the more established methods, which are based on monitoring the transepithelial flux of ions or molecules and provide an average measure across the whole monolayer.
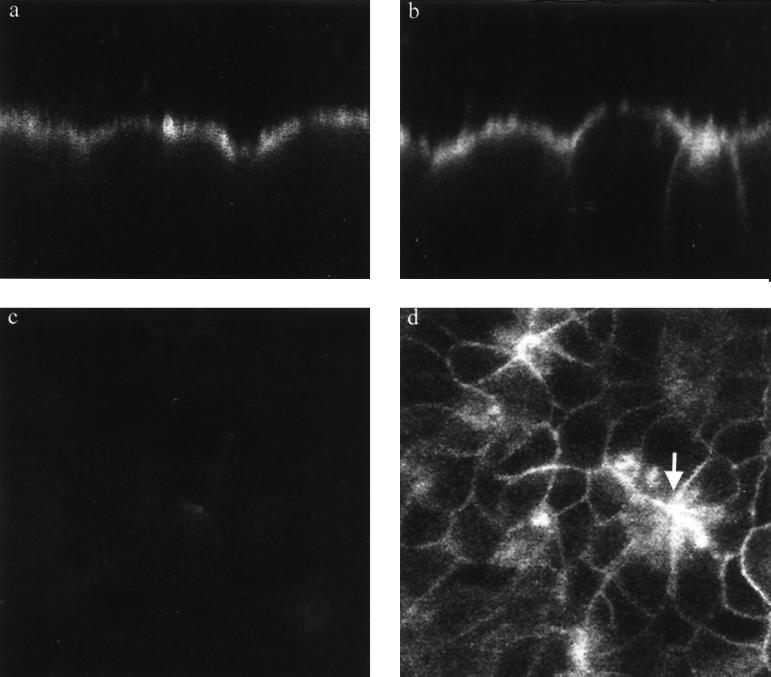
FIG. 1.
Serovar Typhimurium infection causes disruption of the apical-basolateral intramembrane diffusion barrier function of tight junctions in MDCK epithelia. Confocal imaging of BODIPY-sphingomyelin-labeled MDCK epithelia demonstrates retention of fluorescence in the apical membrane of control cell layers, as revealed in an xz (transverse) image (a). Serovar Typhimurium infection for 60 min causes a reduction in the ability of some of the tight junctions to limit apical-basolateral intramembrane diffusion of BODIPY-sphingomyelin, resulting in the appearance of variable mounts of the tracer throughout the lateral membranes of some cells (xz image) (b). A relationship between perijunctional contraction and tight junction dysfunction is revealed in a confocal optical section 10 to 12 μm below the apical surface of the cell layer (d), which reveals relatively high concentrations of dye in the lateral membranes of cells which are contracted as a result of infection (arrow). A similar optical section of uninfected cell layers 10 to 12 μm below the apical surface confirms that there is minimal diffusion of the lipid across tight junctions (c), since little fluorescence is detected in the lateral membranes. All images were obtained under identical imaging parameters with a Leica TCS NT confocal system attached to a Leica DM RBE epifluorescence microscope and equipped with a Kr-Ar mixed-gas laser. Magnification, ×1,010.
Although the localization of aberrant tight junctions indicated that loss of tight junction function is most apparent in contracted and/or distorted cells, this does not provide unequivocal evidence of a causal relationship between contraction and tight junction damage. The apparent relationship might be circumstantial given that the extent of contraction coincides with that of Salmonella invasion (Fig. 2). In order to test whether there is a causal relationship between contraction and tight junction dysfunction, it was necessary to try to dissociate the two phenomena. To do this, we took advantage of a chance observation that the broad-spectrum protein kinase inhibitor staurosporine inhibits perijunctional contraction following serovar Typhimurium infection. The inhibition of serovar Typhimurium-induced cell contraction was clearly illustrated by phalloidin-tetramethyl rhodamine isothiocyanate (TRITC) labeling of F-actin, which reveals the morphology of perijunctional actin (Fig. 3). In agreement with previous reports (33), staurosporine treatment did not significantly alter serovar Typhimurium invasion. A secondary rationale for examining the effects of staurosporine on tight junction modulation by serovar Typhimurium is that protein kinase inhibition protects epithelia from the loss of barrier function induced by a variety of treatments (5, 9), including infection with EPEC (30).
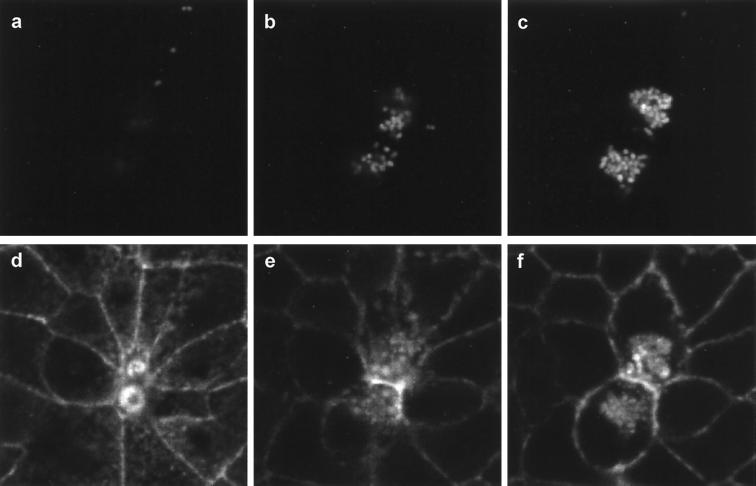
FIG. 2.
Perijunctional contraction is associated with serovar Typhimurium invasion. MDCK monolayers were infected for 60 min with serovar Typhimurium expressing green fluorescent protein (GFP) and then fixed and stained with phalloidin-TRITC to localize F-actin. Dual-channel confocal imaging reveals GFP-expressing bacteria (a to c) and F-actin (d to f) at varying depths within the infected cell layer 2 μm (b,e) and 4 μm (c,f) below the optical section at the apex of the cells (a,d). Images were acquired with a Leica TCS NT confocal system attached to a Leica DM RBE epifluorescence microscope and equipped with a Kr-Ar mixed-gas laser. Note that actin accumulation in the vicinity of invaded bacteria and a small amount of bleedthrough of GFP fluorescence into the TRITC channel allow location of Salmonella (d to f). An optical section at the apex of the cell layer reveals two regions of actin accumulation (corresponding to membrane ruffles on cells) which are contracted (note also the marked stretching of surrounding cells). Optical sections 2 μm (b,e) and 4 μm (c,f) below the apical pole reveal that these two contracted cells have typical profiles beneath the level of the perijunctional actinomyosin ring and have been invaded by several GFP-expressing serovar Typhimurium bacteria. Magnification, ×1,300.
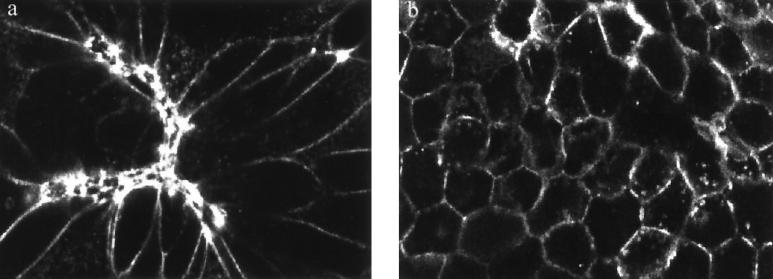
FIG. 3.
Staurosporine inhibits serovar Typhimurium-induced perijunctional actinomyosin contraction. Phalloidin-TRITC staining of MDCK monolayers infected with serovar Typhimurium SL1344 for 60 min without staurosporine treatment (a) or after pretreatment with 200 nM staurosporine (b). Confocal images obtained in the xy plane reveals extensive distortion of MDCK cells after 60 min of infection in the absence of staurosporine (a). Contracted cells are intensely stained with phalloidin-TRITC, and neighboring cells exhibit clear distortion due to the stretching imposed by contraction of infected cells. MDCK monolayers infected with serovar Typhimurium treated with staurosporine (b) reveal a regular cell shape due to the absence of contraction of the perijunctional actin ring. Images were acquired with a Leica TCS NT confocal system attached to a Leica DM RBE epifluorescence microscope and equipped with a Kr-Ar mixed-gas laser. Magnification, ×640.
When experiments on the apical-basolateral diffusion barrier were repeated in the presence of 200 nM staurosporine, it was found that despite the absence of contraction, BODIPY-sphingomyelin diffused across tight junctions throughout the monolayer and labeled the entire lateral membrane (Fig. 4). The overall level of accumulation of fluorescence in the lateral space appeared to exceed that in cell layers infected with serovar Typhimurium in the absence of staurosporine (Fig. 4). Apical-basolateral intramembrane diffusion of BODIPY-sphingomyelin across the tight junctions of staurosporine-treated cells was minimal in the absence of infection (Fig. 4). These data clearly demonstrate that contraction is not a prerequisite for tight junction dysfunction. We also found that the blockage of perijunctional contraction by exposure of cells to 200 nM staurosporine did not reverse changes in epithelial permeability, as measured by changes in TER after 60 min of infection (Fig. 5). Prolonged (120 min) exposure to serovar Typhimurium in the presence of 40 nM staurosporine enhanced the effect of serovar Typhimurium infection on TER (P < 0.05) (Fig. 5). Since it was possible that staurosporine might promote a change in TER by enhancing transcellular ion conductance (as a consequence of its enhancement of membrane ruffling) (33), direct measurements of tight junction permeability were undertaken by measuring transepithelial inulin flux and tight junction cation selectivity as described previously (8). Both methods of assessing tight junction permeability revealed that during 30 min of infection, treatment of MDCK layers with 200 nM staurosporine significantly enhanced (P < 0.01) serovar Typhimurium-induced tight junction dysfunction (Fig. 6).
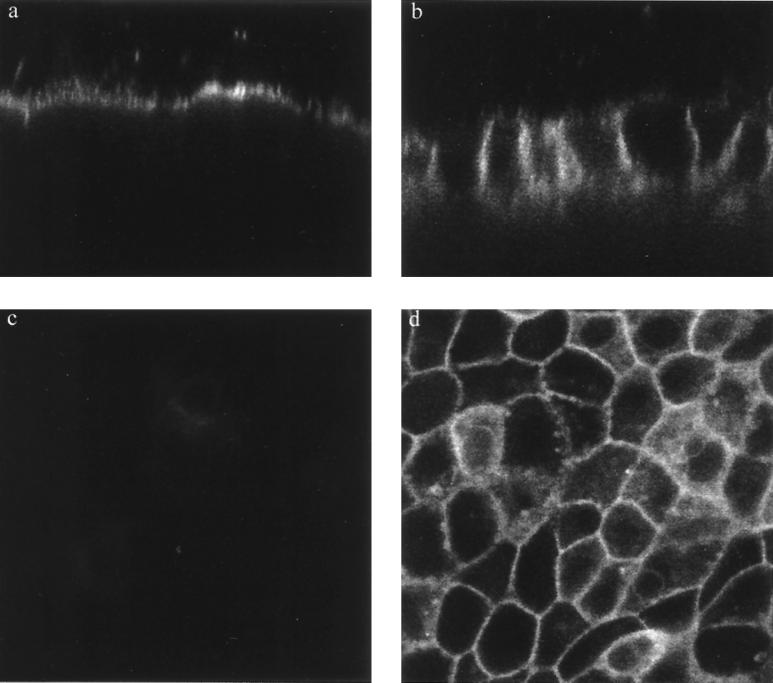
FIG. 4.
Serovar Typhimurium-induced disruption of the apical-basolateral intramembrane diffusion barrier in the presence of staurosporine. Confocal imaging of BODIPY-sphingomyelin labeling reveals the retention of fluorescence in the apical membrane of 200 nM staurosporine-treated uninfected MDCK layers, as revealed in an xz (transverse) image (a). Infection of staurosporine-treated cells with serovar Typhimurium for 60 min impairs the apical-basolateral intramembrane diffusion barrier, resulting in the appearance of BODIPY-sphingomyelin throughout the lateral membranes of all cells (xz image) (b). Cells infected with serovar Typhimurium in the presence of staurosporine do not exhibit distortion that is due to perijunctional contraction but do accumulate BODIPY-sphingomyelin in lateral membranes throughout the cell layer, revealed by xy optical sectioning 8 to 10 μm below the apical surface (d; compare with Fig. 1d). A similar optical section of uninfected, staurosporine-treated cell layers reveals minimal diffusion of the lipid across tight junctions (c). All images were obtained under identical imaging parameters with a Leica TCS NT confocal system attached to a Leica DM RBE epifluorescence microscope and equipped with a Kr-Ar mixed-gas laser. Magnification, ×1,010.
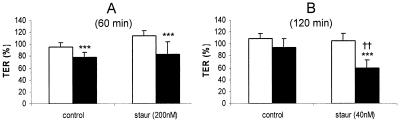
FIG. 5.
Serovar Typhimurium induces a decrease in TER which is not reversed by staurosporine. MDCK layers were infected with serovar Typhimurium at an MOI of 100 following treatment with 200 nM (A) or 40 nM (B) staurosporine (or equivalent concentrations of the carrier, dimethyl sulfoxide). Tight junction permeability was then assessed over a 60-min (A) or 120-min (B) time course, and a comparison was made between uninfected layers (empty bars) and Salmonella-infected layers (solid bars). Staurosporine (200 nM) fails to reverse the drop in TER induced during 60 min of serovar Typhimurium infection (A). Decreasing the staurosporine concentration to 40 nM allowed the experiment to be prolonged while preventing any effect of staurosporine itself on TER. Under these conditions staurosporine enhances the effect of Salmonella on TER (B). All data are expressed as means ± standard deviations (n = 10 to 12 for panel A and n = 4 for panel B). The statistical significance of differences was assessed by Student's unpaired t test with significance set at P values of <0.05. Significant differences between uninfected and infected layers are indicated by three asterisks (P < 0.001), while significant differences between staurosporine-treated and control layers are indicated by double daggers (P < 0.01).
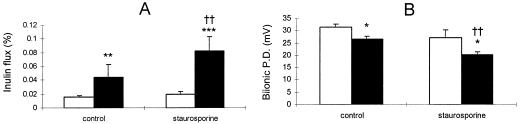
FIG. 6.
Staurosporine treatment enhances the effects of serovar Typhimurium infection on tight junction permeability. MDCK layers were infected with serovar Typhimurium at an MOI of 100 following treatment with 200 nM staurosporine (or equivalent concentrations of the carrier, dimethyl sulfoxide). Tight junction permeability was then assessed over a 30-min time course, and a comparison was made between uninfected layers (empty bars) and Salmonella-infected layers (solid bars). Inulin flux (A) measured by the appearance of apically applied [14C]inulin in the basal bathing medium was significantly increased by Salmonella infection and further enhanced by staurosporine. In separate experiments (B), bi-ionic potential difference (P.D.) (measured following isoosmotic replacement of sodium in the basal medium with choline [8]) was significantly decreased by serovar Typhimurium infection and further diminished by staurosporine treatment. All data are expressed as means ± standard deviations (n = 6 for panel A and n = 3 for panel B). The statistical significance of differences was assessed by Student's unpaired t test with significance set at P values of < 0.05. Significant differences between uninfected and infected layers are indicated by asterisks (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001), while significant differences between staurosporine-treated and control layers are indicated by double daggers (P < 0.01).
These data help to validate the use of BODIPY-sphingomyelin diffusion as an index of tight junction dysfunction, since this measurement and all three alternative indices of tight junction permeability measured were affected in parallel. These experiments also provided no evidence of staurosporine itself having any measurable effect on tight junction permeability over the time courses examined. It was originally hypothesized that the gross distortion of intercellular junctions occurring with perijunctional contraction compromises tight junction function; this suggestion is supported by the finding that in Salmonella-infected epithelia, apical-basolateral BODIPY-sphingomyelin diffusion is most affected in areas of prominent perijunctional contraction (Fig. 1). The fact that staurosporine blocks perijunctional contraction while enhancing transcellular ion and inulin permeability suggests that more than one mechanism contributes to Salmonella-induced epithelial permeability and that there is some degree of redundancy in these effects. It should also be noted that perijunctional contraction induced by Salmonella might limit the effect of dysfunctional tight junctions on epithelial permeability by reducing the proportion of the surface area of the layer occupied by leaky junctions. Alternatively, our observation that staurosporine treatment results in apical-basolateral BODIPYsphingomyelin diffusion throughout the cell layer suggests that staurosporine exacerbates an effect of serovar Typhimurium on tight junction permeability, which appears to be distinct from its effect on perijunctional actinomyosin contraction. It is possible that staurosporine treatment has some effect on intercellular junctions, which by itself is not sufficient to induce measurable changes in permeability but nevertheless renders them susceptible to further insult, e.g., by serovar Typhimurium. Staurosporine can modulate the phosphorylation state of adherens junction proteins in MDCK cells (32), and it is plausible that such an effect might exacerbate the effects of other treatments, such as Salmonella infection, on tight junction barrier functions.
The precise signaling events involved in tight junction modulation by serovar Typhimurium remain to be defined. We have shown that tight junction dysfunction occurs within the first 15 min of Salmonella infection, consistent with its being related to early cell signaling events and possibly being related to bacterial invasion itself rather than to later downstream events, such as cytokine induction. Clearly, the effect of staurosporine in blocking perijunctional contraction suggests that this phenomenon may involve one or more protein kinases. By analogy with the actions of several bacterial toxins, it is possible that tight junction regulation in response to Salmonella infection might involve Rho family GTPases. Recent data have demonstrated that Salmonella translocates several effector proteins into host cells via the type III secretion apparatus encoded in Salmonella pathogenicity island I (SPI1) (16). Some of these proteins are known to regulate actin dynamics by modulating Rho family GTPases or by other means (15, 18, 19, 34, 41). Clearly these SPI1-secreted effector proteins and possibly others yet to be characterized may contribute to the tight junction effects of Salmonella infection. It is also apparent that there is some degree of redundancy in the actions of SPI1-secreted effector proteins, in that mutation of each of their genes has a limited effect on the ability of Salmonella to induce actin reorganization and invade host cells. These factors and the apparent operation of multiple mechanisms leading to tight junction modulation reported here will complicate analysis of the possible role of Salmonella effector proteins and signaling events in Salmonella-induced tight junction dysfunction.
It is known that Salmonella infection of murine intestine in vivo can cause gross disruption of epithelia associated with rapid shedding of enterocytes (7, 25), which must involve the loosening of their intercellular contacts. Here we have demonstrated that epithelial permeability is altered by Salmonella infection even under conditions where overt perijunctional contraction is absent. This is an important observation, since it is not clear whether contraction of infected epithelial cells such as we observed in vitro also occurs during in vivo infection. These new data indicate that even if epithelial cell contraction is not a major feature of in vivo infection, it is likely that Salmonella also induces localized effects on tight junction permeability during intestinal infection. These effects may act synergistically with other conditions, such as inflammatory responses, to promote tight junction dysfunction. Further work is necessary to elucidate how bacteria such as Salmonella modulate tight junction permeability, and the techniques we describe here may help researchers to dissect these signaling mechanisms by using in vitro infection models. This remains an important area of research, since the influence of pathogenic and commensal bacteria on epithelial barrier function has severe implications in the promotion of diarrhea and/or bacterial translocation.
Acknowledgments
We are grateful to A.D. Leard for assistance with cell culture and N.L. Simmons for supplying MDCK strain II cells.
This work was supported by a Wellcome Trust Postdoctoral Fellowship (039684/Z/93/Z) and Royal Society research grant (17996) awarded to M.A.J. C.B.C.-B. was supported by a CAPES Fellowship (1887/91-1) (Brazil) and an ORS Award (9129002) (United Kingdom). Much of this work was performed within the School of Medical Sciences Cell Imaging Facility, University of Bristol, which is funded by a Medical Research Council Infrastructure Award (G4500006).
REFERENCES
- 1.Azghani A O, Gray L D, Johnson A R. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect Immun. 1993;61:2681–2686. doi: 10.1128/iai.61.6.2681-2686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain C, Keller R, Collington G K, Trabulsi L R, Knutton S. Increased levels of intracellular calcium are not required for the formation of attaching and effacing lesions by enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1998;66:3900–3908. doi: 10.1128/iai.66.8.3900-3908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balda M S, Whitney J A, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brumell J H, Steele-Mortimer O, Finlay B B. Bacterial invasion: force feeding by Salmonella. Curr Biol. 1999;9:R277–R280. doi: 10.1016/s0960-9822(99)80178-x. [DOI] [PubMed] [Google Scholar]
- 5.Citi S. Protein kinase inhibitors prevent junction dissociation induced by low extracellular calcium in MDCK epithelial cells. J Biol Chem. 1992;117:169–178. doi: 10.1083/jcb.117.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Citi S, Volberg T, Bershadsky A D, Denisenko N, Geiger B. Cytoskeletal involvement in the modulation of cell-cell junctions by the protein kinase inhibitor H-7. J Cell Sci. 1994;107:683–692. [PubMed] [Google Scholar]
- 7.Clark M A, Hirst B H, Jepson M A. Inoculum composition and Salmonella pathogenicity island 1 regulate M cell invasion and epithelial destruction by Salmonella typhimurium. Infect Immun. 1998;66:724–731. doi: 10.1128/iai.66.2.724-731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collares-Buzato C B, Jepson M A, McEwan G T A, Simmons N L, Hirst B H. Junctional uvomorulin/E-cadherin and phosphotyrosine-modified protein content are correlated with paracellular permeability in Madin-Darby canine kidney (MDCK) epithelia. Histochemistry. 1994;101:185–194. doi: 10.1007/BF00269543. [DOI] [PubMed] [Google Scholar]
- 9.Collares-Buzato C B, Jepson M A, Simmons N L, Hirst B H. Increased tyrosine phosphorylation causes redistribution of adherens junction and tight junction proteins and perturbs paracellular barrier function in MDCK epithelia. Eur J Cell Biol. 1998;76:85–92. doi: 10.1016/S0171-9335(98)80020-4. [DOI] [PubMed] [Google Scholar]
- 10.Fasano A, Baudry B, Pumplin D W, Wasserman S S, Tall B D, Ketley J M, Kaper J B. Vibrio cholerae produces a second enterotoxin which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper J B, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum S E. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Investig. 1995;96:710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feltis B A, Kim A S, Kinneberg K M, Lyerly D L, Wilkins T D, Erlandsen S L, Wells C L. Clostridium difficile toxins may augment bacterial penetration of intestinal epithelium. Arch Surg. 1999;134:1241–1242. doi: 10.1001/archsurg.134.11.1235. [DOI] [PubMed] [Google Scholar]
- 13.Finlay B B, Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 14.Finlay B B, Gumbiner B, Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Sci. 1988;107:221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Galán J E. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–297. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- 16.Galán J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 17.Gerhard R, Schmidt G, Hofmann F, Aktories K. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco-2 cells. Infect Immun. 1998;66:5125–5131. doi: 10.1128/iai.66.11.5125-5131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardt W D, Chen L M, Schuebel K E, Bustelo X R, Galán J E. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- 19.Hayward R D, Koronakis V. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 1999;18:4926–4934. doi: 10.1093/emboj/18.18.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hecht G. Bugs and barriers: enteric pathogens exploit yet another epithelial function. News Physiol Sci. 1995;10:160–166. [Google Scholar]
- 21.Hecht G, Pothoulakis C, Lamont J T, Madara J L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Investig. 1992;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecht G, Koutsouris A, Pothoulakis C, Lamont J T, Madara J L. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 23.Jepson M A, Collares-Buzato C B, Clark M A, Hirst B H, Simmons N L. Rapid disruption of epithelial barrier function by Salmonella typhimurium is associated with structural modification of intercellular junctions. Infect Immun. 1995;63:356–359. doi: 10.1128/iai.63.1.356-359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jepson M A, Lang T F, Reed K A, Simmons N L. Evidence for a rapid, direct effect on epithelial monolayer integrity and transepithelial transport in response to Salmonella invasion. Pflueg Arch Eur J Physiol. 1996;432:225–233. doi: 10.1007/s004240050128. [DOI] [PubMed] [Google Scholar]
- 25.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madara J, Pappenheimer J R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 27.Nusrat A, Giry M, Turner J R, Colgan S P, Parkos C A, Carnes D, Lemichez E, Boquet P, Madara J L. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obiso R J, Jr, Azghani A O, Wilkins T D. The Bacteroides fragilis toxin fragilysin disrupts the paracellular barrier of epithelial cells. Infect Immun. 1997;65:1431–1439. doi: 10.1128/iai.65.4.1431-1439.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 30.Philpott D J, McKay D M, Mak W, Perdue M H, Sherman P M. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect Immun. 1998;66:1680–1687. doi: 10.1128/iai.66.4.1680-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitelka D R, Taggart B. Mechanical tension induces lateral movement of intramembrane components of the tight junction: studies on mouse mammary cells in culture. J Cell Biol. 1983;96:606–612. doi: 10.1083/jcb.96.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratcliffe M J, Rubin L L, Staddon J M. Dephosphorylation of the cadherin-associated p100/p120 proteins in response to activation of protein kinase C in epithelial cells. J Biol Chem. 1997;272:31894–31901. doi: 10.1074/jbc.272.50.31894. [DOI] [PubMed] [Google Scholar]
- 33.Reed K A, Booth T A, Hirst B H, Jepson M A. Promotion of Salmonella typhimurium adherence and membrane ruffling in MDCK epithelia by staurosporine. FEMS Microbiol Lett. 1996;145:233–238. doi: 10.1111/j.1574-6968.1996.tb08583.x. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph M G, Weise C, Mirold S, Hillenbrand B, Bader B, Wittinghofer A, Hardt W D. Biochemical analysis of SopE from Salmonella typhimurium, a highly efficient guanosine nucleotide exchange factor for Rho GTPases. J Biol Chem. 1999;274:30501–30509. doi: 10.1074/jbc.274.43.30501. [DOI] [PubMed] [Google Scholar]
- 35.Scudamore C L, Jepson M A, Hirst B H, Miller H R P. The rat mast cell chymase, RMCP-II, alters epithelial cell monolayer permeability in association with altered distribution of the tight junction proteins ZO-1 and occludin. Eur J Cell Biol. 1998;75:321–330. doi: 10.1016/s0171-9335(98)80065-4. [DOI] [PubMed] [Google Scholar]
- 36.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spitz J, Yuhan R, Koutsourus A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 38.Wells C L, van de Westerlo E M, Jecherok R P, Feltis B A, Wilkins T D, Erlandsen S L. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterology. 1996;110:1429–1437. doi: 10.1053/gast.1996.v110.pm8613048. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z, Nybom P, Magnusson K-E. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol. 2000;2:11–17. doi: 10.1046/j.1462-5822.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- 40.Yuhan R, Koutsouris A, Savkovic S D, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 41.Zhou D, Mooseker M S, Galán J E. An invasion-associated Salmonella protein modulates the actin-bundling activity of plastin. Proc Natl Acad Sci USA. 1999;96:10176–10181. doi: 10.1073/pnas.96.18.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]