Abstract
Background
Children with congenital heart disease (CHD) undergoing open-heart surgery are at risk for developmental impairments with motor delay manifesting first and contributing to parental concerns. Only a few interventional studies aim to improve neuromotor development in infants with CHD with inconclusive results. We thus developed a family-tailored early motor intervention (EMI-Heart), which aims to promote motor development and family well-being in the first year of life after open-heart surgery.
The primary aim described in this protocol is to evaluate feasibility of EMI-Heart. The secondary aim is to describe the difference between the intervention and control group in motor outcomes and family well-being at baseline, post-treatment, and follow-up.
Methods
This prospective, parallel single-center feasibility randomized controlled trial (RCT) will compare EMI-Heart with standard of care in infants with complex CHD. Sixteen infants and their families, randomly allocated to EMI-Heart or the control group, will participate within the first 5 months of life. Infants assigned to EMI-Heart will receive early motor intervention for 3 months. The intervention’s key is to promote infants’ postural control to enhance motor development and partnering with parents to encourage family well-being.
Feasibility outcomes will be (a) clinical recruitment rate and percentage of families completing EMI-Heart, (b) average duration and number of sessions, and (c) acceptability of EMI-Heart using a parental questionnaire post-treatment, and descriptive acceptability of EMI-Heart to the pediatric physiotherapist.
Secondary outcomes of the intervention and control group will be infants’ motor outcomes and questionnaires assessing family well-being at 3–5 months (baseline), at 6–8 months (post-treatment), and at 12 months of age (follow-up).
We will evaluate feasibility using descriptive statistics. Non-parametric statistical analysis of secondary outcomes will assess differences between the groups at baseline, post-treatment, and follow-up.
Discussion
This feasibility RCT will provide information about a newly developed family-tailored early motor intervention in infants with complex CHD. The RCT design will provide a foundation for a future large-scale interventional trial for infants with CHD after open-heart surgery.
Trial registration
This study protocol (version 1.3, 01.02.2022) was approved by the Cantonal Ethics Commission Zurich (BASEC-Nr. 2019–01,787) and is registered by Clinicaltrials.gov (NCTT04666857).
Supplementary Information
The online version contains supplementary material available at 10.1186/s40814-022-01220-y.
Keywords: Congenital heart disease, Open-heart surgery, Early motor intervention, Neuromotor development, Physiotherapy, Family-tailored intervention, Parental and child health-related quality of life, Family well-being
Background
Congenital heart disease (CHD) is one of the most common birth defects, with 8 of 1000 live-born children being affected world-wide [1, 2]. Achievements in prenatal diagnoses and medical care have increased the survival rate of even complex forms of CHD but have also exposed affected infants to a heightened risk of brain injury and developmental disorders [3]. Additional factors related to complex CHD such as abnormal brain development and perioperative white matter injuries contribute to subsequent neurodevelopmental impairments. These neurodevelopmental impairments comprise motor, cognitive, and sensory outcomes [4].
Infants born with complex CHD are at risk for a variety of developmental impairments. Motor development is the first domain in which impairment becomes apparent in the first year of life with a prevalence of 40–60%. Other developmental impairments, such as language disorders and behavioral and learning difficulties, may occur throughout childhood but often only become evident later at school age [5]. Evidence shows that early motor developmental abnormalities persist into childhood, adolescence, and adulthood [6].
Despite strong evidence of motor development impairments in infants with complex CHD, no targeted, specific, or tailored treatment is available. However, there is a clear need for an early motor intervention that aims to prevent problems before they manifest or mitigate existing ones and reduce difficulties later in life [7].
Postural control and sitting
In the first few months of life, typically developing infants spend most of the time they are awake supine, held, or in supported sitting. Time in sitting increases with the ability to learn sitting freely. Motor experiences such as sitting have cumulative consequences, a cascade of effects in other developmental domains such as cognition, social, and language development [8]. Infants’ points of view change in sitting from supine. Infants learn how to control their own body against gravity with hands free to explore toys and their surroundings. They interact variously with objects, people, and their environments [9, 10]. Postural control in sitting also enables the interaction promoting face-to-face exchange and joint attention with their caregivers [11].
Two theories describe the importance of postural control in early motor development. One theory describes postural control as a complex and dynamic process of learning and adapting to diverse environmental forces and tasks [12, 13]. The other theory describes postural control as an innate, genetically determined aspect of behavior that changes with exploration in infancy [14]. Both theories agree that postural control is variable, is affected by many factors, and is a key element in early motor development, including learning to sit independently.
In infants at risk for neuromotor disorders, such as infants with CHD, postural control is reduced. Compared to typically developing infants, they spend more time supine and only start sitting later. Thus, the opportunity to actively explore their body and surroundings is reduced from early infancy. Studies have shown that improvement of postural control in infants at high risk for developmental delay in sitting facilitates their motor and cognitive development [15–18].
Kretch et al. [11] demonstrated that caregivers were most likely to provide learning opportunities when infants were sitting. Their findings suggest that early intervention should focus on improving postural control. Infants with neuromotor delay should be positioned early in supported sitting in a way that allows face-to-face contact with the parents before they can sit independently. This strategy opens new motor and cognitive learning opportunities. Motor learning is an essential part of early motor intervention programs for infants at risk for developmental disorders.
Early intervention
A wide body of literature underlines the importance of early intervention for infants at high risk for neurodevelopmental impairments such as cerebral palsy [15, 19–21]. The World Health Organization even states that it is crucial to identify infants at risk for neurodevelopmental disorders, establish a close relationship between parents and health care professionals, and provide early intervention [22]. Infants with CHD that undergo open-heart surgery together constitute such a population at risk for motor developmental delay.
However, motor interventions that focus on this patient group are sparse. Few interventional studies exist that start in infancy and aim to improve motor development. Cohort studies [23–25] and single case studies [26, 27] have investigated the influence of early physiotherapy for infants with CHD. Although the results are inconclusive due to their low level of evidence and heterogeneity, early motor interventions seem to positively influence motor development in infants with CHD.
A considerable proportion of infants with CHD are unable to tolerate the prone position. This might be due to surgery, lack of prone positioning, discomfort, or parental protection. Dagenais et al. [28] investigated the prone performance in infants after open-heart surgery and concluded that better scores in prone performance of the Alberta Infant Motor Scale [29] predicts earlier onset of walking. Uzark et al. [30] found that infants after open-heart surgery that performed the prone position daily had significantly better motor skills than those who did not. Thus, these studies emphasize the importance of promoting the prone position for infants with CHD.
Parental engagement
Parents offer their children many learning opportunities during their upbringing. Motor delay contributes to parental concerns and difficulties in child–parent wellbeing. In our qualitative study about the experience of parents of infants with CHD, parents reported that they were reluctant to challenge their infants [31]. They feared over-exerting them and watched them constantly. Parental overprotection, which occurs more frequently with infants with CHD, might negatively influence children’s motor development. Reduced physical activity that starts early in life most likely continues. This assumption implies that early support of parents is equally important as early motor support of their infants. Parental attendance and active engagement play a key role in early intervention [32–34].
Depending on the severity of children’s heart disease, parents’ resources, and family support, stress can last beyond children’s hospital stays [35]. This impacts parent–child relationships because parental well-being is crucial for children’s health and adjustment. Our study about the experience parents of children with CHD [31] underpins the importance of involving parents as experts in their children and as partners in decision-making about their care. Parents appreciate medical information that helps them to better understand and support their children’s development and thus provide the best possible outcome for children and their families.
An effective intervention that can prevent maladaptive plasticity of infants’ brains has to (a) start early, (b) occur at high frequency and require the child to be active (c) be playful and goal directed, (d) be tailored to each family separately, and (e) engage caregivers as equal partners [36–38].
There is a lack of an early motor intervention specifically tailored to infants with CHD and their families. Thus, the purpose of the study described in this protocol is to test the feasibility of an early newly developed family-tailored early motor intervention in infants with complex CHD (EMI-Heart) after open-heart surgery. There is equipoise between the intervention and control group therefore we chose a RCT design. The results of this feasibility RCT will lay the foundation for a larger RCT to test the efficacy of this intervention.
Methods/design
Aim
The primary aim of this study is to evaluate feasibility using measures like recruitment and adherence rate, parental acceptability of EMI-Heart, and acceptability to the pediatric physiotherapist providing EMI-Heart. The secondary aim is to describe the difference between the intervention and control group in motor outcomes and measures of family well-being. The results of this feasibility intervention trial will provide the foundation for a larger future RCT.
Study design
This feasibility study is a prospective single-center single-blinded, two-arm parallel RCT that compares EMI-Heart to standard of care for infants with CHD after open-heart surgery.
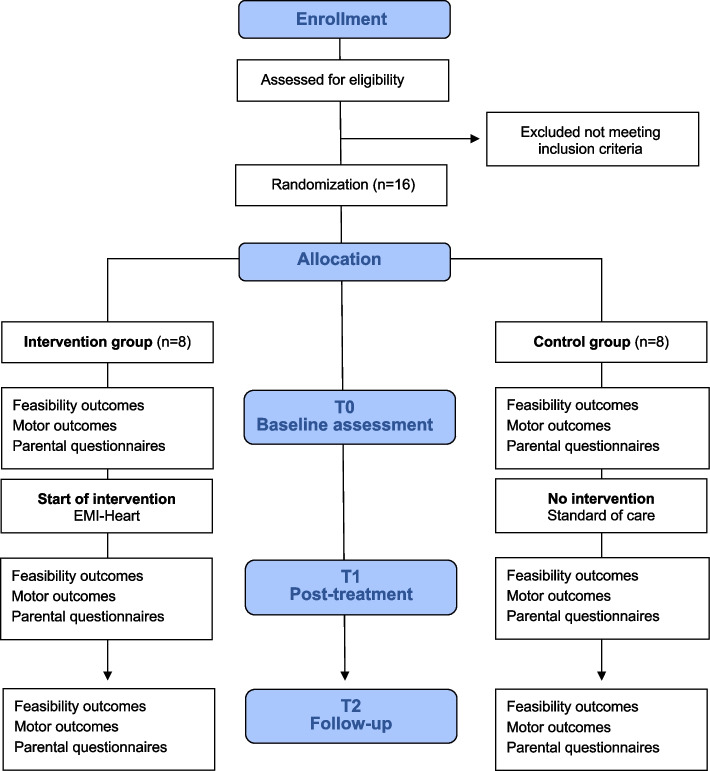
Sixteen participants meeting the eligibility criteria will be recruited over a period of 12 months. Infants will be randomized and assigned to the intervention (n = 8), or control group (n = 8) determined by a computer-generated random sequence. This will ensure that baseline characteristics of each group will be as similar as possible. Groups’ allocation will be concealed. Sealed opaque and numbered envelopes will be sequentially opened to preserve concealment by members of the Children’s Hospital that are not involved in this project. Both the intervention group and the control group will be assessed at baseline (T0), at post-treatment (T1) after nine sessions approximately 3 months later, and at follow-up (T2) at 12 months of age (see Figs. 1 and 2). All assessments will be video recorded. Five assessors (CE, MS, RE, BL, RC) blinded to group allocation and not involved in the intervention will score the video recordings. Assessments will be scored independently, and results will not be shared between assessors. Parents and the pediatric physiotherapist (PT) providing the intervention will not be blinded to the intervention.
Fig. 1.
Flow diagram of the study procedure according to Consort 2010. T0: baseline; T1: post-treatment; T2: follow-up
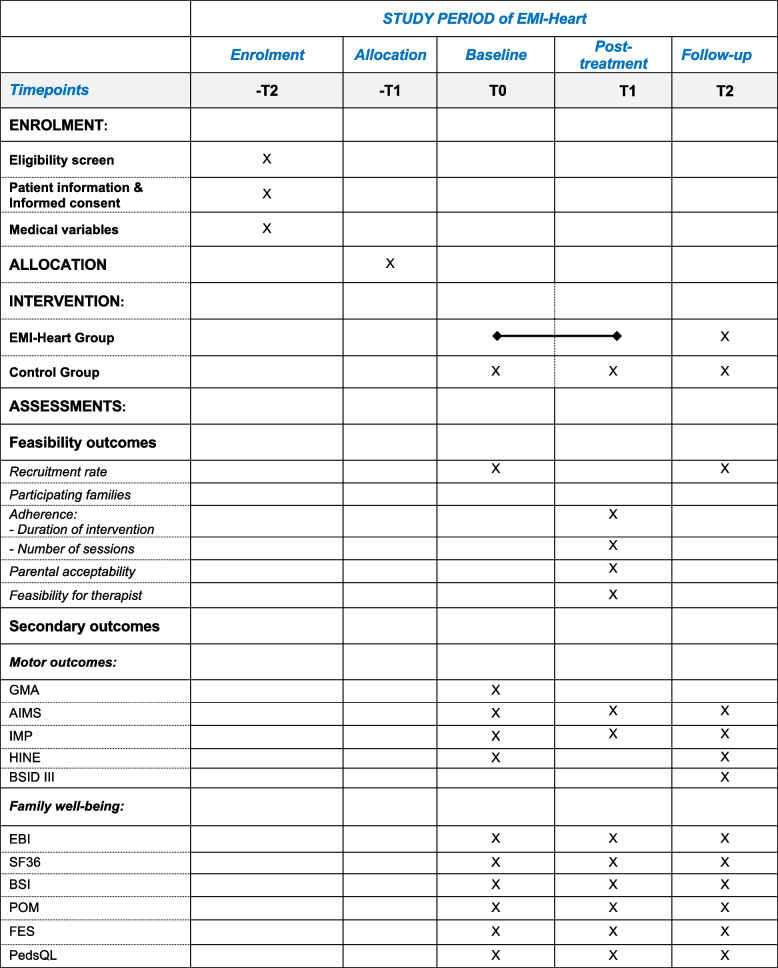
Fig. 2.
Schedule of enrolment, interventions, and assessments according to SPIRIT 2013. IMP (Infant Motor Profile), AIMS (Alberta Infant Motor Scale), GMA (General Movements Assessment), HINE (Hammersmith Infant Neurological Examination), BSID III (Bayley Scales of Infant and Toddler Development), EBI (Eltern Belastungs Inventar), SF36 (Quality of life Short Form 36), BSI (Brief Symptom Inventory), POM (Parental Overprotection Measure), FES (Family Empowerment Scale), PedsQL (Pediatric Quality of Life Inventory)
Study participants and recruitment
All study participants will be assessed for study inclusion and recruited at the University Children’s Hospital Zurich consecutively by members of the Department of Cardiology and the investigator EM before baseline. We will include participants into the trial with the following criteria: (1) infants with CHD (e.g., tetralogy of Fallot, pulmonary atresia, transposition of the great arteries, atrial/ventricular septal defects, double outlet right ventricle), (2) infants born ≥ 37 weeks gestational age, (3) infants aged 3–5 months who underwent open heart surgery with cardiopulmonary bypass once within the first 5 months of life, (4) infants discharged home before the age of 6 months, (5) informed consent of infants’ parents documented by signature, and (6) families living within an hour’s journey from the Children’s Hospital.
We will exclude (1) infants with univentricular heart defects like hypoplastic left-heart syndrome, because they need to undergo several planned open-heart surgeries within the first year of life; (2) infants with syndromes that are often associated with CHD and worse neurodevelopmental outcomes such as trisomy 21, 22q11 microdeletion, CHARGE, Noonan, and the VACTERL association; and (3) large cerebral and clinically manifest lesions, (4) infants whose parents have an inadequate understanding of the German language and are thus unable to comprehend the patient information.
Patient and public involvement
The Swiss parents’ association for the child with heart disease (Elternvereinigung für das herzkranke Kind) will provide us with advice for the conduct of this study and for the recruitment of families. MT, a parental stakeholder, is co-author of this manuscript. In the development of EMI-Heart, we performed interviews with parents of children with CHD who underwent open-heart surgery within the first 6 months of life. The result of this qualitative study describes a variety of burdens and needs parents had experienced and which determines the design of EMI-Heart [31].
All families will be informed of the burden of the intervention and given the option to stop at any stage. All eligible families completing the study will receive an individual report of the results and a general report of study results when data analyses are completed.
Study groups
Intervention group: EMI-Heart
Infants randomly assigned to the intervention group receive EMI-Heart. The investigator EM, a senior pediatric PT, specialized in early motor development will provide all interventions to maximize fidelity of EMI-Heart. The second author TD, a senior pediatric PT with extensive knowledge in early intervention, will support the quality of the content of EMI-Heart and its implementation in practice by discussing video recordings of the intervention with EM. To improve adherence to the intervention EM regularly contacts parents via telephone, text messaging and emails. EMI-Heart will start after hospital discharge and baseline assessments and will last for approximately 3 months, take place once a week or fortnightly for 45–60 min per session. The intervention will consist of nine treatment sessions: three sessions at home, three at the children's hospital, and three online, ideally in an alternating order.
EMI-Heart is based on our qualitative study, which describes parental experience of their infants’ neuromotor development after open-heart surgery. Parents wanted to actively support their infants’ development and be respected as experts of their infants. Therefore, the intervention’s key is to promote postural control to enhance motor development in infancy [13, 39, 40], and partnering between parents and the PT at eye-level to encourage family well-being, as recommended in current research on pediatric rehabilitation [32–34]. EMI-Heart is family-tailored to each unique family. It is adapted to infants’ motor abilities and considers family’s own experiences and wishes. EMI-Heart’s key aims are intertwined with each other and addressed as described below:
Promotion of infants’ postural control
Infants with CHD after open-heart surgery are often not exposed to the prone or the early sitting position due to perceived discomfort and/or parental protection.
The PT creates safe and playful postural activities in prone and early sitting and support parents how to stimulate their infants’ postural activities repeatedly and joyfully in daily life activities. Prone and sitting positions are explored and adjusted to infants’ needs. External support, e.g., towels or cushions placed under infants’ armpits and chest can facilitate head lifting in prone. In early sitting parents’ hands and body, furniture, cushions, and toys are used to stimulate trunk/head control, reaching, and grasping activities.
As soon as infants improve their postural control (e.g., lifting the head with more ease in prone, visible enjoyment, less wobbling of the head, better ability to look around, goal-directed reaching and grasping activities in sitting), PT and parents gradually reduce external support.
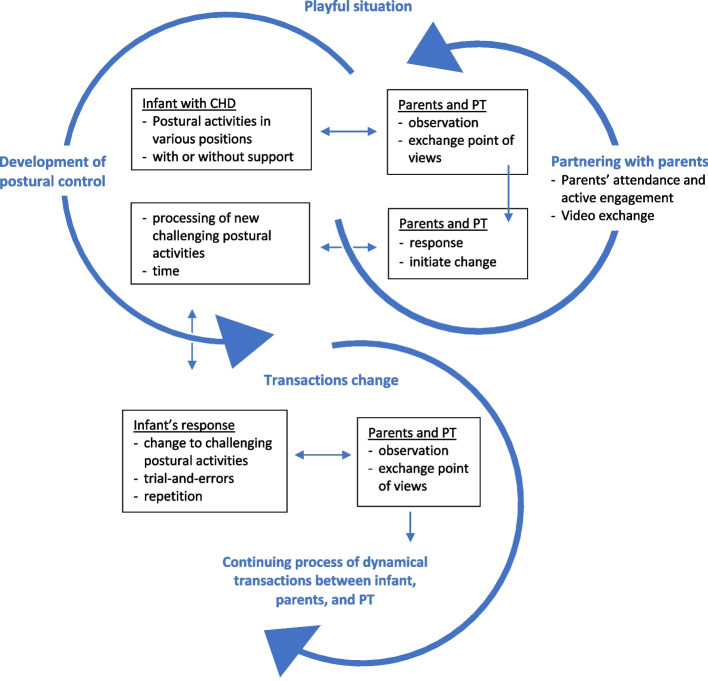
The continuing interplay between infant’s postural activities and responses on parents and the PT’ actions and vice versa are illustrated in Fig. 3. Two dynamic elements are interacting as an ongoing spiral: (1) infant’s postural activities and behavior and (2) partnering between parents and the PT. These elements change constantly due to transactions between the infant, parents, and the PT. These transactions are embedded in daily life activities, which in turn are part of each family’s ecological environment.
Fig. 3.
EMI-Heart, Transactional Model of Change. The spiral illustrates the continuing interplay between infant’s postural activities and responses on parents and the PT’s actions and vice versa. CHD: congenital heart disease, PT: physiotherapist
Partnering with parents
-
a
Parents’ attendance and active engagement
In each session, parents are present and actively engaged. Parents and the PT act as equal partners, share their responsibility and openly discuss their point of views. Parents are their infants’ experts and share their experience and wishes with the PT. The PT in turn shares her/his professional and empirical knowledge, clinical reasoning, and current research evidence in early intervention.
-
b
Encouragement of parents’ confidence and family well-being
In our qualitative study, parents expressed the need to strengthen their confidence and learn how to trust their children’s abilities [31]. The PT uses different strategies to encourage parents’ confidence and family well-being.-
iJoint exploration: parents often feel insecure in handling their infants after open-heart surgery and therefore are reluctant to try out new positions like prone and early sitting. The PT shows parents how they can confidently handle their infants and encourages them to trust their infants’ ability to explore the environment (see “Promotion of infants’ postural control” section). Together the PT and parents jointly explore positions in which both infants and parents feel comfortable.
-
iiPromoting interplay between infants and parents: the PT promotes face-to-face interaction between parents and infants with verbal encouragement. This stimulates joint play and joyful infant-parent interaction. Early sitting with and without support, e.g., does not only enhance active body control against gravity, but also enables infants to explore toys and interact with their parents and the environment.
-
iiiVideo-exchange: all intervention sessions are video-recorded and made available to parents. Additionally, parents are invited to send homemade video-clips of their infants’ daily activities to the PT. Watching those video-recordings enables parents and the PT to see themselves and infants from a distance. This exchange encourages partnering with parents and active engagement. Homemade video-clips enable the PT to see how parents implement EMI-Heart in daily life. Studies showed that video feedback positively promotes parental engagement and increases parental capacity to read and respond to their children’s signals [41, 42].
-
ivOnline sessions in addition to visits at home and at the hospital: Online sessions promote parental engagement as the PT cannot physically interact with the family. Parents show the PT how they stimulate infants’ postural activities in real-life situations. The PT answers parental questions and provides feedback when necessary. This also allows the PT to virtually exchange video-clips of the intervention and demonstrate how well parents are stimulating their infants’ development. Online sessions save travel time, are flexible to plan and are easy to access for both parents and the PT. This is in line with Rosenbaum et al. [43, 44] who described that virtual therapy improved parental skills and understanding of how to support their children.
-
i
Control group: standard of care
Infants randomly assigned to the control group receive standard of care for infants with CHD after open heart surgery, which includes cardiac surveillance, counseling, and screening at the University Children’s Hospital Zurich and standardized developmental check-ups by their pediatrician. Pediatric PT is not normally part of standard of care. However, some infants may receive physiotherapy if they present with obvious signs of motor developmental delay. Infants receiving physiotherapy are usually treated at pediatric outpatient clinics once a week or fortnightly for approximately 3 months.
Outcome measures
The feasibility will be measured by (a) clinical recruitment rate; (b) percentage of families completing EMI-Heart, (c) adherence: average duration and number of sessions used in EMI-Heart; and (d) the acceptability of EMI-Heart using a parental questionnaire post-treatment (see Table 1). We developed a parental acceptability questionnaire based on the core elements of EMI-Heart. It consists of 18 questions with a four-point Likert scale response. The feasibility of the EMI-Heart intervention for the providing pediatric physiotherapist will be described (see Table 1).
Table 1.
Feasibility outcomes
| Description | |
|---|---|
| Recruitment rate | Sum of recruitment rate and reasons of withdrawal |
| Participating families | Percentage of families completing the study |
| Adherence | |
| Duration of the intervention | Time between T0 to T1 expressed in months, weeks |
| Number of intervention sessions | Number of sessions provided per intervention infant |
| Parental acceptability | 18 items Likert scale 0–4 (0 do not agree–4 totally agree) at timepoint T1 (post-treatment) |
| Feasibility for pediatric physiotherapist | Description of the feasibility of EMI-Heart to physiotherapist (setting, travel time, preparation, and follow-up) |
Secondary outcomes of the intervention and control group will be infants’ motor outcome and family well-being, see Table 2. We will assess infants’ motor development and family well-being at timepoints T0, T1, and T2 in both the intervention and control groups. Infants’ motor outcomes include the Infant Motor Profile [45], the Alberta Infant Motor Scale [29] and the Bayley Scales of Infant and Toddler Development third version [46]. Baseline variables include the General Movements Assessment [47, 48] and the Hammersmith Infant Neurological Examination [49, 50]. These validated and reliable assessments are widely used in practice and research. Validated German versions will be used if available. All assessments will be video recorded and evaluated by assessors blinded to group allocation. Parental questionnaires will evaluate parents’ and infants’ health-related quality of life, parental mental health and stress experience, infants’ protection, and parental empowerment. The questionnaires will be provided as a survey to be completed online with Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the University Children’s Hospital Zurich [51, 52]. Medical and cardiac valuables will be derived from the electronic medical charts of the hospital’s data management system.
Table 2.
Secondary outcomes
| Timepoints | ||||
|---|---|---|---|---|
| T0 | T1 | T2 | ||
| Motor assessments | ||||
| Infant Motor Profile [45] | x | x | x | |
| Alberta Infant Motor Scale [29] | x | x | x | |
| General Movement Assessment [47] | x | |||
| Hammersmith Infant Neurological Examination [49] | x | x | ||
| Motor domains of the Bayley Scales of Infant and Toddler Development III [46] | x | |||
| Parental questionnaires assessing family well-being | ||||
| Infants’ quality of life | Pediatric Quality of Life Inventory [53] | x | x | x |
| Parents’ quality of life | Short Form Survey-SF 36 [54, 55] | x | x | x |
| Parental mental health | Brief Symptom Inventory 18 [56] | x | x | x |
| Parental stress experience | Parental Stress Index [57] | x | x | x |
| Infants’ protection | Parental Overprotection Measure [58] | x | x | x |
| Parental empowerment | Family Empowerment Scale [59] | x | x | x |
Sample size and data analysis
Based on previous clinical data of the University Children’s Hospital [60] of the last 3 years and on previous intervention studies [61] approximately 30% of infants undergoing open-heart surgery within the first 5 months of life will meet our inclusion criteria. This corresponds to a sample size of approximately 16 infants who we aim to recruit for our feasibility study. This study will not be powered for statistical hypothesis testing. We are aware that our results will not be generalizable to a wider population. Nonetheless, we decided to keep to this sample size as the Covid pandemic complicates recruitment. Open-heart surgeries are being canceled or postponed leading to the exclusion of initially eligible infants. The study described in this protocol aims to determine the intervention’s feasibility of conducting a larger future multi-center RCT to test the efficacy of this intervention. This study trial will be conducted according to the SPIRIT [62] and the TIDieR statement [63], and reported according to the CONSORT statement [64].
Clinical data will be analyzed with statistics R or the Statistical Package for Social Sciences. Descriptive statistics of the feasibility outcomes will be calculated, including means and SD or medians and IQRs for continuous variables and/or for categorical variables. Recruitment will be measured by summarizing recruitment rate and reasons of withdrawal compared to available patients listed in the screening log. Secondary outcomes will be collected at each timepoint and summarized descriptively for the intervention and the control group. Secondary outcomes will assess differences between the intervention and control group at baseline, post-treatment and follow-up using an intention-to-treat analysis. Non-parametric methods will be used if parametric assumptions are violated.
Discussion
The protocol of this feasibility RCT will provide information about a newly developed family-tailored early motor intervention in infants with complex CHD after open-heart surgery. To the best of our knowledge, no early motor intervention exists that specifically focuses on infants with CHD, aiming to promote infants’ postural control and family well-being. This study will provide preliminary results of the feasibility of EMI-Heart and used outcome measures. Additionally, this study will provide information on the feasibility of home visits and online treatment sessions that will allow interventions to be adjusted to the real-life situations of families. In a future step, we plan to analyze all video recordings of the intervention sessions using a qualitative content analysis. Identifying the transactions between parents, infants, and the therapist will enable the reproducibility of this newly developed intervention.
Supplementary Information
Additional file 2. Parent information and informed consent.
Additional file 3. Study specific monitoring plan.
Additional file 4. Trial Register Data Set.
Acknowledgements
We thank Melanie Ehrler, Alenka Schmid, Flavia Wehrle, Maria Feldmann, Barbara Schnider, Vera Disselhoff, Alexandra Stöckli, Nadja Naef, and Cornelia Hagmann, members of the Children Heart and Developmental research group of the Children’s Hospital for their valuable support in designing the study. Additionally, we thank the team of the pediatric cardiology and the neonatology for their willingness to support us in this study. We also thank Martina Wehrli for her valuable support to prepare our questionnaires in RedCap and Simon Milligan for editing this manuscript.
Data management and safety monitoring
Members of the Children’s Research Center (CRC) of the University Children’s Hospital Zurich will fulfil the monitoring duties prior to the study, after the enrolment of the second and the tenth patient, and at the end of the study. This trial has been considered a non-significant risk device study by the Cantonal Ethics Commission Zurich. Members of the CRC are independent of study sponsor and investigator. Monitoring covers all study documents, source data, and trial master file. The transfer, storage, and evaluation of health-related personal data within the project is carried out in accordance with the Swiss data protection regulations and the Clinical Trials Ordinance. Coded health-related data of participants will be unblinded only, if it is necessary to avert an immediate risk to the health of the participant concerned, if a legal basis exists for breaking the code, if breaking the code is necessary to guarantee the rights of the participant concerned, and the right to revoke consent. A description of this trial’s data management plan, safety monitoring board, and risk–benefits assessment is available in Supplementary Material 1 and 3.
Abbreviations
- CHD
Congenital heart disease
- EM
Elena Mitteregger
- TD
Tineke Dirks
- MT
Manuela Theiler
- BL
Beatrice Latal
- OK
Oliver Kretschmar
- CE
Christa Einspieler
- MS
Maaike Sprong
- RE
Rian Eijsermans
- RC
Rachel Cott
- EMI-Heart
Early motor intervention for infants with complex congenital heart disease
Authors’ contributions
EM, BL, and TD conceptualized the study protocol. EM wrote the manuscript, and TD and BL critically revised it. MT gave her input and feedback as a parental stakeholder. OK contributed his medical expertise and advised on design and recruitment. All authors discussed, read, and approved the final manuscript for publication.
Funding
EM is financially supported by a grant from the Anna Mueller Grocholski-Foundation. This funding source has no role in the design or decision where to submit this protocol of the study.
The other authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials
The datasets that will be used and analyzed will be available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study protocol (version 1.3, 01.02.2022) has been approved by the Cantonal Ethics Commission in Zurich, BASEC-Nr. 2019–01787, amendments 07.01.2020 and 15.02.2022 (Supplementary material 1) and is registered at Clinicaltrials.gov NCTT04666857. Written informed consent will be obtained from all parents of included participants by EM (Supplementary material 2). BL, the trial sponsor and EM, the investigator may terminate the study prematurely according to certain circumstances, e.g., ethical concerns, insufficient participant recruitment, when the safety of participants is doubtful or at risk or when there is early evidence of harm or benefit of the experimental intervention.
Consent for publication
Parents of included participants will be asked to give consent for the publication of this results of the feasibility study. Findings from this study will be published in peer-reviewed journals and presented at national and international conferences, to parent organizations, and to healthcare stakeholders for widespread dissemination of the results.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Der Linde D, Konings EEM, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJM, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011;58(21):2241–7. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48(2):455–463. doi: 10.1093/ije/dyz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marelli A, Miller SP, Marino BS, Jefferson AL, Newburger JW. Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation. 2016;133(20):1951–1962. doi: 10.1161/circulationaha.115.019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyvandi S, Latal B, Miller SP, Mcquillen PS. The neonatal brain in critical congenital heart disease: Insights and future directions. Neuroimage. 2018;2019(185 April 2018):776–82. doi: 10.1016/j.neuroimage.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 5.Latal B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin Perinatol. 2016;43(1):173–185. doi: 10.1016/j.clp.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Bolduc ME, Dionne E, Gagnon I, Rennick JE, Majnemer A, Brossard-Racine M. Motor impairment in children with congenital heart defects: a systematic review. Pediatrics. 2020;146(6):1–16. doi: 10.1542/peds.2020-0083. [DOI] [PubMed] [Google Scholar]
- 7.Cassidy AR, Butler SC, Briend J, Calderon J, Casey F, Crosby LE, et al. Neurodevelopmental and psychosocial interventions for individuals with CHD: a research agenda and recommendations from the Cardiac Neurodevelopmental Outcome Collaborative. Cardiol Young. 2021; 1–12. 10.1017/s1047951121002158. [DOI] [PMC free article] [PubMed]
- 8.Masten AS, Cicchetti D. Developmental cascades. Dev Psychopathol. 2010;22(3):491–495. doi: 10.1017/s0954579410000222. [DOI] [PubMed] [Google Scholar]
- 9.Adolph KE, Hoch JE. Motor Development: Embodied, Embedded, Enculturated, and Enabling. Annu Rev Psychol. 2019;70:141–164. doi: 10.1146/annurev-psych-010418-102836.motor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Needham A, Libertus K. Embodiment in early development. Wiley Interdiscip Rev Cogn Sci. 2011;2(1):117–123. doi: 10.1002/wcs.109. [DOI] [PubMed] [Google Scholar]
- 11.Kretch KS, Koziol NA, Marcinowski EC, Kane AE, Inamdar K, Brown ED, et al. Infant posture and caregiver-provided cognitive opportunities in typically developing infants and infants with motor delay. Dev Psychobiol. 2022;64(1):1–17. doi: 10.1002/dev.22233. [DOI] [PubMed] [Google Scholar]
- 12.Thelen E. Motor development: A new synthesis. Am Psychol. 1995;50(2):79–95. doi: 10.1037/0003-066x.50.2.79. [DOI] [PubMed] [Google Scholar]
- 13.Dusing SC, Harbourne RT. Variability in postural control during infancy: implications for development, assessment, and intervention. Phys Ther. 2010;90(12):1838–1849. doi: 10.2522/ptj.2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadders-Algra M. The Neuronal Group Selection Theory: a framework to explain variation in normal motor development. Dev Med Child Neurol. 2000;42(8):566–572. doi: 10.1017/s0012162200001067. [DOI] [PubMed] [Google Scholar]
- 15.Harbourne RT, Dusing SC, Lobo MA, Mccoy SW, Koziol NA, Hsu LY, et al. START-Play physical therapy intervention impacts motor and cognitive outcomes in infants with neuromotor disorders: a multisite randomized clinical trial. Phys Ther. 2021;101(2):1–11. doi: 10.1093/ptj/pzaa232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inamdar K, Molinini RM, Panibatla ST, Chow JC, Dusing SC. Physical therapy interventions to improve sitting ability in children with or at-risk for cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2021;63(4):396–406. doi: 10.1111/dmcn.14772. [DOI] [PubMed] [Google Scholar]
- 17.Lobo MA, Harbourne RT, Dusing SC, McCoy SW. Grounding early intervention: physical therapy cannot just be about motor skills anymore. Phys Ther. 2013;93(1):94–103. doi: 10.2522/ptj.20120158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen-Willett S, Pleasant M, Jackson B, Needelman H, Roberts H, Mcmorris C. Sitting matters! differences between sitters and nonsitters at 6 months’ adjusted age in infants at-risk and born preterm. Pediatr Phys Ther. 2019;31(3):257–262. doi: 10.1097/pep.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 19.Hadders-Algra M, Boxum AG, Hielkema T, Hamer EG. Effect of early intervention in infants at very high risk of cerebral palsy: a systematic review. Dev Med Child Neurol. 2017;59(3):246–258. doi: 10.1111/dmcn.13331. [DOI] [PubMed] [Google Scholar]
- 20.Morgan C, Darrah J, Gordon AM, Harbourne R, Spittle A, Johnson R, et al. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev Med Child Neurol. 2016;58(9):900–909. doi: 10.1111/dmcn.13105. [DOI] [PubMed] [Google Scholar]
- 21.Novak I, Morgan C, Fahey M, Finch-Edmondson M, Galea C, Hines A, et al. State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep. 2020;20(2):3. doi: 10.1007/s11910-020-1022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization, World Bank. World Report on Disability. Geneva: World Health Organization 2011 (www.who.int/disabilities/world_report/2011. The world report on disability. Disability and Society. 2011. 10.1080/09687599.2011.589198.
- 23.Fourdain S, Simard MN, Dagenais L, Materassi M, Doussau A, Goulet J, et al. Gross Motor Development of Children with Congenital Heart Disease Receiving Early Systematic Surveillance and Individualized Intervention: Brief Report. Dev Neurorehabil; 2020; 1–7. 10.1080/17518423.2020.1711541. [DOI] [PubMed]
- 24.Haseba S, Sakakima H, Nakao S, Ohira M, Yanagi S, Imoto Y, et al. Early postoperative physical therapy for improving short-term gross motor outcome in infants with cyanotic and acyanotic congenital heart disease. Disabil Rehabil. 2018;40(14):1694–701. doi: 10.1080/09638288.2017.1309582. [DOI] [PubMed] [Google Scholar]
- 25.Long SH, Eldridge BJ, Harris SR, Cheung MMH. Challenges in trying to implement an early intervention program for infants with congenital heart disease. Pediatr Phys Ther. 2015;27(1):38–43. doi: 10.1097/pep.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 26.Jara AM, Jacobs JP, Reilly M. Physical therapy management of a critically-ill infant after cardiac surgery: a case report and literature review. J Acute Care Phys Ther. 2018;9(4):163–170. doi: 10.1097/jat.0000000000000084. [DOI] [Google Scholar]
- 27.Gallagher A, Dagenais L, Doussau A, Décarie J, Materassi M, Gagnon K. Significant motor improvement in an infant with congenital heart disease and a rolandic stroke : the impact of early intervention. Dev Neurorehabil. 2017;20(3):165–168. doi: 10.3109/17518423.2015.1132280. [DOI] [PubMed] [Google Scholar]
- 28.Dagenais L, Materassi M, Desnous B, Vinay MC, Doussau A, Sabeh P, et al. Superior performance in prone in infants with congenital heart disease predicts an earlier onset of walking. J Child Neurol. 2018;33(14):894–900. doi: 10.1177/0883073818798194. [DOI] [PubMed] [Google Scholar]
- 29.Darrah J, Piper M, Watt MJ. Assessment of gross motor skills of at-risk infants: predictive validity of the Alberta Infant Motor Scale. Dev Med Child Neurol. 1998;40(7):485–491. doi: 10.1111/j.1469-8749.1998.tb15399.x. [DOI] [PubMed] [Google Scholar]
- 30.Uzark K, Smith C, Yu S, Lowery R, Tapley C, Romano JC, et al. Evaluation of a ‘tummy time’ intervention to improve motor skills in infants after cardiac surgery. Cardiol Young. 2021; 1–6. 10.1017/s1047951121003930. [DOI] [PubMed]
- 31.Mitteregger E, Wehrli M, Theiler M, Logoteta J, Nast I, Seliner B, et al. Parental experience of the neuromotor development of children with congenital heart disease: an exploratory qualitative study. BMC Pediatrics; 2021; 21(1):430. 10.1186/s12887-021-02808-8. [DOI] [PMC free article] [PubMed]
- 32.Mahoney G. Relationship focused intervention (RFI): enhancing the role of parents in children’s developmental intervention. Int J Early Child Spec Educ. 2009;1(1):79–94. doi: 10.20489/intjecse.107978. [DOI] [Google Scholar]
- 33.Phoenix M, Jack SM, Rosenbaum PL, Missiuna C. A grounded theory of parents’ attendance, participation and engagement in children’s developmental rehabilitation services: Part 2. The journey to child health and happiness. Disabil Rehabil. 2020;42(15):2151–60. doi: 10.1080/09638288.2018.1555618. [DOI] [PubMed] [Google Scholar]
- 34.Pighini MJ, Goelman H, Buchanan M, Schonert-Reichl K, Brynelsen D. Learning from parents’ stories about what works in early intervention. Int J Psychol. 2014;49(4):263–270. doi: 10.1002/ijop.12024. [DOI] [PubMed] [Google Scholar]
- 35.Werner H, Latal B, Valsangiacomo Buechel E, Beck I, Landolt MA. The impact of an infant’s severe congenital heart disease on the family: a prospective cohort study. Congenit Hear Dis. 2014;9:203–210. doi: 10.1111/chd.12123. [DOI] [PubMed] [Google Scholar]
- 36.Cioni G, Inguaggiato E, Sgandurra G. Early intervention in neurodevelopmental disorders: underlying neural mechanisms. Dev Med Child Neurol. 2016;58:61–66. doi: 10.1111/dmcn.13050. [DOI] [PubMed] [Google Scholar]
- 37.Guzzetta A. From activity to interactivity: finding the active ingredient of very early intervention. Eur Acad Child Disabil. 2017. http://edu.eacd.org/29th-eacd-annual-meeting-amsterdam-may-17-20-2017-archive. Accessed 14 Jan 2021.
- 38.Peyton C, Sukal Moulton T, Carroll AJ, Anderson E, Brozek A, Davis MM, et al. Starting at Birth: an Integrative, State-of-the-Science Framework for Optimizing Infant Neuromotor Health. Front Pediatr. 2022;9 January:1–8. doi: 10.3389/fped.2021.787196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Dev Psychobiol. 2003;42(4):368–377. doi: 10.1002/dev.10110. [DOI] [PubMed] [Google Scholar]
- 40.Hadders-Algra M, Brogren E, Forssberg H. Ontogeny of postural adjustments during sitting in infancy: variation, selection and modulation. J Physiol. 1996;493(1):273–288. doi: 10.1113/jphysiol.1996.sp021382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juffer F, Bakermans-Kranenburg MJ, van IJzendoorn MH. Pairing attachment theory and social learning theory in video-feedback intervention to promote positive parenting. Curr Opin Psychol. 2017;15:189–94. doi: 10.1016/j.copsyc.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Provenzi L, Giusti L, Caglia M, Rosa E, Mascheroni E, Montirosso R. Evidence and Open Questions for the Use of Video-Feedback Interventions With Parents of Children With Neurodevelopmental Disabilities. Front Psychol. 2020;11 June:1–9. doi: 10.3389/fpsyg.2020.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbaum PL, Silva M, Camden C. Let’s not go back to ‘normal’! lessons from COVID-19 for professionals working in childhood disability. Disabil. Rehabil. 2021;43(7):1022–28. doi: 10.1080/09638288.2020.1862925. [DOI] [PubMed] [Google Scholar]
- 44.Cox SM, Butcher JL, Sadhwani A, Sananes R, Sanz JH, Blumenfeld E, et al. Integrating telehealth into neurodevelopmental assessment: a model from the cardiac neurodevelopmental outcome collaborative. J Pediatr Psychol. 2022;47(6):707–713. doi: 10.1093/jpepsy/jsac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadders-Algra M, Heineman KR. The infant motor profile. 1. Milton Park; New York: Routledge; 2021. [Google Scholar]
- 46.Albers CA, Grieve AJ. Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development– Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoeduc Assess. 2007;25(2):180–90. doi: 10.1177/0734282906297199. [DOI] [Google Scholar]
- 47.Prechtl HF, Einspieler C, Cioni G, Bos AF, Ferrari F, Sontheimer D. An early marker for neurological deficits after perinatal brain lesions. Lancet. 1997;349(9062):1361–1363. doi: 10.1016/s0140-6736(96)10182-3. [DOI] [PubMed] [Google Scholar]
- 48.Einspieler C, Prechtl H. Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev. 2005;11(1):61–67. doi: 10.1002/mrdd.20051. [DOI] [PubMed] [Google Scholar]
- 49.Haataja L, Cowan F, Mercuri E, Bassi L, Guzzetta A, Dubowitz L. Application of a scorable neurologic examination in healthy term infants aged 3 to 8 months. J. Pediatr. 2003;October:546. doi: 10.1067/S0022-3476(03)00393-7. [DOI] [PubMed] [Google Scholar]
- 50.Haataja L, Mercuri E, Regev R, Cowan F, Rutherford M, Dubowitz V, et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J. Pediatr. 1999;135(2 I):153–61. doi: 10.1016/s0022-3476(99)70016-8. [DOI] [PubMed] [Google Scholar]
- 51.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varni JW, Limbers CA, Neighbors K, Schulz K, Lieu JEC, Heffer RW, et al. The PedsQL™ Infant Scales: Feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011;20(1):45–55. doi: 10.1007/s11136-010-9730-5. [DOI] [PubMed] [Google Scholar]
- 54.Roser K, Mader L, Baenziger J, Sommer G, Kuehni CE, Michel G. Health-related quality of life in Switzerland: normative data for the SF-36v2 questionnaire. Qual Life Res. 2019;0(0):0. doi: 10.1007/s11136-019-02161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ware JE, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51(11):903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 56.Franke GH, Jaeger S, Glaesmer H, Barkmann C, Petrowski K, Braehler E. Psychometric analysis of the brief symptom inventory 18 (BSI-18) in a representative German sample. BMC Med Res Methodol. 2017;17(1):1–7. doi: 10.1186/s12874-016-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tröster H. Eltern-Belastungs-Inventar Deutsche Version des Parenting Stress Index (PSI) von R. R. Abidin. Göttingen: Hogrefe Verlag; 2010. [Google Scholar]
- 58.Edwards S. Psychometric properties of a parent-report measure of overprotection of preschool-aged children. Sydney: Macquarie University; 2007.
- 59.Koren PE, DeChillo N, Friesen BJ. Family Empowerment Scale. 1992. [Google Scholar]
- 60.Data management team . Data management of paediatric cardiology and congenital heart surgery of the children’s heart centre at University Children’s Hospital Zurich. 2022. [Google Scholar]
- 61.McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child Care Health Dev. 2010;36(1):110–117. doi: 10.1111/j.1365-2214.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- 62.Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:1–42. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348 March:1–12. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 64.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Parent information and informed consent.
Additional file 3. Study specific monitoring plan.
Additional file 4. Trial Register Data Set.
Data Availability Statement
The datasets that will be used and analyzed will be available from the corresponding author on reasonable request.