Abstract
Immune activation plays a major role in the pathogenesis of schizophrenia, as confirmed by many studies, systematic reviews, and meta-analyses. The important role of neuroinflammation in the formation of the relation between impaired neurobiological processes and schizophrenia psychopathology is being actively discussed. We quantified serum concentrations of 22 cytokines in 236 patients with schizophrenia and 103 mentally and somatically healthy individuals by a multiplex assay. We found higher TGF-α (p = 0.014), IFN-γ (p = 0.036), IL-5 (p < 0.001), IL-6 (p = 0.047), IL-8 (p = 0.005), IL-10 (p <0.001), IL-15 (p = 0.007), IL-1RA (p = 0.007), and TNF-α (p < 0.001) levels in patients with schizophrenia than in healthy individuals. Subgroup analysis revealed a much greater number of statistically significant differences in cytokine levels among females than among males. Patients with a continuous course of schizophrenia showed statistically significantly higher levels of IL-12p70 (p = 0.019), IL-1α (p = 0.046), and IL-1β (p = 0.035) compared with patients with an episodic course. Most cytokines were positively correlated with positive, general, and total PANSS scores. In patients with a duration of schizophrenia of 10 years or more, the level of IL-10 was higher than that in patients with a disease duration of 5 years or less (p = 0.042). Thus, an imbalance in cytokines was revealed in patients with schizophrenia, depending on sex and clinical characteristics of the disease.
Keywords: cytokine, schizophrenia, biomarker, clinical feature
1. Introduction
Schizophrenia is a chronic, heterogeneous, progressive, multifactorial mental disorder of unknown etiology and pathogenesis characterized by positive (e.g., delusions and hallucinations), negative (e.g., anhedonia and social withdrawal), and affective symptoms, as well as cognitive dysfunction. It has been suggested that environmental factors adversely interact with genetic predisposition, thus leading to the development of schizophrenia spectrum disorders [1]. Dopamine, serotonin, and glutamate hypotheses of psychosis have become classic theories of schizophrenia [2]. In recent years, due to the accumulation of a large amount of information, the hypothesis of immune dysregulation in schizophrenia has come onto the scene [3,4,5,6,7,8]. A large study on 36,989 schizophrenia patients and 113,075 controls has revealed associations with genes that play an important role in immunity [9]. A comparative review of serum/plasma biomarkers that can be used to differentiate schizophrenia from healthy individuals indicates that more than 70% of potential markers are involved in the inflammatory response [10]. Uncontrolled activation of proinflammatory cytokines and microglia, along with genetic variants of glutamatergic neurotransmission, may be some of the pathogenetic mechanisms of schizophrenia [11]. Randomized placebo-controlled trials have been conducted to evaluate the effectiveness of adjunctive use of anti-inflammatory drugs in schizophrenia and have yielded inconclusive results [12,13]. A meta-analysis has shown the ability of nonsteroidal anti-inflammatory drugs (celecoxib or acetylsalicylic acid) to reduce the severity of schizophrenia as compared with a placebo, according to PANSS. Nonetheless, those authors explain that these results should be interpreted with caution because these are first-time studies, and the sample size is small [14]. The involvement of the immune system in the pathophysiology of schizophrenia is supported by the following findings: (a) an imbalance between pro- and anti-inflammatory cytokines and an effect of antipsychotic medication on their levels [15,16,17,18]; (b) activation of microglia, as confirmed by animal studies, postmortem brain studies, and positron emission tomography [19,20,21]; (c) higher prevalence of autoantibodies [22,23]; (d) activation of inflammation-related genes in the brain [24,25]; and (e) increased expression of proinflammatory genes in circulating monocytes [26]. Peripheral cytokines can cross the blood–brain barrier in patients with schizophrenia after damage but also bind to specific transporters, penetrate via afferent vagal fibers, and get access through circumventricular organs [27,28]. In addition, it is reported that microglia, astrocytes, neurons, and endothelial cells can produce cytokines [28,29,30]. According to meta-analyses, there is an increase in levels of proinflammatory cytokines in schizophrenia [31,32]. Nevertheless, data on specific cytokines vary. For example, Wei et al. [33] have found no significant changes in the levels of IL-6 in schizophrenia patients; low TNF-α levels in schizophrenia were found to correlate with negative symptoms and in chronic schizophrenia with long-term use of antipsychotics [34,35]. Furthermore, there is no significant increase in IL-1β levels in chronic patients [36], and there are diminished IL-1β and TNF-α levels in first-episode schizophrenia with a disease duration of less than 2 years [37]. Results about T-helper and T-regulatory cytokines are even more inconsistent [32,38]. The discrepancies in the cytokine profile among the studies may be due to various factors: differences in patient characteristics owing to the high heterogeneity of schizophrenia, small sample sizes, differences in the tested parameters between men and women, and not taking into consideration metabolic disorders in the study population. Therefore, there is a need for new research taking into account the possible relationship of clinical features of schizophrenia with serum levels of cytokines.
Previously, we have demonstrated the effect of atypical antipsychotics on peripheral cytokine levels as well as the role of cytokines in the formation of metabolic syndrome in schizophrenia [39,40]. We have also examined the influence of changes in glutamatergic neurotransmission on the onset and course of schizophrenia [41]. Via the promotion of oxidative stress, there is considerable commonality between glutamatergic excitotoxicity and inflammatory damage [5,6,8]. It is, therefore, a logical step to also investigate the relationship between cytokine levels and clinical characteristics of schizophrenia. We hypothesized that patients with schizophrenia have an imbalance between pro- and anti-inflammatory cytokines, and we tried to detect possible correlations between clinical characteristics and the presumed cytokine imbalance.
2. Materials and Methods
2.1. Participants
After signing a voluntary informed consent, 236 patients with schizophrenia (F20 according to the International Classification of Diseases-10 [ICD-10], predominantly with paranoid schizophrenia [F20.0]) were enrolled in the study. The control group consisted of 103 mentally and somatically healthy individuals who provided written informed consent. The control group comes from the same geographical area as the patients with schizophrenia and included mainly students and employees of the Siberian State Medical University (Tomsk, Russia) and Tomsk National Research Medical Center. The age range of the patients and healthy individuals eligible for the study was 18–60 years. Patients with schizophrenia and healthy individuals who had signs of acute or chronic infectious, inflammatory, or autoimmune diseases at the time of enrollment in the study were not allowed to participate in the study. The study did not include persons with comorbid neurological and somatic diseases, which make it difficult to objectively assess the clinical condition and persons who used psychoactive substances (other than antipsychotic medication).
The severity of schizophrenia symptoms was determined using the Positive and Negative Syndrome Scale (PANSS) [42]. This scale consists of a subscale of 7 items assessing the severity of positive symptoms of schizophrenia, a negative subscale of 7 items evaluating symptoms caused by loss of skill, and a subscale of 16 items about general psychiatric symptoms, such as anxiety, tension, and disorientation.
The clinical course of schizophrenia was determined in accordance with ICD-10 criteria (F20.x1–3 for an episodic course and F20.x0 for a continuous course). Mean doses of antipsychotic medication used were converted to chlorpromazine equivalents [43].
2.2. Laboratory Tests
Blood samples were drawn after a 12-h overnight fast on the first days of hospitalization and centrifuged for 30 min at 2000× g and 4 °C to isolate serum; the serum was stored at −80 °C until analysis. The concentrations of 22 cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-5, IL-6, IL-7, IL-8, IL-9, IL-12p40, IL-12p70, IL-15, IL-17A, IFN-α2, IFN-γ, TNF-α, TNF-β, IL-1RA, IL-4, IL-10, IL-13, and TGF-α) in blood serum were determined using the HCYTMAG-60K-PX41 panel of MILLIPLEX® MAP (Merck, Darmstadt, Germany) on multiplex analyzer MAGPIX and Luminex 200 (Luminex, Austin, TX, USA) at the Medical Genomics core facility of Tomsk National Research Medical Center. The obtained data were processed in special software, xPONENT (Luminex, Austin, TX, USA), and the output was exported to the MILLIPLEX Analyst 5.1 software (Merck, Darmstadt, Germany). The final results on cytokine concentrations are presented in pg/mL.
2.3. Statistics
Statistical analysis was performed in SPSS software (version 20) for Windows. We used several methods of group comparisons, such as the Shapiro–Wilk test, the Mann–Whitney U test, the Kruskal–Wallis test, and the χ2 test. For multiple comparisons, the Bonferroni correction was applied. Correlation analysis was performed by means of Spearman’s criteria. Data with p-values of less than 0.05 were considered statistically significant.
3. Results
3.1. The Main Demographic and Clinical Characteristics of Participants
The study included 236 patients with schizophrenia and 103 healthy individuals. There were 121 (51.3%) females among patients with schizophrenia and 51 (53.4%) among the healthy subjects (p = 0.405). The median age (interquartile range) of patients with schizophrenia and healthy individuals was 36 (30; 46) and 35 (27.5; 46.5) years, respectively (p = 0.446). The median age of females with schizophrenia and healthy females was 36 (30; 44) and 42 (31; 52) years, respectively (p = 0.072), and the median age of males with schizophrenia and healthy males was 34 (29.5; 41) and 32 (26.5; 38.5) years, respectively (p = 0.065). The main demographic and clinical characteristics of the patients with schizophrenia are shown in Table 1.
Table 1.
Basic demographic and clinical characteristics of patients with schizophrenia included in the study.
| Parameter | Total (n = 236) |
Females (n = 121) |
Males (n = 115) |
p-Value | |
|---|---|---|---|---|---|
| Age, years | 36 (30; 46) | 36 (30; 44) | 34 (29.5; 41) | 0.380 | |
| Age at disease manifestation, years | 23 (19; 30) | 24.5 (20; 31) | 23 (19; 27) | 0.115 | |
| Duration of disease, years | 12 (5; 19) | 11 (5; 18) | 12 (5; 17) | 0.956 | |
| Clinical course, n (%) | Episodic course | 87 (49.7%) | 52 (55.3%) | 35 (43.2%) | 0.179 |
| Continuous course | 71 (40.6%) | 32 (34.0%) | 39 (48.1%) | ||
| PANSS, positive score | 21 (17; 25) | 21 (17; 26) | 21 (17; 25) | 0.714 | |
| PANSS, negative score | 25 (21; 28) | 24 (21; 28) | 26 (22; 28) | 0.045 | |
| PANSS, general score | 53 (44.5; 57.5) | 53 (47; 58) | 53 (44; 57) | 0.379 | |
| PANSS, total score | 100 (87.5; 109) | 100 (87; 109) | 100 (91; 109) | 0.742 | |
| Duration of antipsychotic therapy, years | 7 (3; 16) | 7 (3; 15) | 7 (3; 15.5) | 0.744 | |
| Total CPZeq | 350 (200; 623) | 342.4 (200; 675) | 387.54 (200; 600) | 0.940 | |
Note: Data are presented as a median (lower quartile; upper quartile); PANSS: Positive and Negative Syndrome Scale; CPZeq: chlorpromazine equivalents. Comparisons between groups were performed by the χ2 test for the clinical course and by the Mann–Whitney U test for the other parameters.
3.2. Serum Concentrations of Cytokines in Patients with Schizophrenia
We found higher TGF-α (p = 0.014), IFN-γ (p = 0.036), IL-5 (p < 0.001), IL-6 (p = 0.047), IL-8 (p = 0.005), IL-10 (p < 0.001), IL-15 (p = 0.007), IL-1RA (p = 0.007), and TNF-α (p < 0.001) levels in patients with schizophrenia compared with healthy individuals (Table 2).
Table 2.
Cytokine levels in the serum of patients with schizophrenia and healthy individuals.
| Parameter | Patients with Schizophrenia | Healthy Individuals | p-Value |
|---|---|---|---|
| Proinflammatory cytokines | |||
| IL-1α | 70.83 (52.61; 105.10) | 61.84 (53.98; 111.89) | 0.660 |
| IL-1β | 3.25 (2.33; 4.48) | 2.85 (1.86; 4.84) | 0.110 |
| IL-2 | 6.11 (4.68; 7.12) | 5.90 (4.83; 8.10) | 0.406 |
| IL-3 | 2.55 (1.31; 3.32) | 3.05 (1.38; 3.70) | 0.116 |
| IL-5 | 3.39 (1.98; 4.40) | 2.04 (1.34; 3.86) | 0.001 * |
| IL-6 | 10.55 (5.28; 15.48) | 6.55 (4.60; 14.68) | 0.047 * |
| IL-7 | 13.09 (9.72; 29.14) | 14.14 (9.72 (29.55) | 0.632 |
| IL-8 | 12.87 (9.26; 20.03) | 10.58 (7.19; 18.53) | 0.005 * |
| IL-9 | 4.87 (3.32; 13.16) | 4.36 (2.81; 14.45) | 0.637 |
| IL-12p40 | 45.83 (38.90; 52.70) | 44.52 (32.37; 56.46) | 0.233 |
| IL-12p70 | 9.62 (6.74; 24.17) | 8.32 (6.67; 22.12) | 0.371 |
| IL-15 | 9.05 (6.26; 11.71) | 7.23 (4.57; 11.42) | 0.007 * |
| IL-17A | 6.58 (4.33; 14.85) | 5.60 (3.74; 14.47) | 0.152 |
| IFN-α2 | 34.00 (17.32; 90.62) | 30.57 (17.50; 87.69) | 0.871 |
| IFN-γ | 14.80 (10.12; 23.01) | 11.76 (8.44; 21.56) | 0.036 * |
| TNF-α | 22.87 (16.94; 28.93) | 18.01 (9.76; 23.40) | <0.001 * |
| TNF-β | 20.87 (6.01; 30.15) | 7.80 (5.01; 33.81) | 0.114 |
| Anti-inflammatory cytokines | |||
| IL-1RA | 47.08 (38.55; 63.58) | 44.79 (33.22; 52.85) | 0.007 * |
| IL-4 | 111.61 (75.13; 159.29) | 92.78 (71.53; 160.49) | 0.351 |
| IL-10 | 12.90 (8.00; 25.67) | 7.93 (5.84; 20.56) | <0.001 * |
| IL-13 | 18.52 (13.18; 24.02) | 17.47 (12.18; 27.70) | 0.717 |
| TGF-α | 5.06 (4.00; 7.06) | 4.61 (2.86; 7.20) | 0.014 * |
Note: Data are presented as a median (lower quartile; upper quartile); comparisons between groups were performed using the Mann–Whitney U test; *: statistically significant differences.
Subgroup analysis revealed a much greater number of statistically significant differences in cytokine levels among females than among males. For instance, in females with schizophrenia, there were statistically significantly greater concentrations of TGF-α (p = 0.006), IFN-γ (p = 0.005), IL-1β (p = 0.038), IL-10 (p < 0.001), IL-12p40 (p = 0.047), IL-15 (p = 0.001), IL-17A (p = 0.037), IL-1RA (p = 0.023), IL-5 (p = 0.006), IL-6 (p = 0.025), IL-8 (p = 0.004), and TNF-α (p < 0.001) and lower IL-3 levels (p = 0.046) compared with healthy age-matched females. Males with schizophrenia had statistically significantly higher levels of IL-10 (p = 0.017), IL-5 (p = 0.048), and TNF-α (p = 0.025) than did healthy age-matched males (Table 3).
Table 3.
Cytokine levels in the serum of patients with schizophrenia and healthy individuals depending on sex.
| Parameter | Females | Males | ||||
|---|---|---|---|---|---|---|
| Patients with Schizophrenia | Healthy Individuals | p-Value | Patients with Schizophrenia | Healthy Individuals | p-Value | |
| Proinflammatory cytokines | ||||||
| IL-1α | 81.16 (54.66; 106.80) | 59.21 (50.20; 94.87) | 0.102 | 63.68 (52.36; 105.10) | 64.84 (57.60; 111.89) | 0.193 |
| IL-1β | 3.68 (2.39; 4.57) | 2.71 (1.58; 4.22) | 0.038 * | 3.10 (2.33; 4.48) | 2.85 (2.41; 4.85) | 0.913 |
| IL-2 | 6.21 (4.91; 7.12) | 5.90 (4.82; 7.95) | 0.907 | 5.63 (4.53; 7.35) | 6.03 (4.85; 8.36) | 0.230 |
| IL-3 | 2.17 (1.23; 3.32) | 3.20 (1.74; 3.69) | 0.046 * | 2.81 (1.32; 3.34) | 2.91 (1.32; 3.70) | 0.886 |
| IL-5 | 3.79 (2.07; 4.53) | 2.05 (1.27; 4.18) | 0.006 * | 3.08 (1.89; 4.27) | 2.03 (1.38; 3.72) | 0.048 * |
| IL-6 | 10.87 (6.23; 15.78) | 6.55 (4.45; 14.93) | 0.025 * | 8.26 (4.99; 15.18) | 6.31 (4.74; 13.58) | 0.590 |
| IL-7 | 15.84 (10.66; 29.91) | 12.00 (9.69 (27.55) | 0.245 | 11.35 (9.39; 28.35) | 16.34 (10.64 (31.42) | 0.070 |
| IL-8 | 12.92 (9.44; 21.21) | 8.88 (6.34; 18.53) | 0.004 * | 12.31 (8.99; 19.31) | 11.48 (8.24; 18.48) | 0.313 |
| IL-9 | 7.77 (3.52; 13.16) | 4.23 (2.54; 14.45) | 0.328 | 4.61 (3.04; 12.30) | 4.43 (2.81; 14.45) | 0.717 |
| IL-12p40 | 45.73 (38.90; 52.70) | 42.10 (28.55; 49.27) | 0.047 * | 45.83 (38.90; 53.29) | 46.29 (37.23; 57.83) | 0.786 |
| IL-12p70 | 15.68 (6.88; 24.63) | 7.99 (6.15; 20.93) | 0.099 | 8.42 (6.71; 23.71) | 8.70 (7.12; 22.79) | 0.645 |
| IL-15 | 9.34 (6.26; 12.01) | 6.44 (3.72; 10.24) | 0.001 * | 8.75 (6.26; 11.27) | 8.07 (6.12; 12.30) | 0.820 |
| IL-17A | 10.72 (4.29; 14.85) | 4.61 (3.25; 15.96) | 0.037 * | 5.86 (4.39; 14.66) | 7.73 (4.61; 12.98) | 0.712 |
| IFN-α2 | 43.38 (18.59; 90.62) | 26.67 (15.99; 82.42) | 0.140 | 28.89 (16.01; 86.21) | 36.33 (23.90; 93.53) | 0.084 |
| IFN-γ | 17.14 (10.00; 23.01) | 9.65 (7.65; 25.88) | 0.005 * | 13.08 (10.12; 23.37) | 13.00 (10.31; 21.04) | 0.925 |
| TNF-α | 22.42 (16.57; 28.66) | 15.27 (8.41; 21.29) | <0.001 * | 23.02 (17.87; 28.93) | 20.26 (12.51; 25.09) | 0.025 * |
| TNF-β | 23.20 (6.44; 30.15) | 7.22 (3.98; 33.82) | 0.090 | 11.92 (6.00; 29.23) | 7.94 (6.39; 30.60) | 0.879 |
| Anti-inflammatory cytokines | ||||||
| IL-1RA | 50.23 (41.23; 67.74) | 43.68 (32.26; 59.45) | 0.023 * | 44.96 (38.13; 57.94) | 43.90 (36.21; 51.25) | 0.176 |
| IL-4 | 130.1 (81.48; 165.30) | 90.57 (65.82; 145.09) | 0.095 | 92.78 (72.14; 152.06) | 95.09 (73.42; 171.82) | 0.671 |
| IL-10 | 18.94 (8.00; 26.41) | 7.43 (5.58; 16.50) | <0.001 * | 11.95 (7.77; 24.56) | 9.04 (6.27; 19.30) | 0.017 * |
| IL-13 | 19.47 (13.75; 24.02) | 16.01 (10.14; 28.06) | 0.122 | 16.35 (12.89; 24.02) | 19.64 (12.97; 26.97) | 0.270 |
| TGF-α | 5.15 (4.09; 7.16) | 4.03 (2.51; 6.14) | 0.006 * | 4.96 (3.77; 7.06) | 4.70 (3.01; 7.93) | 0.456 |
Note: Data are presented as a median (lower quartile; upper quartile); groups were compared by the Mann–Whitney U test; *: statistically significant differences.
3.3. Serum Concentrations of Cytokines and Clinical Characteristics of Schizophrenia
Patients with a continuous course of schizophrenia showed statistically significantly greater levels of IL-12p70 (p = 0.019), IL-1α (p = 0.046), and IL-1β (p = 0.035) compared to patients with an episodic course (Table 4).
Table 4.
Cytokine levels in the serum of patients with schizophrenia depending on the type of clinical course.
| Parameter | Episodic Course (n = 87) |
Continuous Course (n = 71) |
p-Value |
|---|---|---|---|
| Proinflammatory cytokines | |||
| IL-1α | 74.27 (52.48; 105.1) | 94.87 (56.95; 118.65) | 0.046 * |
| IL-1β | 3.69 (2.31; 4.48) | 4.13 (2.94; 5.00) | 0.035 * |
| IL-2 | 6.21 (5.01; 7.12) | 6.67 (5.07; 7.57) | 0.262 |
| IL-3 | 2.1 (1.2; 3.34) | 1.56 (1.26; 3.32) | 0.780 |
| IL-5 | 3.59 (2.02; 4.27) | 4.13 (2.24; 4.67) | 0.084 |
| IL-6 | 11.18 (5.75; 17.27) | 12.12 (6.87; 15.78) | 0.788 |
| IL-7 | 14.43 (9.97; 29.91) | 26.73 (10.33; 32.18) | 0.184 |
| IL-8 | 12.84 (9.41; 21.21) | 12.04 (8.26; 20.21) | 0.544 |
| IL-9 | 8 (3.5; 13.6) | 11.86 (3.84; 13.6) | 0.165 |
| IL-12p40 | 45.83 (37.21;52.7) | 47.55 (42.36; 54.42) | 0.156 |
| IL-12p70 | 16.24 (6.15; 23.71) | 21.86 (7.49; 26.47) | 0.019 * |
| IL-15 | 9.34 (6.26; 11.71) | 10.83 (7.49; 12.3) | 0.092 |
| IL-17A | 8.74 (4.29; 13.73) | 12.23 (4.61; 15.59) | 0.083 |
| IFN-α2 | 52.11 (17.59; 90.62) | 83.26 (23.65; 96.42) | 0.175 |
| IFN-γ | 17.21 (9.97; 23.01) | 19.39 (11.84; 23.73) | 0.106 |
| TNF-α | 23.85 (17.98; 31.16) | 25.20 (20.67; 29.63) | 0.656 |
| TNF-β | 23.67 (6.00; 30.15) | 25.53 (6.90; 35.62) | 0.291 |
| Anti-inflammatory cytokines | |||
| IL-1RA | 49.19 (38.55; 69.55) | 46.02 (38.13; 60.68) | 0.286 |
| IL-4 | 122.08 (77.86; 160.49) | 136.34 (81.48; 170.1) | 0.189 |
| IL-10 | 14.04 (7.85; 26.41) | 23.44 (9.05; 27.89) | 0.087 |
| IL-13 | 19.47 (13.52; 24.02) | 20.24 (13.75; 24.02) | 0.479 |
| TGF-α | 4.67 (3.7; 6.94) | 4.42 (3.44; 5.88) | 0.181 |
Note: Data are presented as a median (lower quartile; upper quartile); groups were compared by the Mann–Whitney U test; *: statistically significant differences.
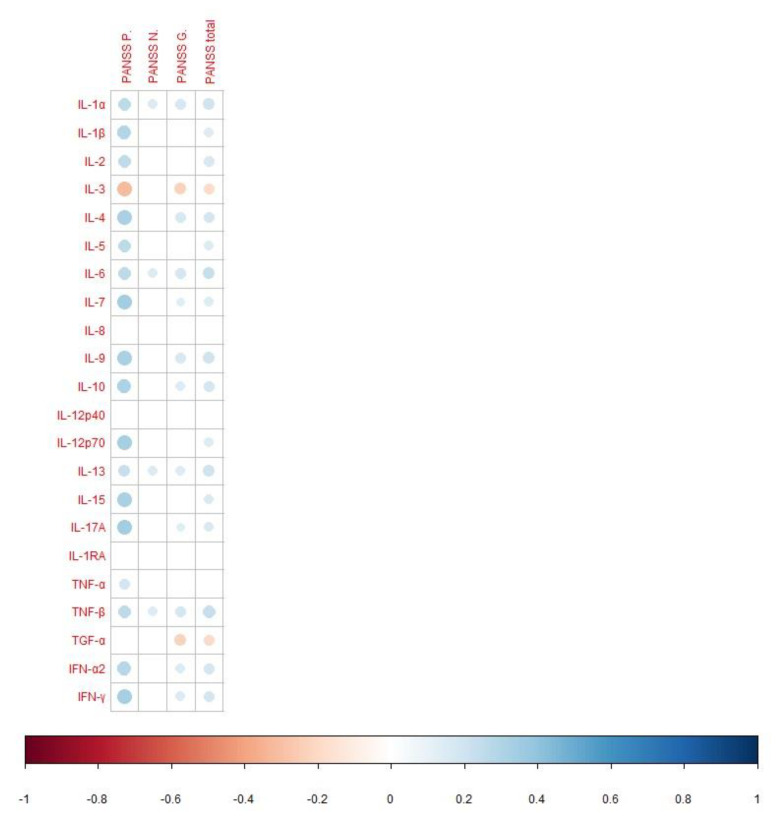
According to the correlation analysis, most cytokines are positively correlated with positive, general, and total PANSS scores. Only IL-8, IL-12p40, and IL-1RA did not statistically significantly correlate with the severity of schizophrenia, according to PANSS (Figure 1).
Figure 1.
Spearman’s correlation analysis of the cytokines and disease severity (according to PANSS) in patients with schizophrenia. PANSS P.: the positive symptom score from PANSS; PANSS N.: the negative symptom score from PANSS; PANSS G.: the general psychopathology score from PANSS; PANSS total: the total PANSS score. In this figure, the red and blue circles refer to significant negative and positive correlations, respectively. The size and color intensity of the circles is proportional to the correlation coefficient. In the legend at the bottom, the intensity of the color shows the coefficients of correlation and their sign (inverse or direct correlation).
In patients with a duration of schizophrenia of 10 years or more, the level of IL-10 was higher than that in patients with a disease duration of 5 years or less (p = 0.042; Table S1). There were no statistically significant differences in the levels of cytokines between groups of the age of schizophrenia onset (Table S2).
4. Discussion
In this work, we found significantly higher serum levels of proinflammatory (IFN-γ, IL-5, IL-6, IL-8, IL-15, and TNF-α) and anti-inflammatory (TGF-α, IL-10, and IL-1RA) cytokines in patients with schizophrenia than in healthy individuals. Overall, the findings are consistent with other studies and meta-analyses about serum cytokines in schizophrenia [31,32]. A considerable number of studies, including meta-analyses, have revealed elevated levels of IL-6 and TNF-α in various groups of patients with schizophrenia [32]. Some of the best-known proinflammatory cytokines belong to the IL-1 family. We found no difference in IL-1α and IL-1β levels between the schizophrenia group and control group. According to Momtazmanesh et al. [32], the literature contains mixed data on blood levels of these cytokines in schizophrenia. IL-1RA competes with IL-1α and IL-1β for binding to the IL-1 receptor and competitively inhibits IL-1 activity [44]. Our findings about elevated serum levels of IL-1RA in patients with schizophrenia are consistent with those of Zhou et al., although they have not detected changes in concentrations of any other cytokines [45]. The increased IL-8 serum concentration in our study is in agreement with the results of a meta-analysis indicating an elevated concentration of this cytokine in chronic schizophrenia [16]. On the other hand, in patients with the first episode of schizophrenia, the level of IL-8 is comparable to the control [46]. Alterations in IFN-γ levels are controversial: several research groups have found no significant alterations of IFN-γ and IL-10 levels, whereas other studies show either increased or decreased levels of IFN-γ in patients with schizophrenia [32]. Elevated levels of IL-5 in the serum of patients with schizophrenia have been demonstrated [47], in line with our results.
Summarizing the above, we can conclude that there is considerable heterogeneity of data regarding blood levels of cytokines in patients with schizophrenia. As mentioned in the Introduction, these discrepancies may be due to the dissimilarity of clinical characteristics among studies, the use of antipsychotic medication, and/or insufficient sample sizes.
In terms of immune-system activation and neuroinflammation, which can influence symptoms and severity of the disease, schizophrenia may be similar to neurological and psychiatric diseases that also feature an imbalance in peripheral cytokines [15,48,49,50]. It is worth underscoring our finding that the aberration of the cytokine profile in schizophrenia is more pronounced in females than in males. In the general population, significant effects of sex and phase of the menstrual cycle on plasma concentration of cytokines have been demonstrated [51]. Some research on patients with schizophrenia indicates statistically significantly greater IL-1β, IL-8, IL-17, IL-23, and TNF-α levels in women but not in men, compared with healthy individuals [52]. Frydecka et al. [53] have shown that the TGFB1 +869T/C gene polymorphism is associated with schizophrenia, especially among females; no differences were found in the severity of schizophrenia symptoms registered by PANSS between males and females with schizophrenia, suggesting that sex is a potentially important factor for group differences in cytokine levels. This phenomenon is probably related to the effects of sex hormones. According to in vivo studies, sex hormones can modulate cytokine production and contribute to sex-related differences in immune responses to health and disease [54,55]. This observation may serve as a theoretical basis for anti-inflammatory treatment of women with schizophrenia.
In our study, patients with a continuous course of schizophrenia had elevated levels of proinflammatory cytokines (IL-12p70, IL-1α, and IL-1β) as compared with patients with an episodic course. In a clinical sense, proinflammatory cytokines—as some of the pathogenesis components—play an important role in clinical polymorphism and the course of schizophrenia through reactivity mechanisms. Malashenkova et al. [56] have reported that episodic schizophrenia and continuous schizophrenia have signs of systemic inflammation. At the same time, the continuous course of schizophrenia is characterized mainly by chronic activation of the humoral response, whereas the episodic one by some activation of cell-mediated immunity. In another work, serum levels of soluble TNF-α receptor 1 were found to be higher in patients with a severe clinical course of schizophrenia [57]. Our study was conducted on a group of patients with a long duration of the illness, which can be considered a limitation of the study on the one hand. On the other hand, this work allows us to use the obtained results as predictors of schizophrenia course along with other determinants [58] because after 5 years of illness, the type of schizophrenia course is already evident [59].
Most of the assayed cytokines were positively correlated with positive, general, or total PANSS scores, thereby reflecting a direct link between immunoinflammation and schizophrenia severity. The fewest relations were observed between cytokine levels and negative symptoms of schizophrenia: only IL-1, IL-6, IL-13, and TNF-β were positively correlated with the negative-symptom PANSS score. Our data are partly consistent with the review by Momtazmanesh et al. [32], according to which there is a correlation between negative symptoms with elevated levels of IL-6, TNF-α, IL-1β, IL-8, IFN-γ, IL-4, and TGF-β. In addition, higher levels of IL-6, IL-1β, IL-33, and IL-17 are associated [32] with more severe positive symptoms. Increased levels of IL-6, IL-33, sIL-2R, IL-17, and TGF-β positively correlate with the general psychopathology subscore of PANSS [32]. The total PANSS score is positively associated with the levels of IL-6, sIL-2R, IL-1β, IFN-γ, IL-13, TGF-β1, and IL-17 [32]. Our findings are also partly consistent with a study that has uncovered correlations between proinflammatory cytokines and positive, general, and total PANSS scores but detected no correlation between cytokine levels and the negative-symptom PANSS score [60]. Our results are in agreement with articles showing correlations between IL-6 and PANSS scores among individuals with schizophrenia, with at-risk mental states, or with another psychotic disorder [61,62]. The link between the upregulation of proinflammatory cytokine IL-10 and ≥10-year duration of schizophrenia may be explained by the activation of compensatory mechanisms. Pedrini et al. [63] have also demonstrated elevated IL-10 levels in patients at ≥10 years after a diagnosis of schizophrenia as compared with healthy individuals. According to a meta-analysis [16], blood levels of IL-6 are significantly positively correlated with disease duration in three studies, although this result is not confirmed by another study. We, too, found no relation between IL-6 levels and schizophrenia duration.
The limitations of our study are as follows: the patients had long-duration schizophrenia, as mentioned above. Therefore, we cannot rule out the long-term impact of antipsychotic medication on the tested cytokines. Evaluation of the long-term effect of antipsychotic medication on the studied parameters was also not possible because the patients had been admitted to the hospital with severe clinical symptoms. Some of the patients were on maintenance treatment. We could glean information about the use of antipsychotics only from the patients’ own words and could not adequately control compliance and drug doses. We have previously evaluated the influence of short-term antipsychotic medication on serum cytokine levels [39].
When assessing the influence of sex-related differences on the levels of cytokines, we did not specify the menstrual cycle, which also plays an important part in the regulation of these parameters. Nonetheless, many women with schizophrenia experience menstrual irregularities, at least partly as an adverse effect of antipsychotic drugs.
From the findings, we can conclude that schizophrenia is characterized by a cytokine imbalance, and manifestations of this imbalance differ depending on sex, the severity of clinical symptoms, and disease duration. Research has shown that adjunctive treatment with acetylsalicylic acid may alleviate schizophrenia symptoms and reduce mortality from cardiovascular disease, which is common in schizophrenia [14]. Our study indirectly confirms the reasons for the heterogeneity of the clinical response to anti-inflammatory therapy in patients with schizophrenia.
5. Conclusions
In patients with schizophrenia, we found a cytokine imbalance that correlates with sex and clinical characteristics of the disease. Although schizophrenia involves changes within the immune system, it is difficult to get a grip on the biological mechanisms involved. A deeper understanding of the disturbances of the cytokine network in schizophrenia may facilitate early diagnosis, prognosis, and personalized treatment.
Acknowledgments
The authors are grateful to Anton Loonen for a discussion of the results. The authors thank Nikolai A. Shevchuk for proofreading and comments.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/life12121972/s1, Table S1: Cytokine levels in the serum of patients with schizophrenia depending on disease duration; Table S2: Cytokine levels in the serum of patients with schizophrenia depending on the onset of disease.
Author Contributions
Conceptualization, S.A.I.; methodology, A.S.B.; validation, E.G.K.; formal analysis, I.A.M.; investigation, A.S.B., I.A.M. and E.G.K.; resources, A.V.S. and S.A.I.; data curation, A.S.B. and E.G.K.; writing—original draft preparation, I.A.M.; writing—review and editing, A.S.B., E.G.K. and S.A.I.; visualization, I.A.M.; supervision, A.V.S., N.A.B. and S.A.I.; project administration, S.A.I.; funding acquisition, S.A.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethical Committee at the Mental Health Research Institute of Tomsk National Research Medical Center, the Russian Academy of Sciences (Protocol # 187, approval on 24 April 2018).
Informed Consent Statement
Informed consent was obtained from all study subjects.
Data Availability Statement
The datasets are available on reasonable request to Svetlana A. Ivanova (ivanovaniipz@gmail.com), after approval from the Board of Directors of the Mental Health Research Institute, in accordance with local guidelines and regulations.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Russian Science Foundation, project No. 22-25-00633.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsuang M. Schizophrenia: Genes and environment. Biol. Psychiatry. 2000;47:210–220. doi: 10.1016/S0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- 2.Stahl S.M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: Dopamine, serotonin, and glutamate. CNS Spectr. 2018;23:187–191. doi: 10.1017/S1092852918001013. [DOI] [PubMed] [Google Scholar]
- 3.Smith R.S., Maes M. The macrophage-T-lymphocyte theory of schizophrenia: Additional evidence. Med. Hypotheses. 1995;45:135–141. doi: 10.1016/0306-9877(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 4.Monji A., Kato T., Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 5.Ermakov E.A., Melamud M.M., Buneva V.N., Ivanova S.A. Immune System Abnormalities in Schizophrenia: An Integrative View and Translational Perspectives. Front. Psychiatry. 2022;13:880568. doi: 10.3389/fpsyt.2022.880568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigenson K.A., Kusnecov A.W., Silverstein S.M. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci. Biobehav. Rev. 2014;38:72–93. doi: 10.1016/j.neubiorev.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Bueno B., Bioque M., Mac-Dowell K.S., Barcones M.F., Martínez-Cengotitabengoa M., Pina-Camacho L., Leza J.C. Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: Toward an integrative inflammatory hypothesis of schizophrenia. Schizophr. Bull. 2014;40:376–387. doi: 10.1093/schbul/sbt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howes O.D., McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: A reconceptualization. Transl. Psychiatry. 2017;7:e1024. doi: 10.1038/tp.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan M.K., Guest P.C., Levin Y., Umrania Y., Schwarz E., Bahn S., Rahmoune H. Converging evidence of blood-based biomarkers for schizophrenia: An update. Int. Rev. Neurobiol. 2011;101:95–144. doi: 10.1016/B978-0-12-387718-5.00005-5. [DOI] [PubMed] [Google Scholar]
- 11.Na K.S., Jung H.Y., Kim Y.K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:277–286. doi: 10.1016/j.pnpbp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Cho M., Lee T.Y., Kwak Y.B., Yoon Y.B., Kim M., Kwon J.S. Adjunctive use of anti-inflammatory drugs for schizophrenia: A meta-analytic investigation of randomized controlled trials. Aust. N. Z. J. Psychiatry. 2019;53:742–759. doi: 10.1177/0004867419835028. [DOI] [PubMed] [Google Scholar]
- 13.Nitta M., Kishimoto T., Müller N., Weiser M., Davidson M., Kane J.M., Correll C.U. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: A meta-analytic investigation of randomized controlled trials. Schizophr. Bull. 2013;39:1230–1241. doi: 10.1093/schbul/sbt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommer I.E., de Witte L., Begemann M. Nonsteroidal anti-inflammatory drugs in schizophrenia: Ready for practice or a good start? A meta-analysis. J. Clin. Psychiatry. 2012;73:414–419. doi: 10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- 15.Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morozova A., Zorkina Y., Pavlov K., Pavlova O., Abramova O., Ushakova V., Mudrak A.V., Zozulya S., Otman I., Sarmanova Z., et al. Associations of Genetic Polymorphisms and Neuroimmune Markers with Some Parameters of Frontal Lobe Dysfunction in Schizophrenia. Front. Psychiatry. 2021;12:655178. doi: 10.3389/fpsyt.2021.655178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upthegrove R., Manzanares-Teson N., Barnes N.M. Cytokine function in medication-naive first episode psychosis: A systematic review and meta-analysis. Schizophr. Res. 2014;155:101–108. doi: 10.1016/j.schres.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Gober R., Ardalan M., Shiadeh S.M.J., Duque L., Garamszegi S.P., Ascona M., Vontell R.T. Microglia activation in postmortem brains with schizophrenia demonstrates distinct morphological changes between brain regions. Brain Pathol. 2022;32:e13003. doi: 10.1111/bpa.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juckel G., Manitz M.P., Brüne M., Friebe A., Heneka M.T., Wolf R.J. Microglial activation in a neuroinflammational animal model of schizophrenia—A pilot study. Schizophr. Res. 2011;131:96–100. doi: 10.1016/j.schres.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Van Berckel B.N., Bossong M.G., Boellaard R., Kloet R., Schuitemaker A., Caspers E., Kahn R.S. Microglia activation in recent-onset schizophrenia: A quantitative (R)-[11C] PK11195 positron emission tomography study. Biol. Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Pearlman D.M., Najjar S. Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr. Res. 2014;157:249–258. doi: 10.1016/j.schres.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Whelan R., St Clair D., Mustard C.J., Hallford P., Wei J. Study of novel autoantibodies in schizophrenia. Schizophr. Bull. 2018;44:1341–1349. doi: 10.1093/schbul/sbx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang Y., Kim J., Shin J.Y., Kim J.I., Seo J.S., Webster M.J., Kim S. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl. Psychiatry. 2013;3:e321. doi: 10.1038/tp.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saetre P., Emilsson L., Axelsson E., Kreuger J., Lindholm E., Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drexhage R.C., Hoogenboezem T.A., Cohen D., Versnel M.A., Nolen W.A., van Beveren N.J., Drexhage H.A. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro-and anti-inflammatory forces. Int. J. Neuropsychopharmacol. 2011;14:746–755. doi: 10.1017/S1461145710001653. [DOI] [PubMed] [Google Scholar]
- 27.Miller A.H., Haroon E., Raison C.L., Felger J.C. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altamura A.C., Buoli M., Pozzoli S. Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: Comparison with schizophrenia. Psychiatry Clin. Neurosci. 2014;68:21–36. doi: 10.1111/pcn.12089. [DOI] [PubMed] [Google Scholar]
- 29.Galic M.A., Riazi K., Pittman Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loonen A.J.M., Ivanova S.A. Circuits regulating pleasure and happiness—Focus on potential biomarkers for circuitry including the habenuloid complex. Acta Neuropsychiatr. 2022:1–11. doi: 10.1017/neu.2022.15. [DOI] [PubMed] [Google Scholar]
- 31.Dawidowski B., Górniak A., Podwalski P., Lebiecka Z., Misiak B., Samochowiec J. The role of cytokines in the pathogenesis of schizophrenia. J. Clin. Med. 2021;10:3849. doi: 10.3390/jcm10173849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Momtazmanesh S., Zare-Shahabadi A., Rezaei N. Cytokine alterations in schizophrenia: An updated review. Front. Psychiatry. 2019;10:892. doi: 10.3389/fpsyt.2019.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei L., Du Y., Wu W., Fu X., Xia Q. Elevation of plasma neutrophil gelatinase-associated lipocalin (NGAL) levels in schizophrenia patients. J. Affect. Disord. 2018;226:307–312. doi: 10.1016/j.jad.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Lv M.H., Tan Y.L., Yan S.X., Tian L., Tan S.P., Wang Z.R. Decreased serum TNF-A-alpha levels in chronic schizophrenia patients on long-term antipsychotics: Correlation with psychopathology and cognition. Psychopharmacology. 2015;232:165–172. doi: 10.1007/s00213-014-3650-y. [DOI] [PubMed] [Google Scholar]
- 35.Turhan L., Batmaz S., Kocbiyik S., Soygur A.H. The role of tumour necrosis factor alpha and soluble tumour necrosis factor alpha receptors in the symptomatology of schizophrenia. Nord. J. Psychiatry. 2016;70:342–350. doi: 10.3109/08039488.2015.1122079. [DOI] [PubMed] [Google Scholar]
- 36.Balõtšev R., Koido K., Vasar V., Janno S., Kriisa K., Mahlapuu R. Inflammatory, cardio-metabolic and diabetic profiling of chronic schizophrenia. Eur. Psychiatry. 2017;39:1–10. doi: 10.1016/j.eurpsy.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Zhu F., Zhang L., Liu F., Wu R., Guo W., Ou J., Zhao J. Altered serum tumor necrosis factor and interleukin-1ß in first-episode drug-naive and chronic schizophrenia. Front. Neurosci. 2018;12:296. doi: 10.3389/fnins.2018.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim Y.K., Myint A.M., Lee B.H., Han C.S., Lee H.J., Kim D.J., Leonard B.E. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:1129–1134. doi: 10.1016/j.pnpbp.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Boiko A.S., Mednova I.A., Kornetova E.G., Gerasimova V.I., Kornetov A.N., Loonen A.J.M., Bokhan N.A., Ivanova S.A. Cytokine Level Changes in Schizophrenia Patients with and without Metabolic Syndrome Treated with Atypical Antipsychotics. Pharmaceuticals. 2021;14:446. doi: 10.3390/ph14050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mednova I.A., Boiko A.S., Kornetova E.G., Parshukova D.A., Semke A.V., Bokhan N.A., Loonen A.J.M., Ivanova S.A. Adipocytokines and Metabolic Syndrome in Patients with Schizophrenia. Metabolites. 2020;10:410. doi: 10.3390/metabo10100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poltavskaya E.G., Fedorenko O.Y., Kornetova E.G., Loonen A.J.M., Kornetov A.N., Bokhan N.A., Ivanova S.A. Study of Early Onset Schizophrenia: Associations of GRIN2A and GRIN2B Polymorphisms. Life. 2021;11:997. doi: 10.3390/life11100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kay S.R., Opler L.A., Fiszbein A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 43.Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D., Zhang S., Li L., Liu X., Mei K., Wang X. Structural insights into the assembly and activation of IL-1beta with its receptors. Nat. Immunol. 2010;11:905–911. doi: 10.1038/ni.1925. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y., Peng W., Wang J., Zhou W., Zhou Y., Ying B. Plasma levels of IL-1Ra are associated with schizophrenia. Psychiatry Clin. Neurosci. 2019;73:109–115. doi: 10.1111/pcn.12794. [DOI] [PubMed] [Google Scholar]
- 46.Frydecka D., Krzystek-Korpacka M., Lubeiro A., Stramecki F., Stanczykiewicz B., Beszlej J.A., Piotrowski p., Kotowicz K., Szewczuk-Bogusławska M., Pawlak-Adamska E., et al. Profiling inflammatory signatures of schizophrenia: A cross-sectional and meta-analysis study. Brain Behav. Immun. 2018;71:28–36. doi: 10.1016/j.bbi.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Maxeiner H.-G., Marion Schneider E., Kurfiss S.-T., Brettschneider J., Tumani H., Bechter K. Cerebrospinal fluid and serum cytokine profiling to detect immune control of infectious and inflammatory neurological and psychiatric diseases. Cytokine. 2014;69:62–67. doi: 10.1016/j.cyto.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Altmann D.M. Neuroimmunology and neuroinflammation in autoimmune, neurodegenerative and psychiatric disease. Immunology. 2018;154:167–168. doi: 10.1111/imm.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai Z., Chen D., Wang L., Zhao Y., Liu T., Yu Y., Cheng Y. Cerebrospinal fluid and blood cytokines as biomarkers for multiple sclerosis: A systematic review and meta-analysis of 226 studies with 13,526 multiple sclerosis patients. Front. Neurosci. 2019;13:1026. doi: 10.3389/fnins.2019.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai K.S.P., Liu C.S., Rau A., Lanctôt K.L., Köhler C.A., Pakosh M., Herrmann N. Peripheral inflammatory markers in Alzheimer’s disease: A systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry. 2017;88:876–882. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]
- 51.O’Brien S.M., Fitzgerald P., Scully P., Landers A., Scott L.V., Dinan T.G. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14:84–90. doi: 10.1159/000107423. [DOI] [PubMed] [Google Scholar]
- 52.O’Connell K.E., Thakore J., Dev K.K. Pro-inflammatory cytokine levels are raised in female schizophrenia patients treated with clozapine. Schizophr. Res. 2014;156:1–8. doi: 10.1016/j.schres.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 53.Frydecka D., Misiak B., Beszlej J.A., Karabon L., Pawlak-Adamska E., Tomkiewicz A., Partyka A., Jonkisz A., Kiejna A. Genetic variants in transforming growth factor-b gene (TGFB1) affect susceptibility to schizophrenia. Mol. Biol. Rep. 2013;40:5607–5614. doi: 10.1007/s11033-013-2662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corcoran M.P., Meydani M., Lichtenstein A.H., Schaefer E.J., Dillard A., Lamon-Fava S. Sex hormone modulation of proinflammatory cytokine and CRP expression in macrophages from older men and postmenopausal women. J. Endocrinol. 2010;206:217. doi: 10.1677/JOE-10-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verthelyi D., Klinman D.M. Sex hormone levels correlate with the activity of cytokine-secreting cells in vivo. Immunology. 2000;100:384–390. doi: 10.1046/j.1365-2567.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malashenkova I.K., Krynskiy S.A., Ogurtsov D.P., Hailov N.A., Zakharova N.V., Bravve L.V., Kostyuk G.P. Immunoinflammatory Profile in Patients with Episodic and Continuous Paranoid Schizophrenia. Consort. Psychiatr. 2021;2:19–31. doi: 10.17816/CP66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noto C., Gadelha A., Belangero S.I., Spindola L.M., Rocha N.P., de Miranda A.S., Teixeira A.L., Cardoso Smith M.A., de Jesus Mari J., Bressan R.A., et al. Circulating levels of sTNF-AR1 as a marker of severe clinical course in schizophrenia. J. Psychiatr. Res. 2013;47:467–471. doi: 10.1016/j.jpsychires.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Häfner H. Onset and early course as determinants of the further course of schizophrenia. Acta Psychiatr. Scand. Suppl. 2000;407:44–48. doi: 10.1034/j.1600-0447.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 59.Jablensky A., Sartorius N., Ernberg G., Anker M., Korten A., Cooper J.E., Day R., Bertelsen A. Schizophrenia: Manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol. Med. Monogr. Suppl. 1992;20:1–97. doi: 10.1017/S0264180100000904. [DOI] [PubMed] [Google Scholar]
- 60.Dimitrov D.H., Lee S., Yantis J., Valdez C., Paredes R.M., Braida N., Walss-Bass C. Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: Potential role for IL-17 pathway. Schizophr. Res. 2013;151:29–35. doi: 10.1016/j.schres.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Stojanovic A., Martorell L., Montalvo I., Ortega L., Monseny R., Vilella E., Labad J. Increased serum interleukin-6 levels in early stages of psychosis: Associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology. 2014;41:23–32. doi: 10.1016/j.psyneuen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Luo Y., He H., Zhang J., Ou Y., Fan N. Changes in serum TNF-A-α, IL-18, and IL-6 concentrations in patients with chronic schizophrenia at admission and at discharge. Compr. Psychiatry. 2019;90:82–87. doi: 10.1016/j.comppsych.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Pedrini M., Massuda R., Fries G.R., de Bittencourt Pasquali M.A., Schnorr C.E., Moreira J.C.F., Gama C.S. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J. Psychiatr. Res. 2012;46:819–824. doi: 10.1016/j.jpsychires.2012.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets are available on reasonable request to Svetlana A. Ivanova (ivanovaniipz@gmail.com), after approval from the Board of Directors of the Mental Health Research Institute, in accordance with local guidelines and regulations.