Abstract
Small interfering RNAs (siRNAs) with N-acetylgalactosamine (GalNAc) conjugation for improved liver uptake represent an emerging class of drugs to treat liver diseases. Understanding how pharmacokinetics and pharmacodynamics translate is pivotal for in vivo study design and human dose prediction. However, the literature is sparse on translational data for this modality, and pharmacokinetics in the liver is seldom measured. To overcome these difficulties, we collected time-course biomarker data for 11 GalNAc–siRNAs in various species and applied the kinetic-pharmacodynamic modeling approach to estimate the biophase (liver) half-life and the potency. Our analysis indicates that the biophase half-life is 0.6–3 weeks in mouse, 1–8 weeks in monkey, and 1.5–14 weeks in human. For individual siRNAs, the biophase half-life is 1–8 times longer in human than in mouse, and generally 1–3 times longer in human than in monkey. The analysis indicates that the siRNAs are more potent in human than in mouse and monkey.
Keywords: GalNAc-conjugated siRNA, KPD modeling, biophase half-life, translation
Introduction
N-acetylgalactosamine (GalNAc)-conjugated small interfering RNAs (siRNAs) are double-stranded molecules containing a sense and an antisense strand, of which the latter elicits the pharmacological effect [1]. These drugs are designed to knock down a certain gene with complementary nucleotide sequences by degrading mRNA after transcription, and as an effect preventing translation. The GalNAc conjugation targets the siRNA specifically to the liver, and GalNAc–siRNAs represent an emerging class of drugs to treat various liver diseases [2].
Understanding how pharmacokinetics and pharmacodynamics translate between species is pivotal to set dose level and dosing schedule in preclinical proof-of-concept studies, to predict the human dose from preclinical data, and to set safety margins. Important translational data in this direction were recently reported in Ref. [3]. However, the literature is generally sparse on translational data for these GalNAc–siRNAs, and pharmacokinetics in the liver is seldom measured.
One way to learn more about translation of GalNAc–siRNAs is to estimate drug pharmacokinetics in the liver, target turnover, and siRNA potency from temporal biomarker data using mathematical dose–response modeling, so-called kinetic-pharmacodynamic (KPD) modeling [4]. Previous study in this direction on oligonucleotides mainly considered antisense oligonucleotides and human data [5]. In this study, we present an analysis of 11 GalNAc–siRNAs in several species. Specifically, we estimate biophase (liver) half-life and potency across species of GalNAc–siRNAs that have reached the clinical phase and for which data are available.
Materials and Methods
We collected and digitized available literature time-course biomarker data from mice, monkeys (rhesus macaque Macaca mulatta or cynomolgus Macaca fascicularis), and humans of the following 11 GalNAc–siRNAs: Revusiran [6–8], Vutrisiran [9], Cemdisiran [10,11], Givlaari (Givosiran) [12,13], Lumasiran [14,15], Fitusiran [16,17], Inclisiran [18–20], ALN-HBV02 [21,22], ARO-APOC3 [23–26], ARO-ANG3 [27–30], and Olpasiran [31]. Compounds for which we could at least find relevant human data were included in the analysis. Group mean data were digitized using MATLAB (R2020a; The MathWorks, Natick, MA) or WebplotDigitizer (Ver. 4.5; Automeris, Pacifica, CA).
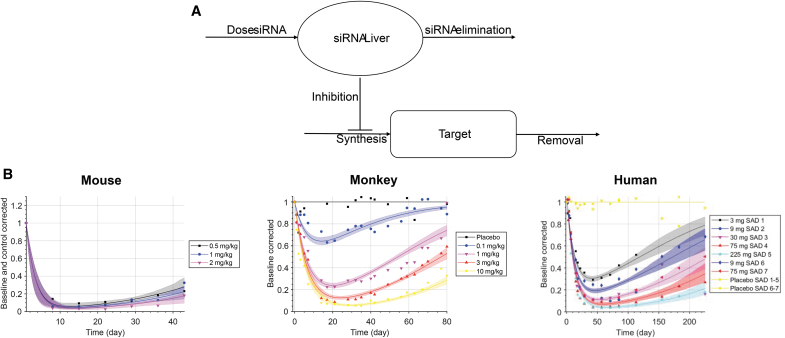
We applied the K-PD modeling approach to estimate the biophase half-life in the target organ and the potency (IDK50, ie, the dose per unit of time that results in 50% reduction in the target mRNA or protein), see Fig. 1. The pharmacokinetics is described by a virtual one-compartment model aimed to represent the biophase. No drug concentration measurements are required, or available, and the model depends solely on the biomarker data for identification of all parameters. We used an indirect-response PD model, where the drug inhibits the zero-order synthesis rate kin of response.
FIG. 1.
(A) Schematic diagram of the K-PD model. The amount of siRNA in the liver is governed by the dose and elimination rate. This amount inhibits the synthesis rate of the target according to the mode of action of siRNAs. Target is eliminated by its natural half-life. (B) Example of K-PD model fits to data for mouse, monkey, and human for different dose levels of Olpasiran. K-PD, kinetic-pharmacodynamic; siRNAs, small interfering RNAs.
In the model, the synthesis rate constant kS of response (R) is inhibited by a virtual infusion rate (IR), expressed in drug amount (A) per time unit, through a sigmoid Emax model:
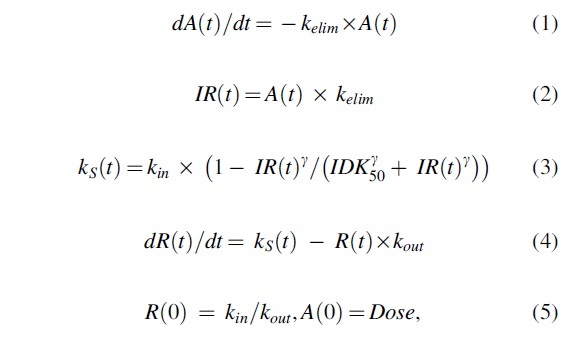
where kelim represents the elimination rate constant from the virtual compartment, kS and kout are the zero-order synthesis and the first-order degradation rate constants of the response R, R(0) is the baseline value of the response, kS is the time-dependent synthesis rate constant, IDK50 represents IR that leads to 50% inhibition of kS, IDK50 represents the apparent in vivo potency of the drug reflecting the ratio of clearance and bioavailability as well as the intrinsic potency of the drug, and γ is the Hill coefficient.
Numerical analyses were performed in MATLAB (R2020a; The MathWorks, Natick, MA). Specifically, the Matlab function fminsearch was used for solving the optimization problems encountered during parameter estimation. Parameter estimation was performed according to a maximum likelihood approach with an additive error model, using the naive-pooled data approach.
Results and Discussion
Mouse, monkey, and human data were collected and digitized for 7, 8, and 11 of the considered GalNAc-conjugated siRNAs, respectively. Model parameters were generally well estimated in terms of confidence intervals (Table 1 and Supplementary Information). For example, Fig. 1B shows the data and model fit for Olpasiran. The analysis indicates that the biophase half-life of GalNAc-conjugated siRNAs is 0.6–3 weeks in mice, 1–8 weeks in monkey, and 1.5–14 weeks in humans.
Table 1.
Parameter Estimation for N-Acetylgalactosamine Small Interfering RNAs
| Species | K-PD model parameter |
siRNA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Revusiran |
Vutrisiran |
Cemdisiran |
Givlaari |
Lumasiran |
Fitusiran |
Inclisiran |
ALN-HBV02 |
ARO-APOC3 |
ARO-ANG3 |
Olpasiran |
|
| Type | WT | AGXT KO | WT | WT | TG | WT | TG | |||||
| Mouse | IDK 50 (mg/[kg·d]) | 7.98e-3 | 2.41e-2 | 2.57e-2 | 6.79e-4 | 5.59e-3 | 4.86e-3 | 1.72e-5 | ||||
| CI (5th, 95th Perc.) | (7.4e-3, 8.7e-3) | (1.5e-2, 4.9e-2) | (2.0e-2, 3.0e-2) | (4.5e-4, 9.2e-4) | (5.1e-3, 6.2e-3) | (3.1e-3, 5.6e-3) | (1.3e-6, 6.9e-4) | |||||
| PK t-half (d) | 15.7 | 6.26 | 11.3 | 21.4 | 10.2 | 16.7 | 4.48 | |||||
| CI (5th, 95th Perc.) | (14, 17) | (4.9, 12) | (7.5, 17) | (15, 34) | (8.3, 13) | (10, 39) | (3.2, 14) | |||||
| PD t-half (d) | 2.11 | 12.6 | 1.19 | 1.54 | 1.82 | 1.17 | 1.64 | |||||
| CI (5th, 95th Perc.) | (1.8, 3.4) | (5.8, 17) | (0.68, 2.3) | (1.2, 2.5) | (1.1, 2.2) | (0.4, 1.7) | (0.98, 2.3) | |||||
| Monkey | IDK 50 (mg/[kg·d]) | 0.281 | 7.06e-3 | 4.91e-2 | 1.36e-2 | 2.06e-2 | 3.09e-2 | 7.36e-3 | 4.79e-3 | |||
| CI (5th, 95th Perc.) | (0.20, 0.37) | (7.9e-6, 8.7e-3) | (4.9e-2, 5.1e-2) | (3.7e-3, 2.5e-2) | (1.5e-2, 2.5e-2) | (2.4e-2, 4.6e-2) | (7.5e-7, 1.6e-2) | (4.1e-3, 5.5e-3) | ||||
| PK t-half (d) | 7.36 | 42.8 | 1.81 | 6.02 | 52.5 | 45.4 | 53.8 | 14.3 | ||||
| CI (5th, 95th Perc.) | (5.6, 9.7) | (30, 4.9e4) | (1.7, 1.9) | (4.2, 7.4) | (34, 83) | (22, 80) | (12, 1.9e5) | (12, 17) | ||||
| PD t-half (d) | 4.91 | 5.26 | 7.08 | 6.01 | 3.70 | 3.46 | 2.37 | 4.56 | ||||
| CI (5th, 95th Perc.) | (3.9, 6.1) | (4.5, 6.3) | (6.9, 7.1) | (5.1, 7.1) | (2.4, 5.0) | (1.8, 4.8) | (0.5, 4.3) | (3.8, 5.2) | ||||
| Human | IDK 50 (mg/[kg·d]) | 0.217 | 3.06e-4 | 1.00e-3 | 5.57e-4 | 6.86e-3 | 3.20e-3 | 5.71e-3 | 2.52e-5 | 6.27e-4 | 4.00e-3 | 1.00e-4 |
| CI (5th, 95th Perc.) | (0.19, 0.25) | (2.5e-4, 3.6e-4) | (7.2e-4, 1.4e-3) | (3.1e-4, 6.8e-4) | (2.9e-3, 1.1e-2) | (2.2e-3, 4.5e-3) | (5.1e-3, 6.2e-3) | (6.1e-8, 1.6e-4) | (5.1e-4, 7.3e-4) | (3.5e-3, 4.5e-3) | (7.2e-5, 1.3e-4) | |
| PK t-half (d) | 12.3 | 121 | 112 | 50.9 | 9.99 | 19.9 | 82.4 | 98.4 | 62.8 | 53.0 | 33.8 | |
| CI (5th, 95th Perc.) | (10, 16) | (93, 163) | (99, 124) | (21, 130) | (6.6, 16) | (16, 25) | (73, 92) | (52, 8.1e4) | (50, 90) | (44, 66) | (27, 42) | |
| PD t-half (d) | 4.82 | 8.16 | 4.98 | 5.25 | 15.9 | 12.3 | 4.07 | 9.49 | 3.79 | 4.43 | 8.41 | |
| CI (5th, 95th Perc.) | (4.1, 5.4) | (7.0, 9.7) | (4.3, 5.7) | (0.60, 12) | (10, 21) | (9.5, 16) | (3.7, 4.5) | (7.2, 12) | (3.2, 4.5) | (3.7, 5.0) | (7.7, 9.2) | |
Summary of estimated parameters for the 10 GalNAc–siRNAs in mouse, monkey, and human. Body weight assumed 70 kg.
AGXT, alanine–glyoxylate aminotransferase-deficient; CI, confidence interval; d, day; KO, knockout; KPD, kinetic-pharmacodynamic; Perc., percentile; PK, pharmacokinetic; TG, transgenic; WT, wildtype.
For individual siRNAs, the biophase half-life is 1–8 times longer in human than in mouse, and generally 1–3 times longer in human than in monkey (Fig. 2A). Givlaari deviates from the general pattern with a 28 times longer biophase half-life in human than in monkey. There is no clear dependency between translation of half-life between species and type of chemistry (indicated by the colors of the markers in Fig. 2A).
FIG. 2.
Predicted ratios between human and mouse and between human and monkey for biophase half-life (A) and potency IDK50 (B) for the analyzed GalNAc–siRNAs. Black: Alnylam's STC; blue: Alnylam's ESC; magenta: Alnylam's ESC-plus (ESC+); red: arrowhead Pharmaceuticals' chemistry. Biophase half-life was calculated as ln(2)/ kelim. ESC, enhanced stabilization chemistry; GalNAc, N-acetylgalactosamine; STC, standard template chemistry.
Potencies in form of IDK50 were relatively similar between mice and monkeys, and greater in humans. Specifically, IDK50 was predicted to be similar or smaller (up to 30-fold) in human than in mouse, and similar or smaller (up to 100-fold) in human than in monkey (Fig. 2B). Similarly to half-life, there is no clear dependency between translation of potency between species and type of chemistry. The higher potency observed in human is likely a result of optimization against the human sequence in the screening phase.
For two estimated half-lives, we could compare the results with previously reported data. First, the predicted biophase half-life of Fitusiran in human of ∼3 weeks compares well with the predicted liver half-life of 20 days in Ref. [32]. Second, the predicted biophase half-life of Givlaari in monkey of 1.8 days compares well with the predicted distributional half-life of 2.1 days from a two-compartment model fit to measured liver concentration data by Ref. [33].
The approach taken is limited by the lack of publicly available biomarker data in some species for certain siRNAs, and by the modeling on group mean data and not on individual data. The approach is supported by two comparisons with available reported data of biophase half-lives. The reported quantitative translational relationships may help guiding in vivo design and human dose predictions of GalNAc-conjugated siRNAs. In conclusion, we present the first systematic translational investigation of biophase (liver) half-life and potency of GalNAc–siRNAs.
Supplementary Material
Author Disclosure Statement
Authors are employed by AstraZeneca AB.
Funding Information
No external funding was received for this article.
Supplemetnary Material
References
- 1. Crooke ST, Baker BF, Crooke RM and Liang XH. (2021). Antisense technology: an overview and prospectus. Nat Rev Drug Discov 20:427–453. [DOI] [PubMed] [Google Scholar]
- 2. Zhang MM, Bahal R, Rasmussen TP, Manautou JE and Zhong. XB (2021). The growth of sirna-based therapeutics: updated clinical studies. Biochem Pharmacol 189:114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chong S, Agarwal S, Agarwal S, Aluri KC, Arciprete M, Brown C, Charisse K, Cichocki J, Fitzgerald K, et al. (2021). The nonclinical disposition and pk/pd properties of galnac-conjugated sirna are highly predictable and build confidence in translation to man. Drug Metab Dispos 50:DMD-MR-2021-000428. [DOI] [PubMed] [Google Scholar]
- 4. Jacqmin P, Snoeck E, van Schaick EA, Gieschke R, Pillai P, Steimer JL and Girard P. (2007). Modelling response time profiles in the absence of drug concentrations: definition and performance evaluation of the k-pd model. J Pharmacokinet Pharmacodyn 34:57–85. [DOI] [PubMed] [Google Scholar]
- 5. Jansson-Löfmark R and Gennemark P. (2018). Inferring half-lives at the effect site of oligonucleotide drugs. Nucleic Acid Ther 28:319–325. [DOI] [PubMed] [Google Scholar]
- 6. Butler JS, Chan A, Costelha S, Fishman S, Willoughby JL, Borland TD, Milstein S, Foster DJ, Goncalves P, et al. (2016). Preclinical evaluation of rnai as a treatment for transthyretin-mediated amyloidosis. Amyloid 23:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajeev K, Zimmermann T, Manoharan M, Maier M, Kuchimanchi S and Charisse. K (2014). Rnai agents, compositions and methods of use thereof for treating transthyretin (ttr) associated diseases. WO2015042564A1.
- 8. Zimmermann TS, Karsten V, Chan A, Chiesa J, Boyce M, Bettencourt BR, Hutabarat R, Nochur S, Vaishnaw A, et al. (2017). Clinical proof of concept for a novel hepatocyte-targeting galnac-sirna conjugate. Mol Ther 25:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Habtemariam BA, Karsten V, Attarwala H, Goel V, Melch M, Clausen VA, Garg P, Vaishnaw AK, Sweetser MT, et al. (2021). Single-dose pharmacokinetics and pharmacodynamics of transthyretin targeting n-acetylgalactosamine-small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin Pharmacol Ther 109:372–382. [DOI] [PubMed] [Google Scholar]
- 10. Kusner LL, Yucius K, Sengupta M, Sprague AG, Desai D, Nguyen T, Charisse K, Kuchimanchi S, Kallanthottathil R, et al. (2019). Investigational rnai therapeutic targeting c5 is efficacious in pre-clinical models of myasthenia gravis. Mol Ther Methods Clin Dev 13:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Badri P, Jiang X, Borodovsky A, Najafian N, Kim J, Clausen VA, Goel V, Habtemariam B and Robbie G. (2021). Pharmacokinetic and pharmacodynamic properties of cemdisiran, an rnai therapeutic targeting complement component 5, in healthy subjects and patients with paroxysmal nocturnal hemoglobinuria. Clin Pharmacokinet 60:365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. EMA. (2020). Assessment report. Givlaari international non-proprietary name: Givosiran. EMEA/H/C/004775/0000. [Google Scholar]
- 13. Sardh E, Harper P, Balwani M, Stein P, Rees D, Bissell DM, Desnick R, Parker C, Phillips J, et al. (2019). Phase 1 trial of an rna interference therapy for acute intermittent porphyria. N Engl J Med 380:549–558. [DOI] [PubMed] [Google Scholar]
- 14. Erbe DV. (2020). Methods for inhibition of hao1 (hydroxyacid oxidase 1 (glycolate oxidase)) gene expression. WO2019014491A1.
- 15. EMA. (2020). Assessment report oxlumo. EMA/568312/2020.
- 16. Sehgal A, Barros S, Ivanciu L, Cooley B, Qin J, Racie T, Hettinger J, Carioto M, Jiang Y, et al. (2015). An rnai therapeutic targeting antithrombin to rebalance the coagulation system and promote hemostasis in hemophilia. Nat Med 21:492–497. [DOI] [PubMed] [Google Scholar]
- 17. Pasi KJ, Rangarajan S, Georgiev P, Mant T, Creagh MD, Lissitchkov T, Bevan D, Austin S, Hay CR, et al. (2017). Targeting of antithrombin in hemophilia a or b with rnai therapy. N Engl J Med 377:819–828. [DOI] [PubMed] [Google Scholar]
- 18. Borodovsky A, Kallanthottathil RG, Fitzgerald K, Frank-Kamenetsky M, Querbes W, Maier M, Charisse K, Kuchimanchi S, Manoharan M, et al. (2014). Pcsk9 sirna compositions and methods of use thereof. WO2014089313A1. [Google Scholar]
- 19. Borodovsky A, Querbes W, Sutherland J, Hutabarat R, Milstein S, Kuchimanchi S, Kuchimanchi R, Charisse K, Yucius K, et al. (2014). Development of monthly to quarterly subcutaneous administration of rnai therapeutics targeting the metabolic diseases genes pcsk9, apoc3 and angptl3. Circulation 130:A11936. [Google Scholar]
- 20. Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay R, Turner T, et al. (2017). Inclisiran in patients at high cardiovascular risk with elevated ldl cholesterol. N Engl J Med 376:1430–1440. [DOI] [PubMed] [Google Scholar]
- 21. Gane E, Lim Y-S, Cloutier D, Shen L, Cathcart A, Ding X, Pang P, Huang S and Yuen R. Safety and antiviral activity of vir-2218, an x-targeting rnai therapeutic, in participants with chronic hepatitis b infection: week 48 follow-up results. Available at: https://investors.vir.bio/static-files/10c2cdad-2e8a-49c1-b8f7-a11d8906bc26 Accessed March 4, 2022.
- 22. Stuart M, Tuyen N, Castoreno A, Liebow A, Vasant J, Maier M and Sepp-Lorenzino L. Preclinical development of an rnai therapeutic drug candidate targeting hepatitis b virus, 2017. Available at: https://www.alnylam.de/wp-content/uploads/2017/09/OTS-2017_Poster-4_Milstein-et-al..pdf Accessed January 27, 2022.
- 23. Clifton P, Sullivan D, Baker J, Schwabe C, Thackwray S, Scott R, Hamilton J, Chang T, Given B, et al. (2020). Pharmacodynamic effect of aro-apoc3, an investigational hepatocyte-targeted rna interference therapeutic targeting apolipoprotein c3, in patients with hypertriglyceridemia and multifactorial chylomicronemia. Circulation 142:A12594. [Google Scholar]
- 24. Schwabe C, Scott R, Sullivan DR, Baker J, Clifton P, Hamilton J, Given B, Melquist S, Knowles J, et al. (2019). Rna interference targeting apolipoprotein c-iii results in deep and prolonged reductions in plasma triglycerides. Circulation 140:E987. [Google Scholar]
- 25. Schwabe C, Scott R, Sullivan D, Baker J, Clifton P, Hamilton J, Given B, San Martin J, Melquist S, et al. (2020). Rna interference targeting apolipoprotein c-iii with aro-apoc3 in healthy volunteers mimics lipid and lipoprotein findings seen in subjects with inherited apolipoprotein c-iii deficiency. Eur Heart J 41:ehaa946.3330. [Google Scholar]
- 26. Li Z, Rui Z, Pei T, Kanner S and Wong. S (2019). Rna interference agents and compositions for inhibiting the expression of apolipoprotein c-iii (apoc3). WO2019051402A1. [Google Scholar]
- 27. Watts GF, Schwabe C, Scott R, Gladding P, Sullivan D, Baker J, Clifton P, Hamilton J, Given B, et al. (2020). Pharmacodynamic effect of aro-ang3, an investigational rna interference targeting hepatic angiopoietin-like protein 3, in patients with hypercholesterolemia. Circulation 142:A15751. [Google Scholar]
- 28. Watts GF, Schwabe C, Scott R, Gladding P, Sullivan DR, Baker J, Clifton P, Hamilton J, Given B, et al. (2019). Rna interference targeting hepatic angiopoietin-like protein 3 results in prolonged reductions in plasma triglycerides and ldl-c in human subjects. Circulation 140:E987–E988. [Google Scholar]
- 29. Li Z, Zhu R and Wong. S (2019). Rnai agents and compositions for inhibiting expression of angiopoietin-like 3 (angptl3), and methods of use. WO2019055633A1.
- 30. Watts G, Schwabe C, Scott R, Gladding P, Sullivan D, Baker J, Clifton P, Hamilton J, Given B, et al. (2020). Rnai inhibition of angiopoietin-like protein 3 (angptl3) with aro-ang3 mimics the lipid and lipoprotein profile of familial combined hypolipidemia. Eur Heart J 41:ehaa946.3331. [Google Scholar]
- 31. Koren MJ, Moriarty PM, Baum SJ, Neutel J, Hernandez-Illas M, Weintraub HS, Florio M, Kassahun H, Melquist S, et al. (2022). Preclinical development and phase 1 trial of a novel sirna targeting lipoprotein(a). Nat Med 28:96–103. [DOI] [PubMed] [Google Scholar]
- 32. Attarwala H, Goel V, Kate M, Akin A and Robbie G. Development of a pharmacokinetic-pharmacodynamic (pk-pd) model of fitusiran, an investigational rnai therapeutic targeting antithrombin for the treatment of hemophilia in patients with and without inhibitors. Available at: https://www.alnylam.de/wp-content/uploads/2017/07/Attarwala_Fitusiran-PKPD_ISTH-2017_09July2017.pdf Accessed January 27, 2022.
- 33. Li J, Liu J, Zhang X, Clausen V, Tran C, Arciprete M, Wang Q, Rocca C, Guan L-H, et al. (2021). Nonclinical pharmacokinetics and absorption, distribution, metabolism, and excretion of givosiran, the first approved n-acetylgalactosamine–conjugated rna interference therapeutic. Drug Metab Dispos 49:572–580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.