Abstract
Background: Long-term sequelae, called Long-COVID (LC), may occur after SARS-CoV-2 infection, with unexplained dyspnoea as the most common symptom. The breathing pattern (BP) analysis, by means of the ratio of the inspiratory time (TI) during the tidal volume (VT) to the total breath duration (TI/TTOT) and by the VT/TI ratio, could further elucidate the underlying mechanisms of the unexplained dyspnoea in LC patients. Therefore, we analysed TI/TTOT and VT/TI at rest and during maximal exercise in LC patients with unexplained dyspnoea, compared to a control group. Methods: In this cross-sectional study, we enrolled LC patients with normal spirometry, who were required to perform a cardio-pulmonary exercise test (CPET) for unexplained dyspnoea, lasting at least 3 months after SARS-CoV-2 infection. As a control group, we recruited healthy age and sex-matched subjects (HS). All subjects performed spirometry and CPET, according to standardized procedures. Results: We found that 42 LC patients (23 females) had lower maximal exercise capacity, both in terms of maximal O2 uptake (VO2peak) and workload, compared to 40 HS (22 females) (p < 0.05). LC patients also showed significantly higher values of TI/TTOT at rest and at peak, and lower values in VT/TI at peak (p < 0.05). In LC patients, values of TI/TTOT at peak were significantly related to ∆PETCO2, i.e., the end-tidal pressure of CO2 at peak minus the one at rest (p < 0.05). When LC patients were categorized by the TI/TTOT 0.38 cut-off value, patients with TI/TTOT > 0.38 showed lower values in VO2peak and maximal workload, and greater values in the ventilation/CO2 linear relationship slope than patients with TI/TTOT ≤ 0.38 (p < 0.05). Conclusions: Our findings show that LC patients with unexplained dyspnoea have resting and exertional BP more prone to diaphragmatic fatigue, and less effective than controls. Pulmonary rehabilitation might be useful to revert this unpleasant condition.
Keywords: COVID-19, spirometry, dyspnoea
1. Introduction
After the resolution of the acute phase, SARS-CoV-2 infection may present important clinical-functional long-term sequelae. The term “Long-COVID” (LC) includes both “persistent symptomatic COVID-disease” and “post-COVID-19 syndrome”. The former considers signs and symptoms related to SARS-CoV-2 lasting between 4 and 12 weeks after the acute phase; the latter refers to signs and symptoms compatible with COVID-19, present for more than 12 weeks after the acute phase, without alternative aetiologies [1]. Respiratory symptoms are the main symptoms during LC, with dyspnoea and fatigue on exertion being the most common complaints [2].
Unexplained dyspnoea is one of the main indications for the cardiopulmonary exercise test (CPET) and several reports have been recently published on the CPET profiles of LC patients [3,4,5,6,7,8]. The first relevant studies in LC patients with unexplained dyspnoea reported a CPET profile of deconditioning, because of an acute inflammatory process, prolonged bed rest, and post-traumatic syndrome and depression [3,4,5]. Subsequently, abnormal ventilatory response to exercise with dysfunctional breathing (DB) was recognized in a range from 29% [7] to 63% [6,8] of the LC patients. Different patient selection criteria may explain the different rates of DB in LC patients, although also the lack of a gold standard to diagnose DB [9,10] might play a role.
A simple and well-known way of analysing the breathing pattern (BP) is to measure the ratio of the inspiratory time (TI) during the tidal volume (VT), to the total breath duration (TI/TTOT) as well as the inspiratory flow, i.e., the ratio of VT to TI [11]. Importantly, TI/TTOT has been termed the duty cycle of the respiratory system and VT/TI has been widely employed as a measure of respiratory drive [11]. So far, an analysis of BP during exercise has only been reported in healthy subjects [12].
We hypothesized that in LC patients with unexplained dyspnoea, the analysis of BP by means of TI/TTOT and VT/TI at rest and during maximal exercise could further elucidate the underlying mechanisms of the symptom. Therefore, we performed CPET in a large cohort of LC patients with normal spirometry and suffering from unexplained dyspnoea, in comparison to a control group. In all subjects, we analysed TI/TTOT and VT/TI along with the traditional CPET parameters.
2. Methods
2.1. Patients
In this cross-sectional study, we prospectively recruited consecutive adult patients with a previous PCR confirmed COVID-19, who were referred for CPET at the Lung Function Unit of the University Hospital of Parma, and at the Cardiac Unit of the “G. da Saliceto” Hospital of Piacenza for unexplained dyspnoea lasting at least 3 months after SARS-CoV-2 infection. We excluded patients with concomitant heart or lung disease, or with an abnormal spirometry. The study was approved by the Ethics Committee of North Emilia (approval number: 131, dated: 18 March 2022). We also enrolled healthy, age, sex and Body Mass Index (BMI, kg/m2)-matched subjects (HS) who had never smoked to serve as a control group; patients were recruited during the routine outpatient clinic according to 1:1 ratio.
2.2. Pulmonary Function and Cardiopulmonary Exercise Test
Pulmonary function tests were performed according to international recommendations [13]. A flow-sensing spirometer connected to a computer for data analysis (Vmax 22 and 6200, Sensor Medics, Yorba Linda, CA, USA) was used for the measurements. Forced vital capacity (FVC) and forced expiratory volume at 1st second (FEV1) were recorded and expressed as percentage of the predicted values, which were obtained from regression equations [14].
CPET was performed according to a standardised procedure [15]. After calibrating the oxygen and carbon dioxide analysers and flow mass sensor, patients were asked to sit on an electromagnetically braked cycle ergometer (Corival PB, Lobe Bv, Groningen, The Netherlands; Cosmed, Rome, Italy) and the saddle was adjusted properly to avoid the maximal extension of the knee. The exercise protocol involved an initial 3 min of rest, followed by unloaded cycling for another 3 min with an increment every minute of 5–20 Watts, according to the patient’s anthropometry, in order to achieve an exercise time in between 8 and 12 min. Patients were asked to maintain a pedalling frequency of 60 rotations/min (rpm) indicated by a digital display placed on the monitor of the ergometer.
Breath-by-breath oxygen uptake (VO2 in mL/kg/min), carbon dioxide production (VCO2 in mL/kg/min), tidal volume (VT in L), respiratory rate (RR in bpm) and minute ventilation (VE in L/min) were recorded during the test (CPX/D; Med Graphics, St. Paul, MN, USA; Quark CPET, Cosmed, Rome, Italy). Patients were continuously monitored by a 12-lead electrocardiogram (Welch Allyn CardioPerfect, Delft, The Netherlands) and a pulse oximeter (Pulse Oximeter 8600, Nonin Medical Inc., MPLS, MN, USA; Cosmed, Rome, Italy). Blood pressure was measured at 2 min intervals. Exercise was stopped according to the standardised criteria [15]. Predicted values were calculated according to equations by Wasserman et al. [16].
Peak workload (in watts) and peak VO2 (in mL/kg/min) were recorded as the mean value of watts and VO2 during the last 20 s of the test. Anaerobic threshold (AT) was non-invasively determined by both V-slope and ventilatory equivalents methods (“dual method approach”) [15] and was expressed as absolute value of VO2 in mL/kg/min.
The breathing reserve (BR, %) was calculated by the formula 1-(peak ventilation/maximum voluntary ventilation) * 100. Maximum voluntary ventilation was obtained by multiplying FEV1 by 40. The ventilatory response during exercise was expressed as a linear regression function by plotting VE against VCO2 obtained every 10 s, excluding data above the ventilatory compensation point [15]. Then, the slope values were obtained from the VE/VCO2 regression line. The end-tidal pressure of CO2 (PETCO2, in mmHg) was measured as mean of PETCO2 during the 3 min rest period and during the last 20 s of the test and was also recorded as the difference between PETCO2 peak and PETCO2 rest (∆PETCO2). At rest and during exercise the pattern of breathing was assessed by recording TI/TTOT and VT/TI.
The cardiovascular response to exercise was expressed as oxygen pulse (O2Pulse) and oxygen uptake efficiency slope (OUES) i.e., the relation between oxygen uptake and ventilation [17] and as the heart rate recovery at peak of exercise (HRR, in bpm) [18]. Dyspnoea induced by incremental exercise was measured at the end of the exercise by a visual analogue scale (VAS), which consisted of a 100 mm horizontal line with the word “none” placed at the left end of the scale and the words “very severe” placed at the right of the scale. The VAS scored from 0 to 100. Dyspnoea perception ratings were then divided by the maximal workload (VAS dyspnoea, in mm/watts) for analysis.
2.3. Statistical Analysis
This is a pilot, cross-sectional study. Due to the explorative nature of the study no formal sample size calculation was performed. Data are reported as mean ± standard deviation (SD), unless otherwise specified. The distribution of variables was assessed by means of a Kolmogorov–Smirnov goodness-of-fit test.
Relationships between variables were assessed by the Pearson’s correlation coefficient (r) and linear regression analysis or Spearman correlation coefficient (rs), when appropriate. Comparisons between variables were determined by unpaired t-test or by Chi-square test, when appropriate. The TI/TTOT cut-off value of 0.38 was chosen a posteriori, since it was the median TI/TTOT value at the peak of exercise in the control group.
Appropriate curve-fitting models were identified to analyse during exercise: TI/TTOT, [Y = (Y0-Plateau) * exp(-K * X) + Plateau, where Y0 is the Y value when X is zero, plateau is the Y value at infinite values, and K is the rate constant expressed in reciprocal of the X axis] and VT/TI [Y = Y Intercept + Slope * X, where Y Intercept is the Y value where the line intersects the Y axis, and slope is the slope of the line, expressed in Y units divided by X units].
A p value of less than 0.05 was taken as significant. Statistical analysis and diagrams were obtained by Prism 8 (©2018 GraphPad Software, La Jolla, CA, USA).
3. Results
Fifty-two LC patients and forty HS controls, respectively, aged between 22 and 66 years and between 26 and 79 years, were studied. In LC patients, spirometry values were in the normal range, although FEV1 values were significantly lower than for HS controls (Table 1).
Table 1.
Subjects’ characteristics.
| Variables | Healthy Controls (No. 40) |
Long COVID Patients (No. 42) |
p |
|---|---|---|---|
| Age (years) | 47 ± 11 | 49 ± 12 | 0.494 |
| Sex (F/M) | 22/18 | 23/19 | 0.983 |
| BMI (Kg/m2) | 25 ± 4 | 26 ± 3 | 0.121 |
| FVC (% pred) | 108 ± 15 | 101 ± 17 | 0.066 |
| FEV1 (% pred) | 106 ± 12 | 99 ± 15 | 0.025 |
| FEV1/FVC (%) | 82 ± 6 | 81 ± 6 | 0.255 |
| VO2 peak (mL/kg/min) | 31 ± 10 | 23 ± 8 | 0.001 |
| VO2 peak (% pred) | 105 ± 27 | 84 ± 21 | 0.001 |
| Workload (Watts) | 181 ± 65 | 123 ± 43 | 0.001 |
| Workload (% pred) | 117 ± 36 | 82 ± 22 | 0.001 |
| AT (mL/kg/min) | 21 ± 10 | 16 ± 8 | 0.030 |
| O2 Pulse rest (mL/bpm) | 4.9 ± 2.4 | 4.1 ± 1.3 | 0.069 |
| O2 Pulse peak (mL/bpm) | 14.6 ± 4.5 | 11.7 ± 3.6 | 0.002 |
| OUES (mL/min) | 2288 ± 687 | 1688 ± 686 | 0.001 |
| HR rest (bpm) | 75 ± 14 | 85 ± 15 | 0.002 |
| HR peak (bpm) | 152 ± 19 | 147 ± 15 | 0.217 |
| HR peak (% pred) | 88 ± 9 | 86 ± 8 | 0.377 |
| HR recovery (bpm) | 25 ± 9 | 20 ± 10 | 0.009 |
| BR (%) | 50 ± 11 | 51 ± 14 | 0.768 |
| VE peak (L/min) | 72 ± 26 | 59 ± 21 | 0.023 |
| Vt rest (L) | 0.74 ± 0.3 | 0.74 ± 0.3 | 0.994 |
| Vt peak (L) | 2.37 ± 0.8 | 1.97 ± 0.5 | 0.007 |
| RR rest (bpm) | 14 ±5 | 14 ± 6 | 0.770 |
| RR peak (bpm) | 31 ± 7 | 31 ± 9 | 0.847 |
| PETCO2 rest (mmHg) | 33 ± 6 | 34 ± 4 | 0.319 |
| PETCO2 peak (mmHg) | 41 ± 5 | 41 ± 5 | 0.921 |
| Δ PETCO2 (mmHg) | 8 ± 6 | 7 ± 5 | 0.468 |
| VE/VCO2 Slope (L) | 26 ± 4 | 30 ± 4 | 0.001 |
| TI/TTot rest | 0.29 ± 0.09 | 0.36 ± 0.09 | 0.001 |
| TI/TTot peak | 0.39 ± 0.05 | 0.42 ± 0.06 | 0.034 |
| VT/TI rest (mL/s) | 685 ± 397 | 605 ± 262 | 0.286 |
| VT/TI peak (mL/s) | 3155 ± 1101 | 2560 ± 850 | 0.008 |
| VAS dyspnoea (mm/watts) | 0.48 ± 0.19 | 0.64 ± 0.22 | 0.022 |
Values are expressed as mean ± SD. Abbreviations: BMI: Body Mass Index, FVC: Forced Vital Capacity, FEV1: Forced Expiratory Volume at 1st Second, VO2: O2 uptake, AT: Anaerobic Threshold, OUES: O2 Uptake Efficiency Slope, HR: Heart Rate, BR: Breathing Reserve, VE: Minute Ventilation, RR: Respiratory Rate, PETCO2: End-Tidal pressure of CO2, TI: Inspiratory Time, TTOT: Tidal Volume duration, VT: Tidal Volume, VAS: Visual Analogue Scale. Bold values indicate statistical significance.
All subjects completed the exercise test without any complications and no subjects were excluded because of poor motivation. The average interval between onset of SARS-CoV-2 infection to CPET was 12 months, ranging from 6 to 15 months. LC patients significantly differed, as compared to HS controls, showing lower values in VO2 at the peak and at the AT, maximal workload (Watts), O2Pulse at the peak, and in OUES values, as well as greater values in VAS dyspnoea (Table 1).
LC had significantly greater values in VE/VCO2 slope than HS controls, but they did not differ in BR and PETCO2 values (Table 1).
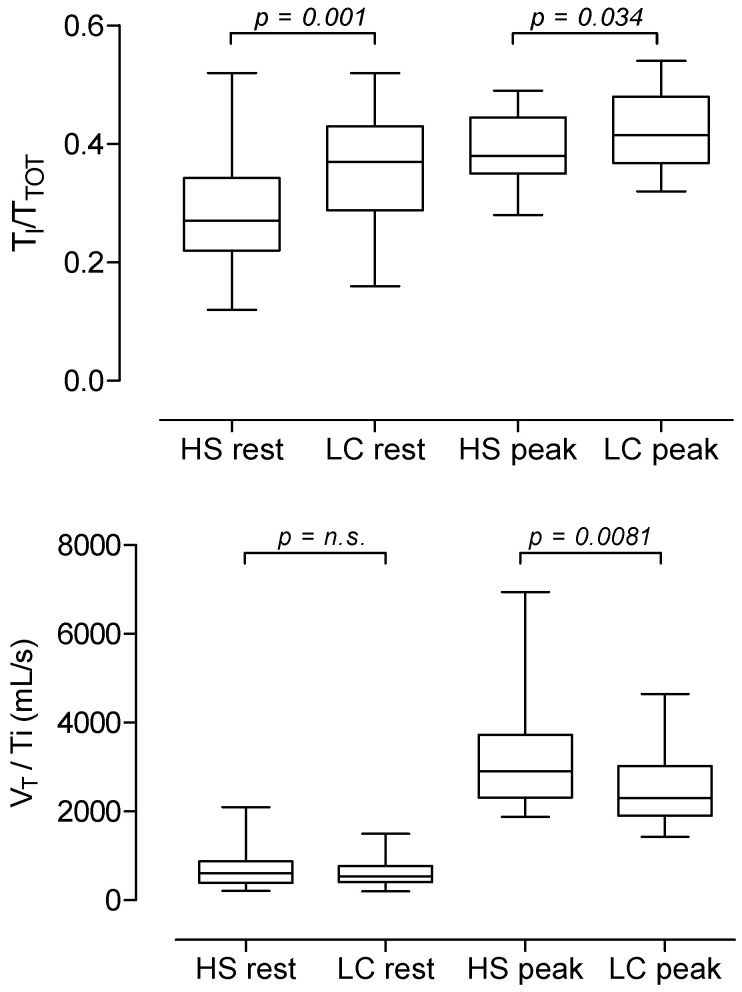
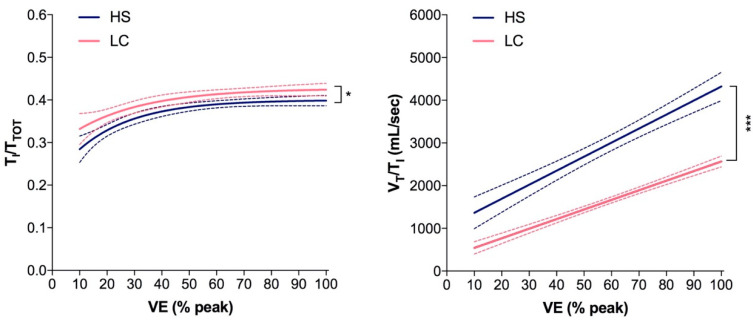
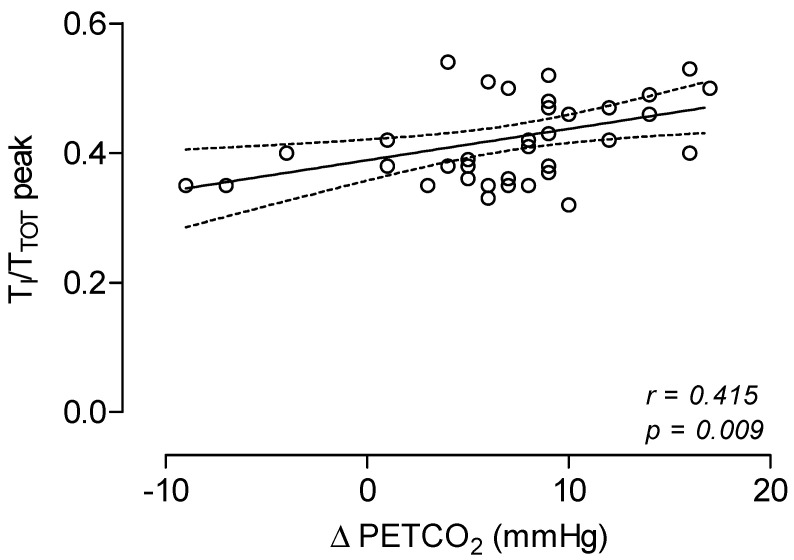
With respect to the breathing pattern analysis, when compared to HS controls, LC patients did not differ in RR both at rest and at the peak but showed lower values in VT at the peak. LC patients showed significantly higher values of TI/TTOT at rest and at the peak of exercise, and lower values in VT/VI at peak (Table 1 and Figure 1). The best fitting curves of data points during exercise of TI/TTOT and VT/VI as plotted to VE (% peak) were significantly different between LC patients and HS controls (Figure 2). In addition, in LC patients, values of TI/TTOT at peak were significantly related to ∆PETCO2 values (Figure 3).
Figure 1.
Mean, SD and range values of TI/TTOT at rest and at peak of exercise in 40 healthy subjects and 42 Long-COVID patients (upper panel) and mean, SD and range values of VT/TI at rest and at peak of exercise in 40 healthy subjects and 42 Long-COVID patients (lower panel).
Figure 2.
Best fitting curves of data points of TI/TTOT (left panel) and VT/VI (right panel) and 95% confidence bands during exercise in 42 Long-COVID patients and 40 healthy subjects. * p < 0.05; *** p < 0.001.
Figure 3.
Relationship between TI/TTOT values at peak of exercise and Δ PETCO2 values in 42 Long-COVID patients.
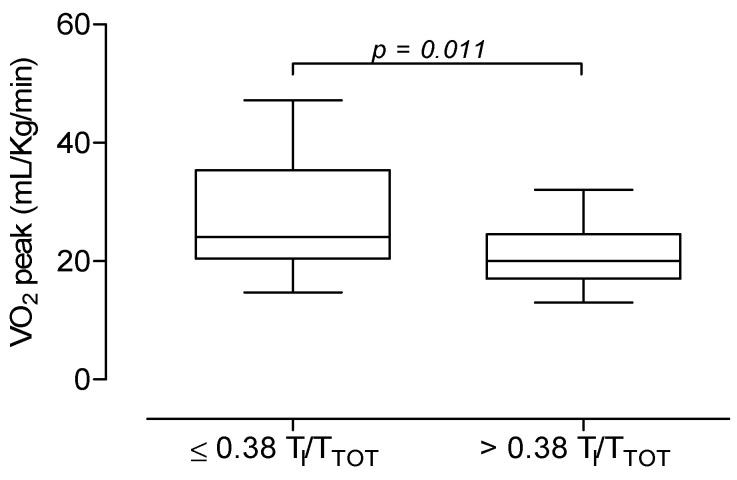
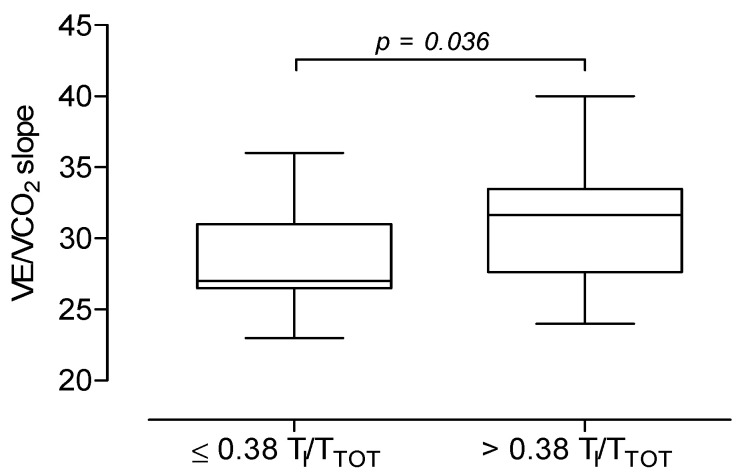
Finally, when LC patients were subdivided according to the TI/TTOT cut-off value of 0.38, 29 out 42 LC patients had TI/TTOT > 0.38 and showed lower values in VO2 peak (21 ± 5 mL/kg/min vs. 27 ± 10 mL/kg/min; p = 0.011) (Figure 4) and in maximal workload (114 ± 28 watts vs. 144 ± 60 watts; p = 0.035), and greater values in VE/VCO2 slope (31 ± 4 L vs. 28 ± 3 L; p = 0.036) than the remaining 13 patients with TI/TTOT ≤ 0.38.
Figure 4.
Mean, SD and range values of VO2 peak (upper panel) and of VE/VCO2 slope (lower panel) in 13 Long-COVID patients with TI/TTOT ≤ 0.38 and in 29 Long-COVID patients with TI/TTOT > 0.38 at peak of exercise. Sing et al. [19] also demonstrated a hyperventilatory response during exercise in all patients. Similarly, in a large cohort of patients at approximately three months after the initial diagnosis of SARS-CoV-2 infection, Motiejunaite et al. [5] found an elevated VE/VCO2 slope in one third of the study participants, suggesting a high incidence of inadequate ventilation on exertion. In the current study, LC patients had higher values in VE/VCO2 slope, as compared to controls, but they did not differ in terms of PETCO2, thereby showing ventilatory inefficiency without hyperventilation.
Importantly, with respect to the ventilatory response, other studies reported significant percentages of LC patients with DB during incremental maximal exercise, with and without hyperventilation [6,7,8]. DB is defined as a neural breathing disorder of the central nervous system, where an abnormal breathing drive results in respiratory discomfort in the absence of underlying cardiopulmonary disease [10]. It is worth of noting that the diagnosis of DB is based on the visual analysis of the plots showing the relationships between VT, RR and VE [10]; therefore, the identification of DB is subjective so that comparison of patients with and without DB may not be reproducible.
In the present study, we measured the resting and exertional breathing patterns in a previously rarely-used way, by analysing the TI/TTOT and VT/TI values in LC patients and in control subjects. Our results agree with the previous ones by Lind and Hesser [12], who studied breathing pattern and lung volumes during maximal exercise in eight young male healthy subjects.
TI/TTOT has been called fractional inspiratory time and has been also defined as the duty cycle of the respiratory system, since the level of stress placed on the respiratory muscles is proportional to TI/TTOT [11]. Therefore, a prolonged TI/TTOT predisposes to respiratory muscle fatigue and is of equal importance to the tension developed by the muscle, as a determinant of diaphragmatic fatigue [20].
During incremental exercise, TI/TTOT increases with increasing minute ventilation [12]. Of interest, in the present study we provided the evidence that LC patients, when compared to HS controls, showed higher values of TI/TTOT both at rest and at the peak of exercise. In addition, in LC patients the change in PETCO2 during exercise was directly related to the duty cycle of the respiratory system.
VT/TI has been termed mean inspiratory flow rate and is considered as a measure of respiratory drive, since it was found to be related to indices of respiratory centre output, such as P0.1 and the ventilatory response to hypercapnia [21]. During incremental exercise VT/TI increases progressively and when related to minute ventilation, the rate of increase in VT/TI decreases as minute ventilation rises [12]. In this study, we found that with the increase in exercise and related hyperpnea, LC patients showed an increase in mean inspiratory flow values, lower than that in HS controls, thereby developing minute ventilation at peak exercise was lower than that developed by HS controls.
Overall, our results suggest that LC patients have a breathing pattern that is more prone to diaphragmatic fatigue and less effective than that of the reference controls. Most of the work of the breath is achieved by the diaphragm. After an illness, especially if requiring mechanical ventilation or in conditions of general physical deconditioning, the diaphragmatic movement may be reduced and use of accessory respiratory muscles may occur [22], thereby resulting in an abnormal breathing pattern and breathlessness perception.
The findings of the present study must be interpreted in the context of limitations. The first limitation is due to the lack of breathing pattern data before the SARS-CoV-2 infection, and therefore no comparison before and after infection can be made. Secondly, we did not measure arterial blood gases, and used PETCO2 to estimate PaCO2 and to exclude hyperventilation syndrome. Furthermore, when using CPET in a non-invasive way, the identification of the primary limitation to exercise can be problematic. However, the present study is, by its nature, a non-invasive study. On the other hand, the strength of this study lies in a well-selected cohort of patients, along with the appropriate group of controls matched for age, gender and BMI. Furthermore, we used an objective approach, based on the measure of TI/TTOT and VT/TI, to investigate the breathing pattern.
In conclusion, we found that patients with previous infection of SARS-CoV-2 who subsequently complained of long-lasting unexplained dyspnoea, showed impairments in resting and on-exertion breathing patterns, along with a CPET profile of deconditioning. Pulmonary rehabilitation, also involving breathing control techniques, might be useful to revert this unpleasant condition.
Author Contributions
Conceptualization, L.C. and A.C.; methodology, L.C., A.F., L.M., G.H., M.A., P.T., G.M., R.P., G.P., M.P. and A.C.; software, L.C., F.D.S. and A.C.; validation, L.C. and A.C.; formal analysis, L.C. and A.C.; investigation, L.C., A.F., F.D.S., L.M., G.H., M.A., P.T., G.M., R.P., G.P. and M.P.; resources, F.D.S. and A.C.; data curation, L.C., A.F., F.D.S., L.M., G.H., M.A., P.T., G.M., R.P., G.P., M.P. and A.C.; writing—original draft, L.C., A.F., M.A. and A.C.; writing—review & editing, L.C., A.F., F.D.S., L.M., G.H., M.A., P.T., G.M., R.P., G.P., M.P. and A.C.; visualization, L.C. and A.C.; supervision, A.F., F.D.S. and A.C.; project administration, A.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of North Emilia (approval number: 131, dated: 18 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. National Institute for Health and Care Excellence (NICE); London, UK: Dec 18, 2020. NICE Guideline, No. 188. ISBN-13: 978-1-4731-3943-5. [PubMed] [Google Scholar]
- 2.Carfì A., Bernabei R., Landi F., Gemelli against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skjørten I., Ankerstjerne O.A.W., Trebinjac D., Brønstad E., Rasch-Halvorsen Ø., Einvik G., Lerum T.V., Stavem K., Edvardsen A., Ingul C.B. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur. Respir. J. 2021;58:2100996. doi: 10.1183/13993003.00996-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinaldo R.R., Mondoni M., Parazzini E.M., Pitari F., Brambilla E., Luraschi S., Balbi M., Papa G.F.S., Sotgiu G., Guazzi M., et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur. Respir. J. 2021;58:2100870. doi: 10.1183/13993003.00870-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motiejunaite J., Balagny P., Arnoult F., Mangin L., Bancal C., Vidal-Petiot E., Flamant M., Jondeau G., Cohen-Solal A., d’Ortho M.-P., et al. Hyperventilation as one of the mechanisms of persistent dyspnoea in SARS-CoV-2 survivors. Eur. Respir. J. 2021;58:2101578. doi: 10.1183/13993003.01578-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancini D.M., Brunjes D.L., Lala A., Trivieri M.G., Contreras J.P., Natelson B.H. Use of Cardiopulmonary Stress Testing for Patients with Unexplained Dyspnea Post-Coronavirus Disease. JACC Heart Fail. 2021;9:927–937. doi: 10.1016/j.jchf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frésard I., Genecand L., Altarelli M., Gex G., Vremaroiu P., Vremaroiu-Coman A., Lawi D., Bridevaux P.O. Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in ‘long COVID’ patients with persistent dyspnoea. BMJ Open Respir. Res. 2022;9:e001126. doi: 10.1136/bmjresp-2021-001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Gruenewaldt A., Nylander E., Hedman K. Classification and occurrence of an abnormal breathing pattern during cardiopulmonary exercise testing in subjects with persistent symptoms following COVID-19 disease. Physiol. Rep. 2022;10:e15197. doi: 10.14814/phy2.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker N., Everard M.L. Getting to grips with ‘dysfunctional breathing’. Paediatr. Respir. Rev. 2015;16:53–61. doi: 10.1016/j.prrv.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Boulding R., Stacey R., Niven R., Fowler S.J. Dysfunctional breathing: A review of the literature and proposal for classification. Eur. Respir. Rev. 2016;25:287–294. doi: 10.1183/16000617.0088-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobin M.J. Breathing pattern analysis. Intensive Care Med. 1992;18:193–201. doi: 10.1007/BF01709831. [DOI] [PubMed] [Google Scholar]
- 12.Lind F., Hesser C.M. Breathing pattern and lung volumes during exercise. Acta Physiol. Scand. 1984;120:123–129. doi: 10.1111/j.1748-1716.1984.tb07381.x. [DOI] [PubMed] [Google Scholar]
- 13.Graham B.L., Steenbruggen I., Miller M.R., Barjaktarevic I.Z., Cooper B.G., Hall G.L., Hallstrand T.S., Kaminsky D.A., McCarthy K., McCormack M.C., et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019;200:E70–E88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quanjer P.H., Tammeling G.J., Cotes J.E., Pedersen O.F., Peslin R., Yernault J.C. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European community for steel and oral official statement of the European respiratory society. Eur. Respir. J. 1993;6:5–40. doi: 10.1183/09041950.005s1693. [DOI] [PubMed] [Google Scholar]
- 15.Ross R.M. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003;167:211–277. doi: 10.1164/ajrccm.167.10.950. [DOI] [PubMed] [Google Scholar]
- 16.Wasserman K., Hansen J.E., Sue D.Y., Casaburi R., Whipp B.J., editors. Principles of Exercise Testing & Interpretation. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 1994. Normal values; pp. 143–162. [Google Scholar]
- 17.Baba R., Nagashima M., Goto M., Nagano Y., Yokota M., Tauchi N., Nishibata K. Oxygen uptake efficiency slope: A new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J. Am. Coll. Cardiol. 1996;28:1567–1572. doi: 10.1016/S0735-1097(96)00412-3. [DOI] [PubMed] [Google Scholar]
- 18.Cole C.R., Blackstone E.H., Pashkow F.J., Snader C.E., Lauer M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 19.Singh I., Joseph P., Heerdt P.M., Cullinan M., Lutchmansingh D.D., Gulati M., Possick J.D., Systrom D.M., Waxman A.B. Persistent Exertional Intolerance After COVID-19: Insights from Invasive Cardiopulmonary Exercise Testing. Chest. 2022;161:54–63. doi: 10.1016/j.chest.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellemare F., Grassino A. Evaluation of human diaphragm fatigue. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982;53:1196–1206. doi: 10.1152/jappl.1982.53.5.1196. [DOI] [PubMed] [Google Scholar]
- 21.Lederer D.H., Altose M.D., Kelsen S.G., Cherniack N.S. Comparison of occlusion pressure and ventilatory responses. Thorax. 1977;32:212–220. doi: 10.1136/thx.32.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gayan-Ramirez G., Decramer M. Effects of mechanical ventilation on diaphragm function and biology. Eur. Respir. J. 2002;20:1579–1586. doi: 10.1183/09031936.02.00063102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available upon request from the corresponding author.