The intestinal mucosa provides both a physiologic and immunologic barrier to a wide range of microorganisms and foreign substances. In general, the mucosal immune system is homeostatic despite the considerable antigenic load in the intestine. When an imbalance does occur in the regulation of this response, gut barrier dysfunction and inflammatory bowel disease are observed. Protozoan parasites that gain access to the host through the mucosal tissue of the alimentary tract may influence the development of such intestinal inflammatory disorders. Gut inflammatory diseases are associated with the production of various inflammatory cytokines including interleukin-1 (IL-1), IL-8, or tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) that may be produced by mucosal epithelial cells or by neighboring cells from the immune system. These immune products may act as chemoattractants (chemokines) for specific inflammatory cells, including macrophages, monocytes, neutrophils, and lymphocytes, that contribute to the mucosal inflammation. Elevated levels of nitric oxide (NO)-derived metabolites have been associated with these Th1-mediated inflammatory disorders. Although this proinflammatory response may be necessary to clear the infection it may invoke pathologic and potentially destructive changes in the tissue. In normal physiologic conditions, a homeostatic balance is maintained and the inflammatory disorders are prevented by downregulation of the immune response in the intestine.
A number of immune mediators are potentially involved in downregulation of the inflammatory response. Two cytokines, in particular, transforming growth factor β (TGF-β) and IL-10, appear to be candidates responsible for the downregulation of NO production. TGF-β is a potent immunoregulatory agent that affects proliferation (1, 82), the state of activation (69, 73) and differentiation (76) of the T-cell response. TGF-β may impair IL-12 production (74) that stimulates IFN-γ synthesis and the proliferation of both T and NK cells. TGF-β alters expression of T-cell costimulatory molecules, in particular, CD40/CD154 interactions that are responsible for the production of IL-12 (91). Recent studies involving genetically impaired mice for some members of the TGF-β family emphasize the role of TGF-β as a master regulator of immune cell function (53).
Another cytokine associated with downregulation is IL-10 that can be produced by a large variety of immune cells, including T lymphocytes, that express the Th2 phenotype, B cells, and macrophages. IL-10 can downregulate IFN-γ synthesis by Toxoplasma gondii-stimulated NK cells (88) and T cells (41) as well as a wide variety of macrophage-derived proinflammatory monokines (66), suggesting a critical role in macrophage effector functions against different pathogens (26). IL-10 inhibits IFN-γ synthesis by NK and Th1 lymphocytes via inhibition of macrophage IL-12 synthesis (34). IL-10 is associated with the downregulation of the expression of costimulatory molecules, which are implicated in the immunopathology observed after T. gondii infection (94).
Intestinal immune homeostasis is dependent upon the successful interaction of several compartments within the intestinal tract. These include organized secondary lymphoid organs, such as mesenteric lymph nodes, Peyer's patches, and leukocytes that are dispersed throughout the intestinal wall and within the mucosa, the intraepithelial lymphocytes (IEL). Epithelial cells or enterocytes lining the alimentary tract serve both as a physiologic barrier separating the lumen from underlying tissues and as a source of immune inflammatory products. These enterocytes or immunocytes play a critical role in mucosal immunophysiology that in part consists of a paracrine network between enterocytes and the underlying immune and inflammatory cells.
PARASITE-ENTEROCYTE INTERACTION
A number of protozoan parasites including Giardia intestinalis and Giardia lamblia, Cryptosporidium parvum, T. gondii, Eimeria spp., and Entamoeba histolytica have been shown to adhere to and to multiply on or within enterocytes (89). Attachment to enterocytes appears essential for colonization of the intestine and is requisite for the induction of host immunity that will lead to enterocyte damage (39). Intestinal inflammation invariably is associated with an increase in epithelial cell proliferation (59). In the small bowel the pathologic consequences of this response include proliferation in the crypt and a decrease in the number of enterocytes in the absorptive villus compartment, which can lead to malabsorption. For the host, this proliferation will presumably rid the intestine of infected and damaged enterocytes that can be quickly replaced.
One mechanism utilized by the host for effective control and removal of intestinal acquired parasites is the induction of NO by the intestinal epithelium. NO is antimicrobial for a wide range of mucosal pathogens (13, 21, 37) and, furthermore, is involved in the regulation of mucosal barrier integrity and vascular tone in the gut (2). NO is produced enzymatically from arginine through the action of NO synthase (NOS), which in many cell types, including intestinal epithelial cells, is expressed in its inducible isoform (81, 97). Expression of inducible NOS is constitutive in the mouse ileum and isolated normal duodenocytes (32) or is inducible in vivo during colonic inflammation or in vitro by cytokines or in response to infection with invasive bacteria (81). In polarized intestinal epithelial cells, the stable NO end products, nitrite and nitrate, are preferentially detected on the apical side, suggesting that relevant targets for epithelial cell-derived NO and its metabolites may be located on the luminal side of the cells (97). Giardia infection of the human intestine is a common protozoan infection and is the cause of self-limited diarrheal disease worldwide. Giardia infection is restricted to the lumen of the intestinal tract. NO inhibits growth, encystation, and excystation of G. lamblia, but has no effect on giardial viability (20). Despite the potent antigiardial activity of NO, G. lamblia is not simply a passive target for host-produced NO but has strategies to evade this potential host defense. In models of human intestinal epithelium, G. lamblia inhibited epithelial NO production by consuming arginine, the crucial substrate used by epithelial NO synthase to form NO.
In C. parvum infection the interaction between the parasite and enterocytes leads to diarrhea, which is characterized by the impairment of glucose-stimulated Na+ absorption, a function principally of villus-absorptive cells (67). When infected, these cells express an increase in their prostaglandin production (3, 47) which can inhibit NaCl absorption and result in secretory diarrhea. In addition to altering epithelial chloride and fluid secretion, increased prostaglandin can upregulate epithelial mucus expression, which could protect the host against further infection and downregulate inflammatory cytokine production by macrophages. Recently, it has been demonstrated that epithelial cells infected with C. parvum undergo caspase-dependent apoptosis, which may further lead to the clinical manifestations associated with enteric infection (72), in particular, the cytolysis of the parasitized mucosa. Parasite may use apoptosis to exit from the infected cell or the infected cell may eliminate the parasite through apoptosis. However, C. parvum has developed strategies to limit apoptosis in order to facilitate growth and maturation in the early period following epithelial cell infection (63).
Intestinal acquired parasites may directly induce the production of chemokines by epithelial cells. These chemokines may be critical to the initiation of the mucosal inflammatory process. C. parvum resides in epithelial cells, and infection of human intestinal epithelial cells in vitro results in upregulated expression and basolateral secretion of C-X-C chemokines IL-8 and GROα (47). These results were expanded in a model of human intestinal xenografts in SCID-HU-INT mice. After C. parvum infection in vivo, human intestinal epithelial cells produced IL-8 in association with TNF-α and IL-1β (87). Unlike enteroinvasive bacteria, the kinetics of increased expression and production of IL-8 and GROα after C. parvum infection was delayed and most marked 16 to 48 h after infection (46, 48). Although acquired by oral ingestion, T. gondii that invades the epithelial cells of the intestine is not usually considered an enteric pathogen. However, inflammatory bowel disease (IBD) has been observed following oral infection in monkeys (15) as well as in rabbits. Similar evidence of IBD has been reported in certain strains of inbred mice following oral infection with T. gondii (55). This hyperinflammatory process is associated with the early mortality of these susceptible mouse strains. Recent studies in our laboratory have suggested that both murine and human enterocytes when infected with T. gondii produce significant quantities of proinflammatory chemokines, among which are IP-10, MCP-1, and MIP-2 (10a). Using an in vitro system, we have determined that parasite-infected enterocytes are chemoattractant for several different cell types, including CD8+ intraepithelial lymphocytes within the mucosal compartment (D. Buzoni-Gatel et al., unpublished observation).
Cell-cell interaction within the intestinal lumen may be altered by protozoan infection. E. histolytica trophozoites colonize the lumen and may disrupt the epithelial tight junction. The principal clinical manifestation of the infection is due to loss of this epithelial barrier with deterioration of normal physiologic function. As the infection progresses, the cytotoxic effects of the parasite as well as molecular changes in the tight junction protein complex (52) may further aggravate the process. The tight junction complex constitutes, after the mucus, the first barrier against the paracellular penetration of intestinal microorganisms. This intercellular barrier is formed by the plasma membrane-spanning proteins claudins (24) and occludin (25) that associate with different peripheral plasma membrane proteins such as the ZO. Tight junction complexes are linked to the actin cytoskeleton (60). Selective disturbance of tight junction complexes by trophozoites from E. histolytica results in the rapid decrease of the transepithelial electrical resistance caused by an increase in paracellular permeability (51, 54, 61).
In addition to alterations in the tight junction, infection with E. histolytica can stimulate the production of IL-8 from human colonic epithelial cells (99) in the absence of cell-cell contact or injury. Experimental models demonstrate that the epithelial cells in response to parasite infection produced IL-1 and IL-8 (85). In vivo, infection of xenografts with E. histolytica trophozoites results in extensive tissue damage associated with infiltration of neutrophils. Human intestinal epithelial cell inflammatory responses to amebic infection were inhibited by the intraluminal administration of an antisense oligonucleotide to the human p65 subunit of NF-κB. This treatment blocked the production of human IL-1β and IL-8 by intestinal epithelial cells and inhibited neutrophil influx into the E. histolytica-infected intestinal xenografts. These data emphasize the role of the intestinal epithelial cell in initiating the inflammatory intestinal response after infection with E. histolytica (86). Other inflammatory molecules, including granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-1α/β mRNAs, were up regulated by E. histolytica infection (44).
Intestinal epithelial cells constitutively express major histocompatibility complex (MHC) class II, and this expression is enhanced in states of inflammation (62). These enterocytes can take up soluble antigens into an endolysosomal pathway, including class II-containing compartment. This suggests that all the mechanisms required for antigen processing and presentation exist within the enterocytes and that MHC class II expressed on murine enterocytes is functional (38). However, intestinal epithelial cells lack the costimulatory B7-1 and B7-2 expression, suggesting that enterocytes are poor antigen-presenting cells compared to dendritic cells or macrophages (5). In an antigen-overloaded environment such as the intestine, the failure of classical class II-mediated activation may be beneficial. Since most intraepithelial lymphocytes (see below) are CD8+, class II restriction may not be involved. There may however be distinct surface molecules and restriction elements that can present processed antigen to CD8 T cells. These include the expression of the class Ib molecule CD1d as well as the gp180 CD8 ligand. However, in spite of the close juxtaposition of IEL and enterocytes, allogenic coculture of these cells fails to result in T-cell activation. Recent data suggests that CD1d and gp180 molecules together may activate a subpopulation of CD8+ regulatory T cells which function to suppress the immune response in an antigen-nonspecific fashion (11). These observations suggest that enterocytes may be a key component in the immune homeostasis in the gut.
PARASITE AND INNATE IMMUNITY
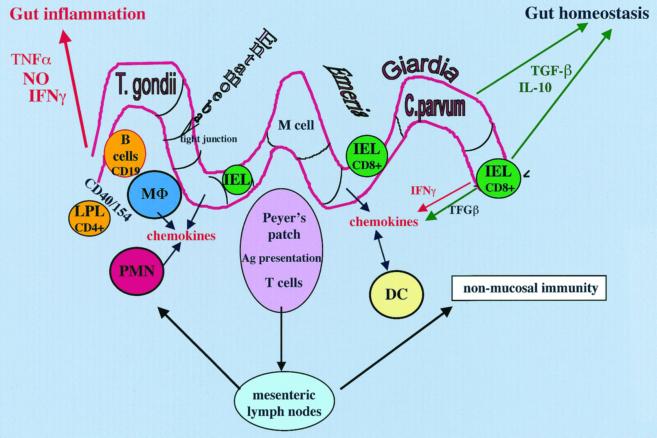
Oral acquisition of a parasite may result in a robust innate mucosal immune response in the infected host (Fig. 1). This response may include activation of neutrophils, tissue macrophages, monocytes, dendritic cells, and eosinophils. Many of these early-phase reactive cells are essential for the protective response as well as the establishment of long-term immunity to the parasite. Induction of chemokines such as IL-8 and GROα play an important role in the chemoattraction and activation of neutrophils. Polymorphonuclear leukocyte (PMN) attraction and accumulation may participate in the development of the intestinal lesion (86) as already illustrated in amebic infection. In those studies, it was demonstrated that depletion in PMN or inhibition of their attraction into the infected intestine results in prevention of the development of intestinal lesions.
FIG. 1.
A model of intestinal mucosal immune response to orally acquired protozoa. After oral infection, some parasites come in direct contact with the enterocytes (Giardia), whereas Entamoeba may disturb the intercellular tight junctions as well as induce lysis of the epithelial cells. Other parasites, such as C. parvum and T. gondii, are more invasive and penetrate into enterocytes or IEL (Eimeria). The infected enterocyte may fend off the microbe by the production of NO as well as various chemokines that participate in the chemoattraction of neutrophils (PMN) and different antigen (Ag)-presenting cells, such as macrophages (MΦ), monocytes, and dendritic cells (DC) from Peyer's patches, mesenteric lymph nodes, or the lamina propria (lamina propria lymphocytes [LPL]). In addition to their effect against parasites, these early inflammatory cells contribute both to the development of the pathogenesis of the intestinal immune process and to the initiation of a long-lasting immunity by activation of T and B cells. Although an essential component to the host defense, IFN-γ that can be produced by intestinal T cells is also the principal cause of inflammation in the intestine. Other cytokines, such as TNF-α, together with IFN-γ may have a synergistic effect. Homeostatic mechanisms to control the hyperinflammatory response are induced. Cells throughout the intestine participate in the control of this response by the secretion of two essential downregulatory cytokines, TGF-β and IL-10.
PMN accumulation is commonly associated with inflamed intestine, this accumulation being due, in part, to a delay in the apoptotic process (35). The lack of apoptosis may result from the activity of G-CSF and GM-CSF (J. Y. Channon, K. Miselis, L. Minns, and L. H. Kasper, submitted for publication). The PMNs are an important source of proinflammatory cytokines, such as IL-1β and TNF-α, and fully participate to the immune response in the intestine. Infection with C. parvum results in a spectrum of pathologic changes in the intestine accompanied by patchy or large neutrophil and mononuclear infiltrates. Neutrophils in mucosal secretions retain their ability to phagocytose and kill pathogens (18). In addition, neutrophils may function to regulate other aspects of the inflammatory response through the secretion of chemokines (57) at the site of infection.
As noted above, parasite-infected epithelial cells produce a variety of chemokines, including MCP-1, which may be involved in macrophage migration to the site of infection. Macrophages are well-known mediators of the host innate response to a large array of microbial pathogens. Macrophages and monocytes can control and kill parasites by both oxidative and nonoxidative mechanisms. Parasite infection of intestinal tissue will lead to a robust inflammatory process and the production of a wide range of cytokines, such as IFN-γ, TNF-α, and IL-1. Intestinal inflammation is characterized by a strong Th1 response and macrophages are important during mucosal inflammation. In situ expression of cell adhesion molecules, such as ICAM-1, may increase recruitment and further sustain the inflammatory process (68). Lamina propria macrophages isolated from patients with IBD display high levels of NF-κB DNA binding activity, accompanied by increased production of IL-1, IL-6, and TNF-α (33, 70). Other cytokines, such as IL-15 and IL-18 (65), enhance IFN-γ production, which further enhances macrophage function.
IFN-γ-activated macrophages exhibit microbicidal activity by production of high levels of NO (17), which is toxic for a variety of orally acquired parasites. IFN-γ-primed macrophages produce TNF-α in response to the Gal-lectin antigen of E. histolytica (84), which in turn promotes NO-mediated cytotoxicity against the parasite. Giardia trophozoites are susceptible to the effect of IFN-γ-activated macrophages (22), presumably through NO-mediated toxicity. Treatment of chicken bone marrow macrophages with IFN-γ inhibits intracellular E. tenella replication via NO or toxic oxygen intermediates (17). T. gondii is sensitive to the presence of NO in the mucosa following oral infection (42). IFN-γ activation can enhance microbiostatic activity independent of the production of NO residues. For example, IFN-γ treatment may lead to the intracellular deprivation of tryptophan and iron, which can be deleterious for T. gondii replication in both macrophages and enterocytes (16, 75). In addition to their role in innate immunity, the macrophages amplify the specific acquired immune response and are necessary for antigen presentation that provides for long-term protection against recurrent infection. However, macrophages may also serve as long-term host cells that facilitate the replication and survival of the pathogens and thereby serve as a vector for the invasion of the parasite (8).
Other first-line defense cells may be recruited to the site of infection. Secretion of chemokines may result in the migration and activation of dendritic cells or lamina propria cells to the site of mucosal infection (40). Peyer's patches represent the primary site for uptake and presentation of ingested antigens in the intestine. There are at least two different populations of dendritic cells in the Peyer's patches. Although there is mounting information indicating that dendritic cells play an important accessory function in response to a number of pathogens, including T. gondii (78, 83), there is currently very little information on the role of mucosal dendritic cells in response to protozoan infection.
One of the unique features of mucosal lymphoid tissue such as Peyer's patches is their capacity to induce Th cells producing type 2 (IL-4, IL-5, and IL-10) and type 3 (TGF-β) cytokines. Induction of the Th cell response is important for immunoglobulin A (IgA) production and generation of regulatory cell-mediated oral tolerance. Despite this ability to generate Th2/Th3 responses in mucosal tissues, distinct Th1 responses occur in the mucosa, particularly following intestinal infection with pathogenic microorganisms such as T. gondii or in IBD (36). The mechanisms that determine the ability of Peyer's patches to generate TH2/Th3 responses yet allow for the differentiation of Th1 response after infection with pathogenic organisms remain uncertain. One possibility is that alteration of the cytokine environment in the intestinal mucosa favors the differentiation of Th2 and Th3 cells, but that pattern is overridden by strong signals from pathogens, such as those that directly induce IL-12 from antigen-presenting cells. Another important factor may be the nature of the resident antigen-presenting cells, compared to the cells that traffic to the infected sites. Resident dendritic cells may differ in their capacities to drive T-cell differentiation.
The role of eosinophils in determining the outcome of parasite infections is mentioned in some studies. Early studies using T. gondii have demonstrated that IgE-bearing eosinophil can be cytotoxic (79). Regarding E. histolytica infection, in vitro data suggest that unlike normal human eosinophils which are destroyed, eosinophils which have been activated by complement and armed with specific IgE antibodies effectively destroy virulent E. histolytica (58). Unfortunately, the clinical relevance of this finding is uncertain since amoebic colitis does not appear to be associated with intestinal eosinophilia.
IEL AND INFECTION
In the intestine, the mucosal immune system consists of organized secondary lymphoid organs, such as mesenteric lymph nodes and Peyer's patches, as well as leukocytes dispersed throughout the intestinal wall and particularly in the mucosa. Mucosal lymphocytes, the IEL, are located between epithelial cells, below the intercellular tight junctions, and express a set of surface receptors different from those of peripheral blood lymphocytes and comprise a phenotypically distinct population. Although some differences exist between humans and animals, more specifically mice, most of the IEL are T lymphocytes and bear an oligoclonal repertoire of T-cell antigen receptor (TCR). The TCR has two forms, αβ and γδ. In the intestinal epithelia of numerous vertebrate hosts, TCR-γδ T cells are often present in large numbers. Up to 90% of IEL are CD4− CD8+, and most of these (60%) express the CD8 homodimer αα. The other population of CD8+ cells bears the CD8 heterodimer αβ (29). Most IEL express the unusual integrin αEβ7, which is involved in adherence to epithelial cells by binding to E-cadherin. Although some IEL may develop within the epithelium (77), it is likely that many IEL traffic from blood vessels present in the lamina propria to the epithelium. The IEL deficiency associated with a lack of β7 expression suggests that αEβ7 is required for entry and/or retention of T cells in the intestinal epithelium (45, 96). T-cell activation results in the accumulation of αEβ7hi cells in the mesenteric lymph nodes, lamina propria, and IEL compartment, suggesting also a role for this molecule in lymphocyte homing. Synthesis of the αE subunit is induced by the TGF-β cytokine (4, 43). This cytokine is abundant in the gut epithelial cells, located in the distal region of the villus, and can induce αE synthesis in T cells following migration into the epithelial microenvironment. Another integrin, α4β7, expressed in low frequency on IEL is evident on lamina propria lymphocytes and on approximately 50% of T cells (6, 71).
Activated lymphocytes expressing α4β7 can bind to several receptors, the most prominent of which is MadCAM-1, a protein expressed by high endothelial venule (HEV) cells in Peyer's patches and mesenteric lymph nodes and the flat endothelium in the lamina propria (6, 92). Studies indicate that the interactions of α4β7 and MadCAM-1 play a major role in lymphocyte homing to Peyer's patches, lamina propria, and mesenteric lymph nodes (31). Diapedesis from the microvasculature occurs in response to the expression of the α4β7 molecule.
Although the migration of T cells into the intestinal epithelium is not fully understood, IEL have in vitro chemotactic activity in response to several different chemokines, including IL-8, RANTES, MCP, MIP, crg-2, and MuMig (murine monokine induced by gamma interferon), all of which can be produced by activated enterocytes (80). Infection of the gut with mucosal pathogens can result in the migration and activation of IEL. Infection with Eimeria vermiformis, results in an increase in the number of recoverable IEL at 3 and 14 days postinfection. The IEL repertoire, and, more precisely, the γδ+ T-cell repertoire, has been shown to be very dynamic postinfection with a naturally occurring epithelialtropic pathogen (23). Following G. lamblia infection in inbred mice, quantitation of T-cell subsets in the intraepithelium (IEL) and lamina propria revealed increased influx of CD8 T cells and Thy1.2+ T cells followed by an increase in CD4 T cells in the lamina propria (95). IEL provide a number of important immunologic functions, including cytotoxic activity (28, 29), secretion of cytokines including IL-2, IL-3, IL5, TNF-α, TGF-β, IL-10, and IFN-γ (30, 49), and modulation of epithelial cell death and regeneration.
Evidence that IEL population in the intestine has a major role in immunity has been obtained from experimental studies of in vivo infection with Toxoplasma and Cryptosporidium spp. Adoptive transfer of T. gondii antigen-primed IEL into the naïve host provide long-term protection following lethal parasite challenge as determined by reduced mortality and decreased number of brain cysts in the recipient. The protective IEL are CD8+α/β+, α/β TCRs and are partially dependent upon the presence of intact γ/δ TCRs as well as endogenous production of IFN-γ (10, 50) in the recipient host. Increased expression of the activated memory T-cell phenotype, in particular Ly-6C, was noted in the protective IEL cell population. T. gondii antigen-primed IEL can traffic to the intestine and stimulate long-term immunity to reinfection. The ability of these cells to traffic to the intestine is dependent upon the expression of the appropriate integrins which if blocked increases susceptibility to parasite challenge. A combined treatment with anti-α4 and anti-αE monoclonal antibodies partially inhibited IEL trafficking and impaired host resistance to T. gondii (9). The predominant functional role of αEβ7 is to retain lymphocytes within or closely apposed to epithelial cells. Since IEL are cytotoxic for T. gondii-infected enterocytes in vitro (12), αEβ7 may play an integral role in that interaction. Intestinal epithelial cells inhibit the proliferative and cytokine responses of intraepithelial T cells (98) and may be involved in control of the extensive intestinal hyperinflammatory response in certain strains of mice (42, 55). We have observed that T. gondii antigen-primed IEL produce substantial amounts of TGF-β. The mucosal inflammatory process observed after oral infection with T. gondii in susceptible C57BL/6 mice is mediated by NO. The inflammation can be reversed when susceptible mice are treated with an NO blocking agent, such as aminoguanidine. Observations to date suggest that local production of IFN-γ perhaps from the CD4 lymphocytes in the lamina propria or NK cells may be responsible for this activity. Supplementation of exogenous TGF-β to susceptible mice reverses the hyperinflammation, whereas treatment of resistant strains such as CBA with a blocking antibody to TGF-β renders them susceptible. In vitro, coculture of antigen-primed IEL can reverse IFN-γ synthesis of primed splenocytes perhaps via a TGF-β-mediated pathway (Kasper et al., submitted).
IL-10 appears to be an important component in maintaining gut homeostasis in response to orally acquired pathogens. Increased mortality of IL-10 knockout (KO) mice is presumed secondary to the abnormally high inflammatory cytokine response during acute toxoplasmosis (27). Mice deficient in IL-10 synthesis exhibit increased inflammation in their intestines following oral infection with T. gondii (90). Mice that have been reconstituted with the gene for IL-10 expressed on the IL-2 promoter regain their ability to control the hyperinflammation (K. Ely, unpublished observation). The source for IL-10 production within the infected intestine is as yet undetermined.
In Cryptosporidium muris infection, immunity can be adoptively transferred to SCID mice using postinfection intestinally derived IEL. Of note however, in contrast to T. gondii infection, protection was associated with the CD4+ T-cell population as opposed to CD8+ (14). When adoptively transferred into SCID mice, primed IEL traffic back to the intestine. IEL from C. muris-infected mice produce significant quantity of IFN-γ in the presence of antigen-presenting cells. Treatment of recipient mice with anti-IFN-γ abrogated the protection (14, 64). This is similar to the observations made following adoptive transfer of T. gondii antigen-primed CD8+. Of note is that transfer of primed CD8+ IEL from wild-type mice into IFN-γ KO mice failed to protect against a T. gondii challenge (50). In contrast, adoptive transfer of IEL from IFN-γ KO mice into the wild type is protective. These data suggest that host IFN-γ is essential for protection. Although less documented, the possible implication of IEL in anti-Giardia immunity has been suggested. Human CD4+ T lymphocytes from the intestine proliferate in response to Giardia infection and produce IFN-γ. Moreover, murine intraepithelial lymphocytes from a Giardia-infected host are cytotoxic to the parasite (19).
IEL may be associated with the parasite life cycle. A study by transmission electron microscopy performed with mice after infection with sporozoites of T. gondii reveals that sporozoites passed through intestinal epithelial cells and infected a number of different cell types within the lamina propria. Sporozoites did not infect intraepithelial lymphocytes, but at 48 h postinfection, IEL could be infected with tachyzoites arising from those that had developed in the lamina propria (89). In chickens, Eimeria multiplication occurs within enterocytes and the sporozoites of some species such as E. tenella enter the epithelium at the villous tip but migrate to the crypt where intracellular development commences. Sporozoites at the villous tip transfer from epithelial cells to IELs and are then translocated within these cells via the lamina propria to the crypt. CD8 cells are mostly the carrier of the sporozoites (56). Eimeria infections induce changes in the intestinal intraepithelial population (7, 23). Growth of the parasite in the intestinal epithelium leads to the development of the host immune response and CD8+ cells, which increased in number after challenge infection, seem to act as effector cells in acquired immunity (93).
CONCLUSION
Many pathogens are acquired via ingestion and invasion of the intestinal tract. Despite the diversity of the extracellular and intracellular pathogens discussed in this review, our current understanding of the mechanisms involved in the immune response indicates that a common exuberant immune response to rid the host of these agents is elicited. This robust inflammatory response is controlled by a series of regulatory mechanisms in most species. When this balance is no longer evident, a lethal inflammation of the intestine may occur, such as IBD or acute ileitis. The delicate balance between these dichotomous responses provides the host with protection against the pathogen yet maintains the integrity of the mucosal surface.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI 19613, AI 30000, and TW01003.
We thank Jacqueline Y. Channon and Franck Mennechet for thoughtful review of the manuscript.
REFERENCES
- 1.Ahuja S S, Paliogianni F, Yamada H, Balow J E, Boumpas D T. Effect of transforming growth factor-beta on early and late activation events in human T cells. J Immunol. 1993;150:3109–3018. [PubMed] [Google Scholar]
- 2.Alican I, Kubes P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol. 1996;270:G225–G237. doi: 10.1152/ajpgi.1996.270.2.G225. [DOI] [PubMed] [Google Scholar]
- 3.Argenzio R A, Liacos J A, Levy M L, Meuten D J, Lecce J G, Powell D W. Villous atrophy, crypt hyperplasia, cellular infiltration, and impaired glucose-Na absorption in enteric cryptosporidiosis of pigs. Gastroenterology. 1990;98:1129–1140. doi: 10.1016/0016-5085(90)90325-u. [DOI] [PubMed] [Google Scholar]
- 4.Austrup F, Rebstock S, Kilshaw P J, Hamann A. Transforming growth factor-beta 1-induced expression of the mucosa-related integrin alpha E on lymphocytes is not associated with mucosa-specific homing. Eur J Immunol. 1995;25:1487–1491. doi: 10.1002/eji.1830250602. [DOI] [PubMed] [Google Scholar]
- 5.Barrett T A, Tatsumi Y, Bluestone J A. Tolerance of T cell receptor gamma/delta cells in the intestine. J Exp Med. 1993;177:1755–1762. doi: 10.1084/jem.177.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin C, Berg E L, Briskin M J, Andrew D P, Kilshaw P J, Holzmann B, Weissman I L, Hamann A, Butcher E C. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–196. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 7.Bessay M, Le Vern Y, Kerboeuf D, Yvore P, Quere P. Changes in intestinal intra-epithelial and systemic T-cell subpopulations after an Eimeria infection in chickens: comparative study between E. acervulina and E. tenella. Vet Res. 1996;27:503–514. [PubMed] [Google Scholar]
- 8.Bogdan C, Rollinghoff M. How do protozoan parasites survive inside macrophages? Parasitol Today. 1999;15:22–28. doi: 10.1016/s0169-4758(98)01362-3. [DOI] [PubMed] [Google Scholar]
- 9.Buzoni-Gatel D, Debbabi H, Moretto M, Dimier-Poisson I H, Lepage A, Bout D T, Kasper L H. Intraepithelial lymphocytes traffic to the intestine and enhance resistance to Toxoplasma gondii oral infection. J Immunol. 1999;162:5846–5852. [PubMed] [Google Scholar]
- 10.Buzoni-Gatel D, Lepage A C, Dimier-Poisson I H, Bout D T, Kasper L H. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J Immunol. 1997;158:5883–5889. [PubMed] [Google Scholar]
- 10a.Burzoni-Gatel, D., H. Debbabi, F. D. Menneclat, J. Martin, A. C. Lepage, J. D. Schwartzman, and L. H. Kasper. Acute inflammatory ileitis after intracellular parasite infection is controlled by TGF-β producing intraepithelial lymphocytes. Gastroenterology, in press. [DOI] [PubMed]
- 11.Campbell N, Yio X Y, So L P, Li Y, Mayer L. The intestinal epithelial cell: processing and presentation of antigen to the mucosal immune system. Immunol Rev. 1999;172:315–324. doi: 10.1111/j.1600-065x.1999.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 12.Chardes T, Buzoni-Gatel D, Lepage A, Bernard F, Bout D. Toxoplasma gondii oral infection induces specific cytotoxic CD8 alpha/beta+ Thy-1+ gut intraepithelial lymphocytes, lytic for parasite-infected enterocytes. J Immunol. 1994;153:4596–4603. [PubMed] [Google Scholar]
- 13.Clark I A, Rockett K A. Nitric oxide and parasitic disease. Adv Parasitol. 1996;37:1–56. doi: 10.1016/s0065-308x(08)60218-3. [DOI] [PubMed] [Google Scholar]
- 14.Culshaw R J, Bancroft G J, McDonald V. Gut intraepithelial lymphocytes induce immunity against Cryptosporidium infection through a mechanism involving gamma interferon production. Infect Immun. 1997;65:3074–3079. doi: 10.1128/iai.65.8.3074-3079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz H H, Henriksen P, Bille-Hansen V, Henriksen S A. Toxoplasmosis in a colony of New World monkeys. Vet Parasitol. 1997;68:299–304. doi: 10.1016/s0304-4017(96)01088-6. [DOI] [PubMed] [Google Scholar]
- 16.Dimier I H, Bout D T. Interferon-gamma-activated primary enterocytes inhibit Toxoplasma gondii: a role for intracellular iron. Immunology. 1998;94:488–495. doi: 10.1046/j.1365-2567.1998.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimier I H, Quere P, Naciri M, Bout D T. Inhibition of Eimeria tenella development in vitro mediated by chicken macrophages and fibroblasts treated with chicken cell supernatants with IFN-gamma activity. Avian Dis. 1998;42:239–247. [PubMed] [Google Scholar]
- 18.Ebenfelt A, Lundqvist H, Dahlgren C, Lundberg C. Neutrophils in mucosal secretion are functionally active. Clin Exp Immunol. 1996;106:404–409. doi: 10.1046/j.1365-2249.1996.d01-846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebert E C. Giardia induces proliferation and interferon gamma production by intestinal lymphocytes. Gut. 1999;44:342–346. doi: 10.1136/gut.44.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckmann L, Laurent F, Langford T D, Hetsko M L, Smith J R, Kagnoff M F, Gillin F D. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J Immunol. 2000;164:1478–1487. doi: 10.4049/jimmunol.164.3.1478. [DOI] [PubMed] [Google Scholar]
- 21.Fang F C. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Investig. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes P D, Assreuy J. Role of nitric oxide and superoxide in Giardia lamblia killing. Braz J Med Biol Res. 1997;30:93–99. doi: 10.1590/s0100-879x1997000100015. [DOI] [PubMed] [Google Scholar]
- 23.Findly R C, Roberts S J, Hayday A C. Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur J Immunol. 1993;23:2557–2564. doi: 10.1002/eji.1830231027. [DOI] [PubMed] [Google Scholar]
- 24.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions [see comments] J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazzinelli R T, Oswald I P, Hieny S, James S L, Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 27.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 28.Gelfanov V, Gelfanova V, Lai Y G, Liao N S. Activated alpha beta-CD8+, but not alpha alpha-CD8+, TCR-alpha beta+ murine intestinal intraepithelial lymphocytes can mediate perforin-based cytotoxicity, whereas both subsets are active in Fas-based cytotoxicity. J Immunol. 1996;156:35–41. [PubMed] [Google Scholar]
- 29.Guy-Grand D, Cuenod-Jabri B, Malassis-Seris M, Selz F, Vassalli P. Complexity of the mouse gut T cell immune system: identification of two distinct natural killer T-cell intraepithelial lineages. Eur J Immunol. 1996;26:2248–2256. doi: 10.1002/eji.1830260942. [DOI] [PubMed] [Google Scholar]
- 30.Guy-Grand D, DiSanto J P, Henchoz P, Malassis-Seris M, Vassalli P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. Eur J Immunol. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Hamann A, Andrew D P, Jablonski-Westrich D, Holzmann B, Butcher E C. Role of α4 integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 32.Hoffman R A, Zhang G, Nussler N C, Gleixner S L, Ford H R, Simmons R L, Watkins S C. Constitutive expression of inducible nitric oxide synthase in the mouse ileal mucosa. Am J Physiol. 1997;272:G383–G392. doi: 10.1152/ajpgi.1997.272.2.G383. [DOI] [PubMed] [Google Scholar]
- 33.Hosokawa T, Kusugami K, Ina K, Ando T, Shinoda M, Imada A, Ohsuga M, Sakai T, Matsuura T, Ito K, Kaneshiro K. Interleukin-6 and soluble interleukin-6 receptor in the colonic mucosa of inflammatory bowel disease. J Gastroenterol Hepatol. 1999;14:987–996. doi: 10.1046/j.1440-1746.1999.01989.x. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages [see comments] Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 35.Ina K, Kusugami K, Hosokawa T, Imada A, Shimizu T, Yamaguchi T, Ohsuga M, Kyokane K, Sakai T, Nishio Y, Yokoyama Y, Ando T. Increased mucosal production of granulocyte colony-stimulating factor is related to a delay in neutrophil apoptosis in inflammatory bowel disease. J Gastroenterol Hepatol. 1999;14:46–53. doi: 10.1046/j.1440-1746.1999.01807.x. [DOI] [PubMed] [Google Scholar]
- 36.Iwasaki A, Kelsall B L. Mucosal immunity and inflammation. I. Mucosal dendritic cells: their specialized role in initiating T cell responses. Am J Physiol. 1999;276:G1074–G1078. doi: 10.1152/ajpgi.1999.276.5.G1074. [DOI] [PubMed] [Google Scholar]
- 37.James S L. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaiserlian D, Vidal K, Revillard J P. Murine enterocytes can present soluble antigen to specific class II- restricted CD4+ T cells. Eur J Immunol. 1989;19:1513–1516. doi: 10.1002/eji.1830190827. [DOI] [PubMed] [Google Scholar]
- 39.Katelaris P H, Naeem A, Farthing M J. Attachment of Giardia lamblia trophozoites to a cultured human intestinal cell line. Gut. 1995;37:512–518. doi: 10.1136/gut.37.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelsall B, Strober W. Dendritic cells of the gastrointestinal tract. Semin Immunopathol. 1997;18:409–420. doi: 10.1007/BF00824050. [DOI] [PubMed] [Google Scholar]
- 41.Khan I A, Matsuura T, Kasper L H. Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int Immunol. 1996;8:887–896. doi: 10.1093/intimm/8.6.887. [DOI] [PubMed] [Google Scholar]
- 42.Khan I A, Schwartzmann J D, Matsuura T, Kasper L H. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci USA. 1997;94:13955–13960. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilshaw P J, Murant S J. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol. 1990;20:2201–2207. doi: 10.1002/eji.1830201008. [DOI] [PubMed] [Google Scholar]
- 44.Kim J M, Jung H C, Im K I, Song I S, Kim C Y. Synergy between Entamoeba histolytica and Escherichia coli in the induction of cytokine gene expression in human colon epithelial cells. Parasitol Res. 1998;84:509–512. doi: 10.1007/BF03356595. [DOI] [PubMed] [Google Scholar]
- 45.Kim S K, Reed D S, Heath W R, Carbone F, Lefrancois L. Activation and migration of CD8 T cells in the intestinal mucosa. J Immunol. 1997;159:4295–4306. [PubMed] [Google Scholar]
- 46.Laurent F, Eckmann L, Savidge T C, Morgan G, Theodos C, Nacirii M, Kagnoff M F. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65:5067–5073. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laurent F, Kagnoff M F, Savidge T C, Naciri M, Eckmann L. Human intestinal epithelial cells respond to Cryptosporidium parvum infection with increased prostaglandin H synthase 2 expression and prostaglandin E2 and F2α production. Infect Immun. 1998;66:1787–1790. doi: 10.1128/iai.66.4.1787-1790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laurent F, McCole D, Eckmann L, Kagnoff M F. Pathogenesis of Cryptosporidium parvum infection. Microbes Infect. 1999;1:141–148. doi: 10.1016/s1286-4579(99)80005-7. [DOI] [PubMed] [Google Scholar]
- 49.Lefrancois L, Fuller B, Huleatt J W, Olson S, Puddington L. On the front lines: intraepithelial lymphocytes as primary effectors of intestinal immunity. Semin Immunopathol. 1997;18:463–475. doi: 10.1007/BF00824053. [DOI] [PubMed] [Google Scholar]
- 50.Lepage A C, Buzoni-Gatel D, Bout D T, Kasper L H. Gut-derived intraepithelial lymphocytes induce long term immunity against Toxoplasma gondii. J Immunol. 1998;161:4902–4908. [PubMed] [Google Scholar]
- 51.Leroy A, De Bruyne G, Verspeelt A, Lauwaet T, Nelis H, Mareel M. Bacterium-assisted invasion of Entamoeba histolytica through human enteric epithelia in two-compartment chambers. Invasion Metastasis. 1997;17:138–148. [PubMed] [Google Scholar]
- 52.Leroy A, Lauwaet T, De Bruyne G, Cornelissen M, Mareel M. Entamoeba histolytica disturbs the tight junction complex in human enteric T84 cell layers. FASEB J. 2000;14:1139–1146. doi: 10.1096/fasebj.14.9.1139. [DOI] [PubMed] [Google Scholar]
- 53.Letterio J J. Murine models define the role of TGF-β as a master regulator of immune cell function. Cytokine Growth Factor Rev. 2000;11:81–87. doi: 10.1016/s1359-6101(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 54.Li E, Stenson W F, Kunz-Jenkins C, Swanson P E, Duncan R, Stanley S L., Jr Entamoeba histolytica interactions with polarized human intestinal Caco-2 epithelial cells. Infect Immun. 1994;62:5112–5119. doi: 10.1128/iai.62.11.5112-5119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liesenfeld O, Kosek J, Remington J S, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lillehoj H S, Trout J M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev. 1996;9:349–360. doi: 10.1128/cmr.9.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lloyd A R, Oppenheim J J. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992;13:169–172. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Osuna M, Arellano J, Kretschmer R R. The destruction of virulent Entamoeba histolytica by activated human eosinophils. Parasite Immunol. 1992;14:579–586. doi: 10.1111/j.1365-3024.1992.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 59.MacDonald T T. Epithelial proliferation in response to gastrointestinal inflammation. Ann N Y Acad Sci. 1992;664:202–209. doi: 10.1111/j.1749-6632.1992.tb39761.x. [DOI] [PubMed] [Google Scholar]
- 60.Madara J L. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- 61.Martinez-Palomo A, Gonzalez-Robles A, Chavez B, Orozco E, Fernandez-Castelo S, Cervantes A. Structural bases of the cytolytic mechanisms of Entamoeba histolytica. J Protozool. 1985;32:166–175. doi: 10.1111/j.1550-7408.1985.tb03033.x. [DOI] [PubMed] [Google Scholar]
- 62.Mayer L, Eisenhardt D, Salomon P, Bauer W, Plous R, Piccinini L. Expression of class II molecules on intestinal epithelial cells in humans. Differences between normal and inflammatory bowel disease [see comments] Gastroenterology. 1991;100:3–12. doi: 10.1016/0016-5085(91)90575-6. [DOI] [PubMed] [Google Scholar]
- 63.McCole D F, Eckmann L, Laurent F, Kagnoff M F. Intestinal epithelial cell apoptosis following Cryptosporidium parvum infection. Infect Immun. 2000;68:1710–1713. doi: 10.1128/iai.68.3.1710-1713.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McDonald V. Gut intraepithelial lymphocytes and immunity to Coccidia. Parasitol Today. 1999;15:483–487. doi: 10.1016/s0169-4758(99)01570-7. [DOI] [PubMed] [Google Scholar]
- 65.Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, Luzza F, Fusco A, Pallone F. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- 66.Moore K W, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 67.Moore R, Tzipori S, Griffiths J K, Johnson K, De Montigny L, Lomakina I. Temporal changes in permeability and structure of piglet ileum after site-specific infection by Cryptosporidium parvum. Gastroenterology. 1995;108:1030–1039. doi: 10.1016/0016-5085(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura S, Ohtani H, Watanabe Y, Fukushima K, Matsumoto T, Kitano A, Kobayashi K, Nagura H. In situ expression of the cell adhesion molecules in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Lab Investig. 1993;69:77–85. [PubMed] [Google Scholar]
- 69.Nelson B J, Ralph P, Green S J, Nacy C A. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol. 1991;146:1849–1857. [PubMed] [Google Scholar]
- 70.Neurath M F, Fuss I, Schurmann G, Pettersson S, Arnold K, Muller-Lobeck H, Strober W, Herfarth C, Buschenfelde K H. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann N Y Acad Sci. 1998;859:149–159. doi: 10.1111/j.1749-6632.1998.tb11119.x. [DOI] [PubMed] [Google Scholar]
- 71.Ni J, Hollander D. Expression of beta 7 integrins and other cell adhesion molecules on mouse lymphocytes and their modulation by a new cytokine, IL-2 receptor-inducing factor. Cell Immunol. 1995;164:150–155. doi: 10.1006/cimm.1995.1154. [DOI] [PubMed] [Google Scholar]
- 72.Ojcius D M, Perfettini J L, Bonin A, Laurent L. Caspase-dependent apoptosis during infection with C. parvum. Microbes Infect. 1999;1:1163–1168. doi: 10.1016/s1286-4579(99)00246-4. [DOI] [PubMed] [Google Scholar]
- 73.Ortaldo J R, Mason A T, O'Shea J J, Smyth M J, Falk L A, Kennedy I C, Longo D L, Ruscetti F W. Mechanistic studies of transforming growth factor-beta inhibition of IL-2-dependent activation of CD3-large granular lymphocyte functions. Regulation of IL-2R beta (p75) signal transduction. J Immunol. 1991;146:3791–3798. [PubMed] [Google Scholar]
- 74.Pardoux C, Asselin-Paturel C, Chehimi J, Gay F, Mami-Chouaib F, Chouaib S. Functional interaction between TGF-beta and IL-12 in human primary allogeneic cytotoxicity and proliferative response. J Immunol. 1997;158:136–143. [PubMed] [Google Scholar]
- 75.Pfefferkorn E R, Guyre P M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984;44:211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plum J, De Smedt M, Leclercq G, Vandekerckhove B. Influence of TGF-beta on murine thymocyte development in fetal thymus organ culture. J Immunol. 1995;154:5789–5798. [PubMed] [Google Scholar]
- 77.Poussier P, Julius M. Intestinal intraepithelial lymphocytes: the plot thickens. J Exp Med. 1994;180:1185–1189. doi: 10.1084/jem.180.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reis e Sousa C, Yap G, Schulz O, Rogers N, Schito M, Aliberti J, Hieny S, Sher A. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 1999;11:637–647. doi: 10.1016/s1074-7613(00)80138-7. [DOI] [PubMed] [Google Scholar]
- 79.Ridel P R, Auriault C, Darcy F, Pierce R J, Leite P, Santoro F, Neyrinck J L, Kusnierz J P, Capron A. Protective role of IgE in immunocompromised rat toxoplasmosis. J Immunol. 1988;141:978–983. [PubMed] [Google Scholar]
- 80.Roberts A I, Bilenker M, Ebert E C. Intestinal intraepithelial lymphocytes have a promiscuous interleukin-8 receptor. Gut. 1997;40:333–338. doi: 10.1136/gut.40.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salzman A L, Eaves-Pyles T, Linn S C, Denenberg A G, Szabo C. Bacterial induction of inducible nitric oxide synthase in cultured human intestinal epithelial cells. Gastroenterology. 1998;114:93–102. doi: 10.1016/s0016-5085(98)70637-7. [DOI] [PubMed] [Google Scholar]
- 82.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur J Immunol. 1994;24:793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 83.Seguin R, Kasper L H. Sensitized lymphocytes and CD40 ligation augment IL-12 production by human dendritic cells in response to T. gondii. J Infect Dis. 1999;179:467–474. doi: 10.1086/314601. [DOI] [PubMed] [Google Scholar]
- 84.Seguin R, Mann B J, Keller K, Chadee K. The tumor necrosis factor alpha-stimulating region of galactose-inhibitable lectin of Entamoeba histolytica activates gamma interferon-primed macrophages for amebicidal activity mediated by nitric oxide. Infect Immun. 1997;65:2522–2527. doi: 10.1128/iai.65.7.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seydel K B, Li E, Swanson P E, Stanley S L., Jr Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seydel K B, Li E, Zhang Z, Stanley S L J. Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology. 1998;115:1446–1453. doi: 10.1016/s0016-5085(98)70023-x. [DOI] [PubMed] [Google Scholar]
- 87.Seydel K B, Zang T, Champion G A, Fichtenbaum C, Swanson P E, Tzipori S, Griffiths J K, Stanley S L. Cryptosporidium parvum infection of human intestinal xenografts in SCID mice induces production of human tumor necrosis factor alpha and interleukin-8. Infect Immun. 1998;66:2379–2382. doi: 10.1128/iai.66.5.2379-2382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sher A, Gazzinelli R T, Oswald I P, Clerici M, Kullberg M, Pearce E J, Berzofsky J A, Mosmann T R, James S L, Morse H C., III Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 89.Speer C A, Dubey J P. Ultrastructure of early stages of infections in mice fed Toxoplasma gondii oocysts. Parasitology. 1998;116:35–42. doi: 10.1017/s0031182097001959. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki Y, Sher A, Yap G, Park D, Neyer L E, Liesenfeld O, Fort M, Kang H, Gufwoli E. IL-10 prevents necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164:5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 91.Takeuchi M, Alard P, Streilein J W. TGF-beta promotes immune deviation by altering accessory signals of antigen-presenting cells. J Immunol. 1998;160:1589–1597. [PubMed] [Google Scholar]
- 92.Tidswell M, Pachynski R, Wu S W, Qiu S Q, Dunham E, Cochran N, Briskin M J, Kilshaw P J, Lazarovits A I, Andrew D P, Butcher E C, Yednock T A, Erle D J. Structure-function analysis of the integrin beta 7 subunit: identification of domains involved in adhesion to MAdCAM-1. J Immunol. 1997;159:1497–1505. [PubMed] [Google Scholar]
- 93.Vervelde L, Vermeulen A N, Jeurissen S H. In situ characterization of leucocyte subpopulations after infection with Eimeria tenella in chickens. Parasite Immunol. 1996;18:247–256. doi: 10.1046/j.1365-3024.1996.d01-94.x. [DOI] [PubMed] [Google Scholar]
- 94.Villegas E N, Wille U, Craig L, Iinsley P S, Rennick D M, Peach R, Hunter C A. Blockade of costimulation prevents infection-induced immunopathology in interleulin-10-deficient mice. Infect Immun. 2000;68:2837–2844. doi: 10.1128/iai.68.5.2837-2844.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vinayak V K, Khanna R, Kum K. Kinetics of intraepithelium and lamina propria lymphocyte responses during Giardia lamblia infection in mice. Microb Pathog. 1991;10:343–350. doi: 10.1016/0882-4010(91)90079-p. [DOI] [PubMed] [Google Scholar]
- 96.Wagner N, Lohler J, Kunkel E J, Ley K, Leung E, Krissansen G, Rajewsky K, Muller W. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 97.Witthoft T, Eckmann L, Kim J M, Kagnoff M F. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am J Physiol. 1998;275:G564–G571. doi: 10.1152/ajpgi.1998.275.3.G564. [DOI] [PubMed] [Google Scholar]
- 98.Yamamoto M, Fujihashi K, Kawabata K, McGhee J R, Kiyono H. A mucosal intranet: intestinal epithelial cells down-regulate intraepithelial, but not peripheral, T lymphocytes. J Immunol. 1998;160:2188–2196. [PubMed] [Google Scholar]
- 99.Yu Y, Chadee K. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology. 1997;112:1536–1547. doi: 10.1016/s0016-5085(97)70035-0. [DOI] [PubMed] [Google Scholar]