Abstract
Introduction: To investigate the prognostic significance of liver tumor markers, the hemoglobin, albumin, lymphocyte, and platelet (HALP) score; neutrophil-to-lymphocyte ratio (NLR); and platelet-to-lymphocyte ratio (PLR), for predicting the specific site of recurrence or metastasis after surgery in patients with intrahepatic cholangiocarcinoma (ICC). Methods: In total, 162 patients with pathologically proven ICC who underwent curative surgery at Sun Yat-sen University Cancer Center between April 2016 and April 2020 were analyzed. Clinicopathological characteristics were collected retrospectively. The Kaplan–Meier method was used to analyze the overall survival (OS) and recurrence-free survival (RFS). Significant clinical factors were examined by univariate analysis and multivariate analysis and analyzed by receiver operating characteristic (ROC) curve analysis. Results: The cutoff values for the HALP score, NLR, and PLR were determined to be 43.63, 3.73, and 76.51, respectively, using the surv_cutpoint function of survminer using RFS as the target variable. In multivariate analysis, vascular invasion, pathology nerve tract invasion, and carbohydrate antigen 19-9 (CA19-9) levels were independent prognostic factors of OS, whereas the tumor number, pathology microvascular invasion, pathology differentiation, CA19-9 levels, and NLR were independent prognostic factors of RFS. For the whole recurrence analysis, the carcinoembryonic antigen (CEA) index exhibited the largest ROC curve area of all (AUC = 0.590), and the alpha-fetoprotein (AFP) index exhibited the smallest ROC curve area (AUC = 0.530). The HALP score exhibited the largest ROC curve area of all in predicting intrahepatic recurrence (AUC = 0.588), the NLR showed the best predictive value in predicting lymph node metastasis (AUC = 0.703), and the AUC of the CA19-9 index was the largest of all variables in predicting distant metastasis (AUC = 0.619). Conclusions: Our study showed that CA19-9, CEA, HALP score, and NLR are easily accessible, reliable, cost-effective indexes for predicting the specific site of recurrence or metastasis after surgery in ICC patients. Patients with high HALP scores and NLR have a higher risk of intrahepatic and lymph node metastasis recurrence.
Keywords: des-gamma-carboxyprothrombin, carbohydrate antigen 19-9, carcinoembryonic antigen, HALP score, NLR, PLR, prognosis
1. Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second-most common primary liver cancer, with an elevated incidence across the world [1]. Surgical resection is currently the only curative treatment strategy. Due to the high malignancy degree with insidious onset, invasive and fast-growing nature, and high rate of lymph node metastasis, most ICC patients are initially diagnosed late and lose the chance of surgical resection. Furthermore, the heterogeneity of ICC renders the early detection of postoperative tumor recurrence a major challenge. About 50% of patients experience recurrence within 2 years after curative resection, and five-year overall survival (OS) ranges from 21% to 35% [2,3,4]. The prognosis of ICC is affected by many factors, such as surgical quality, lymph node metastasis, and postoperative pathological features. Several studies have also reported that systemic nutritional and inflammation status play important roles in the development of cancer [5]. In addition, pretreatment serum tumor markers have important reference values for treatment planning and prognosis prediction.
Tumor markers are essential in the screening, early diagnosis, and differential diagnosis of cancer. The National Comprehensive Cancer Network (NCCN) guideline for ICC recommends laboratory tests of serum levels of alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA). AFP has been widely accepted as a tumor marker for hepatocellular carcinoma in population-based screening and surveillance for many years. Approximately 20% of ICC patients are AFP-positive, and these patients are often misdiagnosed as hepatocellular carcinoma (HCC) before pathological diagnosis [6]. Therefore, a more sensitive and specific tumor marker is needed for diagnosis and prognosis. Accurate clinical classification is the basis of disease diagnosis and treatment. Currently, the most widely used staging system for ICC relies on the Tumor Node Metastasis (TNM) system designed by the American Joint Committee on Cancer (AJCC) [7]. Although the CA19-9 expression levels were not included in the AJCC TNM staging system, they were noted to be an independent prognosis factor in the manual. Mavros et al. confirmed that 57.5% of 429 ICC displayed preoperative CA19-9 levels (>35 U/mL) [2]. This study further confirmed that the expression level of CA19-9 was associated with liver cirrhosis and lymph node metastasis and was positively related to the chance of tumor recurrence. Therefore, CA19-9 is the most widely used tumor marker in the diagnosis and surveillance of ICC. However, a recent meta-analysis suggested that the overall pooled sensitivity of CA19-9 in diagnosing cholangiocarcinoma was 0.72 (0.70–0.75), and specificity was 0.84 (0.82–0.85) [8]. Other related studies have reported that CA19-9 has a wide variation in sensitivity (50–90%) and specificity (54–98%) [9,10]. Because the concentrations of CA19-9 could rise in patients with benign inflammation as well as in other malignancies [11], the single tumor marker of CA19-9 has a high false-negative and high false-positive rate and in the diagnosis of ICC, which cannot achieve the expected effect in clinical application. Thus, additional biomarkers are needed to improve the sensitivity and specificity of ICC diagnoses. CEA, which is known as an important tumor marker in diagnosing and predicting prognosis in many gastrointestinal tumors [12], has also been reported as an independent prognosis factor for cholangiocarcinoma, including ICC [3]. The sensitivity and specificity for CEA values were 53–84% and 50–79% [13,14]. CEA levels have also been reported to be effectively supplementary to CA19-9 levels in predicting survival outcomes for ICC patients after surgery, especially in patients with normal preoperative CA19-9 levels [15]. Furthermore, prothrombin induced by vitamin K absence-II (PIVKA-II), also known as des-gamma-carboxyprothrombin (DCP), has been proven to be not only a specific tumor marker but also a predictor of prognosis for hepatocellular carcinoma (HCC) [16]. According to our previous study, elevated levels of PIVKA-II in HCC patients stand for more aggressive tumor biology with a larger tumor size, increased percentage of vascular invasion, and worse prognosis [17]. We have noticed in clinical practice that positive PIVKA-II is not a rare phenomenon, but the application of PIVKA-II in ICC patients has never been reported in previous publications. As one of the critical tumor markers for liver cancer, whether PIVKA-II can provide useful information on the biological behavior of ICC remains unknown.
If all tumor markers of an ICC patient are negative, searching for some clinical indicators, such as hematological indexes, to predict the survival outcomes of ICC patients is meaningful. Hemoglobin, albumin, lymphocytes, neutrophils, and platelets have been reported to play crucial roles in tumorigenesis and development, but their mechanism is very complex. Elevated hemoglobin, albumin, and lymphocytes may be favorable prognostic factors, while elevated neutrophils and platelets may be unfavorable prognostic factors. Using a single index to predict prognosis could be affected by many biased factors, so combining multiple indexes could reduce the error. Recent studies have identified a variety of inflammatory or nutritional prognosis indexes, including hemoglobin, albumin, lymphocyte, and platelet (HALP) score [18]; neutrophil-to-lymphocyte ratio (NLR) [19]; platelet-to-lymphocyte ratio (PLR) [20]; and so on. However, until now, there has been very little research on how these parameters affect the prognosis of ICC patients.
Therefore, it is informative to inspect the values of liver tumor markers, HALP score, PLR, and NLR in patients from China in a large sample size to predict the prognosis of ICC patients and guide personalized treatment.
2. Materials and Methods
2.1. Patients
A total of 162 patients with pathologically proven ICC who underwent radical surgery at Sun Yat-sen University Cancer Center between April 2016 and April 2020 were enrolled in our study.
The exclusion criteria included (1) pathologically confirmed HCC or combined HCC and ICC, (2) other synchronous malignancies, (3) lack of pretreatment tumor marker data, (4) a post-treatment survival time of less than 1 month, (5) lack of a follow-up assessment, and (6) liver function Child–Pugh C.
We collected clinicopathological data from our hospital information system, and lab test data were collected within three days before surgery. AJCC TNM staging, Child–Pugh classifications, and Barcelona Clinic Liver Cancer (BCLC) staging were calculated accordingly.
2.2. Follow-Up and Data
Patients were followed-up every three months up to two years and every six months until year five. Patients needed to be regularly followed-up with radiological examinations, such as computerized tomography (CT) scans or magnetic resonance imaging (MRI) and chest X-rays or CT. Laboratory tests included blood routines, liver function, and liver tumor markers. The cutoff values of AFP, CA19-9, CEA, and PIVKA-II were set to be 25 ng/mL, 35 U/mL, 5 ng/mL, and 40 mAU/mL, respectively, according to previous studies. If the values of these tumor markers were above the cutoff value, they were considered positive; HALP [hemoglobin (g/L) × albumin (g/L) × lymphocyte (/L)/platelet (/L)] and NLR [neutrophil (/L)/lymphocyte (/L)]; PLR [platelet (/L)/lymphocyte (/L)]. The HALP score, PLR, and NLR optimal cut-off point to separate continuous variables were identified using the surv_cutpoint function of survminer using recurrence-free survival (RFS) as the target variable. Supplement Figure S1 shows that the optimal thresholds of HALP score, PLR, and NLR for predicting ICC recurrence were 43.63, 76.51, and 3.73, respectively. OS was calculated from the date of surgery to the date of death or the last follow-up time. RFS was calculated from the date when the tumor recurrence was diagnosed or the last follow-up time. According to the relapse sites after surgery, recurrence was divided into intrahepatic tumor recurrence, lymph node metastasis, and distant metastasis. Correspondingly, intrahepatic tumor RFS, lymph node metastasis RFS, and distant metastasis RFS were calculated.
2.3. Statistical Analysis
All data were presented as mean 95% confidence intervals (CIs). The Pearson chi-square test was used to analyze categorical variables, and a two-tailed t-test was used to analyze the continuous variables. The Kaplan–Meier technique was employed to estimate the OS and RFS, and the log-rank test was applied to compare them. Significant clinical factors were examined by univariate analysis and multivariate analysis and analyzed by receiver operating characteristic (ROC) curve analyses.
We performed all statistical analyses with SPSS software version 26.0 (IBM Corporation, Armonk, NY, USA) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org (accessed on 1 September 2021)).
3. Results
3.1. Clinicopathological Characteristics
Table 1 outlines the clinicopathological features of the 162 enrolled patients. A total of 100 males (61.7%) with a median age of 56.1 years (range, 32–76 years) and 86 females (37.4%) with a median age of 57.4 years (range, 40–77 years) were included. There were 73 (45.1%) patients whose serum CA19-9 levels were positive (>35 U/mL), which was the highest among the four liver tumor markers. The positive rates of AFP, CEA, and PIVKA-II were 12.3%, 22.2%, and 20.4%, respectively. The patients with negative tumor markers accounted for 35.2%, and patients with single-positive and double- or more positive tumor markers accounted for 34.0% and 30.8%, respectively. The proportion of HBsAg-positive ICC patients was 52.5%. (Table 1).
Table 1.
Demographical characteristics and clinical data of the patients.
| Patient Characteristics | Number | Percentage | |
|---|---|---|---|
| Age | ≥56 | 91 | 56.2% |
| <56 | 71 | 43.8% | |
| Gender | Male | 100 | 61.7% |
| Female | 62 | 37.4% | |
| Tumor numbers | Single | 108 | 66.7% |
| Multiple | 54 | 33.3% | |
| Tumor size | ≤2.0 cm | 5 | 3.1% |
| 2.1–5.0 cm | 70 | 43.2% | |
| >5.0 cm | 87 | 53.7% | |
| Hepatis B surface antigen | Positive | 85 | 52.5% |
| Negative | 77 | 47.5% | |
| Hepatitis C virus antibody | Positive | 1 | 0.6% |
| Negative | 161 | 99.4% | |
| Platelet count (×109/L) | 242.8 ± 87.1 | ||
| Albumin (mg/dL) | 43.6 ± 3.7 | ||
| Alanine aminotransferase (U/L) | 35.4 ± 33.6 | ||
| Aspartate aminotransferase (U/L) | 32.6 ± 27.2 | ||
| Total bilirubin (umol/L) | 13.9 ± 19.9 | ||
| Prothrombin time (s) | 12.0 ± 4.9 | ||
| T stage | 1 | 89 | 54.9% |
| 2 | 58 | 35.8% | |
| 3 | 1 | 0.6% | |
| 4 | 14 | 8.6% | |
| N stage | 0 | 135 | 83.3% |
| 1 | 27 | 16.7% | |
| M stage | 0 | 153 | 94.4% |
| 1 | 9 | 5.6% | |
| AJCC TNM staging system | I | 82 | 50.6% |
| II | 41 | 25.3% | |
| III | 27 | 16.7% | |
| IV | 12 | 7.4% | |
| The number of elevated pretreatment serum liver tumor markers | 0 | 57 | 35.2% |
| 1 | 55 | 34.0% | |
| ≥2 | 50 | 30.8% | |
| Vascular invasion | Yes | 33 | 20.4% |
| No | 129 | 79.6% | |
| Microvascular invasion | Yes | 43 | 26.5% |
| No | 117 | 72.2% | |
| Unknown | 2 | 1.2% | |
| Nerve tract invasion | Yes | 30 | 18.5% |
| No | 130 | 80.2% | |
| Unknown | 2 | 1.2% | |
| Tumor differentiations | Well | 1 | 0.6% |
| Well-moderate | 1 | 0.6% | |
| Moderate | 45 | 27.8% | |
| Moderate to poor | 83 | 51.2% | |
| Poor | 21 | 13.0% | |
| Unknown | 11 | 6.8% | |
| Alpha-fetoprotein (25 ng/mL) | 421.9 ± 3664.4 | ||
| Cancer antigen 199 (35 U/mL) | 715.4 ± 2306.8 | ||
| Carcinoembryonic antigen (5 ng/mL) | 6.76 ± 19.6 | ||
| Protein induced by vitamin K absence or Antagonist-II (40 mAU/mL) | 400.7 ± 4112.3 | ||
| Alpha-fetoprotein (25 ng/mL) | Elevated | 20 | 12.3% |
| Normal | 142 | 87.7% | |
| Cancer antigen 199 (35 U/mL) | Elevated | 73 | 45.1% |
| Normal | 89 | 54.9% | |
| Carcinoembryonic antigen (5 ng/mL) | Elevated | 36 | 22.2% |
| Normal | 126 | 77.8% | |
| Protein induced by vitamin K absence or Antagonist-II (40 mAU/mL) | Elevated | 33 | 20.4% |
| Normal | 129 | 79.6% | |
| Hemoglobin, albumin, lymphocyte, and platelet score | 54.3 ± 30.1 | ||
| Neutrophil-to-lymphocyte ratio | 2.9 ± 2.8 | ||
| Platelet-to-lymphocyte ratio | 140.0 ± 66.6 | ||
| History of cholelithiasis | Yes | 30 | 18.5% |
| No | 132 | 81.5% | |
| Child–Pugh classifications | A | 160 | 98.8% |
| B | 2 | 1.2% | |
| Barcelona Clinic Liver Cancer staging | A | 100 | 61.7% |
| B | 20 | 12.3% | |
| C | 42 | 25.9% | |
| Recurrence | Yes | 94 | 58.0 |
| NO | 68 | 42.0% | |
| Intrahepatic tumor recurrence | Yes | 59 | 36.4% |
| NO | 103 | 63.6% | |
| Lymph node metastasis | Yes | 9 | 5.6% |
| NO | 153 | 94.4% | |
| Distant metastasis | Yes | 15 | 9.3% |
| NO | 147 | 90.7% | |
We then analyzed the correlation between different liver tumor markers and clinicopathological features. Our study found that the AFP level was significantly associated with gender and hepatitis B virus infection status (p < 0.05; Table S1). However, it did not correlate with OS and RFS (p > 0.05). Elevated CA19-9 was associated with decreased albumin levels, more advanced BCLC staging and AJCC staging, and the presence of lymph node metastasis (p < 0.05). Similar to CA19-9, the serum levels of CEA showed statistical significance with total bilirubin levels, BCLC staging, AJCC staging, tumor numbers, OS, RFS, and recurrence (p < 0.05). An increased level of PIVKA-II was related to more advanced BCLC staging and AJCC staging and fewer tumor numbers (p < 0.05). These four liver tumor markers did not exhibit a specific ability in predicting relapse sites (p > 0.05). However, patients in the HALP ≥ 43.63 group showed a greater proportion of intrahepatic recurrence than patients in the HALP < 43.63 group (43.0% vs. 25.8%, p = 0.027). More patients in the NLR ≥ 3.73 group had a higher rate of lymph node metastasis recurrence than patients in the NLR < 3.73 group.
3.2. Overall Survival and Recurrence-Free Survival Analysis
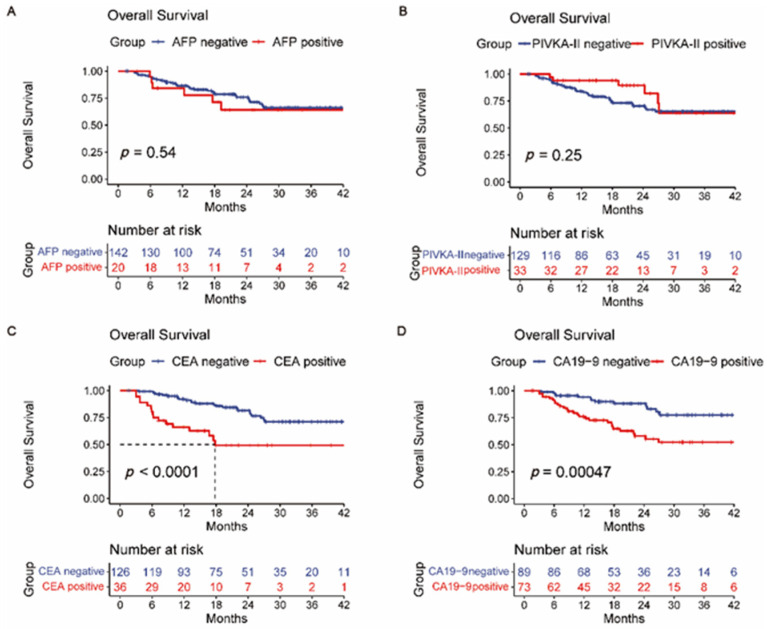
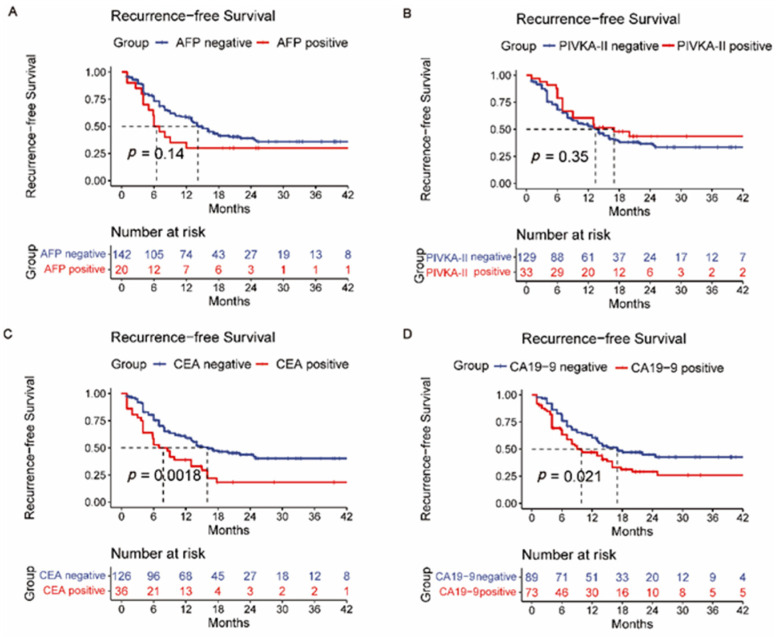
We also analyzed the relationship between four kinds of liver tumor markers and the prognosis of ICC patients (Figure 1A–D and Figure 2A–D). Patients with lower pretreatment CA19-9 and CEA levels had a better longer OS and RFS time (p < 0.05). However, pretreated AFP and PIVKA-II levels were not significant in the OS of the ICC patients. In addition, hepatitis B or C history was not associated with prognosis (Supplement Figures S2A,B and S3A,B). Furthermore, ICC with microvascular invasion (MVI) may have worse surgical outcomes with regard to RFS but not OS (Supplement Figures S2C and S3C). Differing from MVI, nerve tract invasion was significantly associated with OS but did not affect RFS (Supplement Figures S2D and S3D). Survival analysis also indicated that BCLC staging systems had predictive value for the prognosis of ICC (Supplement Figures S2E and S3E). Patients with positive lymph nodes showed poorer prognoses than those with negative lymph nodes (Supplement Figures S2E and S3F).
Figure 1.
Overall survival (OS) curves of grouped intrahepatic cholangiocarcinoma patients based on (A) AFP, (B) PIVKA-II, (C) CEA, and (D) CA19-9.
Figure 2.
Recurrence-free survival (RFS) curves of grouped intrahepatic cholangiocarcinoma patients based on (A) AFP, (B) PIVKA-II, (C) CEA, and (D) CA19-9.
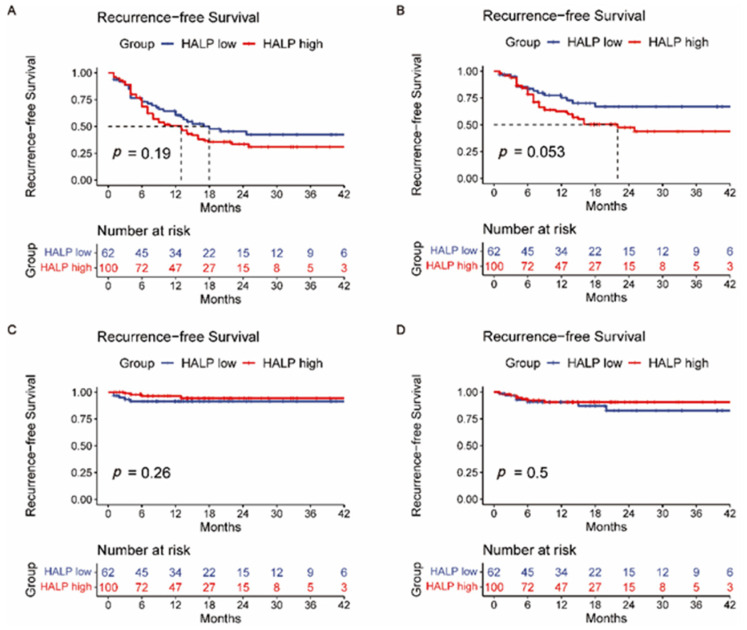
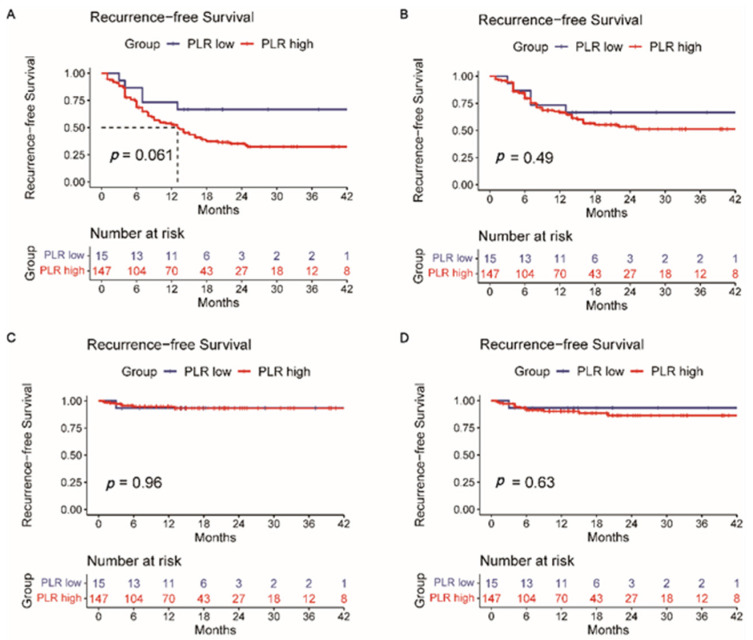
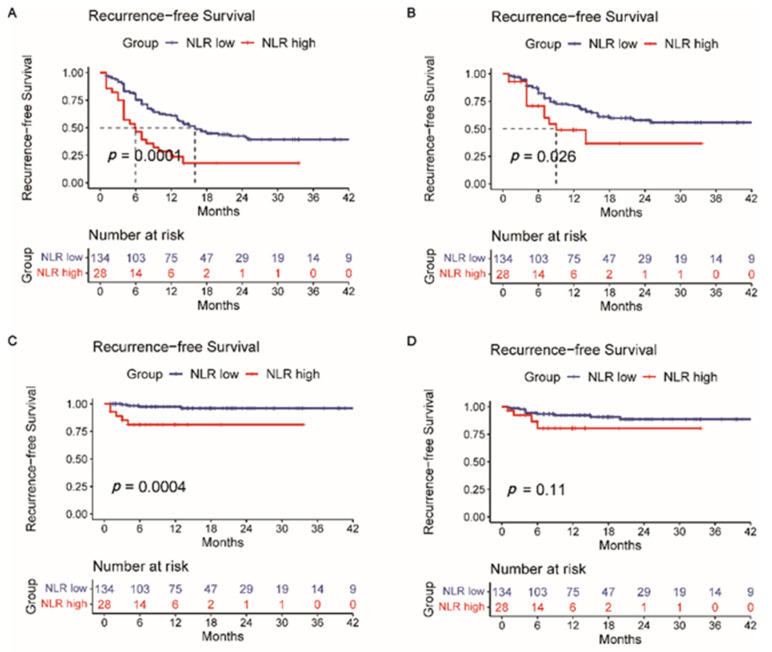
Then, survival analysis for the intrahepatic tumor RFS (Supplement Figure S4), lymph node metastasis RFS (Supplement Figure S5), and distant metastasis RFS (Supplement Figure S6) was calculated correspondingly. Intrahepatic recurrence is most common after liver resection. However, a single liver tumor marker or a different combination of them (AFP + PIVKA-II, AFP + CEA, AFP + CA19-9, PIVKA-II + CEA, PIVKA-II + CA19-9, and CEA + CA19-9) did not indicate reliable predictive power of intrahepatic recurrence (p > 0.05) (Supplement Figure S4). Likewise, this situation also occurred in predicting lymph node metastasis recurrence (p > 0.05) (Supplement Figure S5). Supplement Figure S6 shows that a single CA19-9 or CEA has the potential predictive power of distant metastasis after surgery (p < 0.05). A Kaplan–Meier analysis was also performed to determine the relationship between the HALP score (Figure 3), PLR (Figure 4), NLR (Figure 5), and RFS. Survival analysis revealed that the HALP score could have the potential predictive ability of intrahepatic recurrence (p = 0.053) (Figure 3). The predictive prognosis ability of PLR did not reach statistical significance (p > 0.05) (Figure 4). Moreover, we found that the NLR generated good performance in predicting the survival outcome in intrahepatic recurrence (p = 0.026) and lymph node metastasis (p = 0.0004) (Figure 5).
Figure 3.
The value of HALP score in predicting (A) recurrence-free survival, (B) intrahepatic recurrence, (C) lymph node metastasis, and (D) distant metastasis recurrence.
Figure 4.
The value of PLR in predicting (A) recurrence-free survival, (B) intrahepatic recurrence, (C) lymph node metastasis, and (D) distant metastasis recurrence.
Figure 5.
The value of NLR in predicting (A) recurrence-free survival, (B) intrahepatic recurrence, (C) lymph node metastasis, and (D) distant metastasis recurrence.
3.3. Univariate and Multivariable Cox Regression Analyses in Cohorts
The univariate analyses showed that vascular invasion, pathology nerve tract invasion, CA19-9, CEA, and NLR levels were associated with OS. Tumor number, tumor size, vascular invasion, pathological MVI, pathology differentiation, and CA19-9, CEA, and NLR levels were associated with RFS. In the multivariate study, we demonstrated that vascular invasion, pathology nerve tract invasion, and CA19-9 levels remained independent prognostic factors of OS, and tumor number, MVI, pathology differentiation, and CA19-9 and NLR levels were still independent prognostic factors of RFS (Table 2 and Table 3).
Table 2.
Univariate and multivariate analysis for OS in patients with ICC.
| Variables | Overall Survival Univariate Analysis |
Overall Survival Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (≥56 vs. <56) | 1.725 | 0.916–3.248 | 0.091 | |||
| Gender (male vs. female) | 0.977 | 0.718–1.330 | 0.883 | |||
| Cirrhosis (yes vs. no) | 1.146 | 0.614–2.138 | 0.669 | |||
| Tumor number (multiple vs. single) | 1.757 | 0.953–3.239 | 0.071 | |||
| Tumor size (>5 cm vs. ≤5 cm) | 1.800 | 0.963–3.364 | 0.065 | |||
| Vascular invasion | 2.992 | 1.615–5.543 | <0.001 * | 2.347 | 1.216–4.530 | 0.011 * |
| Pathology microvascular invasion | 1.311 | 0.681–2.523 | 0.418 | |||
| Pathology nerve tract invasion | 2.846 | 1.490–5.437 | 0.002 * | 3.018 | 1.536–5.927 | 0.001 * |
| Pathology differentiation degree (Moderate to poor, poor vs. well, well to moderate, moderate) | 1.483 | 0.719–3.056 | 0.286 | |||
| AFP (≥25 vs. <25) | 1.236 | 0.521–2.935 | 0.631 | |||
| CA19-9 (≥35 vs. <35) | 2.653 | 1.410–4.989 | 0.002 * | 2.071 | 1.030–4.167 | 0.041 * |
| CEA (≥5 vs. <5) | 3.384 | 1.818–6.300 | <0.001 * | 1.965 | 0.880–3.746 | 0.107 |
| PIVKA-II (≥40 vs. <40) | 0.463 | 0.182–1.180 | 0.107 | |||
| History of cholelithiasis | 1.660 | 0.909–3.032 | 0.099 | |||
| Hepatitis B | 0.920 | 0.502–1.687 | 0.788 | |||
| HALP (≥43.63 vs. <43.63) | 1.258 | 0.662–2.391 | 0.483 | |||
| NLR (≥3.73 vs. <3.73) | 0.475 | 0.238–0.946 | 0.034 * | 1.463 | 0.691–3.098 | 0.320 |
| PLR (≥76.51 vs. <76.51) | 0.217 | 0.030–1.580 | 0.132 | |||
Notes: * p < 0.05.
Table 3.
Univariate and multivariate analysis for RFS in patients with ICC.
| Variables | Recurrence-Free Survival Univariate Analysis |
Recurrence-Free Survival Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (≥56 vs. <56) | 1.088 | 0.723–1.636 | 0.686 | |||
| Gender (male vs. female) | 1.172 | 0.948–1.449 | 0.142 | |||
| Cirrhosis (yes vs.no) | 1.277 | 0.844–1.933 | 0.247 | |||
| Tumor number (multiple vs. single) | 2.491 | 1.651–3.759 | <0.001 * | 2.060 | 1.250–3.397 | 0.005 * |
| Tumor size (>5 cm vs. ≤5 cm) | 1.925 | 1.265–2.928 | 0.002 * | 1.315 | 0.813–2.127 | 0.265 |
| Vascular invasion | 2.539 | 1.619–3.982 | <0.001 * | 1.561 | 0.931–2.617 | 0.091 |
| Pathology microvascular invasion | 1.980 | 1.290–3.040 | 0.002 * | 1.800 | 1.121–2.892 | 0.015 * |
| Pathology nerve tract invasion | 1.204 | 0.719–2.015 | 0.481 | |||
| Pathology differentiation degree (moderate to poor, poor vs. well, well to moderate, moderate) | 1.976 | 1.187–3.290 | 0.009* | 1.826 | 1.054–3.163 | 0.032 * |
| AFP (≥25 vs. <25) | 1.514 | 0.858–2.675 | 0.153 | |||
| CA19-9 (≥35 vs. <35) | 1.587 | 1.058–2.380 | 0.025 * | 1.794 | 1.117–2.882 | 0.016 * |
| CEA (≥5 vs. <5) | 1.972 | 1.264–3.074 | 0.003 * | 1.037 | 0.609–1.766 | 0.893 |
| PIVKA-II (≥40 vs. <40) | 0.789 | 0.472–1.320 | 0.789 | |||
| History of cholelithiasis | 1.326 | 0.809–2.173 | 0.263 | |||
| Hepatitis B | 1.056 | 0.704–1.585 | 0.792 | |||
| HALP (≥43.63 vs. <43.63) | 1.319 | 0.857–2.030 | 0.208 | |||
| NLR (≥3.73 vs. <3.73) | 0.400 | 0.246–0.652 | 0.000 * | 2.424 | 1.386–4.239 | 0.002 * |
| PLR (≥76.51 vs. <76.51) | 0.439 | 0.178–1.082 | 0.074 | |||
Notes: * p < 0.05.
To verify the value of liver tumor markers, HALP score, PLR, and NLR on the prognosis of ICC patients, we analyzed the relationship between recurrent sites and the clinicopathological parameters, and the results of univariate analysis are summarized in Table 4. Tumor number, tumor size, vascular invasion, pathology MVI, pathology differentiation degree, and NLR were significantly associated with intrahepatic tumor recurrence on univariate analysis. Notably, the NLR was the only factor significantly associated with lymph node metastasis recurrence (hazard ratio (HR): 7.757, 95% confidence interval (CI0: 2.031–29.619; p = 0.003). In addition, vascular invasion, CEA, and a history of cholelithiasis were associated with a higher rate of distant metastasis recurrence.
Table 4.
Univariate analysis for intrahepatic tumor recurrence, lymph node metastasis, and distant metastasis in patients with ICC.
| Intrahepatic Tumor Recurrence | Lymph Node Metastasis | Distant Metastasis | ||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Univariate Analysis | Univariate Analysis | ||||
| Hazard Ratio (95% CI) |
p-Value | Hazard Ratio (95% CI) |
p-Value | Hazard Ratio (95% CI) |
p-Value | |
| Age (≥56 vs. <56) | 1.320 (0.782–2.229) |
0.298 | 1.635 (0.409–6.538) |
0.487 | 0.549 (0.195–1.542) |
0.255 |
| Gender (male vs. female) | 1.202 (0.918–1.574) |
0.182 | 0.924 (0.478–1.783) |
0.813 | 1.036 (0.617–1.738) |
0.895 |
| Cirrhosis (yes vs. no) | 1.196 (0.711–2.012) |
0.500 | 0.634 (0.170–2.361) |
0.497 | 0.923 (0.335–2.548) |
0.878 |
| Tumor number (multiple vs. single) | 2.461 (1.465–4.135) |
0.001 * | 1.852 (0.494–6.946) |
0.361 | 1.667 (0.588–4.727) |
0.336 |
| Tumor size (>5 cm vs. ≤5 cm) | 1.977 (1.162–3.364) |
0.012 * | 7.836 (0.977–62.857) |
0.053 | 2.971 (0.938–9.408) |
0.064 |
| Vascular invasion | 1.983 (1.094–3.591) |
0.024 * | 0.584 (0.073–4.682) |
0.613 | 3.496 (1.227–9.966) |
0.019 * |
| Pathology microvascular invasion | 1.832 (1.063–3.158) |
0.029 * | 0.438 (0.054–3.562) |
0.440 | 1.301 (0.406–4.170) |
0.658 |
| Pathology nerve tract invasion | 0.794 (0.377–1.676) |
0.546 | 0.600 (0.075–4.799) |
0.630 | 0.354 (0.046–2.690) |
0.315 |
| Pathology differentiation degree (moderate to poor, poor vs. well, well to moderate, moderate) | 1.971 (1.037–3.743) |
0.038 * | 3.919 (0.490–31.359) |
0.198 | 2.911 (0.643–13.173) |
0.165 |
| AFP (≥25 vs. <25) | 1.171 (0.531–2.579) |
0.696 | 0.983 (0.123–7.875) |
0.987 | 1.233 (0.278–5.472) |
0.783 |
| CA19-9 (≥35 vs. <35) | 1.365 (0.818–2.277) |
0.234 | 0.393 (0.082–1.894) |
0.244 | 2.891 (0.986–8.473) |
0.053 |
| CEA (≥5 vs. <5) | 1.302 (0.702–2.414) |
0.403 | 2.012 (0.502–8.059) |
0.323 | 2.920 (1.033–8.255) |
0.043 * |
| PIVKA-II (≥40 vs. <40) | 0.763 (0.962–1.141) |
0.419 | 0.435 (0.054–3.480) |
0.433 | 0.858 (0.242–3.042) |
0.813 |
| History of cholelithiasis | 1.259 (0.668–2.347) |
0.476 | 2.324 (0.581–9.296) |
0.233 | 3.106 (1.105–8.729) |
0.032 * |
| Hepatitis B | 0.976 (0.585–1.627) |
0.925 | 1.068 (0.287–3.980) |
0.922 | 1.741 (0.595–5.099) |
0.312 |
| HALP (≥43.63 vs. <43.63) | 1.732 (0.975–3.078) |
0.061 | 0.478 (0.128–1.782) |
0.272 | 0.703 (0.255–1.941) |
0.703 |
| NLR (≥3.73 vs. <3.73) | 2.020 (1.061–3.847) |
0.032 * | 7.757 (2.031–29.619) |
0.003 * | 2.468 (0.769–7.921) |
0.129 |
| PLR (≥76.51 vs. <76.51) | 1.375 (0.549–3.438) |
0.497 | 0.944 (0.118–7.566) |
0.957 | 1.639 (0.215–12.467) |
0.633 |
Notes: * p < 0.05.
3.4. Comparison of AFP, PIVKA-II, CEA, CA19-9, HALP Score, PLR, and NLR
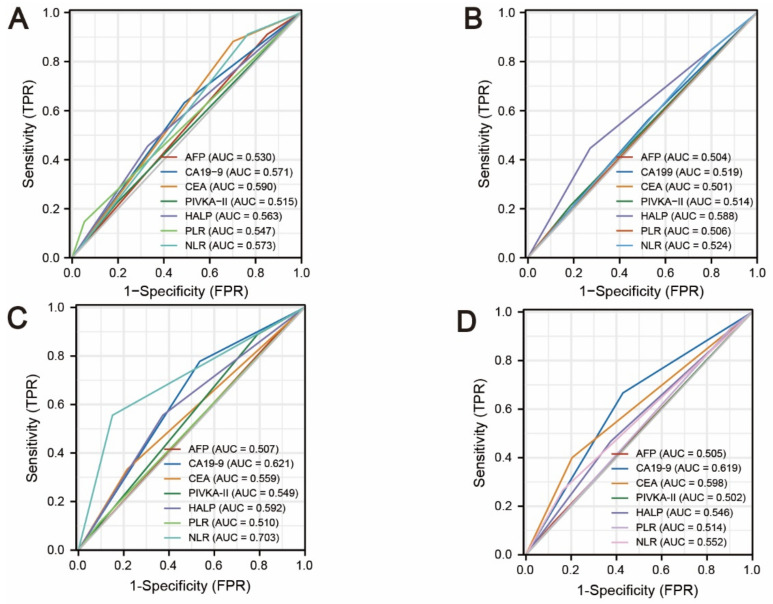
In addition, the predictive accuracy of seven variables (AFP, PIVKA-II, CEA, CA19-9, HALP score, PLR, and NLR), which were readily available before the operation, was analyzed using ROC curves (Figure 6). For the whole recurrence analysis, the CEA index exhibited the largest ROC curve area of all (AUC = 0.590), and the AFP index exhibited the smallest ROC curve area (AUC = 0.530) (Figure 6A). Surprisingly, the HALP score exhibited the largest ROC curve area of all in predicting intrahepatic recurrence (AUC = 0.588) (Figure 6B), and the NLR showed the best predictive value in predicting lymph node metastasis recurrence (AUC = 0.703) (Figure 6C). Last but not least, the AUC of the CA19-9 index was the largest of all variables in predicting distant metastasis recurrence (AUC = 0.619) (Figure 6C).
Figure 6.
The area under the curve (AUC) for HALP, PLR, NLR, AFP, PIVKA-II, CEA, and CA19-9; (A) AUC of recurrence-free survival; (B) AUC of intrahepatic recurrence; (C) AUC of lymph node metastasis; and (D) AUC of distant metastasis recurrence.
4. Discussion
ICC is one of the highest degrees of malignancies, with rising incidence and mortality rates around the world [21,22]. Despite continued advances in new treatment options, the prognosis of ICC patients who cannot undergo curative resection is still unsatisfactory. In general, early diagnosis is necessary for beneficial outcomes, and tumor markers have a noticeable effect on the diagnosis and follow-up of cancer patients. The most commonly used tumor markers for liver cancers include CA19-9, CEA, AFP, and PIVKA-II. Here, we investigated the diagnostic and prognostic value of these four liver tumor markers in 162 ICC patients. Although PIVKA-II has a comparable positive rate with CEA in ICC patients, this is the first study to investigate the application of PIVKA-II in ICC patients.
In this study, the positivity rate of serum CA19-9 levels was about 45.1%, which agrees well with previous studies [2]. Univariate and multivariate Cox proportional hazard regression analyses showed that pretreated serum CA19-9 levels were independently prognostic. Patients with lower CA19-9 levels had better long-term OS and RFS (p < 0.05). According to previous research, preoperative CEA levels were complementary to CA19-9 levels in predicting prognosis in patients with resectable ICC [15], and univariate analysis in our study also revealed that evaluated serum CEA levels were related to poor prognosis. These two tumor markers were previously confirmed and commonly applied in the diagnosis of ICC. However, the NCCN guideline for ICC recommends that the checkup should include CA19-9, CEA, and AFP. The inclusion of AFP was considered to help in the differential diagnosis of the suspected liver mass. Both AFP and PIVKA-II have been used for the early screening and diagnosis of HCC as a routine [23,24], but the evidence regarding ICC is sparse. In our study, the positive rate of PIVKA-II for ICC was about 20.4%, which was close to 22.2% for CEA. The elevated levels of PIVKA-II were positively correlated with gender ratio, higher rate of HBV infection, more advanced BCLC staging, and AJCC staging (Supplement Tables S1 and S2), and these are similar clinicopathologic features that were reported in PIVKA-II-positive HCC [17]. PIVKA-II was reported to be related to abnormal vitamin K metabolism and defects in the post-translational carboxylation of the prothrombin precursor [25,26,27]. The serum levels of PIVKA-II are elevated in disorders of hepatic function and are known as an important serum marker of HCC [28]. However, PIVKA-II is not taken as a routine test in ICC. In the latest National Comprehensive Cancer Network ALL guidelines (NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers), CA19-9, CEA, and AFP were recommended as tests to diagnose ICC [29], while serum PIVKA-II levels were not mentioned. In our study, patients with HBV infection accounted for 66.7% of PIVKA-II-positive patients. Previous studies showed that the clinicopathological similarities between HBV-associated ICC and HBV-associated HCC were numerous [30], and patients with HBV-ICC achieved better outcomes than those without HBV infection [31]. Thus, the features of PIVKA-II-positive patients can be concluded as a more advanced stage with HBV infection, which has opposite effects on the prognosis of ICC. This may be the reason why PIVKA-II-positive patients tended to have a higher degree of malignancy but did not show a worse prognosis than PIVKA-II-negative patients.
It is important to find valid indexes to predict the specific site of recurrence or metastasis after surgery in ICC patients. Patients with only intrahepatic recurrence could have a second chance of surgery, while patients with lymph node metastasis or distant metastasis may lose the chance of receiving a second operation and receive chemotherapy or immunotherapy. Therefore, we also examined the relationship between different recurrence patterns and liver tumor markers. Regrettably, survival analysis for intrahepatic tumor recurrence and lymph node metastasis recurrence showed that single-use or combined use of these four liver tumor markers did not show exhibit reliable prognostic power. In predicting distant metastasis recurrence, CEA did have important prognostic significance in ICC patients (HR: 2.920, 95%CI 1.033–8.255, p = 0.043). Thus, we need to find some novel prognostic indicators for ICC patients, especially for those whose liver tumor markers were all negative.
Tumor is a chronic wasting disease characterized by nutritional, inflammation, and metabolic disorders [32]. An increasing number of researchers have noticed the effect of the inflammatory response and the nutritional status of patients on the prognosis of tumors [33]. The HALP score, PLR, and NLR, which combine convenient and economical blood indicators, have been proven to be effective prognostic factors for multiple tumors [18,19]. However, few related studies have been carried out on ICC patients. Therefore, it is meaningful to study the correlation between liver tumor markers, HALP score, PLR, NLR, and clinicopathological characteristics and prognosis of ICC.
In our study, the HALP score, NLR, and PLR provided a good complement to liver tumor markers. Univariate survival analysis showed that a lower HALP score (HALP score < 43.63) and lower NLR (NLR < 3.73) were associated with lower intrahepatic recurrence risk. In addition, NLR was the only potential index to predict the risk of lymph node metastasis recurrence postoperatively (HR: 7.757, 95% CI 2.031–29.619, p = 0.003). Subjects with a history of cholelithiasis were also more likely to experience distant metastasis recurrence (HR: 3.106, 95% CI 1.105–8.729, p = 0.032), which is consistent with previous research. Cai et al. [34] made a meta-analysis to indicate that bile duct stones, including choledocholithiasis, were an important risk factor for ICC (odds ratio (OR): 11.79, 95% CI 4.17–33.35). Then, the performance was analyzed using ROC curves and areas under ROC curves (AUC). The HALP score exhibited the largest ROC curve area of all in predicting intrahepatic recurrence (AUC = 0.588), and NLR showed the best predictive value in predicting lymph node metastasis (AUC = 0.703). Last but not least, the AUC of the CA19-9 index was the largest of all variables in predicting distant metastasis (AUC = 0.619). Thus, the combination of nutritional and inflammatory indexes could represent a valuable addition to liver tumor markers and the appraisal of recurrence risk before the operation for tailoring individualized treatment.
Although this study is the first to combine the HALP score, PLR, NLR, and liver tumor markers as independent predictive factors in ICC patients, the retrospective single-center study design is a limiting factor of our study. In addition, the cutoff of the HALP score, NLR, and PLR failed to reach a common consensus value due to the limited number of patients, which could affect the accuracy of the results. Moreover, with the emerging studies relating the next-generation sequencing (NGS) results and treatment options, adding genetic profile into consideration is definitely the future trend. We plan to enlarge our cohort and design a multicenter study to further analyze these scores and liver tumor markers in predicting ICC patients’ prognoses.
5. Conclusions
To conclude, CA19-9, CEA, HALP score, and NLR are easily accessible, reliable, and cost-effective indexes for predicting the specific site of recurrence or metastasis after surgery in ICC patients. Patients with high HALP scores and NLR have a higher risk of intrahepatic and lymph node metastasis recurrence.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm12122041/s1, Table S1: The association of demographics and clinicopathologic characteristics with different serum tumor markers; Table S2: The association of demographics and clinicopathologic characteristics with different HALP score, PLR, and NLR; Figure S1: The optimal cut-off values of the HALP score, NLR, and PLR were determined to be 43.63, 3.73, and 76.51 by using thesurv_cutpoint function of survminer using recurrence-free survival as the target variable; Figure S2: Overall survival (OS) curves of grouped intrahepatic cholangiocarcinoma patients based on (A) HbsAg, (B) HCV-Ab, (C) MVI, (D) nerve tract invasion, (E) BCLC staging, and (F) lymph nodes; Figure S3: Recurrence-free survival (RFS) curves of grouped intrahepatic cholangiocarcinoma patients based on (A) HbsAg, (B) HCV-Ab, (C) MVI, (D) nerve tract invasion, (E) BCLC staging, and (F) lymph nodes; Figure S4: Intrahepatic recurrence-free survival curves of grouped intrahepatic cholangiocarcinoma patients based on the (A) AFP, (B) PIVKA-II, (C) CEA, (D) CA19-9, (E) AFP + PIVKA-II, (F) AFP + CEA, (G) AFP + CA19-9, (H) PIVKA-II + CEA, (I) PIVKA-II + CA19-9, (J) CEA + CA19-9, and (K) positive numbers of liver tumor marker; Figure S5: Lymph node metastasis recurrence-free survival curves of grouped intrahepatic cholangiocarcinoma patients based on the (A) AFP, (B) PIVKA-II, (C) CEA, (D) CA19-9, (E) AFP + PIVKA-II, (F) AFP + CEA, (G) AFP + CA19-9, (H) PIVKA-II + CEA, (I) PIVKA-II + CA19-9, (J) CEA + CA19-9, and (K) positive numbers of liver tumor marker; Figure S6: Distant metastasis recurrence-free survival curves of grouped intrahepatic cholangiocarcinoma patients based on the (A) AFP, (B) PIVKA-II, (C) CEA, (D) CA19-9, (E) AFP + PIVKA-II, (F) AFP + CEA, (G) AFP + CA19-9, (H) PIVKA-II + CEA, (I) PIVKA-II + CA19-9, (J) CEA + CA19-9, and (K) positive numbers of liver tumor marker.
Author Contributions
All authors contributed to the study’s conception and design. Conceptualization, D.Z., H.Z. and Y.P.; data curation, Y.Z. (Yumo Zhao), X.W., J.C. and J.W.; methodology, Y.Z. (Yaojun Zhang), Z.Z. and L.X.; project administration, D.Z., H.Z. and Y.P.; writing—original draft, D.Z., H.Z. and Y.P.; writing—review and editing, D.H., M.C. and L.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethical Committee of Sun Yat-Sen University Cancer Center University (RDDA2020000350).
Informed Consent Statement
Because the study used retrospective clinical data obtained after each patient agreed to treatment, patient informed consent is exempt. Individuals cannot be identified based on the information provided. We declare to ensure the confidentiality of patient data.
Data Availability Statement
Not applicable; anonymized data will be supplied upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work is funded by the National Natural Science Foundation of China (No. 81874070, 82103566).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020;70:313. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Mavros M., Economopoulos K., Alexiou V., Pawlik T.J.J. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. JAMA Surg. 2014;149:565–574. doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Li J., Xia Y., Gong R., Wang K., Yan Z., Wan X., Liu G., Wu D., Shi L., et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J. Clin. Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 4.Jiang W., Zeng Z.C., Tang Z.Y., Fan J., Sun H.C., Zhou J., Zeng M.S., Zhang B.H., Ji Y., Chen Y.X. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: The Fudan score. Ann. Oncol. 2011;22:1644–1652. doi: 10.1093/annonc/mdq650. [DOI] [PubMed] [Google Scholar]
- 5.McMillan D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 6.Shen W.F., Zhong W., Xu F., Kan T., Geng L., Xie F., Sui C.J., Yang J.M. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2009;15:5976–5982. doi: 10.3748/wjg.15.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin M.B., Greene F.L., Edge S.B., Compton C.C., Gershenwald J.E., Brookland R.K., Meyer L., Gress D.M., Byrd D.R., Winchester D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA A Cancer J. Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 8.Liang B., Zhong L., He Q., Wang S., Pan Z., Wang T., Zhao Y. Diagnostic accuracy of serum CA19-9 in patients with cholangiocarcinoma: A systematic review and meta-analysis. J. Pharmacol. Exp. Ther. 2015;21:3555–3563. doi: 10.12659/MSM.895040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malaguarnera G., Paladina I., Giordano M., Malaguarnera M., Bertino G., Berretta M. Serum markers of intrahepatic cholangiocarcinoma. Dis. Mark. 2013;34:219–228. doi: 10.1155/2013/196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols J.C., Gores G.J., LaRusso N.F., Wiesner R.H., Nagorney D.M., Ritts R.E., Jr. Diagnostic role of serum CA 19-9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clin. Proc. 1993;68:874–879. doi: 10.1016/S0025-6196(12)60696-X. [DOI] [PubMed] [Google Scholar]
- 11.Singh S., Tang S., Sreenarasimhaiah J., Lara L., Siddiqui A. The clinical utility and limitations of serum carbohydrate antigen (CA19-9) as a diagnostic tool for pancreatic cancer and cholangiocarcinoma. Dig. Dis. Sci. 2011;56:2491–2496. doi: 10.1007/s10620-011-1709-8. [DOI] [PubMed] [Google Scholar]
- 12.Dolscheid-Pommerich R.C., Manekeller S., Walgenbach-Brünagel G., Kalff J.C., Hartmann G., Wagner B.S., Holdenrieder S. Clinical performance of CEA, CA19-9, CA15-3, CA125 and AFP in gastrointestinal cancer using LOCI™-based assays. Anticancer Res. 2017;37:353–359. doi: 10.21873/anticanres.11329. [DOI] [PubMed] [Google Scholar]
- 13.Qin X.L., Wang Z.R., Shi J.S., Lu M., Wang L., He Q.R. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: In comparison with CEA. World J. Gastroenterol. 2004;10:427–432. doi: 10.3748/wjg.v10.i3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loosen S.H., Roderburg C., Kauertz K.L., Koch A., Vucur M., Schneider A.T., Binnebösel M., Ulmer T.F., Lurje G., Schoening W., et al. CEA but not CA19-9 is an independent prognostic factor in patients undergoing resection of cholangiocarcinoma. Sci. Rep. 2017;7:16975. doi: 10.1038/s41598-017-17175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He C., Zhang Y., Song Y., Wang J., Xing K., Lin X., Li S. Preoperative CEA levels are supplementary to CA19-9 levels in predicting prognosis in patients with resectable intrahepatic cholangiocarcinoma. J. Cancer. 2018;9:3117–3128. doi: 10.7150/jca.25339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imamura H., Matsuyama Y., Miyagawa Y., Ishida K., Shimada R., Miyagawa S., Makuuchi M., Kawasaki S. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br. J. Surg. 1999;86:1032–1038. doi: 10.1046/j.1365-2168.1999.01185.x. [DOI] [PubMed] [Google Scholar]
- 17.Pan Y., Sun X., Hu Z., Xie W., Nie K., Fang A., Zhang Y., Fu Y., Chen J., Wang J., et al. Prognostic values of alpha-fetoprotein and des-gamma-carboxyprothrombin in hepatocellular carcinoma in China: An analysis of 4792 patients. J. Hepatocell. Carcinoma. 2021;8:657–670. doi: 10.2147/JHC.S316223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Güç Z.G., Alacacıoğlu A., Kalender M.E., Oflazoğlu U., Ünal S., Yıldız Y., Salman T., Küçükzeybek Y., Tarhan M.O. HALP score and GNRI: Simple and easily accessible indexes for predicting prognosis in advanced stage NSCLC patients. The İzmir oncology group (IZOG) study. Front. Nutr. 2022;9:905292. doi: 10.3389/fnut.2022.905292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouchli M., Reddy S., Gerrard M., Boardman L., Rubio M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma. Review article. Ann. Hepatol. 2021;22:100249. doi: 10.1016/j.aohep.2020.08.067. [DOI] [PubMed] [Google Scholar]
- 20.Mandaliya H., Jones M., Oldmeadow C., Nordman I.I.C. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI) Transl. Lung Cancer Res. 2019;8:886–894. doi: 10.21037/tlcr.2019.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esnaola N.F., Meyer J.E., Karachristos A., Maranki J.L., Camp E.R., Denlinger C.S. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122:1349–1369. doi: 10.1002/cncr.29692. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q., Chen Q., Zhang X., Lu X.L., Du Q., Zhu T., Zhang G.Y., Wang D.S., Fan Q.M. Diagnostic value of gamma-glutamyltransferase/aspartate aminotransferase ratio, protein induced by vitamin K absence or antagonist II, and alpha-fetoprotein in hepatitis B virus-related hepatocellular carcinoma. World J. Gastroenterol. 2019;25:5515–5529. doi: 10.3748/wjg.v25.i36.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G., Lu X., Du Q., Zhang G., Wang D., Wang Q., Guo X. Diagnostic value of the γ-glutamyltransferase and alanine transaminase ratio, alpha-fetoprotein, and protein induced by vitamin K absence or antagonist II in hepatitis B virus-related hepatocellular carcinoma. Sci. Rep. 2020;10:13519. doi: 10.1038/s41598-020-70241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inagaki Y., Tang W., Makuuchi M., Hasegawa K., Sugawara Y., Kokudo N. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des-γ-carboxyprothrombin. Liver Int. Off. J. Int. Assoc. Study Liver. 2011;31:22–35. doi: 10.1111/j.1478-3231.2010.02348.x. [DOI] [PubMed] [Google Scholar]
- 26.Suttie J.W. Recent advances in hepatic vitamin K metabolism and function. Hepatology. 1987;7:367–376. doi: 10.1002/hep.1840070226. [DOI] [PubMed] [Google Scholar]
- 27.Zakhary N.I., Khodeer S.M., Shafik H.E., Abdel Malak C.A. Impact of PIVKA-II in diagnosis of hepatocellular carcinoma. J. Adv. Res. 2013;4:539–546. doi: 10.1016/j.jare.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebman H.A., Furie B.C., Tong M.J., Blanchard R.A., Lo K.J., Lee S.D., Coleman M.S., Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N. Engl. J. Med. 1984;310:1427–1431. doi: 10.1056/NEJM198405313102204. [DOI] [PubMed] [Google Scholar]
- 29.Benson A.B., D’Angelica M.I., Abbott D.E., Anaya D.A., Anders R., Are C., Bachini M., Borad M., Brown D., Burgoyne A., et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. JNCCN. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H.B., Hu J.Y., Hu H.P. Hepatitis B virus infection and intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2014;20:5721–5729. doi: 10.3748/wjg.v20.i19.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q., Li J., Lei Z., Wu D., Si A., Wang K., Wang Y., Wan X., Lau W.Y., Shen F. Prognosis of intrahepatic cholangiocarcinomas with HBV infection is better than those with hepatolithiasis after R0 liver resection: A propensity score matching analysis. Ann. Surg. Oncol. 2017;24:1579–1587. doi: 10.1245/s10434-016-5751-9. [DOI] [PubMed] [Google Scholar]
- 32.Li C., Zhang D., Pang X., Pu H., Lei M., Fan B., Lv J., You D., Li Z., Zhang T. Trajectories of perioperative serum tumor markers and colorectal cancer outcomes: A retrospective, multicenter longitudinal cohort study. EBioMedicine. 2021;74:103706. doi: 10.1016/j.ebiom.2021.103706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reitz D., Gerger A., Seidel J., Kornprat P., Samonigg H., Stotz M., Szkandera J., Pichler M. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients with pancreatic cancer. J. Clin. Pathol. 2015;68:427–433. doi: 10.1136/jclinpath-2014-202451. [DOI] [PubMed] [Google Scholar]
- 34.Cai H., Kong W.T., Chen C.B., Shi G.M., Huang C., Shen Y.H., Sun H.C. Cholelithiasis and the risk of intrahepatic cholangiocarcinoma: A meta-analysis of observational studies. BMC Cancer. 2015;15:831. doi: 10.1186/s12885-015-1870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable; anonymized data will be supplied upon request to the corresponding author.