Abstract
“Screening” is a search for preclinical, asymptomatic disease, including cancer. Widespread cancer screening has led to large increases in early-stage cancers and pre-cancers. Ubiquitous public messages emphasize the potential benefits to screening for these lesions based on the underlying assumption that treating cancer at early stages before spread to other organs should make it easier to treat and cure, using more tolerable interventions. The intuition is so strong that public campaigns are sometimes launched without conducting definitive trials directly comparing screening to usual care. An effective cancer screening test should not only increase the incidence of early-stage preclinical disease but should also decrease the incidence of advanced and metastatic cancer, as well as a subsequent decrease in cancer-related mortality. Otherwise, screening efforts may be uncovering a reservoir of non-progressive and very slowly progressive lesions that were not destined to cause symptoms or suffering during the person's remaining natural lifespan: a phenomenon known as “overdiagnosis.” We provide here a qualitative review of cancer overdiagnosis and discuss specific examples due to extensive population-based screening, including neuroblastoma, prostate cancer, thyroid cancer, lung cancer, melanoma, and breast cancer. The harms of unnecessary diagnosis and cancer therapy call for a balanced presentation to people considering undergoing screening, even with a test of accepted benefit, with a goal of informed decision-making. We also discuss proposed strategies to mitigate the adverse sequelae of overdiagnosis.
Keywords: Cancer overdiagnosis, Screening
1. Screening, a potential benefit with a serious downside: overdiagnosis
1.1. Definitions
Cancer screening is a search for cancer before any symptoms appear. The underlying presumption is that discovering a cancer when it is so small that it is not manifest by any obvious signs or symptoms should make it easier to treat and cure with more tolerable interventions. At a public health level, screening for specific tumor types, especially those with the highest incidence, has become widely emphasized and promoted. At least in theory, any screening test that affords earlier detection would almost certainly improve the balance of benefits and harms of cancer management.1, 2 In practice that is true for some cancer screening tests, but not others. With the emphasis on increasingly sensitive screening tests, it has become evident that they are capable of detecting very slow-growing “cancers” that would never have harmed the person or come to clinical attention during the person's natural lifespan had it not been for the screening test. That is an understudied and underappreciated phenomenon known as “overdiagnosis”, the subject of this paper.
Recently, the U.S. National Library of Medicine (NLM) has added the term “overdiagnosis” to its list of medical subject headings (MeSH), defining it as “the labeling of a person with a disease or abnormal condition that would not have caused the person harm if left undiscovered, creating new diagnoses by medicalizing ordinary life experiences, or expanding existing diagnoses by lowering thresholds or widening criteria without evidence of improved outcomes. Individuals derive no clinical benefit from overdiagnosis, although they may experience physical, psychological, or financial harm.”3 This addition enhances the ability to perform systematic literature searches of overdiagnosis.
It's easy to see why cancer overdiagnosis could change the balance of benefits and harms of a screening test applied to asymptomatic healthy people. It would produce overmedicalization leading to overtreatment and diagnosis “creep”, i.e. shifting thresholds for labeling individuals as sick, even in the absence of symptoms.4, 5, 6 In addition to the physical discomfort from unnecessary treatments, the psychological burden from the knowledge of “having cancer”, being labeled a “patient”, with the accompanying socioeconomic ramifications and financial burden, both personal and societal, for the patient all contribute to the harms of overdiagnosis.3 These overdiagnosis sequelae are in addition to any harms, discomforts and inconvenience of the screening tests themselves. Importantly, overdiagnosis differs from misdiagnosis in that the former is considered a true-positive, revealing lesions that a pathologist consensus would label as cancer or pre-cancer.2, 7
1.2. Requirements for overdiagnosis
1.2.1. Reservoir of subclinical disease/cancer
The absence of symptoms in the context of screen-detected disease implies that there exist subclinical, i.e., occult, lesions in the tissue interrogated, that histologically conform to the definition of “cancer” or “malignancy”, or pre-cancer. Their discovery usually triggers treatment. They are subclinical because they are small and organ confined: the very characteristics that make surgical resection so appealing. Although many screen-detected cancers have lethal potential, many others progress very slowly or are not progressive at all. Histopathology of a formalin-fixed biopsy is merely a snapshot in time, without revealing the dynamic behavior or growth potential of a subclinical lesion.1 If their discovery by screening is actually to confer clinical benefit, their removal or other treatment should pull late-stage life-threatening cancers out of the future into the present as early-stage cancers which are more treatable.8 This process should, in time, at a population level, be reflected in a decline in late-stage cancers and cancer deaths in an amount that is equivalent to the increased incidence of screen-detected early-stage cancers. However, such a shift may be sufficient, but it is not absolutely necessary for screening to confer clinical benefit. A decrease in interval cancers, which develop so fast that they elude screening and emerge between screens, should also occur when more sensitive, effective tests are introduced.9 A common intuition is that any lesion labeled by a pathologist as “cancer” or “pre-cancer” would have progressed if undiscovered. This is part of the reason why cancer has achieved its fearsome reputation as the “emperor of all maladies.”10 However, clinical trials have shown that progression of subclinical lesions is highly variable; it is influenced by cancer site, and underlying biology.11, 12 Unfortunately, the incidence of late-stage cancers has not been shown to decrease in association with a number of commonly used screening tests, making it difficult to attribute mortality trends to screening, especially in the setting of undeniable improvements in systemic therapies for late-stages of disease. Nevertheless, technical advances in screening modalities may further contribute to overdiagnosis by enhancing sensitivity, and thereby exacerbating the discovery of lesions that lack the potential to cause harm.13
1.2.2. Screening that dips into the reservoir of subclinical lesions
A key requirement for overdiagnosis is the existence of a substantial reservoir of such subclinical, or occult, disease, sometimes referred to in the older literature as “pseudodisease”.4, 11 In the absence of an intentional search, these occult lesions would elude detection. The size and natural history of the latent reservoir of subclinical lesions will influence the balance between the benefits and harms of a given screening test. Slower growing lesions are present for a longer period of time and are therefore more likely to be discovered by screening (Fig. 1A). This factor enriches screen-detected cancers with more indolent tumors, while those appearing between screens, “interval” lesions missed by screening, tend to be more aggressive and faster growing. This phenomenon is known as “length-biased sampling” or “length-time bias”. An extreme form of length-biased sampling is overdiagnosis, which occurs in very slow growing and non-progressive tumors (Fig. 1).8, 13, 14 Another contributor to overdiagnosis occurs if a screening test introduces a lengthy lead time between detectability and symptomatic clinical disease. In such cases, patients may die of unrelated causes during the lead time because of competing causes of death, often age-related.
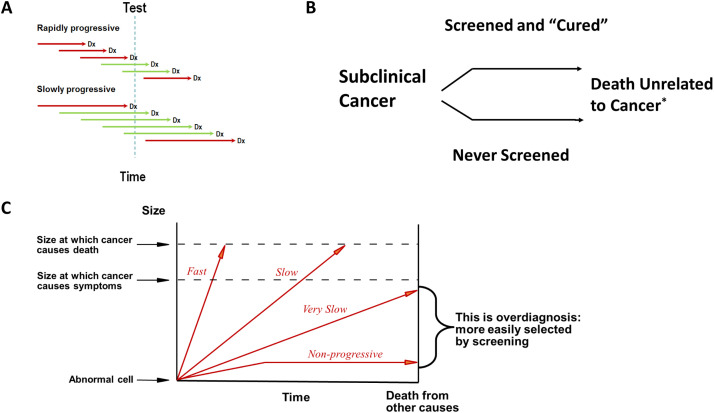
Fig. 1.
(A) Length-time bias/length-biased sampling. Green lines, cancers picked up by screening; Red lines, cancers missed by screening; Dx, time when disease is clinically obvious without testing. (B) Overdiagnosis. *, lifespan unchanged. (C) Cancer overdiagnosis due to tumor heterogeneity. (Courtesy of H. Gilbert Welch, Brigham and Women's Center for Surgery and Public Health).
Evidence supporting a large reservoir of subclinical invasive as well as noninvasive disease in the general population comes from autopsy studies involving prostate, breast, and thyroid cancer.11 In addition, some screen-detected progressive cancers can contribute to overdiagnosis if the patient has co-morbidities or medical conditions that would cause death before any benefit from screening has time to emerge.15 Since cancer is primarily a disease of aging, the risk of cancer overdiagnosis can therefore increase as patients accumulate competing causes of age-related death.16 For all of these reasons, the amount of cancer overdiagnosis is likely to vary from country to country, depending on the prevalence of screening, age structure of the population, and frequency of other medical conditions. At the population level, overdiagnosis produces a large increase in cancer incidence without concomitant reduction in mortality, as shown in Fig. 2.17
Fig. 2.
Overdiagnosis with stable true cancer incidence. (Adapted from H.G. Welch et al., N Engl J Med 381(14):1378–1386, 2019).
1.2.3. Other set-ups for overdiagnosis
The incidental discovery of non-targeted tumors during screening or diagnostic work-ups for other conditions can be a special source of overdiagnosis. These tumors are called incidentalomas. The original exam is unrelated to the incidentally discovered lesion.18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Much of the incidentaloma literature focuses on endocrine organs (e.g., adrenal, parathyroid, pituitary, thyroid glands) but also kidney and lung lesions. Generally, the revelatory technology involves imaging, although even simple physical examination can be implicated, as in the palpation of thyroid nodules during a routine check-up. The anxiety and harms of incidentalomas may be similar to those of classic overdiagnosis.20, 21 A healthy individual has been turned into a patient encumbered now by all the psychological, physical, and financial toxicities associated with disease, often with unclear benefit.
2. Overdiagnosis detected on screening of specific cancer types
2.1. Neuroblastoma
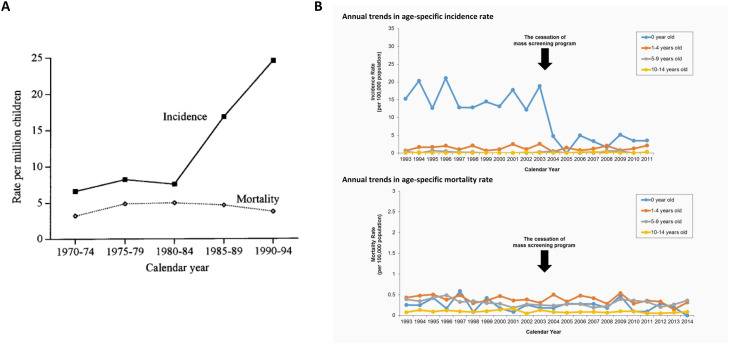
The prototypic example of overdiagnosis is neuroblastoma. Usually appearing as a mass in the neck, chest, abdomen or pelvis of an infant or toddler, neuroblastoma can be deadly.28 The fear associated with this ominous prognosis in an infant encouraged the use of screening to detect early-stage tumors. Screening was deceptively simple, involving collection of urine in which catecholamine metabolites produced by the cancer (vanillylmandelic and homovanillic acid) were detectable. Together, these attributes led to routine inclusion of catecholamine screening of infants in Japan, and a consequent increase in neuroblastoma incidence without a concomitant decrease in mortality (Fig. 3A).29 Screen-detected cancers, even those that are not advanced, are treated aggressively involving surgery and chemotherapy.28 Absence of mortality reduction with screening was also documented in pragmatic trials in Canada and Germany.30, 31, 32 These observations are strongly suggestive of overdiagnosis due to widespread screening.7, 29 Population-based screening was therefore terminated in Japan, with rapid reduction in neuroblastoma incidence and no increase in mortality (Fig. 3B).33, 34
Fig. 3.
(A) Age-standardized incidence and mortality rate of neuroblastoma in Osaka. (Adapted from W. Ajiki et al., Cancer Causes Control 9(6):631–636, 1998) (B) Incidence and mortality rates of neuroblastoma cases before and after cessation of the mass screening program in Japan (Adapted from T. Shinagawa et al., Int J Cancer 140:618–625, 2017).
2.2. Thyroid cancer
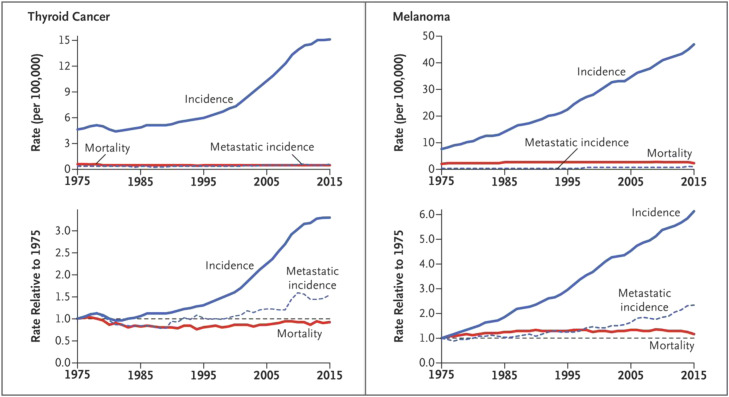
Routine thyroid palpation as part of standard physical examination frequently reveals nodules: up to 21% of thyroid nodules are discovered by palpation,35 further increased by neck ultrasound screening for thyroid cancer.36, 37 This activity has led to an epidemic of thyroid cancers,38, 39 largely confined to the slowest-growing papillary histotype, which comprise up to 87% of the increase.38 In a classic demonstration of overdiagnosis, thyroid cancer-specific mortality was virtually unchanged (Fig. 2).17, 38, 40 But there are clear harms, including unnecessary surgery that can lead to inadvertent parathyroid removal (hypoparathyroidism) and recurrent laryngeal nerve injury (permanent hoarseness).41, 42 Imaging encompassing the thyroid region, although directed to non-thyroid conditions, may also result in thyroid cancer overdiagnosis, a classic case of incidentalomas.22
2.3. Prostate cancer
Following widespread introduction of PSA screening in the 1980s, the incidence of prostate cancer rose dramatically in the US.1, 43 The increase resulted from screening-based detection of a huge reservoir of latent disease that men harbor as they age. Autopsy studies in men who died of causes unrelated to prostate cancer and cystoprostatectomy specimens have documented age-associated prevalence of subclinical prostate cancer in most elderly men.44, 45, 46 These observations, combined with the rising incidence of low-risk tumors, strongly suggest that much of screen-detected prostate cancer is indolent and would likely never have affected the health or life-span of the individual.4
Among 76,693 men in the Prostate, Lung, Colon and Ovarian Cancer Screening Trial (PLCO), follow-up at 7 and 13 years showed a 22% and 12% relative increase in prostate cancer incidence, respectively, with screening versus usual care.47, 48 Yet, prostate-cancer mortality did not differ between the arms at either follow-up. In contrast, the European Study of Screening for Prostate Cancer (ERSPC) trial randomizing 162,387 men to screening every four years or usual care showed a reduction in prostate cancer death: RR = 0.80 (P = 0.04) and RR = 0.79 (P = 0.0001) at 9 and 11 years, respectively, with screening versus control.49, 50 In counterbalance to these benefits there was an approximately 50% higher cumulative incidence of prostate cancer at about 11 years in the men assigned to screening compared to control.50 This suggests harms associated with overdiagnosis that should be weighed against reported benefits. And this demonstrates that screening may be associated with both the benefit of cancer mortality reduction and harm from overdiagnosis.
Influenced by evidence for screening-related prostate cancer overdiagnosis, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine screening for prostate cancer.51 A modified recommendation in 2018 states that “men aged 55 to 69 years make an individual decision about whether to be screened after a conversation with their clinician about the potential benefits and harms.” For men ≥ 70 years the benefits do not outweigh the harms (incontinence, impotence, pain from surgery/radiation), in part due to the harms of overdiagnosis.52
2.4. Lung cancer
Lung cancer screening trials usually target individuals at elevated risk of cancer due to prior cigarette smoking history. In the Mayo Lung Project, lung cancer mortality in 9211 male cigarette smokers was similar with standard chest X-rays (CXRs) plus sputum cytology versus usual care.53 A persistent excess of cases (exclusively early-stage tumors) was observed with screening versus usual care without a reduction in late-stage disease: 583 versus 500, suggesting overdiagnosis. CXR technology, the lung-cancer screen in PLCO, again showed no reduction in lung cancer mortality versus usual care.54, 55 The US National Cancer Institute's (NCI) National Lung Screening Trial (NLST) randomized 53,454 heavy smokers to three annual screenings with low dose/helical computed tomography (LDCT)26,7,22 versus single-view posteroanterior CXR.26,7,32 A 20% relative reduction in lung-cancer mortality with LDCT compared to radiography was initially observed.56 Analysis at 6.4 years follow-up suggested that over 18% of all lung cancers detected by LDCT were potentially overdiagnosed.57 However, at 11.3 years median follow-up, 1701 lung cancers were diagnosed with LDCT and 1681 with CXR: RR = 1.01 (95% CI: 0.95, 1.09). This illustrates the importance of sufficient follow-up. Lung cancer deaths evaluated at a median 12.3-year follow-up, were 1147 and 1236 in the LDCT and CXR arms, respectively (RR = 0.92, 95% CI: 0.85, 1.00).58 Bronchoalveolar carcinomas (BACs) represented most cases of LDCT-associated overdiagnosis,15 reinforcing the notion that a subset of subclinical lung lesions exists that contains indolent, though invasive-appearing, cancers as well as premalignant in situ lesions. As with prostate cancer, small lung nodules found at autopsy in people who died of unrelated causes support the existence of such a reservoir.59
Not all lung cancer screening programs have been restricted to cigarette smokers, and overdiagnosis may be particularly common in Asian female non-smokers who are not at high risk for cancer. A population-based ecological cohort study of LDCT screening among women using the Taiwan Cancer Registry (less than 5% smoking prevalence), demonstrated from 2004 to 2013 more than a six-fold increase in early-stage cancer (from 2.3 to 14.4/100,000; absolute difference, 12.1/100,000). However, the incidence of late-stage cancers did not decline concomitantly (18.7 to 19.3/100,000; absolute difference, 0.6) with the rise in early-stage cancers in this time period, and mortality remained stable despite a 5-year survival that doubled (18% to 40%), suggesting that all the additional cancers represent lung cancer overdiagnosis.60 The authors therefore highlighted the critical need for further study of screening in Asian women. One such trial is ongoing in China.
2.5. Breast cancer
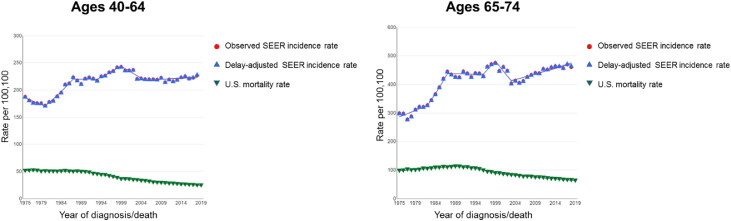
Screening mammography among women ≥ 40 years old increased rapidly from the 1980s through the early 1990s (Fig. 4).11 This was paralleled by an increase in incidence of early-stage breast cancers, with a much smaller decrease in late-stage cancers and virtually no change in metastatic disease, suggesting a trend dominated by overdiagnosis rather than a true stage shift.61 Based on the US Surveillance Epidemiology and End Results (SEER) data 1976–2008, which includes the transition from the era preceding to that following institution of mammographic screening, among women ≥ 40 years a doubling of early-stage breast cancer cases was detected each year (112 to 234 cases per 100,000 women). A concomitant reduction in the rate of late-stage breast cancer was merely 8%. This yielded an estimate by the authors that breast cancer was overdiagnosed in over 70,000 women, constituting 31% of all breast cancers diagnosed.61 Another SEER-based study showed that following introduction of screening mammography, the percentage of small tumors (< 2 cm invasive or in situ) increased from 36% to 68%. Of note, progression rates of ductal carcinoma in situ (DCIS) vary by histologic grade, with higher progression rates associated with high-grade DCIS, although assignment of grade can be subjective. Breast cancer mortality declined but this was attributed largely to improved systemic therapy.62 The effect of the introduction of 3D mammography, with its partial replacement of 2D mammography, has not yet been definitively determined.
Fig. 4.
Breast cancer long-term trends in SEER Incidence (1975–2018) and US mortality (1975–2019). The delay-adjusted incidence curve includes correction of incidence for the most recent historical trends in delayed reporting from SEER sites.
A report of harms associated with breast cancer screening in 29 studies showed overdiagnosis ranging widely, from 0% to 54%, although in randomized trials the range was from 11% to 22%.61,63, 64, 65, 66 This wide variation in range of overdiagnosis estimates and difference in range size between study types has been attributed to the type of data used. Whereas no study based on individual data has yielded an estimate greater than 17%, studies based on aggregated data tended to yield estimates higher than 40%, a difference considered too systematic to be a random observation. Use of aggregated data has been shown to come with biases that can lead to overdiagnosis.65 So-called “lead time-adjusted” statistical models tend to produce estimates in the lower end of this range, while population trend-derived estimates are at the higher end. Although most published statistical models do not incorporate the possibility of a subset of non-progressive tumors, a recent publication has done so, estimating that 1 in 7 cases of screen-detected breast cancer is overdiagnosed.67 In a population of women aged 50 to 74 years (median 56; interquartile range 52–64), among the 15.4% of screen-detected cancers estimated to be overdiagnosed, 6.1% were due to detection of indolent preclinical cancer and 9.3% to detection of progressive preclinical cancer in women who would have died of an unrelated cause prior to clinical breast cancer diagnosis.
Reported rates of overdiagnosis also vary by choice of denominator, each of which carries different implications. The use of the entire screening eligible population provides information about the national burden of overdiagnosis. The use of women in a screening program as the denominator conveys the additional burden of overdiagnosis associated with the offer of screening within an organized context. Restriction of the denominator to women who have actually been screened provides evidence of the burden of overdiagnosis for women who have chosen to be screened.
2.6. Melanoma
Large increases in cutaneous melanoma (but not other types of melanoma) incidence have occurred over recent decades, almost tripling during the 30 years from 1975 to 2005 according to SEER data.11, 68 Much like thyroid cancer, cutaneous melanoma screening is not traditionally dependent on a high-technology intervention, relying mainly on a “naked eye” visual exam.69 However, use of dermoscopy in experienced hands may improve the specificity of diagnosis, with unknown effect on overdiagnosis. Screening trends, reflected by increased skin biopsy rates, have been stimulated by international public health campaigns, particularly in regions with extensive sun exposure, despite the absence of supporting evidence from randomized clinical trials.70, 71 Furthermore, pathologic criteria for diagnosing melanoma were modified in the 1970s and 1980s, and stage migration following introduction of sentinel lymph node biopsies might potentially be responsible for some stage drift.72, 73 Population trends show the classic pattern of overdiagnosis: predominance of early-stage and in situ cancers among the rising number of cases with little or no change in more advanced disease or in mortality (Fig. 2).72, 74, 75, 76 Studies in East Anglia, England and in Australia also documented increasing melanoma incidence that was associated with overdiagnosis.77, 78, 79
3. Mitigating overdiagnosis: How we can limit its harms
3.1. Less screening and more focus on high-risk populations
Identification of a population with elevated risk of progressive cancers may mitigate the proportion of screen-detected cancers that are overdiagnosed.1 Most population-based screening programs attempt this, as for example, by using age thresholds for screening cancers that are more common in older individuals (e.g. colon cancer, prostate cancer, breast cancer, etc.). Other eligibility criteria for screening may include environmental, occupational, and iatrogenic exposures. Candidates for lung cancer screening, for example, may include individuals with current and prior tobacco smoking habits. But overall, current tools to assign risk are crude. There is, therefore, a long way to go in refining this strategy.
3.2. Addressing public over-enthusiasm about screening
The potential benefits of cancer screening are intuitive to patients as well as medical professionals. Public health messages in the US encouraging early detection go back to the early 1900s, leading ultimately to formation of the precursor organization to the American Cancer Society.80 And the health messages have been very effective. In fact, a national survey conducted from 2001 to 2002 revealed that 87% of US adults believed routine cancer screening to be nearly always a good idea and 74% believed that finding cancer early saves lives most or all the time.81 A prominent strategy has been to foster a feeling of vulnerability to cancer followed by an offer of hope.82, 83 A system without negative feedback ensues. Reassurance from a negative screen or gratification from “early”, presumably “curable”, cancer discovery in a positive screen encourage uncritical acceptance of screening.5, 84 In fact, evidence-based guidelines, such as those presented by the USPSTF, are widely resisted by patients as well as physicians, some of whom consider them counterintuitive and reject them even when the possibility of overdiagnosis and attendant harms are explicitly listed.8 In fact, although women surveyed generally expressed knowledge of false positives with screening mammography, far fewer were aware of overdiagnosis and the fact that screening can detect cancers that may never progress (Table 1).85, 86 Women are more aware of the benefits of mammography than the harms.
Table 1.
Perceived benefits and harms of screening mammography in US women ages 40–59*.
| Benefit vs. Harm | Outcome of mammographic screening | Awareness: Have you heard of this before? | Importance in decision to get mammogram: Not at all |
Importance in decision to get mammogram: Very important |
|---|---|---|---|---|
| Benefit | Mortality reduction: mammogram can save lives | 97% | 2% | 67% |
| Harms | False positive: Mammogram can find something that looks like cancer but turns out not to be cancer, i.e. “false alarm” | 75% | 12% | 23% |
| Overdiagnosis: Some beast cancers found by mammograms grow so slowly that they would not have caused any health problems for women in their lifetime. | 27%⁎⁎ | 13% | 22% |
Adapted from J. Yu, et al. JAMA Int Med. 2017;177(9):1381–1382.(87).
An earlier survey indicated that in 1997 only 7% of women were aware of non-progressive breast cancers. L.M. Schwartz, et al. BMJ. 2000;320:1635–1640.(86).
A more nuanced and balanced approach to public messaging as well as education about the existence of overdiagnosis is warranted. There is plenty of room for improvement. In an analysis of media coverage of cancer screening, headlines rarely mentioned concepts of “low-risk”, “overdiagnosis” or “overtreatment” even when the full text content mentioned them.87 A survey of Australian journalists (primarily specializing in health topics) showed that while they were aware of the term overdiagnosis, they found the concept challenging to understand and to communicate, given the prevailing beliefs in the benefits of early detection.88 Overall, their knowledge of the harms of overdiagnosis was limited. Early qualitative evidence suggests that interventions to improve journalists’ understanding of medical research using a Tip Sheet is feasible.89
3.3. Revised terminology
In a 2012 US NCI meeting, a group of experts discussed strategies to mitigate the harms of overdiagnosis and overtreatment.90 One issue addressed the severe psychological stress that comes with the label “cancer patient”.82 The fact that a large percentage of DCIS, for example, is unlikely to progress to invasive cancer led to the suggestion that the terminology be modified to remove the word “carcinoma” (and stage 0 cancer) so that the name is more closely aligned with the growing understanding of the underlying biology by simply referring to them as “intraepithelial neoplasia.”91, 92, 93 As noted above, progression rates are substantial with high-grade DCIS. The terms “cancer” and “carcinoma” would be reserved for lesions likely to progress.94, 95, 96 Some have advanced the term “indolent lesion of epithelial origin (IDLE)”.90 Such an approach to modification in terminology to better suit the underlying biology has already been used in the case of cervical intraepithelial neoplasia (CIN), which used to be called carcinoma in situ; and epithelial tumors of low malignant potential for ovarian lesions. Another suggested approach has been to raise the threshold for labeling a radiologic finding “abnormal.”4, 11, 97 In addition, a recent small qualitative study suggested that women with DCIS or invasive breast cancer appreciated and could benefit from discussion about breast cancer overdiagnosis that went beyond information given by their providers.98
3.4. Better prognostic tools
An important area of research is the development of tools that theoretically could identify overdiagnosis at the molecular level in individual tumors.2, 99, 100 It would then be possible to inform patients with more confidence whether a newly diagnosed tumor has been overdiagnosed or is likely to progress without treatment. A model for this approach is the Oncotype DX Genomic Prostate Score (GPS), an expression array of 17 genes reported to correlate with upgrading on biopsy follow-up during active surveillance for prostate cancer.101 Standard practice decisions for estrogen-receptor positive early-stage breast cancer already employs prognostic categorization based on molecular signatures tested in the Oncotype DX “recurrence score” and other genomic assessments, enabling avoidance of aggressive therapies for cancers scoring as low-risk.102 Comparable molecular assessments of screen-detected lesions scoring as low risk would have the potential to obviate the trend to follow up with invasive, harmful overtreatment.103
4. Conclusions
We emphasize that encouraging a deeper understanding of overdiagnosis is not intended to discourage screening of appropriate individuals in settings that have established mortality reduction using high-level evidence from clinical trials. The goal is to achieve fully informed personal decisions about screening using balanced messages that include a discussion of overdiagnosis when it has been shown to exist for a given screening test.103, 104 Only then can individuals truly map the information to their personal values with a knowledge of the trade-offs involved.
Declaration of competing interest
Dr. Kramer spends 25% of his time on a grant from the Arnold Ventures Foundation on a project devoted to training journalists to critically evaluate medical research publications. Dr. Kramer's affiliation with this foundation and with the Lisa Schwartz Foundation have had no influence on the content or views expressed in this article. Dr. Woloshin also receives funding from the Arnold Foundation (same grant as Dr. Kramer) and is the founder of the Lisa Schwartz Foundation – again, neither Foundation has had any influence on this paper. Dr. Xie is affiliated with Beijing Biostar Pharmaceuticals Co., Ltd. and has no personal or organizational interest in influencing the views of the article.
Acknowledgements
The authors thank Dr. Worta McCaskill-Stevens for reviewing and advising on content and Ms. Carrie Robinson for technical assistance.
Author contributions
B.D., S.W., H.X and B.K. drafted the original manuscript and revised it.
Footnotes
Given their roles as Executive Editor and Associate Editor, respectively, Heng Xie and Barnett S. Kramer had no involvement in the peer-review of this article and have no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Huan He.
References
- 1.Dunn B.K., Kramer B.S. 1st ed. John Wiley & Sons, Inc.; 2017. Chapter 18. Cancer Overdiagnosis, Ramifications and Research Strategies. 2017. [Google Scholar]
- 2.Srivastava S., Koay E.J., Borowsky A.D., et al. Cancer overdiagnosis: a biological challenge and clinical dilemma. Nat Rev Cancer. 2019;19(6):349–358. doi: 10.1038/s41568-019-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woloshin S., Kramer B. Overdiagnosis: it's official. BMJ. 2021;375:n2854. doi: 10.1136/bmj.n2854. [DOI] [PubMed] [Google Scholar]
- 4.Moynihan R., Doust J., Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi: 10.1136/bmj.e3502. [DOI] [PubMed] [Google Scholar]
- 5.Klotz L. Cancer overdiagnosis and overtreatment. Curr Opin Urol. 2012;22(3):203–209. doi: 10.1097/MOU.0b013e32835259aa. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann B., Reid L., Carter S., et al. Overdiagnosis: one concept, three perspectives, and a model. Eur J Epidemiol. 2021;36(4):361–366. doi: 10.1007/s10654-020-00706-4. [DOI] [PubMed] [Google Scholar]
- 7.Coon E.R., Quinonez R.A., Moyer V.A., et al. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013–1023. doi: 10.1542/peds.2014-1778. [DOI] [PubMed] [Google Scholar]
- 8.Kramer B.S., Croswell J.M. Cancer screening: the clash of science and intuition. Annu Rev Med. 2009;60:125–137. doi: 10.1146/annurev.med.60.101107.134802. [DOI] [PubMed] [Google Scholar]
- 9.Brawley O.W., Paller C.J. Overdiagnosis in the age of digital cancer screening. J Natl Cancer Inst. 2021;113(1):1–2. doi: 10.1093/jnci/djaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee S. Scribner: A Division of Simon and Schuster; New York, New York: 2010. The Emperor of All Maladies. A Biography of Cancer. Scribner hardcover ed. 2010 November. [Google Scholar]
- 11.Welch H.G., Black W.C. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 12.Draisma G., Boer R., Otto S.J., et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–878. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 13.Croswell J.M., Ransohoff D.F., Kramer B.S. Principles of cancer screening: lessons from history and study design issues. Semin Oncol. 2010;37(3):202–215. doi: 10.1053/j.seminoncol.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandhu G.S., Andriole G.L. Overdiagnosis of prostate cancer. J Natl Cancer Inst Monogr. 2012;2012(45):146–151. doi: 10.1093/jncimonographs/lgs031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callister M.E.J., Sasieni P., Robbins H.A. Overdiagnosis in lung cancer screening. Lancet Respir Med. 2021;9(1):7–9. doi: 10.1016/S2213-2600(20)30553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodersen J., Voss T., Martiny F., et al. Overdiagnosis of lung cancer with low-dose computed tomography screening: meta-analysis of the randomised clinical trials. Breathe. 2020;16(1) doi: 10.1183/20734735.0013-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch H.G., Kramer B.S., Black W.C. Epidemiologic signatures in cancer. N Engl J Med. 2019;381(14):1378–1386. doi: 10.1056/NEJMsr1905447. [DOI] [PubMed] [Google Scholar]
- 18.Sohaib A. Incidental lesion in oncology patients: kidneys. Cancer Imaging. 2014;14:044. [Google Scholar]
- 19.Pinsky P.F., Dunn B., Gierada D., et al. Incidental renal tumours on low-dose CT lung cancer screening exams. J Med Screen. 2017;24(2):104–109. doi: 10.1177/0969141316657115. [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan J.W., Muntinga T., Grigg S., et al. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. 2018;361:k2387. doi: 10.1136/bmj.k2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganguli I., Simpkin A.L., Lupo C., et al. Cascades of care after incidental findings in a US National Survey of Physicians. JAMA Netw Open. 2019;2(10) doi: 10.1001/jamanetworkopen.2019.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loomans-Kropp H.A., Dunn B.K., Kramer B.S., et al. Thyroid incidentalomas in association with low-dose computed tomography in the National Lung Screening Trial. Am J Epidemiol. 2020;189(1):27–33. doi: 10.1093/aje/kwz219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hock L.M., Lynch J., Balaji K.C. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167(1):57–60. [PubMed] [Google Scholar]
- 24.Mitchell T.L., Pippin J.J., Devers S.M., et al. Incidental detection of preclinical renal tumors with electron beam computed tomography: report of 26 consecutive operated patients. J Comput Assist Tomogr. 2000;24(6):843–845. doi: 10.1097/00004728-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Hara A.K., Johnson C.D., MacCarty R.L., et al. Incidental extracolonic findings at CT colonography. Radiology. 2000;215(2):353–357. doi: 10.1148/radiology.215.2.r00ap33353. [DOI] [PubMed] [Google Scholar]
- 26.Saad A.M., Gad M.M., Al-Husseini M.J., et al. Trends in renal-cell carcinoma incidence and mortality in the United States in the last 2 decades: a SEER-based study. Clin Genitourin Cancer. 2019;17(1):46–57. doi: 10.1016/j.clgc.2018.10.002. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welch H.G. Stumbling onto cancer: avoiding Overdiagnosis of renal cell carcinoma. Am Fam Physician. 2019;99(3):145–147. [PubMed] [Google Scholar]
- 28.Maris J.M. Recent advances in neuroblastoma. N Engl J Med. 2010;362(23):2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajiki W., Tsukuma H., Oshima A., et al. Effects of mass screening for neuroblastoma on incidence, mortality, and survival rates in Osaka. Japan. Cancer Causes Control. 1998;9(6):631–636. doi: 10.1023/a:1008897123707. [DOI] [PubMed] [Google Scholar]
- 30.Woods W.G., Gao R.N., Shuster J.J., et al. Screening of infants and mortality due to neuroblastoma. N Engl J Med. 2002;346(14):1041–1046. doi: 10.1056/NEJMoa012387. [DOI] [PubMed] [Google Scholar]
- 31.Schilling F.H., Spix C., Berthold F., et al. Neuroblastoma screening at one year of age. N Engl J Med. 2002;346(14):1047–1053. doi: 10.1056/NEJMoa012277. [DOI] [PubMed] [Google Scholar]
- 32.Berthold F., Spix C., Erttmann R., et al. Neuroblastoma screening at 1 year of age: the final results of a controlled trial. JNCI Cancer Spectr. 2021;5(4) doi: 10.1093/jncics/pkab041. pkab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katanoda K., Hayashi K., Yamamoto K., et al. Secular trends in neuroblastoma mortality before and after the cessation of national mass screening in Japan. J Epidemiol. 2009;19(5):266–270. doi: 10.2188/jea.JE20090037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinagawa T., Kitamura T., Katanoda K., et al. The incidence and mortality rates of neuroblastoma cases before and after the cessation of the mass screening program in Japan: a descriptive study. Int J Cancer. 2017;140(3):618–625. doi: 10.1002/ijc.30482. [DOI] [PubMed] [Google Scholar]
- 35.Pacini F., Castagna M.G., Brilli L., et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):119. doi: 10.1093/annonc/mds230. Supplvii110. [DOI] [PubMed] [Google Scholar]
- 36.Xu S., Han Y. The overdiagnosis of thyroid micropapillary carcinoma: the rising incidence, inert biological behavior, and countermeasures. J Oncol. 2021;2021 doi: 10.1155/2021/5544232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaccarella S., Franceschi S., Bray F., et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 38.Davies L., Welch H.G. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 39.Roman B.R., Morris L.G., Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes. 2017;24(5):332–336. doi: 10.1097/MED.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakala T., Kellokumpu-Lehtinen P., Kholova I., et al. Rising incidence of small size papillary thyroid cancers with no change in disease-specific survival in Finnish thyroid cancer patients. Scand J Surg. 2012;101(4):301–306. doi: 10.1177/145749691210100415. [DOI] [PubMed] [Google Scholar]
- 41.Welch H.G., Doherty G.M. Saving thyroids - overtreatment of small papillary cancers. N Engl J Med. 2018;379(4):310–312. doi: 10.1056/NEJMp1804426. [DOI] [PubMed] [Google Scholar]
- 42.James B.C., Timsina L., Graham R., et al. Changes in total thyroidectomy versus thyroid lobectomy for papillary thyroid cancer during the past 15 years. Surgery. 2019;166(1):41–47. doi: 10.1016/j.surg.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Catalona W.J., Smith D.S., Ratliff T.L., et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 44.Pettus J.A., Al-Ahmadie H., Barocas D.A., et al. Risk assessment of prostatic pathology in patients undergoing radical cystoprostatectomy. Eur Urol. 2008;53(2):370–375. doi: 10.1016/j.eururo.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 45.Bell K.J., Del Mar C., Wright G., et al. Prevalence of incidental prostate cancer: a systematic review of autopsy studies. Int J Cancer. 2015;137(7):1749–1757. doi: 10.1002/ijc.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimura T., Sato S., Takahashi H., et al. Global trends of latent prostate cancer in autopsy studies. Cancers. 2021;13(2):359. doi: 10.3390/cancers13020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andriole G.L., Crawford E.D., Grubb R.L., 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andriole G.L., Crawford E.D., Grubb R.L., 3rd, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroder F.H., Hugosson J., Roobol M.J., et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 50.Schroder F.H., Hugosson J., Roobol M.J., et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moyer V.A. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 52.Force U.S.P.S.T., Grossman D.C., Curry S.J., et al. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA. 2018;319(18):1901–1913. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 53.Marcus P.M., Bergstralh E.J., Zweig M.H., et al. Extended lung cancer incidence follow-up in the Mayo Lung Project and overdiagnosis. J Natl Cancer Inst. 2006;98(11):748–756. doi: 10.1093/jnci/djj207. [DOI] [PubMed] [Google Scholar]
- 54.Hocking W.G., Hu P., Oken M.M., et al. Lung cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. J Natl Cancer Inst. 2010;102(10):722–731. doi: 10.1093/jnci/djq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oken M.M., Hocking W.G., Kvale P.A., et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306(17):1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 56.Aberle D.R., Adams A.M., Berg C.D., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patz E.F., Jr., Pinsky P., Gatsonis C., et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174(2):269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Lung Screening Trial Research T Lung cancer incidence and mortality with extended follow-up in the national lung screening trial. J Thorac Oncol. 2019;14(10):1732–1742. doi: 10.1016/j.jtho.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dammas S., Patz E.F., Jr., Goodman P.C. Identification of small lung nodules at autopsy: implications for lung cancer screening and overdiagnosis bias. Lung Cancer. 2001;33(1):11–16. doi: 10.1016/s0169-5002(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 60.Gao W., Wen C.P., Wu A., et al. Association of computed tomographic screening promotion with lung cancer overdiagnosis among Asian women. JAMA Intern Med. 2022;182(3):283–290. doi: 10.1001/jamainternmed.2021.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bleyer A., Welch H.G. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 62.Welch H.G., Prorok P.C., O'Malley A.J., et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438–1447. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- 63.Nelson H.D., Tyne K., Naik A., et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. W237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalager M., Adami H.O., Bretthauer M., et al. Overdiagnosis of invasive breast cancer due to mammography screening: results from the Norwegian screening program. Ann Intern Med. 2012;156(7):491–499. doi: 10.7326/0003-4819-156-7-201204030-00005. [DOI] [PubMed] [Google Scholar]
- 65.Chaltiel D., Hill C. Estimations of overdiagnosis in breast cancer screening vary between 0% and over 50%: why? BMJ Open. 2021;11(6) doi: 10.1136/bmjopen-2020-046353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nelson H.D., Pappas M., Cantor A., et al. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive services task force recommendation. Ann Intern Med. 2016;164(4):256–267. doi: 10.7326/M15-0970. [DOI] [PubMed] [Google Scholar]
- 67.Ryser M.D., Lange J., Inoue L.Y.T., et al. Estimation of breast cancer overdiagnosis in a U.S. breast screening cohort. Ann Intern Med. 2022;175(4):471–478. doi: 10.7326/M21-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Welch H.G., Mazer B.L., Adamson AS. The rapid rise in cutaneous melanoma diagnoses. N Engl J Med. 2021;384(1):72–79. doi: 10.1056/NEJMsb2019760. [DOI] [PubMed] [Google Scholar]
- 69.Lee K.J., Betz-Stablein B., Stark M.S., et al. The future of precision prevention for advanced melanoma. Front Med. 2021;8 doi: 10.3389/fmed.2021.818096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolff T., Tai E., Miller T. Screening for skin cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150(3):194–198. doi: 10.7326/0003-4819-150-3-200902030-00009. [DOI] [PubMed] [Google Scholar]
- 71.Weinstock M.A., Lott J.P., Wang Q., et al. Skin biopsy utilization and melanoma incidence among Medicare beneficiaries. Br J Dermatol. 2017;176(4):949–954. doi: 10.1111/bjd.15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weyers W. The 'epidemic' of melanoma between under- and overdiagnosis. J Cutan Pathol. 2012;39(1):9–16. doi: 10.1111/j.1600-0560.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- 73.Rousi E.K., Kallionpaa R.A., Kallionpaa R.E., et al. Increased incidence of melanoma in children and adolescents in Finland in 1990-2014: nationwide re-evaluation of histopathological characteristics. Ann Med. 2022;54(1):244–252. doi: 10.1080/07853890.2022.2026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koh H.K. Cutaneous melanoma. N Engl J Med. 1991;325(3):171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 75.Glusac E.J. The melanoma 'epidemic': lessons from prostate cancer. J Cutan Pathol. 2012;39(1):17–20. doi: 10.1111/j.1600-0560.2011.01848.x. [DOI] [PubMed] [Google Scholar]
- 76.Welch H.G., Woloshin S., Schwartz L.M. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331(7515):481. doi: 10.1136/bmj.38516.649537.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herbert A., Koo M.M., Barclay M.E., et al. Stage-specific incidence trends of melanoma in an English region, 1996-2015: longitudinal analyses of population-based data. Melanoma Res. 2020;30(3):279–285. doi: 10.1097/CMR.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glasziou P.P., Bell K.J., Barratt A.L. Estimating the magnitude of cancer overdiagnosis in Australia. Med J Aust. 2020;213(4):189. doi: 10.5694/mja2.50578. 189e1. [DOI] [PubMed] [Google Scholar]
- 79.Watts C.G., McLoughlin K., Goumas C., et al. Association between melanoma detected during routine skin checks and mortality. JAMA Dermatol. 2021;157(12):1425–1436. doi: 10.1001/jamadermatol.2021.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cantor D. Introduction: cancer control and prevention in the twentieth century. Bull Hist Med. 2007;81(1):1–38. doi: 10.1353/bhm.2007.0001. [DOI] [PubMed] [Google Scholar]
- 81.Schwartz L.M., Woloshin S., Fowler F.J., et al. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 82.Dunn B.K., Srivastava S., Kramer B.S. The word "cancer": how language can corrupt thought. BMJ. 2013;347:f5328. doi: 10.1136/bmj.f5328. [DOI] [PubMed] [Google Scholar]
- 83.Woloshin S., Schwartz L.M., Black W.C., et al. Cancer screening campaigns–getting past uninformative persuasion. N Engl J Med. 2012;367(18):1677–1679. doi: 10.1056/NEJMp1209407. [DOI] [PubMed] [Google Scholar]
- 84.Ransohoff D.F., McNaughton Collins M., Fowler F.J. Why is prostate cancer screening so common when the evidence is so uncertain? A system without negative feedback. Am J Med. 2002;113(8):663–667. doi: 10.1016/s0002-9343(02)01235-4. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz L.M., Woloshin S., Sox H.C., et al. US women's attitudes to false-positive mammography results and detection of ductal carcinoma in situ: cross-sectional survey. West J Med. 2000;173(5):307–312. doi: 10.1136/ewjm.173.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu J., Nagler R.H., Fowler E.F., et al. Women's awareness and perceived importance of the harms and benefits of mammography screening: results from a 2016 national survey. JAMA Intern Med. 2017;177(9):1381–1382. doi: 10.1001/jamainternmed.2017.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nickel B., Moynihan R., Barratt A., et al. Media coverage of calls to rename low-risk cancers: a content analysis. BMJ Open. 2020;10(7) doi: 10.1136/bmjopen-2020-038087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O'Keeffe M., Nickel B., Dakin T., et al. Journalists' views on media coverage of medical tests and overdiagnosis: a qualitative study. BMJ Open. 2021;11(6) doi: 10.1136/bmjopen-2020-043991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Copp T., Dakin T., Nickel B., et al. Interventions to improve media coverage of medical research: a codesigned feasibility and acceptability study with Australian journalists. BMJ Open. 2022;12(6) doi: 10.1136/bmjopen-2022-062706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Esserman L.J., Thompson I.M., Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA. 2013;310(8):797–798. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 91.Veronesi U., Zurrida S., Goldhirsch A., et al. Breast cancer classification: time for a change. J Clin Oncol. 2009;27(15):2427–2428. doi: 10.1200/JCO.2008.21.2647. [DOI] [PubMed] [Google Scholar]
- 92.Ganz P.A. Quality-of-life issues in patients with ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010(41):218–222. doi: 10.1093/jncimonographs/lgq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.NIH State-of-the-Science Conference: Diagnosis and Management of Ductal Carcinoma in Situ (DCIS), September 22-24. (Accessed August 21,2013) 2009 [Available from: http://consensus.nih.gov/2009/dcis.htm.
- 94.Nickel B., Moynihan R., Barratt A., et al. Renaming low risk conditions labelled as cancer. BMJ. 2018;362:k3322. doi: 10.1136/bmj.k3322. [DOI] [PubMed] [Google Scholar]
- 95.Semsarian C.R., Ma T., Nickel B., et al. Do we need to rethink the diagnoses melanoma in situ and severely dysplastic naevus? Br J Dermatol. 2022;186(6):1030–1032. doi: 10.1111/bjd.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma T., Semarian C., Barratt A., et al. Rethinking low-risk papillary thyroid cancers <1cm (Papillary Microcarcinomas): an evidence review for recalibrating diagnostic thresholds and/or alternative labels. Thyroid. 2021;31(11):1626–1638. doi: 10.1089/thy.2021.0274. [DOI] [PubMed] [Google Scholar]
- 97.Smith-Bindman R., Chu P.W., Miglioretti D.L., et al. Comparison of screening mammography in the United States and the United kingdom. JAMA. 2003;290(16):2129–2137. doi: 10.1001/jama.290.16.2129. [DOI] [PubMed] [Google Scholar]
- 98.Pickles K., Hersch J., Nickel B., et al. Effects of awareness of breast cancer overdiagnosis among women with screen-detected or incidentally found breast cancer: a qualitative interview study. BMJ Open. 2022;12(6) doi: 10.1136/bmjopen-2022-061211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crispo A., Barba M., D'Aiuto G., et al. Molecular profiles of screen detected vs. symptomatic breast cancer and their impact on survival: results from a clinical series. BMC Cancer. 2013;13:15–25. doi: 10.1186/1471-2407-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Domingo L., Blanch J., Servitja S., et al. Aggressiveness features and outcomes of true interval cancers: comparison between screen-detected and symptom-detected cancers. Eur J Cancer Prev. 2013;22(1):21–28. doi: 10.1097/CEJ.0b013e328354d324. [DOI] [PubMed] [Google Scholar]
- 101.Kornberg Z., Cowan J.E., Westphalen A.C., et al. Genomic prostate score, PI-RADS version 2 and progression in men with prostate cancer on active surveillance. J Urol. 2019;201(2):300–307. doi: 10.1016/j.juro.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 102.Paik S., Shak S., Tang G., et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 103.Esserman L.J., Study W., Athena I. The WISDOM Study: breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer. 2017;3:34. doi: 10.1038/s41523-017-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Welch H.G., Kramer B. The crazy confluence of Congress, liquid biopsies, Medicare, and health inequities. STAT. 2022 https://www.statnews.com/2022/01/12/medicare-shouldnt-cover-liquid-biopsies-early-cancer-detection/ [Internet]. 2022 March 10[cited 2022. Available from. [Google Scholar]