Abstract
Quorum-sensing systems regulate the expression of virulence factors in a wide variety of plant and animal pathogens, including members of the Enterobacteriaceae. Studies of Shigella virulence gene expression have demonstrated that maximal expression of genes encoding the type III secretion system and its substrates and maximal activity of this virulence organelle occur at high cell density. In these studies, we demonstrate that the expression of ipa, mxi, and spa invasion operons is maximal in stationary-phase bacteria and that conditioned media derived from stationary-phase cultures enhance the expression of these loci. In contrast, expression of virB, a transcription factor essential for the expression of invasion loci, peaks in late log phase; accordingly, virB expression is enhanced by a signal(s) present in conditioned media derived from late-log-phase cultures. Autoinducer 2 (AI-2), a quorum signaling molecule active in late log phase, was synthesized by Shigella species and enteroinvasive Escherichia coli and shown to be responsible for the observed peak of virB expression. However, AI-2 does not influence invasion operon expression and is not required for Shigella virulence, as mutants deficient in AI-2 synthesis are fully virulent. The implications of these findings with regard to both virB and invasion operon expression and the evolution of circuitries governing virulence gene expression are discussed.
The study of single-celled bacteria has historically focused on characteristics of individual cells, overlooking their behavior in clonal or heterogeneous populations. However, studies conducted over the last decade have revealed that the expression of a wide variety of traits in many bacterial species is modulated in response to signals present in dense populations. This population-dependent signaling phenomenon has been termed quorum sensing (14). The salient feature of these systems, signaling molecules that accumulate in the extracellular milieu, exert their influence through cognate receptors to modulate gene expression and the elaboration of a wide variety of phenotypes. Signaling and sensor components have been characterized for a number of systems, and studies have revealed at least three distinct conserved strategies which communicate cell density among both gram-positive and gram-negative bacteria (reviewed in references 10 and 13).
Gram-positive bacteria perceive cell density by using processed peptide signaling molecules that are actively transported outside the cell. The peptides bind and activate cognate two-component receptors that transduce signals and influence target gene expression via phosphorelays. In contrast, many species of gram-negative bacteria use membrane-permeating signaling molecules collectively known as homoserine lactones (HSL). These molecules, which vary in structure depending on the species source, bind a transcriptional activator protein, typically of the LuxR family, that in turn activates operators governing target gene expression. Recently, a third, highly conserved quorum-sensing system used by both gram-positive and gram-negative bacteria was identified (34, 35). This system requires a highly conserved locus, luxS, which has been identified for at least 25 discrete species, including Escherichia coli and Bacillus subtilis. It has been proposed that LuxS acts on a metabolic intermediate to synthesize a unique signaling molecule, termed autoinducer 2 (AI-2), which is likewise conserved in both structure and function. Accordingly, AI-2 can be detected by heterologous species. This feature may enable bacteria to sense and respond to pressures produced by mixed dense populations in niches where space and nutrients may be limited. Kinetic studies of AI-2 action have demonstrated that, unlike that of other quorum-sensing systems, the concentration of this signaling molecule is maximal in late-log or early-stationary-phase cultures and is diminished in stationary phase (34, 35). Evidence for a second E. coli autoinducer molecule that functions in stationary phase has been reported (3). To date, the physical nature of this signaling molecule and the loci required for synthesis and response remain unidentified.
Although the systems used to signal and sense population density are conserved, responses to these environmental cues vary widely, as each niche presents a mélange of unique pressures. The myriad of responses includes bioluminescence, competence, sporulation, antibiotic production, and cessation of growth. In bacterial pathogenesis, quorum-sensing systems regulate virulence factor expression in a variety of organisms, including toxin and alginate production in Pseudomonas aeruginosa (33), type IV secretion in Agrobacterium tumefaciens (26), and exoenzyme production in Burkholderia cepacia (20). Among enteric organisms, HSL-mediated quorum signaling regulates motility in Yersinia pseudotuberculosis (2). It was recently reported that the AI-2 quorum-signaling system regulates the expression of the enteropathogenic and enterohemorrhagic E. coli (EPEC and EHEC, respectively) LEE1 and LEE2 operons (32), which encode the substrates and structural components of a type III secretion system dedicated to promoting intimate adherence to host colonocytes (19, 22). These findings prompted the investigators to hypothesize that the regulatory circuitry governing these EPEC and EHEC virulence factors evolved to take advantage of high AI-2 levels produced by normal intestinal flora that express LuxS. This signal, it is proposed, triggers the pathogen to express adherence factors that direct efficient colonization of the host. Therefore, this unique environmental cue may be exploited by bacterial pathogens to coordinate the expression of virulence factors with transit to desired host niches. Closely coordinated gene expression enhances the virulence potential of these organisms, so that few bacteria need to be ingested to successfully colonize the host. This hypothesis is supported by the low infectious dose of EHEC.
Collectively, these varied studies suggest that the ability to signal, sense, and respond to population density, in either clonal or heterologous consortia, enables bacteria to express factors that enhance their fitness in a wide variety of niches, including aquatic environments, biofilms, and plant or animal tissues. Although the role of quorum sensing has been demonstrated in these varied environments, it is unknown whether these systems regulate virulence gene expression in organisms that exist in intracellular niches. Consequently, we sought to determine whether quorum sensing influences virulence gene expression and virulence in the invasive enteric pathogen Shigella flexneri.
Shigella spp. are the primary agents of bacillary dysentery, an acute inflammatory disease of the human colonic epithelia that results from focal invasion and subsequent radial dissemination of the bacteria (27). Factors central to the invasion of host cells include products of three closely linked operons, ipa, mxi and spa, present on the 230-kb Shigella virulence plasmid. These loci encode both exported factors, which mediate host cell cytoskeletal rearrangements and invasion (the invasion plasmid antigens, IpaA to IpaD), and a type III secretion system (encoded by the mxi and spa operons), dedicated to delivering Ipa proteins to the host cell surface. It is well established that temperature is a key environmental cue exploited by Shigella to sense passage into the human gut, as the expression of these virulence loci is induced >100 fold when bacteria are shifted from 30 to 37°C (18). Factors governing this thermal regulation have been identified and include chromosomally encoded H-NS, which represses virulence gene expression at 30°C, and virulence plasmid-encoded VirF and VirB, which are transcription factors required for the expression of the invasive phenotype at 37°C (9). Current models suggest that H-NS binds the virB operator at 30°C, occluding VirF binding and impairing virB transcription. Upon a shift to 37°C, H-NS is displaced from the virB operator, allowing VirF to bind to virB and induce the expression of VirB, which in turn induces the expression of ipa, mxi, and spa operons.
Recent reports suggest that additional environmental cues, such as cell density, may govern virulence gene expression in Shigella. Bahrani et al. reported that the expression of ipaA∷lacZ and mxiD∷lacZ fusions increased with cell density, peaking in stationary-phase cultures (4). Moreover, the secretion of IpaC increased with cell density and was maximal in stationary-phase bacteria. These observations suggest the action of signaling molecules in stationary-phase cultures (i.e., an HSL class of quorum signaling molecules). In addition, studies with volunteers have established that the infectious dose of Shigella, like that of EHEC, is extremely low and may be as few as 10 organisms (11).
Considering the proposed role of intestinal-flora-derived AI-2 in the optimal temporal expression of virulence genes in EHEC and the close evolutionary relatedness of pathogenic E. coli and Shigella species, this colonization efficiency may reflect the action of AI-2 signaling. Consequently, we hypothesized that quorum-sensing systems influence Shigella virulence gene expression and the elaboration of virulence phenotypes. In this report, we describe the kinetics of Shigella virB, ipa, mxi, and spa operon expression over the growth curve and in the presence of conditioned media containing autoinducer. We also report the luxS-dependent synthesis of AI-2 by S. flexneri 2a as well as virulence phenotypes of mutants defective in AI-2 production.
MATERIALS AND METHODS
Bacterial strains and growth media.
Strains of Shigella, Vibrio harveyi, and E. coli used in this study are described in Table 1. Overnight cultures of Shigella species and E. coli strains were grown in Luria-Bertani broth (LB) at 30°C in a shaking water bath to repress the expression of virulence loci and stabilize the virulence plasmid. Exponential cultures of Shigella species and E. coli strains used in all experiments were established by diluting overnight cultures 100-fold, unless otherwise stated, in fresh LB and incubating them at 37°C in a shaking water bath. V. harveyi strains were cultured in LB–1% NaCl at 30°C in a shaking water bath. Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; spectinomycin; 100 μg/ml; and streptomycin, 100 μg/ml.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristics | Reference or source |
|---|---|---|
| Shigella | ||
| 2457T | Wild-type S. flexneri 2a | 12 |
| 3818T | Wild-type Shigella dysenteriae | Laboratory stock |
| BS103 | Virulence plasmid-cured derivative of 2457T | 21 |
| BS226 | 2457T Φ(spa47∷lacZ+ 11.5)a | 18 |
| BS228 | 2457T Φ(ipaB∷lacZ+ 17.6)a | 18 |
| BS260 | 2457T Φ(mxiA∷lacZ+)b | 1 |
| BS513 | Wild-type Shigella sonnei | Laboratory stock |
| BS534 | 2457T Φ(virB∷lacZ+)a | Laboratory stock |
| BS607 | Wild-type Shigella boydii | Laboratory stock |
| BS620 | 2457T ΔluxS∷aadA | This study |
| BS635 | BS534 ΔluxS∷aadA (P1L4 transduction from BS620) | This study |
| BS636 | 2457T Pbla∷lacZ+ Kanr (P1L4 transduction from MCAmp) | This study |
| BS637 | BS228 ΔluxS∷aadA (P1L4 transduction from BS620) | This study |
| BS638 | BS260 ΔluxS∷aadA (P1L4 transduction from BS620) | This study |
| V. harveyi | ||
| BB152 | luxM∷Tn5 lac; AI-1 negative, AI-2 positive | 5 |
| BB170 | luxN∷Tn5; sensor AI-1 negative, sensor AI-2 positive | 6 |
| E. coli | ||
| ATM266 | Wild-type enteroinvasive isolate | 17 |
| DH5α | AI-2 negative | 16 |
| MC4100 | AI-2 positive | 7 |
| MCAmp | Pbla∷lacZ | 32 |
| SM10λpir | thi-1 thr-1 leuB6 tonA21 lacY1 supE44 recA∷RP4-2-Tc∷Mu Kanr λpir | 31 |
Transcriptional fusion.
Translational fusion.
Molecular methods and strain construction.
Analyses of DNA, plasmid construction, and electroporation of E. coli were performed using manufacturers' suggested conditions or standard protocols described elsewhere (28). All restriction endonucleases and T4 DNA ligase were purchased from New England Biolabs (Beverly, Mass.). PCR was performed with Pfu TURBO DNA polymerase (Stratagene, La Jolla, Calif.) according to the manufacturer's protocols. Templates for DNA sequencing were prepared using an ABI Prism dye terminator cycle sequencing kit. Products were analyzed on an ABI Prism 377 DNA sequencer at the Uniformed Services University of the Health Sciences (USUHS) Biomedical Instrumentation Center. Oligonucleotide synthesis was performed using Applied Biosystems automated solid-phase synthesis with standard chemistry at the USUHS Biomedical Instrumentation Center.
PCR amplification of the S. flexneri luxS promoter and open reading frame was accomplished using upstream and downstream primers derived from regions adjacent to luxS in the E. coli K-12 genome sequence (GenBank accession number U00096). To construct a suicide plasmid that would serve as a substrate for allelic exchange and replace wild-type luxS with a deletion insertion mutation, 1-kb regions upstream and downstream of luxS were amplified from the S. flexneri 2457T chromosome using PCR. By use of restriction sites engineered on the 5′ ends of each primer set, the products were sequentially cloned into pUC19, generating pEBD120. The resulting plasmid insert, which replaces both the luxS promoter and all but 17 bp of the 3′ end of the monocistronic luxS open reading frame with an EcoRI site, allows construction of a deletion mutation that will not affect adjacent gene expression. To facilitate selection of the mutant luxS deletion allele, the aadA gene, which encodes resistance to both streptomycin and spectinomycin, was obtained on an EcoRI fragment from plasmid pUT-Sp/Sm (8) and ligated into the unique EcoRI site of pEBD120. The resulting insert was subcloned into suicide vector pGP704 (24), generating pEBD125. pEBD125 was introduced into E. coli SM10λpir and delivered to S. flexneri by conjugation. Transconjugants harboring the ΔluxS∷aadA allele, resulting from double-crossover recombination, were identified by resistance to streptomycin and spectinomycin and by ampicillin sensitivity and confirmed by subsequent PCR analysis. The chromosomally encoded mutant luxS allele was then transferred to S. flexneri 2457T, BS228, BS260, and BS534 by P1L4 transduction to generate strains BS620, BS637, BS638, and BS635, respectively. Generation of S. flexneri strain BS636, which harbors a single chromosomal copy of the pBR322 β-lactamase promoter fused to lacZ linked to a kanamycin resistance marker, was accomplished by P1L4 transduction using phage lysates grown on E. coli MCAmp (32).
Conditioned-medium assays.
Conditioned media were prepared as described by Sperandio et al. (32). Overnight cultures grown in LB at 30°C were diluted 1:100 in fresh LB–0.5% glucose and cultured in a shaking water bath at 37°C. When the cultures reached an optical density at 600 nm (OD600) of 0.3, the bacteria were again diluted 1:100 in fresh LB–0.5% glucose and incubated in a shaking water bath at 37°C. Cultures were grown to the desired density (OD600 of 1.4 for late-log-phase conditioned media and OD600 of 2.25 for stationary-phase conditioned media), and the bacteria were removed by centrifugation and sterile filtration (0.45-μm-pore-size filters; Corning, Acton, Mass.). Depleted nutrients in the conditioned media were corrected by the addition of 0.1 volume of 5× LB, and the pH was adjusted to 7.0. Aliquots of the conditioned media were stored at −20°C until needed. lacZ fusion reporter strains, cultured overnight under conditions that repress virulence gene expression (i.e., 30°C), were diluted 1:100 in conditioned media, grown at 37°C in a shaking water bath to an OD600 of 0.3, and harvested by centrifugation; the pellets were stored at −20°C until assayed for β-galactosidase activity.
The production of AI-2 was assessed using the V. harveyi bioassay described by Surette and Bassler (34, 35). Overnight cultures of V. harveyi strain BB170, which expresses luminescence in response to AI-2, were diluted 1:100 in autoinducer bioassay medium (4) and cultured in a 30°C shaking water bath. When the culture reached an OD600 of 0.3, the bacteria were again diluted 1:100 in 10% conditioned medium–90% autoinducer bioassay medium and incubated in a 30°C shaking water bath. Cultures were grown to an OD600 of 0.3, and light production was determined using an MLX luminometer (Dynex, Chantilly, Va.).
β-Galactosidase assays.
Reporter strains cultured under the desired conditions were harvested by centrifugation. Cell pellets were resuspended in phosphate-buffered saline and permeabilized with 0.1 volume of chloroform and 0.05 volume of 0.1% sodium dodecyl sulfate. The β-galactosidase activity in each lysate was determined as described previously using o-nitrophenyl-β-d-galactopyranoside as a substrate for permeabilized cells diluted in Z buffer (0.06 M Na2HPO4 · 7H2O, 0.04 M NaH2PO4 · H2O, 0.01 M KCl, 0.001 M MgSO4 · 7H2O, 0.05 M β-mercaptoethanol [pH 7.0]) (23).
Virulence assays.
Invasion assays were performed using a gentamicin protection assay and semiconfluent L2 fibroblast monolayers as previously described (15). Plaque assays were performed using confluent L2 fibroblast monolayers as previously described (25). In vivo virulence was assessed with the Sereny test as previously described (12, 30).
RESULTS
Shigella invasion operon expression increases with culture density.
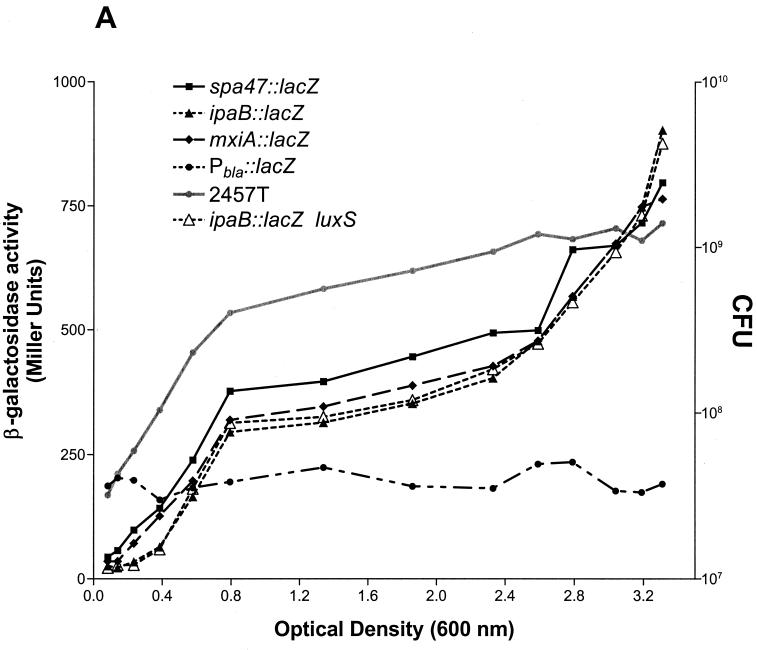
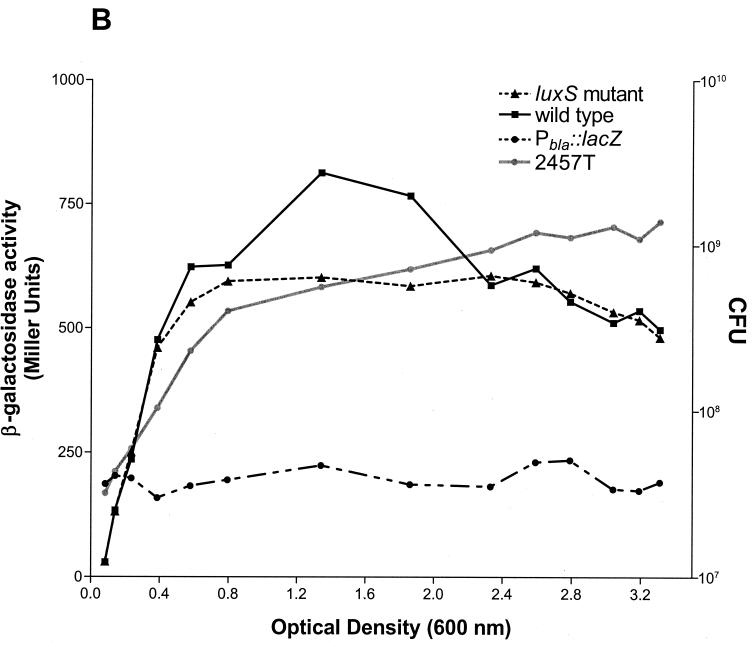
To determine whether the expression of Shigella invasion loci is influenced by cell density, reporter activity was determined over the growth curve for S. flexneri strains carrying lacZ fusions to ipaB, mxiA, and spa47 loci. Strikingly, the kinetics of reporter activity for each of the fusions were essentially identical (Fig. 1A). Moreover, the expression of each fusion increased steadily with population density, as maximal levels of reporter activity were observed in stationary-phase bacteria. This cell density-dependent expression was not observed for the constitutively active Pbla∷lacZ fusion, which is not regulated by quorum sensing and which served as a negative control (32). These observations prompted the examination of virB expression kinetics over the growth curve to determine whether the transcription factor required for induction and expression of the invasion operons was similarly regulated. The expression kinetics of the BS534 virB reporter fusion were dramatically different from those of the invasion operon fusions (Fig. 1B). The expression of the virB fusion was induced earlier and to higher levels than that of the ipa, mxi, and spa fusions. These observations are consistent with the role of VirB in regulating the expression of these loci. Furthermore, reporter activity in strain BS534 peaked in late-log- or early-stationary-phase bacteria (OD600, 1.2 to 2.0) and then quickly dropped to a level which was essentially maintained throughout stationary phase. These findings suggest that the expression of virB and the Shigella invasion operons is influenced by signals active in dense populations.
FIG. 1.
Kinetics of S. flexneri 2a virulence gene expression over the growth curve. (A) Reporter activities of spa47∷lacZ, ipaB∷lacZ, and mxiA∷lacZ fusion strains in the wild-type background (BS226, BS228, and BS260, respectively) and the ipaB∷lacZ fusion strain in a luxS mutant background (BS637) were determined over the growth curve as described in Materials and Methods. (B) Reporter activities of the virB∷lacZ fusion in wild-type S. flexneri 2a (2457T, AI-2 positive) and the isogenic luxS mutant (BS635, AI-2 negative) were determined over the growth curve as described in Materials and Methods. The growth curve of wild-type S. flexneri 2a strain 2457T, plotted as CFU (right y axis), is depicted as a grey line in both panels. The expression kinetics of a non-quorum-responsive reporter fusion (Pbla∷lacZ, strain BS636) are included as a control in both panels. The activity of each reporter fusion was determined in three separate experiments. Results from one representative experiment are shown.
virB and ipaB expression responds to signals present at discrete stages in the growth cycle.
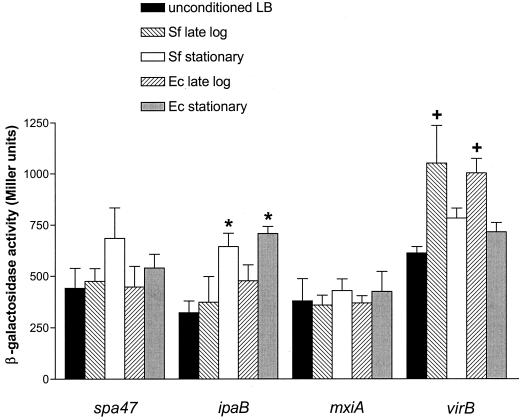
The kinetics of reporter activities over the growth curve suggested that signals present at different stages of the growth cycle influence the expression of the invasion operons and virB (i.e., stationary-phase signals influencing invasion operon expression and late-log- or early-stationary-phase signals influencing virB expression). To determine whether environmental cues present in each phase of growth modulate the expression of these loci, the activity of each reporter fusion was determined for strains grown in fresh media and conditioned media prepared from late-log- or early-stationary-phase or stationary-phase wild-type S. flexneri 2a. Conditioned media derived from late-log-phase cultures significantly enhanced the expression of the virB reporter fusion relative to that seen with unconditioned media or media derived from stationary-phase cultures (Fig. 2). In contrast, the activity of the invasion operon fusions was highest in strains cultured in medium conditioned by stationary-phase cultures. However, the increase was only significant for the ipaB fusion. The expression of the control Pbla∷lacZ reporter was not influenced by growth in conditioned media (data not shown). These findings were consistent with the reporter activity observed over the growth curves and support the idea that the expression of virB and the invasion operons is influenced by signals elicited at different stages of the growth cycle. To determine whether signals present at each phase of growth were unique to S. flexneri 2a, similar analyses were performed using conditioned media derived from E. coli MC4100. Profiles of reporter induction revealed trends similar to those observed with S. flexneri-conditioned media, as stationary-phase E. coli-conditioned media significantly enhanced the expression of the ipaB reporter fusion. In addition, late-log-phase E. coli-conditioned media significantly enhanced the expression of the virB reporter fusion (Fig. 2). These results suggest that E. coli can provide the cell density-dependent environmental cues that influence the expression of Shigella virulence genes.
FIG. 2.
Conditioned media enhance Shigella virulence gene expression. The activities of the lacZ reporter fused to spa47 (BS226), ipaB (BS228), mxiA (BS260), and virB (BS534) in conditioned media derived from late-log-phase (OD600, 1.4) or stationary-phase (OD600, 2.25) cultures of S. flexneri 2457T (Sf) or E. coli MC4100 (Ec) were determined as described in Materials and Methods. The activity of each fusion grown in unconditioned medium is included as a control. An asterisk denotes a significant difference (P < 0.05) in the reporter activity of the ipaB∷lacZ fusion grown in conditioned media derived from stationary-phase cultures relative to conditioned media derived from late-log-phase cultures and unconditioned medium. Values were calculated using an unpaired Student t test. A plus sign denotes a significant difference (P < 0.05) in the reporter activity of the virB∷lacZ fusion grown in conditioned media derived from late-log-phase cultures relative to conditioned media derived from stationary-phase cultures and unconditioned medium. Values were calculated using an unpaired Student t test. Error bars indicate the 95% confidence level. β-gal, β-galactosidase.
Shigellae produce AI-2.
The maximum expression of the virB fusion coincided with the peak activity of the luxS-dependent AI-2 signaling molecule known to mediate quorum sensing during the transition from logarithmic- to stationary-phase growth in a wide variety of bacteria, including E. coli (34, 35). To determine whether S. flexneri harbored luxS, we amplified and sequenced the locus from strain 2457T and found that the S. flexneri gene was 99% homologous to E. coli K-12 luxS at the nucleotide level and contained only three silent mutations (data not shown). In addition, luxS-specific primers amplified products of identical sizes from all other Shigella species and enteroinvasive E. coli (EIEC) (data not shown). To determine whether shigellae and EIEC express luxS and synthesize AI-2, a bioassay was performed with a V. harveyi lux reporter strain that senses environmental AI-2 and responds with the induction of luminescence (6). Conditioned media prepared from late-log- or early-stationary-phase cultures of each species induced levels of luminescence comparable to or higher than those induced by positive controls (Table 2). To determine whether luxS was required for Shigella AI-2 synthesis, an S. flexneri strain harboring a deletion insertion mutation in luxS was constructed and assayed for AI-2 production. As expected, conditioned media derived from this strain failed to induce significant levels of luminescence (Table 2). These findings demonstrate that shigellae and EIEC produce AI-2 and that the production of this signaling factor is luxS dependent.
TABLE 2.
AI-2 synthesis by Shigella species and EIEC
| Source of conditioned mediaa | Fold induction of luminescence in V. harveyi BB170b |
|---|---|
| 2457T (S. flexneri 2a) | 516 |
| BS607 (S. boydii) | 484 |
| 3818T (S. dysenteriae) | 521 |
| BS513 (S. sonnei) | 543 |
| ATM266 (EIEC) | 460 |
| BS620 (S. flexneri 2a ΔluxS∷aadA) | 1 |
| BB152c (V. harveyi, AI-2 positive) | 338 |
| MC4100c (E. coli K-12, AI-2 positive) | 427 |
| DH5αc (E. coli K-12, AI-2 negative) | 1 |
Conditioned media derived from cultures grown in LB–0.5% glucose at 37°C to an OD600 of 1.4 were prepared as described in Materials and Methods.
Fold induction represents the mean of three separate experiments measuring the luminescence of V. harveyi BB170 (sensor AI-1 negative, sensor AI-2 positive) cultured in conditioned media from the designated sources relative to that of V. harveyi BB170 grown in unconditioned LB.
AI-2 production by these strains has been previously characterized (33). Strains served as positive (BB152 and MC4100) and negative (DH5α) control sources of AI-2 sensed by BB170.
Effect of luxS on S. flexneri virulence gene expression.
Since the optimal production of AI-2 coincided with the peak of virB reporter fusion activity, we sought to determine whether the expression of virB during the transition from logarithmic- to stationary-phase growth was dependent on the AI-2 quorum signaling molecule. Consequently, a luxS deletion insertion mutation was transduced into BS534 (virB∷lacZ), and the activity of the virB reporter fusion was determined over the growth curve of the resulting strain. The expression of the reporter fusion in this strain (BS635) was essentially identical to that observed in the wild-type strain during both early-logarithmic and stationary phases of growth. However, the peak of reporter activity observed during the transition from late-log- to early-stationary-phase growth was absent in the luxS mutant (Fig. 1B).
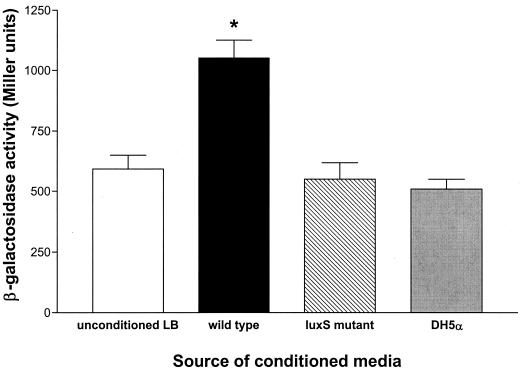
To further examine the possibility that virB expression is influenced by quorum-sensing signals, virB reporter strain BS534 was cultured in conditioned media devoid of AI-2. These conditions, derived from late-log- or early-stationary-phase cultures of the isogenic Shigella luxS mutant, failed to induce the peak of virB reporter expression that coincided with maximal AI-2 activity (Fig. 3). These findings indicate that the maximal expression of virB, an essential Shigella virulence factor, responds to quorum-sensing signals and requires luxS. As virB is known to regulate Shigella invasion operon expression, it was possible that the decrease in virB expression observed in the luxS mutant could have affected invasion gene expression. To examine this possibility, the luxS deletion insertion mutation was transduced into both ipaB and mxiA lacZ reporter strains, generating BS637 and BS638, respectively. The kinetics of reporter activity in each of these strains were identical to those in the wild-type luxS parents (Fig. 1A and data not shown), indicating that AI-2 does not influence ipa or mxi gene expression.
FIG. 3.
virB expression is induced by AI-2. S. flexneri strain BS534 (virB∷lacZ) was cultured in conditioned media derived from late-log-phase cultures (OD600, 1.4) of wild-type S. flexneri 2457T (AI-2 positive) and the isogenic luxS mutant BS620 (AI-2 negative,). The activities of the reporter fusion grown in unconditioned LB medium and conditioned medium derived from E. coli DH5α (AI-2 negative) (33) are included as controls. The asterisk denotes a significant difference (P < 0.05) relative to the results for media devoid of the AI-2 signaling molecule. Values were calculated using an unpaired Student t test. Error bars indicate the 95% confidence level. β-gal, β-galactosidase.
As virB plays a central role in the expression of Shigella invasion phenotypes, the virulence properties of the luxS mutant were examined (Table 3). Neither invasiveness nor the ability to disseminate to adjacent cells, phenotypes essential for Shigella virulence, were altered in the luxS mutant. In addition, no attenuation of virulence in the luxS mutant was observed in an in vivo model, the Sereny test. These findings suggest that although the maximal expression of virB requires AI-2, the quorum signaling molecule is not required for Shigella virulence.
TABLE 3.
Virulence phenotypes of S. flexneri 2aa
| Strain | Description | % Invasionb | % plaquing efficiencyc | Plaque diam (mm)d | Sereny test resulte |
|---|---|---|---|---|---|
| 2457T | Wild type | 0.22 | 1.3 | 1.2 | Positive |
| BS103 | Avirulent control | 0.0013 | <5.8 × 10−3 | ND | ND |
| BS620 | 2457T ΔluxS∷aadA | 0.19 | 1.1 | 1.0 | Positive |
Each value shown represents the average of three independent experiments. ND, not determined.
Calculated by dividing the number of CFU resulting from gentamicin-protected bacteria by the number of bacteria present in the inoculum and multiplying the result by 100.
Calculated by dividing the number of plaques formed on confluent L2 monolayers by the number of bacteria present in the inoculum and multiplying the result by 100.
Calculated by measuring the diameter of the zone cleared on L2 fibroblast monolayers. Each value represents the average for 10 plaques examined in three separate experiments.
Results for the Sereny test represent the ability of the strain to induce keratoconjunctivitis in the guinea pig eye.
DISCUSSION
Quorum sensing systems communicate important information regarding population density and the potential for growth and survival in an environment. A number of bacterial pathogens exploit this information to coordinate virulence gene expression with transit to an environment where these factors permit efficient colonization of host niches. Accordingly, invasive organisms such as Shigella species could send, receive, and respond to signals indicating population density both in the lumen of the intestine prior to invasion (signal derived from normal flora) and in the cytosol of infected cells (signal derived from Shigella). The expression of the ipa, mxi, and spa operons and the transcriptional activator governing their expression (virB) is required for both penetration of host cells (36) and intercellular spread (29). Presumably, if Shigella exploits a quorum signal to regulate virulence gene expression, one or more of these genes would be influenced, as each is required during all phases of the infectious cycle. We observed essentially identical cell density-dependent expression for all three Shigella invasion operons. These findings are consistent with previously reported ipaA and mxiD expression profiles (4) and suggest that the expression of virulence factors essential for both entry of host cells and intercellular spread is influenced by cell density. In addition, coordinate expression of these loci at all phases of the growth curve is consistent with the interwoven roles of factors encoded by these operons and is likely essential for proper function and elaboration of virulence phenotypes. While other environmental factors coincident with dense populations, such as changes in pH, nutrients, and metabolites, may contribute to virulence gene expression in stationary phase, the uniform increase in invasion operon expression suggests a different scenario based on the steady-state expression of virB (see below).
Considering the central role of VirB in invasion operon transcription, it is possible that the circuitry governing this pattern of expression includes the transcription factor. Surprisingly, the kinetics of virB reporter fusion activity suggested that the expression of the locus is enhanced not by stationary-phase signals but instead by signals that peak in activity during the transition from late-log-phase growth to stationary-phase growth. Indeed, a comparison of the reporter activities of the examined loci revealed that the expression of the invasion operons continued to increase in stationary-phase cultures even as virB expression diminished to a level maintained throughout stationary phase. These findings were supported, in part, by conditioned-medium experiments demonstrating that the invasion operons and virB respond to signals elicited at different stages of the growth cycle. The experiments demonstrated that a signaling molecule that closely mirrors AI-2 in temporal activity enhances virB expression and provided the impetus to investigate the roles of luxS and AI-2 in virB expression and Shigella virulence. In contrast, signaling molecules presumed present in stationary-phase-conditioned media did not significantly enhance the expression of mxi or spa reporter fusions. Only the expression of the ipa reporter fusion was significantly, but modestly, enhanced by a factor(s) present in stationary-phase-conditioned media (Fig. 2). It is possible that ipa expression is influenced by the uncharacterized second autoinducer active in stationary-phase E. coli cultures (3). Remarkably, each of these findings is inconsistent with the expression profiles observed over the growth curve. However, a close analysis of the growth curve data suggests a model that may reconcile the apparent inconsistencies.
The kinetics of ipa, mxi, and spa expression over the growth curve suggest a gradual buildup of protein rather than a rapid induction of gene expression brought about by the accumulation of an environmental signal. The patterns of invasion operon expression contrast with the expression kinetics observed for established quorum-responsive genes. The expression of genes in response to cell density is governed by signaling molecules expressed by each bacterium in a population. Accordingly, as the population density increases, the signaling molecule concentration increases. At a sufficiently high population density, essentially a bacterial quorum, signaling molecules accumulate to a threshold concentration and induce a dramatic and rapid increase in the expression of quorum-responsive loci (reviewed in reference 14). This pattern of induction is not observed for the Shigella invasion operons (Fig. 1A), suggesting a different mechanism of high stationary-phase expression that may be based on the observed pattern of virB expression. An apparent steady-state activity of the virB∷lacZ fusion is observed in stationary phase, suggesting that an equilibrium in fusion expression and degradation exists in these bacteria. In contrast, no steady state or decline in reporter activity is observed in the invasion locus lacZ fusions. These results suggest two phenomena. First, less-than-maximal levels of virB produced in stationary phase are sufficient to induce and sustain invasion operon expression. Evidence supporting this hypothesis is provided by the unaltered kinetics of ipa and mxi operon expression in the absence of AI-2 (luxS strains) and, consequently, submaximal virB expression (Fig. 1 and data not shown). Second, a steady increase in invasion operon reporter activity may result from the stability of ipa, mxi, and spa mRNA transcripts, the stability of the VirB protein, or the synergistic stability of these elements. Data supporting this hypothesis are provided by the unwavering activity of the Pbla∷lacZ fusion over the growth curve. These data demonstrate that LacZ is turned over in the cells and indicate that the high levels of reporter activity observed for the ipa, mxi, and spa fusions cannot result from excessive reporter stability. Additional experiments beyond the scope of these studies are required to test this hypothesis.
The data presented indicate that all species of Shigella and EIEC produce the AI-2 quorum signaling molecule. Moreover, we demonstrate that one target of the S. flexneri AI-2 signaling system is VirB, a transcription factor that is essential for the expression of Shigella virulence. Indeed, maximal virB expression requires a functional AI-2 quorum-sensing system. These findings add population density to thermal induction as a second means of controlling virB expression and elaboration of virulence traits. However, our results suggest that this added layer of control does not significantly contribute to the regulation of virulence gene expression. The luxS mutant, which could not express maximal levels of virB, is not attenuated in invasiveness, intercellular spread, or ability to induce a positive Sereny test reaction. These observations suggest that maximal expression of virB is not required for virulence and are consistent with data indicating that steady-state levels of virB expression achieved in the absence of AI-2 are sufficient to induce expression of the invasion operons. Alternatively, AI-2-mediated induction of virB may reflect activity of an AI-2-responsive element epistatic to virB. One possible target of AI-2 signaling that regulates virB expression is H-NS. Studies by Withers and Nordstrom suggest that AI-2 signaling may target conserved cellular processes that influence DNA replication, as conditioned medium containing AI-2 inhibits DNA replication and cell division (37). It is possible that the modulation of virB expression in response to population density indirectly reflects AI-2-mediated modification of H-NS.
These studies define the function of the highly conserved AI-2 quorum-sensing system in the facultative intracellular pathogen S. flexneri. Moreover, we demonstrate that, unlike those in a number of other enteric organisms, the signaling system in Shigella is not exploited to regulate virulence gene expression. These findings are consistent with the ecology of the pathogen. In contrast to EHEC, EPEC, and Vibrio cholerae, which persist in the lumen of the host gut and are continuously exposed to high levels of AI-2 derived from normal flora, Shigella efficiently invades host cells and flourishes in the eukaryotic cytosol. Thus, these organisms likely are exposed to high luminal AI-2 levels for only a short time. Rather than exploit a signal which may be present only in environments shared with intestinal flora, Shigella exploits an environmental cue, temperature, which is uniformly distributed in host tissues both inside the gut lumen and in the colonic epithelial cytosol. This single signal permits high-level expression of virulence factors that are essential for success of the pathogen in multiple host environments. For example, the Ipa, Mxi, and Spa proteins are required in the gut lumen to direct host cell invasion as well as inside cells to promote intercellular spread (29). Therefore, we propose that AI-2 signaling may not be a mechanism used to modulate virulence gene expression for bacterial pathogens that colonize host tissues not occupied by normal flora. This possibility should be considered as investigators pursue therapies that target and diminish virulence gene expression by disrupting AI-2 synthesis (32). These therapies, which may prove effective in controlling colonization by luminal gut pathogens, may not be effective in controlling the invasion and dissemination of organisms, such as Shigella, that colonize intracellular niches and the subepithelial lining of the intestine, both of which are normally free of resident flora.
ACKNOWLEDGMENTS
This work was supported by grant AI24656 from the National Institute of Allergy and Infectious Diseases and grant RO7385 from the Uniformed Services University of the Health Sciences.
We thank Jim Kaper for the gift of E. coli strain MCAmp, Bonnie Bassler for the gift of V. harveyi strains BB152 and BB170, Sara Mixter and Vanessa Sperandio for technical assistance, Michael N. Flora and the USUHS Biomedical Instrumentation Center for DNA sequencing and oligonucleotide synthesis services, and Robin C. Sandlin for thoughtful discussions.
REFERENCES
- 1.Andrews G P, Hromockyj A E, Coker C, Maurelli A T. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate excretion of invasion plasmid antigens. Infect Immun. 1991;59:1997–2005. doi: 10.1128/iai.59.6.1997-2005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson S, Throup J P, Stewart G S, Williams P. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol Microbiol. 1999;33:1267–1277. doi: 10.1046/j.1365-2958.1999.01578.x. [DOI] [PubMed] [Google Scholar]
- 3.Baca-DeLancey R R, South M M, Ding X, Rather P N. Escherichia coli genes regulated by cell-to-cell signaling. Proc Natl Acad Sci USA. 1999;96:4610–4614. doi: 10.1073/pnas.96.8.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahrani F K, Sansonetti P J, Parsot C. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect Immun. 1997;65:4005–4010. doi: 10.1128/iai.65.10.4005-4010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassler B L, Wright M, Showalter R E, Silverman M R. Intercellular signaling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 6.Bassler B L, Wright M, Silverman M R. Multiple signaling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 7.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 8.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorman C J, Porter M E. The Shigella virulence gene regulatory cascade: a paradigm of bacterial gene control mechanisms. Mol Microbiol. 1998;29:677–684. doi: 10.1046/j.1365-2958.1998.00902.x. [DOI] [PubMed] [Google Scholar]
- 10.Dunny G M, Leonard B A B. Cell-cell communication in Gram-positive bacteria. Annu Rev Microbiol. 1997;51:527–564. doi: 10.1146/annurev.micro.51.1.527. [DOI] [PubMed] [Google Scholar]
- 11.DuPont H L, Levine M M, Hornick R B, Formal S B. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989;159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 12.Formal S B, Dammin G J, LaBrec E H, Schneider H. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol. 1958;75:604–610. doi: 10.1128/jb.75.5.604-610.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuqua C, Greenberg E P. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr Opin Microbiol. 1998;1:183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 14.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 15.Hale T L, Formal S B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981;32:137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 17.Harris J R, Wachsmuth I K, Davis B R, Cohen M L. High-molecular-weight plasmid correlates with Escherichia coli enteroinvasiveness. Infect Immun. 1982;37:1295–1298. doi: 10.1128/iai.37.3.1295-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hromockyj A E, Maurelli A T. Identification of Shigella invasion genes by isolation of temperature-regulated inv∷lacZ operon fusions. Infect Immun. 1989;57:2963–2970. doi: 10.1128/iai.57.10.2963-2970.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewenza S, Conway B, Greenberg E P, Sokol P A. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J Bacteriol. 1999;181:748–756. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurelli A T, Blackmon B, Curtiss R., III Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect Immun. 1984;43:397–401. doi: 10.1128/iai.43.1.397-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 24.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires ToxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oaks E V, Wingfield M E, Formal S B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985;48:124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oger P, Kim K S, Sackett R L, Piper K R, Farrand S K. Octopine-type Ti plasmids code for a mannopine-inducible dominant-negative allele of traR, the quorum-sensing activator that regulates Ti plasmid conjugal transfer. Mol Microbiol. 1998;27:277–288. doi: 10.1046/j.1365-2958.1998.00671.x. [DOI] [PubMed] [Google Scholar]
- 27.Parsot C, Sansonetti P J. Invasion and pathogenesis of Shigella infections. In: Miller V L, editor. Bacterial invasiveness. New York, N.Y: Springer-Verlag; 1996. pp. 25–42. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Schuch R, Sandlin R C, Maurelli A T. A system for identifying post-invasion functions of invasion genes: requirement for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol Microbiol. 1999;34:675–689. doi: 10.1046/j.1365-2958.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 30.Sereny B. Experimental Shigella conjunctivitis. Acta Microbiol Acad Sci Hung. 1955;2:293–296. [PubMed] [Google Scholar]
- 31.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 32.Sperandio V, Mellies J L, Nguyen W, Shin S, Kaper J B. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc Natl Acad Sci USA. 1999;96:15196–15201. doi: 10.1073/pnas.96.26.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storey D G, Ujack E E, Rabin H R, Mitchell I. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect Immun. 1998;66:2521–2528. doi: 10.1128/iai.66.6.2521-2528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surette M G, Bassler B L. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:7046–7050. doi: 10.1073/pnas.95.12.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surette M G, Miller M B, Bassler B L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tobe T, Nagai S, Okada N, Adler B, Yoshikawa M, Sasakawa C. Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virB gene on the large plasmid. Mol Microbiol. 1991;5:887–893. doi: 10.1111/j.1365-2958.1991.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 37.Withers H L, Nordstrom K. Quorum-sensing acts at initiation of chromosomal replication in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:15694–15699. doi: 10.1073/pnas.95.26.15694. [DOI] [PMC free article] [PubMed] [Google Scholar]