Abstract
Background: Chronic wounds place a heavy burden on the healthcare system due to the prolonged, continuous need for human resources for wound management. Our aim was to investigate the therapeutic effects of platelet-rich plasma on the treatment of chronic wounds. Methods: The systematic literature search was performed in four databases. Randomized clinical trials reporting on patients with chronic wounds treated with platelet-rich plasma (PRP) were included, comparing PRP with conventional ulcer therapy. We pooled the data using the random effects model. Our primary outcome was the change in wound size. Results: Our systematic search provided 2688 articles, and we identified 48 eligible studies after the selection and citation search. Thirty-three study groups of 29 RCTs with a total of 2198 wounds showed that the odds for complete closure were significantly higher in the PRP group than in the control group (OR = 5.32; CI: 3.37; 8.40; I2 = 58%). Conclusions: PRP is a safe and effective modality to enhance wound healing. By implementing it in clinical practice, platelet-rich plasma could become a widely used, valuable tool as it could not only improve patients’ quality of life but also decrease the healthcare burden of wound management.
Keywords: wound healing, dressing, platelet-rich plasma
1. Introduction
Chronic wounds are common conditions that greatly impact patients’ quality of life [1]. They place a heavy burden on the healthcare system due to the high cost of dressing materials, amputation-related costs, and the prolonged, continuous need for human resources for wound management [2].
The wide range of causes underlying ulceration includes arterial and venous insufficiency, neuropathy, microangiopathy, and several additional factors [3]. Besides treating the underlying cause, the goal of ulcer management is to promote healing through professional wound care; the gold standard methods are smart dressings and compression therapy [4].
Platelet-rich plasma (PRP) is an autologous serum prepared from whole blood by centrifugation, containing high concentrations of platelets, growth factors, and cytokines, which can promote stem cell regeneration and tissue remodeling [5,6]. By potentially shortening the recovery time of ulcers, PRP, as an additional treatment modality, could improve patients’ quality of life and decrease the healthcare burden of wound management.
Although the effects of PRP on wound healing are heavily investigated, the current evidence is inconclusive [7]. Our goal is to investigate the therapeutic effect of PRP on the treatment of chronic wounds by summarizing the latest data in a comprehensive manner by conducting a systematic review and meta-analysis.
2. Materials and Methods
Our study was performed according to the Cochrane Handbook’s recommendations for the Systematic Reviews of Interventions, Version 6.3 [8]. The results are reported following the guidelines of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 Statement [9]. The review protocol was registered on PROSPERO under registration number CRD42021287881 (see https://www.crd.york.ac.uk/prospero, accessed on 28 October 2021); no amendments to the information provided at registration were made.
The systematic literature search was performed in four databases: MEDLINE (via PubMed), Cochrane Library (CENTRAL), Embase, and Web of Science from inception to 29 October 2021. The query (ulcer * OR chronic ulcer OR chronic wound OR diabetic foot) AND (platelet rich plasma OR PRP OR platelet rich plasma gel OR PRPG OR platelet rich in growth factors OR PRGF) was applied to all fields in the search engines. No language or other restrictions were imposed.
Randomized clinical trials (RCTs) reporting on patients with chronic wounds treated with PRP were included, comparing additional PRP treatment with conventional ulcer therapy alone. The following population–intervention–control–outcome (PICO) framework was used:
P—Adult patients with chronic wounds;
I—Platelet-rich plasma (PRP) treatment;
C—Conventional ulcer therapy;
O—Primary outcome: change in wound size (complete closure, reduction of wound area, healing rate); secondary outcomes: healing time, infection, pain, adverse events, amputation, recurrence, and quality of life.
EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) was used for the selection of the articles. Two independent authors (F.A.M. and K.D.K.) screened the publications separately for the title, abstract (Cohen’s Kappa: 0.81), and full text (Cohen’s Kappa: 0.88), and disagreements were resolved by a third author (F.D.).
Two authors (F.A.M. and K.D.K.) independently extracted the data into an Excel spreadsheet (Office 365, Microsoft, Redmond, WA, USA). We collected the following data from the eligible articles: first author, year of publication, study type, study location, number of centers included in the study, study design, demographic data, details of the received treatments, and data regarding our outcomes for statistical analysis. A third reviewer (F.D.) resolved the discrepancies. Secondary outcomes were included if three publications reporting on them were found.
The quality assessment of the outcomes was performed separately by two reviewers (F.A.M. and K.D.K.) using the revised tool for assessing the risk of bias (RoB 2) [10]. A third reviewer (F.D.) resolved any occurring disagreements. To assess the quality of the evidence, we followed the recommendation of the “Grades of Recommendation, Assessment, Development, and Evaluation (GRADE)” workgroup [11].
The statistical analyses were made with R (R Core Team 2022, v4.2.1) [12]. For calculations and plots, we used the meta (Schwarzer 2022, v5.5.0) [13] and dmetar (Cuijpers, Furukawa, and Ebert 2022, v0.0.9000) [14] packages.
For the dichotomous outcomes, the odds ratio (OR) with a 95% confidence interval (CI) was used for the effect measure; to calculate the OR, the total number of patients in each group and those with the event of interest were extracted from each study. Raw data from the selected studies were pooled using a random effect model with the Mantel-Haenszel method [15,16,17]. For the pooled results, the exact Mantel–Haenszel method (no continuity correction) was used to handle zero cell counts [18]. In individual studies, the zero cell count problem was adjusted by treatment arm continuity correction [19]. In the case of continuous outcomes, a standardized mean difference (SMD) with a 95% CI was calculated as the effect size. As different results were used from the same study, a three-level meta-analysis model was used along with estimating an additional within the study heterogeneity variance parameter. The inverse variance weighting method was used to calculate the pooled SMD. To estimate the heterogeneity variance measure, τ2, the restricted maximum-likelihood estimator was applied with a t-distribution-based confidence interval [20].
Between-study heterogeneity was described by Higgins and Thompson’s I2 statistics [21]. As the subgroup analysis, the fixed-effects (plural) model (aka. the mixed-effects model) was used. Common τ values at the subgroup levels were assumed in the subgroup analysis, as we had a limited number of studies in some groups. A “Q” omnibus test (of all levels of the subgroup) was also calculated for comparison of the subgroup’s pooled effect sizes. If the study number for the given outcome was over five, the Hartung–Knapp adjustment [22] was applied (below six studies, no adjustment was applied).
A funnel plot of the logarithm of the effect size and comparison with the standard error for each trial was used to evaluate publication bias. Publication bias was assessed with Egger’s test using the Harbord method [23] to calculate the test statistic. Outlier and influence analyses were carried out following the recommendations of Harrer et al. [20] and Viechtbauer and Cheung [24].
3. Results
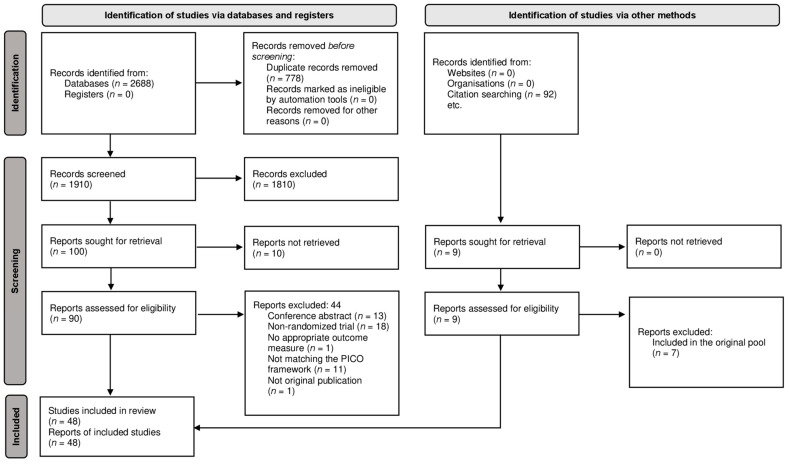
Our systematic search provided a total of 2688 articles; after duplicate removal, we screened 1910 duplicate-free articles. Following the title, abstract, and full-text selection, we identified 46 RCTs matching our PICO framework [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] and two additional articles [71,72] after the citation search. The full text of 10 articles could not be retrieved, even after contacting the authors [73,74,75,76,77,78,79,80,81,82]. The summary of the selection process is shown in Figure 1.
Figure 1.
PRISMA Flow Diagram of the screening and selection process.
We conducted a quantitative analysis of our primary outcome, the change in wound size. The secondary outcomes are detailed in the systematic review section due to the widely varying and poorly defined outcome measures used for their assessment.
The characteristics of the identified RCTs for the systematic review and meta-analysis are detailed in Table 1.
Table 1.
Characteristics of the included studies.
| First Author, Year of Publication | Type of Publication | Study Type | Country | Ulcer Etiology | Outcome |
|---|---|---|---|---|---|
| Abd El-Mabood, 2018 [25] | Journal article | RCT | Egypt | Diabetic | Complete closure, healing rate, infection, and pain |
| Ahmed, 2017 [26] | Journal article | RCT | Egypt | Diabetic | Complete closure, healing rate, and infection |
| Alamdari, 2021 [27] | Journal article | RCT | Iran | Diabetic | Healing time, and amputation |
| Amato, 2020 [28] | Journal article | RCT | Italy | Mixed | Reduction of wound area, complete closure, infection, and pain |
| Anitua, 2008 [29] | Journal article | RCT | Spain | Mixed | Reduction of wound area and infection |
| Burgos-Alonso, 2018 [30] | Journal article | RCT | Spain | Venous | Reduction of wound area, complete closure, infection, pain, adverse events, and quality of life |
| Cardenosa, 2017 [31] | Journal article | RCT | Spain | Venous | Reduction of wound area, pain, and adverse events |
| Chandanwale, 2020 [32] | Journal article | RCT | India | Arterial | Reduction of wound area |
| de Oliveira, 2017 [33] | Journal article | RCT | Brazil | Venous | Reduction of wound area and infection |
| Driver, 2006 [34] | Journal article | RCT | US | Diabetic | Reduction of wound area, healing rate, complete closure, healing time, and adverse events |
| Elbarbary, 2020 [35] | Journal article | RCT | India | Venous | Reduction of wound area, complete closure, healing time, and recurrence |
| Elgarhy, 2020 [36] | Journal article | RCT | India | Venous | Reduction of wound area, complete closure, and healing time |
| Elsaid, 2020 [37] | Journal article | RCT | Egypt | Diabetic | Reduction of wound area, complete closure, and healing time |
| Game, 2018 [38] | Journal article | RCT | UK | Diabetic | Reduction of wound area, complete closure, healing time, infection, pain, amputation, and adverse events |
| Glukhov, 2017 [39] | Journal article | RCT | Russia | Venous | Complete closure, and pain |
| Goda, 2018 1 [41] | Journal article | RCT | Egypt | Diabetic | Healing rate, and complete closure |
| Goda, 2018 2 [40] | Journal article | RCT | Egypt | Venous | Reduction of wound area, and complete closure |
| Gude, 2019 [42] | Journal article | RCT | US | Diabetic | Complete closure, and amputation |
| Helmy, 2021 [43] | Journal article | RCT | Egypt | Venous | Reduction of wound area, complete closure, healing time, pain, adverse events, and recurrence |
| Hongying, 2020 [44] | Journal article | RCT | China | Pressure | Reduction of wound area, and complete closure |
| Kakagia, 2007 [71] | Journal article | RCT | Greece | Diabetic | Reduction of wound area, and complete closure |
| Karimi, 2016 [45] | Journal article | RCT | Iran | Diabetic | Reduction of wound area, complete closure, and amputation |
| Khorvash, 2017 [46] | Journal article | RCT | Iran | Diabetic | Reduction of wound area, infection, pain, and quality of life |
| Kulkarni, 2019 [47] | Journal article | RCT | India | N/A | Reduction of wound area, healing time, and adverse events |
| Li, 2015 [48] | Journal article | RCT | China | Diabetic | Reduction of wound area, complete closure, healing time, infection, amputation, and adverse events |
| Milek, 2019 [49] | Journal article | RCT | Poland | Venous | Reduction of wound area and complete closure |
| Mohammad, 2017 [50] | Journal article | RCT | Iran | Diabetic | Reduction of wound area |
| Moneib, 2018 [51] | Journal article | RCT | Egypt | Venous | Reduction of wound area, complete closure, pain, and adverse events |
| Obolenskiy, 2014 [53] | Journal article | RCT | Russia | Mixed | Complete closure and healing time |
| Obolenskiy, 2017 [52] | Journal article | RCT | Russia | Mixed | Healing rate, complete closure, and healing time |
| Pires, 2021 [54] | Journal article | RCT | Brazil | Venous | Infection |
| Pu, 2019 [55] | Journal article | RCT | China | Arterial | Reduction of wound area, healing rate, and amputation |
| Qin, 2019 [56] | Journal article | RCT | China | Diabetic | Reduction of wound area |
| Rainys, 2019 [57] | Journal article | RCT | Lithuania | N/A | Reduction of wound area, complete closure, infection, and adverse events |
| Ramos-Torrecilla, 2015 [58] | Journal article | RCT | Spain | Pressure | Reduction of wound area, complete closure, and infection |
| Saad Setta, 2011 [59] | Journal article | RCT | Egypt | Diabetic | Complete closure and healing time |
| Saha, 2020 [60] | Journal article | RCT | India | Leprosy | Reduction of wound area, complete closure, and pain |
| Semenic, 2018 [61] | Journal article | RCT | Slovenia | Mixed | Reduction of wound area and adverse events |
| Senet, 2003 [72] | Journal article | RCT | France | Venous | Reduction of wound area, healing rate, complete closure, infection, and adverse events |
| Singh, 2018 [63] | Journal article | RCT | India | Diabetic | Complete closure, healing time, amputation, and adverse events |
| Singh, 2021 [62] | Journal article | RCT | India | Pressure | Reduction of wound area |
| Sokolov, 2017 [64] | Journal article | RCT | Bulgaria | Not defined | Complete closure |
| Somani, 2017 [65] | Journal article | RCT | India | Venous | Reduction of wound area and complete closure |
| Tsachiridi, 2019 [66] | Journal article | RCT | Greece | Pressure | Reduction of wound area and healing rate |
| Tsai, 2019 [67] | Journal article | RCT | US | Mixed | Reduction of wound area |
| Ucar, 2020 [68] | Journal article | RCT | Turkey | Pressure | Reduction of wound area |
| Yang, 2017 [69] | Journal article | RCT | China | Diabetic | Healing rate, healing time, infection, pain, and adverse events |
| Yuvasri, 2020 [70] | Journal article | RCT | India | Venous | Reduction of wound area and complete closure |
3.1. Primary Outcome
The results of the studies assessing the change in wound size are detailed in Table S2 in the Supplementary Materials. Studies evaluating the change in wound size by measuring the baseline and post-treatment wound size or complete closure are included in our quantitative analysis.
3.1.1. Complete Closure
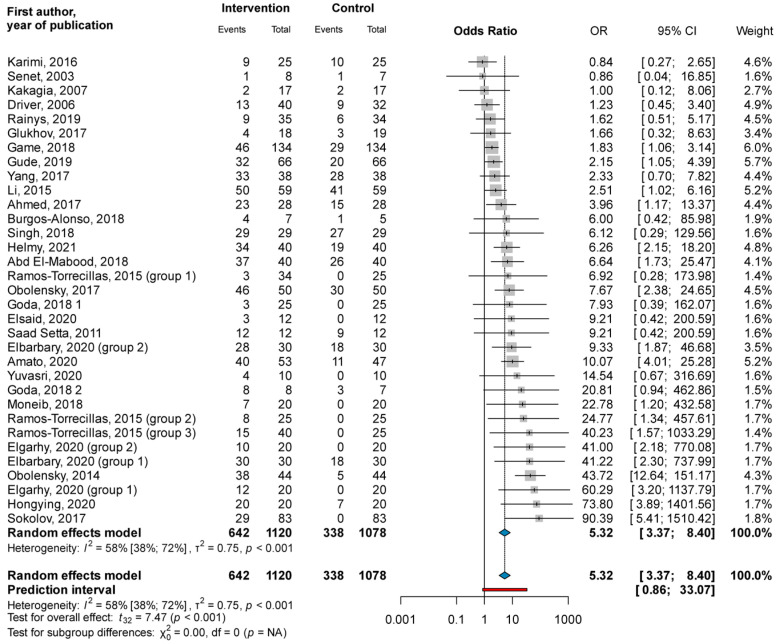
Thirty-three study groups of 29 RCTs with a total of 2198 wounds showed that the odds for complete closure were significantly higher in the PRP group than in the control group (OR = 5.32; CI: 3.37; 8.40; I2 = 58%) (see Figure 2).
Figure 2.
Forest plot for complete closure: platelet-rich plasma compared to conventional ulcer therapy [25,26,28,30,34,35,36,37,38,39,40,41,42,43,44,45,48,51,52,53,57,58,59,63,64,69,70,71,72].
When subgrouping was based on ulcer etiologies, the odds for complete closure were significantly higher in the PRP group than in the control group, both in diabetic foot ulcers (OR = 2.26; CI: 1.50; 3.41; I2 = 12.0%) as well as venous leg ulcers (OR = 8.02; CI: 3.63; 17.71; I2 = 10.0%). The test for the subgroup difference showed a significant difference between the two groups (χ2 = 9.88; df = 1; p = 0.002); the odds for complete closure were significantly higher in venous ulcers than in the diabetic foot ulcers treated with PRP (see Figure S1).
Subgrouping based on the way PRP was applied showed similar results. The odds for complete closure were significantly higher both in the topically applied (OR = 4.74; CI: 2.87; 7.83; I2 = 60%) and injected (OR = 9.42; CI: 3.32; 26.76; I2 = 0%) PRP groups than in the control group, with no significant subgroup difference (χ2 = 2.34; df = 1; p = 0.126) (see Figure S2).
The odds for complete closure were significantly higher in the PRP group than in the control group in the short (OR = 6.03; CI: 3.21; 11.33; I2 = 47%), medium (OR = 3.38; CI: 1.15; 9.89; I2 = 73%), and long (OR = 8.24; CI: 1.66; 40.87; I2 = 0%) follow-up categories, as well with no significant subgroup differences (χ2 = 2.50; df = 3; p = 0.476) (see Figure S3).
3.1.2. Reduction of Wound Area
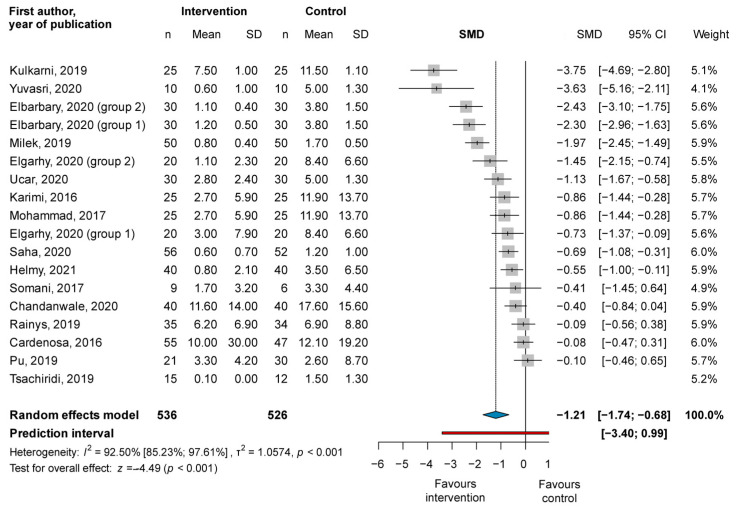
The pooled SMDs from 18 study groups of 16 RCTs with a total of 1062 wounds showed a significant difference between the post-treatment wound size of the PRP and the control groups (SMD = −1.21, CI: −1.74; −0.68; I2 = 92.5%), with the PRP group showing greater improvement (see Figure 3).
Figure 3.
Forest plot for the change of wound size: platelet-rich plasma compared to conventional ulcer therapy [31,32,35,36,43,45,47,49,50,55,57,60,65,66,68,70].
Subgrouping based on ulcer etiology, the application method, and follow-up length showed similar results (see Figures S4–S6). The post-treatment wound size was significantly smaller in the PRP group than in the control group in the diabetic (SMD = −0.68, CI: −1.31; −0.06; I2 = 93.64%), venous (SMD = −1.26, CI: −2.28; −0.24; I2 = 90.76%), topically applied (SMD = −0.94, CI: −1.43; −0.46; I2 = 91.26%), and injected (SMD = −1.03, CI: −1.79; −0.26; I2 = 86.63%) subgroups, as well as in the short follow-up subgroup (SMD = −1.00, CI: −1.64; −0.35; I2 = 89.41%). However, the difference between the PRP and the control groups was not significant in the medium (SMD = −1.38, CI: −2.96; 0.19; I2 = 54.51%) and long (SMD = −0.63, CI: −1.64; 0.37; I2 = 93.88%) follow-up groups. No significant subgroup differences were recorded.
3.2. Secondary Outcomes
The secondary outcomes are summarized in Table 2. Recurrence rates and quality of life are not reported, as less than three studies included them as an outcome.
Table 2.
Main conclusions of the studies assessing the secondary outcomes.
| First Author, Year of Publication | Main Conclusion |
|---|---|
| Healing Time | |
| Alamdari, 2021 [27] | Shorter healing time in the PRP group than in the control group |
| Driver, 2006 [34] | Shorter healing time in the PRP group than in the control group |
| Elbarbary, 2020 [35] | Shorter healing time in the PRP group than in the control group * |
| Elgarhy, 2020 [36] | Shorter healing time in the topical and injected PRP groups than in the control group * |
| Elsaid, 2020 [37] | Shorter healing time in the PRP group than in the control group * |
| Game, 2018 [38] | Shorter healing time in the PRP group than in the control group * |
| Helmy, 2021 [43] | Shorter healing time in the PRP group than in the control group * |
| Kulkarni, 2019 [47] | Shorter healing time in the PRP group than in the control group * |
| Li, 2015 [48] | Shorter healing time in the PRP group than in the control group * |
| Obolenskiy, 2014 [53] | Shorter healing time in the PRP group than in the control group |
| Obolenskiy, 2017 [52] | Shorter healing time in the PRP group than in the control group * |
| Saad Setta, 2011 [59] | Shorter healing time in the PRP group than in the control group * |
| Singh, 2018 [63] | Shorter healing time in the PRP group than in the control group * |
| Yang, 2017 [69] | Shorter healing time in the PRP group than in the control group * |
| Infection Rates | |
| Abd El-Mabood, 2018 [25] | More infection in the control group than in the PRP group * |
| Ahmed, 2017 [26] | More infection in the control group than in the PRP group * |
| Amato, 2020 [28] | More infection in the control group than in the PRP group * |
| Anitua, 2008 [29] | No statistically significant difference between the PRP and the control groups |
| Burgos-Alonso, 2018 [30] | No statistically significant difference between the PRP and the control groups |
| de Oliveira, 2017 [33] | No statistically significant difference between the PRP and the control groups |
| Game, 2018 [38] | No statistically significant difference between the PRP and the control groups |
| Khorvash, 2017 [46] | No statistically significant difference between the PRP and the control groups |
| Li, 2015 [48] | No statistically significant difference between the PRP and the control groups |
| Pires, 2021 [54] | No statistically significant differences in antimicrobial resistance between P. aeruginosa and S. aureus in the PRP and control groups. PRP decreased bacteriological growth or the microbial load and resistance profile in the case of P. aeruginosa |
| Rainys, 2019 [57] | No statistically significant difference between the PRP and the control groups |
| Ramos-Torrecilla, 2015 [58] | No signs of infection were recorded during the study |
| Senet, 2003 [72] | No statistically significant difference between the PRP and the control groups |
| Yang, 2017 [69] | More infection in the control group than in the PRP group * |
| Pain | |
| Abd El-Mabood, 2018 [25] | Pain occurred more frequently in the control group * |
| Amato, 2020 [28] | Pain occurred more frequently in the control group * |
| Burgos-Alonso, 2018 [30] | No statistically significant difference in pain reduction between the PRP and the control groups |
| Cardenosa, 2017 [31] | Pain reduction was higher in the PRP group * |
| Game, 2018 [38] | No statistically significant difference in pain reduction between the PRP and the control groups |
| Glukhov, 2017 [39] | All patients subjectively experienced pain reduction in both groups |
| Helmy, 2021 [43] | All patients subjectively experienced pain reduction in the PRP group |
| Khorvash, 2017 [46] | pain reduction was higher in the PRP group * |
| Moneib, 2018 [51] | All patients subjectively experienced pain reduction in both groups |
| Saha, 2020 [60] | Administration-related pain was reported by 10 participants in the PRP group |
| Yang, 2017 [69] | pain reduction was higher in the PRP group * |
| Amputation Rates | |
| Alamdari, 2021 [27] | No statistically significant difference between the PRP and the control groups |
| Game, 2018 [38] | No statistically significant difference between the PRP and the control group |
| Gude, 2019 [42] | Two amputations in the control group and no amputation in the PRP group |
| Karimi, 2016 [45] | No statistically significant difference between the PRP and the control groups |
| Li, 2015 [48] | Four amputations in the control group one amputation in the PRP group |
| Pu, 2019 [55] | No statistically significant difference between the PRP and the control groups |
| Singh, 2018 [63] | Two amputations in the control group, and no amputation in the PRP group |
| Adverse Events | |
| Burgos-Alonso, 2018 [30] | No statistically significant difference between the PRP and the control groups |
| Cardenosa, 2017 [31] | No adverse events recorded |
| Chandanwale, 2020 [32] | No adverse event in the PRP group |
| Driver, 2006 [34] | No administration related serious adverse event was recorded in either group; one case of Contact dermatitis in the PRP group and one case of maceration in the control group |
| Game, 2018 [38] | No statistically significant difference between the PRP and the control groups |
| Helmy, 2021 [43] | No adverse events recorded |
| Kulkarni, 2019 [47] | No adverse event in the PRP group |
| Li, 2015 [48] | No adverse events were recorded in the PRP group |
| Moneib, 2018 [51] | No adverse events recorded |
| Rainys, 2019 [57] | No statistically significant difference between the PRP and the control groups, and no serious adverse event was recorded |
| Semenic, 2018 [61] | No adverse events recorded |
| Senet, 2003 [72] | No statistically significant difference between the PRP and the control groups |
| Singh, 2018 [63] | No adverse events recorded |
| Yang, 2017 [69] | No statistically significant difference between the PRP and the control groups |
PRP-platelet-rich plasma; * indicates significant difference (p < 0.05).
3.3. Risk of Bias Assessment
The result of the assessment of the risk of bias of the studies included in the meta-analysis and systematic review are detailed in Figures S7–S18 in the Supplementary Materials. None of the studies included in the meta-analysis was at a high risk of bias. In thirty studies [26,28,29,30,32,34,39,42,43,47,48,49,50,51,52,53,56,58,59,61,62,64,65,66,67,68,69,70,71,72], the ‘randomization process’ domain, in twelve studies [28,42,44,48,50,53,56,59,63,65,68,71], the ‘deviations from intended interventions’ domain, in one study [29], the ‘missing outcome data’ domain, in five studies [44,50,56,59,71], the ‘measurement of the outcome’ domain, and in eight studies [26,33,42,57,58,63,65,70], the ‘selection of the reported result’ domain, were rated as ‘some concerns’ for our primary outcome.
3.4. Quality of Evidence
The quality of the evidence for our outcomes is detailed in the Summary of Findings Table (see Table S1 in the Supplementary Materials).
3.5. Publication Bias
The funnel plot assessing the publication bias can be seen in the Supplementary Materials (Figures S19 and S20). No evidence of serious publication bias can be observed in the funnel plot for complete closure; however, the funnel plot for the reduction of the wound area indicates publication bias.
4. Discussion
On the basis of our systematic review and meta-analysis, PRP is an effective add-on treatment modality to enhance wound healing. The odds for complete wound closure were significantly higher in the PRP group than in the control group, and PRP also resulted in a significantly greater reduction of the wound area compared to conventional therapy.
The subgroup analyses, which were conducted to decrease the heterogeneity, showed similar results and also highlighted differences between the ulcer etiologies and PRP application methods. Injected PRP seemed to result in greater improvement than topically applied PRP; however, due to the relatively low sample size of this subgroup, conclusions should be drawn with caution. As for ulcer etiologies, while PRP was superior to conventional therapy regarding complete closure and the reduction of the wound area in diabetic and venous ulcers as well, better results were recorded in the venous ulcer group. The reason for this phenomenon could be that diabetic ulcers are more difficult to heal; however, the fact that PRP was more frequently administered by injection in the venous ulcer group could also be a contributing factor, as we saw better results in the injected PRP subgroup discussed above. PRP was also shown to be effective after short, medium, and long follow-up times regarding complete closure.
Although we did not conduct quantitative analysis on the healing time due to the varying reporting methods of the studies, all the included studies reported shorter healing times in the PRP group than in the conventional therapy group [27,34,35,36,37,38,43,47,48,52,53,59,63,69].
The infection rate is another critical outcome that requires further investigation with more specific criteria for its assessment. Nine studies did not record a significant difference between the PRP and the control groups regarding infection rates [29,30,33,38,46,48,57,58,72], whereas four studies recorded a significantly lower number of infections in the PRP group [25,26,28,69], suggesting that PRP could decrease the risk of infection.
No substantial difference was recorded between the PRP and the control group regarding pain [25,28,30,31,38,39,43,46,51,60,69], amputation rates [38,42,45,48,55,63], and adverse events [30,31,32,34,38,43,47,48,51,57,61,63,69,72].
4.1. Strengths and Limitations
There are several strengths to our study. We summarized the latest data on PRP in wound management in a comprehensive manner, assessing the most objective outcome measure, the change in the wound area. Our results clearly support the superiority of PRP over conventional therapy alone. While previous studies only assessed the efficacy of PRP in different ulcer etiologies separately, we conducted an overall analysis; we believe, as well, that it is crucial to assess the wound-healing properties of PRP in general [7]. We only included RCTs and implemented a rigorous methodology to guarantee the highest possible quality of evidence and conducted a quantitative analysis only on the outcomes that were objectively reported to avoid drawing false conclusions based on poorly recorded secondary outcomes. Our limitations included publication bias and the diversity of the control groups, as a wide range of dressings was used as a part of the conventional therapy.
4.2. Implications for Research
Future studies should report their outcomes uniformly to enable further comprehensive analysis. As the most objective way of assessing the clinical efficacy of PRP in wound management is to record the change in wound size, the baseline and post-treatment wound area should always be reported. However, better reporting guidelines are required that entail detailed descriptive statistics, including the median and interquartile range besides the mean and standard deviation. Additionally, the varying methods used to measure wound size can also lead to further bias: chronic wounds often affect the leg, and simply photographing the wound and measuring it with software does not take into account that wounds often affect the total leg circumference. Also, assessing the wound size by only measuring its width and length can give false results due to the often asymmetrical ulcer areas. We suggest that the most applicable way of precise measurement is tracing the outline of the wound on carbon paper, which can be digitalized and available for further calculations.
In addition to the baseline and post-treatment wound area, the number of completely closed wounds is also a critical outcome measure, showing the clinical efficacy of the treatment; therefore, it should always be reported.
4.3. Implications for Practice
The importance of the early application of research results in clinical practice is undisputable [83]. Due to its wound-healing properties, platelet-rich plasma could become a widely used, valuable tool in chronic wound management. PRP can be administered topically and intralesionally, as well, and can also be applied along with the wide range of available smart dressings. These combinations enable personalized treatment strategies by providing a variety of options for treating physicians.
5. Conclusions
Platelet-rich plasma is a safe and effective modality to enhance wound healing. By implementing it in clinical practice, PRP could become a widely used, valuable tool, as it could improve patients’ quality of life and decrease the healthcare burden of wound management.
Acknowledgments
We would like to thank Norbert M. Wikonkál and Antal Jobbágy for their valuable insights and support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11247532/s1, Table S1: Summary of findings table. Table S2: Characteristics of the studies assessing the change of wound size. Figure S1: Forest plot for complete closure, subgrouping based on ulcer etiologies. Figure S2: Forest plot for complete closure, subgrouping based on PRP application method. Figure S3: Forest plot for complete closure, subgrouping based on follow-up time. Figure S4: Forest plot for wound area reduction, subgrouping based on ulcer etiologies. Figure S5: Forest plot for wound area reduction, subgrouping based on PRP application method. Figure S6: Forest plot for wound area reduction, subgrouping based on follow-up time. Figure S7: Risk of bias assessment of the included studies assessing the change of wound size, using the revised tool for assessing risk of bias in randomized trials (Rob 2). Figure S8: Risk of bias assessment of the included studies assessing the change of wound size, broken down to tools, shown in percentage. Figure S9: Risk of bias assessment of the included studies assessing healing time, using the revised tool for assessing risk of bias in randomized trials (Rob 2). Figure S10: Risk of bias assessment of the included studies assessing healing time, broken down to tools, shown in percentage. Figure S11: Risk of bias assessment of the included studies assessing infection rates, using the revised tool for assessing risk of bias in randomized trials (Rob 2). Figure S12: Risk of bias assessment of the included studies assessing infection rates, broken down to tools, shown in percentage. Figure S13: Risk of bias assessment of the included studies assessing pain, using the revised tool for assessing risk of bias in randomized trials (Rob 2). Figure S14: Risk of bias assessment of the included studies assessing pain, broken down to tools, shown in percentage. Figure S15: Risk of bias assessment of the included studies assessing amputation rates, using the revised tool for assessing risk of bias in randomized trials (Rob 2). Figure S16: Risk of bias assessment of the included studies assessing amputation rates, broken down to tools, shown in percentage. Figure S17: Risk of bias assessment of the included studies assessing adverse events, using the revised tool for assessing risk of bias in randomized trials (Rob 2). Figure S18: Risk of bias assessment of the included studies assessing adverse events, broken down to tools, shown in percentage. Figure S19: Funnel plot for complete closure. Figure S20: Funnel plot for the reduction of wound area.
Author Contributions
F.A.M.: conceptualization, project administration, data curation, visualization, and writing original draft. P.F.: methodology, formal analysis, validation, and visualization. F.D.: conceptualization, data curation and methodology. K.D.K.: conceptualization, data curation, and visualization. L.V.K.: conceptualization and methodology. D.C.: conceptualization, methodology, and supervision. P.H.: conceptualization, methodology, and supervision. A.B.: conceptualization, methodology, supervision, and writing original draft. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
No ethical approval was required for this review, as all data were already published in peer-reviewed journals. No patients were involved in the design, conduction, or interpretation of our study.
Data Availability Statement
The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kapp S., Miller C., Santamaria N. The quality of life of people who have chronic wounds and who self-treat. J. Clin. Nurs. 2018;27:182–192. doi: 10.1111/jocn.13870. [DOI] [PubMed] [Google Scholar]
- 2.Olsson M., Järbrink K., Divakar U., Bajpai R., Upton Z., Schmidtchen A., Car J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019;27:114–125. doi: 10.1111/wrr.12683. [DOI] [PubMed] [Google Scholar]
- 3.Morton L.M., Phillips T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Derm. 2016;74:589–605. doi: 10.1016/j.jaad.2015.08.068. quiz 5–6. [DOI] [PubMed] [Google Scholar]
- 4.Powers J.G., Higham C., Broussard K., Phillips T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Derm. 2016;74:607–625. doi: 10.1016/j.jaad.2015.08.070. quiz 25–26. [DOI] [PubMed] [Google Scholar]
- 5.Hesseler M.J., Shyam N. Platelet-rich plasma and its utility in medical dermatology: A systematic review. J. Am. Acad. Derm. 2019;81:834–846. doi: 10.1016/j.jaad.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Eppley B.L., Woodell J.E., Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast. Reconstr. Surg. 2004;114:1502–1508. doi: 10.1097/01.PRS.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 7.Qu W., Wang Z., Hunt C., Morrow A.S., Urtecho M., Amin M., Shah S., Hasan B., Abd-Rabu R., Ashmore Z., et al. The effectiveness and safety of platelet-rich plasma for chronic wounds: A systematic review and meta-analysis. Mayo Clin. Proc. 2021;96:2407–2417. doi: 10.1016/j.mayocp.2021.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; London, UK: 2022. [Google Scholar]
- 9.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne J.A.C., Savovic J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.Iorio A., Spencer F.A., Falavigna M., Alba C., Lang E., Burnand B., McGinn T., Hayden J., Williams K., Shea B., et al. Use of grade for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 12.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2022. [Google Scholar]
- 13.Schwarzer G. Meta: General Package for Meta-Analysis, Version 6.0-0. R Foundation for Statistical Computing; Vienna, Austria: 2022. [Google Scholar]
- 14.Cuijpers P., Furukawa T., Ebert D.D. dMetar: Companion R Package for the Guide Doing Meta-Analysis in R. R Foundation for Statistical Computing; Vienna, Austria: 2022. [Google Scholar]
- 15.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 16.Robins J., Greenland S., Breslow N.E. A general estimator for the variance of the mantel-haenszel odds ratio. Am. J. Epidemiol. 1986;124:719–723. doi: 10.1093/oxfordjournals.aje.a114447. [DOI] [PubMed] [Google Scholar]
- 17.Thompson S.G., Turner R.M., Warn D.E. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat. Methods Med. Res. 2001;10:375–392. doi: 10.1177/096228020101000602. [DOI] [PubMed] [Google Scholar]
- 18.The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russell Sage Foundation; New York, NY, USA: 2009. [Google Scholar]
- 19.Sweeting M.J., Sutton A.J., Lambert P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 20.Harrer M., Cuijpers P., Furukawa T.A., Ebert D.D. Doing Meta-Analysis with r: A Hands-On Guide. 1st ed. Chapman & Hall/CRC Press; Boca Raton, FL, USA: London, UK: 2021. [Google Scholar]
- 21.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 23.Harbord R.M., Egger M., Sterne J.A.C. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 24.Viechtbauer W., Cheung M.W.L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 25.Abd El-Mabood E.A., Ali H.E. Platelet-rich plasma versus conventional dressing: Does this really affect diabetic foot wound-healing outcomes? Egypt. J. Surg. 2018;37:16–26. doi: 10.4103/ejs.ejs_83_17. [DOI] [Google Scholar]
- 26.Ahmed M., Reffat S.A., Hassan A., Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann. Vasc. Surg. 2017;38:206–211. doi: 10.1016/j.avsg.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Alamdari N.M., Sha A., Mirmohseni A., Besharat S. Evaluation of the efficacy of platelet-rich plasma on healing of clean diabetic foot ulcers: A randomized clinical trial in Tehran, Iran. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15:621–626. doi: 10.1016/j.dsx.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Amato B., Farina M.A., Campisi S., Ciliberti M., di Donna V., Florio A., Grasso A., Miranda R., Pompeo F., Farina E., et al. Cgf treatment of leg ulcers: A randomized controlled trial. Open Med. 2020;14:959–967. doi: 10.1515/med-2019-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anitua E., Aguirre J.J., Algorta J., Ayerdi E., Cabezas A.I., Orive G., Andia I. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008;84:415–421. doi: 10.1002/jbm.b.30886. [DOI] [PubMed] [Google Scholar]
- 30.Burgos-Alonso N., Lobato I., Hernandez I., Sebastian K.S., Rodriguez B., March A.G., Perez-Salvador A., Arce V., Garcia-Alvarez A., Gomez-Fernandez M.C., et al. Autologous platelet-rich plasma in the treatment of venous leg ulcers in primary care: A randomised controlled, pilot study. J. Wound Care. 2018;27:S20–S24. doi: 10.12968/jowc.2018.27.Sup6.S20. [DOI] [PubMed] [Google Scholar]
- 31.Cardenosa M.E., Dominguez-Maldonado G., Cordoba-Fernandez A. Efficacy and safety of the use of platelet-rich plasma to manage venous ulcers. J. Tissue Viability. 2017;26:138–143. doi: 10.1016/j.jtv.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Chandanwale K.A., Mahakalkar C.C., Kothule A.K., Khithani D.V. Management of wounds of peripheral arterial disease using platelet rich plasma. J. Evol. Med. Dent. Sci. 2020;9:2239–2245. doi: 10.14260/jemds/2020/486. [DOI] [Google Scholar]
- 33.de Oliveira M.G., Abbade L.P.F., Miot H.A., Ferreira R.R., Deffune E. Pilot study of homologous platelet gel in venous ulcers. An. Bras. Dermatol. 2017;92:499–504. doi: 10.1590/abd1806-4841.20175496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Driver V.R., Hanft J., Fylling C.P., Beriou J.M. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy/Wound Manag. 2006;52:68–70, 72, 74 passim. [PubMed] [Google Scholar]
- 35.Elbarbary A.H., Hassan H.A., Elbendak E.A. Autologous platelet-rich plasma injection enhances healing of chronic venous leg ulcer: A prospective randomised study. Int. Wound J. 2020;17:992–1001. doi: 10.1111/iwj.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elgarhy L.H., El-Ashmawy A.A., Bedeer A.E., Al-Bahnasy A.M. Evaluation of safety and efficacy of autologous topical platelet gel vs platelet rich plasma injection in the treatment of venous leg ulcers: A randomized case control study. Dermatol. Ther. 2020;33:e13897. doi: 10.1111/dth.13897. [DOI] [PubMed] [Google Scholar]
- 37.Elsaid A., El-Said M., Emile S., Youssef M., Khafagy W., Elshobaky A. Randomized controlled trial on autologous platelet-rich plasma versus saline dressing in treatment of non-healing diabetic foot ulcers. World J. Surg. 2020;44:1294–1301. doi: 10.1007/s00268-019-05316-0. [DOI] [PubMed] [Google Scholar]
- 38.Game F., Jeffcoate W., Tarnow L., Jacobsen J.L., Whitham D., Harrison E.F., Ellender S.J., Fitzsimmons D., Londahl M., LeucoPatch I.I.T.T. Leucopatch system for the management of hard-to-heal diabetic foot ulcers in the uk, denmark, and sweden: An observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6:870–878. doi: 10.1016/S2213-8587(18)30240-7. [DOI] [PubMed] [Google Scholar]
- 39.Glukhov A.A., Aralova M.V. The study of the effectiveness of the drug combination of collagen and platelet-rich plasma for the regional treatment of venous ulcers. Res. J. Pharm. Biol. Chem. Sci. 2017;8:2258–2263. [Google Scholar]
- 40.Goda A.A. Autogenous leucocyte-rich and platelet-rich fibrin for the treatment of leg venous ulcer: A randomized control study. Egypt. J. Surg. 2018;37:316–321. doi: 10.4103/1110-1121.239205. [DOI] [Google Scholar]
- 41.Goda A.A., Metwally M., Ewada A., Ewees H. Platelet-rich plasma for the treatment of diabetic foot ulcer: A randomized, double-blind study. Egypt. J. Surg. 2018;37:178–184. doi: 10.4103/ejs.ejs_139_17. [DOI] [Google Scholar]
- 42.Gude W., Hagan D., Abood F., Clausen P. Aurix gel is an effective intervention for chronic diabetic foot ulcers: A pragmatic randomized controlled trial. Adv. Ski. Wound Care. 2019;32:416–426. doi: 10.1097/01.ASW.0000577140.19174.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helmy Y., Farouk N., Dahy A.A., Abu-Elsoud A., Khattab R.F., Mohammed S.E., Gad L.A., Altramsy A., Hussein E., Farahat A. Objective assessment of platelet-rich plasma (prp) potentiality in the treatment of chronic leg ulcer: Rct on 80 patients with venous ulcer. J. Cosmet. Dermatol. 2021;20:3257–3263. doi: 10.1111/jocd.14138. [DOI] [PubMed] [Google Scholar]
- 44.Hongying J., Liang Z., Xi Y., Jing H., Xiaona X., Zhengyan L., Hongchen H. Effect of platelet-rich plasma on pressure ulcers after spinal cord injury. Chin. J. Tissue Eng. Res. 2020;25:1149–1153. [Google Scholar]
- 45.Karimi R., Afshar M., Salimian M., Sharif A., Hidariyan M. The effect of platelet rich plasma dressing on healing diabetic foot ulcers. Nurs. Midwifery Stud. 2016;5:e30314. doi: 10.17795/nmsjournal30314. [DOI] [Google Scholar]
- 46.Khorvash F., Pourahmad M., Khoshchingol N., Avijgan M., Mohammadi M., Sahebnazar K. Comparing the effects of the platelet-rich plasma gel with wound therapeutic methods on the treatment of diabetic foot. J. Isfahan Med. Sch. 2017;35:1389–1395. [Google Scholar]
- 47.Kulkarni S.R., Chawla A. Study of efficacy of platelet rich plasma dressing in management of chronic non-healing leg ulcers. J. Evol. Med. Dent. Sci. 2019;8:1307–1310. [Google Scholar]
- 48.Li L., Chen D., Wang C., Yuan N., Wang Y., He L., Yang Y., Chen L., Liu G., Li X., et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen. 2015;23:495–505. doi: 10.1111/wrr.12294. [DOI] [PubMed] [Google Scholar]
- 49.Milek T., Nagraba L., Mitek T., Wozniak W., Mlosek K., Olszewski W., Ciostek P., Deszczynski J., Kuchar E., Stolarczyk A. Autologous platelet-rich plasma reduces healing time of chronic venous leg ulcers: A prospective observational study. In: Pokorski M., editor. Advances in Biomedicine. Volume 1176. Springer; Cham, Switzerland: 2019. pp. 109–117. [DOI] [PubMed] [Google Scholar]
- 50.Mohammad A., Rohangiz K., Morteza S., Alireza S., Abolfazl A. Comparison of platelet rich plasma and normal saline dressing effectiveness in the improvement of diabetic foot ulcers. J. Diabet. Nurs. 2017;5:246–255. [Google Scholar]
- 51.Moneib H.A., Youssef S.S., Aly D.G., Rizk M.A., Abdelhakeem Y.I. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: A comparative study. J. Cosmet. Dermatol. 2018;17:495–501. doi: 10.1111/jocd.12401. [DOI] [PubMed] [Google Scholar]
- 52.Obolenskiy V.N., Ermolova D.A., Laberko L.A. Clinical and economic effectiveness of the use of platelet-rich plasma in the treatment of chronic wounds. Wound Med. 2017;19:27–32. doi: 10.1016/j.wndm.2017.09.001. [DOI] [Google Scholar]
- 53.Obolenskiy V.N., Ermolova D.A., Laberko L.A., Semenova T.V. Efficacy of platelet-rich plasma for the treatment of chronic wounds. EWMA J. 2014;14:37–41. [Google Scholar]
- 54.Pires B., de Oliveira B.G.R.B., Bokehi L.C., Luiz R.R., Carvalho B.T.F., Santana R.F., de Souza P.A., de Paula G.R., Teixeira L.A. Clinical and microbiological outcomes associated with use of platelet-rich plasma in chronic venous leg uclers: A randomized controlled trial. J. Wound Ostomy Cont. Nurs. Off. Publ. Wound Ostomy Cont. Nurses Soc. 2021;48:292–299. doi: 10.1097/WON.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 55.Pu D., Lei X., Leng W., Zheng Y., Chen L., Liang Z., Chen B., Wu Q. Lower limb arterial intervention or autologous platelet-rich gel treatment of diabetic lower extremity arterial disease patients with foot ulcers. Ann. Transl. Med. 2019;7:485. doi: 10.21037/atm.2019.07.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin X., Wang J. Clinical study of local injection of autologous platelet-rich plasma in treatment of diabetic foot ulcer. Chin. J. Reparative Reconstr. Surg. 2019;33:1547–1551. doi: 10.7507/1002-1892.201905124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rainys D., Cepas A., Dambrauskaite K., Nedzelskiene I., Rimdeika R. Effectiveness of autologous platelet-rich plasma gel in the treatment of hard-to-heal leg ulcers: A randomised control trial. J. Wound Care. 2019;28:658–667. doi: 10.12968/jowc.2019.28.10.658. [DOI] [PubMed] [Google Scholar]
- 58.Ramos-Torrecillas J., García-Martínez O., de Luna-Bertos E., Ocaña-Peinado F.M., Ruiz C. Effectiveness of platelet-rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biol. Res. Nurs. 2015;17:152–158. doi: 10.1177/1099800414535840. [DOI] [PubMed] [Google Scholar]
- 59.Saad Setta H., Elshahat A., Elsherbiny K., Massoud K., Safe I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: A comparative study. Int. Wound J. 2011;8:307–312. doi: 10.1111/j.1742-481X.2011.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saha S., Patra A.C., Gowda S.P., Mondal N., Rahaman S., Ahmed S.K.S., Debbarma S., Vitthal K.P.K., Sarkar S., Sil A., et al. Effectiveness and safety of autologous platelet-rich plasma therapy with total contact casting versus total contact casting alone in treatment of trophic ulcer in leprosy: An observer-blind, randomized controlled trial. Indian J. Derm. Venereol. Leprol. 2020;86:262–271. doi: 10.4103/ijdvl.IJDVL_571_18. [DOI] [PubMed] [Google Scholar]
- 61.Semenič D., Cirman T., Rožman P., Smrke D.M. Regeneration of chronic wounds with allogeneic platelet gel versus hydrogel treatment: A prospective study. Acta Clin. Croat. 2018;57:434–442. doi: 10.20471/acc.2018.57.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh G., Borah D., Khanna G., Jain S. Efficacy of local autologous platelet-rich plasma in the treatment of pressure ulcer in spinal cord injury patients. Cureus. 2021;13:e18668. doi: 10.7759/cureus.18668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh S.P., Kumar V., Pandey A., Pandey P., Gupta V., Verma R. Role of platelet-rich plasma in healing diabetic foot ulcers: A prospective study. J. Wound Care. 2018;27:550–556. doi: 10.12968/jowc.2018.27.9.550. [DOI] [PubMed] [Google Scholar]
- 64.Sokolov T., Manukova A., Karakoleva S., Valentinov B., Petrova N. Analysis of the results of applying the method platelet-rich plasma (prp) for the treatment of problematic skin wounds. J. IMAB. 2017;23:1460–1465. doi: 10.5272/jimab.2017231.1460. [DOI] [Google Scholar]
- 65.Somani A., Rai R. Comparison of efficacy of autologous platelet-rich fibrin versus saline dressing in chronic venous leg ulcers: A randomised controlled trial. J. Cutan. Aesthetic Surg. 2017;10:8–12. doi: 10.4103/JCAS.JCAS_137_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsachiridi M., Galyfos G., Andreou A., Sianou A., Sigala F., Zografos G., Filis K. Autologous platelet-rich plasma for nonhealing ulcers: A comparative study. Vasc. Spec. Int. 2019;35:22–27. doi: 10.5758/vsi.2019.35.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai H.C., Lehman C.W., Chen C.M. Use of platelet-rich plasma and platelet-derived patches to treat chronic wounds. J. Wound Care. 2019;28:15–21. doi: 10.12968/jowc.2019.28.1.15. [DOI] [PubMed] [Google Scholar]
- 68.Ucar O., Celik S. Comparison of platelet-rich plasma gel in the care of the pressure ulcers with the dressing with serum physiology in terms of healing process and dressing costs. Int. Wound J. 2020;17:831–841. doi: 10.1111/iwj.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang L., Gao L., Lv Y., Wang J. Autologous platelet-rich gel for lower-extremity ischemic ulcers in patients with type 2 diabetes. Int. J. Clin. Exp. Med. 2017;10:13796–13801. [Google Scholar]
- 70.Yuvasri G., Rai R. Comparison of efficacy of autologous platelet-rich fibrin versus unna’s paste dressing in chronic venous leg ulcers: A comparative study. Indian Dermatol. Online J. 2020;11:58–61. doi: 10.4103/idoj.IDOJ_119_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kakagia D.D., Kazakos K.J., Xarchas K.C., Karanikas M., Georgiadis G.S., Tripsiannis G., Manolas C. Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J. Diabetes Its Complicat. 2007;21:387–391. doi: 10.1016/j.jdiacomp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Senet P., Bon F.-X., Benbunan M., Bussel A., Traineau R., Calvo F., Dubertret L., Dosquet C. Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. J. Vasc. Surg. 2003;38:1342–1348. doi: 10.1016/S0741-5214(03)00908-X. [DOI] [PubMed] [Google Scholar]
- 73.Baranovskiy Y.G., Ilchenko F.N., Shapovalova E.Y., Kaliberdenko V.B., Shanmugaraj K., Keerthanaa B. Influence of autologous platelet concentrates on the dynamics of regenerative processes in treatment of trophic ulcers of lower extremities. Indian J. Public Health Res. Dev. 2019;10:1850–1855. [Google Scholar]
- 74.Capoano R., Businaro R., Tesori M.C., Donello C., Lombardo F., Vasco V.R.L., Capriotti L., Corsi M., Raimo T.D., Leopizzi M., et al. Wounds difficult to heal: An effective treatment strategy. Curr. Vasc. Pharmacol. 2017;15:582–588. doi: 10.2174/1570161115666170301122216. [DOI] [PubMed] [Google Scholar]
- 75.Li L., Wang C., Wang Y., He L.P., Yang Y.Z., Chen L.H., Chen D.W., Li X.J., Ran X.W. Impact of topical application of autologous platelet-rich gel on medical expenditure and length of stay in hospitals in diabetic patients with refractory cutaneous ulcers. J. Sichuan Univ. (Med. Sci. Ed.) 2012;43:762–765. [PubMed] [Google Scholar]
- 76.Liu G.Y., Deng X.L., Sun Y., Wang M.Z., Gao J., Gou J. Effect of autologous platelet-rich gel on the treatment of diabetic foot ulcers. J. Xi’an Jiaotong Univ. (Med. Sci.) 2016;37:264–267. [Google Scholar]
- 77.Madhumitha M., Srinivasan S. A study of efficacy of autologous platelet rich plasma in the treatment of chronic diabetic foot ulcers. Int. J. Pharm. Res. 2019;11:190–196. [Google Scholar]
- 78.Martí X., Linares P., Bonell A., Acosta M., Llort C., Lapiedra O. Growth factors used in healing venous ulcers. Chirurgia. 2008;21:17–20. [Google Scholar]
- 79.Slaninka I., Klein L., Čáp R., Hošek F., Guňka I., Šedivý O., Jiška S., Kaška M. Optimizing the treatment procedure in crural ulcers—A pilot study of the surgical method. Rozhl. Chir. 2015;94:69–73. [PubMed] [Google Scholar]
- 80.Smagin M.A., Shumkov O.A., Soluianov M.I., Demura A.U., Smagin A.A., Lykov A.P., Nimaev V.V. Treatment of torpid trophic ulcers in patients of the older age group. Adv. Gerontol. 2020;33:373–378. [PubMed] [Google Scholar]
- 81.Wang L., Liu G., Li Z., Jia B.C., Wang Y. Clinical application of platelet-rich fibrin in chronic wounds combined with subcutaneous stalking sinus. Chin. J. Burn. 2018;34:637–642. doi: 10.3760/cma.j.issn.1009-2587.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 82.Gupta A., Channaveera C., Sethi S., Ranga S., Anand V. Efficacy of intralesional platelet-rich plasma in diabetic foot ulcer. J. Am. Podiatr. Med. Assoc. 2021;111:7. doi: 10.7547/19-149. [DOI] [PubMed] [Google Scholar]
- 83.Hegyi P., Erőss B., Izbéki F., Párniczky A., Szentesi A. Accelerating the translational medicine cycle: The academia europaea pilot. Nat. Med. 2021;27:1317–1319. doi: 10.1038/s41591-021-01458-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.