Abstract
Enterotoxigenic Escherichia coli (ETEC) is capable of invading epithelial cell lines derived from the human ileum and colon. Two separate invasion loci (tia and tib) that direct noninvasive E. coli strains to adhere to and invade cultured human intestine epithelial cells have previously been isolated from the classical ETEC strain H10407. The tib locus directs the synthesis of TibA, a 104-kDa outer membrane glycoprotein. Synthesis of TibA is directly correlated with the adherence and invasion phenotypes of the tib locus, suggesting that this protein is an adhesin and invasin. Here we report the purification of TibA and characterization of its biological activity. TibA was purified by continuous-elution preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Purified TibA was biotin labeled and then shown to bind to HCT8 human ileocecal epithelial cells in a specific and saturable manner. Unlabeled TibA competed with biotin-labeled TibA, suggesting the presence of a specific TibA receptor in HCT8 cells. These results show that TibA acts as an adhesin. Polyclonal anti-TibA antiserum inhibited invasion of ETEC strain H10407 and of recombinant E. coli bearing tib locus clones, suggesting that TibA also acts as an invasin. The ability of TibA to direct epithelial cell adhesion suggests a role for this protein in ETEC pathogenesis.
Enterotoxigenic Escherichia coli (ETEC) is an enteric pathogen that causes watery diarrhea in humans and animals (22). It is a major public health problem, especially in developing countries, where it is responsible for hundreds of thousands of deaths among children under the age of 5 years (28). ETEC infection is acquired by the ingestion of contaminated food or drink. After the initial colonization of the proximal small intestine by fimbrial adhesins, ETEC will secrete at least one of two distinct classes of enterotoxins (heat-labile enterotoxin or heat-stable enterotoxin) (22), which are considered the primary cause of diarrhea. Therefore, ETEC's main virulence factors are thought to be its enterotoxins and colonization factors. However, human and animal studies performed with ETEC strains that have lost the ability to produce enterotoxins indicate that enterotoxins may not be exclusively required for diarrhea (17, 23, 24, 27). This result suggests the presence of previously uncharacterized enterotoxins or other virulence factors.
Although there is currently no direct evidence that ETEC strains invade human intestinal cells in vivo, intestinal biopsies taken from ETEC-infected piglets revealed intracellular bacteria (21). Additionally, it has been shown that the human-specific ETEC strain H10407 is able to adhere to and invade human epithelial cell lines derived from the ileocecum and colon (8). Two chromosomally encoded epithelial cell adherence and invasion determinants (tia and tib) have been cloned from the classical ETEC strain H10407 (8, 9, 11). The tib locus directs the synthesis of a 104-kDa outer membrane glycoprotein called TibA (8, 9, 20). Four genes (tibA, tibB, tibC, and tibD) appear to be required for the synthesis of TibA. TibA is synthesized as a precursor (termed pre-TibA) from the tibA gene. The tibC gene product is required for glycosylation of pre-TibA to form the mature TibA protein (9, 20). This modification is required for tib locus-mediated epithelial cell adherence and invasion (9). The presence of TibA in the outer membrane is directly correlated with the ability of H10407 and recombinant E. coli strains to adhere to and invade epithelial cells (9, 20). Deletion of the tib locus from H10407 eliminates TibA production by that strain and reduces the ability of H10407 to adhere to and invade epithelial cells by about 75% (9). Therefore, it has been proposed that TibA acts as an adhesin and invasin. Here we report the purification of TibA from E. coli strain DH5α bearing a TibA-expressing plasmid. Purified TibA was used to develop an enzyme-linked immunosorbent assay (ELISA)-like binding assay. We demonstrate that purified TibA shows specific binding to cultured human intestinal epithelial cells. Additionally, we show that polyclonal TibA antiserum decreases TibA-mediated bacterial invasion of epithelial cells.
MATERIALS AND METHODS
Bacterial strains, tissue culture cells, and culture conditions.
The human-specific ETEC strain H10407 (serotype O78:H11; CFA/I) (11) was the wild-type strain from which the tib locus was cloned (8). TIB3 is a tib deletion mutant of H10407 (9). DH5α is a laboratory strain of E. coli that was used as a noninvasive control and was the recipient for tib locus-containing plasmids (13). Bacteria were grown in Luria broth (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl [pH 7.6]) at 37°C and 200 rpm. Ampicillin (final concentration, 100 μg/ml) was added to the growth medium of strains bearing recombinant plasmids.
The human ileocecal epithelial cell line HCT8 (ATCC CCL 244) was maintained in RPMI 1640 medium containing 10% fetal bovine serum, 1 mM l-glutamine, and 1 mM sodium pyruvate (Life Technologies). HCT8 cells were grown at 37°C in 6% CO2.
Membrane fractionation.
Outer membrane fractions were isolated as previously described (9, 25). Briefly, Luria broth cultures (500 ml) were grown with shaking at 37°C to late log phase, harvested by centrifugation, and then lysed by two passages through a French press. Inner and outer membranes were separated by sucrose density ultracentrifugation. The protein concentration of membrane fractions was determined by the Bradford method (3) using a kit from Bio-Rad.
Purification of TibA.
TibA was purified from outer membranes of E. coli DH5α(pET109). Outer membrane (4 mg) was solubilized at room temperature in 2% sodium dodecyl sulfate (SDS) for 20 min, followed by 1 h of ultracentrifugation at 100,000 × g at 4°C. The supernatant was then boiled for 10 min in Laemmli sample buffer (17) and loaded onto a discontinuous preparative SDS-polyacrylamide gel (4% stacking gel and 7.5% separating gel) (Mini Prep Cell; Bio-Rad). SDS-polyacrylamide gel electrophoresis (PAGE) was run at 200 V, 6 mA, 2 W, and 4°C until the dye front came off. The flow rate was adjusted to 0.1 ml/min, eluting the proteins in 10 mM HEPES containing 1 mM EDTA. The gel was run for an additional 4 h, draining the eluent. The flow rate was then further decreased to 0.02 ml/min, and 17 fractions (1-ml each) were collected. Thirty microliters of each fraction was subjected to SDS–7.5% PAGE and then stained with Coomassie blue. Fractions with equally high TibA concentrations were pooled and dialyzed against phosphate-buffered saline (PBS)–50 mM potassium phosphate–150 mM NaCl (pH 7.4) containing 0.1% Tween 20 overnight at 4°C. The concentration of purified TibA was determined using the Pierce Micro BCA protein assay. Purified TibA was sequenced by the Edman degradation method (7) using a Hewlett-Packard G1000-A protein sequencer.
Protein electrophoresis.
Electrophoresis of membrane fractions, whole-cell extracts, and purified TibA was performed under denaturing conditions by the method of Laemmli (17). Samples were prepared for electrophoresis by heating in treatment buffer at 95°C for 10 min. For the analysis of whole-cell extracts, 2-ml Luria broth cultures were grown to late log phase with shaking at 37°C, and then 1 ml was harvested by centrifugation and lysed in 200 μl of treatment buffer. Twenty-microliter aliquots were loaded on SDS-PAGE gels. Gels were run for 12 to 14 h at 70 V at room temperature. Gels were stained for proteins with Coomassie blue or for glycoprotein as described below.
For immunoblotting, proteins separated by electrophoresis were transferred to nitrocellulose at 65 V for 1 h, followed by Ponceau S staining to confirm that electroblotting had occurred evenly. Filters were then blocked with casein filler solution (2% casein and 0.1% sodium azide in 10 mM Tris-buffered saline [TBS, pH 7.4]). Blocked filters were probed for 1 h with the indicated serum (1:1,000 dilution in casein filler), washed in Tris-buffered saline containing 0.05% Triton X-100, incubated for 1 h with peroxidase-conjugated protein A (Sigma) (1:2,000 dilution in casein filler), rewashed, and then detected with BM Blue precipitating peroxidase substrate (Roche).
Detection of glycoproteins.
Glycoproteins were detected on nitrocellulose filters using method B of the DIG glycan detection kit according to the manufacturer's recommendations (Roche Molecular Biochemicals) and as described previously (20). Briefly, proteins were separated by SDS-PAGE and blotted to nitrocellulose filters at 60 V for 60 min (26). Subsequently, membranes were washed in PBS (50 mM potassium phosphate, 150 mM NaCl [pH 6.5]), and carbohydrates were oxidized with sodium metaperiodate. Oxidized carbohydrates were labeled with digoxigenin-conjugated hydrazide. Digoxigenin-labeled proteins were visualized using alkaline phosphatase-conjugated antidigoxigenin antibodies, followed by a color reaction with Nitro Blue Tetrazolium–X-phosphate.
Binding assays.
Purified and dialyzed TibA was biotinylated according to the manufacturer's recommendation. Biotin (D-biotinoyl-ɛ-amidocaproic acid-N-hydroxyl succinimide ester; Roche Molecular Biochemicals) that had been dissolved at 1 mg/ml in dimethylformamide was incubated together with purified TibA at a 10:1 molar ratio of Biotin to TibA and a volume ratio of 1:50 (Biotin-TibA) for 4 h at 4°C. The mixture was then dialyzed against PBS containing 0.1% Tween 20 overnight. The same day, 105 HCT8 cells per well were plated in the wells of 96-well plates and incubated overnight at 37°C. After dialysis, the protein concentration of TibA was measured using the Micro BCA protein assay reagent kit (Pierce), and biotinylation was confirmed using dot blot. Confluent HCT8 monolayers were washed once in Earle's balanced salt solution (Life Technologies) and fixed at room temperature in 0.25% glutaraldehyde in Earle's balanced salt solution for 10 min. After two additional washes in PBS containing 0.1% Tween 20 (PBS/T), the wells were blocked in fetal bovine serum (FBS) containing 1% Tween 20 for 1 h at 37°C. After three washes in PBS/T, different concentrations of labeled TibA were added in a final volume of 60 μl of PBS containing 0.1% Tween 20 and 0.1% SDS and incubated for 1 h at 37°C. Monolayers were then washed three times with PBS/T. Peroxidase-linked streptavidin (Roche Molecular Biochemicals) was diluted 1:20,000 in PBS/T, then added in a final volume of 60 μl to each well, and incubated at 37°C for 1 h. Binding was detected by the addition of 100 μl of peroxidase (POD) substrate (ABTS [2,2′-azino-di-(3-ethylbenzthiazoline sulfonate)] buffer system; Roche Molecular Biochemicals) per well, and color development was measured at 405 nm in an ELISA reader.
In competition assays, 4.8 pmol of biotinylated TibA was mixed with the indicated amount of unlabeled TibA. Each mixture was brought to a final volume of 60 μl and final concentrations of 0.1% Tween 20 and 0.1% SDS. After incubation with fixed HCT8 cell monolayers at 37°C for 1 h, the wells were washed, and bound TibA was detected as described above.
Generation and use of polyclonal anti-TibA antiserum.
Ten milligrams of TibA-containing outer membranes purified from E. coli DH5α(pET109) was separated by SDS–7.5% PAGE and then stained with Coomassie blue. From analytical SDS-PAGE results, we estimated that TibA represented 10% of the total outer membrane protein separated by SDS-PAGE. Therefore, a total of 1 mg of TibA was used for all immunizations. The TibA band was then cut out of the gel, crushed, and used to immunize two New Zealand White rabbits by Alpha Diagnostic International, Inc. (San Antonio, Tex.). The animals were injected at weeks 0, 2, 4, 6, 8, 10, and 12, with test bleeds on weeks 7, 9, 11, and 13. The titer of anti-TibA antibodies in the serum was determined by ELISA. Ninety-six-well plates were coated with 500 ng of purified TibA overnight and blocked with 2% casein in TBS. Serial dilutions of the antiserum (1:1,000, 1:2,000, 1:4,000, 1:8,000, and 1:16,000) in PBS/T were added to each well. Bound antibody was detected by protein A coupled to peroxidase (Sigma) and quantified by measuring the optical density at 405 nm (OD405) after development with the ABTS buffer system.
To prepare preabsorbed serum, 1 ml of serum was incubated for 1 h at 37°C with 1 ml of a 1:1 mixture of E. coli strains DH5α and TIB3. Bacteria were then removed by centrifugation twice at 16,000 × g for 10 min, and the serum was used at a final dilution of 1:1,000 for Western immunoblots. For invasion inhibition experiments, antibodies from preabsorbed rabbit polyclonal antiserum and preimmune serum were affinity purified on a protein G column (MabTrap G II; Pharmacia) according to the manufacturer's recommendation. Affinity-purified immunoglobulins were dialyzed against PBS overnight at 4°C. The protein concentration in the dialyzed sample was determined by the Micro BSA assay (Pierce) and adjusted to 25 mg/ml. Purity was confirmed by SDS-PAGE, and the biological activity of the immune serum was detected by ELISA with 500 ng of purified TibA. It was necessary to affinity purify the antibodies because heat -inactivated rabbit serum inhibited bacterial growth, as determined by counts of viable bacteria grown under invasion conditions for 4 h (data not shown). Affinity-purified immunoglobulin G (IgG) from immune and preimmune sera did not inhibit bacterial viability (data not shown).
Invasion assays.
Bacterial invasion of epithelial cells was measured as protection from the bactericidal antibiotic gentamicin (16). Invasion assays were performed as previously described (8). Briefly, approximately 5 × 106 log-phase CFU were added to HCT8 monolayers (approximately 3.5 × 105 cells in 24-well tissue culture plates), which were then incubated at 37°C for 4 h in a 6% CO2 atmosphere. The actual inoculum for each experiment was determined by quantitative plate count. After being washed, the monolayers were incubated for an additional 1 h in tissue culture medium containing gentamicin. The infected monolayers were washed and then lysed in 1.0% Triton X-100 in deionized water, and the bacteria were quantified by plate count. Invasion is expressed as the percentage of organisms surviving exposure to gentamicin relative to the number of bacteria added to the tissue culture wells at the beginning of the assay. Since the results of invasion assays vary on a daily basis, the datum points presented in the figures are average values ± standard deviation for triplicate wells of a single experiment and correlated with values obtained in replicate experiments that were performed at least three times.
In antibody inhibition experiments, preabsorbed and affinity-purified polyclonal rabbit (preimmune or anti-TibA immune) IgG in PBS was added (to reach the indicated dilutions) directly to the tissue culture medium 5 min prior to inoculation of wells with log-phase bacteria.
RESULTS
The tib locus mediates adherence to and invasion of human intestine epithelial cells by recombinant E. coli strains and ETEC strain H10407 (10). Additionally, there is a direct correlation between the presence of TibA in the outer membrane and the adherence and invasion phenotypes of the tib locus (9). These results suggest that TibA acts as an adhesin and may be an invasin. Therefore, we were interested in determining if the TibA protein itself is capable of binding to epithelial cells. To address this question, we purified TibA and examined its ability to bind cultured human ileocecal epithelial cells.
Purification of TibA.
We purified TibA from outer membranes isolated from recombinant E. coli DH5α containing pET109 (9). Plasmid pET109 contains the entire tib locus under its own promoter and overexpresses the TibA protein (9, 20). We purified TibA from recombinant DH5α rather than from the parent ETEC strain because H10407 expresses TibA in low quantities when grown in broth culture (9, 20) and maximally produces TibA only when grown under adherence or invasion assay conditions (E. A. Elsinghorst, unpublished data). Therefore, to obtain the quantity of TibA required for our experiments, E. coli DH5α(pET109) was used as the source of TibA-containing outer membranes.
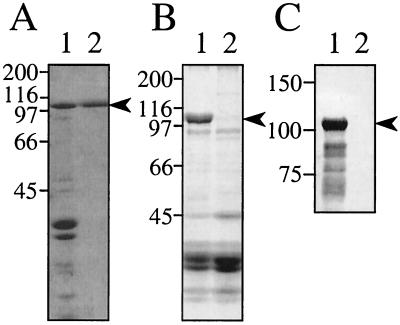
Isolated TibA-containing outer membranes (Fig. 1A, lane 1) were solubilized in 2% SDS and then subjected to preparative SDS-PAGE (Bio-Rad MiniPrep Cell) with elution into HEPES-EDTA buffer. During elution, the flow rate was adjusted to 0.02 ml/min in order to obtain a maximal TibA concentration, as all attempts to concentrate TibA-containing fractions after elution resulted in significant loss of protein. All fractions were then analyzed by SDS-PAGE for the presence of TibA. Only fractions that were free of contaminating proteins were pooled and used for further experiments. Although we used a one-step purification, we are confident that the TibA preparations obtained by this procedure were free of major contaminants of higher or lower molecular mass, based on SDS-PAGE (Fig. 1A, lane 2). Additionally, E. coli DH5α outer membranes do not contain proteins with a molecular mass similar to that of TibA (Fig. 1B). Therefore, based on the single band seen by SDS-PAGE, we had purified TibA to apparent homogeneity. Additional evidence that our TibA preparation was free of contaminating DH5α outer membrane proteins will be discussed below.
FIG. 1.
Purification of TibA. (A) Coomassie blue-stained SDS-PAGE (7.5% polyacrylamide). Lanes: 1, outer membranes (20 μg of protein) from E. coli DH5α(pET109); 2, 5 μg of purified TibA. (B) Coomassie blue-stained SDS-PAGE (7.5% polyacrylamide) of outer membranes prepared from E. coli DH5α bearing either the TibA-expressing plasmid pET109 (lane 1, 20 μg of protein) or pHG165 (the vector for plasmid pET109) (lane 2, 40 μg of protein). (C) SDS-PAGE (7.5% polyacrylamide) gel of the indicated samples was transferred to nitrocellulose and then stained for glycoprotein as described in Materials and Methods. Lanes: 1, purified TibA (5 μg); 2, outer membranes prepared from E. coli DH5α(pHG165) (20 μg of protein). The positions of molecular mass standards (in kilodaltons) are indicated to the left of each panel. The migration of TibA is indicated by an arrowhead to the right of each panel.
To confirm that our purified protein was TibA, we determined its amino-terminal sequence. The sequence (AAYDNQTIGR) was strong and clean, indicating a single pure protein, and exactly matched the amino-terminal sequence previously published for TibA (20). Additionally, we confirmed that our purified protein was glycosylated (Fig. 1C). We previously had found that TibA is the only glycoprotein made by ETEC strain H10407 and that E. coli HB101 and DH5α do not produce glycoproteins in the absence of tib locus plasmids (21) (Fig. 1C). We previously found that the multiple bands seen below the TibA band in glycoprotein stains (such as those observed in Fig. 1C, lane 1) are TibA breakdown products (20).
TibA is an adhesin.
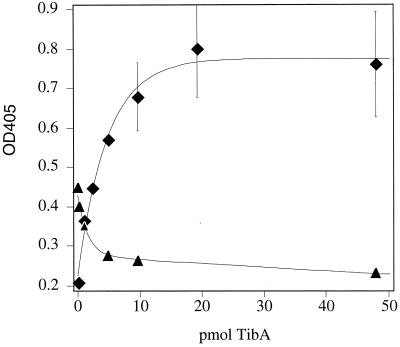
Since the presence of TibA in the outer membrane is directly correlated with the adherence and invasion phenotypes of the tib locus (9), we were interested in determining if purified TibA binds epithelial cells in vitro. Therefore, aliquots of purified TibA were biotinylated and then incubated at increasing concentrations with glutaraldehyde-fixed and FBS-blocked confluent HCT8 (human ileocecum epithelial cell) monolayers. Binding of TibA to epithelial cells was concentration dependent and saturated at approximately 15 pmol, indicating specific binding to the epithelial cells (Fig. 2, diamonds). Binding could not be detected when the various concentrations of labeled TibA were incubated in FBS-blocked wells that lacked HCT8 monolayers. It was reported earlier that purified E. coli outer membrane protein OmpW does not bind to HCT8 monolayers (20a). These results indicate that epithelial cell binding is not a general property of outer membrane proteins and that the observed TibA binding is due to a specific interaction between this protein and the epithelial cells.
FIG. 2.
Binding of purified and biotinylated TibA to HCT8 monolayers. Bound TibA was detected by peroxidase-coupled streptavidin. Color development in each well was measured at 405 nm. Symbols: ♦, saturation curve performed with 0.0, 1.0, 2.4, 4.8, 9.6, 19.2, and 48.0 pmol of TibA per well; ▴, competition curve performed with 4.8 pmol of labeled TibA plus 0.0, 1.0, 4.8, 9.6, or 48.0 pmol of unlabeled TibA. Every experiment was performed at least three times. The extent of biotin labeling varied from day to day, resulting in significant differences in color development. Therefore, both curves shown here represent the average of two experiments performed in duplicate.
The saturable binding of TibA to HCT8 cells suggested that TibA bound to some specific receptor(s) that was limited in number. To further confirm that the binding was specific, increasing amounts of unlabeled TibA were mixed with 4.8 pmol of labeled TibA. Ten picomoles of unlabeled TibA was enough to decrease binding of labeled TibA by 80% (Fig. 2, triangles). The ability of unlabeled TibA to compete with labeled protein for binding to epithelial cells indicates the presence of a limited number of TibA binding sites, such as a specific receptor.
Characterization of anti-TibA antiserum.
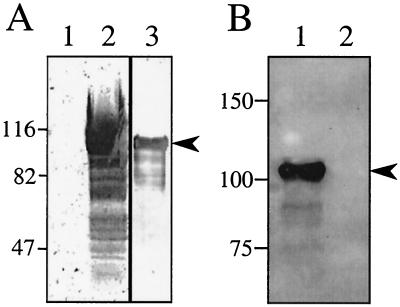
Polyclonal rabbit anti-TibA antiserum was generated using purified TibA as the antigen. In order to test the anti-TibA antiserum in a Western blot, it was preabsorbed with intact E. coli DH5α and the tib deletion mutant of H10407 (TIB3) to remove antibodies that recognized other E. coli outer membrane proteins and lipopolysaccharide. Western blots were then performed in which outer membranes of E. coli strain DH5α(pHG165) and DH5α(pET109) as well as purified TibA were analyzed. The preabsorbed immune serum detected TibA in outer membranes (Fig. 3A, lane 2) as well as purified TibA (Fig. 3A, lane 3), but did not detect any proteins in DH5α(pHG165) outer membranes (Fig. 3A, lane 1). Preimmune serum did not detect TibA.
FIG. 3.
Characterization of anti-TibA antiserum. SDS-PAGE (7.5% polyacrylamide) of the indicated samples was transferred to nitrocellulose and then probed with the indicated sera. (A) Immunoblot performed with preabsorbed and affinity-purified rabbit polyclonal anti-TibA IgG (1:1,000 dilution) as the primary antibody. Lanes: 1, 20 μg of outer membranes from E. coli DH5α(pHG165); 2, 20 μg of outer membranes from E. coli DH5α(pET109); 3, 1 μg of purified TibA. (B) Immunoblot performed with unabsorbed rabbit polyclonal anti-TibA antiserum (1:1,000 dilution) as the primary antibody. Lanes: 1, whole-cell lysate of E. coli DH5α(pET109); 2, whole-cell lysate of E. coli DH5α(pHG165). For all blots, the nitrocellulose membrane was stained with Ponceau S after transfer and before blocking to ensure that transfer was uniform. The migration of molecular mass standards (in kilodaltons) is shown on the left of each panel. The migration of TibA is indicated by an arrowhead to the right of each panel.
As a further confirmation that the purified TibA used in these studies was homogeneous, Western blots were performed using unabsorbed anti-TibA immune serum as a probe against whole-cell extracts of E. coli DH5α bearing either the TibA-expressing plasmid pET109 or the vector plasmid pHG165 as a control. If any DH5α proteins were contaminating the TibA preparation used as the antigen, antibodies against these proteins should recognize the corresponding proteins in the vector control. Unabsorbed anti-TibA antiserum failed to recognize any DH5α proteins with a mass similar to that of TibA (Fig. 3B).
Anti-TibA antibodies inhibit invasion.
The results obtained from binding experiments show that TibA acts as an adhesin. To address the question of whether TibA mediates invasion, antibodies to TibA were added to standard invasion assays. Preabsorbed antibodies purified on a protein G column and log-phase bacteria were added to the wells of a 24-well plate containing confluent HCT8 cells. A 1:10 dilution of anti-TibA antibodies inhibited invasion by recombinant E. coli DH5α carrying tib locus-bearing plasmids to the background levels observed with DH5α alone (Fig. 4A). A 1:10 dilution of anti-TibA antibodies inhibited H10407 invasion by about 70% compared to control wells receiving no antibody (Fig. 4B). The level of H10407 invasion measured in the presence of anti-TibA antibodies was similar to the extent of invasion observed for the H10407 tib locus deletion mutant TIB3 (Fig. 4B). TIB3 has previously been shown to adhere to and invade HCT8 cells at about 25% of the wild-type H10407 level (9) even though it produces the fimbrial adhesin CFA/I at levels identical to those in H10407, as determined by hemagglutination titer and colony immunoblots using anti-CFA/I antiserum. The deletion in TIB3 removes the tibB, tibC, and tibD genes and 70% of the tibA gene, leaving this strain unable to synthesize the TibA protein (9, 20). The residual invasion activity of TIB3 appears to reflect the presence of the tia locus in this strain, as tia deletion derivatives of TIB3 fail to invade HCT8 cells (Elsinghorst, unpublished). The ability of anti-TibA antibodies to inhibit tib locus-mediated invasion indirectly suggests that TibA acts as an invasin. However, these results do not prove that Tia is an invasin, since the effect of anti-Tia antibodies might be indirect.
FIG. 4.
Inhibition of TibA-mediated invasion by anti-TibA antiserum. Invasion assays were performed in the absence of antibodies (▪) or in the presence of preabsorbed and affinity-purified IgG from rabbit preimmune serum or polyclonal anti-TibA antiserum. (A) Invasion of HCT8 cells relative to E. coli DH5α(pET109), representing 100% (actual invasion of this strain in the absence of antibody was 1.30% ± 0.05%). Statistically significant effects of antibody treatment on the invasion efficiency of DH5α(pET109) are indicated by ∗ (P < 0.01) or ∗∗ (P < 0.005) as determined by analysis of variance of three experiments, each performed in triplicate. (B) Invasion of HCT8 cells relative to ETEC strain H10407, representing 100% (actual invasion of this strain in the absence of antibody was 0.24% ± 0.02%). TIB3 is a tib locus deletion mutant of H10407. Data are shown as averages for three replicates. Statistically significant effects of antibody treatment on the invasion efficiency of H10407 are indicated by ∗ (P < 0.01) as determined by analysis of variance of three experiments, each performed in triplicate.
DISCUSSION
It has previously been reported that the chromosomally encoded tib locus from the human-specific ETEC strain H10407 mediates adherence to and invasion of epithelial cells (9). The tib locus directs the synthesis of TibA, a 104-kDa outer membrane glycoprotein (20). It has been shown that the tib locus adherence and invasion phenotypes are directly correlated with the presence of TibA in the outer membrane of recombinant E. coli. Additionally, tib deletion mutants of H10407 are defective in epithelial cell adherence and invasion (9). These results suggest that TibA is responsible for the adherence and invasion phenotypes encoded by the tib locus. Therefore, we have proposed that the TibA protein is a previously uncharacterized afimbrial adhesin that can mediate invasion. In support of the hypothesis that TibA acts as an adhesin, we have previously reported that TibA is homologous to pertactin and AIDA-I, afimbrial adhesins identified in Bordetella pertussis and the pathogenic diffusely adhering E. coli, respectively (1, 5, 20).
To gain direct evidence for its biological activity, we purified TibA from recombinant E. coli bearing a TibA-expressing plasmid. Purified TibA bound to cultured human ileocecal epithelial cells in a specific and saturable manner. Unlabeled TibA could compete with biotin-labeled TibA for binding. These results indicate that TibA is an adhesin that recognizes a specific receptor that is limited in abundance on the surface of HCT8 cells. These experiments do not provide direct evidence that TibA is an invasin. However, we show that anti-TibA polyclonal antibodies inhibit epithelial cell invasion by ETEC strain H10407 and by recombinant E. coli carrying tib locus-bearing plasmids. This result provides indirect evidence that TibA acts as an invasin.
We purified TibA under denaturing conditions. Attempts to purify TibA under nondenaturing conditions, including the use of nonionic detergents to solubilize the outer membrane, were unsuccessful. During these purification attempts, we found TibA to be a highly unstable protein that is very susceptible to proteolytic cleavage. As previously reported, TibA belongs to the autotransporter family of proteins, and its C-terminal 311 residues appear to code for a 14-stranded β-barrel that is inserted in the outer membrane (20). The hydrophobic nature of this region appears to make TibA difficult to purify in a native form. Denaturing conditions increased TibA stability while allowing the protein to retain at least part of its biological activity. As previously reported, TibA must be modified for tib locus-mediated epithelial cell adherence and invasion to occur (9). This result suggests that TibA carbohydrates are involved in its biological activity, potentially contributing to the binding activity of our purified protein in spite of denaturation.
Like many other enteric pathogens, ETEC strain H10407 can express fimbrial and afimbrial adhesins and invasins. The fimbrial adhesins (i.e., CFAs) are thought to mediate the initial adherence to the intestinal epithelium that is required to resist the flushing activity of the intestine. It has previously been shown that CFAs are required for ETEC to cause disease (2, 4, 6, 12, 19). The role of afimbrial adhesins and invasins in ETEC pathogenesis is unclear. Afimbrial adhesins have been found to mediate closer contact between the bacterium and the host cell than fimbrial adhesins (22). This close contact is often required for the more effective delivery of toxins or other effector molecules to the eukaryotic host cell (14, 15). Since enterotoxins appear to be primarily responsible for the water loss associated with ETEC infections, the role of afimbrial adhesins such as TibA may be to provide close contact with the host cell for the most effective delivery of enterotoxin. Additionally, epithelial cell invasion stimulated by a TibA-receptor interaction may contribute to pathogenesis through yet undetermined mechanisms.
ACKNOWLEDGMENTS
This work was supported by a grant from the University of Kansas General Research Fund to E. Elsinghorst. C. Lindenthal was supported by a Fulbright Fellowship.
REFERENCES
- 1.Benz I, Schmidt M A. AIDA-I, the adhesion involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. doi: 10.1111/j.1365-2958.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 2.Black R. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine. 1993;11:100–106. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Cassels F J, Wolf M K. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J Ind Microbiol. 1995;15:214–226. doi: 10.1007/BF01569828. [DOI] [PubMed] [Google Scholar]
- 5.Charles I G, Dougan G, Pickard D, Chatfield S, Smith M, Novotny P, Morrissey P, Fairweather N F. Molecular cloning and characterization of protective outer-membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci USA. 1989;86:3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeGraaf F K, Mooi F R. The fimbrial adhesins of Escherichia coli. Adv Microb Physiol. 1986;28:65–143. doi: 10.1016/s0065-2911(08)60237-4. [DOI] [PubMed] [Google Scholar]
- 7.Edman P. On the mechanism of phenylisothiocyanate degradation of peptides. Acta Chem Scand. 1956;10:761–768. [Google Scholar]
- 8.Elsinghorst E A, Kopecko D J. Molecular cloning of epithelial cell invasion determinants from enterotoxigenic Escherichia coli. Infect Immun. 1992;60:2409–2417. doi: 10.1128/iai.60.6.2409-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsinghorst E A, Weitz J A. Epithelial cell invasion and adherence directed by the enterotoxigenic Escherichia coli tib locus is associated with a 104-kilodalton outer membrane protein. Infect Immun. 1994;62:3463–3471. doi: 10.1128/iai.62.8.3463-3471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans D G, Silver R P, Evans D J, Jr, Chase D G, Gorbach S L. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect Immun. 1975;12:656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleckenstein J M, Kopecko D J, Warren R L, Elsinghorst E A. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect Immun. 1996;64:2256–2265. doi: 10.1128/iai.64.6.2256-2265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaastra W, Svennerholm A M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 14.Hicks S, Frankel G, Kaper J B, Dougan G, Phillips A D. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect Immun. 1998;66:1570–1578. doi: 10.1128/iai.66.4.1570-1578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenny B, Lai L C, Findlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 16.Kihlstrom E. Infection of HeLa cells with Salmonella typhimurium 395 MS and MR10 bacteria. Infect Immun. 1977;17:290–295. doi: 10.1128/iai.17.2.290-295.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Levine M M, Kaper J B, Black R E, Clements M L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine M M. Vaccines against enterotoxigenic Escherichia coli infections. In: Woodrow M M L G C, editor. New generation vaccines. New York, N. Y: Marcell Dekker; 1990. pp. 649–660. [Google Scholar]
- 20.Lindenthal C, Elsinghorst E A. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect Immun. 1999;67:4084–4091. doi: 10.1128/iai.67.8.4084-4091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Mammarappallil J G, Elsinghorst E A. Epithelial cell adherence mediated by the enterotoxigenic Escherichia coli Tia protein. Infect Immun. 2000;68:6595–6601. doi: 10.1128/iai.68.12.6595-6601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moxley R A, Berberov E M, Francis D H, Xing J, Moayeri M, Welch R A, Baker D R, Barletta R G. Pathogenicity of an enterotoxigenic Escherichia coli hemolysin (hlyA) mutant in gnotobiotic piglets. Infect Immun. 1998;66:5031–5035. doi: 10.1128/iai.66.10.5031-5035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sack R B, Kline R L, Spira W M. Oral immunization of rabbits with enterotoxigenic Escherichia coli protects against intraintestinal challenge. Infect Immun. 1988;56:387–394. doi: 10.1128/iai.56.2.387-394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlager T A, Wanke C A, Guerrant R L. Net fluid secretion and impaired villous function induced by colonization of the small intestine by nontoxigenic colonizing Escherichia coli. Infect Immun. 1990;58:1337–1343. doi: 10.1128/iai.58.5.1337-1343.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnaitman C A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970;104:890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Towbin H, Staehelia T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanke C, Guerrant R. Small-bowl colonization alone is cause of diarrhea. Infect Immun. 1987;55:1924–1926. doi: 10.1128/iai.55.8.1924-1926.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. The world health report 1999—making a difference. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]