Abstract
A gene encoding an endopeptidase from Streptococcus parasanguis FW213 has been cloned and shown to have high sequence homology to genes encoding mammalian metalloendopeptidases. The gene, designated S. parasanguis pepO, was cloned into the pET28a expression vector, resulting in a fusion of vector sequences encoding a hexahistidine tag at the carboxyl terminus. The recombinant PepO (rPepO) was expressed in Escherichia coli and purified using an Ni2+ affinity column. Polyclonal antiserum to rPepO was raised in rabbits and used to localize FW213 PepO to the cytosol. Southern hybridization and immunoblot analysis revealed that other oral streptococci contain regions of DNA with homology to pepO and produce a protein with antigenic properties similar to that of FW213 PepO. Enzymatic activity assays indicated that only S. parasanguis species possess the ability to cleave metenkephalin, the natural substrate of the human neutral endopeptidase (NEP). Inhibition assays revealed that S. parasanguis PepO is a member of the M13 category of metalloendopeptidases, which includes NEP and endothelin-converting enzyme 1 (ECE-1), an enzyme involved in the maintenance of vascular tone. Thiorphan and phosphoramidon, two specific inhibitors of this category of endopeptidases, were used to determine that S. parasanguis PepO is more similar to ECE-1 than to NEP.
Streptococcus parasanguis (formerly Streptococcus sanguis) is a member of the mitis group of streptococci (39) and is known to be among the primary colonizers of the human oral cavity (9). Although these bacteria are considered commensal organisms in the oral environment, their prevalence on the tooth surface does contribute to both localized and systemic diseases. The ability of oral streptococci to adhere to the tooth surface, the first step in colonization of the oral cavity, allows for the succession of other microorganisms (20), many of which have the ability to cause pathologies, such as dental carries or soft tissue damage. The oral cavity with its ability to support the survival and growth of a multitude of different microorganisms can also be viewed as a bacterial reservoir. Introduction of organisms into the bloodstream via routine dental hygiene procedures produces transient bacteremias, which as seen with the viridans group streptococci, can result in bacterial endocarditis in people with compromised heart valves (5). It has been demonstrated that S. parasanguis strains that contain a mutation in the fimA operon, an operon that encodes an ATP- binding cassette (ABC) transport system (16), are impaired in their ability to cause endocarditis in a rat model (8). S. parasanguis can adhere to fibrin via the FimA protein (8), suggesting that these bacteria can attach to and colonize fibrin deposits, a component of sterile vegetations located at the site of valve damage.
The oral environment and the organisms it harbors, besides playing a role in bacterial endocarditis, have been implicated in other forms of heart disease (6). In fact, an association of oral health with the development of coronary heart disease has been suggested for many years (25, 26), and the role of bacterial infections in the development of atherosclerosis was investigated as early as the 1930s. One of these early studies indicated that rabbits intravenously inoculated with streptococcus strains and fed high-cholesterol diets developed atherosclerotic-like lesions on their aortas (7). Later experiments revealed that bacteria could be retrieved and cultured from the coronary artery walls of inoculated animals (21). In addition, the fibrin deposits seen on damaged heart valves to which some members of the mitis group of streptococci can adhere are reminiscent of vegetative plaques found in the early stages of atherosclerosis.
In an attempt to identify additional S. parasanguis virulence factors that may play a role in cardiovascular disease, regions surrounding the fimA operon were sequenced and analyzed. As described previously, we identified a gene located 148 nucleotides upstream and divergently transcribed from the fimA operon. This gene, designated S. parasanguis pepO, was determined to have high sequence homology to mammalian metalloendopeptidases, e.g., neutral endopeptidase (NEP) and endothelin-converting enzyme 1 (ECE-1) (17). NEP is involved in regulation of analgesia and aspects of the immune response (31, 38). ECE is the enzyme responsible for processing endothelin-1 (ET-1), the potent vasoconstrictor produced by endothelial cells, into its biologically active form (42). Elevated levels of ET-1 have been associated with hypertension, stroke, and heart failure (30, 37). Recently ET-1 has also been shown to affect mitogenesis and apoptosis in various cell lines and tissues, including smooth muscle cells (23, 41, 43).
In this study pepO and the protein that it encodes were characterized in various oral streptococci strains, including S. parasanguis.
MATERIALS AND METHODS
Bacterial culture.
FW213 is the wild-type S. parasanguis strain used in this study. The pepO allelic replacement mutant, VT1346, was generated by insertion of a kanamycin resistance (Kmr) gene, aphA-3, into pepO as previously described (17). All other streptococci used in this study are listed in Table 1. Streptococci were grown statically in Todd-Hewitt (TH) broth (Difco Laboratories, Detroit, Mich.) in the presence of 5% CO2 at 37°C. Escherichia coli strain JM109 (Promega) was used for cloning and plasmid propagation. E. coli BL21(pLysS) cells were used as expression-competent hosts. E. coli strains were maintained in Luria-Bertani (LB) medium at 37°C with or without the addition of kanamycin (50 μg/ml) and chloramphenicol (34 μg/ml) when required for plasmid selection. Solid medium was prepared by the addition of 1.5% agar to the LB medium.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or Plasmid | Characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| Mitis group streptococci | ||
| FW213 | Wild-type; S. parasanguis | R. Cole (11) |
| VT1346 | pepO mutant | E. Froeliger (17) |
| VT930 | fimA mutant | C. Fenno (15) |
| VT1393 | fap1 mutant | H. Wu (40) |
| VT264 | SPED3; S. gordonii | P. Handley (16) |
| VT527 | Blackburn; S. gordonii | A. Coykendall (16) |
| VT529 | 804; S. sanguis | A. Coykendall (16) |
| VT528 | HPC1; S. sanguis | A. Coykendall (16) |
| VT266 | CR3; S. mitis | P. Handley (16) |
| VT267 | CR311; S. mitis | P. Handley (16) |
| VT522 | MGH413; S. parasanguis | A. Coykendall (16) |
| VT525 | UC Freed; S. parasanguis | A. Coykendall (16) |
| VT523 | MGH145; S. parasanguis | A. Coykendall (16) |
| VT524 | UC4989; S. parasanguis | A. Coykendall (16) |
| E. coli | ||
| JM109 | Cloning and plasmid propagation | Promega |
| BL21(DE3)pLysS | Expression competent host | (32) |
| VT1395 | rPepO expressing cells | This study |
| Plasmids | ||
| pET28a | Expression vector | Novagen |
| pVT1394 | ∼1.9-kb fragment containing the pepO gene ligated into pET28a | This study |
ELISA.
A whole bacterial cell enzyme-linked immunosorbent assay (BactELISA) (12) as well as traditional ELISAs using protein were used to detect proteins present either on the bacterial cell surface or in protein extracts. The presence of surface-bound FimA and Fap1 was determined in FW213 as well as in pepO (VT1346), fimA (VT930), and fap1 (VT1393) mutants using a BactELISA. Bacteria were grown to late-log-growth phase (optical density [OD] of 0.9 at 470 nm) (Spectronic 20D; Milton Roy Company, Rochester, N.Y.) in TH broth, and ∼2 × 108 bacterial cells/ml were suspended in 50 mM sodium carbonate (NaHCO2) buffer, pH 9.6. Aliquots of each sample (100 μl/well) were immobilized onto wells of 96-well microtiter plates by incubation at 37°C overnight. Wells were washed twice with phosphate-buffered saline (PBS) (pH 7.4) and treated with 1% bovine serum albumin (BSA) in PBS for 1 to 2 h at room temperature. Wells were washed twice with PBS and incubated with FimA antiserum (1:2,500 dilution in 1% BSA) or anti-Fap1 mouse monoclonal antibody, MAbF51 (14), (400 ng of MAbF51 monoclonal antibody 14 in 1% BSA) for 1 h at room temperature and were used as probes for the detection of FimA and Fap1 epitopes, respectively. Wells were washed with PBS containing 0.1% polyoxyethelene-sorbitan monolaurate (Tween 20) and treated with a 1:10,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin (Southern Biotechnology Inc., Birmingham, Ala.) in 1% BSA and 0.1% Tween 20 in PBS (PBST) for 1 h. Wells were washed with PBST, and enzymatic activity was determined by incubation with hydrogen peroxide in the presence of o-phenylenediamine in citrate-phosphate buffer, pH 5.0. The reaction was stopped after 5 min by the addition of 4 M sulfuric acid. Color development was quantified by measurement of the absorbance at 490 nm using an EL311 microtiter plate reader (Bio-Tek Inc., Winooski, Vt.).
ELISA was used to detect the presence of PepO-like epitopes in oral streptococci extracts. The procedure is similar to that previously described for the BactELISA except with the following modifications. Soluble whole-cell extracts from each strain tested were suspended in 50 mM sodium carbonate buffer, pH 9.6, and were immobilized (1 μg of total protein; 100 μl/well) on wells of 96-well microtiter plates by incubation at 37°C overnight. PepO antiserum (1:5,000 dilution in 1% BSA) was used as the probe for PepO.
Cloning and expression of recombinant PepO.
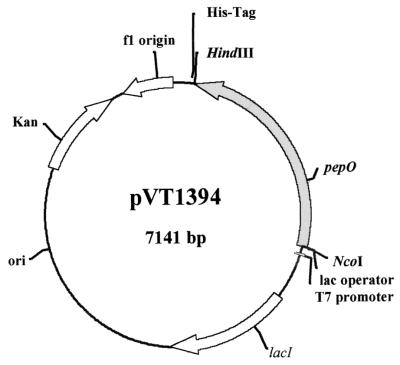
S. parasanguis genomic DNA was isolated using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, Minn.). Amplification of the wild-type pepO gene from S. parasanguis DNA was carried out by PCR using a Perkin-Elmer 9600 thermocycler (Perkin-Elmer Cetus, Norwalk, Conn.) and the GeneAmp PCR reagent kit (Roche Molecular Systems, Inc., Branchburg, N.J.). Primers corresponding to the 5′ (5′-GTCCCCCATGGTACGTTTACAAGATG-3′) and 3′ (5′-CGCCCAAGCTTCCAAATAATCACACGATCCTC-3′) regions of pepO were designed to contain NcoI and HindIII restriction sites (bold type), respectively. The restriction sites were used to facilitate cloning of the amplified gene into the pET28a expression vector (Novagen, Inc., Madison, Wis.). The resultant plasmid, designated pVT1394 (Fig. 1), had the pepO open reading frame under the control of the T7 promoter and generated a carboxy-terminal fusion with sequences coding for a hexahistidine tag. pVT1394 was electroporated into electrocompetent JM109 cells following previously described protocols (3) using a Gene Pulse apparatus (Bio-Rad Laboratories, Hercules, Calif.). The cloned plasmid was isolated from E. coli using Mini or Midi column plasmid purification kits (Qiagen, Inc., Santa Clarita, Calif.). Restriction enzyme digestion (visualized on a 0.8% ethidium bromide agarose gel) and DNA sequence analysis of the plasmid indicated that pepO was in frame and in a proper orientation to allow for the production of the fusion protein. DNA sequence analysis was performed at the Vermont Cancer Center DNA Analysis Facility at the University of Vermont using the Sanger dideoxynucleotide chain termination method as modified for ABI Prism Dye Terminator cycle sequencing using ampliTaq polymerase on an ABI 373A automated DNA sequencer (Perkin-Elmer). pVT1394 was used for electrotransformation of BL21(pLysS) cells. One transformant, designated VT1395, was used to express recombinant PepO. VT1395 was grown to mid-log-growth phase (OD of 0.6 at 600 nm) and induced for 0 to 5 h with 1 mM isopropylthio-β-d-galactoside (IPTG) (GibcoBRL, Grand Island, N.Y.) at 37°C with shaking as suggested by the manufacturer of the pET expression system (Novagen). Expression of the recombinant PepO (rPepO) was determined by Coomassie brilliant blue staining of 12% acrylamide–sodium dodecyl sulfate (SDS) gels (24) loaded with whole-cell extract from induced and uninduced VT1395 cells.
FIG. 1.
Diagram of plasmid pVT1394. The map indicates the location of the cloned pepO gene of S. parasanguis in the expression vector pET28a. The shaded arrow indicates the direction of transcription of the pepO gene. NcoI and HindIII indicate the location of restriction enzyme sites used for insertion of pepO into the vector in frame with sequences encoding the hexahistidine tag.
Preparation of cytosolic and membrane extracts.
Streptococcal cultures were grown to late-log-growth phase in TH broth (OD of 0.9 at 470 nm). Aliquots of each culture (5 ml) were centrifuged at 5,000 × g for 10 min at 4°C. Cell pellets were washed with 20 mM potassium phosphate (KPi) buffer (pH 6), centrifuged, and suspended in 500 μl of 20 mM KPi (pH 6). The suspended cell pellets were transferred to 1.5-ml microcentrifuge tubes containing 0.07 g of acid-washed glass beads (Sigma Chemical, St. Louis, Mo.). Samples were agitated in an FP120 Fast Prep device (Bio 101, Inc., Vista, Calif.) for four 30-s intervals at a speed setting of 6, with 1 min of cooling on ice after the first minute. Tubes were centrifuged at 3,000 × g for 10 min at 4°C to pellet glass beads and intact cells. The supernatant (soluble whole-cell extract) was transferred to fresh tubes, and the previous step was repeated. The soluble extract was transferred to polycarbonate 13- by 51-mm centrifuge tubes (Beckman Coulter, Inc., Fullerton, Calif.) and centrifuged at 40,000 × g for 30 min at 4°C to separate cytosolic and membrane components (18). The cytosolic fractions were transferred to fresh tubes and the membrane fractions were washed with 20 mM KPi (pH 6) and suspended in 500 μl of KPi (pH 6). Both cytosolic and membrane extracts were aliquoted and stored at −80°C. Cytosolic extracts of induced and uninduced VT1395 cells were prepared similarly. Protein concentrations were determined with the use of the bicinchoninic acid protein assay reagent kit (Pierce Chemical Company, Rockford, Ill.) using BSA as the standard.
Purification of rPepO.
For protein purification purposes the manufacturer's recommended method for inducing recombinant protein expression was modified. VT1395 cells were grown to early log growth phase (OD of 0.35 to 0.45 at 600 nm) in 100 ml of LB broth at 24°C with shaking and induced with 0.5 mM IPTG for 3 h. The cells were harvested by centrifugation at 5,000 × g for 10 min, the supernatant was removed, and the cell pellet was frozen at −80°C. The cell pellet was thawed and suspended in 4 ml of binding buffer (5 mM imidazole, 0.5 M NaCl, and 20 mM Tris-HCl [pH 7.9]; Novagen). The suspended cells were frozen and thawed seven times, alternating between a dry-ice ethanol bath and a 37°C water bath. The cell suspension was sonicated (model W-220; HeatSystems-Ultrasonic, Inc., Farmingdale, N.Y.) using a microtip for seven 30-s intervals, with cooling on ice for 15 s between each sonication. The cell lysate was centrifuged for 20 min at 39,000 × g, and the supernatant was filtered through a 0.8/0.2-μm-pore-size filter directly onto a resin column (Novagen) charged with NiSO4 and equilibrated with binding buffer. Recommended conditions for column usage, as described in the pET system manual (Novagen), were followed except that the column was washed with buffer containing 30 mM imidazole and the rPepO was eluted with buffer containing 60 mM imidazole. Elution buffer was exchanged with 20 mM sodium phosphate buffer, pH 7.6, and aliquots were stored at −80°C. Protein concentrations of the eluted fractions were determined by bicinchoninic acid reaction using BSA as a standard. Fractions eluted during the purification process were analyzed by Coomassie brilliant blue and silver staining (Bio-Rad silver stain kit) of 10% polyacrylamide–SDS gels. Proteins present in the eluted fraction were processed for NH2-terminal sequencing by a protocol previously described (29). The NH2-terminal sequence of these proteins was determined by automatic Edman degradation on an Applied Biosystems 475A protein sequencing system equipped with a Blott cartridge in the laboratory of Alex Kurosky (University of Texas Medical Branch at Galveston).
Production of PepO specific antiserum.
Purified rPepO (200 μg/gel) was separated by polyacrylamide gel electrophoresis on a 12% denaturing acrylamide gel. Sections of gel containing rPepO and high-molecular-weight markers (Gibco Life Technologies, Rockville, Md.) were stained briefly with Coomassie brilliant blue while the remaining portions of the gel were kept at 4°C. The stained slices were used to determine the location of the majority of the protein in the unstained gel. Gel slices corresponding to this region were removed, frozen, and stored (−20°C) in two strips of 100 μg of rPepO per acrylamide slice. This preparation was used to immunize rabbits (Cocalico Pharmaceuticals, Reamstown, Pa.) for the production of monospecific polyclonal antiserum.
Western blot analysis of PepO.
Bacterial pellets or extracts (cytosolic and membrane fractions) were suspended in 2× SDS loading dye, boiled for 10 min, centrifuged, loaded at equivalent cell numbers or protein concentrations, and separated by electrophoresis on 12% polyacrylamide–SDS gels. Proteins were transferred to nitrocellulose (Schleicher and Schuell, Keene, N.H.) and examined by Western blot analysis (36) as described previously (40) except that a 1:5,000 dilution of rPepO antiserum was used as a probe for PepO. Goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase at a dilution of 1:10,000 was used as the secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.). Antibody conjugates were detected with a chemiluminescence system as described by the manufacturer (NEN Life Science Products, Boston, Mass.).
Assay of enzyme activity.
Endopeptidase activity of extracts or rPepO was assayed by modification of a procedure of Tan et al. (33). In brief, 1.25 mM metenkephalin (Tyr-Gly-Gly-Phe-Met) (Sigma) was incubated with appropriate amounts of extracts or purified rPepO preparations in 20 mM Tris-HCl (pH 7.0) to a final volume of 50 μl. Samples were incubated at 30°C for 15 min, unless otherwise indicated, and the reactions were stopped by the addition of 10 μl of 30% acetic acid and cooling of the samples to 4°C. Cleavage of metenkephalin was detected by thin-layer chromatography as follows. Samples were centrifuged at 15,000 × g for 5 min, and 10 μl of the mixture was spotted onto a precoated 0.25-cm-thick silica gel 60 plate (Merck, Darmstadt, Germany). l-glycine, l-phenylalanine, l-methionine, l-tyrosine, and the tripeptide Try-Gly-Gly (Sigma) were also spotted onto the plates as markers. The plates were placed in 100 ml of a 4:1:1 (vol/vol/vol) mixture of n-butanol–acetic acid–water as the mobile phase for 3 h. Silica gels were stained with 0.05% fluorescamine in 99% acetone (Sigma) and visualized by UV light.
Inhibition assays involved preincubation of purified rPepO preparations with various concentrations of inhibitors (1,10-phenanthroline, thiorphan, or phosphoramidon; Sigma) for 10 min at 20°C prior to addition of metenkephalin. Enzyme activity was determined as described above.
Conservation of pepO in streptococcal strains.
Chromosomal DNA of streptococcal strains was prepared as described previously for S. parasanguis. Southern hybridization was carried out under low-stringency conditions as recommended by the manufacturer of the ECL kit (direct nucleic acid labeling and detection systems kit; Amersham International, plc., Little Chalfont, Buckinghamshire, England). The probe used for Southern hybridizations was a 1,062-bp PCR-amplified internal fragment of pepO (17).
RESULTS
PepO is not required for cell surface presentation of FimA and Fap1.
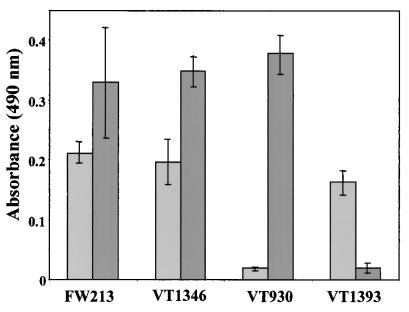
The role of bacterial endopeptidases is as yet unknown. Localization of pepO approximately 150 nucleotides upstream of the fimA operon in S. parasanguis suggested to us that PepO may be involved in the expression of FimA, a cell surface virulence factor associated with endocarditis. Thus, FimA and another cell surface-associated adhesin, Fap1 (14, 40), were examined by BactELISA to determine if there were quantitative changes in their cell surface expression between the wild-type and pepO mutant. No appreciable differences in the cell surface quantity of either FimA or Fap1 were detected between the pepO mutant and the wild type (Fig. 2). This suggests that PepO is not involved in the expression or processing of these proteins for cell surface display, which necessitated biochemical characterization of PepO to aid in the determination of a role for this enzyme in S. parasanguis.
FIG. 2.
BactELISA analysis of FimA and Fap1 on the cell surface. FimA (light gray) and Fap1 (dark gray) were quantified on the cell surface of intact FW213 (wild-type), VT1346 (pepO mutant), VT930 (fimA mutant), and VT1393 (fap1) cells (∼2 × 108 bacterial cells/ml) using FimA antiserum (1:2,500 dilution) or Fap1 monoclonal antibody (400 ng), respectively. Error bars, standard deviations.
Induction and purification of rPepO.
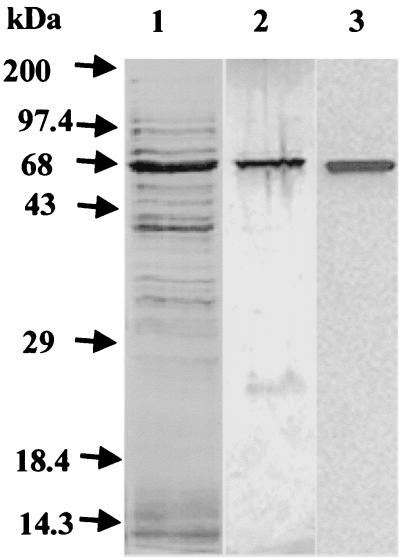
VT1395 E. coli cells, containing the cloned pepO open reading frame, when induced with 0.5 mM IPTG for 3 h at 24°C produced an expected 68-kDa protein that was present primarily in the soluble fraction (Fig. 3, lane 1). Induction at a lower temperature and IPTG concentration avoided formation of inclusion bodies that permitted purification under native conditions. The use of induction conditions (1 mM IPTG and a temperature of 37°C) recommended by the manufacturer of the pET expression system resulted in the production of predominately insoluble protein (data not shown).
FIG. 3.
Analysis of rPepO during induction and purification. IPTG-induced VT1395 soluble cell extracts (3.5 × 107 cells) were electrophoresed on a 12% acrylamide gel and stained with Coomassie brilliant blue (lane 1). Silver staining of a 10% acrylamide gel containing 250 ng of eluted protein (lane 2) and immunoblot analysis of a 10% acrylamide gel containing 250 ng of eluted protein using rPepO antiserum (100 ng/ml) as a probe for rPepO (lane 3) are also shown.
Purification of the His-tagged rPepO from the crude extract by affinity chromatography resulted in 0.264 mg of purified protein from 100 ml of VT1395 cells. Analysis of the purified rPepO by silver staining of 10% acrylamide–SDS gels revealed the presence of a major band at approximately 68 kDa and a minor band at approximately 22 kDa (Fig. 3, lane 2). The 68-kDa band was excised from the gel and used to immunize rabbits as described in Materials and Methods.
Western blot analysis of the fractions indicated that the polyclonal antiserum produced against rPepO reacted specifically with the 68-kDa band corresponding to the recombinant enzyme (Fig. 3, lane 3). The minor 22-kDa band that was present in the silver-stained gel (Fig. 3, lane 2) was not recognized by rPepO antiserum (Fig. 3, lane 3). The 22-kDa protein was determined by NH2-terminal sequencing to be the product of the E. coli chloramphenicol acetyl transferase gene present on the pLysS plasmid in the expression host (data not shown).
PepO is localized to the cytosol of S. parasanguis.
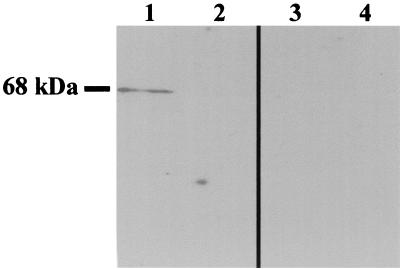
Antiserum directed against rPepO was used to determine the localization of PepO in S. parasanguis. rPepO antiserum recognized a 68-kDa protein in cytosolic extracts of wild-type FW213 (Fig. 4, lane 1) but was not detected in the membrane fraction (Fig. 4, lane 2). As expected, PepO was not detected in any fraction of the mutant (Fig. 4, lanes 3 and 4). Further support for the cytosolic localization of PepO was obtained from enzyme activity assays in which metenkephalin, the natural substrate of NEP, was hydrolyzed by cytosolic but not membrane extracts of FW213 (data not shown). The antiserum to rPepO did not react with other proteins from FW213, confirming the specificity of the antiserum for PepO and indicating that the rPepO antiserum will be a useful tool for investigating the presence of PepO-like proteins in other oral streptococci.
FIG. 4.
Localization of PepO in S. parasanguis by immunoblot analysis. FW213 and VT1346 extracts (1.6 μg of cytosolic or membrane extracts/lane) were probed with PepO antiserum (100 ng/ml). Shown are FW213 cytosolic extract (lane 1), FW213 membrane extract (lane 2), VT1346 cytosolic extract (lane 3), and VT1346 membrane extract (lane 4).
Conservation of pepO.
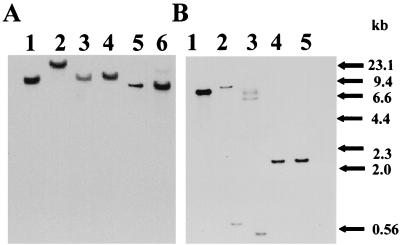
Although members of the oral streptococci share phenotypic similarities and a common phylogeny, they exhibit only a 60% homology on the DNA level with even the most-related species (22). It was of interest to investigate if other members of the oral streptococci also have pepO. Representative strains of various oral streptococcal species were analyzed by Southern blotting under low-stringency conditions. DNA of all strains probed with an internal fragment of S. parasanguis pepO was shown to contain regions of DNA homologous to pepO of FW213 (Fig. 5A, lanes 1 to 6; Fig. 5B, lanes 1 to 5).
FIG. 5.
Southern blot analysis of oral streptococci showing the presence of DNA sequences with homology to pepO. (A) Streptococcal DNA from bacteria representing S. gordonii, S. sanguis, and S. mitis strains probed with an internal fragment of FW213 pepO. Lanes: 1 and 2, S. gordonii strains VT264 and VT527, respectively; 3 and 4, S. sanguis strains VT529 and VT528, respectively; 5 and 6, S. mitis VT266 and VT267, respectively. (B) Streptococcal DNA from bacteria representing S. parasanguis strains probed with an internal fragment of FW213 pepO. S. parasanguis strains VT522 (lane 1), VT525 (lane 2), VT523 (lane 3), VT524 (lane 4), and FW213 (lane 5) are shown.
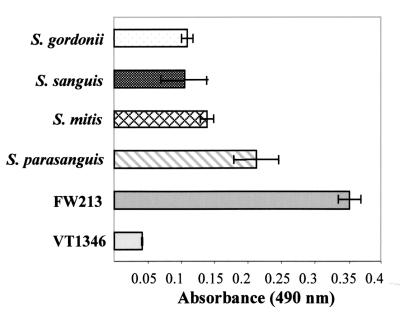
PepO-like proteins are present in other oral streptococci.
The presence of DNA domains with pepO homology in other oral streptococcal strains does not of course indicate that the region is expressed or that a protein product is produced. Thus, soluble whole-cell extracts derived from each of the strains shown to possess DNA with homology to pepO were evaluated in an ELISA for their ability to react with the rPepO antiserum. As shown in Fig. 6, extracts from all of the strains examined reacted to various degrees with the antiserum, indicating that all of them produce PepO-like proteins. This was confirmed by immunoblot analysis using rPepO antiserum, which revealed the presence of an approximately 68-kDa protein corresponding to PepO in each of the strains examined (data not shown).
FIG. 6.
PepO-like proteins in oral streptococci as indicated by ELISA. Soluble whole-cell extracts of oral streptococci representing two strains of S. gordonii, VT264 and VT527; two strains of S. sanguis, VT529 and VT528; two strains of S. mitis, VT266 and VT267; and four strains of S. parasanguis, VT522, VT525, VT523, VT524, and wild-type S. parasanguis FW213 were analyzed by ELISA using rPepO antiserum (1:5,000 dilution) as a probe for PepO-like proteins. The data presented are the averages for each strain.
The expression of PepO in the fimA (VT930) and fap1 (VT1393) mutants was also evaluated in a similar manner. PepO expression was not significantly different from that of wild-type FW213 (data not shown). This further supports our earlier findings that PepO is not involved in the processing of either of these two cells surface adhesins.
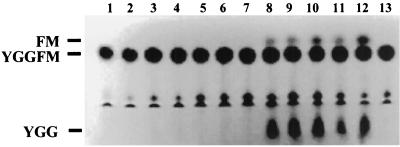
S. parasanguis strains can cleave metenkephalin.
Extracts of each of the streptococcal strains that showed both pepO homology and its homologous protein product were tested for their ability to hydrolyze metenkephalin. Interestingly, although each of the strains examined produced a protein with antigenic properties similar to those of PepO, only S. parasanguis strains were able to cleave metenkephalin under the standard assay conditions (Fig. 7, lanes 8 to 12) as indicated by the presence of the hydrolysis products FM and YGG.
FIG. 7.
Thin-layer chromatography of oral streptococci. Extracts of oral streptococci representing S. gordonii, S. sanguis, S. mitis, and S. parasanguis strains (1 μg of total protein/reaction mixture) were incubated with 1.25 mM metenkephalin for 2 h at 37°C. Shown are the metenkephalin control (lane 1) and extracts of S. gordonii strains VT264 (lane 2) and VT527 (lane 3); S. sanguis strains VT529 (lane 4) and VT528 (lane 5); S. mitis strains VT266 (lane 6) and VT267 (lane 7); S. parasanguis strains VT522 (lane 8), VT525 (lane 9), VT523 (lane 10), VT524 (lane 11), and FW213 (lane 12); and the pepO mutant VT1346 (lane 13).
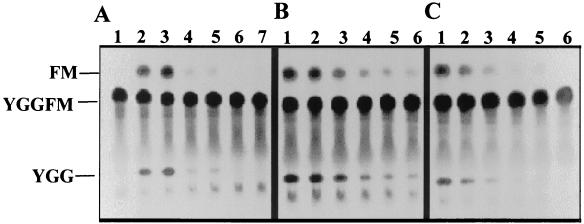
S. parasanguis rPepO is similar to ECE-1.
The effects of various chemical agents on the endopeptidase activity of purified PepO were examined. Purified rPepO possessed the ability to utilize metenkephalin as a substrate (Fig. 8A, lane 2). The divalent cation chelator, 1,10-phenanthroline, which chelates metal ions, such as Fe2+, Cu2+, Mn2+, Zn2+, and Co2+, inhibited rPepO activity (Fig. 8A, lanes 2 to 7). EDTA, which also chelates small metal ions, predominately Mg2+ and Ca2+, also inhibited rPepO activity, but to a lesser degree than 1,10-phenanthroline (data not shown). Two specific inhibitors of NEP-type enzymes, thiorphan (Fig. 8B) (a thiol inhibitor) and phosphoramidon (Fig. 8C) (a Streptomyces-derived natural competitive inhibitor), also inhibited the recombinant enzyme. rPepO was more sensitive to phosphoramidon than to thiorphan, as is seen with ECE-1 (38).
FIG. 8.
Enzyme assay of purified rPepO incubated with inhibitors. Purified rPepO (50 ng) was incubated with 1.25 mM metenkephalin and various concentrations of inhibitors for 15 min at 30°C, and the reactions were stopped by the addition of glacial acetic acid. (A) Lanes: 1, metenkephalin control; 2 to 7, 0, 10, 50, 100, 200, and 400 μM 1,10-phenanthroline, respectively. (B) Lanes 1 to 6, 0, 10, 25, 50, 75, and 100 μM thiorphan, respectively. (C) Lanes 1 to 6, 0, 10, 25, 50, 75, and 100 nM phosphoramidon, respectively.
DISCUSSION
S. parasanguis strain FW213 pepO bears high deduced amino acid sequence homology to the M13 category metalloendopeptidases that have been identified in both bacteria and mammals (17, 30). Additional support for the inclusion of S. parasanguis pepO in this category comes from enzyme activity and inhibition profiles of purified rPepO that were presented here. These studies revealed that rPepO has substrate specificity similar to that of other members of this category in that it could cleave metenkephalin into the expected di- and tri-peptide products. It was also shown that this enzyme is sensitive to the M13 category specific inhibitors, thiorphan (a synthetic enkephalinase inhibitor) and phosphoramidon (a Streptomyces metabolite).
The phylogenetic distribution of bacterial enzymes in this category is quite interesting. As determined by a BLAST search of the National Center for Biotechnology Information nonredundant protein sequence database and database of unfinished genomes (1, 2) only a few gram-negative organisms sequenced to date, such as Porphyromonas gingivalis, Shewanella putrefaciens, Caulobacter crescentus, Haemophilus ducreyi, and Legionella pneumoniae, possess regions of DNA with homology to the M13 metallopeptidases. Of these organisms only P. gingivalis has been shown experimentally to produce an enzyme with catalytic activity similar to the mammalian enzymes of this category (4). The majority of bacteria that have sequence homology to the mammalian M13 metallopeptidases are gram-positive and have an intimate association with mammals. This leads to the suggestion that the gene was acquired via horizontal transfer between bacteria and eukaryotes (17). We have shown using other human oral streptococci that these bacteria also have sequence homology to pepO and produce a protein with antigenic similarity to the FW213 endopeptidase. Only S. parasanguis, however, was able to cleave metenkephalin under the standard assay conditions. This suggests that the enzymatic activity or substrate specificity may be different within the same genus. These differences may be on the nucleotide sequence level or may be due to differential processing of the protein.
Lactococcus lactis has a bacterial metalloendopeptidase also termed PepO that has been highly characterized. The lactococcal enzyme is intracellular (34) and cleaves many of the same substrates as the mammalian endopeptidases, including metenkephalin (13, 33). As presented in this work, S. parasanguis PepO is also intracellular and can cleave metenkephalin. The in vivo substrate of lactococcal PepO is unknown. Originally it was hypothesized that this enzyme played a role in the catalysis of the casein-derived peptides necessary for providing L. lactis with essential amino acids (27). However, the hypothesis was not supported by studies that showed that a mutation in pepO did not alter its growth or its acid production in milk, indicating that PepO function is not essential for survival of L. lactis (28). As with PepO of L. lactis, the function of S. parasanguis PepO remains unknown, as the studies presented in this work did not support our hypothesis of a role for PepO in the processing of FimA or Fap1 on the cell surface.
Unlike their bacterial counterparts, the functions of many of the mammalian M13 category endopeptidases are known. One of these well characterized enzymes is NEP, which is associated with opioid catabolism and response to inflammatory stimuli (13, 31). Another important mammalian metalloendopeptidase is ECE-1, which is involved in regulation of vasodilation. S. parasanguis rPepO was shown here by inhibition studies to be more similar to ECE-1 than to NEP, since it was more sensitive to phosphoramidon than it was to thiorphan. It has been suggested for the same reason that P. gingivalis PepO may also be more akin to ECE-1 than to NEP (4).
Recently, many of the theories on the effect of infectious agents on the development of coronary heart disease that were originally proposed more than 6 decades ago have been revisited due to new seroepidemiological evidence (19). Further evidence that suggests a link between bacterial infection and coronary heart disease is that Chlamydia pneumoniae (35), and P. gingivalis as well as S. sanguis have been identified in atheromas of patients with coronary heart disease (10). The presence of oral streptococci in atherosclerotic plaques, as well as their possession of proteins with homology to ECE-1, leads to the speculation that these oral organisms may play a role in the development of heart disease and warrants further investigation.
ACKNOWLEDGMENTS
We thank Diane Hutchins Meyer for critical review of the manuscript. We also thank Keith Mintz and Hui Wu for their useful commentary on the manuscript.
This work was supported by Public Health Service grant R37-DE11000 from the National Institutes of Health.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: Greene Pub. Associates and Wiley-Interscience; 1988. [Google Scholar]
- 4.Awano S, Ansai T, Mochizuki H, Yu W, Tanzawa K, Turner A J, Takehara T. Sequencing, expression and biochemical characterization of the Porphyromonas gingivalis pepO gene encoding a protein homologous to human endothelin-converting enzyme. FEBS Lett. 1999;460:139–144. doi: 10.1016/s0014-5793(99)01326-5. [DOI] [PubMed] [Google Scholar]
- 5.Baddour L M. Virulence factors among gram-positive bacteria in experimental endocarditis. Infect Immun. 1994;62:2143–2148. doi: 10.1128/iai.62.6.2143-2148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck J, Garcia R, Heiss G, Vokonas P S, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 7.Benson R L, Smith K G, Semonov H. Experimental arteritis and arteriosclerosis associated with streptococcal inoculations. Arch Pathol. 1931;12:924–940. [Google Scholar]
- 8.Burnette-Curley D, Wells V, Viscount H, Munro C L, Fenno J C, Fives-Taylor P, Macrina F L. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect Immun. 1995;63:4669–4674. doi: 10.1128/iai.63.12.4669-4674.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson J, Grahnen H, Jonsson G, Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970;15:1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- 10.Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999;138:S534–S536. doi: 10.1016/s0002-8703(99)70294-2. [DOI] [PubMed] [Google Scholar]
- 11.Cole R M, Calandra G B, Huff E, Nugent K M. Attributes of potential utility in differentiating among “group H” streptococci or Streptococcus sanguis. J Dent Res. 1976;55:A142–A153. doi: 10.1177/002203457605500106011. [DOI] [PubMed] [Google Scholar]
- 12.Elder B L, Boraker D K, Fives-Taylor P M. Whole-bacterial cell enzyme-linked immunosorbent assay for Streptococcus sanguis fimbrial antigens. J Clin Microbiol. 1982;16:141–144. doi: 10.1128/jcm.16.1.141-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdos E G, Skidgel R A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989;3:145–151. [PubMed] [Google Scholar]
- 14.Fachon-Kalweit S, Elder B L, Fives-Taylor P. Antibodies that bind to fimbriae block adhesion of Streptococcus sanguis to saliva-coated hydroxyapatite. Infect Immun. 1985;48:617–624. doi: 10.1128/iai.48.3.617-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenno J C, Shaikh A, Fives-Taylor P. Characterization of allelic replacement in Streptococcus parasanguis: transformation and homologous recombination in a ‘nontransformable’ streptococcus. Gene. 1993;130:81–90. doi: 10.1016/0378-1119(93)90349-8. [DOI] [PubMed] [Google Scholar]
- 16.Fenno J C, Shaikh A, Spatafora G, Fives-Taylor P. The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol Microbiol. 1995;15:849–863. doi: 10.1111/j.1365-2958.1995.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 17.Froeliger E H, Oetjen J, Bond J P, Fives-Taylor P. Streptococcus parasanguis pepO encodes an endopeptidase with structure and activity similar to those of enzymes that modulate peptide receptor signaling in eukaryotic cells. Infect Immun. 1999;67:5206–5214. doi: 10.1128/iai.67.10.5206-5214.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graber K R, Smoot L M, Actis L A. Expression of iron binding proteins and hemin binding activity in the dental pathogen Actinobacillus actinomycetemcomitans. FEMS Microbiol Lett. 1998;163:135–142. doi: 10.1111/j.1574-6968.1998.tb13037.x. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Leatham E W, Carrington D, Mendall M A, Kaski J C, Camm A J. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation. 1997;96:404–407. doi: 10.1161/01.cir.96.2.404. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson H F, Lamont R J. Streptococcal adhesion and colonization. Crit Rev Oral Biol Med. 1997;8:175–200. doi: 10.1177/10454411970080020601. [DOI] [PubMed] [Google Scholar]
- 21.Jones N W, Rogers A L. The incidence of streptococcal infection in cardiovascular sclerosis. Ann Intern Med. 1935;8:834–853. [Google Scholar]
- 22.Kawamura Y, Hou X G, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 23.Komuro I, Kurihara H, Sugiyama T, Yoshizumi M, Takaku F, Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988;238:249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Mackenzie R S, Millard H D. Interrelated effects of diabetes, arteriosclerosis and calculus on alveolar bone loss. J Am Dent Assoc. 1963;66:192–198. [Google Scholar]
- 26.Mattila K J, Nieminen M S, Valtonen V V, Rasi V P, Kesaniemi Y A, Syrjala S L, Jungell P S, Isoluoma M, Hietaniemi K, Jokinen M J. Association between dental health and acute myocardial infarction. Br Med J. 1989;298:779–781. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mierau I, Kunji E R, Leenhouts K J, Hellendoorn M A, Haandrikman A J, Poolman B, Konings W N, Venema G, Kok J. Multiple-peptidase mutants of Lactococcus lactis are severely impaired in their ability to grow in milk. J Bacteriol. 1996;178:2794–2803. doi: 10.1128/jb.178.10.2794-2803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mierau I, Tan P S, Haandrikman A J, Mayo B, Kok J, Leenhouts K J, Konings W N, Venema G. Cloning and sequencing of the gene for a lactococcal endopeptidase, an enzyme with sequence similarity to mammalian enkephalinase. J Bacteriol. 1993;175:2087–2096. doi: 10.1128/jb.175.7.2087-2096.1993. . (Erratum, 175:3918.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mintz K P, Fives-Taylor P M. Identification of an immunoglobulin Fc receptor of Actinobacillus actinomycetemcomitans. Infect Immun. 1994;62:4500–4505. doi: 10.1128/iai.62.10.4500-4505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawlings N D, Barrett A J. Evolutionary families of metallopeptidases. Methods Enzymol. 1995;248:183–228. doi: 10.1016/0076-6879(95)48015-3. [DOI] [PubMed] [Google Scholar]
- 31.Shipp M A, Stefano G B, Switzer S N, Griffin J D, Reinherz E L. CD10 (CALLA)/neutral endopeptidase 24.11 modulates inflammatory peptide-induced changes in neutrophil morphology, migration, and adhesion proteins and is itself regulated by neutrophil activation. Blood. 1991;78:1834–1841. [PubMed] [Google Scholar]
- 32.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 33.Tan P S, Pos K M, Konings W N. Purification and characterization of an endopeptidase from Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1991;57:3593–3599. doi: 10.1128/aem.57.12.3593-3599.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan P S T, Chapot-Chartier M-P, Pos K M, Rousseau M, Boquien C-Y, Gripon J-C, Konings W N. Localization of peptidases in lactococci. Appl Environ Microbiol. 1992;58:285–290. doi: 10.1128/aem.58.1.285-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor-Robinson D, Thomas B J. Chlamydia pneumoniae in atherosclerotic tissue. J Infect Dis. 2000;181:S437–S440. doi: 10.1086/315614. [DOI] [PubMed] [Google Scholar]
- 36.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner A J, Murphy L J. Molecular pharmacology of endothelin converting enzymes. Biochem Pharmacol. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 38.Turner A J, Tanzawa K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. 1997;11:355–364. doi: 10.1096/fasebj.11.5.9141502. [DOI] [PubMed] [Google Scholar]
- 39.Whiley R A, Beighton D. Current classification of the oral streptococci. Oral Microbiol Immunol. 1998;13:195–216. doi: 10.1111/j.1399-302x.1998.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu H, Mintz K P, Ladha M, Fives-Taylor P M. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol Microbiol. 1998;28:487–500. doi: 10.1046/j.1365-2958.1998.00805.x. [DOI] [PubMed] [Google Scholar]
- 41.Wu-Wong J R, Chiou W J, Wang J. Extracellular signal-regulated kinases are involved in the antiapoptotic effect of endothelin-1. J Pharmacol Exp Ther. 2000;293:514–521. [PubMed] [Google Scholar]
- 42.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 43.Zou J, Huang Y, Chen Q, Wang N, Cao K, Hsieh T C, Wu J M. Suppression of mitogenesis and regulation of cell cycle traverse by resveratrol in cultured smooth muscle cells. Int J Oncol. 1999;15:647–651. doi: 10.3892/ijo.15.4.647. [DOI] [PubMed] [Google Scholar]