Abstract
(1) Scant information is available concerning the characteristics that may favour the acquisition of COVID-19 in patients with inflammatory bowel disease (IBD). Therefore, the aim of this study was to assess these differences between infected and noninfected patients with IBD. (2) This nationwide case–control study evaluated patients with inflammatory bowel disease with COVID-19 (cases) and without COVID-19 (controls) during the period March–July 2020 included in the ENEIDA of GETECCU. (3) A total of 496 cases and 964 controls from 73 Spanish centres were included. No differences were found in the basal characteristics between cases and controls. Cases had higher comorbidity Charlson scores (24% vs. 19%; p = 0.02) and occupational risk (28% vs. 10.5%; p < 0.0001) more frequently than did controls. Lockdown was the only protective measure against COVID-19 (50% vs. 70%; p < 0.0001). No differences were found in the use of systemic steroids, immunosuppressants or biologics between cases and controls. Cases were more often treated with 5-aminosalicylates (42% vs. 34%; p = 0.003). Having a moderate Charlson score (OR: 2.7; 95%CI: 1.3–5.9), occupational risk (OR: 2.9; 95%CI: 1.8–4.4) and the use of 5-aminosalicylates (OR: 1.7; 95%CI: 1.2–2.5) were factors for COVID-19. The strict lockdown was the only protective factor (OR: 0.1; 95%CI: 0.09–0.2). (4) Comorbidities and occupational exposure are the most relevant factors for COVID-19 in patients with IBD. The risk of COVID-19 seems not to be increased by immunosuppressants or biologics, with a potential effect of 5-aminosalicylates, which should be investigated further and interpreted with caution.
Keywords: COVID-19, SARS-CoV-2, inflammatory bowel disease, 5-aminosalicylates, immunosuppression
1. Introduction
Knowledge concerning the effect of the SARS-CoV-2 pandemic has grown exponentially. We previously published the largest cohort of patients with inflammatory bowel disease (IBD) and COVID-19 prospectively recruited with 12 months of follow-up in a national study [1]. A high percentage of patients in this cohort had occupational risk or were infected by intrafamilial transmission. We also confirmed that IBD does not worsen the COVID-19 prognosis, even with the use of immunosuppressants and biologics, as was shown elsewhere [2,3]. We demonstrated that COVID-19 affects neither the prognosis of IBD nor its treatment during the acute phase of infection or in the long term. In this sense, severe COVID-19 in patients with IBD is mainly related to older age [4,5] and comorbidities [2,6,7], as it occurs in the general population.
Investigations assessing factors that may favour the acquisition of COVID-19 in patients with IBD (case–control studies of patients with IBD with or without COVID-19) are scarce and needed [8]. Previous case–control studies either included COVID-19 patients from the general population as a control group [5], included a small proportion of COVID-19 cases or were retrospective cohorts [9,10]. The incidence of COVID-19 in patients with IBD is low [11]. This finding does not mean necessarily that patients with IBD are less susceptible to infection by SARS-CoV2, but could merely reflect special measures adopted in patients with a particular risk of infections [12]. Therefore, differences in factors that could influence the acquisition of COVID-19 must be assessed with the same type of population and under the same circumstances. This study complements the findings of our previous study [1] to define which characteristics favour or protect the occurrence of COVID-19 in IBD. Consequently, we sought to investigate, for the first time, two cohorts of patients with IBD, during the same time period and encompassing the first COVID-19 wave.
The present study aimed to assess epidemiological, demographic, and clinical factors that could influence the acquisition of COVID-19 in a large cohort of infected patients with IBD compared with noninfected patients.
2. Materials and Methods
2.1. Design
This study (COVID-19-EII study) was performed in the setting of the ENEIDA project, the Spanish registry of patients with IBD, promoted by the Spanish Working Group on Crohn’s disease and ulcerative colitis (GETECCU) [13]. ENEIDA is a prospectively maintained database that at the moment of study initiation had 60,512 patients with active follow-up (15 July 2020) in 86 hospitals. A total of 73 hospitals with 53,682 patients registered (89% of the entire database) accepted participation in this case–control study.
2.2. Study Population
Cases were all patients with COVID-19, diagnosed between March and July 2020 (during the first COVID-19 wave), who were identified by active search from their IBD unit (systematically addressing all the patients with IBD from each unit by email or phone call) or by direct notification from the emergency department, patient, family physician, or hospitalisation unit. Cases were matched with two controls (1:2) by age (±5 years), type of disease (Crohn’s disease [CD]/ulcerative colitis [UC]), sex, and centre. Both cases and controls came from the ENEIDA registry.
2.3. Definitions
A patient with IBD was considered a case if a COVID-19 diagnosis was made, based on a typical clinical picture that included fever (>38 °C), respiratory symptoms (cough and/or dyspnoea), dysgeusia or anosmia within the epidemiological setting. COVID-19 was confirmed by a positive diagnostic test including serology (IgM or IgG) or polymerase chain reaction (PCR) performed by nasopharyngeal swab for SARS-CoV-2. COVID-19 was considered probable in patients with a typical clinical picture but negative or lacking diagnostic tests.
The control group comprised patients with IBD without a COVID-19 diagnosis, coming from the same centre, during the study period (March–July 2020). The fact of not having COVID-19 was confirmed clinically by each attending physician through direct consultation with the patient, as at that time serologic or PCR tests were performed only for highly suspicious cases. Asymptomatic patients with IBD with positive PCR or serology were excluded from the study and were considered to have been infected with SARS-CoV-2 but without suffering from COVID-19.
Patients were considered to be in adequate compliance with the lockdown measures when they maintained social distance by staying almost exclusively at home since 14 March 2020, the date the Spanish government ordered a total lockdown to prevent the spread of SARS-CoV-2.
2.4. Data Collection
A prospective module hosted on the ENEIDA registry was specifically designed for this study to avoid missing cases and was externally monitored to ensure the correct acquisition of data. The data collected included clinical baseline characteristics such as date of IBD diagnosis and Montreal classification [14], type of IBD, family history of IBD, extraintestinal manifestations, and smoking behaviour at the time of infection. The following comorbidities were specifically registered, taking into account the moment of inclusion in the study as the time frame: chronic renal failure, dementia, chronic obstructive pulmonary disease, HIV, stroke, heart disease, congestive heart failure, diabetes mellitus, dyslipidaemia, arterial hypertension neoplasia, cirrhosis, rheumatological disease and immune-mediated disease, allowing the calculation of the Charlson comorbidity score [15]. These variables were collected at the time of the study (March–July 2022). Variables measuring the exposure risk to SARS-CoV-2 included occupational risk (such as health care workers, basic services such as supermarket cashiers, market clerks or pharmacy workers, teachers, workers of closed institutions, police and firepersons, animal control workers, veterinarians, or conservation and forest technicians), compliance with lockdown measures, social distancing and the route of contagion. The IBD therapeutic regimen was registered during the study period and included 5-aminosalicylates (5-ASA), systemic steroid treatment, immunosuppressants (cyclosporine, methotrexate, thiopurines, tacrolimus and tofacitinib) and biologics (anti-TNF, vedolizumab, and ustekinumab). All the controls were selected after the completeness of the inclusion period of cases (15 July 2020). They were identified by only one investigator blinded to the other characteristics to avoid selection bias.
2.5. Ethical Considerations
Written informed consent was obtained from all the subjects before inclusion in the registry. The Scientific Committee of ENEIDA approved the study on March 2020. It was also approved by the Ethics Committee of Hospital Universitari Mútua Terrassa (coordinating centre).
2.6. Statistical Analysis
Quantitative variables were correlated using the Mann–Whitney test for nonparametric data and Student’s t test for parametric data, while qualitative variables were compared using Fisher’s exact test or Chi2 test, when appropriate. Quantitative variables were compared using Student’s t test and the Mann–Whitney test, and the results were expressed as medians (± interquartile range (IQR) 25–75th percentiles).
Multivariable analysis was performed using conditional logistic regression analysis for case–control studies (COXREG in SPSS), where the presence of COVID-19 was the dependent variable. Variables with p value ≤ 0.1 on univariate analysis were introduced into the model. Because 5-ASA was more frequently used in patients with UC than in those with CD, the model was adjusted for UC diagnosis.
3. Results
Four hundred eighty-two cases with COVID-19 and nine hundred sixty-four controls without COVID-19 were included. The clinical characteristics of the patients with IBD and COVID-19 have been described previously in detail [1].
3.1. Clinical Baseline Characteristics
Table 1 shows the most important clinical characteristics of the cases and controls.
Table 1.
Clinical baseline characteristics regarding inflammatory bowel disease between cases and controls. B1: inflammatory behaviour, B2: stricturing behaviour, B3: penetrating behaviour; L1: ileal, L2: colonic, L3: ileocolonic, L4: upper gastrointestinal tract; E1: proctitis; E2: left colitis; E3: extensive colitis. IQR: interquartile rate; IBD: inflammatory bowel disease.
| Variable | Cases n = 482 |
Controls n = 964 |
Univariate p-Value |
|---|---|---|---|
| Male gender, n (%) | 251 (52) | 501 (52) | 0.97 |
| Age (years) Median (IQR) | 52 (42–61) | 53 (42–62) | 0.75 |
| Type of IBD, n (%) | 0.60 | ||
| Ulcerative colitis | 221 (46) | 471 (49) | |
| Unclassified colitis | 14 (2.9) | 1 (0.1) | |
| Crohn’s disease | 248 (51) | 492 (51) | |
| Crohn’s disease location, n (%) | |||
| L1 | 114 (46) | 232 (47) | 0.79 |
| L2 | 43 (17) | 73 (15) | 0.43 |
| L3 | 88 (36) | 186 (38) | 0.48 |
| L4 (isolated) | 3 (1.2) | 1 (0.2) | 0.08 |
| Crohn’s disease behaviour, n (%) | |||
| B1 | 144 (58) | 302 (31) | 0.75 |
| B2 | 71 (29) | 147 (15) | 0.72 |
| B3 | 47 (19) | 90 (9.3) | 0.89 |
| Perianal | 59 (24) | 164 (17) | 0.85 |
| Ulcerative colitis extent (%) | 0.97 | ||
| E1 | 43 (19) | 85 (17) | |
| E2 | 80 (36) | 183 (37) | |
| E3 | 98 (44) | 203 (41) | |
| Extraintestinal manifestation, n (%) | 125 (26) | 247 (26) | 0.98 |
| Family history of IBD, n (%) | 64 (13) | 122 (25) | 0.02 |
| Smoking behaviour, n (%) | 0.034 | ||
| Active smoker | 53 (11) | 160 (17) | |
| Ex-smoker | 137 (28) | 261 (27) | |
| Never smoker | 268 (56) | 503 (52) |
Cases less frequently had a family history of IBD [13% (64/482) vs. 25% (122/964); p = 0.02] and had a lower proportion of active smokers [11% (53/482) vs. 17% (160/964); p = 0.034] than the controls.
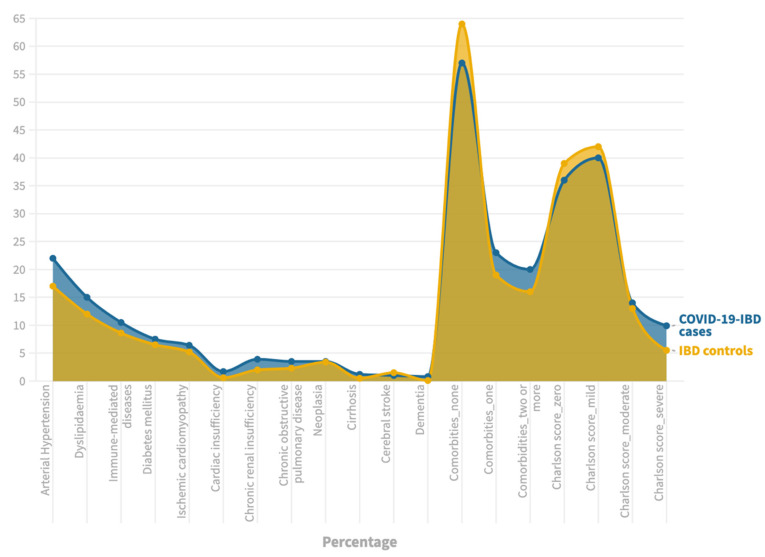
At least one comorbidity was observed in 43% (206/482) of cases and 36% (344/964) of controls (p = 0.01), with 24% (114/482) of cases and 19% (183/964) of controls having a moderate–severe Charlson score (score of three or more) (p = 0.02). The differences within specific comorbidities are shown in Figure 1 and Supplementary Table S1.
Figure 1.
Comorbidities between cases and controls.
3.2. Epidemiological Risk Factors of Exposure
Table 2 summarizes the risk for COVID-19 related to occupational and epidemiological risk factors.
Table 2.
Lockdown and epidemiological risk factors between cases and controls.
| Variable | Cases n = 482 |
Controls n = 964 |
Univariate p-Value |
|---|---|---|---|
| Occupational risk, n (%) | 133 (28) | 101 (10.5) | <0.0001 |
| Healthcare | 85 (18) | 44 (4.5) | |
| Education | 15 (3) | 22 (2.3) | |
| Basic services (market clerks, supermarket cashier, pharmacy) | 18 (3.7) | 28 (2.9) | |
| Police and fireperson | 5 (1) | 2 (0.2) | |
| Closed institutions | 2 (0.4) | 2 (0.2) | |
| Veterinary, animal control worker or conservation and forest technician | 4 (0.8) | 3 (0.3) | |
| Social distance measures since the start of the state of alarm, n (%) | 211 (44) | 574 (60) | 0.154 |
| Sick leave | 73 (15) | 82 (8.5) | <0.0001 |
| Retirement | 60 (12) | 191 (20) | 0.348 |
| Telecommuting | 44 (9.1) | 182 (19) | 0.009 |
| Unemployed | 16 (3.3) | 94 (9.8) | 0.003 |
| Others | 18 (3.7) | 25 (2.6) | 0.016 |
| Total lockdown since the start of the state of alarm, n (%) | 239 (50) | 676 (70) | <0.0001 |
| Sick leave AND total lockdown since the start of the state of alarm, n (%) | 73 (15) | 82 (8.5) | |
| Sick leave WITHOUT total lockdown since the start of the state of alarm, n (%) | 95 (19) | 11 (1) |
Almost one-third of cases (28%) and 10% of controls (p < 0.0001) were at risk because of occupational exposure. Health care professions were the most frequent occupational hazard [18% (85/482)] among cases and involved 4.5% (44/964) of controls]. One hundred thirty-three cases were considered to have an occupational risk. Among them, 96 (78%) were infected during their working activity. Most of these infections [70% (67/96)] occurred in the first month of the pandemic in Spain (March 2020).
Regarding other epidemiological risk factors for COVID-19, 44% of cases and 60% of controls (p = 0.154) declared good adherence to social distancing measures because of mandatory compliance, with no differences between groups. However, cases presented more preventive sick leave than controls [15% (73/482) vs. 8.5% (82/964); p < 0.0001], but a lower proportion of telecommuting [9.1% (44/482) vs. 19% (182/964); p = 0.009] and unemployment [3.3% (16/482) vs. 9.8% (94/964); p = 0.003]. Likewise, cases performed a worse overall total lockdown since the start of the state of alarm [50% (239/482) vs. 70% (676/964); p < 0.0001].
As stated previously, most of the cases were infected in March 2020, despite having had a higher proportion of preventive sick leave at the beginning of the pandemic [64% (47/73) in March 2020 vs. 36% (26/73) in April–July 2020; p = 0.037].
3.3. Treatment of IBD and COVID-19
Table 3 compiles the treatment received for both cases and controls for the period of time between March and July 2020.
Table 3.
Differences in treatment for inflammatory bowel disease between cases and controls.
| Variable | Cases n = 482 |
Controls n = 964 |
Univariate p-Value |
|---|---|---|---|
| 5ASA, n (%) | 204 (42) | 332 (34) | 0.003 |
| Oral (oral and topic) | 125 (26) | 180 (19) | <0.0001 |
| Topical (exclusive) | 6 (1) | 18 (1.9) | 0.051 |
| Monotherapy | 142 (29) | 214 (22) | 0.9 |
| Systemic steroids, n (%) | 26 (5.4) | 35 (3.6) | 0.06 |
| Immunosuppressants (all), n (%) | 319 (66) | 611 (63) | 0.39 |
| Immunosuppressants (in monotherapy), n (%) | 113 (23) | 191 (20) | |
| Azathioprine | 90 (19) | 160 (17) | |
| Mercaptopurine | 8 (1.7) | 7 (0.7) | |
| Cyclosporine | 1 (0.2) | 1 (0.1) | |
| Methotrexate | 9 (1.9) | 11 (1.1) | |
| Tacrolimus | 1 (0.2) | 2 (0.2) | |
| Tofacitinib | 4 (0.8) | 6 (0.6) | |
| Biologics (all), n (%) | 235 (49) | 493 (51) | 0.28 |
| Biologic (in monotherapy), n (%) | 117 (22) | 239 (25) | |
| Anti-TNF | 71 (15) | 134 (14) | |
| Vedolizumab | 25 (5.2) | 50 (5.2) | |
| Ustekinumab | 21 (4.3) | 52 (5.4) | |
| Combotherapy, n (%) | 59 (12) | 148 (15) | |
| Anti-TNF plus thiopurines | 37 (7.7) | 85 (8.8) | |
| Anti-TNF plus methotrexate | 9 (1.9) | 28 (2.9) | |
| Vedolizumab plus thiopurines | 5 (1) | 11 (1.1) | |
| Vedolizumab plus methotrexate | 1 (0.2) | 3 (0.3) | |
| Ustekinumab plus thiopurines | 5 (1) | 15 (1.6) | |
| Ustekinumab plus methotrexate | 2 (0.4) | 6 (0.6) |
No differences were found in the use of immunosuppressants and/or biologics. There was a tendency for greater use of steroids in cases than in controls [5.4% (26/482) vs. 3.6% (35/964); p = 0.06].
The use of 5-ASA was more frequent in cases [42% (204/482)] than controls [34% (332/964); p = 0.003]. However, a difference was found in the use of 5-ASA between patients with CD [14% (106/738)] vs. those with UC [61% (420/693); p < 0.001].
3.4. Risk Factors for COVID-19 in Patients with IBD
Considering the differences found in the univariate analysis (Table 1, Table 2 and Table 3, Supplementary Table S1), a multivariate model was conducted and is presented in Table 4.
Table 4.
Risk factors for symptomatic COVID-19 in inflammatory bowel disease patients. IBD: inflammatory bowel disease. * The cases and controls were paired based on age, sex, disease type and hospital of reference.
| Multivariate Analysis * | ||
|---|---|---|
| Covariates | Adjusted Hazard Ratio (95% Confidence Interval) | p-Value |
| Family history of IBD | 1.15 (0.9–1.5) | 0.291 |
| Active smoking | 0.74 (0.4–1.04) | 0.647 |
| Charlson score | ||
| Mild (one–two) | 1.2 (0.7–2.2) | 0.518 |
| Moderate (three–four) | 2.7 (1.3–5.9) | 0.011 |
| Severe (five or more) | 4.7 (1.7–12.7) | 0.002 |
| Occupational Risk | 2.8 (1.8–4.4) | <0.0001 |
| Total lockdown since the start of the state of alarm | 0.1 (0.09–0.2) | <0.0001 |
| Systemic steroids | 1.6 (0.8–3.1) | 0.203 |
| 5-aminosalycilates | 1.7 (1.2–2.5) | 0.004 |
| Ulcerative colitis | 0.6 (0.08–5.2) | 0.688 |
A moderate (OR: 2.7; 95% CI: 1.3–5.9; p = 0.011) or severe (OR: 4.7; 95% CI: 1.7–12.7; p = 0.002) Charlson score, occupational risk (OR: 2.8; 95% CI: 1.8–4.4; p < 0.0001) and the use of 5-ASA (OR: 1.7; 95% CI: 1.2–2.5; p = 0.004) were independent risk factors for symptomatic COVID-19. Strict adherence to lockdown measures was the only factor protecting patients with IBD from contracting symptomatic infection for SARS-CoV2 (OR: 0.1; 95% CI: 0.09–0.2; p < 0.001).
Because the use of 5-ASA produced conflicting results regarding its potential effect on the association with COVID-19 acquisition and severity [16,17,18,19], we also used an adjusted model considering the Charlson index, corticosteroids, immunomodulators and biologics. The adjusted OR of the use of 5-ASA between cases and controls remained significant after adjusting for these additional factors: 1.8 (95% CI: 1.3–2.2; p < 0.0001).
4. Discussion
We showed that comorbidities and occupational risk were the two most relevant factors for having COVID-19 among patients with IBD in the ENEIDA cohort [13]. Thus, comorbidity is the most relevant risk factor as it is also related to harmful events due to COVID-19 [1,2,4,5,6,7]. Currently, data on the environmental exposure of noninfected patients are limited. Health care providers bore an enormous burden during the pandemic, as revealed in the data coming from Italy and China [20,21]. Almost one-third of cases and 10% of controls (p < 0.0001) had a job position considered to pose a high risk of infection and was the main cause for improper adherence to lockdown measures. Lockdown was demonstrated as the most effective strategy precluding SARS-CoV-2 expansion, also in patients with IBD [22]. Our study found that doing a strict lockdown was the only protective factor for COVID-19 in patients with IBD, regardless of their treatment.
We also confirmed that immunosuppressive treatment does not expose the patient to a greater risk of contagion. Univariate analysis revealed a trend toward greater use of steroids in patients with IBD who were infected (p = 0.06). The use of steroids is critical for other relevant infections in patients with IBD [12]. However, this finding was not replicated in the multivariate analysis (p = 0.203). On the other hand, 5-ASA, irrespective of the type of IBD, was the only drug independently related to symptomatic COVID-19 in patients with IBD.
The use of 5-ASA in patients with IBD and COVID-19 has shown conflicting results and is a debatable issue because the findings represent the first time that a nonimmunosuppressive treatment considered “safe” regarding infection risk was found to be potentially involved in both the acquisition of and/or having severe COVID-19 [23]. The first report of a possible harmful effect of 5-ASA on the COVID-19 outcome came from the first two publications of the SECURE-IBD registry, where associations of 5-ASA with hospitalisation and other severe COVID-19 outcomes were described, even after several and restrictive statistical analyses [17,24]. Nevertheless, the latest report [18] failed to show this relationship. The authors stated that the number of reported cases in the earliest assessments was too low to fully evaluate the association of 5-ASA in COVID-19 evolution. However, further analysis using a machine learning approach showed that 5-ASA was highly associated with COVID-19/IBD mortality [25], but this finding was not confirmed by another similar predictive model [26]. Considering this information, researchers of the SECURE-IBD registry were very cautious and concluded that the association of 5-ASA and COVID-19 was likely due to reporting bias and/or reporting delays [18].
By contrast, several other studies did not find 5-ASA to be related to COVID-19 [3,27]. One of the few IBD-COVID-19 case–control studies performed using a design similar to ours demonstrated that the rate of COVID-19 was similar between patients treated under therapeutic immunosuppression or users of 5-ASA compared to those who were not [9]. A recent case–control study demonstrated that SARS-CoV-2 has no impact on IBD clinical activity in patients under biological therapy, with no difference in the incidence of SARS-CoV-2 infection between users and nonusers of 5-ASA [10]. In two other studies of the Veterans Affairs Health Care System, neither thiopurines nor anti-TNF was associated with an increased risk of COVID-19 in IBD [2], and both vedolizumab and corticosteroids were independently associated with SARS-CoV-2 infection [19]. They reported no harmful COVID-19 events with the use of 5-ASA. However, 5-ASA was used as the reference treatment and was considered the “safest” [19].
Finally, a meta-analysis of 24 studies [28] showed that the risk of hospitalisation, intensive care unit admission and mortality due to COVID-19 in patients with IBD was higher only in patients using steroids or 5-ASA. This meta-analysis speculated that the increased risk of COVID-19 infection related to 5-ASA might reflect a confounding factor due to the use of 5-ASA as a proxy for an underlying UC. In our cohort, although 5-ASA was used differentially between patients with UC or CD, the effect of 5-ASA was independently associated with COVID-19, even when considering the diagnosis of UC in the model.
However, insufficient mechanistic data exist that link the use of 5-ASA and COVID-19. In fact, 5-ASA neither alters intestinal mucosal ACE-2 expression [29] nor changes SARS-CoV-2 infectivity [30]. On the other hand, 5-ASA exerts an anti-inflammatory effect in the colon by binding the peroxisome proliferator–activated receptor gamma (PPAR-γ). This receptor also increases the expression of ACE-2 and inhibits the expression of a transmembrane serine protease (TMPRSS2) relevant for viral entry with ACE-2 internalization and could be a potential mechanism of 5-ASA favouring COVID-19 [31].
Our study has several limitations. First, we did not collect information on the disease activity of the controls at the time of inclusion in the study. Overall, the use of immunosuppressive therapy between cases and controls in our current study was similar; thus, we can assume that there were no significant differences in this respect. Second, we included only symptomatic cases infected with SARS-CoV2. At the time of the first wave, neither PCR nor serology testing was mandatory and was not performed universally, raising the possibility of someone with asymptomatic or mildly symptomatic COVID-19 disease being considered a control. Finally, we must determine whether patients with IBD have a higher risk of contagion or a poorer COVID-19 outcome than the population of reference in population-based studies.
However, our study has crucial strengths. First, the study had national coverage with active participation of almost 90% of the IBD units from the Spanish ENEIDA registry. The universal access to health care within the National Health System in Spain [32] and adherence of most centres to the nationwide certification programme in IBD [33] provided homogeneity to this cohort. Additionally, although national case–control studies series on COVID-19 and IBD have been published previously [2,7,10], our study is the largest cohort of patients with IBD both with and without COVID-19.
5. Conclusions
In conclusion, we have shown that comorbidities and epidemiological risk factors are the most relevant aspects for COVID-19 in patients with IBD. We have also demonstrated that a strict lockdown is the only protective factor against the contagion. Finally, the potential impact of 5-ASA on SARS-CoV2 acquisition and COVID-19 severity should be confirmed or rejected in large-scale studies adjusted for potential effect modifiers and confounders to provide clear therapeutic recommendations. Presently, the risk–benefit of 5-ASA does not favour its withdrawal during the pandemic. Moreover, the findings of our study and others should be a warning not to assume certain apriorisms concerning the risk or harmlessness of certain drugs. The replacement of an effective therapeutic regimen in well-controlled patients by other drugs supposed to be less dangerous or total drug withdrawal may put patients at risk of severe decompensation.
Acknowledgments
The authors would like to thank the centres that registered no cases during the study period but performed an active search of IBD patients with COVID-19: Hospital Sant Joan de Deu, Hospital Sant Jaume de Calella, Hospital de Castellón, Hospital Materno-Infantil de Málaga, Hospital de Granollers, Hospital Joan XXIII, Hospital Niño Jesús, Hospital de Cáceres, Hospital de Vic, and Complejo Hospitalario La Mancha Centro.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm11247540/s1, Table S1: Comorbidities between cases and controls.
Author Contributions
Conceptualization, Y.Z., I.M.-J., I.R.-L. and M.E.; Methodology, Y.Z., I.M.-J. and M.E.; Formal analysis, Y.Z., I.M.-J., I.R.-L. and M.E.; Investigation, Y.Z., I.R.-L., I.V., M.D.M.-A., I.G., J.P.G., F.M. (Francisco Mesonero), O.B., C.T., Á.P.-D., M.P., A.J.L., B.C., M.M., P.M.-M., M.B.-W., J.G., L.B., N.M., T.M.-P., A.L., C.R.-G., S.G.-L., P.V. (Pablo Vega), M.R., L.M., M.C. (María Calvo), M.I., M.B.d.A., B.S., J.B., J.L.P.C., D.B., I.P.-M., M.N.-L., V.H., F.A.-A., F.R.E., S.M., L.R., F.G., F.M. (Fernando Muñoz), G.S., J.O.d.Z., J.M.H., J.L., M.F.G.-S., M.S., M.D., S.E., A.F.C., E.H., L.O., E.I., A.G., P.V. (Pilar Varela), N.R., P.G., A.H.-C., A.B., D.G., E.S., D.C., M.A., J.L.C., Y.G.-L., L.J., M.C. (María Chaparro), A.L.-S.R., C.A., R.P.-S., R.M., S.T.-S., E.R., M.C. (Margalida Calafat), S.O., P.N., F.B., H.A.-G., R.P., P.O. and E.D.; Resources, Y.Z.; Data curation, Y.Z., I.M.-J., I.R.-L. and Pamela Manzano; Writing—original draft, Y.Z.; Writing—review & editing, Y.Z., I.M.-J., I.R.-L., I.V., M.D.M.-A., I.G., J.P.G., F.M. (Francisco Mesonero), O.B., C.T., Á.P.-D., M.P., A.J.L., B.C., M.M., P.M.-M., M.B.-W., J.G., L.B., N.M., T.M.-P., A.L., C.R.-G., S.G.-L., P.V. (Pablo Vega), M.R., L.M., M.C. (María Calvo), M.I., M.B.d.A., B.S., J.B., J.L.P.C., D.B., I.P.-M., M.N.-L., V.H., F.A.-A., F.R.E., S.M., L.R., F.G., F.M. (Fernando Muñoz), G.S., J.O.d.Z., J.M.H., J.L., M.F.G.-S., M.S., M.D., S.E., A.F.C., E.H., L.O., E.I., A.G., P.V. (Pilar Varela), N.R., P.G., A.H.-C., A.B., D.G., E.S., D.C., M.A., J.L.C., Y.G.-L., L.J., M.C. (María Chaparro), A.L.-S.R., C.A., R.P.-S., R.M., S.T.-S., E.R., M.C. (Margalida Calafat), S.O., P.N., F.B., H.A.-G., R.P., P.O., E.D. and M.E.; Supervision, Y.Z. and P.M.; Project administration, Y.Z. and P.M.; Funding acquisition, Y.Z. and M.E. ENEIDA Registry of GETECCU: patients’ platform and data curation. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Scientific Committee of ENEIDA on 16 March 2020. It was also approved by the Ethics Committee of Hospital Universitari Mútua Terrassa (coordinating centre).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Data.
Conflicts of Interest
Y. Zabana has received support for conference attendance, speaker fees, research support and consulting fees from AbbVie, Adacyte, Almirall, Amgen, Falk Pharma, FAES Pharma, Ferring, Janssen, Otsuka, Pfizer, Shire, Takeda, Galapagos, Boehringer Ingelheim and Tillots. I. Marín-Jiménez has received financial support for travelling and educational activities from or has served as an advisory board member to MSD, Pfizer, AbbVie, Amgen, Takeda, Janssen, Sandoz, Shire Pharmaceuticals, Faes Farma, Ferring, Falk Pharma, Tillotts Pharma, Kern Pharma, Fresenius, Chiesi, Galapagos, Gebro Pharma, and Otsuka Pharmaceutical. I. Rodríguez-Lago has received financial support for travelling and educational activities from or has served as an advisory board member to MSD, Pfizer, AbbVie, Takeda, Janssen, Tillotts Pharma, Roche, Celltrion, Ferring, Falk Pharma, Adacyte and Otsuka Pharmaceutical. MD Martín-Arranz has received fees as a speaker, consultant and advisory member for or has received funding from MSD, AbbVie, Hospira, Pfizer, Takeda, Janssen, Shire Pharmaceuticals, Tillots Pharma, and Faes Pharma. J. P Gisbert has served as speaker, consultant, and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene, Gilead/Galapagos, Ferring, Faes Farma, Shire Pharmaceuticals, Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, and Vifor Pharma. F. Mesonero has served as a speaker or has received education funding or advisory fees from MSD, Abbvie, Takeda, Janssen, Pfizer, Kern Pharma, Ferring and Falk Pharma. O. Benítez has received financial support for travelling and educational activities from Janssen, Biogen and MSD. C. Taxonera has served as a speaker, consultant, and advisory board member for or has received research funding from MSD, AbbVie, Pfizer, Takeda, Janssen, Ferring, Faes Pharma, Shire Pharmaceuticals, Falk Pharma, Galapagos, and Tillots. M. Piqueras has received financial support or has served as speaker for Takeda, Abbvie and Janssen. N. Manceñido has been a consultant or speaker for Abbvie, Janssen, Takeda, Ferring, Chiesi, Falk Pharma and Tillotts Pharma. M. Rivero has served as a speaker, consultant and advisory member for Merck Sharp and Dohme, Abbvie, Pfizer, Takeda and Janssen. M. Barreiro has served as a speaker, consultant and advisory member for or has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gillead, Celgene, Pfizer, Sandoz, Biogen, Fresenius, Ferring, Faes Farma, Falk Pharma, Chiesi, Gebro Pharma, Adacyte and Vifor Pharma. F. Argüelles has received financial support for travelling and educational activities from or has served as an advisory board member to MSD, Pfizer, Abbvie, Amgen, Takeda, Janssen, Sandoz, Shire Pharmaceuticals, Ferring and Falk Pharma. J.M. Huguet has participated in educational activities, research projects, scientific meetings or advisory boards sponsored by Merck Sharp Dohme (MSD), Ferring, Abbvie, Janssen, Biogen, Sandoz, Kern Pharma, Faes Farma, Vifor Pharma and Takeda. A. Hernández-Camba has served as speaker, or has received education funding from AbbVie, Takeda, Kern Pharma, Pfizer, Janssen, Adacyte Therapeutics and Ferring. D. Ginard has received support for conference attendance, speaker fees, research support and consulting fees from Abbvie, Adacyte, Jannsen, Takeda, Sandoz, Ferring, Pfizer and Biogen. M. Aceituno has received educational grants from Ferring, Pfizer, Janssen and AbbVie. M. Chaparro has served as a speaker and consultant and has received research or education funding from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Falk Pharma, Tillotts Pharma, Faes Pharma, Biogen and Gilead. H. Alonso-Galán has received financial support for travelling and educational activities from or has served as an advisory board member to Janssen, MSD, Abbvie, Takeda, Ferring, Pfizer and Falk Pharma. E. Domènech has served as a speaker, or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Gilead, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda, Galápagos and Tillots. M. Esteve has received support for conference attendance, speaker fees, research support and consulting fees from Abbvie, Ferring, Janssen, MSD, Otsuka, Pfizer, Takeda and Tillots. L. Bujanda has served as consultant or has received research funding from Ikan Biotech. M Bosca-Watts declares educational activities, research projects, scientific meetings and advisory boards sponsored by MSD, Ferring, Abbvie, Janssen, Biogen and Takeda. M. Navarro-Llavat has received support for conference attendance, speaker fees and research support from Abbvie, MSD, Takeda, Janssen, Ferring, Pfizer, and Biogen. I. Vera, I. Guerra, A. Ponferrada-Díaz, A. Lucendo, B. Caballol, M.Mañosa, P. Martínez-Montiel, J. Gordillo, T. Martínez-Pérez, A. López, C. Rodríguez, S. García-López, P. Vega, L. Melcarne, M. Calvo, M. Iborra, B. Sicilia, J. Barrio, JL. Pérez, D. Busquets, I. Martínez-Pérez, V. Hernández, F. Ramírez Esteso, S. Mejilde, L. Ramos, F. Gomollón, F. Muñoz, G. Suris, J. Ortiz de Zarate, J. Llaó, M. García-Sepulcre, M. Sierra, M. Durà. S. Estrecha, A. Fuentes Coronel, E. Hinojosa, L. Olivan, E. Iglesias, A. Gutiérrez, P. Varela, N. Rull, P. Gilabert, A. Brotons, E. Sesé, D. Carpio, JL Cabriada, Y. González-Lama, L. Giménez, A. López-San Román, C. Alba, R. Plaza-Santos, R. Mena, S. Tamarit-Sebastián, E. Ricart, M. Calafat, S. Olivares, P. Navarro, F. Bertoletti, R. Pajares, P. Olcina, and P. Manzano have no conflicts of interest.
Funding Statement
This study was funded by the Carlos III Health Institute (COV20/00227: Co-IP Dra. Maria Esteve and Dra. Yamile Zabana), FEDER (Fondo Europeo de Desarrollo Regional) and supported by GETECCU. The ENEIDA Registry of GETECCU is supported by Takeda, Pfizer, Galápagos, AbbVie and Biogen.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zabana Y., Marín-Jiménez I., Rodríguez-Lago I., Vera I., Martín-Arranz M.D., Guerra I., Gisbert J.P., Mesonero F., Benítez O., Taxonera C., et al. Nationwide COVID-19-EII Study: Incidence, Environmental Risk Factors and Long-Term Follow-Up of Patients with Inflammatory Bowel Disease and COVID-19 of the ENEIDA Registry. J. Clin. Med. 2022;11:421. doi: 10.3390/jcm11020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan N., Patel D., Xie D., Lewis J., Trivedi C., Yang Y.-X. Impact of Anti-Tumor Necrosis Factor and Thiopurine Medications on the Development of COVID-19 in Patients with Inflammatory Bowel Disease: A Nationwide Veterans Administration Cohort Study. Gastroenterology. 2020;159:1545–1546.e1. doi: 10.1053/j.gastro.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiot A., Rahier J.-F., Baert F., Nahon S., Hart A., Viazis N., Biancone L., Domenech E., Reenears C., Peyrin-Biroulet L., et al. The Impact of COVID-19 on Patients with IBD in a Prospective European Cohort Study. J. Crohn’s Colitis. 2022 doi: 10.1093/ecco-jcc/jjac091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aziz M., Fatima R., Haghbin H., Lee-Smith W., Nawras A. The Incidence and Outcomes of COVID-19 in IBD Patients: A Rapid Review and Meta-analysis. Inflamm. Bowel Dis. 2020;26:E132–E133. doi: 10.1093/ibd/izaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukin D.J., Kumar A., Hajifathalian K., Sharaiha R.Z., Scherl E.J., Longman R.S., Jill Roberts Center Study Group Study Group. Weill Cornell Medicine-Gastrointestinal Study Group Baseline Disease Activity and Steroid Therapy Stratify Risk of COVID-19 in Patients with Inflammatory Bowel Disease. Gastroenterology. 2020;159:1541–1544.e2. doi: 10.1053/j.gastro.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attauabi M., Poulsen A., Theede K., Pedersen N., Larsen L., Jess T., Hansen M.R., Verner-Andersen M.K., Haderslev K.V., Lødrup A.B., et al. Prevalence and Outcomes of COVID-19 Among Patients with Inflammatory Bowel Disease—A Danish Prospective Population-based Cohort Study. J. Crohn’s Colitis. 2021;15:540–550. doi: 10.1093/ecco-jcc/jjaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derikx L.A.A.P., Lantinga M.A., de Jong D.J., van Dop W.A., Creemers R.H., Römkens T.E.H., Jansen J.M., Mahmmod N., West R.L., Tan A.C.I.T.L., et al. Clinical Outcomes of COVID-19 in Patients with Inflammatory Bowel Disease: A Nationwide Cohort Study. J. Crohn’s Colitis. 2021;15:529–539. doi: 10.1093/ecco-jcc/jjaa215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izadi Z., Brenner E.J., Mahil S.K., Dand N., Yiu Z.Z.N., Yates M., Ungaro R.C., Zhang X., Agrawal M., Colombel J.-F., et al. Association Between Tumor Necrosis Factor Inhibitors and the Risk of Hospitalization or Death Among Patients with Immune-Mediated Inflammatory Disease and COVID-19. JAMA Netw. Open. 2021;4:e2129639. doi: 10.1001/jamanetworkopen.2021.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke K.E., Kochar B., Allegretti J.R., Winter R.W., Lochhead P., Khalili H., Colizzo F.P., Hamilton M.J., Chan W.W., Ananthakrishnan A.N. Immunosuppressive Therapy and Risk of COVID-19 Infection in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021;27:155–161. doi: 10.1093/ibd/izaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papa A., Scaldaferri F., Covino M., Tursi A., Furfaro F., Mocci G., Lopetuso L.R., Maconi G., Bibbò S., Fiorani M., et al. Impact of SARS-CoV-2 Infection on the Course of Inflammatory Bowel Disease in Patients Treated with Biological Therapeutic Agents: A Case-Control Study. Biomedicines. 2022;10:843. doi: 10.3390/biomedicines10040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadi Y., Dulai P.S., Kupec J., Mohy-Ud-Din N., Jairath V., Farraye F.A., Kochhar G.S. Incidence, outcomes, and impact of COVID-19 on inflammatory bowel disease: Propensity matched research network analysis. Aliment. Pharmacol. Ther. 2021;55:191–200. doi: 10.1111/apt.16730. [DOI] [PubMed] [Google Scholar]
- 12.Zabana Y., Rodríguez L., Lobatón T., Gordillo J., Montserrat A., Mena R., Beltrán B., Dotti M., Benitez O., Guardiola J., et al. Relevant Infections in Inflammatory Bowel Disease, and Their Relationship with Immunosuppressive Therapy and Their Effects on Disease Mortality. J. Crohn’s Colitis. 2019;13:828–837. doi: 10.1093/ecco-jcc/jjz013. [DOI] [PubMed] [Google Scholar]
- 13.Zabana Y., Panés J., Nos P., Gomollón F., Esteve M., García-Sánchez V., Gisbert J.P., Barreiro-De-Acosta M., Domènech E. The ENEIDA registry (Nationwide study on genetic and environmental determinants of inflammatory bowel disease) by GETECCU: Design, monitoring and functions. Gastroenterol. Hepatol. 2020;43:551–558. doi: 10.1016/j.gastrohep.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg M.S., Satsangi J., Ahmad T., Arnott I.D.R., Bernstein C.N., Brant S.R., Caprilli R., Colombel J.-F., Gasche C., Geboes K., et al. Toward an integrated clinical, molecular and serological classification of in-flammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005;19((Suppl. A)):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y.-Q., Gou R., Diao Y.-S., Yin Q.-H., Fan W.-X., Liang Y.-P., Chen Y., Wu M., Zang L., Li L., et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J. Zhejiang Univ. B. 2014;15:58–66. doi: 10.1631/jzus.B1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attauabi M., Seidelin J., Burisch J. Association between 5-aminosalicylates in patients with IBD and risk of severe COVID-19: An artefactual result of research methodology? Gut. 2021;70:2020–2022. doi: 10.1136/gutjnl-2021-324397. [DOI] [PubMed] [Google Scholar]
- 17.Ungaro R.C., Brenner E.J., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D., Ng S.C., Rahier J.-F., Reinisch W., Steinwurz F., et al. Effect of IBD medications on COVID-19 outcomes: Results from an international registry. Gut. 2021;70:725–732. doi: 10.1136/gutjnl-2020-322539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungaro R.C., Brenner E.J., Agrawal M., Zhang X., Kappelman M.D., Colombel J.-F., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D., et al. Impact of Medications on COVID-19 Outcomes in Inflammatory Bowel Disease: Analysis of More Than 6000 Patients from an International Registry. Gastroenterology. 2022;162:316–319.e5. doi: 10.1053/j.gastro.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan N., Mahmud N., Trivedi C., Reinisch W., Lewis J.D. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut. 2021;70:1657–1664. doi: 10.1136/gutjnl-2021-324356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Lancet COVID-19: Protecting health-care workers. Lancet. 2020;395:922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Qu C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Łodyga M., Maciejewska K., Eder P., Waszak K., Stawczyk-Eder K., Dobrowolska A., Kaczka A., Gąsiorowska A., Stępień-Wrochna B., Cicha M., et al. Social Distancing during COVID-19 Pandemic among Inflammatory Bowel Disease Patients. J. Clin. Med. 2021;10:3689. doi: 10.3390/jcm10163689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strangfeld A., Schäfer M., Gianfrancesco M.A., Lawson-Tovey S., Liew J.W., Ljung L., Mateus E.F., Richez C., Santos M.J., Schmajuk G., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: Results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner E.J., Ungaro R.C., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D., Ng S.C., Rahier J.-F., Reinisch W., Ruemmele F.M., et al. Corticosteroids, But Not TNF Antagonists, Are Associated with Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. 2020;159:481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S., Sheikh S.Z., Furey T.S. A machine learning approach identifies 5-ASA and ulcerative colitis as being linked with higher COVID-19 mortality in patients with IBD. Sci. Rep. 2021;11:16522. doi: 10.1038/s41598-021-95919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sperger J., Shah K.S., Lu M., Zhang X., Ungaro R.C., Brenner E.J., Agrawal M., Colombel J.-F., Kappelman M.D., Kosorok M.R. Development and validation of multivariable prediction models for adverse COVID-19 outcomes in patients with IBD. BMJ Open. 2021;11:e049740. doi: 10.1136/bmjopen-2021-049740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezzio C., Armuzzi A., Furfaro F., Ardizzone S., Milla M., Carparelli S., Orlando A., Caprioli F.A., Castiglione F., Viganò C., et al. Therapies for inflammatory bowel disease do not pose additional risks for adverse outcomes of SARS-CoV-2 infection: An IG-IBD study. Aliment. Pharmacol. Ther. 2021;54:1432–1441. doi: 10.1111/apt.16663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S., Khan A., Chowdhry M., Bilal M., Kochhar G.S., Clarke K. Risk of Severe Coronavirus Disease 2019 in Patients with Inflammatory Bowel Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:1575–1578.e4. doi: 10.1053/j.gastro.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg M., Christensen B., Lubel J.S. Gastrointestinal ACE2, COVID-19 and IBD: Opportunity in the Face of Tragedy? Gastroenterology. 2020;159:1623–1624.e3. doi: 10.1053/j.gastro.2020.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarado D.M., Son J., Thackray L.B., Castro M.F.G., Prasad S., Cui X., Sonnek N.M., Diamond M.S., Ding S., Ciorba M.A. Mesalamine Reduces Intestinal ACE2 Expression Without Modifying SARS-CoV-2 Infection or Disease Severity in Mice. Inflamm. Bowel Dis. 2022;28:318–321. doi: 10.1093/ibd/izab274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magro F., Dias C.C., Morato M. Aminosalicylates and COVID-19: Facts or Coincidences? Gastroenterology. 2021;160:1884–1885. doi: 10.1053/j.gastro.2020.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaparro M., Garre A., Ortiz A.N., Palomares M.T.D.-L., Rodríguez C., Riestra S., Vela M., Benítez J.M., Salgado E.F., Rodríguez E.S., et al. Incidence, Clinical Characteristics and Management of Inflammatory Bowel Disease in Spain: Large-Scale Epidemiological Study. J. Clin. Med. 2021;10:2885. doi: 10.3390/jcm10132885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acosta M.B., Gutiérrez A., Zabana Y., Beltrán B., Calvet X., Chaparro M., Domènech E., Esteve M., Panés J., Gisbert J.P., et al. Inflammatory bowel disease integral care units: Evaluation of a nationwide quality certification programme. The GETECCU experience. United Eur. Gastroenterol. J. 2021;9:766–772. doi: 10.1002/ueg2.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Data.