Abstract
The srtA gene product, SrtA, has been shown to be required for cell wall anchoring of protein A as well as virulence in the pathogenic bacterium Staphylococcus aureus. There are five major mechanisms for displaying proteins at the surface of gram-positive bacteria (P. Cossart and R. Jonquieres, Proc. Natl. Acad. Sci. USA 97:5013–5015, 2000). However, since many of the known surface proteins of gram-positive bacteria are believed to be exported and anchored via the sortase pathway, it was of interest to determine if srtA plays a similar role in other gram-positive bacteria. To that end, the srtA gene in the human oral commensal organism Streptococcus gordonii was insertionally inactivated. The srtA mutant S. gordonii exhibited a marked reduction in quantity of a specific anchored surface protein. Furthermore, the srtA mutant had reduced binding to immobilized human fibronectin and had a decreased ability to colonize the oral mucosa of mice. Taken together, these results suggest that the activity of SrtA plays an important role in the biology of nonpathogenic as well as pathogenic gram-positive cocci.
In order to adhere and multiply within their hosts, gram-positive bacteria such as staphylococci, streptococci, enterococci, and listeriae utilize surface proteins that bind to specific tissues and/or provide strategies for evading the innate and acquired immune response of their host. It appears that the pathway utilized for the secretion, cleavage, and anchoring of surface proteins is universal among these gram-positive cocci, although the mechanistic aspects of this process have been established primarily from studies of protein A, FnbA, and ClfA of Staphylococcus aureus (15, 16). Protein A contains a conserved 5-amino-acid sequence adjacent to a carboxyl-proximal hydrophobic region that is followed by a charged carboxyl-terminus (33). Collectively these motifs are termed the anchor region and appear to be highly conserved in all known surface proteins of gram-positive bacteria (6, 21, 25). The conserved pentapeptide motif (LPXTG, where X represents any amino acid) that had been noted by Fischetti and colleagues (6) was initially implicated in surface protein expression through treatment of Streptococcus pyogenes with muramidase and trypsin and inspection of M protein released from the bacterial surface (23, 24). The released M protein lacked the carboxyl-terminal 19 residues, which included the LPXTG sequence and the remainder of the hydrophobic tail (23, 24). This prompted the original model describing the surface protein expression pathway in gram-positive bacteria (29). A recent survey of surface proteins from staphylococci, streptococci, enterococci, lactococci, and listeriae identified over 60 proteins having the LPXTG sequence (21). Schneewind's group recently identified a cysteine protease, SrtA (also referred to as sortase) that specifically cleaves the LPXTG sequence (16, 31). A homologous gene has been identified in most gram-positive organisms. SrtA was shown to cleave the LPXTG sequence in staphylococcal protein A between the threonine and glycine residues and catalyze the transfer of the processed protein to peptidoglycan precursors (31, 32). Thus SrtA appears to be a multifunctional protease-transpeptidase. These findings correlated with earlier studies in which the structure of the cell wall anchor was described using staphylococcal protein A and a series of fusion proteins to identify both the cleavage site and the linkage site (20, 28). Specifically, protein A, and presumably other staphylococcal surface proteins, is linked to the pentaglycine moiety of the peptidoglycan (28). In line with its apparently essential role in anchoring of protein A, it has been observed that a mutation in the srtA gene of S. aureus resulted in a mutant strain defective in the anchoring of surface proteins in general (16), and the requirement for SrtA was demonstrated by challenge in a mouse model using isogenic srtA mutant and srtA+ strains (15). Mazmanian et al. (15) found that the srtA knockout strain was significantly impaired in colonization of the host and reported a 100-fold reduction in 50% lethal dose. Moreover, the srtA null strain failed to express several surface-associated adherence factors (15).
In the human oral commensal bacterium Streptococcus gordonii, several cell surface polypeptides have been identified that appear to be essential for adhesion and colonization of the oral cavity. Among these are the SspA and SspB proteins that bind salivary agglutinin glycoprotein, type I collagen, and other oral microbial flora (2, 4, 9, 10). Also facilitating colonization are the high-molecular-mass proteins CshA and CshB. These proteins mediate coaggregation between streptococci and actinomycetes and facilitate binding of streptococci to the extracellular matrix glycoprotein fibronectin (Fn) (18). Interestingly, all of these S. gordonii surface proteins contain the cell wall anchor domain LPXTG and, thus, are predicted to require proteolytic cleavage and transpeptidation by SrtA to be anchored to the cell wall (18).
It was therefore of interest to test whether the srtA gene product plays a similarly important role in the biology of S. gordonii as it appears to in S. aureus. In the experiments reported here, we have verified that S. gordonii does in fact contain an srtA gene. Furthermore, it appears that the srtA gene product is required for efficient cell wall anchoring of surface proteins and that in the absence of the srtA gene product, S. gordonii is compromised in its ability to colonize the murine oral cavity. When considered together with previous work on S. aureus, these results support the hypothesis that SrtA is likely to play an essential role in the in vivo survival of gram-positive cocci.
MATERIALS AND METHODS
Bacteriological methods.
Strains, plasmids, and primers used in this study are listed in Table 1. Escherichia coli strains were grown in Luria-Bertani broth or on Luria-Bertani medium containing 1.5% agar. S. gordonii was plated on or cultured in brain heart infusion (BHI) (Difco, Detroit, Mich.) with or without 1.5% agar, respectively. Ampicillin was added at 50 μg/ml for E. coli. Erythromycin was used at 5 μg/ml, kanamycin was used at 500 μg/ml, streptomycin was used at 500 μg/ml, and 5-fluoro-2′-deoxyuridine was used at 50 μg/ml for S. gordonii (selective additives from Sigma, St. Louis, Mo.). Frozen cells of naturally competent S. gordonii SP204(1-1) and GP1223 were prepared and transformed as previously described (22). Standard procedures were used for gene fusions and mutagenesis in E. coli plasmid vectors (14). Chromosomal DNA from S. gordonii strains was prepared as described previously (1).
TABLE 1.
Bacterial strains, plasmids and oligonucleotides
| Strain, plasmid, or oligonucleotide | Relevant marker(s) and characteristics(s) | Reference or source | |
|---|---|---|---|
| Strains | |||
| E. coli INVαF′ | F′ endA1 recA1 hsdR17(rK− mK+) supE44 thi-1 gyrA96 relA1 Φ80lacZΔM15 Δ(lacZYA-argF)U169 | Invitrogen | |
| S. gordonii | |||
| SP204(1-1) | Wild-type V288 with Smr and FudRr | 7 | |
| GP1223 | M protein recombinant strain that expresses M6 protein (S. pyogenes) residues 1 to 16 fused to residues 222 to 441 and contains an aphIII gene, Kmr | 7 | |
| SP-06 | SP204(1-1) srtA knockout mutant | This work | |
| SP-09 | GP1223 srtA knockout mutant | This work | |
| Plasmids | |||
| pCR2.1 | Kmr Ampr | Invitrogen | |
| pVA891 | Escherichia-Streptococcus shuttle vector | K. R. Jones (13) | |
| pCR2.1:B210 | 384-bp srtA B210 fragment cloned into pCR2.1 at EcoRI, Ampr | This work | |
| p891:sgsrtA | srtA B210 fragment cloned into pVA891 at BamHI/SphI, Cmr | This work | |
| Oligonucleotides | |||
| CF4 | 5′-AATAGGGCTCGAGCGGC-3′ | 30 | |
| CF5 | 5′-GGATCCTAATACGACTCACTATAGGGC-3′ | 30 | |
| CF6 | 5′-AATAGGGCTCGAGCGGC-3′ | 30 | |
| CF7 | 5′-ACCTGCCC-(c3-Icaa-CPG spacer)-3′ | 30 | |
| RSO-BamHI | 5′-NNNNNNNNNNGGATCC-3′ | This work | |
| TB206 | 5′-TGTACAAGTGACCAAAGTAAC-3′ | This work | |
| TB210 | 5′-CATAAGTATAAACCTTATTTTTATC-3′ | This work | |
| TB253 | 5′-CCCAAGCTTGTGACCAAAGTAACTAAG-3′ | This work | |
| TB256 | 5′-GCTCTAGAAATAGATTTTCATACCAG-3′ | This work | |
| TB260 | 5′-TTTCTCCTCTTGATAGAGCG-3′ | This work | |
| TB261 | 5′-CGGGATTACAGGAGCCAATG-3′ | This work | |
| TB264 | 5′-TCGGAGCACTGTCCGACCGCTTTGG-3′ | This work | |
| TB265 | 5′-AAGCCAGTATACACTCCGCTAGCGC-3′ | This work | |
| TB266 | 5′-CAGATTGTTACTTTTACAACTG-3′ | This work | |
| TB267 | 5′-CATTAAACTTATGCTTCCTTCC-3′ | This work |
Chromosomal inactivation of srtA gene.
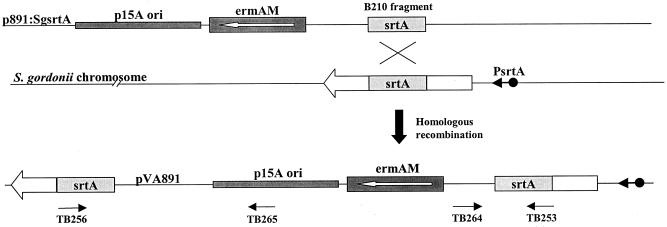
The srtA gene sequences from S. pyogenes, Streptococcus mutans, and Streptococcus pneumonia were aligned, and primers were designed based on the most highly conserved regions. Using restriction site PCR (27, 30), a primary PCR was run using the primers RSO-BamHI and TB206 and chromosomal DNA from S. gordonii SP204(1-1). The PCR conditions were 1 min at 94°C, 2 min at 50°C, and 3 min at 72°C for 30 cycles and another 10 min at 72°C after the last cycle. Then, a nested PCR was performed in the same way except that TB210 (which is internal to TB206) was used and 1 μl of the product of the first PCR was used as the template. The 384-bp amplified product (B210) was purified and cloned into pCR2.1-TOPO vector (Invitrogen, Carlsbad, Calif.) to yield the plasmid pCR2.1:B210. The putative srtA fragment was cut out of pCR2.1:B210 with the restriction enzymes BamHI and SphI and cloned into pVA891 at the respective sites, yielding p891:sgsrtA (Fig. 1). Competent cells of S. gordonii SP204(1-1) and GP1223 were transformed with p891:sgsrtA and generated erythromycin-resistant strains SP-06 and SP-09, respectively. SP-06 and SP-09 were verified by PCR analysis across the plasmid-chromosome junction with the primer pairs TB253-TB264 and TB256-TB265 and by Southern blot analysis.
FIG. 1.
Schematic representation of the plasmid p891:SgsrtA recombining into the S. gordonii chromosome within the srtA locus, resulting in strains SP-06 and SP-09. Primers TB253, TB256, TB264, and TB265 are shown in the location and orientation of their binding.
Cloning of srtA gene.
S. gordonii DNA downstream of the 384-bp B210 region within the srtA gene was cloned by chromosomal walking as described previously (31). Briefly, chromosomal DNA from SP204(1-1) was digested with EcoRV and ligated with adapter primers CF4 and CF7. This adapter-ligated DNA was used as the template for a PCR using primers CF5 and TB261. A consecutive PCR was done using the primers CF6 and TB260 and 1 μl from the first PCR as the template. CF6-TB260 produced a 500-bp product that contained sequence through the carboxy-terminal end of the srtA gene. The combined srtA sequence from the B210 region and the CF6-TB260 PCR product were used to run a BLAST search against the partial S. gordonii sequence available via file transfer protocol (FTP) at The Institute for Genomic Research anonymous FTP web link. Sequence information contained on contig 3458 and contig 1456 in the S. gordonii sequence database was 95% homologous to the srtA gene fragment used in the BLAST search. Based on sequence from the S. gordonii database, primers TB266 and TB267 were designed to amplify the entire srtA gene from S. gordonii SP204(1-1). The srtA gene was cloned into pCR2.1-TOPO vector and sequenced.
Streak blot analysis.
S. gordonii transformants were streaked on the surface of BHI plates by toothpick transfer of colonies from the selection plates. Each plate contained the transformants, an M6+ strain (GP1223) and an M6− strain (SP204 1-1) as controls. The streak blot was performed as previously described (26), using monoclonal antibody (MAb) 10F5 (12), directed against the recombinant M6 protein purified from E. coli.
Competition ELISA.
Overnight cultures of streptococci were back diluted 1:100 in BHI containing the appropriate antibiotics and grown to late log phase (optical density at 650 nm [OD650] = 0.6 to 0.7). A 50-ml culture was harvested by centrifugation (3,500 × g) for 10 min, and the cell pellets were resuspended in 25 ml of phosphate-buffered saline (PBS)-azide (PBS plus 0.02% sodium azide). The bacterial suspensions were placed in a 56°C water bath for 60 min to kill the cells. The cells were centrifuged and washed with 25 ml of PBS-azide. The cell pellets were resuspended in 10 ml of PBS-azide and the OD650 was adjusted to 1.0 with PBS-azide. Ten milliliters of the adjusted suspension was centrifuged, and 9 ml of supernatant was removed by pipette. The pellet was resuspended with the remaining supernatant. Strain preparations were stored at 4°C for up to 1 week. The resulting cell suspensions were used to compete for the binding of MAb 10F5 to recombinant M6 protein expressed in S. gordonii GP1223 in competition enzyme-linked immunosorbent assays (ELISAs), as described by Jones et al. (11, 12). Wild-type strain SP204(1-1) was used as a negative control in the competition ELISA.
Southern blot analysis.
Streptococcal chromosomal DNA was digested with the restriction enzyme PvuII, and the fragments were separated by agarose gel electrophoresis. Following electrophoresis, the DNA fragments were transferred to a Zeta-Probe GT genomic blotting membrane (Bio-Rad, Hercules, Calif.) by capillary transfer. Probes were labeled with [α-32P]dCTP (Amersham Pharmacia, Piscataway, N.J.) using a Rediprime II random prime labeling system (Amersham Pharmacia) as instructed by the manufacturer. The srtA-specific probe consisted of nucleotides 157 to 508 of the srtA gene and was isolated by PCR using primers TB253 and TB256. The ermAM-specific probe was prepared by digesting pVA891 with ClaI and HindIII. The approximately 1.1-kb ermAM fragment was purified following agarose electrophoresis using a Qiaex purification kit (Qiagen, Valencia, Calif.). Hybridization was done according to the manufacturer's instructions. Blots were exposed to X-Omat AR film (Kodak, Rochester, N.Y.) at −70°C for 1 h and developed in a HOPE Micro-Max developer (HOPE X-ray Products, Inc., Warminster, Pa.).
FITC labeling of S. gordonii cells.
The method of fluorescein isothiocyanate (FITC) labeling was adapted from a method described by Falk et al. (5). Wild-type S. gordonii and srtA knockout strains were grown overnight in BHI broth plus appropriate antibiotics. The cells were harvested from the overnight cultures by centrifugation and rinsed two times in 0.15 M NaCl–0.1 M Na+-carbonate buffer, pH 9.0. The cell pellets were resuspended in 10 ml of the same buffer and the OD650 was adjusted to 1.0. A fresh 10-mg/ml FITC stock was made in dimethyl sulfoxide and added to the cells at a 1:100 dilution. The mixture was incubated for 1 h on a rocker in the dark at room temperature. The cells were then washed three times with 1× PBS–0.5% Tween 20 (PBS-T) by centrifugation and resuspension. After the final wash the cells were resuspended in PBS-T and the OD650 was adjusted to 2.0 using PBS-T.
Adherence of streptococci to immobilized Fn.
One hundred microliters of the FITC-labeled S. gordonii cells was loaded onto the first row of a prewashed (PBS-T) Biocoat plate (Becton Dickinson, Franklin Lakes, N.J.) that was coated with human Fn. The cells were serially diluted across the plate, and the plate was incubated for 3 h at 37°C. The plate was washed three times with PBS-T and read on a Spectraflour Plus reader (Tecan U.S., Inc., Research Triangle Park, N.C.). Experiments were repeated at least two times, with each cell dilution done in duplicate.
Colonization studies.
Strains SP204(1-1) and SP-06 were grown to the late exponential phase of growth (OD650) in 50 ml of BHI broth containing appropriate additives. Cultures were placed on ice to stop growth, and 45 ml of each culture was centrifuged at 2,000 × g for 15 min at 4°C. The resulting cell pellet was resuspended in 1.2 ml of BHI, and 50 μl of each strain (25 μl intranasally and 25 μl orally; approximately 109 CFU) was delivered to each of 20 lightly anesthetized (isoflurane) Swiss/CD1 mice (Charles River, Wilmington, Mass.). The pharnyx, teeth, and gingiva of each mouse was swabbed at weekly intervals, and swabs were plated on 5% sheep blood agar containing streptomycin (1 mg/ml), 5-fluoro-2′-deoxyuridine (50 μg/ml), and amphotericin B (2.5 μg/ml). Plates were incubated at 37°C for 2 days prior to CFU determination. A statistical comparison of the colonization levels of the two groups was performed using the Mann-Whitney rank-sum test.
Nucleotide sequence accession number.
The srtA gene was cloned into pCR2.1-TOPO vector and sequenced. This previously unpublished sequence of the srtA gene from S. gordonii has been submitted to GenBank (accession no. AF213261).
RESULTS
Identification of the srtA gene in S. gordonii.
The srtA gene has been identified in S. aureus (16, 31), and database searches have revealed that srtA homologues appear to be present in other gram-positive organisms as well (16). Therefore, to determine if a srtA gene was present in S. gordonii and to obtain the gene for use in allelic exchange experiments, the srtA gene sequences from S. pyogenes, S. mutans, and S. pneumoniae were aligned, and primers TB206 and TB210 were designed to the most highly conserved regions within the srtA gene. These primers were used to run restriction site PCRs with the primer RSO-BamHI and produced a 384-bp PCR product (B210) from the S. gordonii genomic DNA (Fig. 1). Sequencing of this fragment indicated the encoded protein was homologous to the srtA gene product from S. aureus and the other gram-positive srtA enzymes (data not shown). A chromosomal walk downstream of the B210 fragment produced sequence through the 3′ end of the srtA open reading frame. Partial sequence of the S. gordonii genome is available via FTP from The Institute for Genomic Research's anonymous FTP web site. The available sequence was downloaded along with a BLAST search program. The combined srtA sequence fragments from the chromosomal walks were used to run a BLAST search against the S. gordonii sequence database and produced two overlapping sequences that appeared to contain the 5′ end of the srtA gene. Primers TB266 and TB267 were designed in order to amplify the entire srtA gene from SP204(1-1). Analysis of the srtA open reading frame revealed a 252-amino-acid protein with an N-terminal signal sequence, which is similar to the findings of Mazmanian et al. (16). The predicted amino acid sequence suggested that SrtA is a cytoplasmic membrane protein, and alignment with the S. aureus SrtA gene product indicated the cysteine at position 210 within the protein as most likely to be involved in the active site of the enzyme (16). A ClustalW protein alignment using the BCM search launcher (Baylor College of Medicine) comparing the S. gordonii and S. aureus SrtA proteins showed that the enzymes share 25% identity and 38% similarity with the strongest conservation of sequence surrounding the active site cysteine residue. The one striking difference between the two proteins is the presence of a 12-amino-acid extension at the carboxy-terminus of the S. gordonii protein. This sequence extension is not as long or as strongly hydrophobic as that seen with other SrtA homologues such as the recently identified homologues from S. pyogenes, Enterococcus faecalis and S. pneumoniae (data not shown).
Chromosomal inactivation of the srtA gene.
To insertionally inactivate the srtA gene in the two S. gordonii strains SP204(1-1) and GP1223, an internal portion of the srtA gene (B210) was cloned into pVA891. This vector is a deletion derivative of the Escherichia-Streptococcus shuttle vector plasmid pVA838 (13) which has lost the capacity to replicate in streptococci but retains a streptococcal gene encoding erythromycin resistance. The 384-bp B210 srtA fragment corresponding to nucleotides 155 to 538 of the srtA coding sequence was excised from pCR2.1:B210 with BamHI and SphI and cloned into similarly digested pVA891. The new plasmid, p891:sgsrtA, was transformed into S. gordonii SP204(1-1) and GP1223, generating srtA mutant strains SP-06 and SP-09, respectively. Chromosomal DNA from each of the transformants was subjected to PCR analysis using the primer pairs TB253-TB264 and TB256-TB265, producing a 3.5-kb band and a 0.5-kb band, respectively (data not shown), as expected for insertion of the plasmid within the srtA gene in the chromosome (Fig. 1). Southern hybridization analysis of the same chromosomal DNA confirmed interruption of the srtA coding region with the pVA891 sequences (data not shown).
Surface protein expression.
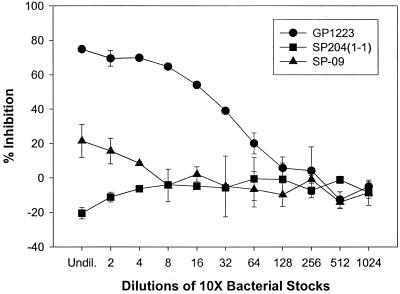
Previous work done by Schneewind and coworkers (16, 31) has shown that the SrtA enzyme appears to be required for surface protein anchoring in gram-positive bacteria. Thus, it was of interest to determine whether the srtA mutants were defective in anchoring surface proteins. We focused on the recombinant M protein-expressing strain GP1223 and the isogenic srtA knockout of GP1223, SP-09, since a MAb to the M6 protein was available for immuno-analysis. These strains have the gene that encodes a fragment of the M protein from serotype 6 (M6) S. pyogenes inserted into the chromosome behind a native promoter (7). The M6 protein contains the LPXTG motif for anchoring the protein to the surface. Streak blot analysis showed that the srtA mutant SP-09 had no apparent M protein anchored to the surface (data not shown) as compared to GP1223. Competition ELISA was used to provide a quantitative comparison of the relative amount of M protein anchored on the surface of these two strains. At the highest concentration of cells the srtA knockout SP-09 showed an approximately 73% reduction in anchored M protein versus the native srtA+ strain GP1223 (Fig. 2).
FIG. 2.
Competition ELISA with S. gordonii recombinant strains expressing surface-anchored M protein versus purified M6 protein. The graph shows percent inhibition of binding of MAb 10F5 to E. coli M6 protein by decreasing concentrations of cells. The strains used are the recombinant M protein-expressing strain GP1223, its isogenic srtA knockout SP-09, and wild-type SP204(1-1), which was used as a negative control. Undil., undiluted. Error bars, standard errors.
Role of SrtA in adherence of streptococcal cells to fibronectin.
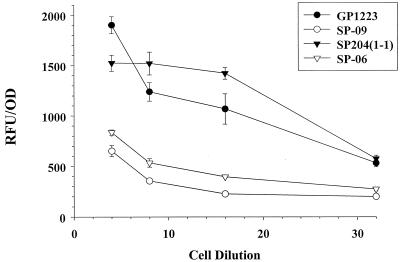
It has been established by McNab and coworkers that S. gordonii binds to immobilized human Fn (17, 18). The cell surface polypeptide CshA mediates the binding of S. gordonii to the extracellular matrix glycoprotein, fibronectin, as well as to other oral bacteria such as Actinomyces naeslundii (17, 18). CshA has a leader peptide directing the export of CshA, amino acid repeat blocks, α-helical structure, and a cell wall anchor LPXTG domain (17). To determine if Fn binding was affected in the srtA mutant strains, we designed a simple Fn binding assay to compare the binding properties of the srtA knockout strains to those of the wild-type strains. FITC-labeled S. gordonii cells were incubated in a 96-well plate that had been precoated with immobilized Fn. The plate was then washed, and the fluorescence of the bound bacteria was read (5). The S. gordonii SrtA-expressing strains SP204(1-1) and GP1223 bound in significantly greater numbers to the Fn than did the isogenic srtA knockout mutant strains SP-06 and SP-09 (P ≤ 0.01 [Student's t test]) (Fig. 3).
FIG. 3.
Fn binding assay shown as relative fluorescence units (RFU) of bound FITC-labeled bacteria versus cell dilution. Adherence assays were carried out in 96-well plates coated with purified Fn. The strains used are as indicated in the inserted legend. Both knockout mutants, SP-06 and SP-09, had significantly reduced binding to fibronectin. Each point represents a mean ± 1 standard error (error bars) (P = 0.01).
Effect of srtA mutation on oral colonization.
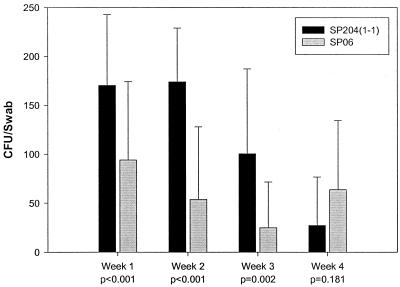
Cell surface-associated polypeptides CshA (described above) and CshB have been shown to be essential for oral streptococci to colonize the murine oral cavity (17, 19). To test the effect of the srtA mutation in SP-06 in this regard, mice were inoculated intranasally and orally with the strains SP204(1-1) and SP-06, and the colonization levels were monitored over 4 weeks. SP-06 showed significantly reduced colonization levels compared to SP204(1-1) during the first 3 weeks (P ≤ 0.002 [Mann-Whitney rank-sum test]) (Fig. 4). Colonization levels of SP204(1-1) dropped off to background levels equivalent with those of SP-06 in week 4 (Fig. 4). The mice were not monitored beyond 4 weeks.
FIG. 4.
Colonization of mice with S. gordonii strains. Groups of 20 mice were inoculated orally and intranasally with S. gordonii srtA+ and srtA mutant strains. Colonization was monitored weekly by swabing the oral cavity of the mice and plating on blood agar plates with appropriate additives. CFUs were determined after incubating the plates for 2 days. The strains used are as indicated in the inserted legend. Error bars, standard deviations.
DISCUSSION
Cell wall-associated proteins of gram-positive bacteria are currently the focus of research attention because of their central role in adhesion (2, 19), invasion, inhibition of phagocytosis, and evasion of host immune defense mechanisms (9). Since most of these surface proteins are thought to utilize the same SrtA-mediated cell wall anchoring mechanism, this pathway provides a potential target for the development of compounds that will inhibit SrtA from sorting and anchoring proteins to the surface of gram-positive bacteria, thereby reducing their virulence and pathogenicity.
Through the results reported here, we have demonstrated the presence of the srtA gene, encoding the SrtA protein, in S. gordonii. SrtA activity was not essential for growth of S. gordonii in culture but was required for the bacterium to exhibit its full adhesive and colonization potential. Schneewind and coworkers discovered the srtA gene in S. aureus and showed that it is responsible for the anchoring of surface proteins to the peptidoglycan of gram-positive bacteria via a transpeptidation process (16). Without the SrtA protein the surface polypeptides are missorted, leaving the bacteria essentially devoid of any surface-anchored proteins. We have extended these observations to a nonpathogenic oral commensal bacterium, S. gordonii, and have shown that isogenic mutants which do not express the SrtA protein show a marked reduction in their ability to express and anchor surface proteins containing the canonical LPXTG motif. Furthermore, these srtA mutants are deficient in adherence to human extracellular matrix proteins (Fn) and are inefficient in colonizing the murine oral cavity. Evidence obtained in this study suggests that SrtA does play a major role in the anchoring of proteins on the surface of bacteria and consequently affects the adherence properties of S. gordonii.
From this study and the previous work of Mazmanian et al. (16) it appears likely that SrtA will play an important role in the in vivo survival of both pathogenic and nonpathogenic gram-positive bacteria. It is clear, for example, that adherence of other gram-positive bacteria, such as the pathogen S. pyogenes, involves expression of multiple surface adhesins containing the LPXTG motif (8). Being able to target the functional expression of these adhesins with an inhibitor should prevent colonization, infection, and spread of disease. Since SrtA is a new and novel anti-infective target, compounds that inhibit SrtA may be useful in fighting human infections caused by gram-positive bacteria that have gained resistance to other antibiotics (31).
ACKNOWLEDGMENTS
We thank Olaf Schneewind and Steven Projan for useful discussion and insightful comments during the course of these experiments.
REFERENCES
- 1.Bollet C, Gevaudan M J, de Lamballerie X, Zandotti C, de Micco P. A simple method for the isolation of chromosomal DNA from gram positive or acid-fast bacteria. Nucleic Acids Res. 1991;19:1955. doi: 10.1093/nar/19.8.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks W, Demuth D R, Gil S, Lamont R J. Identification of a Streptococcus gordonii SspB domain that mediates adhesion to Porphyromonas gingivalis. Infect Immun. 1997;65:3753–3758. doi: 10.1128/iai.65.9.3753-3758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cossart P, Jonquieres R. Sortase, a universal target for therapeutic agents against gram-positive bacteria? Proc Natl Acad Sci USA. 2000;97:5013–5015. doi: 10.1073/pnas.97.10.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demuth D R, Duan Y, Brooks W, Holmes A R, McNab R, Jenkinson H F. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol Microbiol. 1996;20:403–413. doi: 10.1111/j.1365-2958.1996.tb02627.x. [DOI] [PubMed] [Google Scholar]
- 5.Falk P, Roth K A, Boren T, Westblom T U, Gordon J I, Normark S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc Natl Acad Sci USA. 1993;90:2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 7.Franke, C. A., T. C. Bolken, and D. E. Hruby. Genomic organization of recombinant Streptococcus gordonii strain leads to the identification of new intergenic integration site for foreign gene expression. Mol. Microbiol. Biotechnol., in press. [PubMed]
- 8.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes A R, Gilbert C, Wells J M, Jenkinson H F. Binding properties of Streptococcus gordonii SspA and SspB (antigen I/II family) polypeptides expressed on the cell surface of Lactococcus lactis MG1363. Infect Immun. 1998;66:4633–4639. doi: 10.1128/iai.66.10.4633-4639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenkinson H F, Terry S D, McNab R, Tannock G W. Inactivation of the gene encoding surface protein SspA in Streptococcus gordonii DL1 affects cell interactions with human salivary agglutinin and oral actinomyces. Infect Immun. 1993;61:3199–3208. doi: 10.1128/iai.61.8.3199-3208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones K F, Hollingshead S K, Scott J R, Fischetti V A. Spontaneous M6 protein size mutants of group A streptococci display variation in antigenic and opsonogenic epitopes. Proc Natl Acad Sci USA. 1988;85:8271–8275. doi: 10.1073/pnas.85.21.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones K F, Khan S A, Erickson B W, Hollingshead S K, Scott J R, Fischetti V A. Immunochemical localization and amino acid sequences of crossreactive epitopes within the group A streptococcal M6 protein. J Exp Med. 1986;164:1226–1238. doi: 10.1084/jem.164.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macrina F L, Evans R P, Tobian J A, Hartley D L, Clewell D B, Jones K R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983;25:145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- 14.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 15.Mazmanian S K, Liu G, Jensen E R, Lenoy E, Schneewind O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci USA. 2000;97:5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazmanian S K, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 17.McNab R, Forbes H, Handley P S, Loach D M, Tannock G W, Jenkinson H F. Cell wall-anchored CshA polypeptide (259 kilodaltons) in Streptococcus gordonii forms surface fibrils that confer hydrophobic and adhesive properties. J Bacteriol. 1999;181:3087–3095. doi: 10.1128/jb.181.10.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNab R, Holmes A R, Clarke J M, Tannock G W, Jenkinson H F. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect Immun. 1996;64:4204–4210. doi: 10.1128/iai.64.10.4204-4210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNab R, Jenkinson H F, Loach D M, Tannock G W. Cell-surface-associated polypeptides CshA and CshB of high molecular mass are colonization determinants in the oral bacterium Streptococcus gordonii. Mol Microbiol. 1994;14:743–754. doi: 10.1111/j.1365-2958.1994.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 20.Navarre W W, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 21.Navarre W W, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oggioni M R, Pozzi G. A host-vector system for heterologous gene expression in Streptococcus gordonii. Gene. 1996;169:85–90. doi: 10.1016/0378-1119(95)00775-x. [DOI] [PubMed] [Google Scholar]
- 23.Pancholi V, Fischetti V A. Identification of an endogenous membrane anchor-cleaving enzyme for group A streptococcal M protein. Its implication for the attachment of surface proteins in gram-positive bacteria. J Exp Med. 1989;170:2119–2133. doi: 10.1084/jem.170.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pancholi V, Fischetti V A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988;170:2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pozzi G, Contorni M, Oggioni M R, Manganelli R, Tommasino M, Cavalieri F, Fischetti V A. Delivery and expression of a heterologous antigen on the surface of streptococci. Infect Immun. 1992;60:1902–1907. doi: 10.1128/iai.60.5.1902-1907.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pozzi G, Musmanno R A, Lievens P M, Oggioni M R, Plevani P, Manganelli R. Method and parameters for genetic transformation of Streptococcus sanguis Challis. Res Microbiol. 1990;141:659–670. doi: 10.1016/0923-2508(90)90060-4. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar G, Turner R T, Bolander M E. Restriction-site PCR: a direct method of unknown sequence retrieval adjacent to a known locus by using universal primers. PCR Methods Appl. 1993;2:318–322. doi: 10.1101/gr.2.4.318. [DOI] [PubMed] [Google Scholar]
- 28.Schneewind O, Fowler A, Faull K F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 29.Schneewind O, Model P, Fischetti V A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 30.Siebert P D, Chenchik A, Kellogg D E, Lukyanov K A, Lukyanov S A. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 1995;23:1087–1088. doi: 10.1093/nar/23.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ton-That H, Liu G, Mazmanian S K, Faull K F, Schneewind O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc Natl Acad Sci USA. 1999;96:12424–12429. doi: 10.1073/pnas.96.22.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ton-That H, Mazmanian S K, Faull K F, Schneewind O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Sortase catalyzed in vitro transpeptidation reaction using LPXTG peptide and NH(2)-Gly(3) substrates. J Biol Chem. 2000;275:9876–9881. doi: 10.1074/jbc.275.13.9876. [DOI] [PubMed] [Google Scholar]
- 33.Ton-That H, Schneewind O. Anchor structure of staphylococcal surface proteins. IV. Inhibitors of the cell wall sorting reaction. J Biol Chem. 1999;274:24316–24320. doi: 10.1074/jbc.274.34.24316. [DOI] [PubMed] [Google Scholar]