Abstract
Colonization of the gastric mucosa with Helicobacter pylori is associated with a dense infiltration of granulocytes into the lamina propria in the active phase of gastritis. In this study, we investigated the involvement of epithelial cell-derived neutrophil-activating protein 78 (ENA-78) in development of H. pylori-associated gastritis. Antral biopsies from 27 patients with H. pylori-associated gastritis and 25 from H. pylori-negative individuals were first analyzed for ENA-78 and interleukin-8 (IL-8) mRNA by semiquantitative reverse transcription (RT)-PCR. In H. pylori-positive patients, significantly elevated levels were found for both chemokines (P < 0.05). Only IL-8 mRNA levels differed significantly (P < 0.05) in H. pylori-infected individuals who had serum antibodies for cytotoxin-associated protein CagA versus H. pylori-infected CagA-negative persons. Quantification of ENA-78 transcript levels by competitive RT-PCR yielded a significant 45-fold upregulation for ENA-78 transcripts in biopsies of H. pylori-positive versus H. pylori-negative patients (P < 0.05). In contrast to earlier findings with IL-8, the degree of ENA-78 mRNA upregulation was independent of the grade of activity of gastritis. Immunofluorescence studies on tissues of antral biopsies localized ENA-78 protein expression mainly to the gastric epithelium of H. pylori-positive patients, while control tissues were negative. Upregulation of ENA-78 and IL-8 mRNA and protein expression was also observed in an in vitro system using a gastric adenocarcinoma cell line. Only viable H. pylori yielded a strong ENA-78 and IL-8 induction, while H. pylori outer membrane proteins or water-soluble proteins had no significant effect. These data provide evidence for the importance of both IL-8 and ENA-78 in the development and perpetuation of H. pylori-associated gastritis.
Colonization of the gastric mucosa with the gram-negative bacterium Helicobacter pylori leads to the development of chronic type B gastritis. H. pylori infection and subsequent inflammation may also result in more severe diseases such as duodenal and gastric ulcer, B-cell lymphoma of mucosa-associated lymphoid tissue, and Ménétrièr's disease (22, 37). In addition, H. pylori infection has been associated with an increased risk of gastric carcinoma (23, 26). Active H. pylori-associated gastritis is characterized by a dense infiltration of granulocytes and, during the chronic phase, by an infiltration of plasma cells and lymphocytes into the lamina propria (15). Since H. pylori is a noninvasive bacterium, a signal transduction pathway must exist to induce the expression of adhesion molecules on vessel endothelium and thus enable immigration and recruitment of inflammatory cells to the lamina propria. Soluble bacterial products or direct adherence of the bacterium to the epithelium may initiate the signaling cascade (12, 21, 28, 41). Subsequently, mediators secreted by stimulated epithelial cells or professional antigen-presenting cells may be instrumental for the induction of leukocyte emigration.
Recent data provide evidence for the important role of chemokines in mediating leukocyte chemotaxis in vitro and in vivo (2). The fact that granulocytes are major players during the acute phase of H. pylori-associated gastritis predicts the involvement of members of the CXC chemokines, at least during the onset of the disease. Potent neutrophil chemoattractants of this chemokine family, interleukin-8 (IL-8) and epithelial cell-derived neutrophil-activating protein 78 (ENA-78), have been detected as abundant chemotaxins present in certain disease states such as rheumatoid arthritis, chronic pancreatitis, and inflammatory bowel diseases (19, 35, 42). IL-8 expression has also been detected in epithelial surface cells of antral biopsy samples (7) and in supernatants of in vitro-cultivated antral biopsy samples of patients with active H. pylori gastritis (3). Subsequently, it was demonstrated that H. pylori infection upregulates IL-8 mRNA and protein expression in gastric epithelial cell types in vitro and in vivo (10, 13, 16, 31, 39). Little is known about the role of ENA-78 in perpetuating acute and chronic gastritis.
ENA-78 was first isolated from supernatants of stimulated human alveolar type II-like epithelial cells (cell line A549) (36). Significant tissue expression of ENA-78 mRNA has been observed in the intestinal epithelium of patients with inflammatory bowel diseases, such as ulcerative colitis, Crohn's disease, and appendicitis, and in renal tissue of acutely rejected allografts (29, 42). While being as potent as IL-8 in inducing neutrophil responses, ENA-78 tissue expression often does not coincide with the expression of IL-8. This has been observed in Crohn's disease and ulcerative colitis (42). In these diseases, expression of ENA-78 was observed in more than 90% of the preserved epithelial cells, whereas in control tissues ENA-78 was detectable in no more than 30% of the epithelial cells. Thus, ENA-78 may significantly contribute to the activation and recruitment of neutrophils to the inflamed intestinal lamina propria. Recently, ENA-78 mRNA has been observed in tissue samples of H. pylori-associated gastritis (32), and in vitro analysis revealed that purified H. pylori water extract and H. pylori lipopolysaccharide (LPS) stimulated human monocytes to release ENA-78 and IL-8 (8).
The purpose of this study was to quantitate ENA-78 mRNA molecules by competitive reverse transcription-PCR (RT-PCR) and to investigate protein expression of ENA-78 in H. pylori-associated gastritis. These results were then correlated with findings for the formerly investigated neutrophil-attracting chemokine IL-8. Potential differences in the induction pattern for ENA-78 and IL-8 by H. pylori were then studied in an in vitro system using a gastric adenocarcinoma cell line (AGS). The results demonstrate that ENA-78 is an abundant chemokine present in lesions of H. pylori-associated gastritis and thus may contribute significantly to neutrophil infiltration into the gastric lamina propria following H. pylori infection.
MATERIALS AND METHODS
Patients and tissue sampling.
Biopsy samples from 52 patients with dyspepsia were obtained from defined adjacent locations in the antral mucosa during upper gastrointestinal endoscopy. None of the patients had received antibiotics, bismuth compounds, proton pump inhibitors, steroids, or nonsteroidal anti-inflammatory drugs 4 weeks before the examination. Patients with evidence of malignancy, immunosuppression, metabolic disorders, or gastrointestinal hemorrhage and patients with a history of surgery were excluded. Table 1 summarizes patients and grades of gastritis characterized by an updated Sydney system (11). Specimens for histological examination were placed in 3.7% (vol/vol) neutral formalin, and those for immunohistochemistry and subsequent RNA isolation were immediately frozen in liquid nitrogen and stored at −70°C. Heparinized venous blood samples were used for serological testing. Sera were stored at −20°C until analysis.
TABLE 1.
Patient groups and grades of antral gastritis
| Group | Patients (n = 52)
|
Histological gradea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. (male, female) | Mean age (yr) (range) | Active inflammation
|
Chronic inflammation
|
|||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |||
| H. pylori-negative controls | 25 (15, 10) | 51.1 (26–76) | 24 | 1 | 16 | 9 | ||||
| Patients with H. pylori-associated gastritis | 27 (11, 16) | 53.5 (23–70) | 1 | 7 | 16 | 3 | 3 | 13 | 11 | |
0, none; 1, mild; 2, intermediate; 3, high.
Determination of H. pylori status.
The H. pylori status of the patients was determined by enzyme-linked immunosorbent assay as previously described (28). Allocation to H. pylori-positive (n = 27) and negative (n = 25) patient categories was based on the presence or absence of immunoglobulin G (IgG) serum antibodies against H. pylori. The presence and density of H. pylori colonization, as well as the classification of gastritis, were determined histologically as described elsewhere (14). Briefly, the grade of activity of gastritis depended on the neutrophil score, whereas the grade of chronicity of gastritis depended on the mononuclear infiltrate (lymphocytes and plasma cells). All examinations were done in a blinded fashion without knowledge of clinical or endoscopic findings.
Bacteria and cell lines.
The reference strain H. pylori NCTC 11637 was grown under microaerophilic conditions (atmosphere of 9% CO2–11% O2–80% N2) at 37°C on Wilkins-Chalgren anaerobe agar containing 5% horse blood (WCB medium) and DENT-Helicobacter pylori selective supplement (Unipath, Wesel, Germany). After 2 days of incubation, bacterial cells were scraped from agar plates, resuspended in phosphate-buffered saline (PBS), and diluted to a density of 5 × 108 to 1 × 109 cells/ml using the McFarland scale. The exact concentration of the bacterial suspension was determined by viable counting on spread plates of WCB medium after 5 days of incubation. The motility of H. pylori in cultures was confirmed by phase-contrast microscopy before experimental use. The human gastric adenocarcinoma cell line AGS (ATCC CRL 1739) was obtained from the American Type Culture Collection (Manassas, Va.). Cells were grown in RPMI medium (Seromed, Biochrom KG, Germany) supplemented with 2 mM l-glutamine and 5% (vol/vol) fetal calf serum, at 37°C in a water-saturated atmosphere of 95% air–5% CO2. Water-soluble protein (WSP) from H. pylori strain NCTC 11637 was prepared as described elsewhere (28). WSP was used at a concentration of 25 μg/ml, equivalent to a protein concentration of about 106 to 107 bacteria/ml. The outer membrane proteins (OMP) of H. pylori NCTC 11637 were isolated by a modification of the sarcosine protocol as recently described (38) and used at a concentration of 10 μg/ml.
In vitro cytokine stimulation assay.
After trypsinization, AGS cells were resuspended in supplemented RPMI medium at a concentration of 106 cells/ml, seeded into tissue culture petri dishes (2 ml per 35-mm-diameter dish) (Greiner, Solingen, Germany), and incubated for 2 h to allow cell adherence. Monolayers of AGS cells were then stimulated at 37°C and 5% CO2 with bacterial products, viable H. pylori (final concentration of 1 108 CFU/ml), or 2 ml of supplemented RPMI medium (control). Samples were taken after defined time intervals or after 24 h, centrifuged, and stored at −70°C until the assays were carried out. The cell monolayers were harvested by adding 350 μl of lysis buffer (Qiagen, Hilden, Germany), and the suspensions were stored at −70°C until used for RNA isolation. Adherence and confluence of AGS cells were estimated microscopically before sampling. The viable count of H. pylori after 24 h was determined as CFU in WCB medium after a 5-day incubation at 37°C under microaerophilic conditions. Stimulation experiments were carried out in eight-well Permanox chamber slides (Nunc, Naperville, Ill.). AGS cells (400 μl) were added to each chamber, stimulated with viable H. pylori, and after various time intervals acetone fixed and air dried.
RNA extraction and cDNA preparation.
Biopsy specimens were homogenized with an OMNI 2000 homogenizer (Süd-Laborbedarf, Gauting, Germany) in 600 μl of lysis buffer, and total RNA was extracted from the supernatants using RNeasy spin columns (Qiagen). Total RNA was quantitated by measuring the optical density at 260 nm and by gel electrophoresis. cDNA was prepared from 2 μg of total RNA as described previously (28).
PCR.
Oligonucleotide primers (MWG-Biotech, Ebersberg, Germany) were designed such that the expected products were obtained only from cDNA and not from genomic DNA (Table 2). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts were used as internal control for each cDNA preparation. Aliquot of cDNA (3 μl) were amplified by PCR as previously described (28), using a DNA thermal cycler (Perkin-Elmer 480; Cetus Corp., Norwalk, Conn.) or gradient temperature cycler (RoboCycler; Stratagene, Heidelberg, Germany) at the specifications indicated in Table 2. Ten-microliter samples of the amplified products were then subjected to electrophoresis using 1% agarose gels, stained with ethidium bromide, and visualized by UV illumination. Tumor necrosis factor alpha (TNF-α; 10 ng/ml)-stimulated human umbilical vein endothelial cells (HUVEC) were used as a positive control amplification for GAPDH and IL-8, and a plasmid carrying the ENA-78 gene was used for ENA-78. Digital pictures of the agarose gels were densitometrically analyzed using Bio-1D software (LTF-Labortechnik, Wasserburg, Germany).
TABLE 2.
Primer sequences and amplification conditions
| Oligonucleotide | Sequence (5′-3′) | Product size (bp) | Annealing conditions (temp [°C], t [s]) | No. of cycles |
|---|---|---|---|---|
| GAPDH | ||||
| Sense | TGAAGGTCGGAGTCAACGGATTTGGT | 983 | 60, 45 | 22, 28, 35 |
| Antisense | CATGTGGGCCATGAGGTCCACCAC | |||
| ENA-78 | ||||
| Sense | CTGTGTTGAGAGAGCTGCGTTGC | 216 | 60, 45 | 30, 35, 40 |
| Antisense | GTTTTCCTTGTTTCCACCGTCC | |||
| IL-8 | ||||
| Sense | ATTTCTGCAGCTCTGTGTGAA | 255 | 55, 45 | 30, 35, 40 |
| Antisense | TGAATTCTCAGCCCTCTTCAA |
Quantitative RT-PCR.
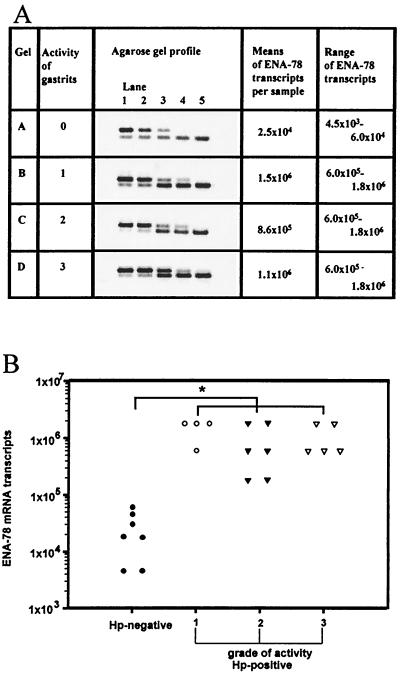
The amount of ENA-78 transcript in patient samples was quantitated via competitive RT-PCR, using an ENA-78 competitor DNA containing a 143-bp insertion within the ENA-78 sequence. The purified and blunt-ended ENA-78 PCR product (216 bp) was ligated into the multiple cloning site of the pCR-Script vector (2,961 bp), using a PCR-polishing kit and pCR-Script cloning kit (Stratagene). Competent Escherichia coli TOP10F′ was used for transformation as described in the protocol for the One Spot kit (Invitrogen, NV Leek, The Netherlands). The resulting plasmid, pCRENA, was then digested with the restriction endonuclease StyI, which has a single recognition sequence within the insert. To obtain an insertion mutation, a polished DNA fragment of 143 bp was ligated into the linearized plasmid pCRENA. Screening for the correct plasmid pCRENAGR (3,320 bp) was performed by ENA-78 amplification. Serial dilutions of the ENA-78 competitor DNA (106 to 102 per sample) and constant amounts of cDNA were then used for quantitation of the ENA-78 transcripts. ENA-78 transcript concentrations in cDNA samples were deduced by comparison of band intensities of the sample amplicons (216 bp) and the competitor amplicons (355 bp) (see Fig. 3A).
FIG. 3.
Quantitative RT-PCR analysis of ENA-78 transcripts in antral biopsy samples of patients with H. pylori-associated gastritis. (A) Competitive RT-PCR was carried out with 0.2 μg of RNA and serial dilutions of ENA-78 competitor DNA. Lanes 1 to 5 contained decreasing concentrations of competitor DNA (upper bands), 10−1 to 10−3 amol (gel A) and 10 to 10−1 amol (gels B to D). Representative agarose gel profiles of each grade of activity of gastritis are shown. (B) Correlation of ENA-78 mRNA levels of negative controls with biopsy samples exhibiting different grades of activity of gastritis. A statistically significant elevation of ENA-78 mRNA transcripts was obtained in all tissue samples tested with stage 1 to 3 H. pylori-associated gastritis (∗, P < 0.05).
Immunofluorescence studies.
Detection of ENA-78 protein was performed on air-dried, acetone-fixed cryostat (4-μm) sections of antral biopsy samples or acetone-fixed stimulated AGS cells in eight-well Permanox chamber slides. Immunohistochemistry was carried out by a modification of a procedure described earlier (1). Fixed cryostat sections were rehydrated with acetone, PBS, and blocking solution (3% bovine saline albumin, 50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 0.02% NaAcid). After 1 h of incubation in blocking solution, affinity-purified rabbit anti-human ENA-78 antibody (33) at 10 μg/ml was added, and incubation continued for 3 h at room temperature. After two washes in PBS, slides were incubated with anti-rabbit IgG-Cy3 conjugate for 1 h at room temperature. As a negative control, an affinity-purified IgG fraction of normal rabbit antiserum was used as primary reagent, followed by an anti-rabbit Cy3 conjugate. ENA-78 antiserum did not cross-react with members of the chemokine families, as described elsewhere (33). Immunostained sections were examined with a Leitz Diaplan microscope equipped with a Ploemopak fluorescence assembly and a digital charge-coupled device camera. Controls, including incubation with secondary antibody alone, were negative.
CagA analysis.
The cytotoxin-associated gene product CagA was determined with an immunoblot H. pylori IgG kit (Mikrogen, Munich, Germany). This in vitro test is based on electrophoretically separated antigens on a test strip, which is incubated with diluted human serum. Binding of anti-H. pylori IgG can be visualized by a secondary reaction with peroxidase-conjugated anti-human IgG. CagA appears as a band at 120 kDa.
Statistical analysis.
Statistical analyses were performed by the Wilcoxon or Student t test. A P value of <0.05 was considered statistically significant.
RESULTS
Semiquantitative RT-PCR analysis.
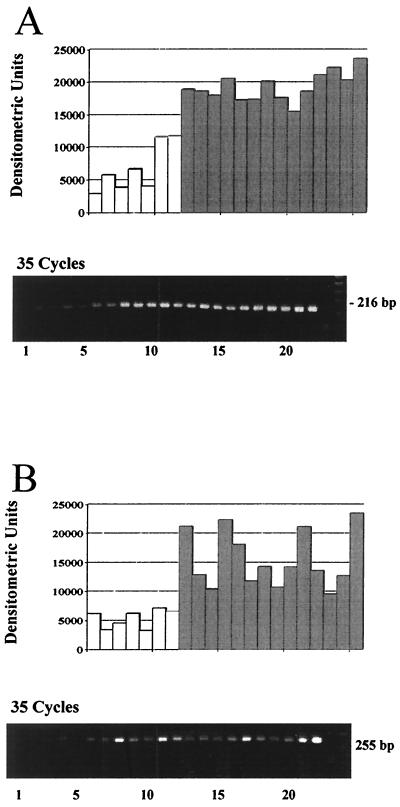
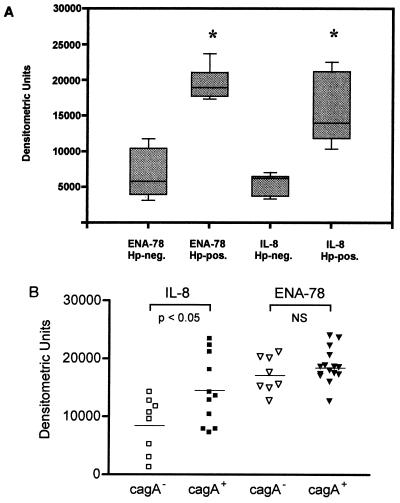
Gastric biopsy samples of 7 H. pylori negative individuals and of 15 patients with H. pylori-associated gastritis were analyzed by RT-PCR for the expression of ENA-78 and IL-8 mRNA. Amplification for 35 cycles yielded strong signals for ENA-78 (216 bp) and IL-8 (255 bp) transcripts in all biopsy samples of patients with H. pylori-associated gastritis (Fig. 1A and B, lower panels, samples 8 to 21). Gel bands were analyzed densitometrically; to ensure equal sample loading, individual samples were corrected for GAPDH expression. The semiquantitative analysis demonstrated that all H. pylori-negative biopsy samples contained lower levels of IL-8 and ENA-78 transcripts than the H. pylori-positive specimens (Fig. 1A and B, upper panels). IL-8 transcript levels showed a striking variation in the individual biopsy samples tested, while the same tissue samples exhibited relatively constant levels of ENA-78 transcripts (Fig. 1A and B, upper panels, samples 8 to 21). The semiquantitative analysis of IL-8 and ENA-78 transcript levels for all tested H. pylori-positive biopsy samples (n = 27) and negative control samples (n = 25) is shown in Fig. 2A. Statistical analysis revealed significantly elevated levels for both CXC chemokines, ENA-78 and IL-8, in antral biopsy samples of patients with H. pylori infection compared to biopsy samples of H. pylori-negative individuals (P < 0.05).
FIG. 1.
Semiquantitative RT-PCR analysis of ENA-78 (A) and IL-8 (B) mRNA in human gastric biopsy samples. (A) Lanes: 1 to 7, antral biopsy samples of H. pylori-negative individuals; 8 to 12, antral biopsy samples of patients with H. pylori-associated active gastritis, grade 1; 13 to 20, antral biopsy samples of patients with H. pylori-associated active gastritis, grade 2; 21, antral biopsy samples of patients with H. pylori-associated active gastritis, grade 3; 22, positive control DNA; 23, negative control; 24, DNA length marker. (B) Lanes: 1 to 7, gastric tissue from uninfected persons; 8 to 12, antral biopsy samples of patients with H. pylori-associated active gastritis, grade 1; 13 to 19, antral biopsy samples of patients with H. pylori-associated active gastritis, grade 2; 20 and 21, antral biopsy samples of patients with H. pylori-associated active gastritis, grade 3; 22, TNF-α-stimulated HUVEC; 23, negative control; 24, DNA length marker. Representative gels of three experiments are shown. White bars, biopsy samples of H. pylori-negative individuals; shaded bars, biopsy samples of H. pylori-positive individuals.
FIG. 2.
(A) Expression of ENA-78 and IL-8 mRNA in antral biopsy samples of patients with H. pylori-associated gastritis, demonstrated by densitometric analysis of semiquantitative RT-PCR analysis from 27 patients with H. pylori infection (Hp-pos.) compared to 25 control samples (Hp-neg.). Results were plotted with 10th, 25th, 75th, and 90th percentiles as vertical boxes with error bars. Medians are marked in each group. (∗, P < 0.05). (B) Antral IL-8 and ENA-78 mRNA expression in gastric biopsy samples of CagA-positive (n = 15) and CagA-negative (n = 8) patients with H. pylori infection.
Correlation with H. pylori protein CagA.
The presence of the cytotoxin-associated protein CagA was investigated by measuring the serum IgG status of patients with H. pylori-associated gastritis. This serologic assay also identifies patients with H. pylori CagA−/CagA+ mixed infections and those with H. pylori who have lost the pathogenicity islands. Chemokine IL-8 and ENA-78 expression in antral biopsy samples was determined by semiquantitative PCR. Statistical analysis of ENA-78 transcript levels in gastric tissue of CagA-positive (n = 15, median = 18,740) versus CagA-negative (n = 8, median = 17,210) persons did not demonstrate any significant differences due to the fact that mRNA levels were similarly elevated in all H. pylori-positive biopsy samples (Fig. 2B). In contrast, IL-8 mRNA levels differed significantly (P < 0.05), demonstrating higher levels of antral IL-8 transcripts in persons who had serum antibodies for cytotoxin-associated protein CagA. Three CagA-positive and CagA-negative antral biopsy samples of each two to four persons per grade of gastritis were also analyzed by competitive RT-PCR. No significant differences were noticed between ENA-78 RNA levels of CagA+ versus CagA− H. pylori strains (data not shown). Thus, an association between CagA positivity of the H. pylori strain and specific chemokine expression was observed only with IL-8 mRNA expression.
Quantification of transcript levels by competitive RT-PCR.
The competitive RT-PCR technique was applied to correlate precise levels of ENA-78 mRNA with the grade of activity of gastritis. Quantitation and determination of mean ENA-78 mRNA transcript levels were obtained from three antral biopsy samples of each four to seven persons per grade of activity of gastritis (seven of grade 0, four of grade 1, six of grade 2, and five of grade 3) (Fig. 3A). The detection limit of the competitive PCR was at 3 × 102 molecules per sample. Using constant amounts (0.2 μg) of tissue-derived RNA), the following mean ENA-78 transcript levels were determined: gastritis grade 0 (control tissue), 2.5 × 104; grade 1, 1.5 × 106; grade 2, 8.6 × 105; and grade 3, 1.1 × 106 (Fig. 3B). Transcript levels determined for gastritis grades 1 to 3 did not significantly differ. Transcript levels in biopsy samples of control tissue ranged from 4.5 × 103 to 6 × 104. Thus, tissue from H. pylori-associated gastritis (all grades) contained 45-fold more ENA-78 transcripts than control tissues. This elevation was statistically significant (P < 0.05).
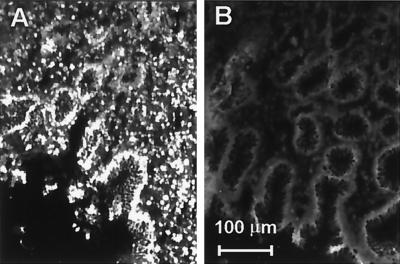
ENA-78 expression in gastric mucosa.
Tissues of antral biopsy samples from patients with various grades of activity of H. pylori-associated gastritis were analyzed by immunofluorescence for the expression of ENA-78 protein. Using an affinity-purified rabbit anti-ENA-78 antibody, positivity was mainly localized to the epithelium of biopsy samples of H. pylori positive patients (Fig. 4A). Some ENA-78-expressing cells with the typical morphology of monocytes were also seen in the lamina propria. H. pylori-negative samples stained only weakly for ENA-78 (Fig. 4B).
FIG. 4.
Immunofluorescence staining with affinity-purified polyclonal anti-ENA-78 antibody and phase-contrast image of the same cryostat sections. (A) Antral biopsy of patient with H. pylori-associated gastritis; (B) antral biopsy of negative control individual.
H. pylori induces ENA-78 mRNA in AGS cells.
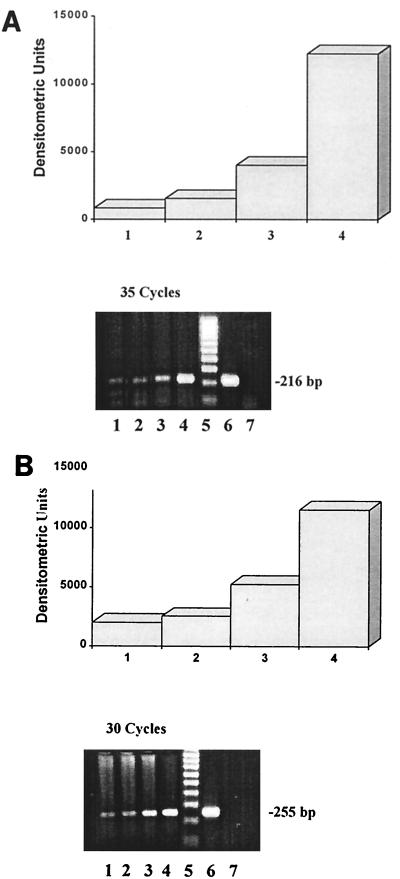
To gain further insight into how H. pylori or its products may induce a chemokine response, we tested its effect on induction of ENA-78 and IL-8 transcription in the gastric adenocarcinoma cell line AGS. Cells were stimulated with viable H. pylori (108 CFU/ml), OMP (10 μg/ml), or WSP (25 μg/ml) for 24 h at 37°C. As shown by RT-PCR, a significant increase in ENA-78 and IL-8 mRNA transcription was obtained in AGS cells stimulated with viable H. pylori (Fig. 5A and B, lanes 4). In contrast, only a nonsignificant increase in ENA-78 and IL-8 mRNA production was detected with OMP (Fig. 5A, lane 3), while WSP stimulation had no effect on chemokine induction (Fig. 5A and B, lanes 2).
FIG. 5.
RT-PCR amplification and densitometric analysis of ENA-78 (A) and IL-8 (B) mRNA of human gastric adenocarcinoma (AGS) cells. Electrophoresis was carried with amplicons obtained from unstimulated AGS cells (lane 1) and cells stimulated with WSP (25 μg/ml; lane 2), OMP (10 μg/ml; lane 3), or viable H. pylori (lane 4). Lane 5, DNA standard; lane 6, positive control ENA-78 plasmid DNA (A) or TNF-α-stimulated HUVEC (B); lane 7, negative control.
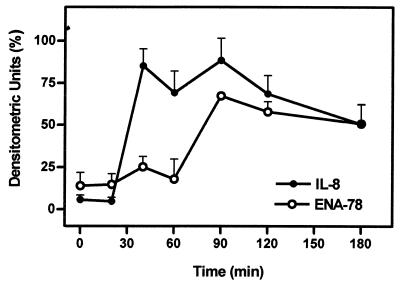
To analyze possible differences in the induction patterns of ENA-78 and IL-8 mRNA by viable H. pylori, time course experiments were carried out. RNA from stimulated gastric epithelial cells was isolated after various time intervals and semiquantitatively analyzed by RT-PCR (Fig. 6). The result demonstrates that IL-8 mRNA is rapidly induced, reaching saturating transcript levels 40 min postinduction. In contrast, upregulation of ENA-78 mRNA was delayed by about 50 min, reaching plateau mRNA concentrations at around 90 min after induction by viable H. pylori. Azide-treated H. pylori also induced ENA-78 mRNA production, while separation of viable H. pylori from the AGS cells by a filter membrane did not demonstrate ENA-78 mRNA upregulation (data not shown).
FIG. 6.
Kinetics of ENA-78 and IL-8 mRNA induction in human AGS cells stimulated with viable H. pylori. RNA was isolated from AGS cells at the time points indicated and subjected to RT-PCR. Amplified products were subjected to gel electrophoresis using 1% agarose gels, and the resulting bands were analyzed by densitometry. Means and standard errors (indicated by error bars) of three experiments are shown.
H. pylori induces ENA-78 protein expression in gastric epithelial cells.
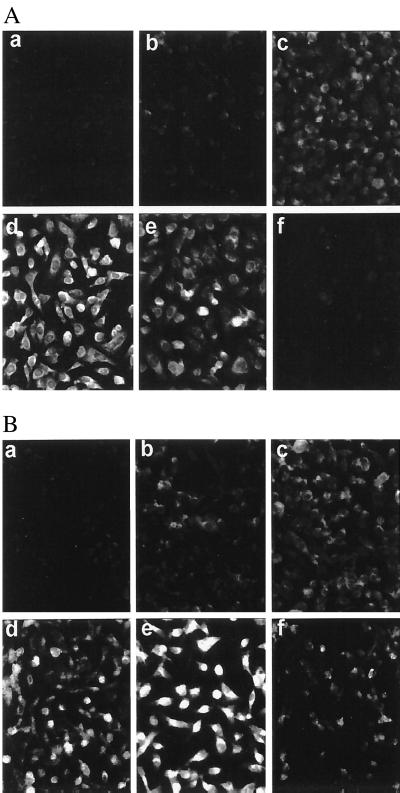
ENA-78 protein expression in H. pylori-induced AGS cells was tested by immunofluorescence staining at 0, 4, 13, 22, 38, and 62 h after bacterial challenge (Fig. 7B). In untreated AGS cells, ENA-78 immunoreactivity was almost absent, while a striking fluorescence was observed after stimulation with viable H. pylori. Maximal fluorescence occurred at 22 to 38 h postinduction. At 62 h there were still many cells expressing ENA-78 immunoreactivity, indicating long-lasting ENA-78 protein expression. Control cultures stimulated with IL-1β demonstrated a similar transient ENA-78 expression, with a maximal fluorescence occurring at about 22 h postinduction. With IL-1β used as a stimulus, practically no ENA-78 expression remained at 62 h (Fig. 7A).
FIG. 7.
Immunofluorescence staining of ENA-78 in stimulated AGS cells cultivated on chamber slides. AGS cells were stimulated with IL-1 (20 ng/ml) (A) or viable H. pylori (100 bacteria per AGS cell) (B). Cells were acetone fixed after incubation at 37°C for 0 (a), 4 (b), 13 (c), 22 (d), 38 (e), and 62 (f) h. Staining was carried out as described in Materials and Methods (original magnification, ×200).
DISCUSSION
The close association of gastric neutrophils with H. pylori-associated incidence of ulceration indicates that neutrophil infiltration is not a transient phenomenon but likely persists throughout H. pylori infection (34). Thus, formation of neutrophil chemoattractants may be expected not only during the initial colonization of the gastric mucosa with H. pylori but during the entire course of infection. IL-8, a prominent member of the CXC chemokine family, was first reported to be a chemoattractant for neutrophils in type B gastritis (8, 28). More recent studies have also implicated elevated levels of ENA-78 mRNA with H. pylori-associated gastritis (32). In the present study we demonstrate that in contrast to IL-8, ENA-78 transcript levels do not significantly correlate with the CagA+ phenotype and that upregulation of ENA-78 is delayed and independent on the gastritis grade.
To compare the two potent neutrophil chemoattractants and evaluate differences in the expression of ENA-78 in tissues exhibiting various grades of gastritis, exact amounts of mRNA levels were determined by competitive RT-PCR. This technique has the advantages that quantification is independent of the many variables that affect amplification, and it is more sensitive than Northern blotting or RNase protection assays. Overall, a highly significant 45-fold upregulation of ENA-78 transcripts was observed in H. pylori-positive samples. The analysis of biopsy samples expressing different degrees of gastritis did not demonstrate significant differences in ENA-78 mRNA levels. This is in contrast to former studies on quantification of IL-8 mRNA (28) and protein (40) in which a correlation of IL-8 expression and activity grade of H. pylori-associated gastritis was observed. A recent study found a significant correlation of ENA-78 mRNA levels and neutrophil infiltration in the antral mucosa with H. pylori infection (32). The discrepancy with our results may be explained by the difference in techniques used to quantify RNA levels. While we determined absolute mRNA levels by competitive RT-PCR, their results are based on relative mRNA levels evaluated by semiquantitative PCR.
Our results show that the degrees of maximal mRNA upregulation in biopsy samples of patients with H. pylori-associated gastritis are similar for the two chemokines ENA-78 and IL-8. However, absolute amounts of transcripts vary significantly, due to the higher basal levels of ENA-78 mRNA present in H. pylori-negative samples. Constitutive basal expression of ENA-78 has been observed before in adherent human monocytes, endothelial cells, and alveolar type II-like epithelial cells (35). Detection of ENA-78 immunoreactivity in cryostat sections of antral biopsies of patients with H. pylori-associated gastritis revealed predominant ENA-78 expression in the epithelium, while earlier results localized IL-8 expression to the lamina propria and the epithelium (7). A similar differential expression for the two CXC chemokines was also observed in tissue specimens of patients with inflammatory bowel disease (42).
Recent studies on diversity among H. pylori strains demonstrated that CagA-positive strains were associated with increased gastric IL-8 mRNA and IL-8 protein production (6, 9, 27, 38). In accordance with these reports, our studies revealed that CagA positivity correlated significantly with increased IL-8 transcript levels. In contrast, all H. pylori-positive biopsy samples expressed ENA-78 mRNA at similar high levels, yielding only a nonsignificant surplus of mRNA expression in CagA-positive patients. This is in contrast to findings reported by Shimoyama et al. (32), who observed statistically significantly elevated expression of ENA-78 mRNA in CagA-positive versus CagA-negative antral biopsy samples. This discrepancy may be explained only partially by differences in patient material or technical procedures. We used a commercial serologic test system to determine the CagA antibodies in the serum of the patients, while Shimoyama et al. used PCR methodology to detect CagA mRNA in tissue samples. They also showed a difference in ENA-78 expression between biopsy samples from antrum and corpus. Since we have used only antral biopsy samples in our studies, we cannot address this finding with respect to our results. It is of interest, however, that we obtained the difference in antral IL-8 and ENA-78 expression in H. pylori-infected patients by using RNA originating from the same biopsy samples, thus excluding tissue sample variations. Differences in gene expression of IL-8 and ENA-78 have also been observed earlier in mucosal epithelial cells, as well as other cell types (42, 30).
The expression pattern of ENA-78 by viable H. pylori or products thereof was investigated in vitro using a human gastric epithelial cell line, AGS. These cells expressed ENA-78 when induced with viable H. pylori but not with WSP or OMP isolated from H. pylori. Thus, upregulation of ENA-78 mRNA by H. pylori required adherence of bacteria to the epithelial cells, similar to the upregulation of IL-8 as described earlier (28). In these studies we have shown that WSP, cytoplasmic proteins, total proteins, OMP, and LPS from H. pylori NCTC 11637 and Tx30a did not increase IL-8 secretion by AGS or Kato III cells above constitutive levels. However, responsiveness to bacterial products may depend on the cell lines used. A recent study demonstrated IL-8 induction by H. pylori water extracts in MKN45 gastric cancer cells (17). The nature of the inducing substance in these water extracts has not been identified but is likely a nonprotein substance of low molecular weight.
While delayed production of ENA-78 mRNA is not observed with all cell types (35), the kinetics of IL-8 and ENA-78 induction by viable H. pylori in AGS cells differed significantly. IL-8 mRNA was rapidly induced, reaching peak levels at around 40 min, while ENA-78 mRNA induction was delayed, reaching maximal levels at 90 min after challenge with the bacteria. Differential expression of IL-8 and ENA-78 has been also observed in human monocytes (30) and in the human intestinal epithelial cell line Caco-2 (18). In LPS-stimulated monocytes, steady-state ENA-78 mRNA peaked at 20 to 28 h, while IL-8 mRNA was maximal at 8 to 12 h after stimulation. Similarly, IL-1β-stimulated Caco-2 cells exhibited maximal levels for IL-8 and ENA-78 mRNA at about 3 and 8 h, respectively. The cell-type-specific induction of IL-8 and ENA-78 mRNA may depend on the expression of specific transcription factors and the presence of corresponding regulatory elements in the upstream areas of the two promoters regions. While an NF-κB binding site seems to be essential for the induction of both IL-8 and ENA-78 transcription, putative AP-2-like binding sites have been detected only in the ENA-78 promoter region (5). Transcription factor AP-2 has been shown to play a role in epidermal cell-specific expression of keratin genes (20).
As recently demonstrated by Nufer et al. (24), differential regulation of chemokine activity may also be exerted at the protein level. ENA-78 truncated at the N terminus yielded variants with potencies in neutrophil chemotaxis up to eightfold higher than observed with wild-type ENA-78. Chymotrypsin and neutrophil cathepsin G efficiently produced the stable and highly potent variant ENA(9-78). In contrast, activity of IL-8 is rapidly lost upon exposure to cathepsin G in vitro (25). Thus, proteases may differentially modulate the activity of the two neutrophil-activating chemokines ENA-78 and IL-8. N-terminal truncation of variant ENA(9-78) by one amino acid to ENA(10-78) decreased its potency for chemotaxis about 14-fold, while a similar cleavage in IL-8 yielded a variant with highest potency (4, 24). The results obtained with cathepsin G may reflect a role for N-terminal proteolysis as a significant mechanism in regulating the inflammatory process in vivo.
In summary, our data demonstrate the important role of neutrophil-activating chemokine ENA-78, in addition to IL-8, as an inflammatory mediator in H. pylori-associated gastritis. ENA-78 mRNA and protein expression are highly upregulated in infected mucosal epithelial cells; moreover, due to its delayed and long-lasting production, ENA-78 may significantly contribute to the recruitment of granulocytes, not only during the acute phase but also during the chronic phase of the disease.
ACKNOWLEDGMENTS
This work was supported by a research grant to R.A.H. from the Chiles Foundation, Portland, Oreg., and in part by Swiss National Science Foundation grant 3100-045538.95.
We thank Linda Buechi for excellent technical assistance.
REFERENCES
- 1.Allmann-Iselin I, Car B D, Zwahlen R D, Mueller-Schupbach R, Wyder-Walther M, Steckholzer U, Walz A. Bovine ENA, a new monocyte-macrophage derived cytokine of the interleukin-8 family. Structure, function, and expression in acute pulmonary inflammation. Am J Pathol. 1994;145:1382–1389. [PMC free article] [PubMed] [Google Scholar]
- 2.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 3.Bliss C M, Jr, Golenbock D T, Keates S, Linevsky J K, Kelly C P. Helicobacter pylori lipopolysaccharide binds to CD14 and stimulates release of interleukin-8, epithelial neutrophil-activating peptide 78, and monocyte chemotactic protein 1 by human monocytes. Infect Immun. 1998;66:5357–5363. doi: 10.1128/iai.66.11.5357-5363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark-Lewis I, Schumacher C, Baggiolini M, Moser B. Structure-activity relationships of interleukin-8 determined using chemically synthesized analogs. Critical role of NH2-terminal residues and evidence for uncoupling of neutrophil chemotaxis, exocytosis, and receptor binding activities. J Biol Chem. 1991;266:23128–23134. [PubMed] [Google Scholar]
- 5.Corbett M S, Schmitt I, Riess O, Walz A. Characterization of the gene for human neutrophil-activating peptide 78 (ENA-78) Biochem Biophys Res Commun. 1994;205:612–617. doi: 10.1006/bbrc.1994.2709. [DOI] [PubMed] [Google Scholar]
- 6.Crabtree J E, Covacci A, Farmery S M, Xiang Z, Tompkins D S, Perry S, Lindley I J, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree J E, Wyatt J I, Trejdosiewicz L K, Peichl P, Nichols P H, Ramsay N, Primrose J N, Lindley I J D. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabtree J E, Peichl P, Wyatt J I, Stachl U, Lindley I J. Gastric interleukin-8 and IgA IL-8 autoantibodies in Helicobacter pylori infection. Scand J Immunol. 1993;37:65–70. doi: 10.1111/j.1365-3083.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree J E, Xiang Z, Lindley I J D, Tompkins D S, Rappuoli R, Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by a CagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48:967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe S E, Alvarez L, Dytoc M, Hunt R H, Muller M, Sherman P, Patel J, Jin Y, Ernst P B. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 11.Dixon M F, Genta R M, Yardley J H, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Enders G, Brooks W, von Jan N, Lehn N, Bayerdorffer E, Hatz R. Expression of adhesion molecules on human granulocytes after stimulation with Helicobacter pylori membrane proteins: comparison with membrane proteins from other bacteria. Infect Immun. 1995;63:2473–2477. doi: 10.1128/iai.63.7.2473-2477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X-G, Chua A, Fan X-J, Keeling P W N. Increased gastric production of interleukin-8 and tumour necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol. 1995;48:133–136. doi: 10.1136/jcp.48.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatz R A, Rieder G, Stolte M, Bayerdorffer E, Meimarakis G, Schildberg F W, Enders G. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology. 1997;112:1908–1919. doi: 10.1053/gast.1997.v112.pm9178683. [DOI] [PubMed] [Google Scholar]
- 15.Hatz R A, Brooks W P, Krämling H-J, Enders G. Stomach immunology and Helicobacter pylori infection. Curr Opin Gastroenterol. 1992;8:993–1001. [Google Scholar]
- 16.Huang J, O'Toole P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassai K, Yoshikawa T, Yoshida N, Hashiramoto A, Kondo M, Murase H. Helicobacter pylori water extract induces interleukin-8 production by gastric epithelial cells. Dig Dis Sci. 1999;44:237–242. doi: 10.1023/a:1026629812245. [DOI] [PubMed] [Google Scholar]
- 18.Keates S, Keates A C, Mizoguchi E, Bhan A, Kelly C P. Enterocytes are the primary source of the chemokine ENA-78 in normal colon and ulcerative colitis. Am J Physiol. 1997;273:G75–G82. doi: 10.1152/ajpgi.1997.273.1.G75. [DOI] [PubMed] [Google Scholar]
- 19.Koch A E, Kunkel S L, Harlow L A, Mazarakis D D, Haines G K, Burdick M D, Pope R M, Walz A, Strieter R M. Epithelial neutrophil activating peptide-78: a novel chemotactic cytokine for neutrophils in arthritis. J Clin Investig. 1994;94:1012–1018. doi: 10.1172/JCI117414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leask A, Byrne C, Fuchs E. Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci USA. 1991;88:7948–7952. doi: 10.1073/pnas.88.18.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai U E, Perez-Perez G I, Allen J B, Wahl S M, Blaser M J, Smith P D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller S, Seifert E, Stolte M. Simultaneous MALT-type lymphoma and early adenocarcinoma of the stomach. Z Gastroenterol. 1999;37:153–157. [PubMed] [Google Scholar]
- 23.Nomura A, Stemmermann G N, Chyou P H, Kato I, Perez-Perez G I, Blaser M J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 24.Nufer O, Corbett M, Walz A. Amino-terminal processing of chemokine ENA-78 regulates biological. Biochemistry. 1999;38:636–642. doi: 10.1021/bi981294s. [DOI] [PubMed] [Google Scholar]
- 25.Padrines M, Wolf M, Walz A, Baggiolini M. Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett. 1994;352:231–235. doi: 10.1016/0014-5793(94)00952-x. [DOI] [PubMed] [Google Scholar]
- 26.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 27.Peek R M, Jr, Miller G G, Tham K T, Perez-Perez G I, Zhao X, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Investig. 1995;73:760–770. [PubMed] [Google Scholar]
- 28.Rieder G, Hatz R A, Moran A P, Walz A, Stolte M, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622–3630. doi: 10.1128/iai.65.9.3622-3630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmouder R L, Streiter R M, Walz A, Kunkel S L. Epithelial-derived neutrophil-activating factor-78 production in human renal tubule epithelial cells and in renal allograft rejection. Transplantation. 1995;59:118–124. doi: 10.1097/00007890-199501150-00021. [DOI] [PubMed] [Google Scholar]
- 30.Schnyder-Candrian S, Walz A. Neutrophil-activating protein ENA-78 and IL-8 exhibit different patterns of expression in lipopolysaccharide- and cytokine-stimulated human monocytes. J Immunol. 1997;158:3888–3894. [PubMed] [Google Scholar]
- 31.Sharma S A, Tummuru M K R, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimoyama T, Everett S M, Dixon M F, Axon A T R, Crabtree J E. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J Clin Pathol. 1998;51:765–770. doi: 10.1136/jcp.51.10.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strieter R M, Kunkel S L, Burdick M D, Lincoln P M, Walz A. The detection of a novel neutrophil-activating peptide (ENA-78) using a sensitive ELISA. Immunol Investig. 1992;21:589–596. doi: 10.3109/08820139209069393. [DOI] [PubMed] [Google Scholar]
- 34.Taha A S, Dahill S, Morran C, Hudson N, Hawkey C J, Lee F D, Sturrock R D, Russell R I. Neutrophils, Helicobacter pylori, and nonsteroidal anti-inflammatory drug ulcers. Gastroenterology. 1999;116:254–258. doi: 10.1016/s0016-5085(99)70120-4. [DOI] [PubMed] [Google Scholar]
- 35.Walz A, Schmutz P, Mueller C, Schnyder-Candrian S. Regulation and function of the CXC chemokine ENA-78 in monocytes and its role in disease. J Leukoc Biol. 1997;62:604–611. doi: 10.1002/jlb.62.5.604. [DOI] [PubMed] [Google Scholar]
- 36.Walz A, Burgener R, Car B, Baggiolini M, Kunkel S L, Strieter R M. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991;174:1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood D W, Block K P. Helicobacter pylori: a review. Am J Ther. 1998;5:253–261. doi: 10.1097/00045391-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- 39.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand J Gastroenterol. 1995;30:1153–1159. doi: 10.3109/00365529509101624. [DOI] [PubMed] [Google Scholar]
- 40.Yamaoka Y, Kita M, Kodama T, Sawai N, Tanahashi T, Kashima K, Imanishi J. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. 1998;42:609–617. doi: 10.1136/gut.42.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida N, Granger D N, Evans D J, Jr, Evans D G, Graham D Y, Anderson D C, Wolf R E, Kvietys P R. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993;105:1431–1440. doi: 10.1016/0016-5085(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 42.Z'Graggen K, Walz A, Mazzucchelli L, Strieter R M, Mueller C. The C-X-C chemokine ENA-78 is preferentially expressed in intestinal epithelium in inflammatory bowel disease. Gastroenterology. 1997;113:808–816. doi: 10.1016/s0016-5085(97)70175-6. [DOI] [PubMed] [Google Scholar]