Abstract
It is generally accepted that protective immunity against tuberculosis is generated through the cell-mediated immune (CMI) system, and a greater understanding of such responses is required if better vaccines and diagnostic tests are to be developed. γδ T cells form a major proportion of the peripheral blood mononuclear cells (PBMC) in the ruminant system and, considering data from other species, may have a significant role in CMI responses in bovine tuberculosis. This study compared the in vitro responses of αβ and γδ T cells from Mycobacterium bovis-infected and uninfected cattle. The results showed that, following 24 h of culture of PBMC with M. bovis-derived antigens, the majority of γδ T cells from infected animals became highly activated (upregulation of interleukin-2R), while a lower proportion of the αβ T-cell population showed activation. Similar responses were evident to a lesser degree in uninfected animals. Study of the kinetics of this response showed that γδ T cells remained significantly activated for at least 7 days in culture, while activation of αβ T cells declined during that period. Subsequent analysis revealed that the majority of activated γδ T cells expressed WC1, a 215-kDa surface molecule which is not expressed on human or murine γδ T cells. Furthermore, in comparison with what was found for CD4+ T cells, M. bovis antigen was found to induce strong cellular proliferation but relatively little gamma interferon release by purified WC1+ γδ T cells. Overall, while the role of these cells in protective immunity remains unclear, their highly activated status in response to M. bovis suggests an important role in antimycobacterial immunity, and the ability of γδ T cells to influence other immune cell functions remains to be elucidated, particularly in relation to CMI-based diagnostic tests.
Bovine tuberculosis, a zoonotic disease caused by infection with Mycobacterium bovis (45), is a major economic problem in a number of countries (12) and a serious public health risk in others (18). While a spectrum of immune responses to bovine tuberculosis has been characterized (45, 54), it is generally accepted that protective immunity is mediated through the cellular immune system. A detailed understanding of these responses is essential for the development of better control methods.
It has been suggested that all major T-cell subsets are involved in immune responses to mycobacteria (6, 31, 43). Studies involving experimental Mycobacterium tuberculosis infection, including gene deletion mutations and adoptive transfer experiments in the murine model, have shown that both αβ and γδ T cells have roles in such immune responses (35, 49, 51). To date, much attention has focused on αβ T-cell functions, but there has been increasing evidence that human and murine γδ T cells become potently stimulated by various mycobacterial antigens, including heat shock (24, 48) and other protein antigens (5, 9), along with nonpeptide phosphate-rich low-molecular-weight compounds (17, 20, 57).
In the ruminant system, γδ T cells constitute up to 75% of peripheral blood mononuclear cells (PBMC) in young animals (40) and up to 40% of the circulating population in adults (15, 41, 60). In contrast, only 7% of human PBMC and 2 to 3% of murine PBMC typically express the γδ T-cell receptor (TCR1) (10, 30). Furthermore, a unique feature of ruminant γδ T cells is the expression of a 215-kDa surface molecule identified as WC1 on the majority of TCR1+ PBMC (15, 42, 61). The extracellular portion of this molecule has 11 repeats of a cysteine-rich domain and belongs to the scavenger receptor cysteine-rich family of proteins, which also includes CD5 and CD6 (61). It has been suggested that WC1 is a possible ligand for E-selectin (59) and that it may control the tissue-specific homing of γδ T cells (62). More recently, it has been proposed that WC1 regulates interleukin-2 (IL-2)-dependent γδ T cells and their proliferation through induction of reversible growth arrest (34, 56). However, the precise functions of this molecule and indeed of γδ T cells are still not clearly defined.
Given the apparent role of γδ T cells in human and murine tuberculosis, the greater numbers of these cells found in ruminants suggest that they could have an important role in bovine tuberculosis, which requires further investigation. A range of functions, including proliferation and natural killer and cytotoxic activity, have been documented for bovine γδ T cells in response to mitogens and parasite, bacterial, and viral antigens (1, 14, 15, 16, 19), and it is likely that such functions could be involved in the defense against M. bovis. Furthermore, it has previously been demonstrated that the WC1+ cells are among the first T cells to show changes in circulating numbers and antigen responsiveness following experimental M. bovis infection of cattle (53).
The aim of the present study was to investigate the in vitro responsiveness of γδ T-cell subsets to mycobacterial antigens using a model of bovine tuberculosis established in the natural host.
MATERIALS AND METHODS
Experimental animals.
Two groups of Friesian cross, male calves (approximately 6 months of age) obtained from herds with no history of M. bovis infection for at least 5 years were used in this study. All animals were screened for lymphocyte proliferation and for gamma interferon (IFN-γ) production against a range of M. bovis and control antigens to confirm disease-free status. Animals were selected on the basis of a negative response to M. bovis antigens and low reaction with Mycobacterium avium. Group 1 contained three experimentally infected (I1, I2, and I3) calves and three uninfected age-matched controls (U1, U2, and U3). Group 2 contained four experimentally infected animals (I4, I5, I6, and I7) and two uninfected animals (U4 and U5). Animals for experimental infection were inoculated with 106 CFU of a field strain of M. bovis (T/91/1378) by intranasal instillation and housed in strict isolation (44). Animals were sampled at weeks 3 to 10 for lymphocyte functional studies and were confirmed as diseased by gross postmortem examination (weeks 40 to 41 postinfection [p.i.]) and M. bovis culture.
Mycobacterial antigens.
M. bovis sonic extract (MBSE) was prepared as previously described (52). Briefly, M. bovis (T/91/1378) was grown to mid-log phase in Middlebrook 7H9 medium. Bacteria were harvested by centrifugation, washed in phosphate-buffered saline (PBS) and subjected to ultrasonication. MBSE was clarified by centrifugation, filter sterilized (0.22-μm-pore-size filter), and stored at −70°C. M. bovis culture filtrate (CF) was prepared by culturing M. bovis (T/91/1378) in Sauton's specific protein-free medium for 21 days at 37°C in air. Bacteria were removed by centrifugation, and the supernatant was filter sterilized and concentrated 100-fold by gas pressure in an ultrafiltration cell containing a 10-kDa-cutoff membrane (Amicon Ltd., Stonehouse, Gloucestershire, United Kingdom).
Antibodies.
Monoclonal antibodies (MAbs) CC8 (anti-CD4), CC63 (anti-CD8), CC15 (anti-WC1; isotype, immunoglobulin G2A [IgG2a]), and CC30 (anti-CD4; isotype, IgG1) (26, 28, 42), obtained from the European Collection of Animal Cell Cultures (Porton Down, Wiltshire, United Kingdom), were prepared as hybridoma culture supernatants and used at 1/10 dilution for flow-cytometric analysis (FCA) and magnetic (magnetically activated cell sorting [MACS]) labeling. GB21A (anti-γδ TCR/TCR1; isotype, IgG2b) and CACT116A (anti-CD25/IL-2R; isotype, IgG1) (38, 39) purified antibodies were obtained from Veterinary Medical Research and Development Inc. (Pullman, Wash.) and used at 1/100 dilution for FCA labeling. For flow cytometry, primary MAbs were detected using secondary goat anti-mouse (GAM) isotype-specific conjugates (IgG2a-fluorescein isothiocyanate [FITC], IgG2b-FITC, IgG2b-biotin, and IgG1-phycoerythrin [PE]). The GAM-IgG2b-biotin was detected using streptavidin-SpectralRed (Southern Biotechnology Associates, Inc., Birmingham, Ala.).
Magnetic purification of CD4+ and WC1+ T cells.
PBMC were separated from heparinized blood samples over Ficoll-Paque as described previously (52). CD4+ and WC1+ cells were labeled with antibovine MAb CD4 (CC8) or WC1 (CC15) (1/10 dilution) and positively selected with GAM microbeads using the Miltenyi Biotec (Bergisch Gladbach, Germany) MACS system as described previously (36).
Positively selected cells were washed (once in PBS) and resuspended at 106 cells/ml in T-cell culture media (TCM) (RPMI 1640 supplemented with 10 mM HEPES buffer, 2 mM l-glutamine, 5% fetal calf serum (Gibco, Paisley, United Kingdom), and 25 μg of gentamicin sulfate (Sigma, Poole, United Kingdom). Purified T-cell subsets were routinely found to be >96% pure, as determined by flow cytometry, and >98% viable. Purified WC1+ cell preparations were also checked and found to be negative for contamination with CD4+ cells using MAb CC30.
Preparation of APC.
Isolated PBMC from each animal were resuspended at 107 cells/ml and incubated with mitomycin C (50 μg/ml) (Sigma) at 37°C for 30 min. The antigen-presenting cells (APC) were washed three times with PBS by centrifugation and resuspended in TCM at 106 cells/ml.
Lymphocyte proliferation assay (LPA).
Proliferation assays were performed using PBMC or purified T-cell subsets with autologous APC. PBMC were prepared at 106 cells/ml, while purified CD4+ and WC1+ T cells were resuspended at a concentration of 1.5 × 105 cells/ml with APC added at 105 cells/ml. Cell suspensions (200 μl/well) were dispensed into 96-well, flat-bottom microtiter plates (Nunc, Roskilde, Denmark). Antigens were added to triplicate wells at a previously determined optimal concentration (4 μg/ml), and an equal volume of PBS was added to control wells. The cultures were incubated for 5 days, pulsed with 0.25 μCi of [3H]thymidine (Amersham International, Amersham, United Kingdom), and harvested, and the incorporated radiolabel was measured by liquid scintillation as previously described (52) and recorded as counts per minute. Results are expressed as either mean total counts per minute or mean net counts per minute (net counts per minute = antigen counts per minute − control PBS counts per minute).
Detection of in vitro activation of T cells by FCA.
Cultures were established for each animal to determine the phenotype of T cells becoming activated in the presence of an antigen. PBMC were resuspended at 106 cells/ml in TCM, and 7-ml cultures were maintained in 25-cm2 tissue culture flasks (Costar Corp., Cambridge, Mass.). Cultures were stimulated with MBSE (4 μg/ml) or an equivalent volume of PBS (control) and incubated in 6% CO2 at 37°C. After specified periods, cells from PBMC cultures were harvested and labeled for two-color (CC8, CC63, or CC15 and CACT116A) and three-color (CC15, GB21A, and CACT116A) FCA. Cells (106 per test) were pelleted in U-well microtiter plates, resuspended in 25 μl of MAbs diluted in PBS containing 10% (vol/vol) heat-inactivated normal rabbit serum (PBS-NRS), and incubated for 30 min at 4°C. The cells were washed twice in PBS containing 0.1% (wt/vol) sodium azide (Sigma). Cells were then resuspended in 25 μl of the appropriate GAM isotype-specific conjugates (IgG2a-FITC, IgG1-PE, IgG2b-biotin; used at 1/500 in PBS-NRS), incubated, and washed as described above. For tertiary labeling of IgG2b-biotin, cells were incubated with 25 μl of streptavidin-SpectralRed (1/500 in PBS-NRS). Following final washes, cells were fixed in 400 μl of 1% (wt/vol) paraformaldehyde (Sigma) in PBS.
FCA was performed using a FACS Vantage (Becton Dickinson, Oxford, United Kingdom) equipped with an Innova Enterprise ion laser (Coherent Laser Group, San Jose, Calif.). Lymphocytes were identified on the basis of forward and side scatter and gated appropriately (36). Green (FITC), orange (PE), and red (SpectralRed) log integral signals were obtained from the gated population. Ten thousand cells were counted for each sample, and analyses were performed using LYSYS II software (Becton Dickinson).
IFN-γ enzyme-linked immunosorbent assay.
Cultures of PBMC or T-cell subsets (CD4+ or WC1+) with autologous APC were set up as for LPA above with the PBS control or M. bovis antigens. Following 96 h of incubation, 100 μl of supernatant was aspirated from duplicate wells and assayed for IFN-γ using an enzyme immunoassay (Commonwealth Serum Laboratories Ltd., Parkville, Victoria, Australia), performed as specified by the manufacturer. Results were expressed as optical densities at 450 nm (OD450) or OD indices (ODI) (37) (ODI = OD for the antigen/OD for the PBS control). An ODI of >2 and also greater than the ODI for APC controls was considered positive.
Statistical analysis.
The interaction between infection status (where applicable) and the effects of PBS or MBSE on the activation status within each T-cell subset as measured by two-color FCA, proliferation, and IFN-γ release following culture was investigated by analysis of variance using GENSTAT statistical software (Clarendon Press, Oxford, United Kingdom).
RESULTS
Antigen-specific CMI responses in M. bovis-infected cattle.
From 21 days after experimental infection, group 1 animals (I1, I2, and I3) had consistent cell-mediated immune (CMI) responses to MBSE and CF as measured by LPA and IFN-γ release, while the three noninfected control cattle (U1, U2, and U3) did not show significant responsiveness (Table 1). During the initial stages of this response, FCA was used to analyze the in vitro antigenic activation status of T-cell subsets by measuring the expression of IL-2R (CD25).
TABLE 1.
Antigen responsiveness of PBMC from uninfected and M. bovis-infected animals stimulated with control or M. bovis antigens as measured by lymphocyte proliferation and IFN-γ production from 21 to 63 days p.i.a
| Animal | Lymphocyte proliferation (cpm) in response to:
|
IFN-γ production (OD450) in response to:
|
||||
|---|---|---|---|---|---|---|
| PBS | MBSE | CF | PBS | MBSE | CF | |
| Infected | ||||||
| I1 | 435 ± 112 | 69,588 ± 8,805 | 78,440 ± 9,402 | 0.09 ± 0.02 | 1.87 ± 0.14 | 2.06 ± 0.24 |
| I2 | 398 ± 120 | 64,854 ± 9,997 | 46,414 ± 9,854 | 0.10 ± 0.01 | 2.36 ± 0.20 | 2.49 ± 0.19 |
| I3 | 516 ± 186 | 45,452 ± 3,531 | 29,599 ± 9,579 | 0.10 ± 0.01 | 3.01 ± 0.21 | 3.16 ± 0.26 |
| Control | ||||||
| U1–U3 | 395 ± 92 | 646 ± 445 | 703 ± 369 | 0.10 ± 0.01 | 0.13 ± 0.05 | 0.116 ± 0.02 |
Experimental animals before M. bovis infection and control animals before the experiment displayed mean responses of <700 cpm and <0.10 OD450 units in response to PBS, MBSE, CF, and avian and bovine PPD for lymphocyte proliferation and IFN-γ production, respectively. Results for infected cattle are means ± standard errors of the mean (SEM) of four repeated experiments for each animal. Results for control (uninfected) cattle are means ± SEM of the responses for three animals from four repeated experiments.
In vitro activation of T-cell subsets.
Initially, cultures of PBMC from the animals of group 1 were stimulated with PBS (control) or MBSE for 24 h prior to FCA. As Fig. 1 demonstrates, distinct levels of T-cell subset activation were observed in response to MBSE, with a high degree of activation within the CD4+ and WC1+ populations of the infected animals. A relatively low degree of CD25 expression on CD8+ T cells was observed.
FIG. 1.
Representative two-color flow-cytometric dot plots of phenotype (CD4+ or WC1+; FL1) and CD25 (IL-2R) expression (FL2) of PBMC from an individual M. bovis-infected animal following 24 h of culture with PBS (control; a and b) or MBSE (c and d). Percentages of cells with the subset phenotype activated (upper right) were calculated by dividing the percentage of CD4+ (or WC1+) CD25+ cells by the percentage of CD4+ (or WC1+) cells and multiplying by 100.
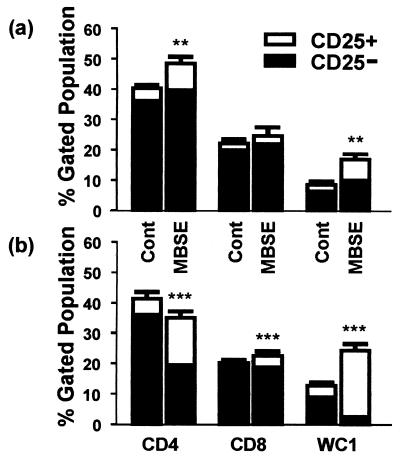
When the analyses were repeated on several occasions for all group 1 animals, it was found that MBSE also caused an increase in the proportion of cells expressing CD25 for the uninfected animals (Fig. 2a). For those control animals, 10 and 18% of the CD4+ subset were CD25+ within the PBS and MBSE cultures, respectively, while 26 and 39% of WC1+ cells in PBS- and MBSE-stimulated cultures, respectively, were CD25+. Approximately 10% of CD8+ cells from uninfected animals, stimulated with either PBS or MBSE, were observed to express CD25. However, much larger proportions of T cells in the PBMC from infected animals were activated. In particular, there were notable levels of activation within the CD4+ and WC1+ subsets (Fig. 2b). The levels of CD25 expression in the CD4+ subset from infected animals were 13% for PBS cultures and 45% for MBSE cultures, while within the WC1+ population 28 (PBS) and 90% (MBSE) of T cells were CD25+. A higher level of CD8 T-cell subset activation in response to MBSE (17% CD25+) was observed for infected animals than for control animals.
FIG. 2.
Proportions of activated (CD25+) and nonactivated (CD25−) CD4+, CD8+, and WC1+ T-cell subsets within the total lymphocyte gated population as determined by FCA of uninfected (a) and M. bovis-infected (b) animals following 24 h of culture with PBS (Cont) or MBSE. Results are means ± standard errors of the means for three uninfected and three infected cattle (group 1). Experiments were repeated on five occasions. Statistical comparisons between PBS control and MBSE cultures for the proportion of activated cells within each T-cell subset are shown (∗∗, P < 0.01; ∗∗∗, P < 0.001.
Statistical analysis revealed that the levels of activation seen in the CD4+, CD8+, and WC1+ populations from infected animals in response to MBSE were significantly higher than those seen in the PBS control cultures (P < 0.001). While MBSE also induced a significant degree of activation in the CD4+ and WC1+ populations from control animals, compared to the PBS control, this was at a lower level of significance (P < 0.01). No significant activation of CD8+ cells in response to MBSE was observed, compared to that in response to PBS, for uninfected animals (P > 0.05).
Most importantly, when the infected and uninfected animals were compared, the activation studies revealed large differences in the MBSE responses of CD4+ and WC1+ T-cell subsets. The results indicated that, compared with control animals, animals which were infected with M. bovis had highly significantly greater levels of CD25 expression in WC1+ (P < 0.001) and CD4+ (P < 0.01) populations but did not have significantly greater activation of CD8+ cells (P > 0.05).
Kinetics of in vitro activation of T cells.
The analysis of T-cell subset activation at 24 h of culture for group 1 infected animals showed the predominant involvement of CD4+ and WC1+ cells. This was also found to be the case with animals of group 2 from as early as 28 days p.i. (data not shown). Subsequently, time course analysis was performed with the group 2 infected animals to define the kinetics of T-cell subset activation in vitro from 0 to 168 h following stimulation with MBSE.
This experiment confirmed strong activation of CD4+ and WC1+ cells (Fig. 3a and b), along with a high degree of activation in the total TCR1+ population (Fig. 3c). These data suggest that, in terms of initial in vitro T-cell kinetics, CD4+ T cells may become activated slightly in advance of γδ T cells. Significant differences in activation status between PBS control and MBSE cultures were detected at 18 h for CD4+ cells (P < 0.05) (Fig. 3a). The activation profiles of the total TCR1+ population and the WC1+ subset mirrored each other, showing significant levels of activation by 24 h (P < 0.01) (Fig. 3b and c). Beyond 24 h, the TCR1+ and WC1+ subsets maintained a high-level activation status until 168 h (P < 0.001), while CD4+ T-cell activation declined after 24 h, although remaining significantly elevated until 96 h (P < 0.01) of culture (Fig. 3a to c). CD8+ cells constituted a relatively small percentage of the total population of CD25+ cells but had statistically significant activation at 18 and 48 h only (P < 0.01 and P < 0.05, respectively) (Fig. 3d).
FIG. 3.
Kinetics of αβ and γδ T-cell activation. Shown are percentages of T-cell subsets coexpressing CD25 as determined by FCA at various times after 0 to 168 h of culture with PBS (Cont) or MBSE. Results are means ± standard errors of the means for four M. bovis-infected cattle (group 2). Statistical comparisons between control and MBSE cultures at each time point revealed the first significant differences in activation at 18 (αβ T cells; P < 0.05; a and d) and 24 h (γδ T cells; P < 0.01; b and c). Beyond 24 h, significant activation of CD4+ (a) cells was apparent up to 144 h (P < 0.01 or P < 0.001), significant activation of CD8+ cells was apparent at 48 h only (P < 0.05) (d), and significant activation of γδ T cells (TCR1+ and WC1+) was apparent at all time points (P < 0.01 or P < 0.001) (b and c).
Differential antigen responsiveness of WC1+ and WC1− γδ T cells.
The kinetics study revealed that the activation profile of the TCR1+ (total γδ T-cell) population was mirrored by the activation profile of the WC1+ subset (Fig. 3b and c) suggesting a possible relationship between WC1+ expression and activation. Three-color FCA of γδ T-cell subset phenotype and activation status following 24 h of culture was performed to investigate coexpression of CD25 and WC1+ on TCR1+ cells. Data which are representative of the overall mean results from repeated experiments using group 2 infected animals are shown in Fig. 4. The results demonstrate how the main populations of activated γδ (TCR1+) T cells in both control and MBSE-stimulated cultures were WC1+ (approximately 84%), whereas a much smaller number of nonactivated γδ T cells expressed WC1+ (Fig. 4c and f), particularly in MBSE-stimulated cultures. Analysis of the overall data clearly shows that the majority of the predominant γδ population of WC1+ cells within M. bovis antigen-stimulated cultures exist in a highly activated state (Table 2), making up a large proportion of the highly activated cells within the total PBMC culture (Fig. 4).
FIG. 4.
Expression of WC1 on activated and nonactivated TCR1+ T cells. Shown are representative flow-cytometric dot plots of TCR1 (FL1) and CD25 (IL-2R) expression (FL2) of PBMC from an M. bovis-infected animal following 24 h of culture with PBS (control) or MBSE and corresponding histograms of WC1 expression (FL3). (a) Control (PBS) CD25 versus TCR1 expression dot plot. (b and c) Histograms showing WC1 expression of R2 and R3 gated cells within the dot plot (a), respectively. (d) Dot plot of CD25 and TCR1 coexpression in response to the MBSE antigen. (e and f) Histograms of WC1 expression by cells of gate R2 (e) and gate R3 (f) within the dot plot (d).
TABLE 2.
In vitro activation (IL-2R expression) of γδ T-cell subsets from M. bovis-infected animals (group 2) following stimulation with MBSE in short-term culture
| Phenotype | % of total PBMC activateda in cultures stimulated with:
|
|
|---|---|---|
| PBS (control) | MBSE | |
| TCR1+ WC1+ CD25+ | 4.4 ± 0.2 | 14.4 ± 1.2 |
| TCR1+ WC1− CD25+ | 1.2 ± 0.2 | 3.3 ± 0.3 |
Results are means ± standard errors of the means for three repeated experiments.
Functional studies of WC1+ and CD4+ T cells.
The flow-cytometric studies clearly demonstrated that CD4+ and γδ T cells from infected animals were highly activated by MBSE. Furthermore, the data suggested that the WC1+ subset of γδ T cells was more antigen responsive than the WC1− subset. Thus, CD4+ and WC1+ T cells were purified by indirect labeling using paramagnetic beads and then cultured with mitomycin C-treated autologous PBMC as APC in order to measure proliferative responses and IFN-γ secretion in response to M. bovis antigens.
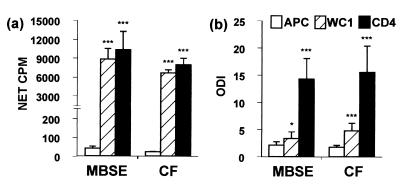
MBSE and CF induced strong proliferation of both CD4+ and WC1+ T cells (Fig. 5a). With equivalent numbers of sorted cells, it was found that incorporation of [3H]thymidine (in terms of counts per minute) into proliferating WC1+ T cells was only slightly lower than that for the CD4+ population. APC responses to both antigens were equivalent to background counts per minute (Fig. 5a). No specific proliferation was observed using sorted cells from uninfected animals stimulated with MBSE and CF (net counts per minute <100). Comparison of the responses of T cells from control and infected animals showed significant proliferation of purified WC1+ and CD4+ cells in response to MBSE and CF antigens (P < 0.001).
FIG. 5.
Proliferation and IFN-γ release by purified CD4+ or WC1+ T cells and APC only from M. bovis-infected animals in response to MBSE and CF antigens. CD4+ and WC1+ cells were cultured in the presence of autologous mitomycin C-treated PBMC as APC. APC alone were included as controls. (a) Lymphocyte proliferation expressed as mean net counts per minute in excess of control counts per minute (net counts per minute = antigen counts per minute − control PBS counts per minute). (b) IFN-γ release expressed as ODI (ODI of >2 was considered positive). Results are means ± standard errors of the means for four M. bovis-infected cattle (group 2). Experiments were repeated four times. Results of statistical comparisons between PBS control and MBSE- or CF-stimulated cultures for T-cell proliferation and IFN-γ production are shown (∗, P < 0.05; ∗∗∗, P < 0.001).
Measurement of IFN-γ release revealed that MBSE and CF induced purified CD4+ T cells from infected animals to secrete high levels of IFN-γ (ODI > 14), while much lower levels were detected in WC1+ cultures (ODI < 5) (Fig. 5b). No specific release of IFN-γ was observed using sorted CD4+ or WC1+ T cells from uninfected animals stimulated with the M. bovis antigens (ODI < 2). Comparison of the responses of T cells from control and infected animals revealed that CD4+ T cells from infected animals released significant levels of IFN-γ in response to both antigens (P < 0.001), while purified WC1+ T cells only released significant levels of IFN-γ in response to CF (P < 0.05) (data not shown).
DISCUSSION
There has been growing interest in the role of γδ T cells during immune responses, especially in relation to tuberculosis. An increasing body of evidence has shown human and murine γδ T cells responding to mycobacterial antigens (3, 20, 23, 50, 58), possibly displaying many of the effector functions described for αβ T cells (32). The present investigation used a natural host disease model to study the responses of bovine γδ T cells, in comparison with those of αβ T cells, to mycobacterial antigens. The results have indicated a high activation status of bovine γδ in response to M. bovis antigens within this experimental model. The significance of these responses in terms of protective immunity and CMI-based diagnostic tests requires further investigation.
Initial two-color FCA of PBMC T-cell subset activation following 24 h of culture with MBSE revealed powerful activation of CD4+ and, in particular, WC1+ T cells. While such activation was greatest in infected cattle, it also occurred to a lesser degree in uninfected animals. This was possibly as a consequence of using MBSE, which, like purified protein derivative (PPD), is a very complex mixture of antigens, containing many undefined components. The use of such complex antigens could potentially have resulted in some nonspecific lymphocyte activation in the short-term cultures. Alternatively, the high proportion of WC1+ CD25+ cells observed in uninfected control animals in response to MBSE (Fig. 2) may suggest a high frequency of precursors within the WC1+ population which are capable of responding to M. bovis antigens. There may also be a degree of cross-reactivity with antigens shared with environmental mycobacterial species, resulting in some γδ T-cell activation.
Doherty et al. (21) studied the tuberculin skin reaction in cattle and demonstrated WC1+ γδ T cells to be the dominant lymphocyte population in the earliest perivascular infiltrate (6 to 24 h) after intradermal injection of PPD, followed by increasing αβ T-cell infiltration. Early infiltration of γδ- and IL-2R (CD25+)-bearing T cells (21) into the skin test site, together with the present findings of rapid activation and proliferation in response to M. bovis antigens, suggests that γδ cells could possibly have a direct or indirect effect on CMI-based diagnostic tests. It may be, for example, that bovine γδ T cells contribute significantly to the development of the skin test response, especially as they may exist in a preactivated state (16) and may become rapidly activated in response to MBSE. Additionally, it has been shown that WC1+ cells are involved in recruiting other cells to sites in response to M. bovis antigens (55). However, the exact mechanism by which bovine γδ cells become activated is the subject of ongoing studies.
Following 24 h of culture in the absence of antigens, there was a relatively high level of CD25 expressed by γδ T cells (up to 40%), and it has been reported previously that WC1+ cells express CD25 constitutively (16). A ready availability of IL-2R may imply a particular sensitivity to IL-2, and it has been suggested that these cells have a lower threshold for induction of proliferation (34). Thus, there exists the possibility that the level of activation seen within the WC1+ population may be partly due to nonspecific stimulation by IL-2 secreted by CD4+ cells responding to an antigen(s). Indeed, the present study of the kinetics of in vitro activation indicated that CD4+ cells became activated in advance of γδ T cells. Significant CD4+ T-cell activation occurred after 12 to 18 h of culture and was followed after 24 h of culture by rises in the level of activation within the total γδ T-cell population (TCR1+), mirroring the response profile of the WC1+ subpopulation. Previous investigations with sheep have shown that concanavalin A induced maximal activation of CD4+ T cells at 24 to 48 h of culture, while WC1+ T cells become activated after only 12 h of culture (11). The present observation of a slightly faster rate of activation of CD4+ T cells may simply be a feature of the bovine system but may also reflect differences in antigen versus mitogen stimulation.
Elloso et al. (22) demonstrated the human γδ T-cell proliferative response in response to malarial antigens to be dependent on CD4+ T-cell secretion of cytokines that signal through IL-2R. In the bovine system it has been reported that purified WC1+ T cells require the addition of exogenous IL-2 for proliferation in response to Thileria annulata-infected autologous cells but that IL-2 alone induces a limited WC1+ proliferative response (16). Other studies have also reported that IL-2 alone induces minimal proliferation of bovine WC1+ cells, implying that IL-2 is required as a secondary signal for activation of γδ cells (15, 29), driving them to proliferation (16). In the present study, the high degree of proliferation of purified WC1+ T cells in response to MBSE and CF was observed only in infected animals, indicating the specificity of the response to M. bovis antigens, with a possible requirement for IL-2 and/or other cytokines supplied by other cells. While the mitomycin C-treated PBMC acted as APC and a possible source of cytokines (including IL-2) within the sorted LPA cultures, it should be noted that little IFN-γ production was detected in APC or WC1/APC cultures from infected animals following stimulation with M. bovis antigen. Although IL-2 levels were not measured within this set of experiments, any activation and proliferation of bovine γδ T cells which were detected following stimulation with M. bovis antigens and influenced by IL-2 are likely to reflect in vivo responses to mycobacterial antigens, where several populations of T cells will be involved. Therefore, results in this paper highlight the activation status of γδ T cells in terms of bovine tuberculosis. Clearly the mechanisms involved in their activation and proliferation and the effect of cytokines influencing their responses (such as IL-2) should be a major area for further investigation, allowing the biological significance of this population of T cells to be fully appreciated.
Interestingly, in the present study both MBSE and CF induced strong proliferation of WC1+ T cells. Culture filtrates are known to be rich in immunodominant secretory antigens, which are considered important in early responses (2). Such antigens have been shown to be strong inducers of IFN-γ production in skin tuberculin test reactor cattle (37). The IFN-γ pathway is known to be crucial to the development of protective immunity against mycobacteria in mice (33), and, in humans, mutations in the gene for the IFN-γ receptor result in a much greater susceptibility to mycobacterial infection (46). Here it was observed that CD4+ T cells from infected cattle were major producers of IFN-γ in response to both CF and MBSE, and we have previously shown bovine CD8+ cells to be an important source of IFN-γ in M. bovis infection (36). Human M. tuberculosis-reactive γδ T-cell lines have been observed to produce greater amounts of IFN-γ than αβ T-cell lines (4). In this study the purified WC1+ T-cell cultures from infected animals were found to release small quantities of IFN-γ in response to the M. bovis antigens. Collins et al. (16) found that WC1+ cells did not produce IFN-γ mRNA, although the message has been observed in bovine γδ T-cell lines (47).
The potent activation and proliferation of WC1+ T cells induced by M. bovis antigens demonstrate clearly their involvement in the CMI response to M. bovis, but their role in antimycobacterial immunity remains undefined. It has been demonstrated previously that bovine γδ T cells influence the proliferative responsiveness of CD4+ cells in Mycobacterium paratuberculosis infection (14). Other studies have indicated a role for WC1+ cells in the modulation of antibody responses (27), and the expression of CD40L on bovine γδ cells may be involved in macrophage activation and may facilitate helper activity in response to B cells (25).
It has recently been suggested that bovine WC1− and WC1+ cells represent functionally distinct subsets of γδ T cells which preferentially home to different tissue locations (39). In addition, it has been shown using a SCID-bo mouse model that WC1+ T cells play a pivotal, early role in the recruitment of various cell types to sites of M. bovis infection (55). The data presented here indicate that the WC1+ population of PBMC form the main population of mycobacterially activated bovine γδ T cells, implying that WC1+ expression has an important role in the antigen responsiveness and function of these cells.
Recent reports have suggested that the function of WC1 is to regulate γδ T-cell proliferation through growth arrest (56). This growth arrest was found to correlate with tyrosine phosphatase activation and could be reversed through signalling via the TCR-CD3 complex (34). It was postulated that such a mechanism may control IL-2 responsiveness, while retaining the antigen sensitivity of WC1+ T cells (56). WC1 gene families in other species have been described (61), and WC1 expression on porcine γδ T cells has been demonstrated (13). In humans and mice there appear to be WC1 genes, with human genomic WC1 sequences showing 85% homology to those of bovine WC1 (62). However, while γδ T-cell subsets defined by the expression of distinctly rearranged γδ TCRs exist in other species (7, 8), these have not been associated with the expression of any other specific surface molecules. Therefore, as there is no evidence of a human or rodent WC1 homologue, it is possible that bovine γδ T cells (in particular the WC1+ cells) have additional, potentially important roles.
In conclusion the results of this study clearly demonstrate that γδ T cells, and in particular the WC1+ cells, from M. bovis-infected cattle become highly activated and proliferate strongly in vitro in response to M. bovis antigenic stimulation. Along with previous reports of early infiltration of WC1+ cells in response to PPD (21), recruitment of cells to sites of infection by WC1+ cells (55), and the influence of WC1+ cells on other lymphocyte functions (14, 27), the present findings imply that γδ cells play a central role in immune responses to bovine tuberculosis. While their role in protective immunity requires further investigation, some consideration should also be given to the responses of bovine γδ T cells in response to M. bovis antigens in terms of their influence on the performance of diagnostic tests that are dependent on CMI responses, in particular the skin test. Although purified WC1+ γδ cells do not appear to be major producers of IFN-γ, their influence on the ability of other T cells to produce cytokines and respond to mycobacterial antigens has not yet been fully investigated.
ACKNOWLEDGMENTS
We thank Deirdre Fitzpatrick for statistical analyses and S. D. Neill and D. P. Mackie for their assistance.
This work was supported by the Department of Agriculture and Rural Development for Northern Ireland (DARD) and a Department of Agriculture Ph.D. studentship.
REFERENCES
- 1.Amadori M, Archetti I, Verardi R, Berneri C. Role of distinct population of bovine γδ T cells in the immune response to viral agents. Viral Immunol. 1995;8:81–91. doi: 10.1089/vim.1995.8.81. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Askgaard D, Gottschau A, Bennedsen J, Nagai S, Heron I. Identification of immunodominant antigens during infection with Mycobacterium tuberculosis. Scand J Immunol. 1992;36:823–831. doi: 10.1111/j.1365-3083.1992.tb03144.x. [DOI] [PubMed] [Google Scholar]
- 3.Balaji K N, Boom W H. Processing of Mycobacterium tuberculosis bacilli by human monocytes for CD4+ αβ and γδ T cells: role of particulate antigen. Infect Immun. 1998;66:98–106. doi: 10.1128/iai.66.1.98-106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes P F, Grisso C L, Abrams J S, Band H, Rea T H, Modlin R L. γδ T lymphocytes in human tuberculosis. J Infect Dis. 1992;165:506–512. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 5.Batoni G, Esin S, Harris R A, Källenhius G, Svenson S B, Andersson R, Campa M, Wigzell H. γδ+ and CD4+ αβ+ human T cell subset responses upon stimulation with various Mycobacterium tuberculosis soluble extracts. Clin Exp Immunol. 1998;112:52–62. doi: 10.1046/j.1365-2249.1998.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batoni G, Esin S, Pardini M, Bottai D, Senesi S, Wigzell H, Campa M. Identification of distinct lymphocyte subsets responding to subcellular fractions of Mycobacterium bovis bacille Calmette-Guerin (BCG) Clin Exp Immunol. 2000;119:270–279. doi: 10.1046/j.1365-2249.2000.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binns R M, Duncan I A, Powis S J, Hutchings A, Butcher G W. Subsets of null and γδ T cell receptor+ T lymphocytes in the blood of young pigs identified by specific monoclonal antibodies. Immunology. 1992;77:219–227. [PMC free article] [PubMed] [Google Scholar]
- 8.Bonneville M, Janeway C A, Jr, Ito K, Haser W, Ishida I, Nakanishi N, Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of γδ T cells. Nature. 1988;336:479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- 9.Boom W H, Balaji K N, Nayak R, Tsukaguchi K, Chervenak K A. Characterization of a 10- to 14-kilodalton protease-sensitive Mycobacterium tuberculosis H37Ra antigen that stimulates human γδ T cells. Infect Immun. 1994;62:5511–5518. doi: 10.1128/iai.62.12.5511-5518.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucy R P, Chen C-L H, Cooper M D. Tissue localisation and CD8 accessory molecule expression of T γδ cells in humans. J Immunol. 1989;142:3045–3049. [PubMed] [Google Scholar]
- 11.Bujdoso R, Lund B T, Evans C W, McConnell I. Different rates of interleukin 2 receptor expression by ovine γ/δ and α/β T cells. Vet Immunol Immunopathol. 1993;39:109–114. doi: 10.1016/0165-2427(93)90170-9. [DOI] [PubMed] [Google Scholar]
- 12.Caffery J P. Status of bovine tuberculosis eradication programmes in Europe. Vet Microbiol. 1994;40:1–4. doi: 10.1016/0378-1135(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 13.Carr M M, Howard C J, Sopp P, Manser J M, Parsons K R. Expression on porcine γδ lymphocytes of a phylogenetically conserved surface antigen previously restricted in the expression to ruminant γδ T lymphocytes. Immunology. 1994;81:36–40. [PMC free article] [PubMed] [Google Scholar]
- 14.Chiodini R A, Davis W C. The cellular immunology of bovine paratuberculosis: the predominant response is mediated by cytotoxic gamma/delta T lymphocytes which prevent CD4+ activity. Microb Pathog. 1992;13:447–463. doi: 10.1016/0882-4010(92)90012-d. [DOI] [PubMed] [Google Scholar]
- 15.Clevers H, MacHugh N D, Bensaid A, Dunlap S, Baldwin C L, Kaushal A, Iams K, Howard C J, Morrison W I. Identification of a bovine surface antigen uniquely expressed on CD4−CD8− T-cell receptor γδ T lymphocytes. Eur J Immunol. 1990;20:809–817. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- 16.Collins R A, Sopp P, Gelder K I, Morrison W I, Howard C J. Bovine γδ TcR+ T lymphocytes are stimulated to proliferate by autologous Theileria annulata-infected cells in the presence of interleukin-2. Scand J Immunol. 1996;44:444–452. doi: 10.1046/j.1365-3083.1996.d01-332.x. [DOI] [PubMed] [Google Scholar]
- 17.Constant P, Poquet Y, Peyrat M-A, Davodeau F, Bonneville M, Fournie J-J. The antituberculous Mycobacterium bovis BCG vaccine is an attenuated mycobacterial producer of phosphorylated nonpeptidic antigens for human γδ T cells. Infect Immun. 1995;63:4628–4633. doi: 10.1128/iai.63.12.4628-4633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daborn C J, Grange J M. HIV/AIDS and its implications for the control of animal tuberculosis. Br Vet J. 1993;149:405–417. doi: 10.1016/S0007-1935(05)80107-1. [DOI] [PubMed] [Google Scholar]
- 19.Daubenberger C, Taracha E, Gaidulis L, Davis W, McKeever D. Bovine γδ T-cell responses to the intracellular protozoan parasite Theileria parva. Infect Immun. 1999;67:2241–2249. doi: 10.1128/iai.67.5.2241-2249.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieli F, Sireci G, Di Sano C, Romano A, Titone L, Di Carlo P, Ivanyi J, Fournie J, Salerno A. Ligand-specific αβ and γδ T cell responses in childhood tuberculosis. J Infect Dis. 2000;181:294–301. doi: 10.1086/315180. [DOI] [PubMed] [Google Scholar]
- 21.Doherty M L, Bassett H F, Quinn P J, Davis W C, Kelly A P, Monaghan M L. A sequential study of the bovine tuberculin reaction. Immunology. 1996;87:9–14. [PMC free article] [PubMed] [Google Scholar]
- 22.Elloso M M, van der Heyde H C, Troutt A, Manning D D, Weidanz W P. Human γδ T cell subset-proliferative response to malarial antigen in vitro depends on CD4+ T cells or cytokines that signal through components of the IL-2R. J Immunol. 1996;157:2096–2102. [PubMed] [Google Scholar]
- 23.Esin S, Batoni G, Kallenius G, Gaines H, Campa M, Svenson S B, Andersson R, Wigzell H. Proliferation of distinct human T cell subsets in response to live, killed or soluble extracts of Mycobacterium tuberculosis and Myco. avium. Clin Exp Immunol. 1996;104:419–425. doi: 10.1046/j.1365-2249.1996.d01-691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Y-X, Cranfill R, Vollmer M, van der Zee R, O'Brien R L, Born W. In vivo response of murine γδ T cells to a heat shock protein derived peptide. Proc Natl Acad Sci USA. 1993;90:322–326. doi: 10.1073/pnas.90.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirano A, Brown W C, Tuo W, Estes D M. Kinetics of expression and subset distribution of expression of the TNF superfamily members CD40 ligand and Fas ligand on T lymphocytes in cattle. Vet Immunol Immunopathol. 1998;61:251–263. doi: 10.1016/s0165-2427(97)00155-4. [DOI] [PubMed] [Google Scholar]
- 26.Howard C, Morrison I. Leukocyte antigens in cattle, sheep and goats. Proceedings of the 1st International Workshop on Leukocyte Antigens in Cattle, Sheep and Goats. Vet Immunol Immunopathol. 1991;27:17–34. [PubMed] [Google Scholar]
- 27.Howard C J, Sopp P, Parsons K R, Finch J. In vivo depletion of BoT4 (CD4) and of non-T4/T8 lymphocyte subsets in cattle with monoclonal antibodies. Eur J Immunol. 1989;19:757–764. doi: 10.1002/eji.1830190428. [DOI] [PubMed] [Google Scholar]
- 28.Howard C J, Naessens J. Summary of workshop findings for cattle. Vet Immunol Immunopathol. 1993;39:25–48. doi: 10.1016/0165-2427(93)90161-v. [DOI] [PubMed] [Google Scholar]
- 29.Howard C J, Sopp P, Brownlie J, Parsons K R, Kwong L-S, Collins R A. Afferent lymph veiled cells stimulate proliferative responses in allogenic CD4+ and CD8+ T cells but not γδ TCR+ T cells. Immunology. 1996;88:558–564. doi: 10.1046/j.1365-2567.1996.d01-680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itohara S, Nakanishi N, Kanagawa O, Kubo R, Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor γδ—analysis of γδ cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci USA. 1989;86:5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jirillo E, Munro I, Tortorella C, Antonaci S. The immune response to mycobacterial infection. Med Sci Res. 1989;17:929–934. [Google Scholar]
- 32.Kaufmann S H E. γδ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawamura I, Tsukada H, Yoshikawa H, Fujita M, Nomoto K, Mitsuyama M. IFN-γ-producing ability as a possible marker for the protective T cells against Mycobacterium bovis BCG in mice. J Immunol. 1992;148:2887–2893. [PubMed] [Google Scholar]
- 34.Kirkham P A, Takamatsu H-H, Parkhouse R M E. Growth arrest of γδ T cells induced by monoclonal antibody against WC1 correlates with activation of multiple tyrosine phosphatases and dephosphorylation of MAP kinase erk2. Eur J Immunol. 1997;27:717–725. doi: 10.1002/eji.1830270321. [DOI] [PubMed] [Google Scholar]
- 35.Ladel C H, Daugelat S, Kaufmann S H E. Immune responses to Mycobacterium bovis bacille Calmette Guerin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 36.Liébana E, Girvin R M, Welsh M, Neill S D, Pollock J M. Generation of CD8+ T-cell responses to Mycobacterium bovis and mycobacterial antigen in experimental bovine tuberculosis. Infect Immun. 1999;67:1034–1044. doi: 10.1128/iai.67.3.1034-1044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lightbody K A, Girvin R M, Pollock D A, Mackie D P, Neill S D, Pollock J M. Recognition of a common mycobacterial T-cell epitope in MPB59 of Mycobacterium bovis. Immunology. 1998;93:314–322. doi: 10.1046/j.1365-2567.1998.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacHugh N D, Taracha E L, Toye P G. Reactivity of workshop antibodies with COS cell transfectants expressing bovine CD antigens. Vet Immunol Immunopathol. 1993;39:61–67. doi: 10.1016/0165-2427(93)90164-y. [DOI] [PubMed] [Google Scholar]
- 39.MacHugh N D, Mburu J K, Carol M J, Wyatt C R, Orden J A, Davis W C. Identification of two distinct subsets of bovine γδ T cells with unique cell surface phenotype and tissue distribution. Immunology. 1997;92:340–345. doi: 10.1046/j.1365-2567.1997.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackay C R, Maddox J F, Brandon M R. Three distinct subpopulations of sheep T lymphocytes. Eur J Immunol. 1986;16:19–25. doi: 10.1002/eji.1830160105. [DOI] [PubMed] [Google Scholar]
- 41.Mackay C R, Hein W R. A large proportion of bovine T cells express the γδ T cell receptor and show a distinct tissue distribution and surface phenotype. Int Immunol. 1989;1:540–545. doi: 10.1093/intimm/1.5.540. [DOI] [PubMed] [Google Scholar]
- 42.Morrison W I, Davis W C. Differentiation antigens expressed predominantly on CD4− CD8− T lymphocytes (WC1, WC2) Vet Immunol Immunopathol. 1991;27:71–76. doi: 10.1016/0165-2427(91)90082-n. [DOI] [PubMed] [Google Scholar]
- 43.Munk M E, Gatrill A J, Schoel B, Gulle H, Pfeffer K, Wagner H, Kaufmann S H E. Immunity to mycobacteria: possible role of alpha/beta and gamma/delta T lymphocytes. Acta Pathol Microbiol Immunol Scand. 1990;98:669–673. doi: 10.1111/j.1699-0463.1990.tb04987.x. [DOI] [PubMed] [Google Scholar]
- 44.Neill S D, Hanna J, O'Brien J J, McCracken R M. Excretion of Mycobacterium bovis by experimentally-infected cattle. Vet Rec. 1988;123:340–345. doi: 10.1136/vr.123.13.340. [DOI] [PubMed] [Google Scholar]
- 45.Neill S D, Pollock J M, Bryson D B, Hanna J. Pathogenesis of Mycobacterium bovis infection in cattle. Vet Microbiol. 1994;40:41–52. doi: 10.1016/0378-1135(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 46.Newport M J, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 47.Norimine J, Ramiya V, Amills M, Olmstead C A, Lewin H A. Cytokine mRNA expression in bovine γδ T cell lines. FASEB J. 1998;12:3603. [Google Scholar]
- 48.O'Brien R L, Fu Y-X, Cranfill R, Dallas A, Ellis C, Reardon C, Lang J, Carding S R, Kubo R, Born W. Heat shock protein Hsp60-reactive γδ cells: a large, diversified T-lymphocyte subset with highly focused specificity. Proc Natl Acad Sci USA. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orme I M, Collins F M. Protection against Mycobacterium tuberculosis infection by adoptive transfer. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orme I M, Miller E S, Roberts A D, Furney S K, Griffin J P, Dobos K M, Chi D, Rivoire B, Brennan P J. T lymphocytes mediating protection and cellular cytolosis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–196. [PubMed] [Google Scholar]
- 51.Orme I M, Andersen P, Boom W H. Cell responses to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 52.Pollock J M, Douglas A J, Mackie D P, Neill S D. Identification of bovine T-cell epitopes for three Mycobacterium bovis antigens: MPB70, 19,000 MW and MPB57. Immunology. 1994;82:9–15. [PMC free article] [PubMed] [Google Scholar]
- 53.Pollock J M, Pollock D A, Campbell D G, Girvin R M, Crockard A D, Neill S D, Mackie D P. Dynamic changes in circulating and antigen-responsive T-cell subpopulations post-Mycobacterium bovis infection in cattle. Immunology. 1996;87:236–241. doi: 10.1046/j.1365-2567.1996.457538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ritacco V, López B, De Kantor I N, Barrera L, Errico F, Nader A. Reciprocal cellular and humoral immune responses in bovine tuberculosis. Res Vet Sci. 1991;50:365–367. doi: 10.1016/0034-5288(91)90143-c. [DOI] [PubMed] [Google Scholar]
- 55.Smith R A, Kreeger J M, Alvarez A J, Goin J C, Davis W C, Whipple D L, Estes D M. Role of CD8+ and WC-1+ γ/δ T cells in resistance to Mycobacterium bovis infection in the SCID-bo mouse. J Leukoc Biol. 1999;65:28–34. doi: 10.1002/jlb.65.1.28. [DOI] [PubMed] [Google Scholar]
- 56.Takamatsu H-H, Kirkham P A, Michael R, Parkhouse E. A γδ T cell specific surface receptor (WC1) signalling G0/G1 cell cycle arrest. Eur J Immunol. 1997;27:105–110. doi: 10.1002/eji.1830270116. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka Y, Morita C T, Tanaka Y, Nieves E, Brenner M B, Bloom B R. Natural and synthetic non-peptide antigens recognised by human γδ T-cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 58.Tsukaguchi K, Balaji N K N, Boom W H. CD4+ αβ T cell and γδ T cell responses to Mycobacterium tuberculosis. Similarities and differences in antigen recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- 59.Walcheck B, Watts G, Jutila M A. Bovine γ/δ T cells bind E-selectin via a novel glycoprotein receptor: first characterization of a lymphocyte/E-selectin interaction in an animal model. J Exp Med. 1993;178:853–863. doi: 10.1084/jem.178.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson R A, Zolnai A, Rudas P, Frenyo L V. T-cell subsets in blood and lymphoid tissues obtained from fetal calves, maturing calves and adult bovine. Vet Immunol Immunopathol. 1996;53:49–60. doi: 10.1016/0165-2427(95)05543-6. [DOI] [PubMed] [Google Scholar]
- 61.Wjingaard P L J, Metzelaar M J, MacHugh N D, Morrison W I, Clevers H C. Molecular characterisation of the WC1 antigen expressed specifically on bovine CD4− CD8− γδ T lymphocytes. J Immunol. 1992;149:3273–3277. [PubMed] [Google Scholar]
- 62.Wjingaard P L J, MacHugh N D, Metzelaar M J, Romberg S, Bensaid A, Pepin L, Davis W C, Clevers H C. Members of the novel WC1 gene family are differentially expressed on subsets of bovine CD4−CD8− γδ T lymphocytes. J Immunol. 1994;152:3476–3482. [PubMed] [Google Scholar]