Abstract
Plasmodium falciparum-infected erythrocytes (PfIEs) present P. falciparum erythrocyte membrane protein 1 proteins (PfEMP1s) on the cell surface, via which they cytoadhere to various endothelial cell receptors (ECRs) on the walls of human blood vessels. This prevents the parasite from passing through the spleen, which would lead to its elimination. Each P. falciparum isolate has about 60 different PfEMP1s acting as ligands, and at least 24 ECRs have been identified as interaction partners. Interestingly, in every parasite genome sequenced to date, at least 75% of the encoded PfEMP1s have a binding domain for the scavenger receptor CD36 widely distributed on host endothelial cells and many other cell types. Here, we discuss why the interaction between PfIEs and CD36 is optimal to maintain a finely regulated equilibrium that allows the parasite to multiply and spread while causing minimal harm to the host in most infections.
Keywords: Plasmodium falciparum, malaria, sequestration, cytoadhesion, endothelial cell receptor, CD36
1. Introduction
Despite progress in malaria control, malaria remains one of the most important infectious diseases worldwide. In 2020, about 267 million malaria cases, including 409,000 deaths, were recorded [1]. Concerning all malaria parasites, the deadliest, Plasmodium falciparum, has a complex life cycle that alternates between Anopheles mosquitoes and humans. The asexual cycle that takes place in humans consists of the liver stage (multiplication of the parasite in hepatocytes) and the intraerythrocytic cycle (multiplication of the parasite in erythrocytes). Each intraerythrocytic cycle lasts approximately 48 h, during which the merozoite that invaded the erythrocyte develops through the ring stage to the trophozoite and finally the schizont. At the end of the intraerythrocytic cycle, the newly formed merozoites are released, ready to invade new erythrocytes. Some merozoites develop into gametocytes, which must be taken up by female Anopheles mosquitoes to complete their sexual development. The complexity of the parasite’s life cycle and its masterful ability to evade its elimination by the host immune system challenge our efforts to combat the disease.
To survive in the human host, P. falciparum has evolved unique mechanisms, two of which, called sequestration and antigenic variation, rely mainly on a highly diverse protein family, the P. falciparum erythrocyte membrane protein 1 (PfEMP1). The PfEMP1s, which the parasite exposes on the surface of its host cell from the trophozoite stage onwards, have at least a dual function. First, they bind to various endothelial cell receptors (ECRs) on the walls of blood vessels (sequestration), thereby disappearing from the peripheral circulation and bypassing removal by the spleen. Unlike other plasmodial species, the deformability of P. falciparum infected erythrocytes (PfIEs) decreases as the parasite matures, so that circulating trophozoites and schizonts would be retained in the spleen and removed from circulation by resident macrophages [2,3,4,5,6]. Second, PfEMP1 represents the main target of the humoral immune response [7], but due to the presence of numerous copies of var genes encoding PfEMP1, the parasite can sequentially present different PfEMP1 variants on the surface of its host cell and use them for sequestration. The ability to alter the presented PfEMP1 by antigenic variation enables the parasite to stay one step ahead of the immune system and maintain long-lasting, chronic infections, e.g., for bridging dry seasons [8,9,10,11,12].
2. The PfEMP1 Family
The PfEMP1 family is encoded by about 45–90 var genes per parasite genome [12]. Expression of the var genes is mutually exclusive in ring-stage parasites, such that only a single PfEMP1 variant is present on the surface of trophozoite- or schizont-stage PfIEs at any given time [13,14] for review [10]. Mutually exclusive expression relies on very complex mechanisms. These are based on both epigenetic regulation and cis-acting DNA elements and RNA transcripts involved in var gene activation and silencing (for review [10]).
The var genes and their encoding PfEMP1s vary greatly from parasite to parasite, and recombination constantly generates new variants, so there is an enormous repertoire of var genes in nature [13,14,15,16]. The molecular masses of PfEMP1s range from 150 to 400 kDa. These proteins consist of an intracellular acidic terminal segment (ATS domain), a transmembrane domain, and a variable, extracellularly exposed region responsible for receptor binding. This extracellular region contains a single N-terminal segment (NTS; main classes A, B, and pam) and a variable number of different Duffy binding-like domains (DBL; main classes α–ζ and pam) and cysteine-rich interdomain regions (CIDR; main classes α–δ and pam) [17,18,19,20]. Approximately two-thirds of var genes localize in the subtelomeric regions of the chromosomes. Most of the subtelomeric and central localized var genes are located in regions of electron-dense heterochromatin at the nuclear periphery, with the active var gene shifting to the region of lower electron density [14]. Depending on the chromosomal localization, the upstream sequence, and the direction of transcription of the var genes, PfEMP1s can be classified as A, B, C, or E [17,21,22,23].
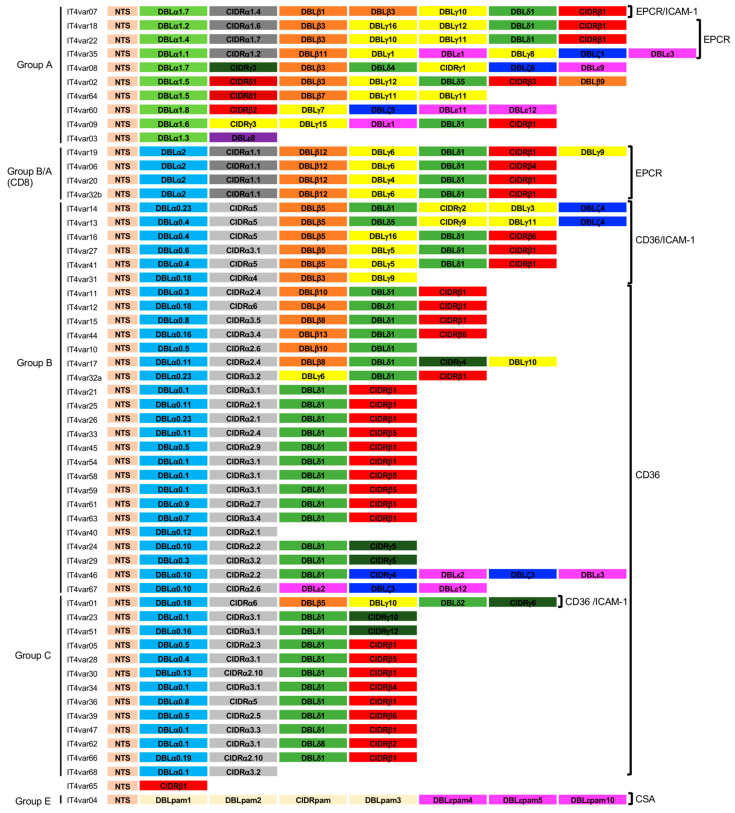
A few conserved, strain-transcendent var variants have been described: var1, var2csa (group E), and var3. The var1 gene occurs in two variants in the parasite population, var1-3D7 and -IT, is often conserved as a pseudogene, and the encoded protein may not be presented on the erythrocyte surface [12,24]. VAR2CSA has an atypical domain architecture, mediates binding to chondroitin sulfate A (CSA) in the placenta, and is, thus, important in pregnancy-associated malaria [25]. VAR3 proteins are very short PfEMP1s with unknown receptor binding phenotypes [26]. Analysis of 399 different PfEMP1 sequences from seven P. falciparum genomes allowed the identification of 23 domain cassettes (DCs) that could be important for protein folding and binding to human ECRs, as well as for reflecting recombination breakpoints [17]. About 10% of PfEMP1s variants belong to group A and are usually longer proteins with a head structure that includes a DBLα1 and either a CIDRα1 domain (CIDRα1.4–7) that binds to the endothelial protein C receptor (EPCR) or a CIDRβ/γ/δ domain with unknown receptor binding phenotype [12]. Groups B and C make up the majority of PfEMP1s (at least 75%) and typically have DBLα0-CIDRα2–6 head structures that bind to CD36, followed by only two additional extracellular domains (DBLδ1, CIDRβ/γ). A subset of chimeric B-type proteins (group B/A, also known as DC8-containing proteins) has a DBLα2 domain (chimeric DBLα0/1 domain) and an EPCR-binding CIDRα1.1 or CIDRα1.8 domain typically attached to a complement component C1q receptor (C1qR)-binding DBLβ12 domain [27,28,29,30,31,32,33,34]. Thus, the head structure confers mutually exclusive binding properties to either EPCR (14%), CD36 (72%), CSA (3%), or to one or more unknown ECRs via the CIDRβ/γ/δ domains (10%) or VAR3 (1%) [35]. Concerning the C-terminal to the head structure, most PfEMP1s have additional DBL domains, of which certain subsets of the DBLβ domains bind intercellular adhesion molecule-1 (ICAM-1) [36,37] or C1qR [38]. As an example, the PfEMP1 repertoire of the P. falciparum isolate IT4 is shown in Figure 1.
Figure 1.
PfEMP1 repertoire of the P. falciparum isolate IT4, adapted from [17]. ECR binding phenotype [36]. Color code: light brown: N-terminal segment (NTS); bright green: Duffy binding-like (DBL)α1; light blue: DBLα2, DBLα0; dark grey: Cys rich inter-domain regions (CIDR)α1; light grey: CIDRα2–6; dark green: CIDRγ; orange: DBLβ; yellow: DBLγ; green: DBLδ; pink: DBLε; blue: DBLζ; purple: DBLε; IT4var04: light yellow: DBL/CIDRpam: pink: DBLεpam.
3. Knobs—Anchor Point for PfEMP1s
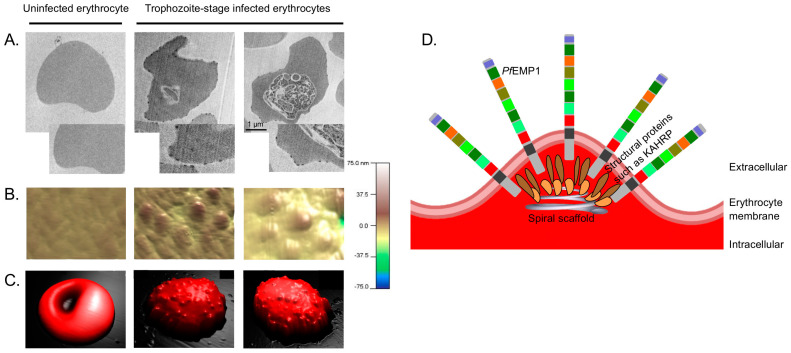
PfEMP1s are concentrated in nanoscale, electron-dense protrusions of the plasma membrane of PfIEs, the so-called knobs. They are formed in erythrocytes about 16 h after parasite invasion and reach their highest density 20 h after infection [39,40]. Single knobs have a hemispherical ellipsoid shape with a minor axis of 20 nm and a major axis of 120 nm [41]. Knobs are composed of various submembrane structural proteins, including the major protein of this structure, knob-associated histidine-rich protein (KAHRP). These consist of PfEMP3, the ring-infected red cell antigen (RESA), the mature parasite-infected red cell surface antigen (MESA)/PfEMP2, and Pf332 [41,42]. The knobs consist of a highly organized skeleton made of a spiral structure located beneath specialized areas of the erythrocyte membrane (Figure 2) [43]. The arrangement of PfEMP1s in a cluster near the top of the knobs is assumed to increase the binding capacity of PfIEs, especially under flow conditions (see below) [44,45,46,47].
Figure 2.
The knobs of PfIEs. (A) Transmission electron micrographs of uninfected and synchronised trophozoite-stage P. falciparum culture 24–28 h post-infection. The parasites were cultivated in the presence of human serum (10%), and the PfIEs were subjected to gelatin sedimentation to enrich knobby PfIEs [40,48]. (B,C) atomic force microscopic three-dimensional images of the surface of uninfected and trophozoite-stage PfIEs [40]. Images in (B) show magnifications directly from the membrane surface of the erythrocytes shown in (C). (D) Simplified schematic illustration of the structure of knobs.
4. Endothelial Cell Receptors (ECRs)
At least 24 ECRs were described as binding partners for PfIEs. These include EPCR, gC1qR, ICAM-1, and CD36, mentioned above, as well as platelet endothelial cell adhesion molecule-1 (PECAM-1), CSA (adhesion to placental epithelium) [49], heparan sulphate, hyaluronic acid, neuronal cell adhesion molecule (NCAM), P-selectin, E-selectin, vascular cell adhesion molecule-1 (VCAM-1), thrombospondin, fractalkine, ανβ3- and αVβ6-integrin, fibronectin, CD9, CD151, multidrug resistance protein 1 and 2, erythropoietin receptor 1, and tumour necrosis factor receptor (TNFR) 1 and 2 [6,33,37,48,50,51,52,53]. To date, only a few ECRs have been shown to interact via PfEMP1, and PfEMP1 binding domains have only been identified for CD36, ICAM-1, EPCR, PECAM-1, and gC1qR [18,27,32,33,54].
5. Cytoadhesion of PfIEs
Cytoadhesion of PfIEs to ECRs in the vascular bed of organs, such as the brain, heart, lung, stomach, skin, and kidney is a central component of the pathogenesis of malaria [3,4,5,6,55,56,57]. In addition to the blockage of capillaries by the cytoadhesion of the PfIEs, there is also an increased production of inflammatory cytokines, endothelial dysfunction, and increased vascular permeability in the affected tissue [58,59]. As a result of the immune response triggered by the growth and sequestration of the parasites, patients develop fever, headache, muscle pain, and rigor [3,60,61,62,63]. Depending on the age and immune status of the patient, severe, fatal complications such as cerebral malaria (CM), lung damage, kidney failure, acidosis, and severe anemia may occur [3,62,63]. Both children and adults can be affected by cerebral malaria, but while severe malaria puts children at higher risk for anemia and convulsions, liver dysfunction and kidney failure are more common in adults. In addition, the clinical picture of severe malaria clearly depends on age, with mortality increasing significantly with age [64].
6. Pathology Induced by Cytoadhesion
Different PfEMP1s have different binding properties to ECRs and are associated with different clinical outcomes (see review [4]). Several studies have shown that severe malaria is associated with the expression of group A and B/A PfEMP1s and, in particular, with variants possessing EPCR binding capacities [65,66,67,68,69]. In contrast, infections dominated by CD36-binding parasites show mild disease courses [33,35,68,70,71,72].
Since PfEMP1s are multi-domain proteins, it has already been shown that some variants can mediate adhesion to multiple ECRs (dual binder) [36,37,73,74]. Examples include PfEMP1 variants that interact with ICAM-1 and EPCR or CD36. Dual binding to ICAM-1 and EPCR specifically enhances the binding of PfIEs to endothelial cells (ECs) under physiologically higher shear stresses. Expression of these variants has been associated with an increased risk of developing CM, including induction of brain swelling and disruption of the blood-brain barrier [36,74,75,76]. Less is known about the role of dual ICAM-1 and CD36 binding PfEMP1s, mainly of group B, but ICAM-1 and CD36 have been shown to work together to enhance the binding of PfIEs to microvascular cells [27,37,66,77,78].
7. ECR-Specific Expression in Relation to the Origin of the Endothelial Cells
ECs derived from different organs presenting different ECRs on their cell surface. It is known that EPCR and ICAM-1 are presented on brain ECs and are mainly bound by DC8- and group A PfEMP1s [33,36,74,75,79]. Ortolan and colleagues have recently shown that the same PfEMP1s that cytoadhere to brain ECs also bind EPCR intestinal and renal ECs. In this context, it is suggested that a binding axis between the brain, gut, and kidney may contribute to the multi-organ complications of severe malaria [80]. In contrast, CD36 is not presented on brain ECs, or only in very low amounts, and was also not detected on intestinal and peritubular renal ECs [81,82]. Thus, in contrast to EPCR, CD36 seems to occur mainly in the microvascular beds of non-vital organs. As mentioned above, several studies have linked EPCR- or dual EPCR- and ICAM-1-binding PfEMP1s to severe malaria, which most likely occurs in individuals without preformed immunity [33,68,72,79,83,84,85]. For example, Wichers and colleagues recently demonstrated a clear association between the expression of PfEMP1s, which have an EPCR-binding phenotype, with first-time infection and severe malaria [84]. Transcripts for CD36-binding variants were found more frequently in parasites from non-severely infected and pre-exposed patients.
Interestingly, however, in the same study, CD36-binding variants are overrepresented in all groups of adult malaria patients analyzed, even in severe cases and in first-time infected individuals [84], which is in stark contrast to the pattern seen in severely ill children [70]. The authors speculate that this could be the reason for multisystemic disease symptoms in adult malaria patients. Alternatively, parasites in these less ill adult patients compared to children could have a less dominant expression of EPCR-binding PfEMP1 [84]. Further studies also showed that parasite cytoadhesion to CD36 correlates with the development of mild malaria [70,85,86]. Accordingly, both factors, the already acquired immunity and the age of patients, seem to favor the expression of CD36-binding variants.
8. Hierarchy of var Expression during the Human Blood Phase
Independent analyses of first-generation blood-stage parasites from malaria-naive human volunteers infected with P. falciparum sporozoites have shown remarkably consistent expression of a broad repertoire of var genes, primarily type B (P. falciparum strain NF54: [87,88,89,90]; unpublished data for P. falciparum strain 7G8). This broad expression pattern is modulated by existing host immunity. In African pre-exposed individuals, the expression of many variants at the parasite population level is reduced to very few or a single B-type, possibly reflecting gaps in the host antibody repertoire [91]. In severe disease, expression of var genes shifts toward group A or A/B for unknown reasons, resulting in expression of PfEMP1 with EPCR and/or ICAM-1 or a yet unknown binding domain [65,84,92,93,94,95,96]. Group A PfEMP1s are therefore thought to possess binding phenotypes that confer a selective advantage for parasites to replicate asexually, e.g., by decreasing splenic clearance, but at the same time favors the development of severe malaria [31]. In this context, it has been shown that antibodies for the EPCR binding domains (CIDRα1.1/4–8) are acquired faster and earlier in life than those to the CD36 binding domains (CIDRα2–6) in endemic areas and that this is associated with protection against severe malaria, including CM [31,65,95,97,98]. This raises the question of the evolutionary advantage of A-type expression for the parasite since cytoadhesion in vital organs via EPCR and ICAM-1 may lead to rapid death of infected individuals and thus not to transmission of the parasite to the mosquito. On the other hand, the rapid development of the protection of individuals from severe malaria could therefore be an advantage for the parasites, as they would be less likely to harm their hosts in the event of re-infection [93]. Later in the course of asymptomatic infection, PfIEs appear to have altered cytoadherence properties, as more developed, “older” parasites circulate in the blood than in symptomatic cases. This observation suggests that these parasites either express a lower total amount of PfEMP1 on the host cell surface or a different set of PfEMP1 variants with less adhesive binding domains [99]. Since it is already known that chromosomal location determines the on-and-off rate of var genes [100], it would be plausible that parasites in primary infections initiate expression of the most telomeric B-type var genes, then tend to express A-types during severe disease, but in the case of long-lasting asymptomatic infections may then express centrally located C-types, which are known to have very slow off rates in comparison to subtelomeric var genes. The concept of an initial high var gene switching rate to establish infection and a slower switching rate of later expressed genes to maintain infection was already proposed 20 years ago [101,102].
9. P. falciparum and CD36
Looking at the PfEMP1 family, the question arises why, depending on the parasite genome, between 75–85% of var genes encode PfEMP1s, which have a CIDRα2–6 domain for CD36 binding [17,27,31]. Interestingly, the CIDRα domains were shown to be present only in the P. falciparum-containing branch (clade B) of the Laverania subgenus. This could indicate that the binding to CD36 provides a selective advantage for P. falciparum [103]. What kind of selection advantage this was is yet unclear.
What advantage does the parasite have in retaining this large number of CD36-binding PfEMP1 variants in its genome? Additionally, what is the difference between the individual variants or, more generally, between CD36 binding mediated by group B or C PfEMP1s?
10. CD36
CD36 is a pattern recognition receptor (PRR) that belongs to the class B scavenger receptor family. It is a glycoprotein present in many tissues and involved in several key processes. These include lipid processing and uptake, thrombostasis, glucose metabolism, immune function, angiogenesis, and fat taste (for review [104,105,106,107,108]. CD36 is found on platelets, mononuclear phagocytes, adipocytes, hepatocytes, myocytes, some epithelia and, as mentioned above, expressed on the endothelia of liver, spleen, skin, lung, muscle, and adipose tissue [81,82,109]. On microvascular ECs, CD36 is a receptor for thrombospondin-1 and related proteins and functions as a negative regulator of angiogenesis. At least 60 variants have been described in the coding region of the CD36 gene. The mutations of CD36 caused by gene variants can also influence the adhesion of PfIEs and ECs. This could directly influence the severity of a malaria infection via the degree of cytoadhesion. There are several studies on this, but with contradictory results [110]).
11. CD36 Binding PfEMP1 Variants—Benefits for Parasite and Host
Several observations may help explain why a large number of CD36-binding PfEMP1 variants is not only beneficial for parasite development, but may also be an advantage for the infected host.
The parasite targets a region of CD36 that is essential for its physiological role in fatty acid uptake because mutation of F153 disrupts the interaction of CD36 with CIDRα2–6 but also abolishes the binding of CD36 to oxidized LDL particles. This reduces the likelihood that the human host can escape from PfEMP1 binding by altering its CD36 [28].
In contrast to the EPCR binding surface of CIDRα1 domains, which protrudes and is a structure that is likely to be well recognized by antibodies, the CD36 binding site is concave, and the conserved hydrophobic residues are hidden in a pocket, so maybe they are less easily recognized. In addition, the binding site is surrounded by a sequence-diverse protein surface containing a flexible loop that may make antibody recognition less likely. This unique interaction site of the parasite with CD36, which protects essential residues from exposure to the immune system, appears to allow the parasite to utilize an antigenically diverse set of CIDRα2–6 for cytoadhesion to CD36 to be protected from splenic clearance [28].
CD36 is found in cells of the innate and adaptive immune system [104,105,106,107,108]. It has been shown that PfIEs can adhere to dendritic cells (DCs). This attachment inhibits maturation of these cells and their ability to stimulate T cells. Thus, the parasite can trigger dysregulation of the immune system. This favors the development of the parasite by impairing the host immune system’s ability to clear the infection [108,111,112,113,114]. However, there is also an observation that the mechanism of DC inhibition by PfIEs may be independent of PfEMP1 and CD36 [115].
The previously determined hierarchy of var expression upon parasite entry into human blood begins with group B and suggests that most parasites bind to CD36, as they all encode a CD36-binding phenotype. Most infected individuals, including those who are not immune, do not develop severe malaria, and cytoadhesion of PfIEs occurs in extensive microvascular beds in tissues other than the brain (skin, muscle, adipose tissue). Therefore, cytoadhesion in such non-vital tissues could promote survival and transmission of the parasite while minimizing host damage and death [87,88,89,90].
Antibody-induced selective binding and internalization of CD36 do not result in proinflammatory cytokine production by human macrophages. Interestingly, CD36-mediated phagocytosis of PfIEs also did not result in cytokine secretion by primary macrophages [116]. However, CD36-mediated binding of PfIEs increases the likelihood of phagocytosis by macrophages. This can lead to a reduction in parasitemia, but also allows the parasite to maintain a viable infection without causing too much damage to the host through high parasitemia [108,114,117,118].
DCs react to P. falciparum very early during infection and can, thus, influence the development of immunity. Internalization of PfIEs by DCs and subsequent pro-inflammatory cytokine production of DCs, NK, and T cells depends on CD36. Notably, plasmacytoid DCs regulate innate and adaptive immunity to malaria via the production of proinflammatory cytokines. As this effect is particularly evident at low levels of parasitemia, the role of CD36 for malaria immunity appears to take place early during infection and to promote the development of protective immunity against malaria [118,119].
All these observations underline the importance of CD36 for malaria. During long co-evolution, a fine balance has evolved between host and parasite, allowing the parasite to multiply but harming the host as little as possible.
12. Binding Phenotypes of PfIEs
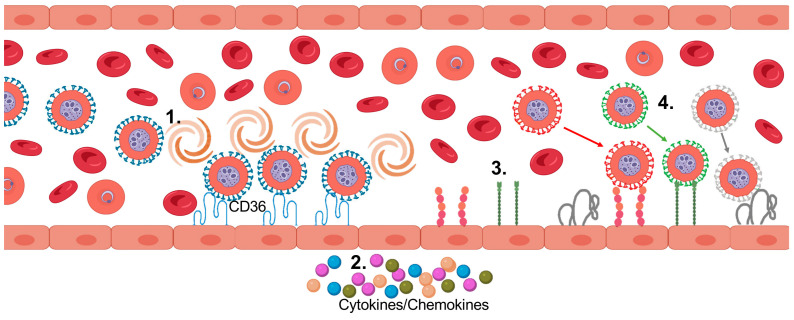
Cytoadhesion of PfIEs is divided into the three phases: “tethering”, “rolling”, and “immobilization”, comparable to leukocyte diapedesis [120,121,122]. However, the dynamics of cytoadhesion of PfIEs to the vascular endothelium is controversial. For example, some authors describe cytoadhesion to ICAM-1 as rolling, and to CD36 as stationary, or vice versa [123,124,125,126,127]. However, there is increasing evidence that PfIEs are very likely to roll over CD36 [126,127,128,129,130,131]. Recently, the binding phenotype for different ECRs was investigated using a laminar flow system with transgenic Chinese hamster ovary (CHO) cells carrying different ECRs on their surface [127]. Rolling was observed upon interaction with CD36, and the rolling behavior of disc-shaped PfIEs at the trophozoite stage (flipping) differed from the rolling behavior of round-shaped PfIEs at the schizont stage (continuous rolling) (Figure 3). Moreover, PfIEs in the schizont stage roll more stably than PfIEs in the trophozoite stage at different shear stresses [127]. The rolling motion of PfIEs was also seen on transgenic mouse fibroblasts presenting CD36 [128] and on recombinant CD36 instead of transgenic eukaryotic cells [129]. As described above, the dermal endothelium has large amounts of CD36. Rolling movements of PfIEs have also been found on dermal ECs, as well as on human skin grafts, on which large amounts of CD36 are found [126,128,130,131]. Additionally, last but not least, the rolling CD36 binding phenotype was also confirmed by in silico modeling [132,133]. However, depending on the experimental setup, the parasite isolates used, and the parasite stage, different velocities were measured at similar shear forces. For trophozoite-stage parasites confronted with recombinant CD36, average velocities between 140 µm/min to 680 µm/min were measured at a shear force of 1.6 Pa, depending on the isolate [129]. When transgenic CHO cells presenting CD36 on the surface were used instead of recombinant CD36 in a similar experimental setup, average velocities ranging from 11 µm/min to 33 µm/min, i.e., a 12–20 fold lower value, were measured, also depending on the parasite stage and isolate [127]. If PfIEs cytoadhere for approximately 30 h during their intraerythrocytic development, they travel distances between 25–122 cm or 2–6 cm, respectively, depending on the experimental setup [127,129]. In both cases, however, the probability of passing over the spleen and being removed accordingly is low.
Figure 3.
Presumed sequence of sequestration of PfIEs to the vascular endothelium. 1. Adhesion and rolling over CD36. 2. Over time: endothelial activation, cytokine release. 3. Cytokine/chemokine-induced presentation of various receptors (e.g., ICAM-1, P-selectin, CD9). 4. Adhesion of PfEMP1s with different binding phenotypes (created with BioRender).
Further studies showed that initial contact of PfIEs to CD36 under flow conditions activates Scr-family kinases, leading to dephosphorylation of CD36 via p130CAS signaling. This increases the binding affinity of PfIEs to CD36 and, thus, leads to increased adhesion of the PfIEs. This mechanism also leads to actin cytoskeletal remodeling and subsequent CD36 clustering, which further increases PfIE adhesion [128,131,134]. It is postulated that a small number of strongly adherent PfIEs activate the endothelium, and thus enhance the cytoadhesion of most parasites [131]. However, the binding mode of PfIEs also seems to be strongly dependent on the respective ECR. For ICAM-1, CD9, P-selectin, as well as CSA, stationary binding, instead of rolling, was observed under flow conditions [127] (Figure 3). Stationary binding to ICAM-1 was also demonstrated in an earlier study [126]. However, while binding to CD36 occurred at shear forces below 4 dyn/cm2, binding to ICAM-1, CD9, P-selectin, and CSA occurred mostly at lower shear forces (from 2 dyn/cm2) [127].
Of note, the origin and environment of the ECR studied (recombinant or presented on eukaryotic cells) also seems to be important for characterising the binding phenotype. Antia and colleagues observed a rolling binding type for PfIEs, with an average rolling velocity of about 10 µm/s at 1–2 kPa and of 1–3 µm/s when recombinant ICAM-1 or CD36 was used, respectively [123]. Interestingly, in the same study, stationary binding of PfIEs, as also described by Lubiana and colleagues [127], was observed on transgenic CHO cells presenting ICAM-1 [123]. However, the binding showed large variations. Thus, the PfIEs came to a standstill for a few seconds, but were then also able to detach from the CHO cells again [123].
13. Importance of Knobs for Cytoadhesion
It is well established that knobs play a crucial role in the cytoadhesion of P. falciparum [45,47,48,127,135]. Among other findings, PfIEs from patients with hemoglobin S (HbS) or hemoglobin C (HbC; both hemoglobin mutations protect against severe complications and death from malaria [136,137,138]) have been found to exhibit reduced cytoadhesion to microvascular endothelial cells [139,140]. The results suggest that HbS and HbC alter the erythrocyte membrane in a manner that inhibits the transport and/or docking of parasite proteins and impairs the ability of the parasite to remodel the surface of its host cell. This also leads to the fact that the knobs can no longer be formed correctly, and PfEMP1 is also no longer presented correctly [139,140].
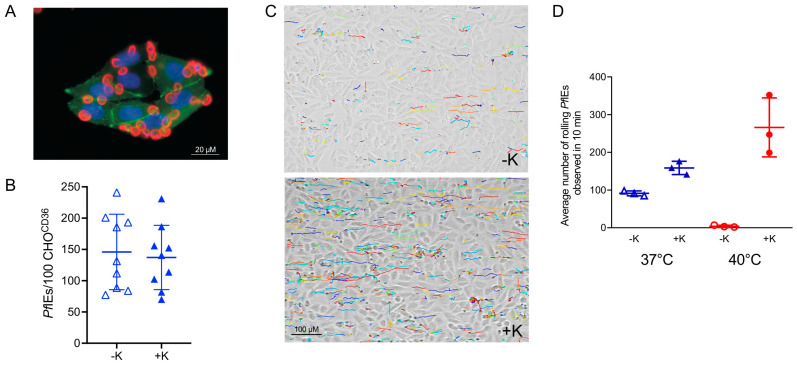
In the absence of knobs, parasite adhesion to CD36 was observed only under static conditions, but not under flow conditions simulating the situation in human blood [47,141]. In the absence of knobs on the surface of PfIEs, the rolling distance is shortened compared to knob-positive PfIEs [127]. The more stable rolling described above, and the rolling over longer distances of the schizont stage, is most likely related to the uniform coverage of the surface with knobs [130]. Furthermore, the adhesion force seems to be lower in the schizonts than in the trophozoites [142]. The comparison of knob-negative and knob-positive PfIEs suggests that the presence of knobs stabilises the ligand-receptor interaction due to the concentrated amount of PfEMP1 on the knob surface (Figure 4) [46,127].
Figure 4.
Cytoadhesion of knobby and knobless PfIEs to transgenic CHO cells presenting CD36 on the cell surface (CHOCD36) under static and flow conditions and at different temperatures. (A) Adhesion of PfIEs (red; anti-glycophorin A) to CHOCD36 cells (blue: nucleus (DAPI), green: cell surface (CD36-GFP fusion protein) under static binding conditions. (B) Cytoadhesion of knobbless (−K) and knobby (+K) PfIEs to CHOCD36 cells. (C) Trajectories showing the rolling binding behavior of knobless (−K) and knobby (+K) PfIEs to CHOCD36 cells. (D) Average number of knobless (−K) and knobby (+K) PfIEs adhering to CHOCD36 cells at 37 °C (blue) and 40 °C (red) at a shear stress of 0.9 dyn/cm2 [127].
Another important observation could be made under fever conditions. Only knob-positive PfIEs were able to bind to different ECRs (CSA, CD36) at 40 °C with preserved ECR-specific binding mode (rolling or stationary) (Figure 4) [48,127]. Measurement of the binding force between PfIEs and CSA by force spectroscopy showed a decrease in binding force at febrile temperatures, but the number of bound PfIEs increased. It was hypothesized that this increase in binding is due to non-specific binding despite the decrease in force [143]. Again, however, a study showed that, at febrile temperatures, binding affinities to CD36 and ICAM-1 decreased [144].
In summary, there is strong evidence that the presence of knobs on the surface of PfIEs is an essential prerequisite for the parasite circulating in the bloodstream to adhere to the endothelium even under febrile conditions. An evolutionary pressure for the formation of knobs on PfIEs in the human host is therefore operative.
14. Conclusions
CD36 is the main receptor for PfIE cytoadhesion to the vascular endothelium. Due to the rolling behavior and the resulting short contact of parasites with CD36 on the ECs, these ECs may not or are only slightly activated. Likely, only B group PfEMP1s with a particularly strong binding affinity to CD36 or dual binding properties, as well as the increase in parasitemia and the accompanying stimulation of the immune system and the release of proinflammatory cytokines, lead to an activation of the endothelium and thus also to the presentation of other ECRs such as ICAM-1 or P-selectin. Finally, PfIEs with different binding phenotypes can also adhere, with static binding leading to further activation of the endothelium and the immune system.
Further observations highlight the role of CD36 in P. falciparum infection. (i) A large number of PfEMP1s containing a CD36 binding domain [17,27,31]; (ii) the binding of PfIEs to DCs via CD36, which inhibits their cell maturation and ability to stimulate NK and T cells [108,111,112,113,114]; (iii) CD36-mediated phagocytosis of PfIEs does not result in cytokine secretion by macrophages [116] (however, it may result in a reduction in parasitemia [108,114,117,118]); (iv) in the early stage of infection, internalization of CD36-binding PfIEs by DCs leads to increased cytokine production and activation of NK and T cells, which promotes the establishment of protective immunity [118,119].
Thus, CD36 is of great importance for establishing a finely regulated equilibrium between the parasite and the host, whereby the parasite can multiply and spread while the host experiences little damage.
Author Contributions
Writing—original draft preparation, A.B., N.G.M. and I.B.; writing—review and editing, A.B., N.G.M., J.A., J.C., M.d.P.M.T., A.M., L.K.R., H.T., Y.W., T.G. and I.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The work was supported by Deutsche Forschungsgemeinschaft (BR 1744/20-1), Joachim Herz Siftung (Jakob Cronshagen), the Leibniz Center Infection (Maria del Pilar Martinez Tauler), the Chinese Scholarship Council (Yifan Wu), Jürgen Manchot Stiftung (Hanifeh Torabi), and the German Center for Infection Research (DZIF, Agnes Murk).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . World Malaria Report 2021. WHO; Geneva, Switzerland: 2021. [Google Scholar]
- 2.Saul A. The role of variant surface antigens on malaria-infected red blood cells. Parasitol. Today. 1999;15:455–457. doi: 10.1016/S0169-4758(99)01534-3. [DOI] [PubMed] [Google Scholar]
- 3.Phillips M.A., Burrows J.N., Manyando C., van Huijsduijnen R.H., Van Voorhis W.C., Wells T.N.C. Malaria. Nat. Rev. Dis. Prim. 2017;3:17050. doi: 10.1038/nrdp.2017.50. [DOI] [PubMed] [Google Scholar]
- 4.Craig A.G., Khairul M.F., Patil P.R. Cytoadherence and severe malaria. Malays. J. Med. Sci. 2012;19:5–18. [PMC free article] [PubMed] [Google Scholar]
- 5.Newbold C., Craig A., Kyes S., Rowe A., Fernandez-Reyes D., Fagan T. Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int. J. Parasitol. 1999;29:927–937. doi: 10.1016/S0020-7519(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 6.Rowe J.A., Claessens A., Corrigan R.A., Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: Molecular mechanisms and therapeutic implications. Expert Rev. Mol. Med. 2009;11:e16. doi: 10.1017/S1462399409001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J.A., Howell K.B., Reiling L., Ataide R., Mackintosh C.L., Fowkes F.J., Petter M., Chesson J.M., Langer C., Warimwe G.M., et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J. Clin. Investig. 2012;122:3227–3238. doi: 10.1172/JCI62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller L.H., Good M.F., Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 9.Nyarko P.B., Claessens A. Understanding Host-Pathogen-Vector Interactions with Chronic Asymptomatic Malaria Infections. Trends Parasitol. 2021;37:195–204. doi: 10.1016/j.pt.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Deitsch K.W., Dzikowski R. Variant Gene Expression and Antigenic Variation by Malaria Parasites. Annu. Rev. Microbiol. 2017;71:625–641. doi: 10.1146/annurev-micro-090816-093841. [DOI] [PubMed] [Google Scholar]
- 11.Petter M., Duffy M.F. Antigenic Variation in Plasmodium falciparum. Results Probl. Cell Differ. 2015;57:47–90. doi: 10.1007/978-3-319-20819-0_3. [DOI] [PubMed] [Google Scholar]
- 12.Otto T.D., Assefa S.A., Bohme U., Sanders M.J., Kwiatkowski D., Pf3k c., Berriman M., Newbold C. Evolutionary analysis of the most polymorphic gene family in falciparum malaria. Wellcome Open Res. 2019;4:193. doi: 10.12688/wellcomeopenres.15590.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss T.S., Healer J., Marty A.J., Duffy M.F., Thompson J.K., Beeson J.G., Reeder J.C., Crabb B.S., Cowman A.F. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439:1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- 14.Kyes S.A., Kraemer S.M., Smith J.D. Antigenic variation in Plasmodium falciparum: Gene organization and regulation of the var multigene family. Eukaryot. Cell. 2007;6:1511–1520. doi: 10.1128/EC.00173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasternak N.D., Dzikowski R. PfEMP1: An antigen that plays a key role in the pathogenicity and immune evasion of the malaria parasite Plasmodium falciparum. Int. J. Biochem. Cell Biol. 2009;41:1463–1466. doi: 10.1016/j.biocel.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Kraemer S.M., Smith J.D. A family affair: Var genes, PfEMP1 binding, and malaria disease. Curr. Opin. Microbiol. 2006;9:374–380. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Rask T.S., Hansen D.A., Theander T.G., Gorm Pedersen A., Lavstsen T. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes—Divide and conquer. PLoS Comput. Biol. 2010;6:e1000933. doi: 10.1371/journal.pcbi.1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith J.D., Craig A.G., Kriek N., Hudson-Taylor D., Kyes S., Fagan T., Pinches R., Baruch D.I., Newbold C.I., Miller L.H. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: A parasite adhesion trait implicated in cerebral malaria. Proc. Natl. Acad. Sci. USA. 2000;97:1766–1771. doi: 10.1073/pnas.040545897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith J.D., Subramanian G., Gamain B., Baruch D.I., Miller L.H. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 2000;110:293–310. doi: 10.1016/S0166-6851(00)00279-6. [DOI] [PubMed] [Google Scholar]
- 20.Gardner M.J., Hall N., Fung E., White O., Berriman M., Hyman R.W., Carlton J.M., Pain A., Nelson K.E., Bowman S., et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraemer S.M., Smith J.D. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol. Microbiol. 2003;50:1527–1538. doi: 10.1046/j.1365-2958.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer S.M., Kyes S.A., Aggarwal G., Springer A.L., Nelson S.O., Christodoulou Z., Smith L.M., Wang W., Levin E., Newbold C.I., et al. Patterns of gene recombination shape var gene repertoires in Plasmodium falciparum: Comparisons of geographically diverse isolates. BMC Genom. 2007;8:45. doi: 10.1186/1471-2164-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavstsen T., Salanti A., Jensen A.T., Arnot D.E., Theander T.G. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar. J. 2003;2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyes S.A., Christodoulou Z., Raza A., Horrocks P., Pinches R., Rowe J.A., Newbold C.I. A well-conserved Plasmodium falciparum var gene shows an unusual stage-specific transcript pattern. Mol. Microbiol. 2003;48:1339–1348. doi: 10.1046/j.1365-2958.2003.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowe J.A., Kyes S.A. The role of Plasmodium falciparum var genes in malaria in pregnancy. Mol. Microbiol. 2004;53:1011–1019. doi: 10.1111/j.1365-2958.2004.04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C.W., Lavstsen T., Bengtsson D.C., Magistrado P.A., Berger S.S., Marquard A.M., Alifrangis M., Lusingu J.P., Theander T.G., Turner L. Evidence for in vitro and in vivo expression of the conserved VAR3 (type 3) Plasmodium falciparum erythrocyte membrane protein 1. Malar. J. 2012;11:129. doi: 10.1186/1475-2875-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson B.A., Welch T.L., Smith J.D. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol. Microbiol. 2003;47:1265–1278. doi: 10.1046/j.1365-2958.2003.03378.x. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh F.L., Turner L., Bolla J.R., Robinson C.V., Lavstsen T., Higgins M.K. The structural basis for CD36 binding by the malaria parasite. Nat. Commun. 2016;7:12837. doi: 10.1038/ncomms12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo M., Lee H.C., Kotaka M., Niang M., Gao X., Iyer J.K., Lescar J., Preiser P. The C-terminal segment of the cysteine-rich interdomain of Plasmodium falciparum erythrocyte membrane protein 1 determines CD36 binding and elicits antibodies that inhibit adhesion of parasite-infected erythrocytes. Infect. Immun. 2008;76:1837–1847. doi: 10.1128/IAI.00480-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller L.H., Hudson-Taylor D., Gamain B., Saul A.J. Definition of the minimal domain of CIDR1alpha of Plasmodium falciparum PfEMP1 for binding CD36. Mol. Biochem. Parasitol. 2002;120:321–323. doi: 10.1016/S0166-6851(02)00011-7. [DOI] [PubMed] [Google Scholar]
- 31.Smith J.D., Rowe J.A., Higgins M.K., Lavstsen T. Malaria’s deadly grip: Cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2013;15:1976–1983. doi: 10.1111/cmi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau C.K., Turner L., Jespersen J.S., Lowe E.D., Petersen B., Wang C.W., Petersen J.E., Lusingu J., Theander T.G., Lavstsen T., et al. Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell Host Microbe. 2015;17:118–129. doi: 10.1016/j.chom.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner L., Lavstsen T., Berger S.S., Wang C.W., Petersen J.E., Avril M., Brazier A.J., Freeth J., Jespersen J.S., Nielsen M.A., et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. 2013;498:502–505. doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baruch D.I., Ma X.C., Singh H.B., Bi X., Pasloske B.L., Howard R.J. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: Conserved function with variant sequence. Blood. 1997;90:3766–3775. doi: 10.1182/blood.V90.9.3766. [DOI] [PubMed] [Google Scholar]
- 35.Mkumbaye S.I., Wang C.W., Lyimo E., Jespersen J.S., Manjurano A., Mosha J., Kavishe R.A., Mwakalinga S.B., Minja D.T.R., Lusingu J.P., et al. The Severity of Plasmodium falciparum Infection Is Associated with Transcript Levels of var Genes Encoding Endothelial Protein C Receptor-Binding P. falciparum Erythrocyte Membrane Protein 1. Infect. Immun. 2017;85 doi: 10.1128/IAI.00841-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennartz F., Adams Y., Bengtsson A., Olsen R.W., Turner L., Ndam N.T., Ecklu-Mensah G., Moussiliou A., Ofori M.F., Gamain B., et al. Structure-Guided Identification of a Family of Dual Receptor-Binding PfEMP1 that Is Associated with Cerebral Malaria. Cell Host Microbe. 2017;21:403–414. doi: 10.1016/j.chom.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janes J.H., Wang C.P., Levin-Edens E., Vigan-Womas I., Guillotte M., Melcher M., Mercereau-Puijalon O., Smith J.D. Investigating the host binding signature on the Plasmodium falciparum PfEMP1 protein family. PLoS Pathog. 2011;7:e1002032. doi: 10.1371/journal.ppat.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magallon-Tejada A., Machevo S., Cistero P., Lavstsen T., Aide P., Rubio M., Jimenez A., Turner L., Valmaseda A., Gupta H., et al. Cytoadhesion to gC1qR through Plasmodium falciparum Erythrocyte Membrane Protein 1 in Severe Malaria. PLoS Pathog. 2016;12:e1006011. doi: 10.1371/journal.ppat.1006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quadt K.A., Barfod L., Andersen D., Bruun J., Gyan B., Hassenkam T., Ofori M.F., Hviid L. The density of knobs on Plasmodium falciparum-infected erythrocytes depends on developmental age and varies among isolates. PLoS ONE. 2012;7:e45658. doi: 10.1371/journal.pone.0045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilly A.K., Thiede J., Metwally N., Lubiana P., Bachmann A., Roeder T., Rockliffe N., Lorenzen S., Tannich E., Gutsmann T., et al. Type of in vitro cultivation influences cytoadhesion, knob structure, protein localization and transcriptome profile of Plasmodium falciparum. Sci. Rep. 2015;5:16766. doi: 10.1038/srep16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alampalli S.V., Grover M., Chandran S., Tatu U., Acharya P. Proteome and Structural Organization of the Knob Complex on the Surface of the Plasmodium Infected Red Blood Cell. Proteom. Clin. Appl. 2018;12:e1600177. doi: 10.1002/prca.201600177. [DOI] [PubMed] [Google Scholar]
- 42.Maier A.G., Cooke B.M., Cowman A.F., Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat. Rev. Microbiol. 2009;7:341–354. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- 43.Watermeyer J.M., Hale V.L., Hackett F., Clare D.K., Cutts E.E., Vakonakis I., Fleck R.A., Blackman M.J., Saibil H.R. A spiral scaffold underlies cytoadherent knobs in Plasmodium falciparum-infected erythrocytes. Blood. 2016;127:343–351. doi: 10.1182/blood-2015-10-674002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gruenberg J., Allred D.R., Sherman I.W. Scanning electron microscope-analysis of the protrusions (knobs) present on the surface of Plasmodium falciparum-infected erythrocytes. J. Cell Biol. 1983;97:795–802. doi: 10.1083/jcb.97.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cutts E.E., Laasch N., Reiter D.M., Trenker R., Slater L.M., Stansfeld P.J., Vakonakis I. Structural analysis of P. falciparum KAHRP and PfEMP1 complexes with host erythrocyte spectrin suggests a model for cytoadherent knob protrusions. PLoS Pathog. 2017;13:e1006552. doi: 10.1371/journal.ppat.1006552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez C.P., Karathanasis C., Sanchez R., Cyrklaff M., Jager J., Buchholz B., Schwarz U.S., Heilemann M., Lanzer M. Single-molecule imaging and quantification of the immune-variant adhesin VAR2CSA on knobs of Plasmodium falciparum-infected erythrocytes. Commun. Biol. 2019;2:172. doi: 10.1038/s42003-019-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crabb B.S., Cooke B.M., Reeder J.C., Waller R.F., Caruana S.R., Davern K.M., Wickham M.E., Brown G.V., Coppel R.L., Cowman A.F. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89:287–296. doi: 10.1016/S0092-8674(00)80207-X. [DOI] [PubMed] [Google Scholar]
- 48.Dorpinghaus M., Furstenwerth F., Roth L.K., Bouws P., Rakotonirinalalao M., Jordan V., Sauer M., Rehn T., Pansegrau E., Hohn K., et al. Stringent Selection of Knobby Plasmodium falciparum-Infected Erythrocytes during Cytoadhesion at Febrile Temperature. Microorganisms. 2020;8:174. doi: 10.3390/microorganisms8020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boeuf P., Hasang W., Hanssen E., Glazier J.D., Rogerson S.J. Relevant assay to study the adhesion of Plasmodium falciparum-infected erythrocytes to the placental epithelium. PLoS ONE. 2011;6:e21126. doi: 10.1371/journal.pone.0021126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esser C., Bachmann A., Kuhn D., Schuldt K., Forster B., Thiel M., May J., Koch-Nolte F., Yanez-Mo M., Sanchez-Madrid F., et al. Evidence of promiscuous endothelial binding by Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2014;16:701–708. doi: 10.1111/cmi.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chesnokov O., Merritt J., Tcherniuk S.O., Milman N., Oleinikov A.V. Plasmodium falciparum infected erythrocytes can bind to host receptors integrins alphaVbeta3 and alphaVbeta6 through DBLdelta1_D4 domain of PFL2665c PfEMP1 protein. Sci. Rep. 2018;8:17871. doi: 10.1038/s41598-018-36071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siano J.P., Grady K.K., Millet P., Wick T.M. Short report: Plasmodium falciparum: Cytoadherence to alpha(v)beta3 on human microvascular endothelial cells. Am. J. Trop Med. Hyg. 1998;59:77–79. doi: 10.4269/ajtmh.1998.59.77. [DOI] [PubMed] [Google Scholar]
- 53.Metwally N.G., Tilly A.K., Lubiana P., Roth L.K., Dorpinghaus M., Lorenzen S., Schuldt K., Witt S., Bachmann A., Tidow H., et al. Characterisation of Plasmodium falciparum populations selected on the human endothelial receptors P-selectin, E-selectin, CD9 and CD151. Sci. Rep. 2017;7:4069. doi: 10.1038/s41598-017-04241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bengtsson A., Joergensen L., Rask T.S., Olsen R.W., Andersen M.A., Turner L., Theander T.G., Hviid L., Higgins M.K., Craig A., et al. A novel domain cassette identifies Plasmodium falciparum PfEMP1 proteins binding ICAM-1 and is a target of cross-reactive, adhesion-inhibitory antibodies. J. Immunol. 2013;190:240–249. doi: 10.4049/jimmunol.1202578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milner D.A., Jr., Valim C., Carr R.A., Chandak P.B., Fosiko N.G., Whitten R., Playforth K.B., Seydel K.B., Kamiza S., Molyneux M.E., et al. A histological method for quantifying Plasmodium falciparum in the brain in fatal paediatric cerebral malaria. Malar. J. 2013;12:191. doi: 10.1186/1475-2875-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milner D.A., Jr., Lee J.J., Frantzreb C., Whitten R.O., Kamiza S., Carr R.A., Pradham A., Factor R.E., Playforth K., Liomba G., et al. Quantitative Assessment of Multiorgan Sequestration of Parasites in Fatal Pediatric Cerebral Malaria. J. Infect. Dis. 2015;212:1317–1321. doi: 10.1093/infdis/jiv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor T.E., Fu W.J., Carr R.A., Whitten R.O., Mueller J.S., Fosiko N.G., Lewallen S., Liomba N.G., Molyneux M.E. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 58.Lyke K.E., Burges R., Cissoko Y., Sangare L., Dao M., Diarra I., Kone A., Harley R., Plowe C.V., Doumbo O.K., et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raacke M., Kerr A., Dorpinghaus M., Brehmer J., Wu Y., Lorenzen S., Fink C., Jacobs T., Roeder T., Sellau J., et al. Altered Cytokine Response of Human Brain Endothelial Cells after Stimulation with Malaria Patient Plasma. Cells. 2021;10:1656. doi: 10.3390/cells10071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hasday J.D., Bannerman D., Sakarya S., Cross A.S., Singh I.S., Howard D., Drysdale B.E., Goldblum S.E. Exposure to febrile temperature modifies endothelial cell response to tumor necrosis factor-alpha. J. Appl. Physiol. 2001;90:90–98. doi: 10.1152/jappl.2001.90.1.90. [DOI] [PubMed] [Google Scholar]
- 61.Oakley M.S., Gerald N., McCutchan T.F., Aravind L., Kumar S. Clinical and molecular aspects of malaria fever. Trends Parasitol. 2011;27:442–449. doi: 10.1016/j.pt.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Cunnington A.J., Riley E.M., Walther M. Microvascular dysfunction in severe Plasmodium falciparum Malaria. J. Infect. Dis. 2013;207:369–370. doi: 10.1093/infdis/jis681. [DOI] [PubMed] [Google Scholar]
- 63.Gazzinelli R.T., Kalantari P., Fitzgerald K.A., Golenbock D.T. Innate sensing of malaria parasites. Nat. Rev. Immunol. 2014;14:744–757. doi: 10.1038/nri3742. [DOI] [PubMed] [Google Scholar]
- 64.Dondorp A.M., Lee S.J., Faiz M.A., Mishra S., Price R., Tjitra E., Than M., Htut Y., Mohanty S., Yunus E.B., et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin. Infect. Dis. 2008;47:151–157. doi: 10.1086/589287. [DOI] [PubMed] [Google Scholar]
- 65.Jensen A.T., Magistrado P., Sharp S., Joergensen L., Lavstsen T., Chiucchiuini A., Salanti A., Vestergaard L.S., Lusingu J.P., Hermsen R., et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 2004;199:1179–1190. doi: 10.1084/jem.20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avril M., Tripathi A.K., Brazier A.J., Andisi C., Janes J.H., Soma V.L., Sullivan D.J., Jr., Bull P.C., Stins M.F., Smith J.D. A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proc. Natl. Acad. Sci. USA. 2012;109:E1782–E1790. doi: 10.1073/pnas.1120534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Claessens A., Adams Y., Ghumra A., Lindergard G., Buchan C.C., Andisi C., Bull P.C., Mok S., Gupta A.P., Wang C.W., et al. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc. Natl. Acad. Sci. USA. 2012;109:E1772–E1781. doi: 10.1073/pnas.1120461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lavstsen T., Turner L., Saguti F., Magistrado P., Rask T.S., Jespersen J.S., Wang C.W., Berger S.S., Baraka V., Marquard A.M., et al. Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc. Natl. Acad. Sci. USA. 2012;109:E1791–E1800. doi: 10.1073/pnas.1120455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duffy F., Bernabeu M., Babar P.H., Kessler A., Wang C.W., Vaz M., Chery L., Mandala W.L., Rogerson S.J., Taylor T.E., et al. Meta-analysis of Plasmodium falciparum var Signatures Contributing to Severe Malaria in African Children and Indian Adults. mBio. 2019;10:e00217-19. doi: 10.1128/mBio.00217-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jespersen J.S., Wang C.W., Mkumbaye S.I., Minja D.T., Petersen B., Turner L., Petersen J.E., Lusingu J.P., Theander T.G., Lavstsen T. Plasmodium falciparum var genes expressed in children with severe malaria encode CIDRalpha1 domains. EMBO Mol. Med. 2016;8:839–850. doi: 10.15252/emmm.201606188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bertin G.I., Lavstsen T., Guillonneau F., Doritchamou J., Wang C.W., Jespersen J.S., Ezimegnon S., Fievet N., Alao M.J., Lalya F., et al. Expression of the domain cassette 8 Plasmodium falciparum erythrocyte membrane protein 1 is associated with cerebral malaria in Benin. PLoS ONE. 2013;8:e68368. doi: 10.1371/journal.pone.0068368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernabeu M., Danziger S.A., Avril M., Vaz M., Babar P.H., Brazier A.J., Herricks T., Maki J.N., Pereira L., Mascarenhas A., et al. Severe adult malaria is associated with specific PfEMP1 adhesion types and high parasite biomass. Proc. Natl. Acad. Sci. USA. 2016;113:E3270–E3279. doi: 10.1073/pnas.1524294113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Q., Heddini A., Barragan A., Fernandez V., Pearce S.F., Wahlgren M. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J. Exp. Med. 2000;192:1–10. doi: 10.1084/jem.192.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avril M., Bernabeu M., Benjamin M., Brazier A.J., Smith J.D. Interaction between Endothelial Protein C Receptor and Intercellular Adhesion Molecule 1 to Mediate Binding of Plasmodium falciparum-Infected Erythrocytes to Endothelial Cells. mBio. 2016;7:e00615-16. doi: 10.1128/mBio.00615-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernabeu M., Gunnarsson C., Vishnyakova M., Howard C.C., Nagao R.J., Avril M., Taylor T.E., Seydel K.B., Zheng Y., Smith J.D. Binding Heterogeneity of Plasmodium falciparum to Engineered 3D Brain Microvessels Is Mediated by EPCR and ICAM-1. mBio. 2019;10:e00420-19. doi: 10.1128/mBio.00420-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adams Y., Olsen R.W., Bengtsson A., Dalgaard N., Zdioruk M., Satpathi S., Behera P.K., Sahu P.K., Lawler S.E., Qvortrup K., et al. Plasmodium falciparum erythrocyte membrane protein 1 variants induce cell swelling and disrupt the blood-brain barrier in cerebral malaria. J. Exp. Med. 2021;218:e20201266. doi: 10.1084/jem.20201266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCormick C.J., Craig A., Roberts D., Newbold C.I., Berendt A.R. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J. Clin. Investig. 1997;100:2521–2529. doi: 10.1172/JCI119794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray C., McCormick C., Turner G., Craig A. ICAM-1 can play a major role in mediating P. falciparum adhesion to endothelium under flow. Mol. Biochem. Parasitol. 2003;128:187–193. doi: 10.1016/S0166-6851(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 79.Storm J., Jespersen J.S., Seydel K.B., Szestak T., Mbewe M., Chisala N.V., Phula P., Wang C.W., Taylor T.E., Moxon C.A., et al. Cerebral malaria is associated with differential cytoadherence to brain endothelial cells. EMBO Mol. Med. 2019;11:e9164. doi: 10.15252/emmm.201809164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ortolan L.S., Avril M., Xue J., Seydel K.B., Zheng Y., Smith J.D. Plasmodium falciparum Parasite Lines Expressing DC8 and Group A PfEMP1 Bind to Brain, Intestinal, and Kidney Endothelial Cells. Front. Cell Infect. Microbiol. 2022;12:813011. doi: 10.3389/fcimb.2022.813011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swerlick R.A., Lee K.H., Wick T.M., Lawley T.J. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J. Immunol. 1992;148:78–83. [PubMed] [Google Scholar]
- 82.Turner G.D., Morrison H., Jones M., Davis T.M., Looareesuwan S., Buley I.D., Gatter K.C., Newbold C.I., Pukritayakamee S., Nagachinta B., et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 83.Kessler A., Dankwa S., Bernabeu M., Harawa V., Danziger S.A., Duffy F., Kampondeni S.D., Potchen M.J., Dambrauskas N., Vigdorovich V., et al. Linking EPCR-Binding PfEMP1 to Brain Swelling in Pediatric Cerebral Malaria. Cell Host. Microbe. 2017;22:601–614.e605. doi: 10.1016/j.chom.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wichers J.S., Tonkin-Hill G., Thye T., Krumkamp R., Kreuels B., Strauss J., von Thien H., Scholz J.A., Smedegaard Hansson H., Weisel Jensen R., et al. Common virulence gene expression in adult first-time infected malaria patients and severe cases. eLife. 2021;10:e69040. doi: 10.7554/eLife.69040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tuikue Ndam N., Moussiliou A., Lavstsen T., Kamaliddin C., Jensen A.T.R., Mama A., Tahar R., Wang C.W., Jespersen J.S., Alao J.M., et al. Parasites Causing Cerebral Falciparum Malaria Bind Multiple Endothelial Receptors and Express EPCR and ICAM-1-Binding PfEMP1. J. Infect. Dis. 2017;215:1918–1925. doi: 10.1093/infdis/jix230. [DOI] [PubMed] [Google Scholar]
- 86.Ochola L.B., Siddondo B.R., Ocholla H., Nkya S., Kimani E.N., Williams T.N., Makale J.O., Liljander A., Urban B.C., Bull P.C., et al. Specific receptor usage in Plasmodium falciparum cytoadherence is associated with disease outcome. PLoS ONE. 2011;6:e14741. doi: 10.1371/journal.pone.0014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bachmann A., Petter M., Krumkamp R., Esen M., Held J., Scholz J.A., Li T., Sim B.K., Hoffman S.L., Kremsner P.G., et al. Mosquito Passage Dramatically Changes var Gene Expression in Controlled Human Plasmodium falciparum Infections. PLoS Pathog. 2016;12:e1005538. doi: 10.1371/journal.ppat.1005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C.W., Hermsen C.C., Sauerwein R.W., Arnot D.E., Theander T.G., Lavstsen T. The Plasmodium falciparum var gene transcription strategy at the onset of blood stage infection in a human volunteer. Parasitol. Int. 2009;58:478–480. doi: 10.1016/j.parint.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 89.Milne K., Ivens A., Reid A.J., Lotkowska M.E., O’Toole A., Sankaranarayanan G., Munoz Sandoval D., Nahrendorf W., Regnault C., Edwards N.J., et al. Mapping immune variation and var gene switching in naive hosts infected with Plasmodium falciparum. eLife. 2021;10:e62800. doi: 10.7554/eLife.62800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pickford A.K., Michel-Todo L., Dupuy F., Mayor A., Alonso P.L., Lavazec C., Cortes A. Expression Patterns of Plasmodium falciparum Clonally Variant Genes at the Onset of a Blood Infection in Malaria-Naive Humans. mBio. 2021;12:e0163621. doi: 10.1128/mBio.01636-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bachmann A., Bruske E., Krumkamp R., Turner L., Wichers J.S., Petter M., Held J., Duffy M.F., Sim B.K.L., Hoffman S.L., et al. Controlled human malaria infection with Plasmodium falciparum demonstrates impact of naturally acquired immunity on virulence gene expression. PLoS Pathog. 2019;15:e1007906. doi: 10.1371/journal.ppat.1007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kyriacou H.M., Stone G.N., Challis R.J., Raza A., Lyke K.E., Thera M.A., Kone A.K., Doumbo O.K., Plowe C.V., Rowe J.A. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol. Biochem. Parasitol. 2006;150:211–218. doi: 10.1016/j.molbiopara.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warimwe G.M., Keane T.M., Fegan G., Musyoki J.N., Newton C.R., Pain A., Berriman M., Marsh K., Bull P.C. Plasmodium falciparum var gene expression is modified by host immunity. Proc. Natl. Acad. Sci. USA. 2009;106:21801–21806. doi: 10.1073/pnas.0907590106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bachmann A., Predehl S., May J., Harder S., Burchard G.D., Gilberger T.W., Tannich E., Bruchhaus I. Highly co-ordinated var gene expression and switching in clinical Plasmodium falciparum isolates from non-immune malaria patients. Cell Microbiol. 2011;13:1397–1409. doi: 10.1111/j.1462-5822.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- 95.Cham G.K., Turner L., Lusingu J., Vestergaard L., Mmbando B.P., Kurtis J.D., Jensen A.T., Salanti A., Lavstsen T., Theander T.G. Sequential, ordered acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 domains. J. Immunol. 2009;183:3356–3363. doi: 10.4049/jimmunol.0901331. [DOI] [PubMed] [Google Scholar]
- 96.Obeng-Adjei N., Larremore D.B., Turner L., Ongoiba A., Li S., Doumbo S., Yazew T.B., Kayentao K., Miller L.H., Traore B., et al. Longitudinal analysis of naturally acquired PfEMP1 CIDR domain variant antibodies identifies associations with malaria protection. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cabrera A., Neculai D., Kain K.C. CD36 and malaria: Friends or foes? A decade of data provides some answers. Trends Parasitol. 2014;30:436–444. doi: 10.1016/j.pt.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 98.Turner L., Lavstsen T., Mmbando B.P., Wang C.W., Magistrado P.A., Vestergaard L.S., Ishengoma D.S., Minja D.T., Lusingu J.P., Theander T.G. IgG antibodies to endothelial protein C receptor-binding cysteine-rich interdomain region domains of Plasmodium falciparum erythrocyte membrane protein 1 are acquired early in life in individuals exposed to malaria. Infect. Immun. 2015;83:3096–3103. doi: 10.1128/IAI.00271-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andrade C.M., Fleckenstein H., Thomson-Luque R., Doumbo S., Lima N.F., Anderson C., Hibbert J., Hopp C.S., Tran T.M., Li S., et al. Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nat. Med. 2020;26:1929–1940. doi: 10.1038/s41591-020-1084-0. [DOI] [PubMed] [Google Scholar]
- 100.Frank M., Dzikowski R., Amulic B., Deitsch K. Variable switching rates of malaria virulence genes are associated with chromosomal position. Mol. Microbiol. 2007;64:1486–1498. doi: 10.1111/j.1365-2958.2007.05736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paget-McNicol S., Gatton M., Hastings I., Saul A. The Plasmodium falciparum var gene switching rate, switching mechanism and patterns of parasite recrudescence described by mathematical modelling. Parasitology. 2002;124:225–235. doi: 10.1017/S0031182001001160. [DOI] [PubMed] [Google Scholar]
- 102.Gatton M.L., Cheng Q. Investigating antigenic variation and other parasite-host interactions in Plasmodium falciparum infections in naive hosts. Parasitology. 2004;128:367–376. doi: 10.1017/S0031182003004608. [DOI] [PubMed] [Google Scholar]
- 103.Otto T.D., Gilabert A., Crellen T., Bohme U., Arnathau C., Sanders M., Oyola S.O., Okouga A.P., Boundenga L., Willaume E., et al. Genomes of all known members of a Plasmodium subgenus reveal paths to virulent human malaria. Nat. Microbiol. 2018;3:687–697. doi: 10.1038/s41564-018-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pepino M.Y., Kuda O., Samovski D., Abumrad N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014;34:281–303. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Silverstein R.L., Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Y., Zhang J., Cui W., Silverstein R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022;219:e20211314. doi: 10.1084/jem.20211314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang M., Silverstein R.L. CD36 signaling in vascular redox stress. Free Radic. Biol. Med. 2019;136:159–171. doi: 10.1016/j.freeradbiomed.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Serghides L., Smith T.G., Patel S.N., Kain K.C. CD36 and malaria: Friends or foes? Trends Parasitol. 2003;19:461–469. doi: 10.1016/j.pt.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Canton J., Neculai D., Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 110.Xu X., Zheng X., Zhu F. CD36 gene variants and their clinical relevance: A narrative review. Ann. Blood. 2021;6:34. doi: 10.21037/aob-21-49. [DOI] [Google Scholar]
- 111.Urban B.C., Willcox N., Roberts D.J. A role for CD36 in the regulation of dendritic cell function. Proc. Natl. Acad. Sci. USA. 2001;98:8750–8755. doi: 10.1073/pnas.151028698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Urban B.C., Ferguson D.J., Pain A., Willcox N., Plebanski M., Austyn J.M., Roberts D.J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 113.Wu X., Gowda N.M., Gowda D.C. Plasmodium falciparum: Differential merozoite dose requirements for maximal production of various inflammatory cytokines. Exp. Parasitol. 2011;127:202–207. doi: 10.1016/j.exppara.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patel S.N., Serghides L., Smith T.G., Febbraio M., Silverstein R.L., Kurtz T.W., Pravenec M., Kain K.C. CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J. Infect. Dis. 2004;189:204–213. doi: 10.1086/380764. [DOI] [PubMed] [Google Scholar]
- 115.Elliott S.R., Spurck T.P., Dodin J.M., Maier A.G., Voss T.S., Yosaatmadja F., Payne P.D., McFadden G.I., Cowman A.F., Rogerson S.J., et al. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect. Immun. 2007;75:3621–3632. doi: 10.1128/IAI.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Erdman L.K., Cosio G., Helmers A.J., Gowda D.C., Grinstein S., Kain K.C. CD36 and TLR interactions in inflammation and phagocytosis: Implications for malaria. J. Immunol. 2009;183:6452–6459. doi: 10.4049/jimmunol.0901374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGilvray I.D., Serghides L., Kapus A., Rotstein O.D., Kain K.C. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: A role for CD36 in malarial clearance. Blood. 2000;96:3231–3240. doi: 10.1182/blood.V96.9.3231. [DOI] [PubMed] [Google Scholar]
- 118.Gowda N.M., Wu X., Kumar S., Febbraio M., Gowda D.C. CD36 contributes to malaria parasite-induced pro-inflammatory cytokine production and NK and T cell activation by dendritic cells. PLoS ONE. 2013;8:e77604. doi: 10.1371/journal.pone.0077604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thylur R.P., Wu X., Gowda N.M., Punnath K., Neelgund S.E., Febbraio M., Gowda D.C. CD36 receptor regulates malaria-induced immune responses primarily at early blood stage infection contributing to parasitemia control and resistance to mortality. J. Biol. Chem. 2017;292:9394–9408. doi: 10.1074/jbc.M117.781294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simon S.I., Goldsmith H.L. Leukocyte adhesion dynamics in shear flow. Ann. Biomed. Eng. 2002;30:315–332. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- 121.Kunkel E.J., Dunne J.L., Ley K. Leukocyte arrest during cytokine-dependent inflammation in vivo. J. Immunol. 2000;164:3301–3308. doi: 10.4049/jimmunol.164.6.3301. [DOI] [PubMed] [Google Scholar]
- 122.Helms G., Dasanna A.K., Schwarz U.S., Lanzer M. Modeling cytoadhesion of Plasmodium falciparum-infected erythrocytes and leukocytes-common principles and distinctive features. FEBS Lett. 2016;590:1955–1971. doi: 10.1002/1873-3468.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Antia M., Herricks T., Rathod P.K. Microfluidic modeling of cell-cell interactions in malaria pathogenesis. PLoS Pathog. 2007;3:e99. doi: 10.1371/journal.ppat.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Flatt C., Mitchell S., Yipp B., Looareesuwan S., Ho M. Attenuation of cytoadherence of Plasmodium falciparum to microvascular endothelium under flow by hemodilution. Am. J. Trop Med. Hyg. 2005;72:660–665. doi: 10.4269/ajtmh.2005.72.660. [DOI] [PubMed] [Google Scholar]
- 125.Li A., Lim T.S., Shi H., Yin J., Tan S.J., Li Z., Low B.C., Tan K.S., Lim C.T. Molecular mechanistic insights into the endothelial receptor mediated cytoadherence of Plasmodium falciparum-infected erythrocytes. PLoS ONE. 2011;6:e16929. doi: 10.1371/journal.pone.0016929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yipp B.G., Hickey M.J., Andonegui G., Murray A.G., Looareesuwan S., Kubes P., Ho M. Differential roles of CD36, ICAM-1, and P-selectin in Plasmodium falciparum cytoadherence in vivo. Microcirculation. 2007;14:593–602. doi: 10.1080/10739680701404705. [DOI] [PubMed] [Google Scholar]
- 127.Lubiana P., Bouws P., Roth L.K., Dorpinghaus M., Rehn T., Brehmer J., Wichers J.S., Bachmann A., Hohn K., Roeder T., et al. Adhesion between P. falciparum infected erythrocytes and human endothelial receptors follows alternative binding dynamics under flow and febrile conditions. Sci. Rep. 2020;10:4548. doi: 10.1038/s41598-020-61388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ho M., Hoang H.L., Lee K.M., Liu N., MacRae T., Montes L., Flatt C.L., Yipp B.G., Berger B.J., Looareesuwan S., et al. Ectophosphorylation of CD36 regulates cytoadherence of Plasmodium falciparum to microvascular endothelium under flow conditions. Infect. Immun. 2005;73:8179–8187. doi: 10.1128/IAI.73.12.8179-8187.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herricks T., Avril M., Janes J., Smith J.D., Rathod P.K. Clonal variants of Plasmodium falciparum exhibit a narrow range of rolling velocities to host receptor CD36 under dynamic flow conditions. Eukaryot. Cell. 2013;12:1490–1498. doi: 10.1128/EC.00148-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dasanna A.K., Lansche C., Lanzer M., Schwarz U.S. Rolling Adhesion of Schizont Stage Malaria-Infected Red Blood Cells in Shear Flow. Biophys. J. 2017;112:1908–1919. doi: 10.1016/j.bpj.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yipp B.G., Robbins S.M., Resek M.E., Baruch D.I., Looareesuwan S., Ho M. Src-family kinase signaling modulates the adhesion of Plasmodium falciparum on human microvascular endothelium under flow. Blood. 2003;101:2850–2857. doi: 10.1182/blood-2002-09-2841. [DOI] [PubMed] [Google Scholar]
- 132.Fedosov D.A., Caswell B., Karniadakis G.E. Wall shear stress-based model for adhesive dynamics of red blood cells in malaria. Biophys. J. 2011;100:2084–2093. doi: 10.1016/j.bpj.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fedosov D.A., Lei H., Caswell B., Suresh S., Karniadakis G.E. Multiscale modeling of red blood cell mechanics and blood flow in malaria. PLoS Comput. Biol. 2011;7:e1002270. doi: 10.1371/journal.pcbi.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davis S.P., Amrein M., Gillrie M.R., Lee K., Muruve D.A., Ho M. Plasmodium falciparum-induced CD36 clustering rapidly strengthens cytoadherence via p130CAS-mediated actin cytoskeletal rearrangement. FASEB J. 2012;26:1119–1130. doi: 10.1096/fj.11-196923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Horrocks P., Pinches R.A., Chakravorty S.J., Papakrivos J., Christodoulou Z., Kyes S.A., Urban B.C., Ferguson D.J., Newbold C.I. PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J. Cell Sci. 2005;118:2507–2518. doi: 10.1242/jcs.02381. [DOI] [PubMed] [Google Scholar]
- 136.May J., Evans J.A., Timmann C., Ehmen C., Busch W., Thye T., Agbenyega T., Horstmann R.D. Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA. 2007;297:2220–2226. doi: 10.1001/jama.297.20.2220. [DOI] [PubMed] [Google Scholar]
- 137.Modiano D., Luoni G., Sirima B.S., Simpore J., Verra F., Konate A., Rastrelli E., Olivieri A., Calissano C., Paganotti G.M., et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 138.Agarwal A., Guindo A., Cissoko Y., Taylor J.G., Coulibaly D., Kone A., Kayentao K., Djimde A., Plowe C.V., Doumbo O., et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000;96:2358–2363. doi: 10.1182/blood.V96.7.2358. [DOI] [PubMed] [Google Scholar]
- 139.Fairhurst R.M., Baruch D.I., Brittain N.J., Ostera G.R., Wallach J.S., Hoang H.L., Hayton K., Guindo A., Makobongo M.O., Schwartz O.M., et al. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435:1117–1121. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 140.Cholera R., Brittain N.J., Gillrie M.R., Lopera-Mesa T.M., Diakite S.A., Arie T., Krause M.A., Guindo A., Tubman A., Fujioka H., et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc. Natl. Acad. Sci. USA. 2008;105:991–996. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arman M., Adams Y., Lindergard G., Rowe J.A. A method for positive and negative selection of Plasmodium falciparum platelet-mediated clumping parasites and investigation of the role of CD36. PLoS ONE. 2013;8:e55453. doi: 10.1371/journal.pone.0055453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu X., Efremov A.K., Li A., Lai L., Dao M., Lim C.T., Cao J. Probing the cytoadherence of malaria infected red blood cells under flow. PLoS ONE. 2013;8:e64763. doi: 10.1371/journal.pone.0064763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carvalho P.A., Diez-Silva M., Chen H., Dao M., Suresh S. Cytoadherence of erythrocytes invaded by Plasmodium falciparum: Quantitative contact-probing of a human malaria receptor. Acta Biomater. 2013;9:6349–6359. doi: 10.1016/j.actbio.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lim Y.B., Thingna J., Cao J., Lim C.T. Single molecule and multiple bond characterization of catch bond associated cytoadhesion in malaria. Sci. Rep. 2017;7:4208. doi: 10.1038/s41598-017-04352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.