Abstract
Plant-based natural products have been used as a source for therapeutics since the dawn of civilization. According to the World Health Organization (WHO), more than 80% of the world’s population relies on traditional medicine for their primary healthcare. Numerous natural extracts, widely known in Traditional Chinese Medicine, Indian Ayurveda medicine and other practices, have led to the modern discovery and development of new drugs. Plants continuously interact with their environment, producing new compounds and ever-changing combinations of existing ones. Interestingly, some of the compounds have shown lower therapeutic activity in comparison to the extract they were isolated from. These findings suggest that the higher therapeutic activity of the source extract was due to the synergistic effect of several compounds. In other words, the total therapeutic potential of the extract cannot be explained only by the sum of its parts alone. In traditional medicine, most herbal remedies are based on a mixture of plants, and it is the interaction between different constituents that amplifies their therapeutic potential. Considering the significant influence traditional medicine has on human healthcare, knowing and studying the synergistic effect of compounds is paramount in designing smart therapeutic agents.
Keywords: natural products, synergistic effect, therapeutic, extracts, bioactive compounds, in vitro and in vivo studies
1. Introduction
Plants have formed the basis for traditional medicine systems in many cultures for thousands of years, and traditional medicine will continue to play an important part in health care worldwide. There is enormous potential in natural products, and less than 10% of the world’s biodiversity has been evaluated for biological activity. Phytochemicals with unique structural diversity have long been the primary source of potential drug leads, and many of them have become official drug candidates. Natural constituents and their derivatives have been recognised since ancient times as a source of therapeutic agents and the treatments of ailments, hence becoming an integral part of traditional medicine systems in various parts of the world. Numerous natural constituents (e.g., phenolics, terpenoids, and alkaloids) are attributed with antioxidant, antiseptic, antimicrobial, anti-inflammatory, antiviral, cytotoxic, neuroprotective, and other bioactivities. Due to the wide range of activities, plants and their bioactive molecules are used as natural therapeutics and significantly contribute to the production of commercial drugs. Despite a long history of medicinal use throughout the world, significant utilisation of plants is still limited due to the lack of ethnobotanical information. A combination of natural compounds is usually known to be non-toxic and have synergistic effects.
Based on their role in metabolism, organic compounds can be divided into two major groups: primary and secondary metabolites. While primary metabolites can be defined as “those molecules that are involved in the biosynthetic pathways of essential components of living cells, such as amino acids in proteins, nucleotides in nucleic acids, sugars as an energy resource and in polysaccharides, or phospholipids as major constituents of cell membranes” [1], secondary metabolites have often been considered those that were not necessary and that were, in essence, by-products of the primary metabolism. However, decades of research into these metabolites [1,2] have shown that, while they have a somewhat limited distribution (i.e., characteristic for specific taxa), they possess a myriad of different specific roles; hence, the term specialised metabolites has been proposed [1,2].
While all living organisms produce these metabolites, bacteria, plants, and fungi are the most important. This is probably due to their sedentary lifestyle. While animals can adapt to the environment through behaviour and avoid unfavourable conditions or engage other organisms in many different ways, microorganisms, plants, and fungi cannot. Plants, and to a lesser extent, fungi, are well known for their vast metabolomic diversity [1,3,4,5,6]. No one knows the number of different metabolites produced by plants, but taking into account the estimated number of plant taxa and, at times, massive genomes (especially in polyploid taxa), as well as the ability of some enzymes to produce more than one specialised metabolite [7,8], the number of estimated metabolites may very well exceed 200,000 [1].
This review compiles the phytochemicals present in medicinal plants, with a focus on the synergistic effect between extracts and isolated components, as well as their biological properties (in vitro and in vivo). The significance of phytochemicals in anticipation of traditional medicine and their synergistic effects are described herein. Moreover, much work is being done to explore natural products as antimicrobial, anti-inflammatory cytotoxic, antiprotozoal, and antifungal agents. This review gives the highlights of in vitro and in vivo studies of the combination of natural products, especially plants (extracts/essential oils and their individual bioactive components). The aim is to present the interaction between metabolites, whether they originate from a single plant or plant combinations (two or more plants). Beyond herbal combinations, as a part of this review, we went further and gave an interesting overview of different living organisms’ products, for example, plant-plus-fungal extract combinations or bacterial-products-plus-plant-essential-oils, with their medicinal properties, which is a new insight into this evolving topic.
2. Natural Products
Specialised metabolites can be divided into several groups based on their chemical nature. The three most numerous groups are terpenoids, alkaloids, and phenolics. There are other groups, but their distribution is fairly narrow–e.g., glucosinolates (Brassicaceae, Capparaceae and some other families), organic disulfides in the Amaryllidaceae family, unusual fatty acids in certain gymnosperms and angiosperms, cyanogenic glucosides in particular members of the Rosaceae family, etc. [1,3,4].
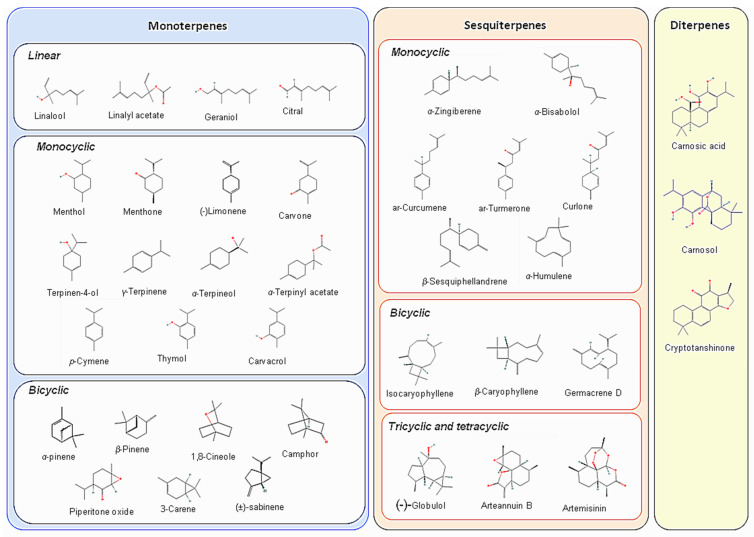
Terpenoids are by far the most diverse group of natural compounds, counting over 30,000 compounds (Figure 1). They can be classified based on the number of isoprene units (C5) they possess: Hemiterpenes consist of a single isoprene unit, monoterpenes of two, sesquiterpenes of three, diterpenes of four, triterpenes of six and polyterpenes of seven or more isoprene units. Terpenoids are universally present in all taxa, though sometimes only in small quantities [3,9,10,11]. They play an important role in plant–environment interactions, e.g., attracting pollinators, deterring herbivores, or protecting plants from infections caused by microorganisms [3,12,13,14,15,16,17,18]. They are non-polar compounds, often produced in specialised glands on the surface of plant organs or in resin ducts [12,13,14,19,20]. Most of the studied compounds are part of complex, volatile mixtures called essential oils (EOs) or oleoresins [1,3,9,21].
Figure 1.
Most-common terpenoid compounds.
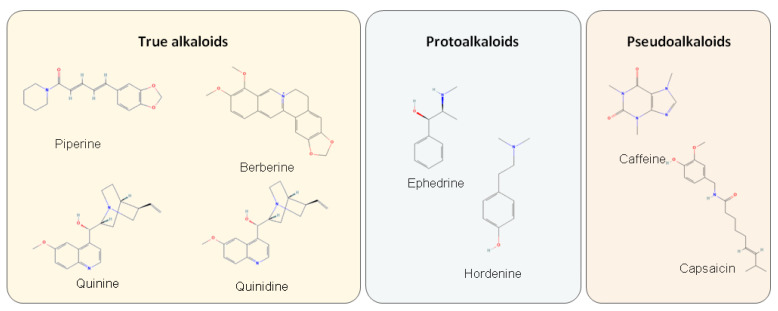
Alkaloids are the second-largest group of natural products, counting over 20,000 different structures (Figure 2). Alkaloids are organic heterocyclic nitrogen compounds soluble in water. The nitrogen in their structure is usually derived from amino acids, though not in all groups. Based on their biosynthetic pathway, they are divided into true alkaloids, protoalkaloids and pseudoalkaloids. Both true alkaloids and protoalkaloids are synthesised from amino acids, although protoalkaloids do not contain heterocyclic nitrogen. Pseudoalkaloids are, however, synthesised differently, e.g., from terpenes or other specialised metabolites. These compounds protect plants from herbivores–they are bitter tasting and oftentimes toxic to mammals and other animals [1,3,9,22,23,24]. While significant, these compounds have limited distribution and can be found only in certain families, (e.g., Solanaceae, Papaveraceae, Cycadaceae etc.) [25].
Figure 2.
Three groups of alkaloids.
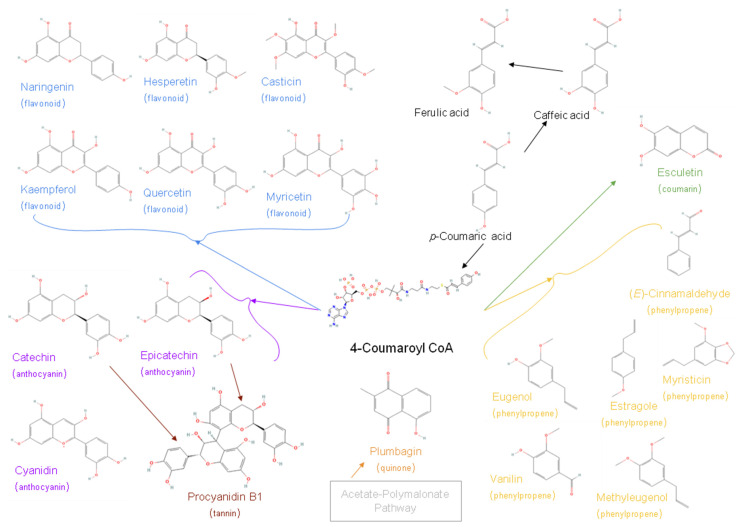
Phenolics are the third most diverse group of specialised metabolites. They contain over 10,000 different compounds that can be divided into several groups: flavonoids, tannins, and coumarins [1,3,9,26,27] (Figure 3). These compounds differ significantly in their structure and biological role, though they all start their biosynthetic pathway from 4-Coumaroyl CoA. Flavonoids are universally present in all plants. They have a 15-carbon skeleton, organised in two phenyl and one heterocyclic oxygen ring. The structural diversity is based on the chemical nature and the position(s) of substituents on different rings, so their polarity varies from more non-polar (aglycones) to more polar (glycosides) compounds. Flavonoids are involved in plant-pollinator interaction (colouring compounds), photoprotection and antimicrobial protection [1,3,6,9,23,28]. Tannins are polymeric phenolics found in all plants where they play a role in protection from herbivores and might help regulate plant growth [3,23,29]. Coumarins are a group of aromatic benzopyrones consisting of fused benzene and alpha pyrone rings [9,30,31]. Due to their astringent taste, they play a role in protection from predation and pathogenic microorganisms [1,9].
Figure 3.
Some of the most common phenolic compounds.
Medicinal Plants and Phytochemicals
Even before scientists were able to identify different specialised metabolites, plants have been the staple of traditional medicines around the world [32,33,34]. Records of the use of plants in medicine go back about 5000 years, most notably in India and China. Even today, in a modern society dominated by Western medicine, medicinal plants and traditional medicine have their important place. According to the World Health Organization (WHO), more than 80% of the world’s population relies on traditional medicine for their primary healthcare [35]. Numerous natural extracts, widely known in Traditional Chinese Medicine and Ayurveda, led to the modern discovery and development of new drugs. Thousands of years of experience found in ethnobotanical manuscripts, coupled with an ever-evolving scientific approach in the study of these medicinal properties, is a significant opportunity for discovery.
Phytochemicals present in medicinal plants have long been studied [2,36,37]. While having a significant role in plants’ adaptation to the continuously changing environment, these compounds also have a multitude of medicinal properties. Aromatic plants and their essential oils (EOs) have a long history of use in traditional medicine, so it is not surprising that perhaps one of the most studied groups of medicinal phytochemicals is essential oil. Essential oils are complex nonpolar mixtures of volatile organic compounds extracted from all parts of plants, mainly through distillation. These mixtures can have up to 300 different compounds. While in many cases, monoterpenes, sesquiterpenes and volatile diterpenes dominate essential oils, other volatile organic compounds are also found–various carbohydrates, fatty acid derivatives, aldehydes, ketones and esters, among many [7,12,13,15,16,19,20,38,39,40,41,42]. These compounds have various structures–some are acyclic compounds, and others are cyclic or have aromatic rings. The most common compounds include α–pinene, β–pinene, limonene, α–terpinene, β–terpinene, ɣ–terpinene, p-cymene, thymol, carvacrol, pulegone and piperiton as cyclic compounds and linalool, geraniol and citronellol as acyclic. Owing to their larger molecules, even greater structural variability exists among sesquiterpenes (C15). Some of the most common ones are α–bisabolene, β–caryophyllene, caryophyllene oxide, germacrene D, eugenol etc. [7,12,13,14,15,16,19,21,39,41,43]. The plenitude of literature on the medicinal properties of essential oils is in part due to their abundance, unique composition, and molecular complexity [44,45,46,47,48]. Additionally, other extrinsic factors, like geographical location, phenophase, time of year, sun exposure/orientation, local climate, soil type, and innumerable other factors, can significantly impact the essential oil’s chemical composition [7,13,14,16,19,36,47]. This inherent variability in essential oil composition both benefits plants and influences their medicinal properties. This plethora of different structures means that each compound in this mixture can have a distinct biological property because it will react differently with the substrate. Various medicinal properties have been reported so far for essential oils and their components, e.g., antioxidant, antimicrobial, antifungal, antitumor, anti-inflammatory, analgesic, etc. [40,44,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63].
Other phytochemicals are obtained by solvent extraction using various methods. These plant extracts may contain more than a hundred individual constituents at varying levels of abundance [33,64,65,66,67,68,69,70,71,72,73,74,75]. However, most of these compounds belong to phenolics, i.e., flavonoids, phenolic acids, tannins, coumarins, non-volatile terpenes (e.g., diterpenes, triterpenes), etc. The obtained extracts’ variation is based on the myriad of different extraction methods and the solvent or solvent combinations used. Thus, different ratios of plant specialised metabolites can be found in the obtained extracts from a single herb. Different medicinal properties have been reported for plant extracts containing phenolics. Flavonoid-rich extracts have shown antibacterial activity, antioxidant properties, anti-inflammatory, antiallergic, vasodilatory, enzyme-inhibitory effects and antitumor activity [9,62,64,65,66,67,68,69,76,77,78]. Phenolic acids are in the focus of current research due to their ability to counteract the consequences of ageing, i.e., the development of cardiovascular diseases, degenerative disorders, and cancer [67]. At the same time, coumarins have shown antimicrobial, antifungal and anti-inflammatory properties [9,77]. A diverse group of compounds, collectively referred to as cannabinoids due to their property to join the cannabinoid receptors of the body and brain, have been extracted from Cannabis sativa and other plants (Echinacea, Acmella oleracea, Helichrysum umbraculigerum, Radula marginata) [79,80]. These compounds, among many others, exert antitumor properties [79,80]. Medicinal properties of plant extracts rich in non-volatile terpenoids (e.g., carnosic acid, carnosol, cassane, norcassane) have also been investigated. These extracts show antioxidative, antimicrobial, antifungal, antitumor and anti-inflammatory activities [76,81,82,83].
Plant extracts may contain a hundred or more individual phytochemicals that vary in their abundance, so the chemical characterisation of these extracts and identification of their components is paramount in understanding their medicinal properties. Until relatively recently, researchers have too often attempted to attribute the medicinal property of an extract to the most abundant compound(s). However, follow-up studies on the medicinal properties of individual compounds extracted and purified have shown either lower activity or, in some cases, even toxicity. Recently, many studies have indicated that the overall activity of extracts is the result of interactions between their components [33]. Indeed, traditional phytotherapy is based on the combination of various medicinal plants [33,34,84,85]. The blend of extracts has proven to have a synergistic effect on their therapeutic properties. In addition, the combination of phytochemicals can reduce the toxicity of individual components in the extract. This effect is reached when components contained in plant extract minimize the negative effects by destroying toxic-acting compound or inhibiting its negative activity and thus providing better activity when added to the original raw drug. Using one of four known methods for preparation of Radix Aconiti, the level of toxicity can be reduced to 0.2% [86]. Rhus hirta extract, when combined with 5-fluorouracil (chemotherapeutic drug), reduced the toxicity of the drug in vitro, possibly due to presence of antioxidants in the extract [33]. Juniperus communis and Solanum xanthocarpum when combined in lower doses noticeably reduced hepatotoxicity in vivo studies [34]. The abovementioned interaction is achieved when the compounds affect different target sites or increase the solubility of one another, thus enhancing their bioavailability [33,34,84,85,86].
The evaluation of interactions between multiple phytochemicals has gained popularity in recent times. Compounds in extracts can have synergistic, additive, or antagonistic activity [33,87]. Additive and non-interactive combinations indicate that the combined effect of two substances is just a summation effect. Conversely, an antagonistic interaction results in a less-than-additive effect. When the interaction of two or more different compounds or extracts shows a larger positive effect than that of individual components, this positive interaction is considered a synergism. There are several methods deployed in the study of the synergistic effect of compound/extract combinations, though one of the most used are: the general isobole equation [33], fractional inhibitory concentration indices (FICIs) [88], and combination index (CI) [76]. Additionally, several authors have used different statistical methods to assess which compounds from extracts influence different biological activities [60,64,77].
3. Plants Extracts vs. Isolated Components and Their Synergistic Effect
3.1. Whole Extract vs. Individual Compounds
Initial studies have shown that the essential oils with a higher abundance of certain compounds also show higher bioactivity. However, the activity of the dominant compounds when tested individually was consistently lower or on par with EO/extract (Table 1). For example, the essential oil of four Mentha species has shown higher antimicrobial activity connected with the relative abundance of menthol, menthone, piperitenone oxide and carvone [61]. Some seasonal variation in the essential oil composition was also noticed that affected antimicrobial activity, but inconsistently so. In M. longifolia, the main compound was piperitenone oxide. The relative abundance of this compound changed from 40% in summer to 64% in winter. However, this did not significantly influence the antimicrobial properties of the oil. The authors also tested the antimicrobial activity of isolated compounds (menthol, menthone, piperitenone oxide and carvone), which was somewhat lower than the total essential oil’s. The essential oil of Thymus vulgaris and Origanum vulgare was also tested for antioxidant and antimicrobial activity [89]. These essential oils were dominated by thymol and carvacrol, respectively. Both oils showed strong antioxidative and antimicrobial activity, but their respective dominant compounds, when tested individually, showed significantly lower activity [89]. Essential oil of Pimenta spp. isolated from leaves and berries showed higher antibiofilm activity in comparison to the dominant compound (eugenol) [90]. The authors concluded that the monoterpene hydrocarbons, which in general exert a less antibacterial effect in comparison to oxygenated compounds, attribute synergistically to dominant compounds and attribute to the higher activity of the whole essential oil. However, the nature of the dominant compound is not the only factor. When studying antimicrobial activities of Thymus pulegioides essential oil was dominated by α–terpinyl acetate. In almost all cases, essential oil demonstrated higher antimicrobial activity than pure α–terpinyl acetate itself [91]. Kerekes et al. [92] studied the antimicrobial effect of three essential oils (Cinnamomum zeylanicum, Origanum majorana, Thymus vulgaris) and their major components (trans-cinnamaldehyde, terpinen-4-ol and thymol). Thymol showed the most promising effect in single-species biofilms, while thyme oil demonstrated better antibacterial activity in polymicrobial bacterial cultures. Antifungal properties of essential oil of Artemisia pedemontana subsp. assoana were assessed [93]. While the essential oil dominated by 1,8-cineole and camphor showed antifungal properties, main compounds tested individually or in combinations did not, attesting to the synergistic effect of minor compounds in the essential oil as well. Hossan et al. [88] tested hexane, ethyl acetate and ethanol extracts of 18 medicinal plants used by the Khyang tribe in Bangladesh for their antimicrobial effects against different pathogenic bacteria, including methicillin-resistant strains. Most of the extracts tested showed antimicrobial properties, even against the methicillin-resistant Staphylococcus aureus strain. Cinnamaldehyde, eugenol and gallic acids were also tested for their antimicrobial effect, and in most tested organisms showed lower activity than the extracts.
Table 1.
Interactions between natural compounds in extracts.
| Activity | Plant Species | Main Component(s) | Tested Compounds | X * | Ref. |
|---|---|---|---|---|---|
| Anti-cancer | Agasrache rugosa | Methyl chavicol, Limonene, Anisaldehyde | Methyl chavicol, Limonene, Anisaldehyde | S/I | [94] |
| Anti-diabetic | Cinnamomum zeylanicum | (E)-cinnamaldehyde, (E)-cinnamyl acetate | (E)-Cinnamaldehyde, (E)-Cinnamyl acetate | A | [95] |
| Eucalyptus camaldulensis | p-Cymene, 1,8-Cineole | p-Cymene, 1,8-Cineole, 1-(S)-α–Pinene | I | [96] | |
| S | |||||
| Laurus nobilis | 1,8-Cineole, α–Pinene | 1,8-Cineole, α–Pinene, Limonene | S | [97] | |
| Anti- inflammatory | Bupleurum fruticescens | β-Caryophyllene, α-Pinene | β-Caryophyllene, α-Pinene | S | [98] |
| Piper chaba | 6-shogaol, Piperine, 6-Gingerol | 6-shogaol, Piperine, 6-Gingerol | S | [99] | |
| Piper interruptum | |||||
| Piper sarmentosum | |||||
| Plumbago indica | |||||
| Zingiber officinale | |||||
| Antimalarial | Croton zehntneri | Estragole | Estragole | S | [100] |
| Lippia sidoides | Thymol | Thymol | A | [100] | |
| Vanillosmopsis arborea | α-Bisabolol | α-Bisabolol | I/A | [100] | |
| Antimicrobial | Agastache rugosa | Methyl Chavicol Limonene | Methyl Chavicol, Limonene, Anisaldehyde | S | [94] |
| Angelica keiskei | Berberine, Magnolol | Berberine, Magnolol, Cryptotanshinone, α-Mangostin | A | [101] | |
| Caryophyllus aromaticus | Eugenol (76%) | Eugenol | S/A | [102] | |
| Cinnamomum cassia | Cinnamaldehyde, Eugenol | S | [88] | ||
| Cinnamomum zeylanicum | (E)-cinnamaldehyde, (E)-cinnamyl acetate | (E)-cinnamaldehyde, (E)-cinnamyl acetate | S/I | [92,95] | |
| Citrus sinensis | D-Limonene | D-Limonene, α–Pinene, Linalool, α–Terpineol, Citral, 3-Carene, Decanal | S | [103] | |
| Croton zehntneri | Estragole | Estragole | I | [100] | |
| Curcuma longa (rhizomes) | α–Zingiberene, β–Sesquiphellandrene, ar-Turmerone | α–Zingiberene, β–Sesquiphellandrene, ar-Curcumene, Curlone, ar-Turmerone | S | [47] | |
| Eucalyptus globulus | (+)-Aromadendrene, 1,8-Cineole | (+)-Aromadendrene, (-)-Globulol, 1,8-Cineole | S | [104] | |
| Lavender angustifolia | Linalool, Linalyl acetate | Linalool, Linalyl acetate | S | [105] | |
| Lippia sidoides | Thymol | Thymol | I/A | [100] | |
| Marchantia polymorpha | Terpenes, Oils, Sugars | Marchantin A | A | [106] | |
| Melaleuca alternifolia | Terpinen-4-ol Eucalyptol | Terpinen-4-ol | I | [50] | |
| Mentha arvensis | Eugenol | S | [88] | ||
| Mentha arvensis | Menthol | Menthol, Menthone, Carvone | S | [61] | |
| Mentha longifolia | Piperitone oxide | Piperitone oxide | S | [61] | |
| Mentha piperita | Menthone, Menthyl acetate, Limonene, | Menthol, Menthone, Carvone | S | [61] | |
| Mentha spicata | Carvone | Carvone, Menthone | S | [61] | |
| Ocimum basilicum | Estragole | Estragole | S | [102] | |
| Origanum majorana | Terpinen-4-ol | Terpinen-4-ol | S | [92] | |
| Origanum vulgare | Carvacrol | thymol, carvacrol | S | [89] | |
| Perilla frutescens | Perillaldehyde, Limonene | Perillaldehyde, Limonene | S | [107] | |
| Pimenta dioica (berry) | Eugenol | Eugenol | A | [90] | |
| Pimenta racemosa (berry and leaf) | β-Myrcene, Eugenol | Eugenol | S | [90] | |
| Pistacia lentiscus var. chia | α–Pinene, Myrcene | fractions | S | [108] | |
| Salvia hispanica | Camphor | Camphor | S/A | [102] | |
| Satureja hortensis | Carvacrol | Carvacrol | I/A | [102] | |
| Terminalia bellirica | Gallic acid | S/A | [88] | ||
| Thapsia villosa | (R)-(+)-Limonene, Methyleugenol | (R)-(+)-Limonene, Methyleugenol | S | [109] | |
| Thymus pulegioides | α–Terpinyl acetate | α–Terpinyl acetate | S | [91] | |
| Thymus vulgaris | Thymol, Carvacrol | Thymol | S/I | [89,92,102] | |
| Vanillosmopsis arborea | α-Bisabolol | α-Bisabolol | I | [100] | |
| Anti-trypanosomal | Curcuma longa (rhizomes) | α–Zingiberene, β–Sesquiphellandrene, ar-Turmerone | α–Zingiberene, β–Sesquiphellandrene, ar-Curcumene, Curlone, ar-Turmerone | S | [47] |
| Immunomodulatory | Glycyrrhiza spp. | Glycyrrhizin | S | [110] | |
| Hypericum perforatum (flower) | 3-methoxy-2,3-dimethylcyclobutene, cis-p-Menth-3-en-1,2-diol | 6-methyl-3,5-heptadien-2-one, β–Caryophyllene | S | [53] | |
| (leaves) | Germacrene D, β-Caryophyllene, Terpinen-4-ol | Germacrene D, α–Humulene, β–Caryophyllene | S/A | [53] | |
* X—Interaction: A—additive, I—indifferent, S—synergistic.
A synergistic effect was detected for other medicinal properties as well. For example, essential oil from the flowers of Agastache rugosa expressed dose-dependent higher anti-mutagenic activity against the AS52 cell line (Chinese hamster ovary cells) in comparison to any of the three individual components (estragole, limonene and anisaldehyde). Additionally, anisaldehyde was the least abundant compound in the essential oil, but showed the highest anti-mutagenic activity of the three tested compounds [94]. Navel orange essential oil, dominated by D-limonene, showed high antioxidative activity in several in vitro antioxidant tests [103]. When tested against A549 cells (human lung cancer) and 22RV1 cells (human prostate cancer), essential oil showed dose-dependent antiproliferative and apoptosis activity. This activity was several folds higher than the individual components tested (i.e., linalool, δ–3-carene, α–terpineol, decanal, citral, D-limonene and α–pinene) [103]. Similar results were also reported for anti-diabetic, skin-whitening and antioxidative activities of Cinnamomum zeylanicum and its main component–trans-cinnamaldehyde (81.4%) [95]. Di Martille et al. [50] studied the antitumor effect of Melaleuca alternifolia essential oil and its main component–terpinen-4-ol on six melanoma cell lines. Even though terpinen-4-ol exhibited an antitumor effect, the synergistic effect with other compounds in the essential oil was lower. The essential oil of Eucalyptus camaldulensis is dominated by p-cymene, 1,8-cineole, α–pinene [96]. While both EO and individual components showed α–amylase inhibition activity, the EO’s was superior, demonstrating synergistic effects of compounds present in the whole EO. Pharmacokinetic interactions occur, for example, between constituents of Artemisia annua tea that enables more rapid absorption of artemisinin from plant extract than of the pure drug. Some plant extracts may have an immunomodulatory effect as well as a direct anti-plasmodial effect, while others contain multidrug resistance inhibitors. Some plant constituents are added mainly to attenuate the side effects of others (for example, ginger to prevent nausea) [111].
3.2. Whole Extract vs. Fractions
Rostro-Atlanis et al. [56] studied Origanum vulgare essential oil. This oil was dominated by monoterpene hydrocarbons, namely o-cymene and ɣ–terpinene. The essential oil was fractionated into five fractions using fractional distillation. The fractions differed both in the overall composition and dominant compounds, namely o-cymene, ɣ–terpinene, and carvacrol. Monoterpene hydrocarbons dominated the first three fractions, while oxygenated monoterpenes dominated the last two. Two oxygenated monoterpenes (thymol and carvacrol) were absent in the first two fractions. These fractions showed different antioxidative and antimicrobial activity due to the composition. Not surprisingly, the first two fractions demonstrated poor antioxidant activity. Still, the activity of the third fraction, which had at least some amount of thymol and carvacrol, was several folds higher. The highest activity was reported for the last fraction, rich in carvacrol and β–caryophyllene. Interestingly, the last two fractions had higher activity than the entire essential oil, owing to a relatively lower abundance of monoterpenes and, possibly, some antagonistic effect of compounds present in the mixture. In a similar study of Thymus pectinatus essential oil [112], antioxidative and antimicrobial activity crude essential oil, essential fractions dominated (>80%) by a single compound and individual compounds (thymol, carvacrol, borneol, p-cymene) were assessed. As in previous cases, the essential oil showed significantly higher activity than any of its dominant components or fractions dominated by a single component, thus attesting to the synergistic effect of the whole essential oil.
3.3. When 1 + 1 Is Not Equal to 2?
The relationships between components in plant extracts are not always synergistic, and the multitude of individual components in these mixtures make it hard to properly assess the contribution of each of the components. When testing antimicrobial activity of Nigella sativa essential oil against Listeria monocytogenes strains, EO sometimes displayed higher activity than individual compounds, and sometimes lower, e.g., N. sativa EO had higher activity than carvacrol, while p-cymene always showed lower activity than the EO [57]. Another study of essential oils of Curcuma longa, C. zedoaria, Zingiber officinale, and Litsea cubeba showed high anti-trypanosomal activity and low cytotoxicity compared to standard pharmaceuticals. When individual compounds representing the major components in their EOs were tested, some showed the same or similar anti-trypanosomal activity but higher cytotoxicity. In comparison, others like curlone showed much higher activity than Eos, while evincing significantly lower cytotoxicity at the same time [47]. Hammer et al. [113] tested the antifungal activity of tea tree essential oil (Melaleuca alternifolia) and its components. The EO and almost all of the individual components showed antifungal activity. However, antifungal activity was much higher for the whole oil compared to most of the individual components. Terpinen-4-ol, the dominant compound in EO, showed higher activity than EO itself but lower than α–terpineol, α–pinene, and β–pinene (all minor compounds) against Candida albicans ATCC10231. When studying the effect of hexane and methanol extracts and their fractions of Juniperus phoenicea leaves, Keskes et al. [78] reported that the terpene-rich hexane extract had a higher α–amylase inhibition activity and no antioxidative activity. In comparison, flavonoid-rich methanol extract showed higher antioxidant and lower α–amylase inhibitory activity. The bioactivity of the fractions of methanol extract was higher, and the individual compound (amentoflavone) was even higher.
3.4. Synergy of Compounds
The initial hypothesis that the dominant compound in a plant extract is responsible for its medicinal properties is being replaced by the synergistic effect hypothesis. An increasing number of studies have suggested that interaction between different components in herbal extracts shows much better activity than any individual component. Barring multivariate statistical analyses (e.g., [60]), one of the most utilised methods of assessing this hypothesis was creating binary and ternary combinations of compounds or fractions and systematically testing their synergistic effects (Table 2). García-García et al. [114] discovered the most active binary (e.g., thymol and carvacrol) and ternary (e.g., thymol, carvacrol, and eugenol) combinations against Listeria innocua. Bassolé and Juliani [115] reviewed the antimicrobial properties of mixtures of individual compounds. Some combinations, like thymol/eugenol, carvacrol/eugenol, carvacrol/cymene, and carvacrol/linalool, showed synergistic effects against different bacteria. However, some binary blends displayed antagonistic or pure additive effects (e.g., carvacrol/myrcene or cinnamaldehyde/eugenol, respectively). Several authors [56,89,116] reported the synergistic effect of thymol and carvacrol on their antimicrobial activity. When studying synergistic effects of binary and ternary combinations, the ratios of the compounds is also important. Some showed the highest synergistic effects in 1:1 proportion, e.g., cinnamaldehyde/thymol, thymol/carvacrol or α–pinene/limonene. Others exerted the most increased synergistic effects in different proportions, e.g., 1,8-cineole/(+)-limonene in ratio 9:1 or cinnamaldehyde/eugenol in ratio 1:4 and 1:8 [115] (and references cited therein). The combination of compounds belonging to different chemical classes also produces synergistic effects. For example, a combination of 3-caffeoylquinic acid (phenolic acid) and artemisinin (terpenoid) in ratio 1:3 exerts a synergistic antimicrobial effect [116]. Two flavonoids, epigallocatechin gallate and quercetin, as well as quercetin-3-glucoside, punicalagin, ellagic acid, and myricetin in different proportions and combinations, have been shown to exert synergism in their antimicrobial activity against methicillin-resistant Staphylococcus aureus [117,118]. Chen et al. [119] studied the cytotoxicity and antihyperglycemic effect of both the main alkaloids from Rhizoma coptidis and minor compounds like ferulic acid and choline. Co-administration of the main alkaloids and minor constituents displayed lower cytotoxicity and synergistic anti-hyperglycemic effects.
Table 2.
Synergism between natural compounds.
| Activity | Studied Components | Model/Test | Ratio | Type | Ref. |
|---|---|---|---|---|---|
| Anti-inflammatory | esculetin + hesperetin + curcumin | PGE2 release | in vitro | [77] | |
| NO, IL-6, TNF-a, IL-1β, IL-6 | 12:13:09 | in vitro | |||
| croton oil-induced mouse ear edema | 337:191:60 | in vivo | |||
| quercetin + curucumine | COX-2 expression, NFκβ activation and NO levels | in vitro | [120] | ||
| Antidiabetic | berberine + ferulic acid | HepG2 cells | in vitro | [119] | |
| chlorogenic acid + ferulic acid | Uptake of 2DG | in vitro | [121] | ||
| (E)-cinnamaldehyde + (E)-cinnamyl acetate | α–Amylase | 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8 | in vitro | [95] | |
| Antimicrobial | 1,8-cineole + camphor | Aspergillus niger | 1:1 | in vitro | [93] |
| (+)limonene + (-)limonene | Cryptococcus neoformans | 1:1 | [122,123] | ||
| Moraxella catarrhalis | |||||
| Pseudomonas aeruginosa | |||||
| Staphylococcus aureus | |||||
| artemisinin + 3-caffeoylquinic acid | Plasmodium falciparum | 1:10-100 | [33] | ||
| artemisinin + arteannuin b | |||||
| artemisinin + casticin | 1:10-100 | ||||
| artemisinin + rosmarinic acid | |||||
| berberine + flavonoid 3,3′-dihydroxy-5,7,4′-trimethoxy-6,8-c-dimethoxyflavone | Staphylococcus aureus | [124] | |||
| berberine + piperine | Staphylococcus aureus | [124] | |||
| capric acid + thymol, carvacrol, resorcylic acid, eugenol, trans-cinnamaldehyde | Escherichia coli | 1:1 | [125] | ||
| caprylic acid + vanillin | Cronobacter sakazakii, Salmonella typhimurium | 2:3 | [125] | ||
| carvacrol + thymol + eugenol | Listeria innocua | [114] | |||
| carvacrol + thymol | Listeria innocua | ||||
| carvacrol + cymene, eugenol, linalool | Bacillus cereus, | in vitro | [115] | ||
| Escherichia coli, | |||||
| Listeria monocytogenes | |||||
| cinnamaldehyde + carvacrol | Salmonella typhimurium, | 1:1, 1:2 | in vitro | [115,125] | |
| Escherichia coli | |||||
| cinnamaldehyde + thymol | Escherichia coli, | 1:1, 1:2 | in vitro | [115,125] | |
| Salmonella typhinurium | |||||
| eugenol + linalool, menthol | Enterobacter aerogenes, Escherichia coli, | in vitro | [115] | ||
| Pseudomonas aeruginosa | |||||
| lauric acid + resorcylic acid, carvacrol, thymol | Escherichia coli | 1:2 | [125] | ||
| limonene + 1,8-cineole | Pseudomonas aeruginosa, Staphylococcus aureus | in vitro | [115] | ||
| menthol + geraniol, thymol | Bacillus cereus, | in vitro | [115] | ||
| Staphylococcus aureus | |||||
| nisin + linalool | Bacillus cereus | in vitro | [126] | ||
| nisin + p-coumaric acid | Salmonella typhimurium | in vitro | [126] | ||
| polygodial + perillaldehyde | Bacillus subtilis, | [106] | |||
| Candida albicans, | |||||
| Mucor mucedo, | |||||
| Pseudomonas aeruginosa, Penicillium chrysogenum, Saccharomyces cerevisiae, Salmonella choleraesuis | |||||
| thymol + carvacrol, eugenol | Escherichia coli, | 1:1 | in vitro | [115,125] | |
| Salmonella typhinurium | |||||
| gallate + quercetin | MethicillinResistant Staphylococcus aureus (MRSA) | 1:1, 2:1 | in vitro | [117] | |
| quercetin3-glucoside + myricetin | Staphylococcus aureus | 3:1, 1:3, 1:7 | in vitro | [118] | |
| quercetin3-glucoside + punicalagin | Staphylococcus aureus | 1:3, 1:7 | in vitro | ||
| myricetin + punicalagin | Staphylococcus aureus | 1:3, 1:7 | in vitro | ||
| ellagic acid + punicalagin | Staphylococcus aureus | 7:1, 3:1, 1:3, 1:7 | in vitro | ||
| ellagic acid + quercetin3-glucoside | Staphylococcus aureus | 3:1, 1:3, 1:7 | in vitro | ||
| Antiproliferative | carnosic acid (CA) carnosol (CAR), betulinic acid (BA) and ursolic acid (UA), combinations: CA+CAR, CA+BA, CA+UA, CAR+UA, and CAR+BA | HT-29 cells | in vitro | [81] | |
| Anti-neurodegenerative | (E)-cinnamaldehyde + (E)-cinnamyl acetate | Tyrosinase inhibition assay | 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8 | in vitro | [95] |
| AChE inhibition | 1:9 | in vitro | |||
| Inhibition of Aβ1-42 aggregation | 8:2, 7:3, 6:4, 2:8, 1:9 | in vitro | |||
| 1,8-cineole + α–pinene, 1,8-cineole + caryophyllene oxide, 1,8-cineole + camphor | Inhibition of AChE | 1:10 | in vitro | [127] | |
| Antioxidative | (E)-cinnamaldehyde + (E)-cinnamyl acetate | Phosphomolybdenum, FRAP, CUPRAC | 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9 | in vitro | [95] |
| malvidin-3-o-glucoside + pelargonidin-3-o-glucoside, catechin, epicatechin, myricetin, quercetin, quercetin-3-glucoside | FRAP | 1:1 | in vitro | [128] | |
| pelargonidin-3-o-glucoside + epicatechin, myricetin, kaempferol, quercetin, quercetin-3-glucoside | FRAP | 1:1 | in vitro | [128] | |
| catechin + myricetin, quercetin, quercetin-3-glucoside | FRAP | 1:1 | in vitro | [128] | |
| epicatechin + myricetin, quercetin, quercetin-3-b-glucoside | FRAP | 1:1 | in vitro | [128] | |
| rosmarinic acid + quercetin, caffeic acid | AAPH-induced oxidation | 0-4:0-1:0-5 | in vitro | [129] | |
| quercetin + rutin, catechin, p-coumaric acid, cyanidin | Liposome oxidation test, Inhibition of platelet function, ORAC | 0.5-1:0.25-0.5, 5:25, 1:1 | in vitro | [130] | |
| p-coumaric acid + catechin | ORAC | 1:1 | in vitro | [131] | |
| kaempferol + myricetin, quercetin, quercetin-3-glucoside | DPPH, FRAP | 1:1 | in vitro | [128] | |
| hesperidin + chlorogenic acid, myricetin, naringenin | ORAC | 1:1 | in vitro | [132] | |
| Antitumor/anticancer | Cannabidiol + Cannabigerol | leukemia (CEM)HL60, breast MCF-7 | 1:1 | in vitro | [133,134] |
| Cannabidiol + Δ9-tetrahydrocannabinol | acute lymphocytic leukemia (CEM)HL60, glioblastoma cell lines U251, SF26 | 1:1 | in vitro | [133,135] | |
| Quercetin + Curcumin | Chronic myeloid leukemia cell line K562, breast cancer, ovarian cancer | in vitro | [136,137,138] | ||
| berberine + d-limonene | human gastric carcinoma cell line MGC803 | in vitro | [139] | ||
| β–caryophyllene + α–humulene, isocaryophyllene | human breast adenocarcinoma cell line MCF-7 | 1:3 | in vitro | [140] |
Carnosic acid and carnosol, two diterpenes from Rosmarinus officinalis extract, were tested against colon cancer cells [141]. The authors attributed the bioactivity of the extract to these two compounds and their interaction. To test the synergistic effect, Pérez-Sanchez et al. [81] used these two compounds and two more triterpenes, betulinic acid and ursolic acid in single treatments and in pairwise combinations. Individual components showed a dose-dependent antiproliferative effect which was always lower than that of the entire R. officinalis extract. Most of the pairwise combinations between di- and tri-terpenes showed additivity or mild synergy, which would explain the better results of the R. officinalis extract. On the other hand, the triterpene combination always brought antagonism between them. Another strong example is the synergism between curcumin and piperine. Curcumin (diferuloylmethane) alone, isolated from Curcuma longa, has low oral bioavailability due to glucuronidation in the small intestine. Piperine originated from black pepper (Piper nigrum) enhances the bioavailability of curcumin by 2000% in humans, due to an inhibition of this glucuronidation and slowing the gastrointestinal transit [111]. Cai et al. [77] investigated the anti-inflammatory potential of Ipomoea stolonifera butanol extract, as well as the individual compounds from the extract (scopoletin, umbelliferone, esculetin, hesperetin and curcumin) and their combinations. The results from in vitro analysis show that esculetin, curcumin and hesperetin were the principal constituents that had synergistic effects when used at the optimal ratio. Additionally, the principal constituents were found to work synergistically in the in vivo mouse ear oedema model at low doses.
While synergism can be assumed in many cases of plant extracts, it is very hard to prove, since hundreds of compounds can be mixed in innumerable ways. Working with complex mixtures consisting of numerous compounds, it is arduous, if not impossible, to test the synergism between them all. Multivariate statistics can provide a helping tool in this. For example, Ivanov et al. [64] studied cytotoxic, wound healing, antioxidant, antidiabetic, antimicrobial and antibiofilm capacities of various inflorescence extracts of Salvia nemorosa. Statistical analysis was performed to assess the correlation between individual compounds in extracts and their medicinal properties. In most of the bioassays, high correlations were found between several of the components and bioactivity, suggesting a synergistic effect between compounds in extract. On the other hand, Rostro-Alanis et al. [56] used the multivariate analysis to find the correlation between the antioxidant and antimicrobial activities with the terpenes in essential oil, namely thymol, carvacrol, β–caryophyllene, and α–humulene. Similarly, Buriani et al. [60] used principal component analysis (PCA) to study the cytotoxic activity of essential oils from Pistacia lentiscus, P. lentiscus var. chia, P. terebinthus, P. vera, and P. integerrima on human tumor cell lines (human adenocarcinoma cell lines: MCF-7 (breast), 2008 (ovarian), and LoVo (colon)). The multivariate approach highlighted the presence of different cooperating clusters of bioactive phytochemicals, which represent the base for further research.
Another way to analyse bioactivity synergism is to fractionate the plant extracts and test the obtained fractions for pharmaceutical properties. However, in this approach, some important compounds might be missed. These missed compounds might not have medicinal properties of their own, but in fact facilitate or enable the activity of the active compound. Junio et al. [142] used broth microdilution antimicrobial checkerboard assay to evaluate the synergy of a crude flavonoid-rich extract of Hydrastis canadensis in the presence of berberine (alkaloid) at a range of concentrations. The alkaloid berberine can be found in various amounts in the extracts of H. canadensis. The crude extract was fractionated and tested with and without the presence of additional berberine. In the fraction that contained three flavonoids that did not possess antimicrobial properties (sideroxylin, 8-desmethyl-sideroxylin, and 6-desmethyl-sideroxylin), a 16-fold decrease of the MICs was observed. Since Hydrastis canadensis root extracts are rich in alkaloids and the leaves in flavonoids, the authors concluded that an extract mixing two parts of the plant would be a more effective antimicrobial agent against S. aureus.
4. Biological Properties: In Vitro and In Vivo Assays
The majority of traditional pharmaceuticals rely on the combination of multiple medicinal plants. In fact, the traditional theory of treatment of diseases is with formulae containing several herbs, in which the medicinal property of one herb is prolonged or enhanced by the other, or the negative effect of one is decreased by the action of the other [85]. In other words, while testing the activity of individual components and their individual interactions is one aspect of synergy study, there is another way–testing synergy of blended plant extracts (Table 3). To validate the traditional use of some plants, in vitro/in vivo bioassays are used.
Table 3.
Synergistic effect of plant extract with other natural compounds or extracts.
| Activity | Botanical Extract | Plant Species/Natural Product Combination | Ratio | Type | Ref. |
|---|---|---|---|---|---|
| Anti-Inflammatory | Extract + Extract | Astragalus membranaceus + Rehmannia glutinosa | 2:1 | in vitro | [143] |
| Boswellia carterii + Commiphora myrrha | in vivo | [144] | |||
| Coptis chinensis + Phellodendron amurense | in vivo | [145] | |||
| Piper chaba + P. sarmentosum + P. interruptum + Plumbago indica + Zingiber officinale | n/a | in vitro | [99] | ||
| Antibacterial | Essential oil + essential oil | Anethum graveolens + Foeniculum/Salvia/Rosmarinus/Thymus | n/a | in vitro | [146] |
| Aniba rosaeodora, Thymus vulgaris | n/a | in vitro | [84] | ||
| Blepharis ogadensis + Blepharis cuspidata | 1:1 | in vitro | [147] | ||
| Cinnamomum zeylanicum + Syzygium aromaticum | n/a | in vitro | [84,115] | ||
| Cinnamomum zeylanicum + Petroselimum sativum | n/a | in vitro | [148] | ||
| Cuminun cyminum + Cinnamomum zeylanicum | n/a | in vitro | |||
| Cuminum cyminum + Coriandrum sativum | n/a | in vitro | [84] | ||
| Cymbopogon + Juniperus/Foeniculum/Rosa/Rosmarinus/Salvia | n/a | in vitro | [146] | ||
| Cymbopogon citratus + Cymbopogon giganteus | 2:1 | in vitro | [115] | ||
| Eucalyptus camaldulensis + Mentha pulegium + Rosmarinus officinalis | 3:4:2 | in vitro | [49] | ||
| Garlic + Bay | n/a | in vitro | [148] | ||
| Juniperus + Foeniculum/Mentha/Rosmarinus/Salvia | n/a | in vitro | [146] | ||
| Lavandula angustifolia + Cinnamomum zeylanicum/Daucus carota/Juniperus virginiana/Thymus vulgaris | n/a | in vitro | [84] | ||
| Lippia multiflora + Mentha piperita, Origanum basilicum | 16:1, 5:3, 8:1 | in vitro | [115] | ||
| Melissa officinalis + Thymus vulgaris | n/a | in vitro | [84] | ||
| Mentha piperita + Ocimum basilicum | n/a | in vitro | [84] | ||
| Mentha pulegium + Rosmarinus officinalis | 06:04 | in vitro | [49] | ||
| Ocimum basilicum + Citrus bergamia | n/a | in vitro | [84] | ||
| Origanum vulgare + Citrus bergamia, Ocimum basilicum, Rosmarinus officinalis | 1:16, 1:8 | in vitro | [84,115] | ||
| Salvia + Rosmarinus, Foeniculum, Mentha, Rosa | n/a | in vitro | [146] | ||
| Satureja hortensis + Origanum vulgare subsp. hirtum | 2:1 | in vivo | [149] | ||
| Thymus schimper + Blepharis cuspidata, B. ogadensis, Melaleuca alternifolia, Pimpinella anisum | 1:1 | in vitro | [54,84,147] | ||
| Thymus capitatus + Cinnamomum zeylanicum | n/a | in vitro | [148] | ||
| Thymus capitatus + Cuminun cyminum | n/a | in vitro | |||
| Thymus capitatus + Garlic | n/a | in vitro | |||
| Thymus capitatus + Petroselimum sativum | n/a | in vitro | |||
| Thymus capitatus + Rosmarinus officinalis | n/a | in vitro | |||
| Essential oil + Essential oil fractions | Anethum graveolens | n/a | in vitro | [150] | |
| Coriandrum | n/a | in vitro | [150] | ||
| Coriandrum (F9) + Eucalyptus (F2) | n/a | in vitro | [150] | ||
| Eucalyptus | n/a | in vitro | [150] | ||
| Pistacia lentiscus var. chia | n/a | in vitro | [108] | ||
| Thymus vulgaris | n/a | in vitro | [151] | ||
| EO + MT104b | Cinnamomum cassia | n/a | in vitro | ||
| EO + nisin | Origanum vulgare | n/a | in vitro | [152] | |
| Thymus vulgaris | n/a | in vitro | [152] | ||
| Zingiber officinale var. rubrum/nisin | n/a | in vitro | [153] | ||
| EO + pediocin | Satureja montana | n/a | in vitro | [152] | |
| Extract + Extract | Elephantorrhiza elephantina + Pentanisia prunelloides | in vitro | [154] | ||
| Hypoxis hemerocallidea (different plant organs) | n/a | in vitro | [155] | ||
| Merwilla plumbea (different plant organs) | n/a | in vitro | |||
| Tulbaghia violacea (different plant organs) | n/a | in vitro | |||
| Extract + berberine | Hydrastis canadensis | n/a | in vitro | [142] | |
| Antifungal | Essential oil + Essential oil | Cymbopogon martini + Chenopodium ambrosioides | 1:1 | in vitro | [156] |
| in vivo | |||||
| Lavandula angustifolia + Andropogon muricatus, Angelica archangelica, Artemisia dracunculus, Canarium luzonicum, Carum carvi, Citrus aurantium, C. grandis, C. sinensis, C. medica limonum, Cinnamomum zeylanicum, Commiphora myrrha, Cupressus sempervirens, Cymbopogon nardus/Daucus carota, Eucalyptus globulus, Foeniculum dulce, Hyssopus officinalis, Juniperus virginiana, Litsea cubeba, Melaleuca alternifolia, Myrtus communis, Origanum majorana, Pinus sylvestris, Piper nigrum, Pogostemon patchouli, Rosmarinus officinalis, Santalum album, Styrax benzoin, Tagetes patula | n/a | in vitro | [84] | ||
| Origanum + Coriandrum sativum, Cassia, Cinnamum | 1:1, 4:1, 2:1 | in vitro | [157] | ||
| Rosa + Cassia | 4:1 | in vitro | [157] | ||
| Salvia officinalis + Thymus vulgaris | n/a | [58] | |||
| Syzygium aromaticum + Brassica nigra | 9:2 | in vitro | [158] | ||
| 10:1 | in vivo | ||||
| Syzygium aromaticum + Rosmarinus officinalis | 1:5, 1:7, 1:9 | in vitro | [115] | ||
| Essential oil + Fraction | Coriandrum sativum | n/a | in vitro | [150] | |
| Eucalyptus | n/a | in vitro | |||
| Antioxidative | Extract + Extract | Astragalus membranaceus + Glycyrrhiza uralensis | 01:01 | in vitro | [159] |
| Camellia sinensis, Cinnamomum cassia, Ginkgo biloba, Phyllanthus emblica, Punica granatum, Vitis vinifera | 5:3:3:3:3:3 | in vitro | [160] | ||
| Salvia officinalis + Ganoderma (fungus) | 7:3 | in vitro | [76] | ||
| Rhus hirta + Rubus strigosus | 1:1 | in vitro | [161] | ||
| Essential oil + Essential oil | Apium graveolens + Thymus vulgaris + Coriandrum sativum | 6:2:2 | in vitro | [162] | |
| Coriandrum sativum + Cuminum cyminum | 1:1 | in vitro | [163] | ||
| Zingiber officinale + Cinnamomum verum + Elettaria cardamomum | 1:7:2 | in vitro | [164] | ||
| Essential oil + natural compound | Santalum sp + α-santalol | 10:1 | in vivo | [165] | |
| Antimalarial | Extract + Extract | Mitragyna inermis + Feretia apodanthera, Guiera senegalensis | in vitro | [144] | |
| Nauclea latifolia + Feretia apodanthera, Guiera senegalensis, Mitragyna inermis | |||||
| Lawsonia inermis + Tithonia diversifolia | 1:1 | in vitro | [166] | ||
| in vivo | |||||
| Antitumor/Anticancer | Extract + Quercetin | Lycopodium clavatum | 10 µL: 50 µM | in vitro | [167] |
| Extract + Extract | Coptis chinensis + Evodia rutaecarpa | 6:1 | in vitro | [168] | |
| in vivo | |||||
| Corydalis + Curucuma | in vitro | [169] | |||
| Curcuma longa + Rosmarinus officinalis | in vivo | [170] | |||
| propolis + bee venom | 7:5 | in vitro | [171] | ||
| Vigna radiata + Vigna unguiculata. subsp. unguiculata + Sauropus androgynus | in vitro | [172] | |||
| Cytotoxicity | EO + EO | Cymbopogon citratus + Cymbopogon giganteus | in vitro | [173] | |
| Salvia officinalis + Thymus vulgaris | n/a | in vitro | [58] | ||
| Anti-neurodegenerative | Extract + Extract | Polygala tenuifolia + Panax ginseng + Poria cocos + Acorus tatarinowii | 3:2:3:2 | in vivo | [174] |
| Salvia officinalis + Ganoderma | 7:3 | in vitro | [76] |
4.1. In Vitro Assays
In the past decade, much attention has been paid to in vitro antimicrobial activity screening methods. Several bioassays, such as microdilution method, disk-diffusion, well diffusion and broth or agar dilution, are the processes mainly used to prove bioactivity of plant extracts and components isolated. Various specialised plant metabolites from different vascular and nonvascular plants are defense mechanisms against infections caused by pathogenic microorganisms, and their antimicrobial activity has been proved in a number of scientific articles [106,175,176,177,178,179,180,181,182,183]. Plant-based natural products (essential oils, plant extracts and their antimicrobial compounds) found an interesting application in food preservations as natural food antimicrobial preservatives [177,178,180,184,185,186,187,188,189,190]. While this review deals mostly with medicinal properties, the molecular mechanisms that govern antimicrobial activity are universal, and thus conclusions on the medicinal aspect can also be drawn. The antimicrobial properties are based on the different modes of action of specialised metabolites. For example, thymol and carvacrol are small molecules that can easily overcome lipid barriers. Studies on the mechanisms of their action show that they are able to integrate themselves into the lipid layer of the cell membrane, thus increasing the surface curvature. The hydrophilic part of the molecule interacts with the polar part of the membrane, while the hydrophobic part sinks into the membrane. This destabilizes the lipid layer, decreases its elasticity and increases its fluidity, which leads to increased permeability to potassium and hydrogen ions [191]. On the other hand, baicalin, a flavonoid from Scutellaria amoena, inhibits β–lactamase, an enzyme that catalyses the hydrolysis of penicillins, cephalosporins and other β–lactam antibiotics [192]. Alternatively, α–pinene, while having low antimicrobial activity, blocks the efflux pump responsible for the ejection of toxic/antibiotic compounds from bacterial cells. The same effect was reported for carnosol, carnosic acid, capsaicin and reserpin [192]. Checkerboard, graphical and time-kill methods are the most widely used procedures to assess the interaction of essential oil components [115].
Éva György et al. [146] investigated in vitro antimicrobial properties of different essential oils (thyme, lemongrass, juniper, oregano, sage, fennel, rosemary, mint, rosehips, and dill) and their combinations against some phytopathogenic bacterial strains (Pseudomonas hibiscicola, Brevibacillus agri, Enterobacter ludwigii, Curtobacterium herbarum, Acinetobacter beijerinckii, Acinetobacter calcoaceticus, Achromobacter xylosoxidans, Staphylococcus succinus, and Staphylococcus sciuri). The authors concluded that a synergistic effect was observed in the case of five combinations of essential oils. The most pronounced antimicrobial activity was detected in the case of oregano, while the most promising combination of the essential oil tested was thyme and dill which showed synergism [146].
García-Díeza et al. [148], investigated selected EOs isolated from aromatic and medicinal herbs and spices and their synergistic effect based on antimicrobial activity against food pathogenic bacteria Salmonella spp., Listeria monocytogenes, Escherichia coli and Staphylococcus aureus. The authors highlighted that almost all combinations of EOs (thyme, cinnamon, rosemary, cumin, garlic, bay, black pepper, lemon, parsley, and nutmeg) studied displayed a synergic effect against foodborne pathogens. In particular, Thymus EO presented the lowest individual MIC, but in combination decreased the MIC of the other EOs. Cinnamon EO also improved the reduction of the individual MICs of the EOs of cumin and parsley. The authors suggest the potential use of EO mixtures to control foodborne pathogens.
Three medicinal plant species (Merwilla plumbea, Hypoxis hemerocallidea, and Tulbaghia violacea) are used in South Africa to treat some infectious diseases [155]. Individual extracts and their combinations were tested in vitro against Candida albicans and four bacterial species (2 Gram+ and 2 Gram-). They found that the proportional combination of dichloromethane and petroleum ether extracts of Merwilla plumbea bulb showed the strongest synergistic effect against Staphylococcus aureus. When studying antimicrobial activity against S. aureus and E. coli, a combination of emulsified pomelo peel oil and chitosan (polysaccharide) were added in broths of different pH values. Synergistic effects of oil and chitosan (Figure 4) were shown, but only at certain pH [193]. Kachkoul et al. [49] studied the synergistic antimicrobial effect of combinations of three essential oils (Mentha pulegium, Eucalyptus camaldulensis, and Rosmarinus officinalis) against three antibiotic-resistant bacterial strains (Proteus mirabilis, Klebsiella pneumoniae, and Staphylococcus aureus). Individually, the essential oils of M. pulegium and R. officinalis did not show any activity against S. aureus. However, when in combination with another essential oil, the synergistic effect of the combination was recorded.
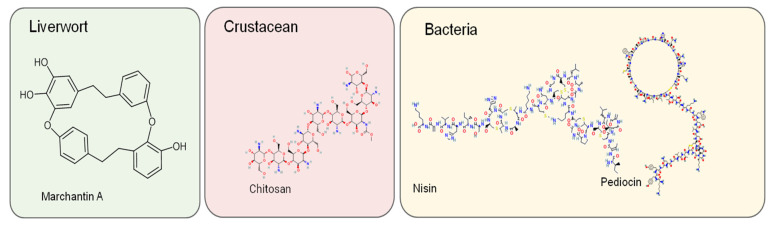
Figure 4.
Some of the studied natural products not originating from vascular plants.
Combinations of essential oils obtained from Blepharis cuspidata, Boswellia ogadensis, and Thymus schimperi against multidrug-resistant Escherichia coli, Klebsiella pneumoniae and Methicillin-resistant Staphylococcus aureus showed synergistic effects in in vitro tests [147]. In a recent review of antimicrobial activity of essential oil combinations, Cho et al. [125] found that the EO blends showed mainly synergistic or antagonistic effects, rather than additive. Loose et al. [54] studied the antibacterial activity of combinations of essential oils from Cymbopogon flexuosus, Melaleuca alternifolia, M. leucadendron var. cajuputi, and Thymus vulgaris against different resistant uro-pathogenic species in artificial urine (Proteus mirabilis, Klebsiella pneumoniae and Staphylococcus aureus). By using checkerboard assays, potential synergistic activity combining different essential oils together were determined. While not all blends resulted in synergy, Melaleuca alternifolia/Thymus vulgaris blend showed an 8-fold increase in antimicrobial activity. Combinations of marjoram and thyme EOs showed antibacterial activities against tested strains. Thus, the lowest MIC of EO combinations of the mixture were Mentha pulegium (29.38%), Eucalyptus camaldulensis (45.37%) of and Rosmarinus officinalis (25.25%) against P. mirabilis and combinations of M. pulegium (60.61%) and Rosmarinus officinalis (39.39%) against Klebsiella pneumoniae. Aside from the antibacterial effect, other in vitro studies of the synergy of different extracts combinations have been studied. For example, Parrish et al. [157] tested the combinations of pairs of sub-inhibitory concentrations of selected 65 essential oils against clinical dermatophytes. Combinations with oregano essential oil were synergistic with EOs of cilantro, cassia, and cinnamon bark. Additionally, rose and cassia EOs were found to completely inhibit one dermatophyte. Additionally, antioxidant activities of combined ethyl acetate extract fractions of Astragalus membranaceus and Glycyrrhiza uralensis showed comparatively better results than any of the individual extracts [159].
Pharmacodynamic synergy has been demonstrated between the Cinchona alkaloids and between various plant extracts traditionally combined. Cinchona alkaloids (quinine and quinidine-isolated from C. calisaya and then chemically synthesised) has evolved from bark extracts to chemical synthesis and controlled clinical trials. The molecular mechanisms of the antimalarial activities of cinchona alkaloids are investigated through hemoglobin digestion in digestive vacuoles of plasmodium parasites to hematin. The effects of quinine and quinidine are investigated not only to treat malaria but also to treat atrial fibrillation, restless leg syndrome, Alzheimer’s disease, and epilepsy [194]. However, the use of quinine and quinidine is under a question mark due to their toxicity [194].
4.2. In Vivo Studies
Apart from in vitro studies which refer to manipulations of organs, tissue or cells under controlled artificial conditions, in vivo studies are often directed for observing the effects of an experiment on a living organism [195]. In spite of the fact that in vivo studies are more expensive and more difficult to control, in many scientific articles, both, in vitro and in vivo experiments are performed. In vivo studies are more suitable for the understanding of the mechanisms of drug-induced toxicity due to their lower structural and functional heterogeneity. For all in vivo methods (antimicrobial, antioxidant, cytotoxic) the natural products that are to be tested are usually administered to the testing animals (mice, rats, etc.) at a definite dosage range. After a determined period of time, the animals are usually sacrificed and blood or tissues are used for the specific assay.
There are numerous examples of in vivo antimicrobial testing using natural products as active components. Kim et al. [196] investigated the antibacterial activities of 20 natural compounds originating from the plants against pathogenic enteric bacterial strains. The experimental design was made by combinations of in vitro (microdilution and biofilm assays) and in vivo tests (mouse macrophages, reactive oxygen species (ROS) assays). The authors determined a dose-dependent bactericidal and biofilm inhibitory activity of two compounds, honokiol and magnolol, against Vibrio cholerae. Combined in vitro and in vivo results suggested that flavonoids and polyphenolic compounds found in food may have protective activity against cholera infection [196].
Kuropakornpong et al. [99], tested a traditional remedy from Thailand consisting of five species: Piper chaba Hunt., Piper sarmentosum Roxb., Piper interruptum Opiz., Plumbago indica Linn., and Zingiber officinale Roscoe. This study investigated the anti-inflammatory activity of plant extracts and their compounds against PGE2 production in murine macrophage (RAW 264.7) cell line and two in vivo models of anti-inflammatory studies. Extract of this natural product was administered both topically and orally to rats inhibited with inflammation induced by ethyl phenylpropionate (rat ear oedema model) and carrageenan (hind paw oedema model). Results obtained from this work proved traditional remedies and also gave possibilities for the development of phytopharmaceutical products for broader use.
While in vivo studies with natural products are mainly connected to the cytotoxic activity, antiprotozoal, antifungal and other activities are also tested. For example, lavender EO and its components linalool, and linalyl acetate are tested for antitumor activities in vitro on human prostate cancer PC-3 and DU145 cell lines using flow cytometry to study apoptosis induction and cell cycle arrest. For in vivo experiments on mice PC-3 cell line was used to establish subcutaneous xenograft tumors in nude mice. Results confirmed that all three natural products, EO and both compounds showed a stronger inhibitory effect on PC-3 cells than on DU145 cells [105]. Toledo et al., [197] investigated in vitro and in vivo anti-Candida activity of Cymbopogon nardus EO in the microemulsion. The experimental in vivo study in a mouse model was performed in female mice. In vivo vulvovaginal candidiasis assay showed that the use of the microemulsion significantly improved the action of the EO.
In vivo antimalarial tests on mice were performed to check the activity of two essential oils (Cymbopogon citratus and Ocimum gratissimum). At concentrations of 200, 300 and 500 mg/kg of mouse per day, the essential oil of C. citratus produced the highest activity with the respective percentages of suppression of parasitaemia: 62.1%, 81.7% and 86.6%. However, before any application of this natural product, toxicity investigation is necessary [198]. Investigation of potential safety and toxicity of natural products are important for in vivo analysis and application for pre-clinical and clinical use of natural products. Dahham et al. [199] tested in vivo toxicity and antitumor activity of essential oils from agarwood (Aquilaria crassna). In this study, dose of the EO at 2000 mg/kg/day was given to mice, screened for two weeks, and confirmed as a safe concentration for further preclinical studies. Some in vivo studies, however, proved a therapeutic arsenal against malaria based on traditional medicine. Mota et al. [100] assessed the in vitro activity of EOs obtained from Vanillosmopsis arborea, Lippia sidoides and Croton zehntneri, aromatic plants well known as Brazilian medicinal plants used in ethnomedicine against the human malaria parasite Plasmodium falciparum. The toxicity of these oils was assessed in healthy mice and in vitro cytotoxicity was determined at different concentrations against HeLa cells and mice macrophages. The authors investigated individual components of EO (α-bisabolol, estragole, and thymol) and concluded that EOs and compounds showed good activity against P. falciparum in vitro and in vivo. Another in vivo study showed that the combined lower doses of Juniperus communis and Solanum xanthocarpum improved the hepatoprotective effect in rats [200].
4.3. Beyond Herbal Combinations
Combinations of natural products do not end with herbal blends. Ganoderma lucidum (fungus) and Salvia officinalis have a long history of use in the prevention and treatment of numerous health problems. For example, Ćilerdžić et al. [76] studied the antioxidant and acetylcholinesterase (AChE) and tyrosinase (TYR) inhibition of a fungus-plant ethanol extract. The activity of AChe and TYR is related to the development of neurodegenerative diseases like Alzheimer’s disease. They found strong synergism in antioxidative activity in fungus-plant (ratio 7:3 for dry mixture, 3:7 for extract mixture), and synergism in tyrosinase inhibition activity at 1:1 ratio. Another example of a different origin combination can be found in the combination of water extract of propolis with bee venom in antitumor activity against two breast cancer cell lines, MCF-7 and Hs578T [171]. Drigla et al. found that the synergic effect at higher bee venom concentrations was, respectively, five and two times more pronounced than the two treatments alone.
As a new generation of antimicrobials, bacteriocins received significant attention, either alone or combined with other natural products (mainly essential oils and their components). An interesting combination occurs between bacterial products such as nisin (bacteriocin produced by a group of Gram-positive bacteria Lactococcus and Streptococcus sp.) and essential oils. Nissa et al. [152] investigated the antimicrobial activity of nisin and red ginger essential oil (Zingiber officinale var. rubrum) and their combination against foodborne pathogens and food spoilage microorganisms. The authors concluded that nisin and red ginger EO had a synergistic effect against Bacillus cereus and fungicidal effect against Aspergillus niger at concentration 62.5 IU/mL of nisin and 1% of the EO. Another group of authors [151] studied a combination of thyme EO at different concentrations (0.3%, 0.6%, or 0.9) and nisin (500 or 1000IU/g), and their combination against Listeria monocytogenes. Results showed synergistic activity against the pathogen. The most promising among treatments was the combination of EO at 0.6% and nisin at concentration 1000IU/g. Bag et al. [126], studied synergistic antibacterial and antibiofilm efficacy of nisin and EOs components (linalool and p-coumaric) against bacteria Bacillus cereus and Salmonella typhimurium. The results provide evidence that p-coumaric acid with nisin is very effective against biofilms of both bacteria.
Turgis et al. [152] reported the synergistic effect of combined antimicrobial agents on pathogenic bacteria and concluded that combinations of EOs and bacteriocins can act synergistically or additionally to eliminate foodborne pathogens and spoilage bacteria. In the article mentioned, the authors tested six EOs (Origanum vulgare, Cinnamomum cassia, Brassica hirta, Thymus vulgaris, Satureja montana, and Cymbopogon nardus) and four bacteriocins (nisin, pediocin, and two other) against five pathogenic bacteria and two spoilage bacteria.
5. Conclusions
Many natural products, which have been widely used as medicinal agents in traditional medicines throughout human history, are now available in commercial supplements and promoted for general health benefits or for prevention and treatment of specific diseases or food supplements, and even additives. Identification of potent herbal mixtures, isolation and identification of compounds and their individual medicinal properties is the first and crucial step for further research aiming at better understanding the underlying mechanisms of their action. This insight, on the other hand, will help us to predict the properties of herbal mixtures and put standards into practice that will ensure the quality control of traditional pharmaceuticals that is needed. This need is being met presently, but the constant rise continues in the number of synergistic studies of different plant extracts.
Although the search for natural products has intensified, several obstacles still slow down its progress. These obstacles can be found in different areas. Some are in the origin of natural-products-bearing-plants–variation in the quality of natural products could be related to plant variety, agronomic practice, and processing [28,150,195]. Then there are those related to analytical techniques or methods for identifying and characterising the activities of compounds, because botanical extracts contain thousands of individual compounds. It is frequently challenging to assign activity to individual components [101,150,195]. Also, there is a problem with methods of application of natural products, particularly of essential oils; since these compounds have low water solubility, strong organoleptic flavour, and low stability, they require protection from oxygen, light, moisture, and heat. These properties limit their intended usage [51,150,169].
The efficacy and safety of products for humans and animals require numerous in vitro and in vivo studies. In vivo studies (which are costly) are often difficult to control. Also, there are ethical issues and extrapolation of data from animals to humans in terms of physiology, biochemistry, genetics, and behaviour. Several EC directives cover food supplements and herbal medicine products that guide medical product identification, such as detailed data on physiological vs. pharmacological and health vs. disease conditions on dose/concentration bases [195].
Plant extracts are especially significant in fighting antimicrobial resistance. The uncontrollable use of antibiotics has led to an increase in the number of antibiotic-resistant strains. While sometimes not as effective as an antibiotic, plant extracts still bear some advantages. They are complex mixtures of different natural compounds that affect, kill, or inhibit the growth of bacteria and other pathogenic microorganisms using different mechanisms. Since different mechanisms are responsible for their antimicrobial mode of action, bacteria are less likely to develop resistance to them. Investigations should be carried out both on their mode of action and their cytotoxicity.
One of the main reasons for the isolation and identification of active natural compounds and investigation of their synergistic effect, in the pharmaceutical development process, is the elimination of potentially toxic compounds. Synergy can occur through a variety of mechanisms, for example, through multi-target effects or through modulation of drug transport, permeation, and bioavailability. Synergy can also lead to the elimination of adverse effects, or, in the case of microorganisms, it can circumvent disease resistance mechanisms [33]. Synergism should be investigated at different levels, from in vitro studies, whose role is screening, to in vivo and, consequently, clinical studies of the most promising herbal combinations. Much more clinical research is needed on pharmacodynamic synergy to prove interaction between plant components and also, resistance reversal and attenuation of side effects.
One could expect promising results and solutions from a number of ongoing investigations in many areas (e.g., pharmacy, agronomy, and food science) for which natural preparations are consistent, and clinical studies well-defined. Furthermore, advances in genomics, informatics and associated contemporary ‘omics technologies and their combination with ethnomedical and ethnobotanical studies of traditional medicines will contribute considerably to the speed of discovery, analysis, and development of improved medicines and new drugs [37].
Author Contributions
Conceptualization, N.R. and P.D.M., writing—original draft preparation, N.R., D.B. and T.D., writing—review and editing, P.D.M. and D.B., visualization, T.D. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Ministry of Science and Technological Development of Republic of Serbia (grant number 451-03-68/2022-14/200113).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tissier A., Ziegler J., Vogt T. Specialized plant metabolites: Diversity and biosynthesis. In: Krauss G.-J., Nies D.H., editors. Ecological Biochemistry. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2014. pp. 14–37. [Google Scholar]
- 2.Davies J. Specialized microbial metabolites: Functions and origins. J. Antibiot. 2013;66:361–364. doi: 10.1038/ja.2013.61. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan B.B., Gruissem W., Jones R.L. Biochemistry & Molecular Biology of Plants. American Society of Plant Physiologists; Rockville, MD, USA: 2009. Natural products (secondary metabolites) pp. 1251–1268. [Google Scholar]
- 4.Barbero F., Maffei M. Biodiversity and chemotaxonomic significance of specialized metabolites. In: Arimura G., Maffei M., editors. Plant Specialized Metabolism. CRC Press; Boca Raton, FL, USA: Taylor & Francis Group; Boca Raton, FL, USA: 2016. pp. 35–76. [Google Scholar]
- 5.Bach T.J., Rohmer M., editors. Isoprenoidys. Springer; New York, NY, USA: 2013. [Google Scholar]
- 6.Grotewold E. The Science of Flavonoids. Springer; New York, NY, USA: 2006. [Google Scholar]
- 7.Rajčević N., Janaćković P., Dodoš T., Tešević V., Marin P.D. Essential-oil variability of Juniperus deltoides RP Adams along the east Adriatic coast–How many chemotypes are there? Chem. Biodivers. 2015;12:82–95. doi: 10.1002/cbdv.201400122. [DOI] [PubMed] [Google Scholar]
- 8.Kampranis S.C., Ioannidis D., Purvis A., Mahrez W., Ninga E., Katerelos N.A., Anssour S., Dunwell J.M., Degenhardt J., Makris A.M., et al. rational conversion of substrate and product specificity in a Salvia monoterpene synthase: Structural insights into the evolution of terpene synthase function. Plant Cell Online. 2007;19:1994–2005. doi: 10.1105/tpc.106.047779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta P.D., Birdi T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017;8:266–275. doi: 10.1016/j.jaim.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang W., Chen X., Li Y., Guo S., Wang Z., Yu X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020;15:1934578X20903555. doi: 10.1177/1934578X20903555. [DOI] [Google Scholar]
- 11.Bergman M.E., Davis B., Phillips M.A. Medically Useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules. 2019;24:3961. doi: 10.3390/molecules24213961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodoš T., Janković S., Marin P.D., Rajčević N. Essential oil composition and micromorphological traits of Satureja montana L., S. subspicata Bartel ex Vis., and S. kitaibelii Wierzb. ex Heuff. plant organs. Plants. 2021;10:511. doi: 10.3390/plants10030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajčević N., Dodoš T., Novaković J., Boršić I., Janaćković P., Marin P.D. Differentiation of north-western Balkan Juniperus communis L. (Cupressaceae) Populations–ecological and chemophenetic implications. J. Essent. Oil Res. 2020;32:562–570. doi: 10.1080/10412905.2020.1805038. [DOI] [Google Scholar]
- 14.Rajčević N., Nikolić B., Marin P.D. Different responses to environmental factors in terpene composition of Pinus heldreichii and P. peuce: Ecological and chemotaxonomic considerations. Arch. Biol. Sci. 2019;71:629–637. doi: 10.2298/ABS190705045R. [DOI] [Google Scholar]
- 15.Novaković J., Rajčević N., Garcia-Jacas N., Susanna A., Marin P.D., Janaćković P. Capitula essential oil composition of seven Centaurea species (sect. Acrocentron, Asteraceae)–Taxonomic implication and ecological significance. Biochem. Syst. Ecol. 2019;83:83–90. doi: 10.1016/j.bse.2019.01.010. [DOI] [Google Scholar]
- 16.Dodoš T., Rajčević N., Janaćković P., Vujisić L., Marin P.D. Essential oil profile in relation to geographic origin and plant organ of Satureja kitaibelii Wierzb. ex Heuff. Ind. Crops Prod. 2019;139:111549. doi: 10.1016/j.indcrop.2019.111549. [DOI] [Google Scholar]
- 17.Ludwiczuk A., Skalicka-Woźniak K., Georgiev M.I. Pharmacognosy. Elsevier; Amsterdam, The Netherlands: 2017. Terpenoids; pp. 233–266. [Google Scholar]
- 18.Boncan D.A.T., Tsang S.S.K., Li C., Lee I.H.T., Lam H.-M., Chan T.-F., Hui J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020;21:7382. doi: 10.3390/ijms21197382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolic B., Ljujic J., Bojovic S., Mitic Z., Rajcevic N., Tesevic V., Marin P. Headspace volatiles isolated from twigs of Picea omorika from Serbia. Arch. Biol. Sci. 2020;72:445–452. doi: 10.2298/ABS200511038N. [DOI] [Google Scholar]
- 20.Eggersdorfer M. Terpenes. In: Wiley-VCH Verlag GmbH & Co. KGaA, editor. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2000. pp. 26–205. [Google Scholar]
- 21.Potente G., Bonvicini F., Gentilomi G.A., Antognoni F. Anti-candida activity of essential oils from Lamiaceae plants from the Mediterranean area and the Middle East. Antibiotics. 2020;9:395. doi: 10.3390/antibiotics9070395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dey P., Kundu A., Kumar A., Gupta M., Lee B.M., Bhakta T., Dash S., Kim H.S. Recent Advances in Natural Products Analysis. Elsevier; Amsterdam, The Netherlands: 2020. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids) pp. 505–567. [Google Scholar]
- 23.Othman L., Sleiman A., Abdel-Massih R.M. Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front. Microbiol. 2019;10:911. doi: 10.3389/fmicb.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurek J. Alkaloids: Their Importance in Nature and Human Life. BoD–Books on Demand; Norderstedt, Germany: 2019. [Google Scholar]
- 25.Hegnauer R. Biochemistry, distribution and taxonomic relevance of higher plant alkaloids. Phytochemistry. 1988;27:2423–2427. doi: 10.1016/0031-9422(88)87006-7. [DOI] [Google Scholar]
- 26.Dai X., Liu Y., Zhuang J., Yao S., Liu L., Jiang X., Zhou K., Wang Y., Xie D., Bennetzen J.L. Discovery and characterization of tannase genes in plants: Roles in hydrolysis of tannins. New Phytol. 2020;226:1104–1116. doi: 10.1111/nph.16425. [DOI] [PubMed] [Google Scholar]
- 27.Naikoo M.I., Dar M.I., Raghib F., Jaleel H., Ahmad B., Raina A., Khan F.A., Naushin F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. Plant Signal. Mol. 2019:157–168. doi: 10.1016/B978-0-12-816451-8.00009-5. [DOI] [Google Scholar]
- 28.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aires A. Tannins: Structural Properties, Biological Properties and Current Knowledge. BoD–Books on Demand; Norderstedt, Germany: 2020. [Google Scholar]
- 30.Stringlis I.A., De Jonge R., Pieterse C.M. The age of coumarins in plant–microbe interactions. Plant Cell Physiol. 2019;60:1405–1419. doi: 10.1093/pcp/pcz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robe K., Izquierdo E., Vignols F., Rouached H., Dubos C. The coumarins: Secondary metabolites playing a primary role in plant nutrition and health. Trends Plant Sci. 2021;26:248–259. doi: 10.1016/j.tplants.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Petrovska B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012;6:1. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caesar L.K., Cech N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019;36:869–888. doi: 10.1039/C9NP00011A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan H., Ma Q., Cui H., Liu G., Zhao X., Li W., Piao G. How can synergism of traditional medicines benefit from network pharmacology? Molecules. 2017;22:1135. doi: 10.3390/molecules22071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bodeker G., Ong C.-K. WHO Global Atlas of Traditional, Complementary and Alternative Medicine. Volume 1. World Health Organization; Geneva, Switerzland: 2005. [Google Scholar]
- 36.Powers C.N., Satyal P., Mayo J.A., McFeeters H., McFeeters R.L. Bigger data approach to analysis of essential oils and their antifungal activity against Aspergillus niger, Candida albicans, and Cryptococcus neoformans. Molecules. 2019;24:2868. doi: 10.3390/molecules24162868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngo L.T., Okogun J.I., Folk W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013;30:584. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajčević N., Janaćković P., Bojović S., Tešević V., Marin P.D. Variability of the Needle Essential oils of Juniperus deltoides RP Adams from different populations in Serbia and Croatia. Chem. Biodivers. 2013;10:144–156. doi: 10.1002/cbdv.201200190. [DOI] [PubMed] [Google Scholar]
- 39.Janaćković P., Rajčević N., Gavrilović M., Novaković J., Giweli A., Stešević D., Marin P.D. Essential oil composition of five Artemisia (Compositae) species in regards to chemophenetics. Biochem. Syst. Ecol. 2019;87:103960. doi: 10.1016/j.bse.2019.103960. [DOI] [Google Scholar]
- 40.Xanthis V., Fitsiou E., Voulgaridou G.-P., Bogadakis A., Chlichlia K., Galanis A., Pappa A. Antioxidant and cytoprotective potential of the essential oil Pistacia lentiscus var. chia and its major components myrcene and α-pinene. Antioxidants. 2021;10:127. doi: 10.3390/antiox10010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajčević N.F., Labus M.G., Dodoš T.Z., Novaković J.J., Marin P.D. Juniperus phoenicea var. turbinata (Guss.) Parl. leaf essential oil variability in the Balkans. Chem. Biodivers. 2018;15:e1800208. doi: 10.1002/cbdv.201800208. [DOI] [PubMed] [Google Scholar]
- 42.Rajčević N., Dodoš T., Novaković J., Kuzmanović N., Janaćković P., Marin P. Are environmental factors responsible for essential oil chemotype distribution of Balkan Juniperus communis var. saxatilis populations? Plant Biosyst. 2022:1–19. doi: 10.1080/11263504.2022.2089764. [DOI] [Google Scholar]
- 43.Novaković J., Rajčević N., Milanovici S., Marin P.D., Janaćković P. Essential oil composition of Centaurea atropurpurea and Centaurea orientalis inflorescences from the Central Balkans–Ecological significance and taxonomic implications. Chem. Biodivers. 2016;13:1221–1229. doi: 10.1002/cbdv.201600029. [DOI] [PubMed] [Google Scholar]
- 44.Raut J.S., Karuppayil S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014;62:250–264. doi: 10.1016/j.indcrop.2014.05.055. [DOI] [Google Scholar]
- 45.Bhalla Y., Gupta V.K., Jaitak V. Anticancer activity of essential oils: A review: Anticancer activity of essential oils. J. Sci. Food Agric. 2013;93:3643–3653. doi: 10.1002/jsfa.6267. [DOI] [PubMed] [Google Scholar]
- 46.Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Alternat. Med. 2016;2016:3012462. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le T., Beaufay C., Nghiem D., Pham T., Mingeot-Leclercq M.-P., Quetin-Leclercq J. Evaluation of the anti-trypanosomal activity of vietnamese essential oils, with emphasis on Curcuma longa L. and its components. Molecules. 2019;24:1158. doi: 10.3390/molecules24061158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajcevic N., Dodos T., Novakovic J., Janackovic P., Marin P. Essential oil composition and antioxidant activity of two Juniperus communis L. varieties growing wild in Serbia. Zb. Matice Srp. Prir. Nauk. 2016:197–205. doi: 10.2298/ZMSPN1631197R. [DOI] [Google Scholar]
- 49.Kachkoul R., Benjelloun Touimi G., Bennani B., El Habbani R., El Mouhri G., Mohim M., Sqalli Houssaini T., Chebaibi M., Koulou A., Lahrichi A. The synergistic effect of three essential oils against bacteria responsible for the development of Lithiasis infection: An optimization by the mixture design. Evid. Based Complement. Alternat. Med. 2021;2021:1–17. doi: 10.1155/2021/1305264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Martile M., Garzoli S., Sabatino M., Valentini E., D’Aguanno S., Ragno R., Del Bufalo D. Antitumor effect of Melaleuca alternifolia essential oil and its main component terpinen-4-ol in combination with target therapy in melanoma models. Cell Death Discov. 2021;7:127. doi: 10.1038/s41420-021-00510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiriac A.P., Rusu A.G., Nita L.E., Chiriac V.M., Neamtu I., Sandu A. Polymeric carriers designed for encapsulation of essential oils with biological activity. Pharmaceutics. 2021;13:631. doi: 10.3390/pharmaceutics13050631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Sayed E., Gad H.A., El-Kersh D.M. Characterization of Four Piper Essential oils (GC/MS and ATR-IR) coupled to chemometrics and their anti- Helicobacter pylori activity. ACS Omega. 2021;6:25652–25663. doi: 10.1021/acsomega.1c03777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schepetkin I., Özek G., Özek T., Kirpotina L., Khlebnikov A., Quinn M. Chemical composition and immunomodulatory activity of Hypericum perforatum essential oils. Biomolecules. 2020;10:916. doi: 10.3390/biom10060916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loose M., Pilger E., Wagenlehner F. Anti-bacterial effects of essential oils against uropathogenic bacteria. Antibiotics. 2020;9:358. doi: 10.3390/antibiotics9060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spyridopoulou K., Fitsiou E., Bouloukosta E., Tiptiri-Kourpeti A., Vamvakias M., Oreopoulou A., Papavassilopoulou E., Pappa A., Chlichlia K. Extraction, Chemical composition, and anticancer potential of Origanum onites L. essential oil. Molecules. 2019;24:2612. doi: 10.3390/molecules24142612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rostro-Alanis M.d.J., Báez-González J., Torres-Alvarez C., Parra-Saldívar R., Rodriguez-Rodriguez J., Castillo S. Chemical composition and biological activities of oregano essential oil and its fractions obtained by vacuum distillation. Molecules. 2019;24:1904. doi: 10.3390/molecules24101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mouwakeh A., Telbisz Á., Spengler G., Mohácsi-Farkas C., Kiskó G. Antibacterial and resistance modifying activities of Nigella sativa essential oil and its active compounds against Listeria monocytogenes. In Vivo. 2018;32:737–743. doi: 10.21873/invivo.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alexa E., Sumalan R., Danciu C., Obistioiu D., Negrea M., Poiana M.-A., Rus C., Radulov I., Pop G., Dehelean C. Synergistic antifungal, allelopatic and anti-proliferative potential of Salvia officinalis L., and Thymus vulgaris L. essential oils. Molecules. 2018;23:185. doi: 10.3390/molecules23010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leyva-López N., Gutiérrez-Grijalva E., Vazquez-Olivo G., Heredia J. Essential oils of oregano: Biological Activity beyond their antimicrobial properties. Molecules. 2017;22:989. doi: 10.3390/molecules22060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buriani A., Fortinguerra S., Sorrenti V., Dall’Acqua S., Innocenti G., Montopoli M., Gabbia D., Carrara M. Human adenocarcinoma cell line sensitivity to essential oil phytocomplexes from Pistacia species: A multivariate approach. Molecules. 2017;22:1336. doi: 10.3390/molecules22081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hussain A.I., Anwar F., Nigam P.S., Ashraf M., Gilani A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species: Biological activities of Mentha essnetial oils. J. Sci. Food Agric. 2010;90:1827–1836. doi: 10.1002/jsfa.4021. [DOI] [PubMed] [Google Scholar]
- 62.Álvarez-Martínez F.J., Barrajón-Catalán E., Micol V. Tackling antibiotic resistance with compounds of natural origin: A comprehensive review. Biomedicines. 2020;8:405. doi: 10.3390/biomedicines8100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Utchariyakiat I., Surassmo S., Jaturanpinyo M., Khuntayaporn P., Chomnawang M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016;16:158. doi: 10.1186/s12906-016-1134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanov M., Božunović J., Gašić U., Drakulić D., Stevanović M., Rajčević N., Stojković D. Bioactivities of Salvia nemorosa L. inflorescences are influenced by the extraction solvents. Ind. Crops Prod. 2022;175:114260. doi: 10.1016/j.indcrop.2021.114260. [DOI] [Google Scholar]
- 65.Veličković I., Žižak Ž., Rajčević N., Ivanov M., Soković M., Marin P.D., Grujić S. Prunus spinosa L. leaf extracts: Polyphenol profile and bioactivities. Not. Bot. Horti Agrobot. Cluj-Napoca. 2021;49:12137. doi: 10.15835/nbha49112137. [DOI] [Google Scholar]
- 66.Stojković D., Drakulić D., Schwirtlich M., Rajčević N., Stevanović M., Soković M.D., Gašić U. Extract of herba Anthrisci cerefolii: Chemical profiling and insights into its anti-glioblastoma and antimicrobial mechanism of actions. Pharmaceuticals. 2021;14:55. doi: 10.3390/ph14010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Velickovic I., Zizak Z., Rajcevic N., Ivanov M., Sokovic M., Marin P., Grujic S. Examination of the polyphenol content and bioactivities of Prunus spinosa L. fruit extracts. Arch. Biol. Sci. 2020;72:105–115. doi: 10.2298/ABS191217004V. [DOI] [Google Scholar]
- 68.Stojković D., Gašić U., Drakulić D., Zengin G., Stevanović M., Rajčević N., Soković M. Chemical profiling, antimicrobial, anti-enzymatic, and cytotoxic properties of Phlomis fruticosa L. J. Pharm. Biomed. Anal. 2020:113884. doi: 10.1016/j.jpba.2020.113884. [DOI] [PubMed] [Google Scholar]
- 69.Stojković D., Drakulić D., Gašić U., Zengin G., Rajčević N., Soković M. Ononis spinosa L. an edible and medicinal plant: UHPLC-LTQ Orbitrap/MS chemical profiling and biological activities of the herbal extract. Food Funct. 2020;11:7138–7151. doi: 10.1039/D0FO01595D. [DOI] [PubMed] [Google Scholar]
- 70.Dodoš T., Rajčević N., Tešević V., Marin P.D. Chemodiversity of epicuticular n -alkanes and morphological traits of natural populations of Satureja subspicata Bart. ex Vis. along Dinaric Alps–Ecological and taxonomic aspects. Chem. Biodivers. 2017;14:e1600201. doi: 10.1002/cbdv.201600201. [DOI] [PubMed] [Google Scholar]
- 71.Dodoš T., Rajčević N., Tešević V., Matevski V., Janaćković P., Marin P.D. Composition of leaf n-alkanes in three Satureja montana L. subspecies from the Balkan Peninsula: Ecological and taxonomic aspects. Chem. Biodivers. 2015;12:157–169. doi: 10.1002/cbdv.201400112. [DOI] [PubMed] [Google Scholar]
- 72.Rajčević N., Janaćković P., Dodoš T., Tešević V., Bojović S., Marin P.D. Leaf n-alkanes as characters differentiating coastal and continental Juniperus deltoides populations from the Balkan Peninsula. Chem. Biodivers. 2014;11:1042–1052. doi: 10.1002/cbdv.201300363. [DOI] [PubMed] [Google Scholar]
- 73.Rajčević N., Janaćković P., Dodoš T., Tešević V., Marin P.D. Biogeographic variation of foliar n-alkanes of Juniperus communis var. sxatilis Pallas from the Balkans. Chem. Biodivers. 2014;11:1923–1938. doi: 10.1002/cbdv.201400048. [DOI] [PubMed] [Google Scholar]
- 74.Rajčević N., Dodoš T., Novaković J., Janaćković P., Marin P.D. Epicuticular Wax Variability of Juniperus deltoides R.P. Adams from the Central Balkan–Ecology and chemophenetics. Biochem. Syst. Ecol. 2020;89:104008. doi: 10.1016/j.bse.2020.104008. [DOI] [Google Scholar]
- 75.Dodoš T., Rajčević N., Janaćković P., Novaković J., Marin P.D. Intra- and interpopulation variability of Balkan endemic–Satureja kitaibelii based on n-alkane profile. Biochem. Syst. Ecol. 2019;85:68–71. doi: 10.1016/j.bse.2019.05.008. [DOI] [Google Scholar]
- 76.Ćilerdžić J., Alimpić Aradski A., Stajić M., Vukojević J., Duletić-Laušević S. Do Ganoderma lucidum and Salvia officinalis Extracts exhibit synergistic antioxidant and antineurodegenerative effects? J. Food Meas. Charact. 2019;13:3357–3365. doi: 10.1007/s11694-019-00258-6. [DOI] [Google Scholar]
- 77.Cai C., Chen Y., Zhong S., Zhang Y., Jiang J., Xu H., Shi G. Synergistic effect of compounds from a chinese herb: Compatibility and dose optimization of compounds from n-butanol extract of Ipomoea stolonifera. Sci. Rep. 2016;6:27014. doi: 10.1038/srep27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keskes H., Belhadj S., Jlail L., El Feki A., Damak M., Sayadi S., Allouche N. LC-MS–MS and GC-MS analyses of biologically active extracts and fractions from Tunisian Juniperus phoenice leaves. Pharm. Biol. 2017;55:88–95. doi: 10.1080/13880209.2016.1230139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bauer R., Woelkart K., Salo-Ahen O.M. CB Receptor Ligands from Plants. Curr. Top. Med. Chem. 2008;8:173–186. doi: 10.2174/156802608783498023. [DOI] [PubMed] [Google Scholar]
- 80.Tomko A.M., Whynot E.G., Ellis L.D., Dupré D.J. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in Cannabis. Cancers. 2020;12:1985. doi: 10.3390/cancers12071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pérez-Sánchez A., Barrajón-Catalán E., Ruiz-Torres V., Agulló-Chazarra L., Herranz-López M., Valdés A., Cifuentes A., Micol V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019;9:808. doi: 10.1038/s41598-018-37173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakagawa S., Hillebrand G.G., Nunez G. Rosmarinus officinalis L. (Rosemary) extracts containing carnosic acid and carnosol are potent quorum sensing inhibitors of Staphylococcus aureus virulence. Antibiotics. 2020;9:149. doi: 10.3390/antibiotics9040149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maurya R., Ravi M., Singh S., Yadav P.P. A review on cassane and norcassane diterpenes and their pharmacological studies. Fitoterapia. 2012;83:272–280. doi: 10.1016/j.fitote.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Orchard A., van Vuuren S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid. Based Complement. Alternat. Med. 2017;2017:1–92. doi: 10.1155/2017/4517971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J., Liu J., Shen F., Qin Z., Jiang M., Zhu J., Wang Z., Zhou J., Fu Y., Chen X., et al. Systems pharmacology analysis of synergy of TCM: An example using saffron formula. Sci. Rep. 2018;8:380. doi: 10.1038/s41598-017-18764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wagner H., Ulrich-Merzenich G. Synergy Research: Approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 87.Hyldgaard M., Mygind T., Meyer R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hossan M.S., Jindal H., Maisha S., Samudi Raju C., Devi Sekaran S., Nissapatorn V., Kaharudin F., Su Yi L., Khoo T.J., Rahmatullah M., et al. Antibacterial Effects of 18 medicinal plants used by the Khyang Tribe in Bangladesh. Pharm. Biol. 2018;56:201–208. doi: 10.1080/13880209.2018.1446030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gavaric N., Mozina S.S., Kladar N., Bozin B. Chemical profile, antioxidant and antibacterial activity of Thyme and Oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. Plants. 2015;18:1013–1021. doi: 10.1080/0972060X.2014.971069. [DOI] [Google Scholar]
- 90.Ismail M.M., Samir R., Saber F.R., Ahmed S.R., Farag M.A. Pimenta oil as a potential treatment for Acinetobacter baumannii wound infection: In vitro and in vivo bioassays in relation to its chemical composition. Antibiotics. 2020;9:679. doi: 10.3390/antibiotics9100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vaičiulytė V., Ložienė K., Švedienė J., Raudonienė V., Paškevičius A. α-Terpinyl acetate: Occurrence in essential oils bearing Thymus pulegioides, phytotoxicity, and antimicrobial effects. Molecules. 2021;26:1065. doi: 10.3390/molecules26041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kerekes E.B., Vidács A., Takó M., Petkovits T., Vágvölgyi C., Horváth G., Balázs V.L., Krisch J. Anti-biofilm effect of selected essential oils and main components on mono-and polymicrobic bacterial cultures. Microorganisms. 2019;7:345. doi: 10.3390/microorganisms7090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sainz; Andrés; Martínez-Díaz; Bailén; Navarro-Rocha; Díaz; González-Coloma Chemical composition and biological activities of Artemisia pedemontana subsp. assoana essential oils and hydrolate. Biomolecules. 2019;9:558. doi: 10.3390/biom9100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim M.H., Chung W.T., Kim Y.K., Lee J.H., Lee H.Y., Hwang B., Park Y.S., Hwang S.J., Kim J.H. The effect of the oil of Agastache rugosa O. Kuntze and three of its components on human cancer cell lines. J. Essent. Oil Res. 2001;13:214–218. doi: 10.1080/10412905.2001.9699669. [DOI] [Google Scholar]
- 95.Sihoglu Tepe A., Ozaslan M. Anti-Alzheimer, Anti-diabetic, skin-whitening, and antioxidant activities of the essential oil of Cinnamomum zeylanicum. Ind. Crops Prod. 2020;145:112069. doi: 10.1016/j.indcrop.2019.112069. [DOI] [Google Scholar]
- 96.Basak S.S., Candan F. Chemical composition and in vitro antioxidant and antidiabetic activities of Eucalyptus camaldulensis Dehnh. essential oil. J. Iran. Chem. Soc. 2010;7:216–226. doi: 10.1007/BF03245882. [DOI] [Google Scholar]
- 97.Sahin Basak S., Candan F. Effect of Laurus nobilis L. essential oil and its main components on α-glucosidase and reactive oxygen species scavenging activity. Iran. J. Pharm. Res. IJPR. 2013;12:367–379. [PMC free article] [PubMed] [Google Scholar]
- 98.Martin S., Padilla E., Ocete M.A., Galvez J., Jiménez J., Zarzuelo A. Anti-Inflammatory activity of the essential oil of Bupleurum fruticescens. Planta Med. 2007;59:533–536. doi: 10.1055/s-2006-959755. [DOI] [PubMed] [Google Scholar]
- 99.Kuropakornpong P., Itharat A., Panthong S., Sireeratawong S., Ooraikul B. In vitro and in vivo anti-inflammatory activities of Benjakul: A potential medicinal product from Thai traditional medicine. Evid. Based Complement. Alternat. Med. 2020;2020 doi: 10.1155/2020/9760948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mota M.L., Lobo L.T.C., Galberto da Costa J.M., Costa L.S., Rocha H.A.O., Rocha e Silva L.F., Pohlit A.M., de Andrade Neto V.F. In vitro and in vivo antimalarial activity of essential oils and chemical components from three medicinal plants found in northeastern Brazil. Planta Med. 2012;78:658–664. doi: 10.1055/s-0031-1298333. [DOI] [PubMed] [Google Scholar]
- 101.Caesar L.K., Kellogg J.J., Kvalheim O.M., Cech R.A., Cech N.B. Integration of biochemometrics and molecular networking to identify antimicrobials in Angelica keiskei. Planta Med. 2018;84:721–728. doi: 10.1055/a-0590-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dawidowicz A.L., Olszowy M. Does antioxidant properties of the main component of essential oil reflect its antioxidant properties? The comparison of antioxidant properties of essential oils and their main components. Nat. Prod. Res. 2014;28:1952–1963. doi: 10.1080/14786419.2014.918121. [DOI] [PubMed] [Google Scholar]
- 103.Yang C., Chen H., Chen H., Zhong B., Luo X., Chun J. Antioxidant and anticancer activities of essential oil from Gannan Navel orange peel. Molecules. 2017;22:1391. doi: 10.3390/molecules22081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mulyaningsih S., Sporer F., Zimmermann S., Reichling J., Wink M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010;17:1061–1066. doi: 10.1016/j.phymed.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Y., Chen R., Wang Y., Qing C., Wang W., Yang Y. In vitro and in vivo efficacy studies of Lavender angustifolia essential oil and its active constituents on the proliferation of human prostate cancer. Integr. Cancer Ther. 2017;16:215–226. doi: 10.1177/1534735416645408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ivković I., Bukvički D., Novaković M., Ivanović S., Stanojević O., Nikolić I., Veljić M. Antibacterial properties of thalloid liverworts Marchantia polymorpha L., Conocephalum conicum (L.) Dum. and Pellia endiviifolia (Dicks.) Dumort. J. Serbian Chem. Soc. 2021;12:1249–1258. doi: 10.2298/JSC210728084I. [DOI] [Google Scholar]
- 107.Nakatsu T., Lupo A.T., Chinn J.W., Kang R.K.L. Studies in Natural Products Chemistry. Volume 21. Elsevier; Amsterdam, The Netherlands: 2000. Biological activity of essential oils and their constituents; pp. 571–631. [Google Scholar]
- 108.Koutsoudaki C., Krsek M., Rodger A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus var. chia. J. Agric. Food Chem. 2005;53:7681–7685. doi: 10.1021/jf050639s. [DOI] [PubMed] [Google Scholar]
- 109.Pinto E., Gonçalves M.-J., Cavaleiro C., Salgueiro L. Antifungal activity of Thapsia villosa essential oil against Candida, Cryptococcus, Malassezia, Aspergillus and Dermatophyte species. Molecules. 2017;22:1595. doi: 10.3390/molecules22101595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uto T., Morinaga O., Tanaka H., Shoyama Y. Analysis of the synergistic effect of glycyrrhizin and other constituents in licorice extract on lipopolysaccharide-induced nitric oxide production using knock-out extract. Biochem. Biophys. Res. Commun. 2012;417:473–478. doi: 10.1016/j.bbrc.2011.11.143. [DOI] [PubMed] [Google Scholar]
- 111.Rasoanaivo P., Wright C.W., Willcox M.L., Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011;10:1–12. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vardar-Ünlü G., Candan F., Sökmen A., Daferera D., Polissiou M., Sökmen M., Dönmez E., Tepe B. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. var. pectinatus (Lamiaceae) J. Agric. Food Chem. 2003;51:63–67. doi: 10.1021/jf025753e. [DOI] [PubMed] [Google Scholar]
- 113.Hammer K.A., Carson C.F., Riley T.V. Antifungal Activity of the Components of Melaleuca alternifolia (Tea tree) oil. J. Appl. Microbiol. 2003;95:853–860. doi: 10.1046/j.1365-2672.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 114.García-García R., López-Malo A., Palou E. Bactericidal action of binary and ternary mixtures of carvacrol, thymol, and eugenol against Listeria innocua. J. Food Sci. 2011;76:M95–M100. doi: 10.1111/j.1750-3841.2010.02005.x. [DOI] [PubMed] [Google Scholar]
- 115.Bassolé I.H.N., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suberu J.O., Gorka A.P., Jacobs L., Roepe P.D., Sullivan N., Barker G.C., Lapkin A.A. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract–Possible synergistic and resistance mechanisms. PLoS ONE. 2013;8:e80790. doi: 10.1371/annotation/57ae25b0-d2c8-444b-ab62-f047c5f3e01e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Betts J.W., Sharili A.S., Phee L.M., Wareham D.W. In vitro activity of epigallocatechin gallate and quercetin alone and in combination versus clinical isolates of methicillin-resistant Staphylococcus aureus. J. Nat. Prod. 2015;78:2145–2148. doi: 10.1021/acs.jnatprod.5b00471. [DOI] [PubMed] [Google Scholar]
- 118.Tomás-Menor L., Barrajón-Catalán E., Segura-Carretero A., Martí N., Saura D., Menéndez J.A., Joven J., Micol V. The promiscuous and synergic molecular interaction of polyphenols in bactericidal activity: An opportunity to improve the performance of antibiotics? Phytother. Res. 2015;29:466–473. doi: 10.1002/ptr.5296. [DOI] [PubMed] [Google Scholar]
- 119.Chen H.-Y., Ye X.-L., Cui X.-L., He K., Jin Y.-N., Chen Z., Li X.-G. Cytotoxicity and antihyperglycemic effect of minor constituents from rhizoma Coptis in HepG2 Cells. Fitoterapia. 2012;83:67–73. doi: 10.1016/j.fitote.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 120.Güran M., Şanlıtürk G., Kerküklü N.R., Altundağ E.M., Süha Yalçın A. combined effects of quercetin and curcumin on anti-inflammatory and antimicrobial parameters in vitro. Eur. J. Pharmacol. 2019;859:172486. doi: 10.1016/j.ejphar.2019.172486. [DOI] [PubMed] [Google Scholar]
- 121.Prabhakar P.K., Doble M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine. 2009;16:1119–1126. doi: 10.1016/j.phymed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 122.Bassolé I.H.N., Lamien-Meda A., Bayala B., Tirogo S., Franz C., Novak J., Nebié R.C., Dicko M.H. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules. 2010;15:7825–7839. doi: 10.3390/molecules15117825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Vuuren S., Viljoen A.M. Antimicrobial activity of limonene enantiomers and 1, 8-cineole alone and in combination. Flavour Fragr. J. 2007;22:540–544. doi: 10.1002/ffj.1843. [DOI] [Google Scholar]
- 124.Britton E.R., Kellogg J.J., Kvalheim O.M., Cech N.B. Biochemometrics to identify synergists and additives from botanical medicines: A case study with Hydrastis canadensis (Goldenseal) J. Nat. Prod. 2018;81:484–493. doi: 10.1021/acs.jnatprod.7b00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cho T.J., Park S.M., Yu H., Seo G.H., Kim H.W., Kim S.A., Rhee M.S. Recent advances in the application of antibacterial complexes using essential oils. Molecules. 2020;25:1752. doi: 10.3390/molecules25071752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bag A., Chattopadhyay R.R. Synergistic antibacterial and antibiofilm efficacy of nisin in combination with p-coumaric acid against food-borne bacteria Bacillus cereus and Salmonella typhimurium. Lett. Appl. Microbiol. 2017;65:366–372. doi: 10.1111/lam.12793. [DOI] [PubMed] [Google Scholar]
- 127.Savelev S., Okello E., Perry N.S.L., Wilkins R.M., Perry E.K. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003;75:661–668. doi: 10.1016/S0091-3057(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 128.Hidalgo M., Sánchez-Moreno C., de Pascual-Teresa S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010;121:691–696. doi: 10.1016/j.foodchem.2009.12.097. [DOI] [Google Scholar]
- 129.Peyrat-Maillard M., Cuvelier M.-E., Berset C. Antioxidant activity of phenolic compounds in 2, 2′-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidation: Synergistic and antagonistic effects. J. Am. Oil Chem. Soc. 2003;80:1007–1012. doi: 10.1007/s11746-003-0812-z. [DOI] [Google Scholar]
- 130.Becker E.M., Ntouma G., Skibsted L.H. Synergism and antagonism between quercetin and other chain-breaking antioxidants in lipid systems of increasing structural organisation. Food Chem. 2007;103:1288–1296. doi: 10.1016/j.foodchem.2006.10.034. [DOI] [Google Scholar]
- 131.Reber J.D., Eggett D.L., Parker T.L. Antioxidant capacity interactions and a chemical/structural model of phenolic compounds found in strawberries. Int. J. Food Sci. Nutr. 2011;62:445–452. doi: 10.3109/09637486.2010.549115. [DOI] [PubMed] [Google Scholar]
- 132.Freeman B.L., Eggett D.L., Parker T.L. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. J. Food Sci. 2010;75:C570–C576. doi: 10.1111/j.1750-3841.2010.01717.x. [DOI] [PubMed] [Google Scholar]
- 133.Scott K.A., Dalgleish A.G., Liu W.M. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int. J. Oncol. 2017;51:369–377. doi: 10.3892/ijo.2017.4022. [DOI] [PubMed] [Google Scholar]
- 134.Schoeman R., Beukes N., Frost C. Cannabinoid combination induces cytoplasmic vacuolation in MCF-7 breast cancer cells. Molecules. 2020;25:4682. doi: 10.3390/molecules25204682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marcu J.P., Christian R.T., Lau D., Zielinski A.J., Horowitz M.P., Lee J., Pakdel A., Allison J., Limbad C., Moore D.H., et al. Cannabidiol enhances the inhibitory effects of δ 9 -tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010;9:180–189. doi: 10.1158/1535-7163.MCT-09-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mutlu Altundağ E., Yılmaz A.M., Serdar B.S., Jannuzzi A.T., Koçtürk S., Yalçın A.S. Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia (K562) cells: II. Signal transduction pathways involved. Nutr. Cancer. 2021;73:703–712. doi: 10.1080/01635581.2020.1767167. [DOI] [PubMed] [Google Scholar]
- 137.Kundur S., Prayag A., Selvakumar P., Nguyen H., McKee L., Cruz C., Srinivasan A., Shoyele S., Lakshmikuttyamma A. synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. J. Cell. Physiol. 2019;234:11103–11118. doi: 10.1002/jcp.27761. [DOI] [PubMed] [Google Scholar]
- 138.Arzuman L., Beale P., Chan C., Yu J.Q., Huq F. Synergism from combinations of Tris (benzimidazole) monochloroplatinum (ii) chloride with capsaicin, quercetin, curcumin and cisplatin in human ovarian cancer cell lines. Anticancer Res. 2014;34:5453–5464. [PubMed] [Google Scholar]
- 139.Zhang X.-Z., Wang L., Liu D.-W., Tang G.-Y., Zhang H.-Y. Synergistic Inhibitory effect of berberine and d -limonene on human gastric carcinoma cell line MGC803. J. Med. Food. 2014;17:955–962. doi: 10.1089/jmf.2013.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Legault J., Pichette A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2010;59:1643–1647. doi: 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- 141.Borrás-Linares I., Pérez-Sánchez A., Lozano-Sánchez J., Barrajón-Catalán E., Arráez-Román D., Cifuentes A., Micol V., Carretero A.S. A bioguided identification of the active compounds that contribute to the antiproliferative/cytotoxic effects of Rosemary extract on colon cancer cells. Food Chem. Toxicol. 2015;80:215–222. doi: 10.1016/j.fct.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 142.Junio H.A., Sy-Cordero A.A., Ettefagh K.A., Burns J.T., Micko K.T., Graf T.N., Richter S.J., Cannon R.E., Oberlies N.H., Cech N.B. Synergy-directed fractionation of botanical medicines: A case study with Goldenseal (Hydrastis canadensis) J. Nat. Prod. 2011;74:1621–1629. doi: 10.1021/np200336g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Azas N., Laurencin N., Delmas F., Di Giorgio C., Gasquet M., Laget M., Timon-David P. Synergistic in vitro antimalarial activity of plant extracts used as traditional herbal remedies in Mali. Parasitol. Res. 2002;88:165–171. doi: 10.1007/s004360100454. [DOI] [PubMed] [Google Scholar]
- 144.Park E., Rhee H.I., Jung H., Ju S.M., Lee Y., Lee S., Hong S., Yang H., Yoo M., Kim K.S. Antiinflammatory effects of a combined herbal preparation (RAH13) of Phellodendron amurense and Coptis chinensis in animal models of inflammation. Phytother. Res. 2007;21:746–750. doi: 10.1002/ptr.2156. [DOI] [PubMed] [Google Scholar]
- 145.Su S., Hua Y., Wang Y., Gu W., Zhou W., Duan J., Jiang H., Chen T., Tang Y. Evaluation of the anti-inflammatory and analgesic properties of individual and combined extracts from Commiphora myrrha, and Boswellia carterii. J. Ethnopharmacol. 2012;139:649–656. doi: 10.1016/j.jep.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 146.György É., Laslo É., Kuzman I.H., Dezső András C. The Effect of essential oils and their combinations on bacteria from the surface of fresh vegetables. Food Sci. Nutr. 2020;8:5601–5611. doi: 10.1002/fsn3.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gadisa E., Weldearegay G., Desta K., Tsegaye G., Hailu S., Jote K., Takele A. Combined antibacterial effect of essential oils from three most commonly used Ethiopian traditional medicinal plants on multidrug resistant bacteria. BMC Complement. Altern. Med. 2019;19:24. doi: 10.1186/s12906-019-2429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.García-Díez J., Alheiro J., Pinto A.L., Falco V., Fraqueza M.J., Patarata L. Synergistic Activity of essential oils from herbs and spices used on meat products against food borne pathogens. Nat. Prod. Commun. 2017;12:1934578X1701200236. doi: 10.1177/1934578X1701200236. [DOI] [PubMed] [Google Scholar]
- 149.Harmati M., Gyukity-Sebestyen E., Dobra G., Terhes G., Urban E., Decsi G., Mimica-Dukić N., Lesjak M., Simin N., Pap B. Binary mixture of Satureja hortensis and Origanum vulgare subsp. hirtum essential oils: In vivo therapeutic efficiency against Helicobacter pylori infection. Helicobacter. 2017;22:e12350. doi: 10.1111/hel.12350. [DOI] [PubMed] [Google Scholar]
- 150.Delaquis P. Antimicrobial Activity of individual and mixed fractions of Dill, Cilantro, Coriander and Eucalyptus essential oils. Int. J. Food Microbiol. 2002;74:101–109. doi: 10.1016/S0168-1605(01)00734-6. [DOI] [PubMed] [Google Scholar]
- 151.Solomakos N., Govaris A., Koidis P., Botsoglou N. The antimicrobial effect of Thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008;25:120–127. doi: 10.1016/j.fm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 152.Turgis M., Vu K.D., Dupont C., Lacroix M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res. Int. 2012;48:696–702. doi: 10.1016/j.foodres.2012.06.016. [DOI] [Google Scholar]
- 153.Nissa A., Utami R., Sari A.M., Nursiwi A. Combination Effect of nisin and red ginger essential oil (Zingiber officinale var. rubrum) against foodborne pathogens and food spoilage microorganisms. AIP Conf. Proc. 2018;2014:020023. doi: 10.1063/1.5054427. [DOI] [Google Scholar]
- 154.Ncube B., Finnie J., Van Staden J. In vitro antimicrobial synergism within plant extract combinations from three South African medicinal bulbs. J. Ethnopharmacol. 2012;139:81–89. doi: 10.1016/j.jep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 155.Mpofu S., Tantoh Ndinteh D., van Vuuren S.F., Olivier D.K., Krause R.W.M. Interactive efficacies of Elephantorrhiza elephantina and Pentanisia prunelloides extracts and isolated compounds against gastrointestinal bacteria. S. Afr. J. Bot. 2014;94:224–230. doi: 10.1016/j.sajb.2014.07.002. [DOI] [Google Scholar]
- 156.Parrish N., Fisher S.L., Gartling A., Craig D., Boire N., Khuvis J., Riedel S., Zhang S. Activity of various essential oils against clinical dermatophytes of Microsporum and Trichophyton. Front. Cell. Infect. Microbiol. 2020;10:545913. doi: 10.3389/fcimb.2020.545913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Prasad C.S., Shukla R., Kumar A., Dubey N. In vitro and in vivo antifungal activity of essential oils of Cymbopogon martini and Chenopodium ambrosioides and their synergism against dermatophytes. Mycoses. 2010;53:123–129. doi: 10.1111/j.1439-0507.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- 158.Aguilar-González A.E., Palou E., López-Malo A. Antifungal activity of essential oils of Clove (Syzygium aromaticum) and/or Mustard (Brassica nigra) in vapor phase against Gray Mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015;32:181–185. doi: 10.1016/j.ifset.2015.09.003. [DOI] [Google Scholar]
- 159.Li M., Xu Y., Yang W., Li J., Xu X., Zhang X., Chen F., Li D. In vitro synergistic anti-oxidant activities of solvent-extracted fractions from Astragalus membranaceus and Glycyrrhiza uralensis. LWT-Food Sci. Technol. 2011;44:1745–1751. doi: 10.1016/j.lwt.2011.02.017. [DOI] [Google Scholar]
- 160.Jain D.P., Pancholi S.S., Patel R. Synergistic antioxidant activity of green tea with some herbs. J. Adv. Pharm. Technol. Res. 2011;2:177–183. doi: 10.4103/2231-4040.85538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang S., Zhu F., Marcone M.F. Synergistic interaction of Sumac and Raspberry mixtures in their antioxidant capacities and selective cytotoxicity against cancerous cells. J. Med. Food. 2015;18:345–353. doi: 10.1089/jmf.2013.0171. [DOI] [PubMed] [Google Scholar]
- 162.Crespo Y.A., Bravo Sánchez L.R., Quintana Y.G., Cabrera A.S.T., Bermúdez del Sol A., Mayancha D.M.G. evaluation of the synergistic effects of antioxidant activity on mixtures of the essential oil from Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L. using simplex-lattice design. Heliyon. 2019;5:e01942. doi: 10.1016/j.heliyon.2019.e01942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bag A., Chattopadhyay R.R. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS ONE. 2015;10:e0131321. doi: 10.1371/journal.pone.0131321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jafarizadeh-Malmiri H., Anarjan N., Berenjian A. Developing three-component ginger-cinnamon-cardamom composite essential oil nanoemulsion as natural food preservatives. Environ. Res. 2022;204:112133. doi: 10.1016/j.envres.2021.112133. [DOI] [PubMed] [Google Scholar]
- 165.Misra B.B., Dey S. Evaluation of in vivo anti-hyperglycemic and antioxidant potentials of α-santalol and sandalwood oil. Phytomedicine. 2013;20:409–416. doi: 10.1016/j.phymed.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 166.Afolayan F.I.D., Adegbolagun O.M., Irungu B., Kangethe L., Orwa J., Anumudu C.I. Antimalarial actions of Lawsonia inermis, Tithonia diversifolia and Chromolaena odorata in combination. J. Ethnopharmacol. 2016;191:188–194. doi: 10.1016/j.jep.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 167.Banerjee A., Pathak S., Jothimani G., Roy S. Antiproliferative effects of combinational therapy of Lycopodium clavatum and quercetin in colon cancer cells. J. Basic Clin. Physiol. Pharmacol. 2020;31 doi: 10.1515/jbcpp-2019-0193. [DOI] [PubMed] [Google Scholar]
- 168.Sui H., Liu X., Jin B.-H., Pan S.-F., Zhou L.-H., Yu N.A., Wu J., Cai J.-F., Fan Z.-Z., Zhu H.-R. Zuo Jin Wan, A traditional Chinese herbal formula, reverses p-GP-mediated MDR in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2013;2013:957078. doi: 10.1155/2013/957078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gao J.-L., He T.-C., Li Y.-B., Wang Y.-T. A Traditional Chinese medicine formulation consisting of rhizoma Corydalis and rhizoma Curcumae exerts synergistic anti-tumor activity. Oncol. Rep. 2009;22:1077–1083. doi: 10.3892/or_00000539. [DOI] [PubMed] [Google Scholar]
- 170.Makaremi S., Ganji A., Ghazavi A., Mosayebi G. Inhibition of tumor growth in CT-26 colorectal cancer-bearing mice with alcoholic extracts of Curcuma longa and Rosmarinus officinalis. Gene Rep. 2021;22:101006. doi: 10.1016/j.genrep.2020.101006. [DOI] [Google Scholar]
- 171.Drigla F., Balacescu O., Visan S., Bisboaca S.E., Berindan-Neagoe I., Marghitas L.A. Synergistic effects induced by combined treatments of aqueous extract of propolis and venom. Med. Pharm. Rep. 2016;89:104–109. doi: 10.15386/cjmed-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nguyen M.-N.T., Ho-Huynh T.-D. Selective cytotoxicity of a Vietnamese traditional formula, Nam Dia Long, against MCF-7 cells by synergistic effects. BMC Complement. Altern. Med. 2016;16:220. doi: 10.1186/s12906-016-1212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bayala B., Bassole I.H.N., Maqdasy S., Baron S., Simpore J., Lobaccaro J.-M.A. Cymbopogon citratus and Cymbopogon giganteus essential oils have cytotoxic effects on tumor cell cultures. Identification of citral as a new putative anti-proliferative molecule. Curr. Trends Oxysterols Relat. Sterols. 2018;153:162–170. doi: 10.1016/j.biochi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 174.Guo S., Wang J., Wang Y., Zhang Y., Bi K., Zhang Z., Li Q. Study on the multitarget synergistic effects of Kai-Xin-San against Alzheimer’s disease based on systems biology. Oxid. Med. Cell. Longev. 2019;2019:1707218. doi: 10.1155/2019/1707218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bukvicki D., Gottardi D., Veljic M., Marin P.D., Vannini L., Guerzoni M.E. Identification of volatile components of liverwort (Porella cordaeana) extracts using GC/MS-SPME and their antimicrobial activity. Molecules. 2012;17:6982–6995. doi: 10.3390/molecules17066982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bukvicki D.R., Tyagi A.K., Gottardi D.G., Veljic M.M., Jankovic S.M., Guerzoni M.E., Marin P.D. Assessment of the chemical composition and in vitro antimicrobial potential of extracts of the liverwort Scapania aspera. Nat. Prod. Commun. 2013;8:1313–1316. doi: 10.1177/1934578X1300800932. [DOI] [PubMed] [Google Scholar]
- 177.Bukvički D., Stojković D., Soković M., Vannini L., Montanari C., Pejin B., Savić A., Veljić M., Grujić S., Marin P.D. Satureja horvatii essential oil: In vitro antimicrobial and antiradical properties and in situ control of Listeria monocytogenes in pork meat. Meat Sci. 2014;96:1355–1360. doi: 10.1016/j.meatsci.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 178.Bukvicki D., Stojkovic D., Sokovic M., Nikolic M., Vannini L., Montanari C., Marin P.D. Potential application of Micromeria dalmatica essential oil as a protective agent in a food system. LWT-Food Sci. Technol. 2015;63:262–267. doi: 10.1016/j.lwt.2015.03.053. [DOI] [Google Scholar]
- 179.Bukvicki D., Ciric A., Sokovic M., Vannini L., Nissen L., Novakcivic M., Vujisic L., Asakawa Y., Marin P.D. Micromeria thymifolia essential oil suppresses quorum-sensing signaling in Pseudomonas aeruginosa. Nat. Prod. Commun. 2016;11:1903–1906. doi: 10.1177/1934578X1601101232. [DOI] [PubMed] [Google Scholar]
- 180.Bukvicki D., Giweli A., Stojkovic D., Vujisic L., Tesevic V., Nikolic M., Sokovic M., Marin P.D. Cheese supplemented with Thymus algeriensis oil, a potential natural food preservative. J. Dairy Sci. 2018;101:3859–3865. doi: 10.3168/jds.2017-13714. [DOI] [PubMed] [Google Scholar]
- 181.Bukvicki D., Gottardi D., Prasad S., Novakovic M., Marin P.D., Tyagi A.K. The healing effects of spices in chronic diseases. Curr. Med. Chem. 2020;27:4401–4420. doi: 10.2174/0929867325666180831145800. [DOI] [PubMed] [Google Scholar]
- 182.Tyagi A.K., Bukvicki D., Gottardi D., Veljic M., Guerzoni M.E., Malik A., Marin P.D. Antimicrobial potential and chemical characterization of Serbian liverwort (Porella arboris-vitae): SEM and TEM observations. Evid. Based Complement. Alternat. Med. 2013;2013:382927. doi: 10.1155/2013/382927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yongabi K.A., Novakovic M., Bukvicki D., Reeb C., Asakawa Y. Management of diabetic bacterial foot infections with organic extracts of liverwort Marchantia debilis from Cameroon. Nat. Prod. Commun. 2016;11:1333–1336. doi: 10.1177/1934578X1601100938. [DOI] [PubMed] [Google Scholar]
- 184.Bukvicki D., Gottardi D., Tyagi A.K., Veljic M., Marin P.D., Vujisic L., Guerzoni M.E., Vannini L. Scapania nemorea liverwort extracts: Investigation on volatile compounds, in vitro antimicrobial activity and control of Saccharomyces cerevisiae in fruit juice. LWT-Food Sci. Technol. 2014;55:452–458. doi: 10.1016/j.lwt.2013.09.029. [DOI] [Google Scholar]
- 185.Patrignani F., Siroli L., Serrazanetti D.I., Gardini F., Lanciotti R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015;46:311–319. doi: 10.1016/j.tifs.2015.03.009. [DOI] [Google Scholar]
- 186.Siroli L., Patrignani F., Serrazanetti D.I., Tabanelli G., Montanari C., Tappi S., Rocculi P., Gardini F., Lanciotti R. Efficacy of natural antimicrobials to prolong the shelf-life of minimally processed apples packaged in modified atmosphere. Food Control. 2014;46:403–411. doi: 10.1016/j.foodcont.2014.05.049. [DOI] [Google Scholar]
- 187.Siroli L., Baldi G., Soglia F., Bukvicki D., Patrignani F., Petracci M., Lanciotti R. Use of essential oils to increase the safety and the quality of marinated pork loin. Foods. 2020;9:987. doi: 10.3390/foods9080987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Picone G., Laghi L., Gardini F., Lanciotti R., Siroli L., Capozzi F. Evaluation of the effect of carvacrol on the Escherichia coli 555 metabolome by using 1H-NMR spectroscopy. Food Chem. 2013;141:4367–4374. doi: 10.1016/j.foodchem.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 189.Siroli L., Patrignani F., Serrazanetti D.I., Vernocchi P., Del Chierico F., Russo A., Torriani S., Putignani L., Gardini F., Lanciotti R. Effect of Thyme essential oil and Lactococcus lactis CBM21 on the microbiota composition and quality of minimally processed Lamb’s Lettuce. Food Microbiol. 2017;68:61–70. doi: 10.1016/j.fm.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 190.Sado Kamdem S.L., Belletti N., Tchoumbougnang F., Essia-Ngang J.J., Montanari C., Tabanelli G., Lanciotti R., Gardini F. Effect of mild heat treatments on the antimicrobial activity of essential oils of Curcuma longa, Xylopia aethiopica, Zanthoxylum xanthoxyloides and Zanthoxylum leprieurii against Salmonella enteritidis. J. Essent. Oil Res. 2015;27:52–60. doi: 10.1080/10412905.2014.982873. [DOI] [Google Scholar]
- 191.Kowalczyk A., Przychodna M., Sopata S., Bodalska A., Fecka I. Thymol and thyme essential oil—New insights into selected therapeutic applications. Molecules. 2020;25:4125. doi: 10.3390/molecules25184125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ayaz M., Ullah F., Sadiq A., Ullah F., Ovais M., Ahmed J., Devkota H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Biol. Interact. 2019;308:294–303. doi: 10.1016/j.cbi.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 193.Chen G.-W., Lin Y.-H., Lin C.-H., Jen H.-C. Antibacterial activity of emulsified Pomelo (Citrus grandis Osbeck) peel oil and water-soluble chitosan on Staphylococcus aureus and Escherichia coli. Molecules. 2018;23:840. doi: 10.3390/molecules23040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Eyal S. The Fever tree: From malaria to neurological diseases. Toxins. 2018;10:491. doi: 10.3390/toxins10120491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Petronilho S., Maraschin M., Coimbra M.A., Rocha S.M. In vitro and in vivo studies of natural products: A challenge for their valuation. The case study of Chamomile (Matricaria recutita L.) Ind. Crops Prod. 2012;40:1–12. doi: 10.1016/j.indcrop.2012.02.041. [DOI] [Google Scholar]
- 196.Kim H.-I., Kim J.-A., Choi E.-J., Harris J.B., Jeong S.-Y., Son S.-J., Kim Y., Shin O.S. In vitro and in vivo antimicrobial efficacy of natural plant-derived compounds against Vibrio cholerae of O1 El tor inaba serotype. Biosci. Biotechnol. Biochem. 2015;79:475–483. doi: 10.1080/09168451.2014.991685. [DOI] [PubMed] [Google Scholar]
- 197.Gaspar de Toledo L., Dos Santos Ramos M.A., Bento da Silva P., Rodero C.F., de Sá Gomes V., Noronha da Silva A., Pavan F.R., da Silva I.C., Bombarda Oda F., Flumignan D.L., et al. Improved in vitro and in vivo anti-Candida albicans activity of Cymbopogon nardus essential oil by its incorporation into a microemulsion system. Int. J. Nanomed. 2020;15:10481–10497. doi: 10.2147/IJN.S275258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tchoumbougnang F., Zollo P.H.A., Dagne E., Mekonnen Y. In vivo antimalarial activity of essential oils from Cymbopogon citratus and Ocimum gratissimum on mice infected with Plasmodium berghei. Planta Med. 2005;71:20–23. doi: 10.1055/s-2005-837745. [DOI] [PubMed] [Google Scholar]
- 199.Dahham S.S., Hassan L.E.A., Ahamed M.B.K., Majid A.S.A., Majid A.M.S.A., Zulkepli N.N. In vivo toxicity and antitumor activity of essential oils extract from agarwood (Aquilaria crassna) BMC Complement. Altern. Med. 2016;16:236. doi: 10.1186/s12906-016-1210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Singh H., Prakash A., Kalia A., Majeed A.B.A. Synergistic hepatoprotective potential of ethanolic extract of Solanum xanthocarpum and Juniperus communis against Paracetamol and Azithromycin induced liver injury in rats. J. Tradit. Complement. Med. 2016;6:370–376. doi: 10.1016/j.jtcme.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]