Abstract
Parasexual genetic analysis of Candida albicans utilized the dominant selectable marker that conferred resistance to mycophenolic acid. We cloned and sequenced the IMH3r gene from C. albicans strain 1006, which was previously identified as resistant to mycophenolic acid (MPA) (A. K. Goshorn and S. Scherer, Genetics 123:213–218, 1989). MPA is an inhibitor of IMP dehydrogenase, an enzyme necessary for the de novo biosynthesis of GMP. G. A. Kohler et al. (J. Bacteriol. 179:2331–2338, 1997) have shown that the wild-type IMH3 gene, when expressed in high copy number, will confer resistance to this antibiotic. We demonstrate that the IMH3r gene from strain 1006 has three amino acid changes, two of which are nonconservative, and demonstrate that at least two of the three mutations are required to confer resistance to MPA. We used this gene as a dominant selectable marker in clinical isolates of C. albicans and Candida tropicalis. We also identified the presence of autonously replicating sequence elements that permit autonomous replication in the promoter region of this gene. Finally, we found the excision of a φ-type long terminal repeat element outside the IMH3 open reading frame of the gene in some strains. We used the IMH3r allele to disrupt one allele of ARG4 in two clinical isolates, WO-1 and FC18, thus demonstrating that a single ectopic integration of this dominant selectable marker is sufficient to confer resistance to MPA.
Candida albicans, an asexual, diploid yeast, has emerged as the primary fungal pathogen of medical importance (18). This polymorphic yeast normally exists as a harmless commensal. However, in patients immunocompromised due to AIDS, organ transplantation, or chemotherapy, C. albicans can cause significant morbidity and mortality. Despite the increasing clinical importance of C. albicans and other Candida spp., we lack a clear understanding of Candida pathogenesis and the etiology of candidiasis (4). Much of this lack of understanding of the basic biology of Candida is due to the facts that it is diploid and has no known sexual cycle, although recent evidence suggests that a genetic system may exist (11, 17). Many of the classical genetic approaches that have been successful in elucidating the pathogenicity of bacteria and of some species of phytopathogenic fungi cannot be successfully applied to Candida. In particular, it has been difficult to demonstrate unequivocally the role of particular genes in the pathogenic process (16).
Presently, the use of molecular techniques such as transformation has circumvented some of the problems of genetic analysis (24). The Ura-blaster cassette, which was originally developed by Alani et al. (1) for the sequential disruption of genes in Saccharomyces cerevisiae, was adopted by Irwin and Fonzi (5) for use in C. albicans. This procedure utilizes a selectable marker (in this case, the URA3 gene, encoding orotidine 5′-monophosphate [OMP] decarboxylase) flanked by direct repeats of heterologous sequence (the Salmonella hisG gene). This cassette is used to disrupt or replace the gene of interest. The construct is then used for disruption of the intended gene by homologous integration into a ura3 strain, and cells are selected on the basis of URA3 prototrophy. Mitotic recombination between the hisG repeat sequences results in a return to uridine auxotrophy, with the presence of a hisG “footprint” disrupting one allele of the gene of interest. This process is then repeated to disrupt the second allele. To date, most molecular studies of C. albicans biology have utilized the Ura-blaster approach in a ura3 strain, CAI-4, derived from a clinical strain, SC5314 (5). Because use of the Ura-blaster cassette requires ura3 auxotrophy, its use is limited to laboratory strains and precludes the study of clinical isolates.
Recently Lay et al. (16) found that many strains harboring genetic lesions induced by the Ura-blaster technique showed variable OMP decarboxylase activities, usually reduced compared to the wild-type progenitor strain SC5314 (16). Variation in the levels of OMP decarboxylase may cause problems in studies of virulence (13). It was suggested that position effects (15) can reduce the levels of OMP decarboxylase activity to less than wild-type levels. This finding calls into question previous studies in which decreased virulence is attributed to disruption of the targeted gene, although some laboratories have not been able to repeat the variation in OMP dehydrogenase (W. A. Fonzi et al., personal communication). Lay et al. (16) called for the development of a different selectable marker, one that does not influence virulence.
A variety of selectable markers have been used in fungi. However, until now no dominant selectable marker has been found to be useful in Candida. There are two major reasons for this: (i) Candida species in general, and C. albicans in particular, have been found to be naturally resistant to most selectable markers available, including hygromycin, benomyl, cycloheximide, mitomycin C, and tunicamycin (B. Magee, personal communication); and (ii) at least 11 Candida species read the CUG codon as serine instead of leucine (21), rendering potential transgenic resistance genes and/or reporter genes nonfunctional in these yeasts (24). Mycophenolic acid (MPA) resistance (Mpar), conferred by a mutation of the IMH3 gene, which encodes IMP dehydrogenase, is a selectable phenotype that has been successfully used in C. albicans for selection in spheroplast fusion (9) and, more recently, as a reporter for virulence gene activation in vivo (26) or as a selectable marker if used in high copy number (14). This gene was cloned by Kohler et al. (14) from strain SS and found to be 2,908 bp in size. Herein we report on the isolation, sequence, and use of the dominant IMH3r allele, which confers Mpar in both C. albicans and C. tropicalis. Finally, we demonstrate that the ectopic integration of a single copy of the IMH3r allele is sufficient to confer Mpar and can be used as a selectable marker for gene disruption.
MATERIALS AND METHODS
Strains and culture conditions.
Strains used in this study and their relevant genotypes are described in Table 1. C. albicans strains were maintained in YEPD medium (1% yeast extract, 2% Bacto Peptone, 2% dextrose, 1.5% plant agar (Sigma, St. Louis, Mo.). 5-fluoro-orotic acid (5-FOA) medium contains 0.42% g of yeast nitrogen base without amino acids, 1.2% dextrose, and 1.5% agar, with 60 mg of uridine and 0.6 g of 5-FOA dissolved in dimethyl sulfoxide added to the cooled medium. For selection and testing of Mpar, we used minimal medium (mm) consisting of 0.67% yeast nitrogen base without amino acids, 2% dextrose, and concentrations of MPA (Sigma) in ethanol ranging from 1 to 10 μg/ml.
TABLE 1.
C. albicans strains used in this study
| Strain(s) | Parent strain | Genotype | Reference(s) or source |
|---|---|---|---|
| FC18 | Clinical isolate of C. albicans | Wild type | |
| WO-1 | Clinical isolate of C. albicans | Wild type | 23 |
| SC5314 | Clinical isolate of C. albicans | Wild type | 5 |
| CAI-4 | SC5314 | Δura3::imm434/Δura3::imm434 | 5 |
| 1006 | ura3/ura3 arg4/arg4 ser/ser lys1/lys1 IMH3r | 9 | |
| 16F, R1b, R3b, 36 | Clinical isolates of C. dublinensis | Wild type | 26, 27 |
| 660, 678 | Clinical isolate of C. tropicalis | Wild type | Scherer strain collection |
| 669 | Clinical isolate of C. pseudotropicalis | Wild type | Scherer strain collection |
| 688 | Clinical isolate of C. parapsilosis | Wild type | Scherer strain collection |
| 653 | Clinical isolate of C. krusei | Wild type | Scherer strain collection |
Construction of p3408.
The following oligomers were designed corresponding to the published sequence of the IMH3 gene (14), with XhoI (underlined) restriction sites added to the previously identified XbaI sites (bold underlined): B15106 (5′CTCGAGTCTAGATGTTTATGATACTAAG-3′) and C15107 (5′CTCGAGTCTAGAACTCAGTATATCTTCA-3′).
PCR.
PCR amplification was carried out in a 50-μl reaction volume containing 10 mM Tris-Cl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 0.01% gelatin, 20 μM deoxynucleoside triphosphates, 5 μM each oligonucleotide primer, 50 ng of genomic DNA, and 5 U of Taq polymerase (Boehringer Mannheim Biochemicals). After an initial incubation time of 94°C for 5 min, the samples were cycled 30 times as follows: 94°C for 1 min, 55°C for 1 min, and 70°C for 1 min. A 10-min extension at 72°C completed the reaction. Aliquots of the reactions were fractionated on 0.7% TAE (Tris-acetate-EDTA) agarose, and fragments were cloned using a TA cloning kit (Invitrogen, Carlsbad, Calif.) to create p3408 (derived from strain 1006), pIMH3C (derived from strain CAI-4), and pIMH3W (derived from strain WO-1). Plasmids were then sequenced by ATG Technologies (Minneapolis, Minn.). Plasmid p3408 was digested with XhoI and fractionated on 0.7% TAE-agarose. The 2.7-kb fragment containing the IMH3 gene was excised and cloned into XhoI-linearized pABSKII to yield p3417.
Transformation and selection for Mpar.
Two-milliliter cultures of C. albicans strains FC18, WO-1, and CAI-4, and C. tropicalis strains 660 and 678 from the Scherer strain collection (http://alces.med.umn.edu/Candida/strains), were grown overnight in YEPD broth at 30°C with shaking at 200 rpm. Samples (0.2 ml) of cells were pelleted, washed with water, recentrifuged, and resuspended in freshly made OSB (0.8 ml of 50% polyethylene glycol 8000, 0.2 ml 1 M lithium acetate, 25 mg dithiothreitol, 25 μl of carrier DNA [10 mg of sonicated salmon sperm DNA per ml]). Samples containing FC18 and WO-1 were transformed with p3408 DNA (2.5 μg) or pIMH3W (2.5 μg); CAI-4 was transformed with 2.5 μg of p3417 in duplicate (Table 2). Cells (including no-DNA controls) were incubated at 43.5°C for 45 min. Strains FC18 and WO-1 and one-half of the CAI-4 transformation were plated onto MM with 5 μg of MPA; the second-half of the CAI-4 transformation was plated onto MM. Plates were incubated at 30°C. Colonies appeared within 3 days. (Due to the labile nature of MPA, plates were made 24 h prior to the transformation experiment to ensure accurate levels of the drug.)
TABLE 2.
Plasmids used in this study
| Plasmid | Marker | Description | Reference |
|---|---|---|---|
| p1129 | None | ARG4 cloned from p1076 in pUC18 | 10 |
| p3408 | IMH3R | IMH3 cloned from strain 1006 | This study |
| pABSKII | URA3 | pBSKII vector with ARS2, URA3, and blue/white selection | Unpublished |
| p3417 | URA3/IMH3r | IMH3r cloned into polylinker of pABSKII | This study |
| p3394 | IMH3R | IMH3r inserted into MscI-SpeI site of p1129 | This study |
Genomic DNA isolation and analysis.
For DNA isolation, 1 ml of log-phase cells grown in YEPD was centrifuged. The supernatant was removed and replaced with 0.5 ml of TENTS buffer (10 mM Tris-Cl [pH 7.5], 1 mM EDTA, 100 mM NaCl, 2% Triton X-100, 1% [wt/vol] sodium dodecyl sulfate), 0.2 g of acid-washed glass beads, and 0.5 ml of phenol-chloroform (1:1, vol/vol). The mixture was vortexed for 4 min and then centrifuged for 5 min. The supernatant was transferred to a new tube and precipitated with 0.1 volume of ammonium acetate and 2 volumes of 95% ethanol. The solution was centrifuged for 5 min, the supernatant was removed, and the pellet was washed with 70% ethanol and allowed to dry. The pellet was resuspended in 50 μl of Tris-EDTA.
Segregation of Mpar with URA3.
Prototrophic and Mpar CAI-4-derived strains (URA3/ura3 Mpar) were streaked onto MM–5 μg of MPA, MM, and YEPD and grown at 30°C for 3 days. Colonies from these plates were then picked onto plates containing 5-FOA, MM–5 μg of MPA, MM, or YEPD to test for plasmid stability. Two days later, colonies growing on 5-FOA were plated onto MM–5 μg of MPA and MM to examine segregation of Ura3 and Mpar.
Examination of mutations necessary to confer Mpar.
The IMH3r allele was separated into two subclones by PCR; each subclone contained one of the exons and part of the intron. No overlap exists between the intron sequences. Primers B15106 (above) and B17359 (5′CATATGTTAAAGAATCATCAGAGTAATAGTATAATGTGATA-3′) were used to subclone exon 1; C15107 (above) and B17360 (5′CATATGAAAATTTATTAACTTTCGTTCTACCGATA-3′) were used to subclone exon 2. XhoI restriction sites added to the oligonucleotides are underlined. The subclones were used to transform strains, selecting for Mpar. The exon subclones were used both individually and together.
Ectopic integration and gene disruption with IMH3r.
Plasmid p1129, containing the 3.3-kb ARG4 gene subcloned into pUC18 (10), was digested with MscI and SpeI and blunt ended with T4 polymerase to delete a 1.6-kb region encompassing the entire open reading frame (ORF) of ARG4. The IMH3r allele was excised from p3408 by digestion with EcoRI and blunt ended with T4 polymerase. The fragments were ligated to create p3394. FC18 and WO-1 were transformed with p3394 as described above. Mpar transformants were restreaked onto MM–MPA (10 μg/ml) to confirm resistance. DNA was extracted as previously described, and digested with BamHI and SacI. Gels were blotted with a Zeta-probe nylon membrane as instructed by the manufacturer (Bio-Rad, La Jolla, Calif.). Radioactive probes of IMH3 or ARG4 were generated with a Rediprime kit (Amersham, Piscataway, N.J.).
Nucleotide sequence accession number.
The IMHr sequence reported here has been assigned GenBank accession number AF249293.
RESULTS
The IMH3r gene from the Mpar strain 1006 possesses three mutations and a deletion.
We performed PCR to clone the IMH3r alleles from strains 1006 and CAI-4, using primers designed from the previously reported IMH3 sequence (14). Both the Mpa-susceptible strain CAI-4 and the Mpar strain 1006 yielded a 2,704-bp fragment, as opposed to the 2,908-bp fragment expected based on the previously reported sequence (14). Sequence analysis and subsequent BLAST searches of GenBank sequences confirmed that the 2.7-kb fragment contained the IMH3 gene and its previously identified sequence motifs, including two exons separated by an intron of 248 nucleotides (nt) (14). The DNA sequences differ at several places; however, the putative amino acid sequences encoded by the two alleles that we sequenced are identical to the published sequence, except for one conservative and two nonconservative changes that occur within the ORF in the gene from 1006: I47V, S102A, and G482D. The first two of these mutations are in exon 1, and the third mutation is in exon 2. BLAST (2) analysis was performed on the approximately 200-bp sequence in the noncoding region of the 3′ end of the second exon. This sequence is found in strain SS but is missing in the noncoding region at the 3′ end of the gene in 1006 and CAI-4. The analysis identified the missing nucleotides as a potential φ-like long terminal repeat (LTR) element (8); it appears to have been excised from strains 1006 and CAI-4 since these strains possess a GTAATA footprint at nt 2676 in these strains which may be indicative of excised of LTR-like elements (8).
An IMH3 allele from 1006 is able to confer Mpar in C. albicans and C. tropicalis.
Kohler et al. (14) previously demonstrated that the insertion of IMH3 on a high-copy-number plasmid was capable of conferring Mpar. We wished to determine whether overexpression of the IMH3 allele from the already Mpar strain 1006 was capable of conferring resistance as well. We had preliminary evidence that 1006 was heterozygous at the IMH3 locus (data not shown). The IMH3r allele was cloned into pABSKII, a Bluescript-based plasmid containing both ARS2 and URA3 to create p3417. CAI-4 was transformed with p3417, and the transformants were selected for URA3 prototrophy, Mpar (in the presence of uridine), or both. The URA3 prototrophic colonies were restreaked onto MM–MPA (10 μg/ml) to examine Mpar. The Mpar colonies were restreaked onto MM to examine prototrophy. Cosegregation of URA3 prototrophy and Mpar was 100%. Twenty-four colonies that were identified as Ura+ and Mpar (eight from each selection protocol) were plated onto 5-FOA. The resulting 5-FOA-resistant colonies were then screened Mpar. None of the colonies tested was resistant, even when the concentration of MPA was lowered to 1 μg/ml. Therefore, the resistance was due to the IMH3 allele carried on the plasmid. This allele was designated IMH3r.
To determine whether single-copy integration could confer Mpar in other Candida species, the IMH3r allele was amplified by PCR, and the product was cloned into pCR2.1 to create p3408. This plasmid, containing IMH3r, was linearized and used to transform the clinical isolates in Table 1 (Fig. 1). Selection for Mpar was consistently achieved with C. tropicalis and C. albicans. Other Candida species tested were naturally Mpar at concentrations as high as 20 μg/ml under the conditions tested. Colonies of putative C. albicans and C. tropicalis transformants were restreaked onto MM–MPA (10 μg/ml) to confirm that they were Mpar. Resistant colonies of C. tropicalis were screened by PCR using the primers that were used to clone the IMH3r allele, and a 2.7-kb fragment was obtained. The primers failed to produce a product in the untransformed control strains of C. tropicalis (Fig. 2).
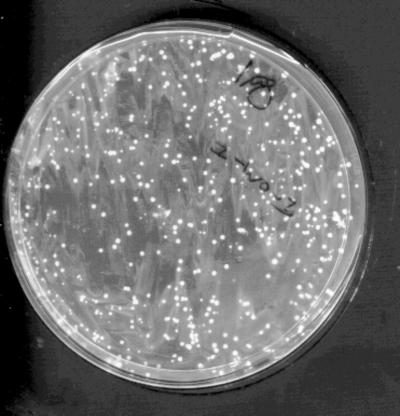
FIG. 1.
Typical selection for Mpar after transformation with p3408. WO-1 was transformed with uncut p3408 and plated onto medium containing MPA (1 μg/ml). The plates were incubated at 30°C for 3 days. Transformants were picked and replated onto MPA at 1, 10, and 20 μg/ml. Almost all colonies grew under all conditions tested.
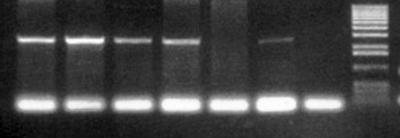
FIG. 2.
Plasmid p3408 is sufficient to confer Mpar in clinical isolates of C. tropicalis. Primers used to clone the IMH3r allele were used to detect the presence of the IMH3r allele in C. tropicalis strain 678 transformed with p3408 (Lanes 1 to 6, transformants). Sequence similarity between the IMH3 allele of C. tropicalis and the primers used was not sufficient to detect the 2.7-kb IMH3 homolog in untransformed C. tropicalis (lane 7). Lane 8, 1.kb Plus DNA ladder (Gibco-BRL, Grand Island, N.Y.).
The IMH3r allele possesses an ARS element.
Southern blot analysis of the Mpar strains of C. albicans transformed with plasmid p3408 showed that the plasmid was replicating autonomously (Fig. 3), suggesting the presence of an unidentified autonomously replicating sequence (ARS) element. Analysis of the sequence of the IMH3r allele reveals the presence of putative ARS elements at nt 7 (5′GTTTATGATAC3′), based on similarity to the previously identified ARS consensus sequence (5′TTTATGTTT3′) (3), and at positions 101 (5′ATTTAATTTTC3′) and 358 (5′TTTTTCGCTTTTT3′), based on identified ARS sequences of C. maltosa (23). Although relatively stable, plasmid p3408 was lost when placed under extended nonselective conditions (data not shown).
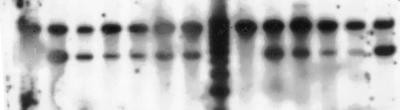
FIG. 3.
The IMH3r allele possesses an ARS. FC18 and WO-1 were transformed with uncut p3408 and selected on MPA (1 μg/ml). Mpar transformants were replated onto MPA at 1, 5, 10, and 20 μg/ml. Colonies were picked from the second set of plates and grown overnight in YEPD. DNA was extracted, digested with HindIII, subjected to gel electrophoresis, and blotted. Blots were hybridized with a radiolabeled IMH3 probe. The bands at 11.0 kb are the genomic copies. The 6.7-kb bands correspond to the size of the linearized p3408 plasmid.
Mutations in both exons are required to confer Mpar.
Because the IMH3r allele contains several sequence changes in the promoter and three mutations affecting the amino acid sequence, we wished to determine which mutations were sufficient to confer Mpar. We therefore used PCR to obtain fragments of the gene. A 1.1-kb fragment which included the promoter and exon 1 with half of the intron and a 1.6-kb fragment which contained the remainder of the intron and exon 2 were constructed (Fig. 4). The PCR products were used separately and together to transform FC18 and WO1 to Mpar. The 2.7-kb PCR product, containing the complete gene, was used as a positive control. Neither fragment alone was sufficient to confer Mpar, although both the positive control and cotransformation with the combined PCR fragments yielded resistant transformants (although the number of Mpar yeast cells were greatly reduced in cotransformation with the two exons compared to the positive control). This demonstrates that both exons are necessary for Mpar and that neither alone is sufficient to confer resistance.
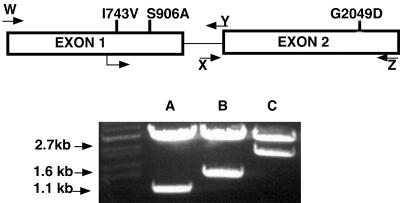
FIG. 4.
Structure of the IMH3r allele and location of primer pairs. The IMH3 gene consists of two exons, of 1.1 and 1.6 bp, respectively, and an intron of 248 bp. The mutations which distinguish the IMH3r allele from the wild-type gene are shown in their approximate locations. The primers which were used to amplify the exons separately are shown as arrows. Amplification of the gene was carried out with the following primers: for exon 1, B15106(W) and B17359(Y); for exon 2, C15107(Z) and B17360(X).
IMH3r is capable of conferring Mpar by single-copy integration.
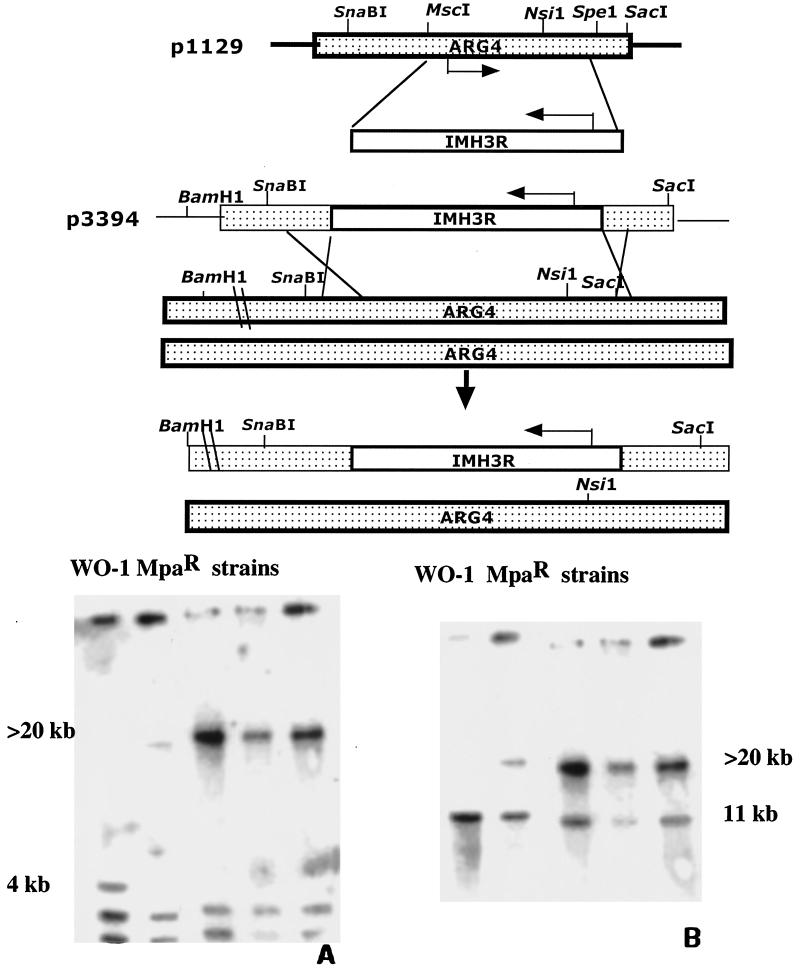
Despite the ARS element on the fragment encoding the IMH3r allele, we found several transformants of CAI-4 in which Mpar was mitotically stable. However, due to the sequence similarity of IMH3r and the wild-type IMH3, it was difficult to determine whether these stable resistant cells were the result of ectopic integration, insertion, or gene conversion at the chromosomal IMH3 locus. To determine whether the IMH3r allele could confer resistance in a single, ectopically integrated copy, we targeted the IMH3r allele to the ARG4 locus (10). IMH3r was used to replace the sequence between the MscI and SpeI sites of p1129 (10), which contains the ARG4 ORF (Fig. 5, top), to create p3394. Strains FC18 and WO-1 were transformed with EcoRI-linearized p3394 and selected for Mpar, and transformants were analyzed by Southern blot analysis. Blots A and B in Fig. 5 demonstrate that gene replacement with IMH3r occurred at the ARG4 locus in strain WO-1; similar results were observed in strain FC18 (data not shown). Of the 12 transformants tested in each strain, 100% homologous integration occurred at the ARG4 locus. This is the first report describing the use of a dominant selectable marker for gene disruption in clinical isolates.
FIG. 5.
Disruption of one allele of ARG4 with IMH3. (Top) A 1.6-kb fragment of the ARG4 ORF in plasmid p1129 was replaced with the IMH3r allele to create p3394. SnaBI-linearized p3394 was used to transform both FC18 and WO-1. Transformants were selected on the basis of Mpar. (Bottom) DNA from p3394-transformed WO-1 was extracted and digested with BamHI and NsiI, electrophoresed, and blotted. Blots were hybridized with a radiolabeled probe of p1129 (ARG4) (A) or IMH3 (B). These blots demonstrate that the IMH3r allele integrated ectopically at the ARG4 locus. They further demonstrate that one copy of the IMH3r allele is sufficient to confer Mpar.
DISCUSSION
C. albicans is the most common fungal pathogen of humans, yet research into its pathogenesis has been greatly hampered by the fact that it is both asexual and diploid. Before the advent of molecular genetics and then genomics, parasexual genetics through spheroplast fusion was the only tool available for genetic analysis (12, 19, 22). Goshorn and Scherer (9) isolated strain 1006, an Mpar isolate, for use in spheroplast fusions with prototrophic clinical isolates. Their work showed that the Mpar phenotype, probably mediated through an altered IMH3 gene, could be exploited as a dominant selectable marker. We have identified and cloned the dominant IMH3r allele and demonstrated that it confers Mpar. We present molecular proof that IMH3r is sufficient to confer Mpar since resistance cosegregates with uridine prototrophy when the IMH3r allele is carried on an autonomously replicating plasmid that bears a URA3 marker as well. The IMH3r allele contains three mutations. Two of these mutations, a nonconservative mutation that results in S102A and a conservative change that results in I47V, occur in exon 1. A second nonconservative change G482D, occurs in exon 2, and both exons (and hence at least two of the mutations) are required to confer Mpar. This is consistent with the findings of Goshorn and Scherer (9) that spontaneous resistance to MPA is a low-frequency occurrence and that Mpar is a stable phenotype. Finally, we used IMH3r to disrupt one allele at the ARG4 locus in two clinical isolates, thus demonstrating that a single allele is sufficient to confer Mpar and that this selection strategy can be used with clinical isolates.
Due to the absence of dominant selectable markers, most gene disruption experiments in C. albicans use the Ura-blaster cassette and CAI-4, a Ura− (hence avirulent) mutant. Although extremely useful, the Ura-blaster technique has drawbacks. In addition to the issues recently raised about the effect (if any) of reintroduction of URA3 at different loci (16), the requirement for URA auxotrophy precludes the molecular manipulation of clinical isolates that may have unique phenotypes such as drug resistance (20, 30) and phenotypic switching (25).
In principle, the IMH3r allele is ideal for gene disruption in prototrophic strains. It confers resistance to an antibiotic to which most, if not all, clinical isolates are sensitive. Furthermore, it is a single gene of less than 3.0 kb and is easy to insert into the gene to be disrupted. Finally, spontaneous resistance is extremely rare. However, there are two virtually identical alleles in the normal C. albicans genome. Thus, IMH3r preferentially integrates at the IMH3 locus with a very high frequency. This high frequency of homologous integration precludes its use in oligonucleotide-mediated gene disruption approaches (data not shown). To obviate this problem, we are currently examining the IMH3 genes from several fungi which appear to be naturally Mpar in order to determine whether they confer Mpar.
Another interesting feature of the IMH3r allele is the fact that it replicates autonomously in relatively low copy number and contains several ARS-like elements. We identified three ARS elements: at nt 7 (5′GTTTATGATAC3′), based on similarity to the previously identified C. albicans ARS consensus sequence of (5′TTTATGTTT3′) (3), and at positions 101 (5′ATTTAATTTTC3′) and 358 (5′TTTTTCGCTTTTT3′), based on the ARS element identified in C. maltosa (23). Furthermore, this plasmid did not appear to multimerize like other ARS element-bearing plasmids of C. albicans (data not shown). We are currently comparing this ARS element to other previously identified ARS elements in the hope of developing a better ARS plasmid for use in Candida studies.
This marker can be used to transform C. tropicalis as well as C. albicans. In our hands, C. dubliniensis, C. parapsilosis, and C. krusei were naturally Mpar under the conditions described here; inconsistent results were obtained upon transforming C. glabrata with our construct. Recently Staib et al. (27) described the transformation of C. dubliniensis using electroporation to introduce another Mpar allele of IMH3 into a C. dubliniensis strain. We have no explanation for the discrepancy except that the heterogeneity of clinical isolates of asexual fungi may mean that no generalizations can be made about the sensitivity or resistance to MPA of a species as a whole. It will be interesting to determine whether the IMH3r allele that they used (27) is the same as the one we have isolated and whether the difference in results is attributable to differences in transformation techniques. Previously, Kohler et al. (14) found that IMH3 is constitutively expressed under conditions requiring the biosynthesis of purine, such as the minimal medium described here. For this reason, more complete medium represses biosynthesis of the IMH3 gene and prevents selection. Further support for this phenomenon is provided by Goshorn and Scherer (9), who found that exogenous application of guanine to minimal medium acts as a competitive inhibitor of MPA (a guanine analog) and prevents selection.
The IMH3r allele can be used for purposes other than gene disruption. A similar allele, coupled with the FLP recombination system, has been used for in vivo expression studies to demonstrate the expression of particular genes in vivo (26). The IMH3r allele should also be useful in studies of population biology, since it will allow one to mark a particular strain so that it can be followed in a mixed population. This application may very well be useful for in vitro and in vivo competition studies. Finally, the IMH3r allele would be ideal for haploinsufficiency studies similar to those performed on diploid Saccharomyces cerevisiae (7). Work to examine the usefulness of the IMH3r allele in a similar study for C. albicans, using strain SC5314, is under way.
Although the IMH3r allele is a dominant selectable marker and will confer a selectable phenotype when only one allele is present, it is not sufficient by itself to achieve the goal of gene disruption in prototrophic clinical isolates, since in principle both genes need to be inactivated to achieve the deficient phenotype. Although a haploinsufficient phenotype has often been seen for Candida genes, and a single selectable marker is sufficient to test for haploinsufficiency (6), two dominant selectable markers are needed for complete gene disruption. There are two ways to achieve this: either one can find a second marker, or one can disrupt the first allele with the marker under a regulatable promoter, so that specific conditions render the transformant sensitive once more to the selection. We are currently examining other potential markers for C. albicans. Additionally, we are constructing an inducible promoter fusion of IMH3r. A construct containing the ORF of IMH3r fused to an inducible promoter would be used to disrupt one allele of a gene with selection under conditions that induce promoter-driven expression. For the second disruption, a construct containing the IMH3r gene under the control of its own promoter would be used, permitting sequential selection with a single marker. We are currently developing and testing this approach.
ACKNOWLEDGMENTS
This work was supported by USPHS grants AI16567, AI35109, and AI46351 awarded to P.T.M.
We are grateful to Suzanne Grindle, Bebe Magee, Judith Berman, and Stew Scherer for helpful discussions. We also acknowledge the assistance of Amy Post, a participant in the Life Sciences Summer Undergraduate Research Program.
REFERENCES
- 1.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altshul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Cannon R D, Jenkinson H F, Shepherd M G. Isolation and nucleotide sequence of an autonomously replicating sequence (ARS) element functional in Candida albicans and Saccharomyces cerevisiae. Mol Gen Genet. 1990;221:210–218. doi: 10.1007/BF00261723. [DOI] [PubMed] [Google Scholar]
- 4.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 5.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gale C A, Bendel C M, McClellan M, Hauser M, Becker J M, Berman J, Hostetter M K. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 7.Giaever G, Shoemaker D D, Jones T W, Liang H, Winzeler E A, Astromoff A, Davis R W. Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat Genet. 1999;21:278–283. doi: 10.1038/6791. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin T J, Poulter R T. Multiple LTR-retrotransposon families in the asexual yeast Candida albicans. Genome Res. 2000;10:174–191. doi: 10.1101/gr.10.2.174. [DOI] [PubMed] [Google Scholar]
- 9.Goshorn A K, Scherer S. Genetic analysis of prototrophic natural variants of Candida albicans. Genetics. 1989;123:667–673. doi: 10.1093/genetics/123.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoyer L L, Magee B B, Rikkerink E H, Scherer S. The ARG4 gene of Candida albicans. Gene. 1994;142:213–218. doi: 10.1016/0378-1119(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 11.Hull C M, Raisner R M, Johnson A D. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- 12.Kakar S N, Magee P T. Genetic analysis of Candida albicans: identification of different isoleucine-valine, methionine, and arginine alleles by complementation. J Bacteriol. 1982;151:1247–1252. doi: 10.1128/jb.151.3.1247-1252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirsch D R, Whitney R R. Pathogenicity of Candida albicans auxotrophic mutants in experimental infections. Infect Immun. 1991;59:3297–3300. doi: 10.1128/iai.59.9.3297-3300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler G A, White T C, Agabian N. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J Bacteriol. 1997;179:2331–2338. doi: 10.1128/jb.179.7.2331-2338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurenson P, Rine J. Silencers, silencing, and heritable transcriptional states. Microbiol Rev. 1992;56:543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lay J, Henry L K, Clifford J, Koltin Y, Bulawa C E, Becker J M. Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect Immun. 1998;66:5301–5306. doi: 10.1128/iai.66.11.5301-5306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magee B, Magee P T. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller M A. The use of molecular techniques for epidemiologic typing of Candida species. Curr Top Med Mycol. 1992;4:43–63. doi: 10.1007/978-1-4612-2762-5_2. [DOI] [PubMed] [Google Scholar]
- 19.Poulter R, Jeffery K, Hubbard M J, Shepherd M G, Sullivan P A. Parasexual genetic analysis of Candida albicans by spheroplast fusion. J Bacteriol. 1981;146:833–840. doi: 10.1128/jb.146.3.833-840.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos M A, Tuite M F. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans. Nucleic Acids Res. 1995;23:1481–1486. doi: 10.1093/nar/23.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarachek A, Rhoads D D, Schwarzhoff R H. Hybridization of Candida albicans through fusion of protoplasts. Arch Microbiol. 1981;129:1–8. doi: 10.1007/BF00417169. [DOI] [PubMed] [Google Scholar]
- 23.Sasnauskas K, Jomantiene R, Lebediene E, Lebedys J, Januska A, Janulaitis A. Molecular cloning and analysis of autonomous replicating sequence of Candida maltosa. Yeast. 1992;8:253–259. doi: 10.1002/yea.320080403. [DOI] [PubMed] [Google Scholar]
- 24.Scherer S, Magee P T. Genetics of Candida albicans. Microbiol Rev. 1990;54:226–241. doi: 10.1128/mr.54.3.226-241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slutsky B, Buffo J, Soll D R. High-frequency switching of colony morphology in Candida albicans. Science. 1985;230:666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- 26.Staib P, Kretschmar M, Nichterlein T, Kohler G, Michel S, Hof H, Hacker J, Morschhauser J. Host-induced, stage-specific virulence gene activation in Candida albicans during infection. Mol Microbiol. 1999;32:533–546. doi: 10.1046/j.1365-2958.1999.01367.x. [DOI] [PubMed] [Google Scholar]
- 27.Staib P, Michel S, Kohler G, Morschhauser J. A molecular genetic system for the pathogenic yeast Candida dubliniensis. Gene. 2000;242:393–398. doi: 10.1016/s0378-1119(99)00512-0. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan D J, Westerneng T J, Haynes K A, Bennett D E, Coleman D C. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 29.Timmins E M, Howell S A, Alsberg B K, Noble W C, Goodacre R. Rapid differentiation of closely related Candida species and strains by pyrolysis-mass spectrometry and Fourier transform-infrared spectroscopy. J Clin Microbiol. 1998;36:367–374. doi: 10.1128/jcm.36.2.367-374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]