Summary
Background
The ongoing outbreak of SARS-CoV-2 Omicron BA.2 infections in Hong Kong, the model city of universal masking of the world, has resulted in a major public health crisis. Although the third vaccination resulted in strong boosting of neutralization antibody, vaccine efficacy and correlate of immune protection against the major circulating Omicron BA.2 remain to be investigated.
Methods
We investigated the vaccine efficacy against the Omicron BA.2 breakthrough infection among 470 public servants who had received different SARS-CoV-2 vaccine regimens including two-dose BNT162b2 (2 × BNT, n = 169), three-dose BNT162b2 (3 × BNT, n = 168), two-dose CoronaVac (2 × CorV, n = 34), three-dose CoronaVac (3 × CorV, n = 67) and third-dose BNT162b2 following 2 × CorV (2 × CorV+1BNT, n = 32). Humoral and cellular immune responses after three-dose vaccination were further characterized and correlated with clinical characteristics of BA.2 infection.
Findings
During the BA.2 outbreak, 27.7% vaccinees were infected. The timely third-dose vaccination provided significant protection with lower incidence rates of breakthrough infections (2 × BNT 46.2% vs 3 × BNT 13.1%, p < 0.0001; 2 × CorV 44.1% vs 3 × CorV 19.4%, p = 0.003). Investigation of immune responses on blood samples derived from 90 subjects in three-dose vaccination cohorts collected before the BA.2 outbreak revealed that the third-dose vaccination activated spike (S)-specific memory B cells and Omicron cross-reactive T cell responses, which correlated with reduced frequencies of breakthrough infections and disease severity rather than with types of vaccines. Moreover, the frequency of S-specific activated memory B cells was significantly lower in infected vaccinees than uninfected vaccinees before vaccine-breakthrough infection whereas IFN-γ+ CD4 T cells were negatively associated with age and viral clearance time. Critically, BA.2 breakthrough infection boosted cross-reactive memory B cells with enhanced cross-neutralizing antibodies to Omicron sublineages, including BA.2.12.1 and BA.4/5, in all vaccinees tested.
Interpretation
Our results imply that the timely third vaccination and immune responses are likely required for vaccine-mediated protection against Omicron BA.2 pandemic. Although BA.2 conferred the highest neutralization resistance compared with variants of concern tested before the emergence of BA.2.12.1 and BA.4/5, the third dose vaccination-activated S-specific memory B cells and Omicron cross-reactive T cell responses contributed to reduced frequencies of breakthrough infection and disease severity. Neutralizing antibody potency enhanced by BA.2 breakthrough infection in vaccinees with prior 3 doses of CoronaVac or BNT162b2 may reduce the risk of infection against ongoing BA.2.12.1 and BA.4/5.
Funding
Hong Kong Research Grants Council Collaborative Research Fund, Health and Medical Research Fund, Wellcome Trust, Shenzhen Science and Technology Program, the Health@InnoHK, Innovation and Technology Commission of Hong Kong, China, National Program on Key Research Project, Emergency Key Program of Guangzhou Laboratory, donations from the Friends of Hope Education Fund and the Hong Kong Theme-Based Research Scheme.
Keywords: SARS-CoV-2, COVID-19, Omicron, BA.2, Breakthrough infection, Neutralizing antibody, T cell response
Research in context.
Evidence before this study
The emergence of variants of concern (VOCs), especially the Omicron variants, has substantially threatened the vaccine efficacy by reducing the neutralizing effectiveness of antibodies produced from SARS-CoV-2 vaccinations. Although several studies have demonstrated that the boost immunization (the third dose) is helpful in reducing the infection and hospitalization rates during the Omicron BA.2 prevalence, vaccine efficacy and its correlation- with the immune protection against Omicron BA.2 remain- unclear.
Added value of this study
By investigating public servants who had received either two doses or three doses CoronaVac (CorV) or BNT162b2 (BNT) vaccines, we found that the timely third-dose vaccination resulted in significant lower incidence rates of BA.2 infection (e.g. 2 × BNT: 46.2% vs 3 × BNT: 13.1%, p < 0.0001; 2 × CorV: 44.1% vs 3 × CorV: 19.4%, p = 0.009). Retrospective study of immune responses of sample collected right before BA.2 outbreak among three dose vaccinees showed that the timely third-dose vaccination was able to activate spike-specific memory B and Omicron cross-reactive T cell responses, which correlated with reduced frequencies of breakthrough infections and disease severity rather than with types of vaccines. Moreover, BA.2 breakthrough infection and the fourth vaccination enhanced cross-neutralizing antibodies to Omicron sublineages by recalling prior vaccine-induced memory B cells.
Implications of all the available evidence
Our results show the correlation between vaccine-induced immune responses and BA.2 breakthrough infection to highlight the importance of spike-specific activated memory B cells and cross-reactive T cell responses for protection and illness reduction. BA.2 breakthrough infection and the fourth vaccination-enhanced neutralizing antibody may help to reduce the risk for infection of ongoing BA.2.12.1 and BA.4/5.
Introduction
To fight the ongoing SARS-CoV-2 pandemic, over 10 billion doses of COVID-19 vaccines under emergency use authorization (EUA) have been administered globally, which has significantly reduced the rates of hospitalization, disease severity and death.1, 2, 3, 4, 5 Unfortunately, the emergence of variants of concern (VOCs), especially the Omicron variants, has substantially threatened the vaccine efficacy.6 We recently reported that waning anti-Omicron neutralizing antibody and T cell responses especially among CoronaVac-vaccinees might pose a risk to vaccine-breakthrough infections in Hong Kong.7 Although the third heterologous BNT162b2 vaccination after 2-dose CoronaVac generates high neutralizing antibody responses against ancestral and Omicron BA.1 than the third homologous CoronaVac booster,8,9 vaccine efficacy and its correlation- with the immune protection against the major circulating Omicron BA.2 strains remains to be investigated.10, 11, 12 In addition, it remains unclear if BA.2 breakthrough infection would reduce the risk against ongoing BA.2.12.1 and BA.4/5 reinfection by enhancing cross-reactive neutralizing antibody potency.
Methods
Human subjects
This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (Ref No. UW 21-452). A total of 481 participants were recruited in this study. Written informed consent and questionnaire of vaccination and infection were obtained from these subjects. Patients provided the information of symptom onset date, type of symptoms, hospitalization, duration of illness and the date of viral negative conversion as summarized in Table 1. The vaccination record was officially registered by professional medical staff in the governmental system called “LeaveHomeSafe”. The diagnosis of SARS-CoV-2 infection was confirmed by results of rapid antigen test and PCR, as well as quarantine records enforced strictly by law. Peripheral blood mononuclear cells (PBMCs) from 90 randomly selected-participants who had the third vaccination were isolated from fresh blood samples before SARS-CoV-2 infection using Ficoll–Paque density gradient centrifugation in our BSL-3 laboratory at the same day of blood collection. The majority of purified PBMCs were used for immune cell phenotyping whereas plasma samples were subjected to antibody testing. The rest of the cells were cryopreserved in freezing medium (Synth-a-Freeze Cryopreservation Medium, ThermoFisher Scientific) at 5 × 106 cells/mL at −150 °C. Subjects included in the study were required to complete vaccination (all dose) for at least 7 days, to allow the manifestation of the delayed immune response to vaccination.
Table 1.
Demographic characteristics of breakthrough infection among 470 vaccinees.
| Vaccinations |
2 × BNT (n = 169) |
3 × BNT (n = 168) |
2 × CorV (n = 34) |
3 × CorV (n = 67) |
2 × CorV+1 × BNT (n = 32) |
|
|---|---|---|---|---|---|---|
| Infection rate % (No. Patient/Total No.) | 46.2% (78/169) | 13.1% (22/168) | 44.1% (15/34) | 19.4% (13/67) | 6.3% (2/32) | |
| Patients | (n = 78) | (n = 22) | (n = 15) | (n = 13) | (n = 2) | |
| Age, year (Ranges in parentheses) | 32 (24–58) | 40 (27–60) | 41 (24–64) | 50 (20–62) | 47.5 (37–58) | |
| Gender | Male (% patients of all participants) | 60 (48.8%) | 14 (12.3%) | 9 (42.9%) | 8 (18.2%) | 2 (7.1%) |
| Female (% patients of all participants) | 18 (39.1%) | 8 (14.8%) | 6 (46.2%) | 5 (21.7%) | 0 (0%) | |
| Median interval days between latest vaccination and symptom onset (ranges in parentheses) | 227 (140–332) | 48.5 (10–111) | 237 (52–341) | 56 (7–109) | 25.5 (10–41) | |
| Asymptomatic rate % (No. Asymptomatic patient/No. Total patient) | 3.8% (3/78) | 0% (0/22) | 0% (0/15) | 0% (0/13) | 0% (0/2) | |
| Disease severity | Mild | Mild | Mild | Mild | Mild | |
| Number of symptoms (ranges in parentheses) | 4 (0–6) | 3 (1–5) | 3 (1–6) | 2 (1–5) | 3.5 (3–5) | |
| Presentation to hospital % (No. Patients presenting to hospital/No. Total patient) | 19.2% (15/78) | 4.5% (1/22) | 20% (3/15) | 15.4% (2/13) | 50% (1/2) | |
| Duration of illness, days (Ranges in parentheses) | 7 (0–19) | 7.5 (2–19) | 8 (6–21) | 8 (2–14) | 9.5 (2–17) | |
| The interval days between symptom onset and two negative RAT 48 h apart | 8 (1–20) | 9 (6–13) | 8 (6–12) | 9 (3–14) | 8 (5–11) | |
A total of 481 subjects were joined the survey. To allow the manifestation of the delayed immune response to vaccination, 11 subjects were excluded for analysis (Three 2 × CorV and six 3 × BNT subjects who developed symptoms by infection less than 7 days post latest vaccination; One 3 × CorV and one 3 × BNT subjects who donated their blood sample less than 7 days post third vaccination). Values displayed are medians, with ranges in parentheses.
Enzyme-linked immunosorbent assays (ELISA)
Plasma IgG binding antibodies to Spike were quantitated by ELISA using WHO International Standard as standard. Briefly, different recombinant trimeric Spike proteins derived from SARS-CoV-2 VOCs (Sino Biological) were diluted to final concentrations of 1 μg/mL, then coated onto 96-well plates (Corning 3690) and incubated at 4 °C overnight. Plates were washed with PBST (PBS containing 0.05% Tween-20) and blocked with blocking buffer (PBS containing 5% skim milk or 1% BSA) at 37 °C for 1 h. Two-fold serial dilution of WHO international standard (from 20 BAU/mL to 0.15625 BAU/mL) and plasma samples (400-fold diluted) were added to the plates and incubated at 37 °C for 1 h. Wells were then incubated with a secondary goat anti-human IgG labeled with horseradish peroxidase (HRP) (1:5000 Invitrogen) TMB substrate (SIGMA). Optical density (OD) at 450 nm was measured by SkanIt RE6.1 with VARIOSKAN Lux (Thermo Scientific).
Pseudotyped viral neutralization assay
To determine the neutralizing activity of subject's plasma, the plasma was inactivated at 56 °C for 30 min prior to a pseudotyped viral entry assay. In brief, different SARS-CoV-2 pseudotyped viruses were generated through co-transfection of 293T cells with 2 plasmids, pSARS-CoV-2 S and pNL4-3Luc_Env_Vpr, carrying the optimized SARS-CoV-2 S gene and a human immunodeficiency virus type 1 backbone, respectively. At 48 h post-transfection, viral supernatant was collected and frozen at −150 °C. Serially diluted plasma samples (from 1:20 to 1:14,580) were incubated with 200 TCID50 of pseudovirus at 37 °C for 1 h. The plasma-virus mixtures were then added into pre-seeded HEK293T-hACE2 cells. After 48 h, infected cells were lysed, and luciferase activity was measured using Luciferase Assay System kits (Promega) in a Victor3-1420 Multilabel Counter (PerkinElmer). The 50% inhibitory concentrations (IC50) of each plasma specimen were calculated to reflect anti-SARS-CoV-2 potency.
Antigen-specific B cells
To characterize the SARS-CoV-2 Spike-specific B cells, PBMCs from each vaccinee were first stained with an antibody cocktail contained dead cell dye (Zombie aquae), CD3-Pacific Blue, CD14-Pacific Blue, CD56-Pacific Blue, CD19-BV785, IgD-BV605, IgG-PE, CD27-BV711, CD21-PE/Cy7, CD38-Percp/Cy5.5, CD11C-APC/Fire750 and His-tag Spike protein. Cells were then washed with FACS buffer (PBS with 2% FBS) and further stained with the secondary antibodies including APC anti-His and DyLight 488 anti-his antibodies. Stained cells were acquired by FACSAriaIII Flow Cytometer (BD Biosciences) and analyzed with FlowJo software (v10.6) (BD Bioscience).
Intracellular cytokine staining (ICS)
To measure antigen-specific T cell responses, PBMCs were stimulated with 2 μg/mL Spike peptide pool (15-mer overlapping by 11) from SARS-CoV-2 ancestral or Omicron variant, or 2 μg/mL nucleocapsid protein (NP) peptide pool in the presence of 0.5 μg/mL anti-CD28 and anti-CD49d mAbs (Biolegend). Cells were incubated at 37 °C for 9 h and BFA was added at 3 h post incubation, as previously described.11 PMA/ionomycin stimulation was included as positive control. Cells were then washed with staining buffer (PBS containing 2% FBS) and stained with mAbs against surface markers, including dead cell dye (Zombie aqua), CD3-Pacific Blue, CD4-Percp/Cy5.5, CD8-APC/Fire750, CD45RA-BV711, CCR7-BV785, CXCR5-APC, CCR6-BV605. For intracellular staining, cells were fixed and permeabilized with BD Cytofix/Cytoperm (BD Biosciences) prior to staining with the mAbs against IFN-γ-PE, TNF-α-AF488 and IL-2-PE-Cy7. Stained cells were acquired by FACSAriaIII Flow Cytometer (BD Biosciences) and analyzed with FlowJo software (v10.6) (BD Bioscience). Results were subtracted from percentage of unstimulated control.
Correlation plots and heatmap visualizations
Correlograms plotting the Spearman rank correlation coefficient (r), between all parameter pairs were generated with the corrplot package (v0.9.2)13 running under R (v4.2.1) in RStudio (2022.02.0+443). Spearman rank two-tailed P values were calculated using corr.test (psych v1.8.12) and graphed (ggplot 2 v3.1.1) based on ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Statistical analysis
Statistical analysis was performed using PRISM 8.0. For between-group or multiple-group categorical values comparison, two-sided chi-square tests or fisher's exact tests were used. Unpaired Student's t tests were used to compare group means of GMT and cell frequencies between two groups. The statistic details are depicted in the respective legends. A P value < 0.05 was considered significant.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, interpretation, writing of the report.
Results
Demographic characteristics of breakthrough infection among 470 vaccinees
Considering sociodemographic characteristics and exposure risk may affect vaccine efficacy. In this study, we focus on 7247 subjects who are public servants working for Hong Kong Government with comparable exposure risks. During the time from January to March 2022 (Omicron BA.2 was first found in mid-January 2022 and reached the peak in the early March as dominant strain in Hong Kong10,14), 5995 (82.7%) and 1012 (14%) study subjects had received two and three doses of vaccinations, respectively, resulting in an overall vaccination rate of 96.7%. During the recent fifth wave of COVID-19 in Hong Kong since the end of January 2022,10 a total of 481 (6.6%) subjects joined the survey. To allow the manifestation of the delayed immune response to vaccination, eleven subjects were excluded for analysis (Three 2 × CorV and six 3 × BNT subjects who developed symptoms by infection less than 7 days post latest vaccination; One 3 × CorV and one 3 × BNT subjects who donated their blood sample less than 7 days post third vaccination). Therefore, 470 (6.5%) subjects were included in our follow-up study. These subjects had received 2-dose BNT162b2 (2 × BNT, n = 169), 3-dose BNT162b2 (3 × BNT, n = 168), 2-dose CoronaVac (2 × CorV, n = 34), 3-dose CoronaVac (3 × CorV, n = 67) or a heterologous booster dose of BNT162b2 after two prior doses of CoronaVac (2 × CorV+1 × BNT, n = 32) (Table 1). Among these 470 subjects, a total of 130 (130/470, 27.7%) infections were confirmed by governmental reverse transcriptase-polymerase chain reaction (RT-PCR) or lateral flow-based rapid antigen test (RAT) during the study period. Gender difference in infection was not observed. Patients in 2 × BNT were relatively younger than 3 × BNT (2 × BNT vs 3 × BNT: median 32 years vs median 40 years, p < 0.0001), likely indicating the hesitation for taking the third dose BNT162b2 among younger people. Patients who received two dose BNT162b2 were significantly younger than patients who received two dose CoronaVac (2 × CorV vs 2 × BNT: median 41 years vs median 32 years, p = 0.0006 (Table 1 and Supplementary Table S1), in line with elderly people's preference of taking CoronaVac with less side effects. Moreover, a shorter median interval between latest vaccination and symptom onset was noticed for 3 × BNT compared to 2 × BNT (2 × BNT vs 3 × BNT: median 227 days vs median 48.5 days, p < 0.0001) and for 3 × CorV compared to 2 × CorV (2 × CorV vs 3 × CorV: median 237 days vs median 56 days, p < 0.0001), respectively (Table 1 and Supplementary Table S1).
Infections were found in both 2 × BNT and 2 × CorV groups with comparable incidence rates of 46.2% (78/169) and 44.1% (15/34) (p = 0.828), respectively. For the third dose vaccination groups, however, both third homologous BNT162b2 (3 × BNT: 22/168, 13.1%, p < 0.0001) and CoronaVac vaccination (3 × CorV: 13/67, 19.4%, p = 0.009) showed significantly reduced infection rate compared to 2 × BNT and 2 × CorV, respectively. The third heterologous BNT162b2 vaccination group (2 × CorV+1 × BNT) exhibited the lowest incident rate of 6.3% compared to the 2 × CorV group (p < 0.0001). No statistical significance was found in the infection rates between any 3 dose groups, although 3 × BNT and 2 × CorV+1 × BNT showed lower infection rates than 3 × CorV (Table 1 and Supplementary Table S1). Notably, most infected subjects developed mild disease, presenting three major symptoms including fever, cough and/or sore throat. Asymptotic infections were only found in 2 × BNT groups with a low frequency of 3.8% (3/78) (Table 1). The hospitalization rate was lower for 3 × BNT (4.5%) than that of 3 × CorV (15.4%) patients. Comparable illness duration was observed in 2 × BNT (median 7 days) and 3 × BNT (median 7.5 days) than those of 2 × CorV (median 8 days) and 3 × CorV (median 8 days). There was no significant difference in terms of duration time for viral antigen conversion to negativity between any groups (Table 1 and Supplementary Table S1). These results suggested that the timely third dose vaccination by both BNT162b2 and CoronaVac reduced the incident rate of BA.2 infection.
Activation of Spike-specific memory B cells by the third vaccination
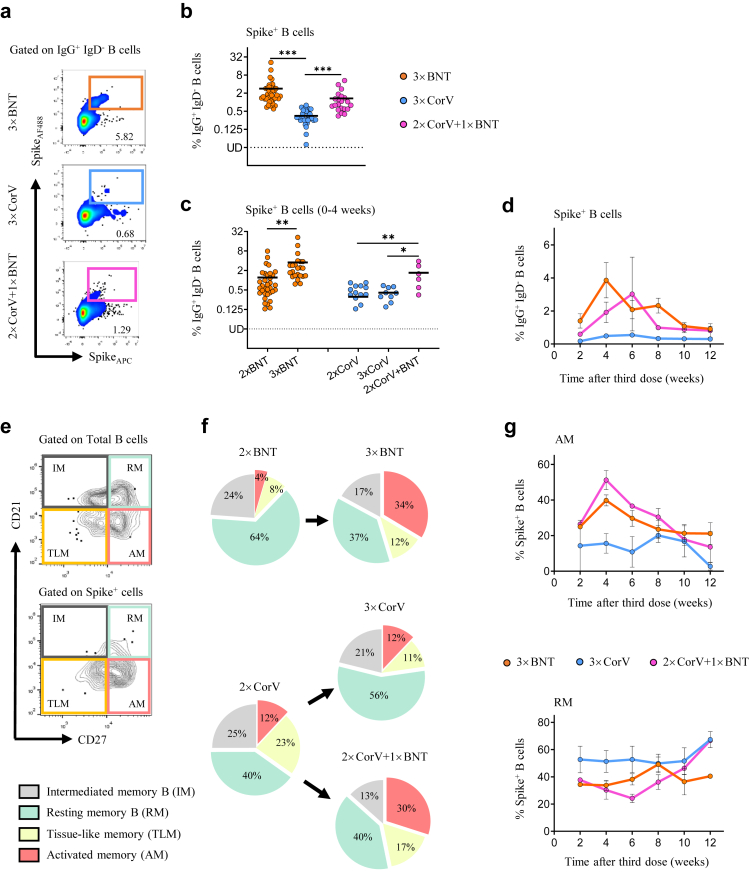
To characterize the third dose vaccination-induced immune responses, we were able to obtain 90 blood samples donated by subjects with 3 doses of vaccines in the same cohort including 41 from 3 × BNT, 28 from 3 × CorV and 21 from 2 × CorV+1 × BNT at median 23, 56 and 47 days after the last vaccination, respectively, on January 27, 2022, right before BA.2 outbreak in Hong Kong 10,14 (Supplementary Table S2). Considering that memory B cell responses contribute to long-term immunological protection against COVID-19, we measured the frequency of Spike (S)-specific B cells (gated on CD19+ IgG+ IgD− cells) after the third dose vaccination (Fig. 1a). We found that the third dose of BNT162b2, either 3 × BNT (mean 2.83%) or 2 × CorV+1 × BNT (mean 1.33%), induced significant higher frequency of S-specific B cells than 3 × CorV (mean 0.35%) (Fig. 1b). The significant boost effect of S-specific B cells was not observed by the third dose of CoronaVac (Fig. 1c). Moreover, S-specific B cells elicited by the third dose of BNT162b2 reached the peak around 4–6 weeks and lasted for 3 months with a higher mean frequency than that of 3 × CorV (Fig. 1d). Further phenotypical analysis (Fig. 1e) showed that the third dose of BNT162b2 resulted in elevated frequency of activated memory B cells (AM, CD21−CD27+) compared with 2 × BNT or 2 × CorV whereas the third dose of CoronaVac enhanced the frequency of resting memory (RM) B cells (Fig. 1f). The frequency of AM reached the peak at 4 weeks after the third booster and subsequently declined, accompanied by proportional increase of RM, in both 3 × BNT and 2 × CorV+1 × BNT groups whereas AM remained unchanged in the 3 × CorV group around two months (Fig. 1g). These results demonstrated that S-specific memory B cells were predominantly activated by the third dose of BNT162b2 but not significantly by the third dose of CoronaVac. However, the third BNT162b2 vaccination following 2 doses of CoronaVac-boosted S-specific B cells was comparable to those induced by three doses of BNT162b2, indicating that BNT162b2 can recall and augment CoronaVac-induced memory B cells.
Fig. 1.
Activation of Spike-specific memory B cells by the third dose vaccination. (a) Representative flow cytometry plots showing staining patterns of SARS-CoV-2 Spike probes on memory B cells (IgD− IgG+ CD19+). (b) Quantified results depict the percentage of Spike+ B cells in 3 × BNT (orange), 3 × CorV (blue) and 2 × CorV+1 × BNT (purple) groups after the third dose vaccination. (c) Comparisons of Spike+ B cell frequency between 2-dose (sample collected at median 28 days after second vaccination for 2 × BNT and 2 × CorV groups) and 3-dose (sample collected at median 16, 20 and 18.5 days after third vaccination for 3 × BNT, 3 × CorV and 2 × CorV+1 × BNT groups, respectively) cohorts within 4 weeks after the last vaccinations. Statistics were generated by using 2-tailed Student's t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. (d) Cross-sectional analysis of Spike-specific B cells by time after third dose vaccination. The connection lines indicate the mean value. (e) Phenotypes of Spike-specific B cells were defined by using CD21 and CD27 markers. (f) Pie chart showed the proportion of activated (AM), tissue-like (TLM) memory, intermediate memory (IM) and resting-memory (RM) B cells. (g) Cross-sectional analysis of the percentage of AM (upper) and RM (bottom) in the Spike-specific B cells by time after third vaccination. The connection lines indicate the mean value.
The titer and breadth of neutralizing antibodies (NAbs) against a full panel of current SARS-CoV-2 VOCs
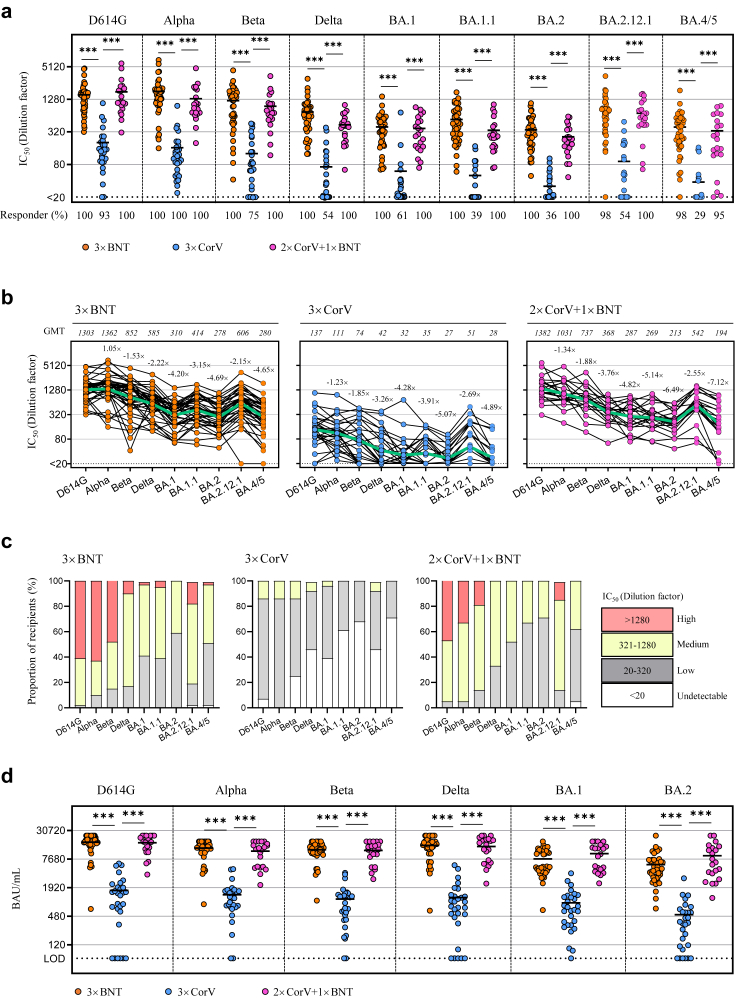
We then measured the titer and breadth of neutralizing antibodies (NAbs) against a full panel of current SARS-CoV-2 VOCs including D614G, Alpha, Beta, Delta and five Omicron variants (BA.1, BA.1.1, BA.2, BA.2.12.1 and BA.4/5) using the pseudovirus assay as we previously described.7 We included data from subjects who previously received 2 × BNT or 2 × CorV at the activation (0–4 weeks) and memory (4–15 weeks) periods for comparison7 (Supplementary Table S2). In line with significantly higher frequencies of S-specific B cells, both 3 × BNT- and 2 × CorV+1 × BNT-vaccinees displayed significantly stronger geometric mean 50% neutralizing titers (GMT) than 3 × CorV against all variants tested (Fig. 2a). The overall fold of neutralization resistance followed the order of Alpha < Beta < Delta < Omicron lineages in all three vaccine groups. Omicron BA.2 and BA.4/5 were more resistant than other VOCs with comparable reduction fold of GMT while BA.2.12.1 showed a downward resistance compared to BA.2 among all vaccinees (Fig. 2b). According to the criteria that convalescent plasma with NAb titer >1:320 was useful for SARS-CoV-2 therapy 15 and considering that the prophylactic administration of convalescent plasma at 1:320 dilution hardly prevents SARS-CoV-2 infection in the hamster model,16 we used 1:320 as the threshold to define NAb titer: less than 1:320 as “Low”, 1:320–1:1280 as “Medium” and above 1: 1280 as “High” for proportion analysis (Fig. 2c). We found that 61% of 3 × BNT and 48% of 2 × CorV+1 × BNT vaccinees had high neutralization activity (>1280) against D614G whereas none of 3 × CorV vaccinees showed similar activities (Fig. 2c). For BA.2, neither 3 × BNT nor 2 × CorV+1 × BNT vaccinees had high neutralization activity, but 41% of 3 × BNT and 29% of 2 × CorV+1 × BNT vaccinees still had medium neutralization activity (321–1280). Strikingly, 68% of 3 × CorV vaccinees showed undetectable neutralization antibodies against BA.2. Similar proportion of GMT magnitude was observed in all vaccine groups against BA.4/5 (Fig. 2c). We also compared the binding antibody titers using different VOC spike protein as the coating antigen. Since spike-specific IgG titers were correlated positively with the neutralizing potency,7,11 we found that plasma binding titers of various VOCs in 3 × BNT and 2 × CorV+1 × BNT groups were dramatically higher than those in 3 × CorV group (Fig. 2d). However, as vaccine-induced NAbs wane over time,7 we further compared the NAb titer between 2-dose and 3-dose vaccinees at the similar time post-vaccination (Supplementary Tables S3 and S4). The third dose of BNT162b2 induced significant higher NAb titers against all VOCs in 3 × BNT and 2 × CorV+1 × BNT groups compared to the 2-dose groups at both 0–4 weeks (activation) (Supplementary Table S3) and >4 weeks (memory) (Supplementary Table S4) after vaccination. In contrast to weak boost effects by the third dose of CoronaVac in the 3 × CorV group, 10.1–26.1-fold and 9.7–27.5-fold enhancements against Omicron variants at activation and memory phases were observed after the third heterologous BNT162b2 (2 × CorV+1 × BNT), similar to the boost effects in the 3 × BNT group (Supplementary Tables S3 and S4). Apart from the significantly increased NAb titers, the responder rates of anti-BA.2 raised from 33% to 100%, from 0% to 38% and from 0% to 100% at 0–4 weeks; from 39% to 100%, from 0% to 35% and from 0% to 100% at >4 weeks in 3 × BNT, 3 × CorV and 2 × CorV+1 × BNT groups, respectively, post the last vaccination. Consistently, BA.2 exhibited the most resistant profile to the boost effect, especially in 3 × CorV (Supplementary Table S5). These results demonstrated that the third heterologous BNT162b2 vaccination in 2 × CorV+1 × BNT made significant improvement on not only bringing the anti-Omicron responder rate to 100% but also enhancing NAb titers close to 3 × BNT at both 0–4 and >4 weeks (Supplementary Table S3–S5).
Fig. 2.
The titer and breadth of neutralizing antibodies (NAbs) against a full panel of current SARS-CoV-2 VOCs. (a) The geometric mean titers (GMT) of neutralizing antibody (IC50 represents serum dilution required to achieve 50% virus neutralization) against nine SARS-CoV-2 strains were measured by pseudovirus-based assay among 3 × BNT (orange), 3 × CorV (blue) and 2 × CorV+1 × BNT vaccinees (purple) after the third dose vaccination. Numbers under the x-axis indicate the responder rates (IC50 > 20 termed ‘responder’). (b) GMT of neutralizing antibody was depicted on the top of Figure. The green lines indicate the change of GMT among variants. Numbers on the top of dots indicate the fold change of different VOC relative to D614G. Each symbol represents an individual donor with a line indicating the mean of each group. (c) Proportion of four neutralizing antibody magnitudes among vaccinees. (d) Levels of anti-Spike IgG (BAU/mL) of all vaccinated subjected are shown as mean ± SEM. Dotted line represents value of 64.5 BAU/mL used as the limit of detection (LOD). Statistics were generated by using 2-tailed Student's t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Spike-specific CD4 and CD8 T cell responses
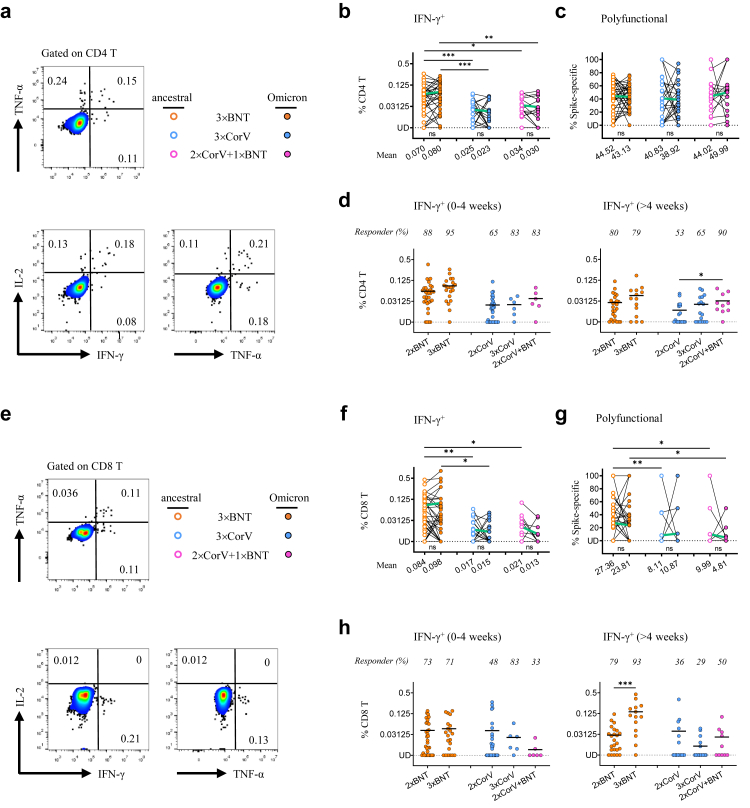
T cell responses may play an important role in control of SARS-CoV-2 infection,11,12,17 CD4 and CD8 T cell responses to viral Spike (S) and nucleocapsid protein (NP) were determined by measuring intracellular IFN-γ, TNF-α and IL-2 (Fig. 3a and 3e). Since many amino acid mutations were found in Omicron Spike protein, we measured ancestral and Omicron S-specific T cell responses in parallel. Significantly higher mean frequencies of S-specific IFN-γ+ CD4 T cells were found in 3 × BNT (ancestral: 0.070% and Omicron: 0.080%) than those in 3 × CorV (ancestral: 0.025% and Omicron: 0.023%) and in 2 × CorV+1 × BNT (ancestral: 0.034% and Omicron: 0.030%) (Fig. 3b). No significant differences of S-specific IFN-γ+ and polyfunctional CD4 T cells were found between ancestral and Omicron (Fig. 3b and 3c). There were also no significant differences between 2 × BNT and 3 × BNT, and between 2 × CorV and 3 × CorV at activation period (Fig. 3d, left). However, the third BNT162b2 vaccination in the 2 × CorV+1 × BNT group recalled significant higher frequency of S-specific IFN-γ+ cells and responder rate than those in the 3 × CorV group at the memory phase (Fig. 3d, right). In addition, significantly higher mean frequencies of S-specific IFN-γ+ CD8 T cells were found in 3 × BNT (ancestral: 0.084% and Omicron: 0.098%) than those in 3 × CorV (ancestral: 0.017% and Omicron: 0.015%) and in 2 × CorV+1 × BNT (ancestral: 0.021% and Omicron: 0.013%) (Fig. 3f). The frequency of S-specific polyfunctional CD8 T cells were relatively higher in 3 × BNT than those in 3 × CorV and 2 × CorV+1 × BNT (Fig. 3g). Significant increase of S-specific IFN-γ+ CD8 T cells was not observed in 3 × BNT compared to that in 2 × BNT at the acute phase (Fig. 3h, left) but was observed at the memory one (Fig. 3h, right). CoronaVac, however, did not show similar activities. Besides the Spike, weak nucleocapsid protein (NP)-specific IFN-γ+ CD4 and CD8 T cells were observed in 3 groups although more CD4 T cell responders (67%) were found in 3 × CorV (Supplementary Figure S1), indicating the possible pre-existing cross-reactive NP-specific T cell responses in unexposed donors.18 Considering that S-specific circulating T follicular helper cells (cTFH, CD45RA−CXCR5+CD4+) are associated with potent NAb responses,19 we found that the frequency of IFN-γ+ cTFH cells were low with mean 0.033–0.048%, 0.01–0.023% and 0.017–0.059% in 3 × BNT, 3 × CorV and 2 × BNT+1 × CorV groups, respectively (Supplementary Fig. S2a and S2b). However, the responder rate was higher in 3 × BNT (20–22%) and 2 × BNT+1 × CorV (14–24%) than that of 3 × CorV (7–10%) (Supplementary Figure S2b). These results indicated that the third dose of BNT162b2 vaccination significantly improved S-specific IFN-γ+, polyfunctional and memory T cells in 3 × BNT but not in 2 × CorV+1 × BNT and 3 × CorV.
Fig. 3.
Spike-specific CD4 and CD8 T cell responses. PBMCs were stimulated with the Spike peptide pools from ancestral or Omicron SARS-CoV-2 prior to intracellular cytokine staining assay. Representative flow cytometry plots showing single positive of IFN-γ+ or TNF-α+ or IL-2+ as well as the polyfunctional cells with ≥2 cytokines among CD4+ (a) and CD8+ (e) T cells. Paired analysis of the frequencies of IFN-γ-producing CD4+ (b) and CD8+ (f) T cells as well as the frequencies of polyfunctional CD4+ (c) and CD8+ (g) T cells to ancestral (open dots) or Omicron (solid dots) Spike among the 3 × BNT (orange), 3 × CorV (blue) and 2 × CorV+1 × BNT (purple) vaccinees. The mean frequencies were depicted under the x-axis. The frequencies of IFN-γ-producing CD4+ (d) and CD8+ (h) T cell to ancestral Spike among 2 × BNT, 3 × BNT, 2 × CorV, 3 × CorV and 2 × CorV+1 × BNT vaccinees at 0–4 weeks (left) and >4 weeks (right) periods after vaccinations. Undetected (UD): % of IFN-γ+ cells<0.00781%. The green lines in b, c, f, g indicate the change of mean responses to ancestral and Omicron Spike. The responses are depicted as the background-subtracted percentage of S-specific T cells (Background subtraction refers to the subtraction of the values of the negative control sample from the peptide-stimulated sample). The responder rates were depicted on the top of dots (% of IFN-γ+ cells>0.00781% termed ‘responder’ after subtracted from percentage of unstimulated control). Each symbol represents an individual donor with a line indicating the mean of each group. Statistics were generated by using 2-tailed Student's t test. Ns: no significance, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Associations among humoral, cellular immune response and breakthrough infection features
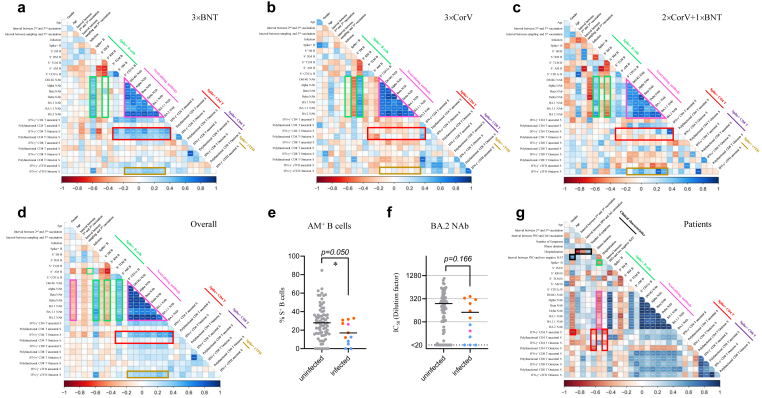
Immune correlation analysis was subsequently conducted for 23 antigen-specific measurements together with gender, age, time interval between second and third vaccinations, sampling time after third dose of vaccination and infection. Consistent with the kinetics of AM proportion, S-specific AM correlated negatively with time after the third dose of vaccination in the 2 × CorV+1 × BNT group (Fig. 4c). Positive correlations between S-specific B cells and NAbs were observed in both 3 × BNT and 2 × CorV+1 × BNT groups while the RM was positively associated with NAbs in the 3 × CorV group (Fig. 4a–c, green rectangle). Consistently, significant positive correlations were found in NAbs titers against all 7 viral variants tested (Fig. 4a–c, purple triangles). Since the third dose vaccination by BNT162b2 or CoronaVac did not improve S-specific CD4 T cell responses among 2 × CorV vaccinees, positive correlations among S-specific CD4 T cells, S-specific B cells and NAbs were limited to the 3 × BNT group (Fig. 4a, red rectangle). However, positive correlations between S-specific cTFH cells and NAbs were observed in 3 × BNT and 2 × CorV+1 × BNT, but not in 3 × CorV (Fig. 4a–c, yellow rectangles). Interestingly, in the 3 × BNT group, Omicron S-specific CD4 T cell and cTFH responses exhibited stronger correlation with S-specific B cell and the broadly NAbs than those with ancestral S-specific CD4 T cell and cTFH responses (Fig. 4a, yellow rectangle). We then combined all three groups for overall analysis (Fig. 4d). Strong positive correlations were consistently found in NAbs titers against all 7 viral variants (Fig. 4d, purple triangle). Both age and S-specific RM B cells were negatively correlated with NAb activity (Fig. 4d, purple rectangle) whereas S-specific AM B cells were positively correlated with neutralizing activity (Fig. 4d, green rectangle). Moreover, the frequency of S-specific AM B cells was significantly lower in infected vaccinees than uninfected vaccinees before vaccine-breakthrough infection (Fig. 4e) whereas the anti-BA.2 NAb titer did not achieve statistical significance (Fig. 4f). Notably, few vaccinees (2/12, 16.7%) with NAb titer higher than 1:320 became infected. We further analyzed the relationships between immune responses and clinical characteristics among our study subjects who were subsequently infected by BA.2 (Fig. 4g). NAb titer was negatively correlated with hospitalization rate (Fig. 4g, purple rectangle), indicating the importance of NAb in reducing COVID-19 severity. Age was positively correlated with viral negative conversion time, likely suggesting a longer viral clearance time among older patients (Fig. 4g, black square). Notably, IFN-γ+ CD4 T cells were negatively associated with age and viral negative conversion time (Fig. 4g, red squares). In addition, hospitalization was negatively correlated with the interval between second and third dose of vaccinations and with the interval between third dose of vaccination and symptom onset, likely suggesting the importance of the optimal timing for the third dose vaccination (Fig. 4g, black rectangle). These results demonstrated that the third dose vaccination-induced NAbs and T cell response contributed to reducing risk of severe clinical outcomes after breakthrough infection.
Fig. 4.
Associations among humoral, cellular immune response and breakthrough infection features. Correlogram of immune responses among 3 × BNT (a), 3 × CorV (b), 2 × CorV+1 × BNT (c) and overall (d) vaccinees. Comparison of AM+ B cell frequency on Spike-specific B cells (e) and neutralizing titer against BA.2 (f) between uninfected and infected vaccinees. Uninfected vaccinees, infected 3 × BNT vaccinees, infected 3 × CorV vaccinees and infected 2 × CorV+1 × BNT vaccinees were presented as grey, orange, blue and purple dots, respectively. Statistics were generated by using 2-tailed Student's t test. ∗p < 0.05. (g) Correlogram of clinical characteristics and immune responses among patients. Spearman rank order correlation values (r) are shown from red (−1.0) to blue (1.0); r values are indicated by color and square size. p values are indicated by white asterisks. The green rectangles denote SARS-CoV-2 Spike-specific B cell features, the purple triangle and rectangles denote anti-SARS-CoV-2 variants' neutralizing antibody features, the red rectangles denote the SARS-CoV-2 Spike-specific CD4 T cell features, the yellow rectangle denotes the SARS-CoV-2 Spike-specific cTFH features and the black rectangles denotes clinical characteristic features.
Immune responses after Omicron BA.2 breakthrough infection and the fourth vaccination
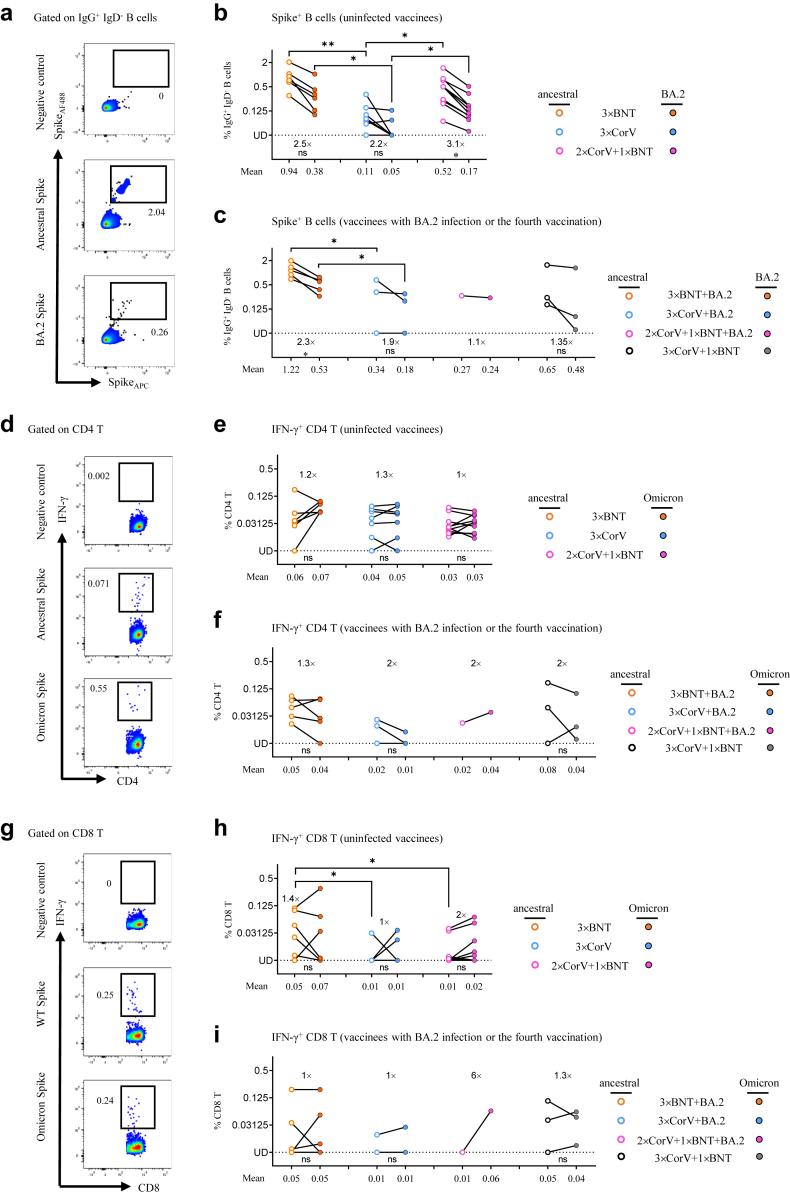
Rapidly recalled antibody and T cell responses were observed in vaccine breakthrough infections by SARS-CoV-2 variants.17,20, 21, 22 At median 137 (range 122–164) days post symptom onset (Supplementary Table S6), we able to harvest the blood sample from five 3 × BNT, three 3 × CorV and one 2 × CorV+1 × BNT subject who had a BA.2 breakthrough infections. Six 3 × BNT, seven 3 × CorV and ten 2 × CorV+1 × BNT subjects who never had infection were also included. For comparison, we also included three subjects who received the fourth vaccination with BNT162b2 following three-dose CoronaVac (3 × CorV+1 × BNT) (Supplementary Table S6). We first measured the frequency of S-specific B cells and found that BA.2 S-specific B cells were consistently lower than ancestral S-specific B cells among all vaccinees no matter with or without BA.2 infection (2.2–3.1-fold and 1.1–2.3-fold difference among uninfected and infected vaccinees, respectively) (Fig. 5a–c). Among uninfected vaccinees, the frequency of BA.2 S-specific B cells in 3 × CorV group (mean 0.05%) was significantly lower than those in 3 × BNT (mean 0.38%) and 2 × CorV+1 × BNT (mean 0.17%) groups (Fig. 5b). Although BA.2 infection increased BA.2 S-specific B cells in 3 × CorV (mean 0.18%), it was still significantly lower than those in 3 × BNT group (mean 0.53%) and lower than 3 × CorV+1 × BNT group (mean 0.48%) without significance (Fig. 5c). In contrast to B cell response, all vaccinees showed similar CD4 and CD8 T cell responses to ancestral and Omicron Spike, and BA.2 infection did not boost a higher T cell response than uninfected vaccinees (Fig. 5d–i). Moreover, uninfected and infected 3 × CorV showed lower T cell responses than those in 3 × BNT and 3 × CorV+1 × BNT without significance (Fig. 5f and i). Particularly, markedly higher CD8 T cells were found in 3 × BNT uninfected vaccinees than those in 3 × CorV and 2 × CorV+1 × BNT uninfected vaccinees even at a long term after vaccination (>4 months) (Fig. 5h). These results indicated that BA.2 infection boosted cross-reactive B cells rather than T cells to ancestral and Omicron Spike.
Fig. 5.
Immune responses after Omicron BA.2 breakthrough infection and the fourth vaccination.(a) Representative flow cytometry plots showing staining patterns of SARS-CoV-2 ancestral or BA.2 Spike probes on memory B cells (IgD- IgG+ CD19+) (b and c) Quantified results depict the percentage of ancestral (empty) and BA.2 (solid) Spike+ B cells in uninfected (b) and infected (c) 3 × BNT (orange), 3 × CorV (blue), 2 × CorV+1 × BNT (purple) and 3 × CorV+1 × BNT (grey) groups. The numbers above the x-axis indicate the fold-change in frequency of positive B cells to ancestral and BA.2 Spike. The numbers under x-axis indicate the mean frequencies of ancestral or BA.2-specific B cells. Undetected (UD): % of Spike+ cells<0.03125% (d and g) Representative flow cytometry plots showing the IFN-γ+ cells among CD4+ (d) and CD8+ (g) T cells to negative control, ancestral Spike and Omicron Spike peptide pools. Quantified results depict the percentage of ancestral (empty) and Omicron (solid)-specific IFN-γ+ cells in uninfected (e and h) and infected (f and i) 3 × BNT (orange), 3 × CorV (blue), 2 × CorV+1 × BNT (purple) and 3 × CorV+1 × BNT (grey) groups. The numbers above the figures indicate the fold-change in frequency of positive T cells to ancestral and BA.2 Spike. The numbers under x-axis indicate the mean frequencies of ancestral or Omicron-specific IFN-γ+ cells T cells. Undetected (UD): % of IFN-γ+ cells<0.00781%. Each symbol represents an individual donor. Statistics were generated by using 2-tailed Student's t test. Ns: no significance, ∗p < 0.05; ∗∗p < 0.01.
Neutralizing antibody titer after BA.2 breakthrough infection and the fourth vaccination
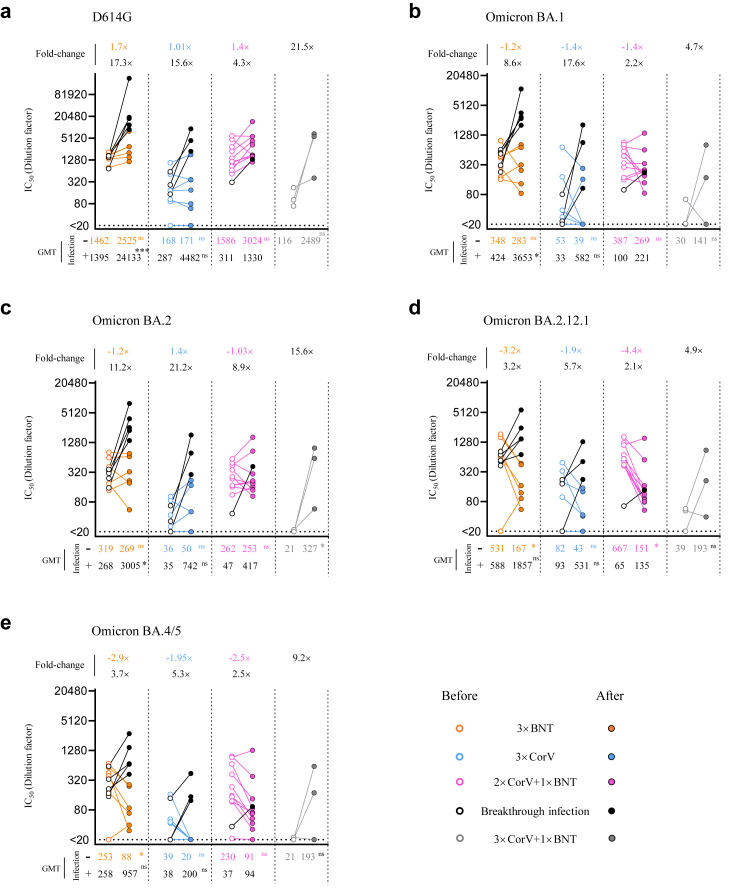
Since broadly neutralizing activity would be boosted by an increased number of exposures to SARS-CoV-2 antigens (vaccination or infection) among vaccinees,17,21,23,24 pairwise comparison of neutralizing activity was analyzed using the plasma sample collected before and after BA.2 breakthrough infection. Three-dose and 4-dose uninfected vaccinees were also included (Supplementary Table S6). Consistent to our previous findings in two-dose vaccinees,7 NAb titer of uninfected vaccinees waned over time, especially against BA.2.12.1 and BA.4/5 (Fig. 6a–e), but the waning effect was not observed in NAbs against D614G (Fig. 6a). However, 100% and 90% of the uninfected 3 × BNT and 2 × CorV+1 × BNT vaccinees were maintained measurable NAbs against all Omicron variants whereas more uninfected 3 × CorV vaccinees (4/7) reduced neutralizing capacity against Omicron BA.4/5. Notably, the fourth vaccination could boost higher NAbs titers and responder rates for 3 × CorV vaccinees (Fig. 6a–e). Moreover, different 3-dose vaccinees after BA.2 breakthrough infection and 3 × CorV+1 × BNT vaccinees consistently exhibited a stronger GMT against BA.1 (3 × BNT: 3653, 3 × CorV: 582 and 2 × CorV+1 × BNT: 221) and BA.2 (3 × BNT: 3005, 3 × CorV: 742 and 2 × CorV+1 × BNT: 417) than those against BA.2.12.1 (3 × BNT: 1857, 3 × CorV: 531 and 2 × CorV+1 × BNT: 135) and BA.4/5 (3 × BNT: 957, 3 × CorV: 200 and 2 × CorV+1 × BNT: 94) (Fig. 6a–e). This boost effect by BA.2 breakthrough infection was more profound among 3 × CorV vaccinees with the highest fold-change (up to 21.2-fold increased for BA.2) in GMT against Omicron sublineages (Fig. 6a–e). Consistent to the frequency of S-specific B cells (Fig. 5b and c), boost neutralizing activity by BA.2 infection or the fourth vaccination showed much higher affinity to D614G than to the Omicron sublineages (Fig. 6a–e). The results indicated that BA.2 breakthrough infection and the fourth vaccination enhanced cross-neutralizing antibodies to Omicron sublineages in all vaccinees by recalling prior vaccine-induced memory B cells.
Fig. 6.
Neutralizing antibody titer after BA.2 breakthrough infection and the fourth vaccination.(a–e) The neutralizing antibody (IC50 represents serum dilution required to achieve 50% virus neutralization) against five SARS-CoV-2 strains were measured by pseudovirus-based assay among uninfected and infected 3 × BNT (orange), 3 × CorV (blue), 2 × CorV+1 × BNT (purple) and 3 × CorV+1 × BNT (grey) before (empty dots) and after (solid dots) BA.2 infection or the fourth vaccination. Black dots and lines represent the breakthrough infection sample in each group. Numbers on the figure top indicate the fold-change in NAb titer between samples collected before and after BA.2 infection or the fourth vaccination. Numbers under the x-axis indicate the geometric mean titers (GMT). Statistics were generated by using 2-tailed Student's t test. ∗p < 0.05; ∗∗∗p < 0.001; ns: not significant.
Discussion
Clinical trials have demonstrated that a third heterologous booster vaccination by EUA SARS-CoV-2 mRNA vaccines (BNT162b2 and mRNA-1273) increased NAb titer accompanied by better prevention and lower disease severity than the initial two doses with BBIP-CorV or CoronaVac during the Gamma and Delta epidemics.25, 26, 27, 28, 29 After the emergence of the Omicron variants, some cohort studies reported that Omicron BA.1 infection was associated with milder disease and shorter duration of clinical symptoms than Delta infection.30, 31, 32, 33, 34, 35 The third vaccination was helpful in reducing the infection and hospitalization rates during the Delta and Omicron BA.1 prevalence in other countries.25,36,37 Till now, the association between immune responses induced by the third vaccination and Omicron BA.2 breakthrough infection remains unknown. In this study, we investigated the immune responses of vaccinees after they received the third vaccination right before the explosive fifth wave of SARS-CoV-2 epidemic caused by Omicron BA.2 in Hong Kong, where the universal masking has been stringently sustained since the begining of the pandemic.10,14 We also followed up the infection status and clinical outcomes of our study subjects during the wave period. We found that the third dose of either BNT162b2 or CoronaVac led to significantly lower infection rates than those who received the standard 2-dose vaccination regimen, particularly in the heterologous 2 × CorV+1 × BNT group. Furthermore, the third BNT162b2 resulted in significantly higher rates of asymptomatic and lower rates of hospitalization than the 3 × CorV group. Our findings, therefore, provided critical knowledge on understanding the role of third vaccination-induced immune responses in protection against the globally spreading Omicron BA.2 infections.
Omicron BA.2 has higher transmissibility and immune evasion than Omicron BA.1,38,39 explaining its rapid spread in Hong Kong and other places.40,41 Since the end of January 2022, BA.2 has quickly dominated the fifth wave of SARS-CoV-2 epidemic in Hong Kong, with a shorter doubling time of 1.28 days than 1.6–2.8 days of BA.1.10 It was unexpected observing 27.7% breakthrough infections among our vaccinees in Hong Kong, a model city of universal masking in the world. BA.2 shares 21 mutations in the Spike with BA.1. Although Q496S and N501Y mutations are missing in the BA.2 S-BRD domain, unique S371F, T376A, D405N and R408S mutations have been found.39 Due to these mutations, we and others 20,39 demonstrated that NAb titers against BA.2 showed 0.97–1.18 and 1.14–1.42 time lower than those against BA.1 at 0–4 weeks and >4 weeks after third vaccination by BNT162b2 or CoronaVac. Also, we consistently found that BA.2 confers the highest NAb resistance compared with other VOCs including BA.1 and BA.1.1 before emergence of BA.4/5. While 59–71% and 29–41% BNT162b2 booster recipients had low (IC50: 20–320) and median (IC50: 321–1280) NAbs against BA.2, 68% CoronaVac booster recipients had undetectable (IC50 < 20) NAbs. Surprisingly, although the third BNT162b2 vaccination boosted higher anti-BA.2 NAb titer and responder rate as well as more S-specific T cell responses than the third CoronaVac, there was no significant difference in incidence of breakthrough infections between 3 × BNT and 3 × CorV. Firstly, the majority of our vaccinees, including 3 × BNT and 3 × CorV, have low neutralizing antibody titers at the time of exposure, rendering them susceptible to BA.2 breakthrough infection. Ten of twelve vaccinees who had IC50 < 320 NAb against BA.2 became infected, which is consistent to the animal study that the prophylactic administration of convalescent plasma at 1:320 dilution hardly prevents SARS-CoV-2 infection in hamster model.16 Secondly, both intramuscular CoronaVac and BNT162b2 vaccines hardly induce enough mucosal neutralizing antibody or T cell responses for prevention,42 as Omicron replicates faster and stronger than wild type and Delta variant in the nasal and bronchial compartments but less efficiently in the lung parenchyma.43, 44, 45 Critically, although CoronaVac displays lower immunogenicity than BNT162b2, it still induced memory B cell and T cell responses that can be recalled quickly for protection as demonstrated among 2 × CorV vaccinees with BNT booster and 3 × CorV vaccinees with BA.2 breakthrough infection. Therefore, recalled immune responses, especially the comparable T cell responses in different vaccinations groups, were invoked by the BA.2 breakthrough infection for protection.
Three doses of either CoronaVac or BNT162b2 vaccines provided similar protection against Omicron infection-induced disease severity outcomes.46,47 In our study, BA.2 infection-mediated immune activation was likely more profound among 3 × CorV vaccinees, resulting in significantly reduced infection and hospitalization rates as compared with 2 × CorV vaccinees. When all vaccinees were analyzed together, we found that S-specific activated memory B cell subset was a significant factor in protecting BA.2 infection because these AM B cells could differentiate into long-lived plasma cells48 and are associated with expansion of memory B cells, and the re-establishment of B cell memory after the third vaccination.23,49 Moreover, recalled T cell responses could be another protective factor because they might recognize mutated viral variants without significantly reduced potency.50 We and others previously demonstrated that both BNT162b2 and CoronaVac-induced T cell responses cross-reacted to Omicron S peptides with comparable activities to ancestral S peptides.51,52 Since S-specific T cells are associated with the control and clearance of the ongoing infection,12 potent T cell responses correlated with fewer hospitalization among patients who had received the third vaccination.
While we were studying the BA.2 variant, BA.2.12.1, BA.4, and BA.5 variants have emerged with increased resistance as compared to previous VOCs to vaccine-induced NAbs due to L452R/Q and F486V mutations in the Spike.53, 54, 55 We confirmed that BA.2 breakthrough infection and the fourth vaccination enhanced neutralizing antibody titers against BA.2.12.1 and BA.4/5. This finding explains why vaccinees after BA.1/BA.2 breakthrough infections were relatively at lower risk of BA.4/5 infection than individuals infected with a pre-Omicron VOCs.56 However, since BA.2 breakthrough infections mainly recalled vaccine-induced ancestral Spike-specific memory B cells, boosting broadly reactive immune responses remains necessary to prevent further viral escape mutations and variant-associated reinfection.54,57, 58, 59
There are some limitations in our study. Firstly, most of our breakthrough infections were confirmed by self-RAT, thus the effect of different vaccine regimens on controlling viral loads could not be determined. Secondly, it should be noted that the median interval time between the latest vaccination and symptom onset for the 2 × BNT (227 days) and 2 × CorV (237 days) groups was significantly longer than those for 3 dose vaccination groups, including 3 × BNT (48.5 days), 3 × CorV (56 days) and 2 × CorV+1 × BNT (25.5 days). Although NAb potency wanes over time,7 we and others consistently found that timely boost vaccination not only restore reduced NAb titers but also broaden the breadth of NAbs, which is able to cross-neutralize VOCs including Omicron.8,23,49,60. Thirdly, only one sample was obtained from 2 × CorV+1 × BNT vaccinees with BA.2 breakthrough infections. Future studies remain necessary to define the correlate of immune protection associated with the heterologous BNT162b2 boost vaccination.
In summary, we report that 3 × BNT and 3 × CorV provided better protection against SARS-CoV-2 BA.2 than 2 × BNT and 2 × CorV. High frequencies of S-specific activated memory B cells and cross-reactive T cell responses induced by the timely third vaccination are critical for BA.2 prevention and illness reduction during the Omicron BA.2 breakthrough infection. Enhanced NAbs boosted by the BA.2 breakthrough infection or the fourth vaccination may help to reduce the risk of infection against ongoing BA.2.12.1 and BA.4/5.
Contributors
Z.C. conceived and supervised the study. R.Z. and Z.C. designed the experiments, analyzed the data, and wrote the manuscript. Z.C., R.Z., X.L., Y.-C.K., H.-Y.K., I.F.-N.H., and K.-Y.Y coordinated donor recruitment and specimen collection. R.Z., N.L., H.H., D.Y., Q.P. prepared the clinical sample. R.Z., N.L. and H.H. performed the flow cytometry analysis. R.Z., N.L. and D.Y. performed the pseudoviral neutralization assay. Z.D. did the correlation analysis.
Data sharing statement
The authors declare that the data supporting the findings of this study are available from the corresponding author upon request.
Declaration of interests
We declare no competing interests.
Acknowledgements
We sincerely thank Mr. Tsz-Tat Chan and Mr. Mark Wai-Kwan Woo for helping with the survey and all subjects for participating the study. We thank Mr. Shek-Hong Kwan for editorial work. This study was supported by the Hong Kong Research Grants Council Collaborative Research Fund (C7156-20G, C1134-20G and C5110-20G) and Health and Medical Research Fund (19181012, COVID1903010 and COVID190123); Wellcome Trust (P86433); Shenzhen Science and Technology Program (JSGG20200225151410198 and JCYJ20210324131610027); the Health@InnoHK, Innovation and Technology Commission of Hong Kong and the China National Program on Key Research Project (2020YFC0860600, 2020YFA0707500 and 2020YFA0707504); Emergency Key Program of Guangzhou Laboratory (EKPG22-01) and donations from the Friends of Hope Education Fund. Z.C.’s team was also partly supported by the Hong Kong Research Grants Council Theme-Based Research Scheme (T11-706/18-N and T11-709/21-N).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2022.100660.
Contributor Information
Runhong Zhou, Email: zhourh@hku.hk.
Zhiwei Chen, Email: zchenai@hku.hk.
Appendix. ASupplementary data
SARS-CoV-2 NP-specific T cell responses. PBMCs from vaccinees were subjected to the intracellular cytokine staining assay against NP peptide pool. IFN-γ+ cells were gated on CD4 (a) and CD8 (b) T cells, respectively. Quantified results depict the percentage of IFN-γ+ cells as background subtracted data from the same sample stimulated with negative control (anti-CD28/CD49d only). Each symbol represents an individual donor with a line indicating the mean of each group among the 3 × BNT (orange), 3 × CorV (blue) and 2 × CorV+1 × BNT (purple) vaccinees. The mean frequency of IFN-γ+ cells and responder rates were depicted under x-axis (% of IFN-γ+ cells>0.00781% termed ‘responder’ after subtracted from percentage of unstimulated control). Undetected (UD): % of IFN-γ+ cells<0.00781%
SARS-CoV-2 Spike-specific cTFH responses. PBMCs from vaccinees were subjected to the intracellular cytokine staining assay against Spike peptide pools from ancestral or Omicron SARS-CoV-2. (a) IFN-γ+ cells were gated on cTFHs. (b) Quantified results depict the percentage of IFN-γ+ cells as background subtracted data from the same sample stimulated with negative control (anti-CD28/CD49d only). Each symbol represents an individual donor with a line indicating the mean of each group to ancestral (open dots) or Omicron (solid dots) Spike among the 3 × BNT (orange), 3 × CorV (blue) and 2 × CorV+1 × BNT (purple) vaccinees. The mean frequency of IFN-γ+ cells and responder rates were depicted under x-axis. Undetected (UD): % of IFN-γ+ cells<0.00781%. Statistics were generated by using 2-tailed Student's t test. Ns: no significance. Ns: no significance
References
- 1.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanriover M.D., Doganay H.L., Akova M., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia S., Zhang Y., Wang Y., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Raddad L.J., Chemaitelly H., Butt A.A., National Study Group for C-V Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385(2):187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Q., Zhou R., Wang Y., et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Then E., Lucas C., Monteiro V.S., et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28:481–485. doi: 10.1038/s41591-022-01705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng S.M.S., Mok C.K.P., Leung Y.W.Y., et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022;28:486–489. doi: 10.1038/s41591-022-01704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng V.C., Ip J.D., Chu A.W., et al. Rapid spread of SARS-CoV-2 Omicron subvariant BA.2 in a single-source community outbreak. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou R., To K.K., Wong Y.C., et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53(4):864–877.e5. doi: 10.1016/j.immuni.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei T., Sikmo V. Package ‘‘corrplot’’: visualization of a correlation matrix (Version 0.84) 2017. https://githubcom/taiyun/corrplot
- 14.Mefsin Y.M., Chen D., Bond H.S., et al. Epidemiology of infections with SARS-CoV-2 Omicron BA.2 variant, Hong Kong, January-March 2022. Emerg Infect Dis. 2022;28(9):1856–1858. doi: 10.3201/eid2809.220613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wajnberg A., Amanat F., Firpo A., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haagmans B.L., Noack D., Okba N.M.A., et al. SARS-CoV-2 neutralizing human antibodies protect against lower respiratory tract disease in a hamster model. J Infect Dis. 2021;223(12):2020–2028. doi: 10.1093/infdis/jiab289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou R., To K.K., Peng Q., et al. Vaccine-breakthrough infection by the SARS-CoV-2 omicron variant elicits broadly cross-reactive immune responses. Clin Transl Med. 2022;12(1) doi: 10.1002/ctm2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Bert N., Tan A.T., Kunasegaran K., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 19.Juno J.A., Tan H.X., Lee W.S., et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med. 2020;26(9):1428–1434. doi: 10.1038/s41591-020-0995-0. [DOI] [PubMed] [Google Scholar]
- 20.Yu J., Collier A.Y., Rowe M., et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 variants. N Engl J Med. 2022;386(16):1579–1580. doi: 10.1056/NEJMc2201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suntronwong N., Yorsaeng R., Puenpa J., et al. COVID-19 breakthrough infection after inactivated vaccine induced robust antibody responses and cross-neutralization of SARS-CoV-2 variants, but less immunity against Omicron. Vaccines (Basel) 2022;10(3):391. doi: 10.3390/vaccines10030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koutsakos M., Lee W.S., Reynaldi A., et al. The magnitude and timing of recalled immunity after breakthrough infection is shaped by SARS-CoV-2 variants. Immunity. 2022;55(7):1316–13126.e4. doi: 10.1016/j.immuni.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muecksch F., Wang Z., Cho A., et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature. 2022 doi: 10.1038/s41586-022-04778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walls A.C., Sprouse K.R., Bowen J.E., et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell. 2022;185(5):872–880.e3. doi: 10.1016/j.cell.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Accorsi E.K., Britton A., Fleming-Dutra K.E., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and delta variants. JAMA. 2022;327(7):639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng G., Wu Q., Pan H., et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2021;22:483–495. doi: 10.1016/S1473-3099(21)00681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moghnieh R., Mekdashi R., El-Hassan S., et al. Immunogenicity and reactogenicity of BNT162b2 booster in BBIBP-CorV-vaccinated individuals compared with homologous BNT162b2 vaccination: results of a pilot prospective cohort study from Lebanon. Vaccine. 2021;39(46):6713–6719. doi: 10.1016/j.vaccine.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa Clemens S.A., Weckx L., Clemens R., et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerqueira-Silva T., Katikireddi S.V., de Araujo Oliveira V., et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med. 2022;28:838–843. doi: 10.1038/s41591-022-01701-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menni C., Valdes A.M., Polidori L., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M.K., Lee B., Choi Y.Y., et al. Clinical characteristics of 40 patients infected with the SARS-CoV-2 Omicron variant in korea. J Korean Med Sci. 2022;37(3):e31. doi: 10.3346/jkms.2022.37.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolter N., Jassat W., Walaza S., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maslo C., Friedland R., Toubkin M., Laubscher A., Akaloo T., Kama B. Characteristics and Outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA. 2022;327(6):583–584. doi: 10.1001/jama.2021.24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jassat W., Abdool Karim S.S., Mudara C., et al. Clinical severity of COVID-19 patients admitted to hospitals during the Omicron wave in South Africa. medRxiv. 2022:38–42. doi: 10.1016/j.ijid.2021.12.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houhamdi L., Gautret P., Hoang V.T., Fournier P.E., Colson P., Raoult D. Characteristics of the first 1119 SARS-CoV-2 Omicron variant cases, in marseille, France, November-December 2021. J Med Virol. 2022;94(5):2290–2295. doi: 10.1002/jmv.27613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon S.K., Hegmann K.T., Thiese M.S., et al. Protection with a third dose of mRNA vaccine against SARS-CoV-2 variants in frontline workers. N Engl J Med. 2022 doi: 10.1056/NEJMc2201821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson M.G., Natarajan K., Irving S.A., et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and Omicron variant predominance - VISION network, 10 states. MMWR Morb Mortal Wkly Rep. August 2021-January 2022;71(4):139–145. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes C.O., Jette C.A., Abernathy M.E., et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588(7839):682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iketani Sho, Liu Lihong, Guo Yicheng, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamasoba D., Kimura I., Nasser H., et al. Virological characteristics of SARS-CoV-2 BA.2 variant. bioRxiv. 2022:1540–1555. doi: 10.1016/j.chom.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyngse F.P., Kirkeby C.T., Denwood M., et al. Transmission of SARS-CoV-2 Omicron VOC subvariants BA.1 and BA.2: evidence from Danish households. medRxiv. 2022 doi: 10.1038/s41467-022-33498-0. 2022.01.28.22270044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou D., Chan J.F., Zhou B., et al. Robust SARS-CoV-2 infection in nasal turbinates after treatment with systemic neutralizing antibodies. Cell Host Microbe. 2021;29(4):551–563.e5. doi: 10.1016/j.chom.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui K.P.Y., Ho J.C.W., Cheung M.C., et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 44.Hui K.P.Y., Ng K.C., Ho J.C.W., et al. Replication of SARS-CoV-2 Omicron BA.2 variant in ex vivo cultures of the human upper and lower respiratory tract. EBioMedicine. 2022;83 doi: 10.1016/j.ebiom.2022.104232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shuai H., Chan J.F., Hu B., et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature. 2022;603(7902):693–699. doi: 10.1038/s41586-022-04442-5. [DOI] [PubMed] [Google Scholar]
- 46.McMenamin M.E., Nealon J., Lin Y., et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–1443. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan V.K.C.B., Wan E.Y.F.P., Ye X.M., et al. Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: a case-control study. Emerg Microbes Infect. 2022:1–48. doi: 10.1080/22221751.2022.2114854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau D., Lan L.Y., Andrews S.F., et al. Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol. 2017;2(7) doi: 10.1126/sciimmunol.aai8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goel R.R., Painter M.M., Lundgreen K.A., et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell. 2022;185(11):1875–1887.e8. doi: 10.1016/j.cell.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scully C., Georgakopoulou E.A., Hassona Y. The immune system: basis of so much health and disease: 3. Adaptive immunity. Dent Update. 2017;44(4) doi: 10.12968/denu.2017.44.4.322. 322-4, 327. [DOI] [PubMed] [Google Scholar]
- 51.Gao Y., Cai C., Grifoni A., et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat Med. 2022;28:472–476. doi: 10.1038/s41591-022-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keeton R., Tincho M.B., Ngomti A., et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603(7901):488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q., Guo Y., Iketani S., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Y., Yisimayi A., Jian F., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuekprakhon A., Nutalai R., Dijokaite-Guraliuc A., et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185(14):2422–2433.e13. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malato J., Ribeiro R.M., Leite P.P., et al. Risk of BA.5 infection among persons exposed to previous SARS-CoV-2 variants. N Engl J Med. 2022;387:953–954. doi: 10.1056/NEJMc2209479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaku C.I., Bergeron A.J., Ahlm C., et al. Recall of preexisting cross-reactive B cell memory after Omicron BA.1 breakthrough infection. Sci Immunol. 2022;7(73) doi: 10.1126/sciimmunol.abq3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quandt J., Muik A., Salisch N., et al. Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci Immunol. 2022 doi: 10.1126/sciimmunol.abq2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Zelm M.C. Immune memory to SARS-CoV-2 Omicron BA.1 breakthrough infections: to change the vaccine or not? Sci Immunol. 2022;7(74) doi: 10.1126/sciimmunol.abq5901. [DOI] [PubMed] [Google Scholar]
- 60.Chu L., Vrbicky K., Montefiori D., et al. Immune response to SARS-CoV-2 after a booster of mRNA-1273: an open-label phase 2 trial. Nat Med. 2022;28(5):1042–1049. doi: 10.1038/s41591-022-01739-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SARS-CoV-2 NP-specific T cell responses. PBMCs from vaccinees were subjected to the intracellular cytokine staining assay against NP peptide pool. IFN-γ+ cells were gated on CD4 (a) and CD8 (b) T cells, respectively. Quantified results depict the percentage of IFN-γ+ cells as background subtracted data from the same sample stimulated with negative control (anti-CD28/CD49d only). Each symbol represents an individual donor with a line indicating the mean of each group among the 3 × BNT (orange), 3 × CorV (blue) and 2 × CorV+1 × BNT (purple) vaccinees. The mean frequency of IFN-γ+ cells and responder rates were depicted under x-axis (% of IFN-γ+ cells>0.00781% termed ‘responder’ after subtracted from percentage of unstimulated control). Undetected (UD): % of IFN-γ+ cells<0.00781%
SARS-CoV-2 Spike-specific cTFH responses. PBMCs from vaccinees were subjected to the intracellular cytokine staining assay against Spike peptide pools from ancestral or Omicron SARS-CoV-2. (a) IFN-γ+ cells were gated on cTFHs. (b) Quantified results depict the percentage of IFN-γ+ cells as background subtracted data from the same sample stimulated with negative control (anti-CD28/CD49d only). Each symbol represents an individual donor with a line indicating the mean of each group to ancestral (open dots) or Omicron (solid dots) Spike among the 3 × BNT (orange), 3 × CorV (blue) and 2 × CorV+1 × BNT (purple) vaccinees. The mean frequency of IFN-γ+ cells and responder rates were depicted under x-axis. Undetected (UD): % of IFN-γ+ cells<0.00781%. Statistics were generated by using 2-tailed Student's t test. Ns: no significance. Ns: no significance