Abstract
Evidence that telomere length (TL) and dynamics can be interpreted as proxy for ‘life stress’ experienced by individuals stems largely from correlational studies. We tested for effects of an experimental increase of workload on telomere dynamics by equipping male great tits (Parus major) with a 0.9 g backpack for a full year. In addition, we analysed associations between natural life‐history variation, TL and TL dynamics. Carrying 5% extra weight for a year did not significantly accelerate telomere attrition. This agrees with our earlier finding that this experiment did not affect survival or future reproduction. Apparently, great tit males were able to compensate behaviourally or physiologically for the increase in locomotion costs we imposed. We found no cross‐sectional association between reproductive success and TL, but individuals with higher reproductive success (number of recruits) lost fewer telomere base pairs in the subsequent year. We used the TRF method to measure TL, which method yields a TL distribution for each sample, and the association between reproductive success and telomere loss was more pronounced in the higher percentiles of the telomere distribution, in agreement with the higher impact of ageing on longer telomeres within individuals. Individuals with longer telomeres and less telomere shortening were more likely to survive to the next breeding season, but these patterns did not reach statistical significance. Whether successful individuals are characterized by losing fewer or more base pairs from their telomeres varies between species, and we discuss aspects of ecology and social organisation that may explain this variation.
Keywords: birds, fitness, longitudinal, senescence, TRF
1. INTRODUCTION
Individual variation in phenotypic quality, as evidenced for example by consistent variation in reproductive success, is ubiquitous in natural systems. Unfortunately, inferring such quality differences from phenotypic traits such as size or physiology is usually difficult, in particular in birds, where quality differences usually depend at most weakly on size. Telomeres, a repeated sequence of non‐coding DNA at the ends of linear chromosomes, functioning in the protection and stabilization of the genome (Blackburn, 1991), have emerged in recent years as a potential biomarker of phenotypic quality. This is based on the accumulation of studies in wild animals that find associations between telomere length and fitness components. More specifically, individuals with longer telomeres have higher survival rates in a range of species (Wilbourn et al., 2018). The relation between telomere length and reproductive success has been studied less intensively, and the findings are mixed. Nevertheless, the general tendency is a positive relation between telomere length and reproductive success (Eastwood et al., 2019; Pauliny et al., 2006; Plot et al., 2012; Le Vaillant et al., 2015). Also in humans a positive relation was reported between telomere length and reproductive success in indigenous rural communities in Guatemala (Barha et al., 2016). However, in common terns the opposite was found: individuals which were more successful in reproduction had shorter telomeres, which was attributed to the longitudinal finding that more successful individuals lost more base pairs with age (Bauch et al., 2013, 2014). In agreement with these findings, two brood size manipulation studies found that parents raising enlarged broods lost more base pairs with age (Sudyka, 2019).
The associations between telomere length and fitness components suggest that telomere studies may provide a tool (e.g. as biomarker) to study the mechanism underlying variation in phenotypic quality and the mechanisms mediating life‐history trade‐offs (Sudyka, 2019). However, most studies of the causes and consequences of telomere length variation and dynamics are correlational, making inference about causality difficult, if not impossible. The brood size manipulation studies (op cit.) are an important exception. But also in these studies it is not clear what the brood size manipulation changes exactly, because caring parents can respond to an increase in brood demand in different ways. For example, parents can adjust their daily energy expenditure to increase provisioning rate (e.g. Tinbergen & Verhulst, 2000), but alternatively, they could increase their foraging yield and provisioning without working harder, by for instance taking more risk during foraging with respect to predators and parasites (Houston et al., 1993). These different responses could each affect telomere dynamics, but in very different ways. Hence, instead of manipulating brood size we aimed to manipulate workload more directly by letting birds carry extra mass for a whole year to examine its effect on telomere shortening rate.
In addition to the effect of carrying extra mass on telomere dynamics we investigated the correlations between TL and the two key fitness components: reproductive success and survival. We used two approaches to quantify the association between fitness components and telomeres, that address different questions: (i) cross‐sectionally, correlating telomere length and reproductive output or survival in the same year. (ii) Longitudinally, relating telomere dynamics to variation in reproductive success and survival (Van de Pol & Verhulst, 2006). The longitudinal analyses provide information that is complementary to the cross‐sectional analysis. For example, when cross‐sectional analyses reveal that short telomeres are associated with high success, this may be due to successful individuals losing more base pairs annually than less successful individuals, which can be tested in a longitudinal analysis (see e.g. Bauch et al., 2013). Likewise, only a longitudinal analysis can test whether low survival in individuals with short telomeres can be attributed to telomere length per se or to telomere attrition independent of absolute length (see e.g. Boonekamp et al 2014).
2. MATERIALS AND METHODS
2.1. Study population and data collection
We studied great tits on Vlieland, an island in the Dutch Wadden Sea, in the period 2011–2015. Breeding activity was monitored through regular nest visits, recording number of eggs and young at each visit. Parents were caught with spring traps while feeding nestlings (8–11 days old, hatching day =day 0) and subsequently nestlings were ringed. In winter, birds were caught while roosting in the nest boxes. Individual great tits were identified by ring number and unringed individuals were ringed. The overall capture probability of individuals known to be alive was 88% in our population (Atema et al., 2016), hence we were able to estimate survival based on catches of the adults in both winter and spring.
To quantify the association between telomeres and reproductive success we used data from first broods only (only three out of 109 males had a second brood; note that we measured telomeres in males only). Furthermore, we did not investigate associations with time of breeding and clutch size, because these traits are known to be under female control with no influence of the partner in our study population (Van Noordwijk et al., 1981; Postma & van Noordwijk, 2005). We verified that this also holds in the years of our study by comparing the repeatability of clutch size and hatch date in females and males (total population, years 2010–2014), and found both to be substantially higher in females (Table S1). Based on these findings we used the number of fledglings and recruits (fledglings recaptured as breeding bird in a later year) as measures of reproductive success.
Blood samples were taken from the brachial vein and stored in 2% EDTA at 4–7°C for up to three weeks. Subsequently, samples were snap‐frozen in 40% glycerol buffer and stored at −80°C.
2.2. Backpack experiment
In order to manipulate workload, we equipped male great tits in the years 2011 – 2013 with a small backpack that they carried during 1 year, after which they were recaptured when feeding nestlings and the backpack was removed. The experiment included three treatments: (i) control without a backpack (n = 97), (ii) "light" with an empty backpack of 0.1 g as a control for possible effects of the harness (n = 68) and (iii) ‘heavy’ with a backpack of 0.9 g (~5% of body mass; n = 80). Further details can be found in Atema et al. (2016), where we show there were no long‐term fitness consequences of the manipulation, although there were short‐term effects of the backpacks on male state: males carrying backpacks slightly increased their mass, and were less successful in securing a nest box as roosting site in winter (Atema et al., 2016).
2.3. Telomere terminal restriction fragment analyses
We quantified telomere length using terminal restriction fragment as described by Salomons et al. (2009) and Atema et al. (2019). Briefly, DNA was extracted in agarose plugs and subsequently DNA in half a plug was digested with a mixture of three restriction enzymes, cutting DNA sequence except for telomere sequence (5’‐TTAGGG‐3’). The restricted DNA and the 32P end‐labelled size standards were size separated with pulsed field gel electrophoreses. Gels were subsequently dried and the single‐stranded overhangs of telomeres were hybridized with 32P‐labelled oligonucleotide (5’‐CCCTAA‐3’)4.
An image of the distribution of telomere lengths was retrieved by phosphor imaging. Gel images were analysed using the open source software IMAGEJ v. 1.38x. In Atema et al. (2019) we showed that in adults only class II, but not ultra‐long class III telomeres shorten with age, and hence class II telomeres are most relevant to use as a biomarker. We therefore limited the analyses in the present study to Class II telomeres (average range 3–18 kb). Telomere length varies within a sample, due to TL differences between chromosomes and cells, and we previously showed that in great tits and other bird species (jackdaws Corvus monedula: Salomons et al., 2009; common tern Sterna hirundo: Bauch et al., 2014) the higher percentiles within the distribution lose more bp / year than the lower percentiles (Atema et al., 2019). Next to average telomere length of the telomere distribution we therefore calculated the telomere length per percentile (10th–90th) of the class II distribution. See Atema et al. (2019) for details, where the telomere data are presented on which the current study is based.
2.4. Statistical analysis
Data were analysed using mixed‐effects models in R (R Core Team, 2019) and we tested the specific hypotheses explained above, that is the model selection procedure compared only models with and without the treatment effects. All models included random terms to correct for between gel variation and for individual identity when repeated measurements were included. All models included age as fixed effect and longitudinal models included also initial telomere length and the interaction of age with initial telomere length if significant as fixed effects.
To test for effects of the backpack experiment on telomere length, we included added mass as a continuous variable to the model, since this approach explained variation caused by the treatment best in our earlier analyses of the fitness consequences of the backpack experiment (Atema et al., 2016). Added mass was coded zero for all baseline samples, and the added mass (0, 0.1 or 0.9) for subsequent samples. We further included sampling season as factor (0 = spring; 1 = winter), age at manipulation and the time elapsed between the baseline sample and the follow‐up sample (note that age at manipulation and elapsed time since manipulation add up to age at sampling). To investigate the relation between telomere length and reproduction, we included number of offspring either as continuous variable or as binomial factor (unsuccessful, 0 offspring =0; successful, ≥1 offspring = 1) because complete and partial brood failure are likely to have different causes. To quantify the relation between telomere length and survival, we included the binomial factor survived to the model (died = 0; survived = 1). We considered an individual to have not survived when it was not seen or recaptured in the 1–3 years after 1 April of the next year. In the analyses, we refer to the breeding season in which the backpack was added as spring 1, and the next breeding season as spring 2.
3. RESULTS
3.1. Backpack experiment
Initial telomere length did not differ significantly between the three experimental groups (F2,71.8 = 0.33, p = .72). Because the aim of the study was to evaluate long‐term effects of carrying a backpack, the analyses below are limited to individuals that were resampled in the subsequent winter and / or in spring 2. We found no significant effect of the added mass on telomere loss during the treatment period (F1,113.5 = 0.01, p = .9, Figure 1) when pooled over the recaptures in winter and spring 2, and neither was there a significant interaction between treatment and time elapsed since the start of the treatment (F1,111.5 = 0.04, p = .8). Testing for an effect of added mass on telomere shortening separately for recaptures in winter or spring 2 did not change this result (both p > .4). Thus, we concluded that the added mass did not significantly accelerate telomere dynamics.
FIGURE 1.
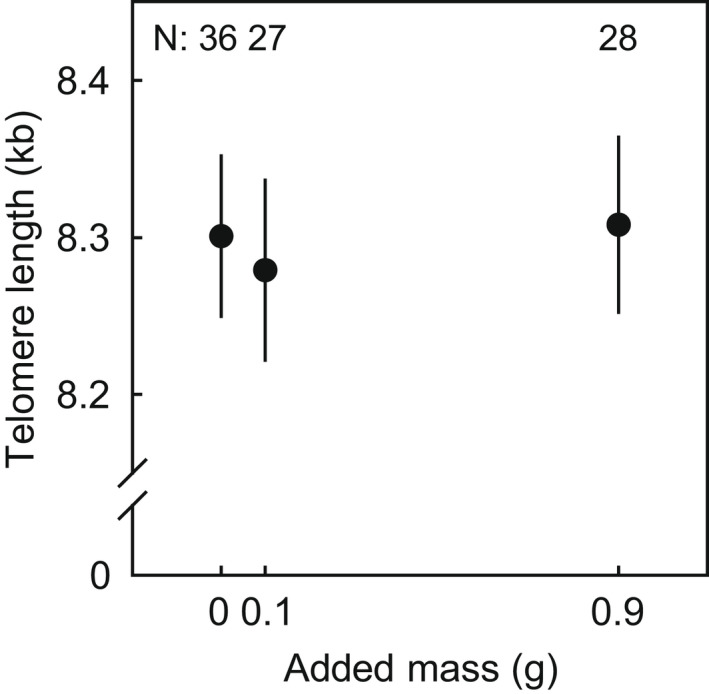
Telomere length of males after carrying additional mass (X‐axis) for one year. Plotted values are estimated means ± standard error of Class II telomere length, and numbers in the graph indicate the number of males
The rate of telomere shortening rate appeared slightly higher in the winter subset of samples than in the spring subset (model estimates respectively: 55 ± 44 bp/year and 76 ± 40 bp/year), but not significantly so (F1,57.7 = 0.16, p = .69). Likewise, among individuals which were successfully sampled on all three occasions there was no difference in telomere shortening rate from spring 1 to winter and winter to spring 2 (F1,33.0 = 0.06, p = .80).
3.2. Reproduction
Neither the number of fledglings, nor the number of recruits was affected by the backpack experiment (Atema et al., 2016), and hence we ignored the experiment in the following analyses. Telomere length was not associated with reproductive success in that same year in terms of number of fledglings or recruits (Table 1a and b) When, for example, more successful individuals lose fewer base pairs per year than less successful individuals, the association between telomere length and reproductive success may become stronger with age. We therefore analysed the correlation between telomere length and number of recruits separately for males of 1 year and males older than 1 year. We did this for recruit production only, because in this stage of the breeding cycle the effects of ageing were found to be strongest in great tits in our study population (Bouwhuis et al., 2010). However, there was no correlation between telomere length and the number of recruits in either one year old males (slope: 36.07 ± 180.39; F1,60.9 = 0.040, p = .84) or in males older than one year (slope: −22.64 ± 33.13; F1,30.6 = 0.47, p = .50).
TABLE 1.
Models testing for an association between class II telomere length (bp) and reproductive success while controlling for age (years)
| Model | Fixed effect | Estimate (s.e.) | F ratio | p‐value |
|---|---|---|---|---|
| Cross‐sectional | ||||
| (a) | Age | −301.0 (118.8) | 6.42 | .013 |
| No.# fledglings | 60.9 (49.6) | 1.51 | .22 | |
| (b) | Age | −269.0 (118.9) | 5.12 | .026 |
| No.# recruits | −16.4 (113.8) | 0.02 | .89 | |
| Longitudinal | ||||
| (c) | Age | −137.2 (54.9) | 6.25 | .013 |
| No.# fledglings | 3.83 (7.5) | 0.26 | .61 | |
| (d) | Age | −162.3 (38.6) | 17.68 | <.0001 |
| No.# recruits | 59.1 (25.9) | 5.21 | .024 | |
Models (a) and (b) are cross‐sectional, with respectively number of fledglings and recruits as estimates of reproductive success. Models (c) and (d) are longitudinal, with reproductive success coded as zero in spring 1, and as the number of fledglings or recruits produced in spring 1 at all later captures. This coding yields estimates of the effect of the number of offspring produced while controlling for age. As random effect gel identity was included in all models and individual identity in the longitudinal models. N = 103 in tables (a) and (b), and N = 231 in tables (c) and (d).
In the longitudinal analyses, we investigated telomere dynamics in the year following reproduction (including winter captures). We found no association between the number of fledglings and subsequent telomere dynamics (Table 1c), but individuals who produced more recruits lost fewer base pairs in that year (Table 1d). Based on the model estimates, individuals that were unsuccessful in producing recruits lost on average 177.6 ± 36.4 bp/year, whereas successful individuals lost on average only 4.1 ± 39.6 bp/year (Figure 2).
FIGURE 2.
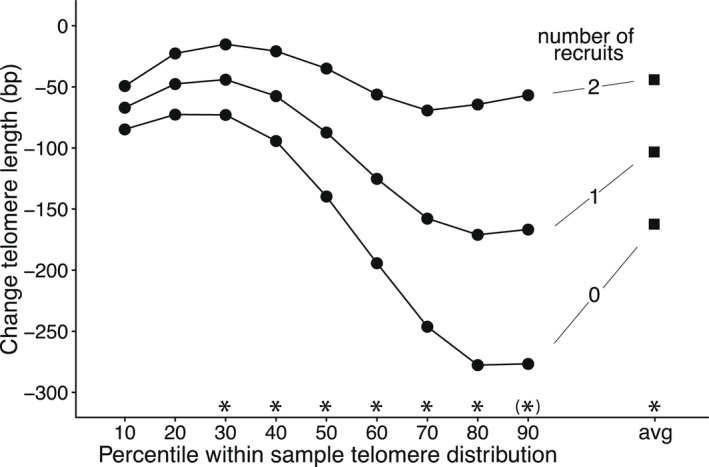
Reproductive success (number of recruits) and change in telomere length from spring 1 to spring 2 at percentiles 10 – 90 within the sample telomere distribution, and at the average of sample telomere distribution (indicated with "avg" on the x‐axis). Lines from bottom to top for males producing 0, 1 or 2 recruits respectively. For clarity, standard errors are not shown, but these are presented in Table 2 and Table S2 for the average and the different percentiles respectively. Significant association of the change in telomere length with number of recruits marked with * for p < .05 and (*) for p < .10
The difference in telomere dynamics between individuals producing recruits or not, was more pronounced in the longer telomeres of the telomere distribution (Figure 2; interaction recruits x percentile: F1,1045 = 37.82, p < .0001; including sample identity as random effect).
3.3. Survival
Controlling for age, birds that survived from spring 2 to the next breeding season had 127 bp longer telomeres (Figure 3), but this modest difference was not statistically significant (Table 2a). To investigate the link between telomere attrition and survival we compared telomere attrition from spring 1 to spring 2 between survivors and non‐survivors. We restricted the dataset to samples taken in spring of year 1 and 2 (i.e. ignored samples taken in winter), and found that surviving individuals lost on average ± s.e. 60.2 ± 100.6 bp/year, whereas non‐surviving individuals lost 125.7 ± 45.4 bp/year, but this two‐fold difference did not reach statistical significance (Table 2b). As can be seen in Figure 3b, a few individuals show much larger changes in telomere length than all other individuals. When we removed the two most extreme observations from the analysis we estimate that survivors lost on average −0.5 ± 85.3 bp while non‐survivors lost 151.7 ± 38.0 bp, and this difference approached statistical significance (p = .07). This finding leads us to suggest that telomere shortening may be a better predictor of survival than absolute telomere length, but obviously a larger data set is required to confirm this statistically.
FIGURE 3.
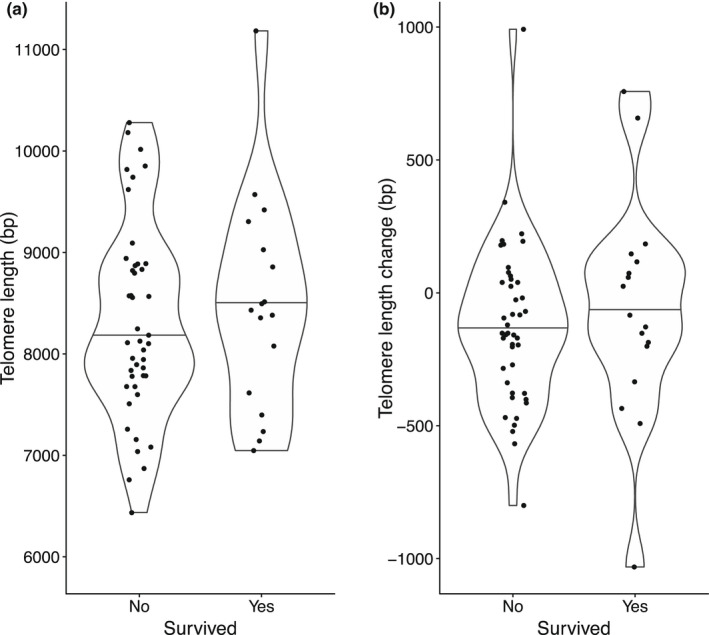
Survival to spring 3 in relation to (a). class II telomere length in spring 2 and (b). telomere dynamics (telomere length spring 2 – telomere length spring 1). Violin plots with horizontal line indicating median. N = 61
TABLE 2.
Survival in relation to (a). telomere length in spring 2 and (b). telomere dynamics (telomere length spring 2–telomere length spring 1). The variable "survived" was 1 for survivors and 0 for non‐survivors. N = 61
| Variable | Estimate (s.e.) | F ratio | p‐value | |
|---|---|---|---|---|
| (a) | Age (years) | −56.0 (119.4) | 0.37 | .64 |
| survived | 126.7 (294.0) | 0.19 | .67 | |
| (b) | survived | 65.5 (95.9) | 0.47 | .49 |
4. DISCUSSION
Carrying 5% additional mass during one whole year seems a severe challenge, but we found no effect of this manipulation on telomere dynamics. On the one hand, this was surprising, because of the nature of the manipulation and because our earlier analysis of the experiment on other traits revealed that males responded to the manipulation by changing their mass, and males with heavy backpacks appeared less successful in competition over roosting sites (Atema et al., 2016). On the other hand, it agrees with our finding that these effects did not translate into long‐term consequences for reproduction or survival (Atema et al., 2016). Apparently great tits have a way to avoid or compensate for costs of the additional mass, for example by a combination of reducing time spent flying, carrying lower energy reserves, and choosing better foraging sites at the expense of increased predation risk. Cumulatively, these mechanisms should reduce the residual reproductive value, but not necessarily in every year, because such effects will be strongly dependent on for example current food availability, predator abundance and climatic conditions.
Our backpack treatment was comparable to the weight of transmitters used in great tits (e.g. Snijders et al., 2014). Altogether we showed that equivalent devices to the backpacks can be used in small songbirds without long‐term consequences on fitness or telomere shortening and presumably ageing. We are aware of one comparable handicap experiment, in adult Adélie penguins, that also found no significant effect on telomere attrition (Beaulieu et al., 2011). Indeed, a recent meta‐analysis did not find a significant overall effect of diverse stressors on TL in wild populations (Chatelain et al., 2019), and our findings fit this pattern.
Previous cross‐sectional comparisons of the association between telomere length and reproductive success have yielded mixed results (review: Sudyka, 2019), sometimes even contrasting between the sexes within a species (Bauch et al., 2020). In our study, neither the number of fledglings nor the number of recruits was correlated with telomere length. However, a longitudinal comparison revealed that successful males (with recruits) lost fewer base pairs compared to unsuccessful individuals. The positive relationship in great tits could be due to the male being of better quality and/or having a better territory, and agrees with the finding that individuals that are more successful at reproduction also have higher survival rates than less successful individuals (e.g. Lane et al., 2010; Olsson et al., 2001) also in great tits (Bouwhuis et al., 2009). Alternatively, more successful males may have been mated with females that provided more parental care, resulting into the high reproductive success and leaving the male with more resources for somatic maintenance.
When analysing the association between reproductive success and telomere dynamics in more detail, by dividing the telomere distribution in percentiles, it appeared that the positive correlation between recruit production and telomere shortening was strongest in the longer telomeres within individuals. A similar pattern was found in common terns (Bauch et al., 2014), which is to our best knowledge the only other species in which this was investigated. This finding agrees with our expectation, because the longest telomeres also shorten at the highest rate in great tits and other species (Andrews et al., 2021; Atema et al., 2019; Bauch et al., 2014; Kimura et al., 2007; Salomons et al., 2009), and it confirms our interpretation that the longer telomeres within individuals are more sensitive to environmental conditions and hence more informative read‐out parameters of experienced life‐stress (Bauch et al., 2014).
The non‐significant correlation between reproductive success and telomere length in the cross‐sectional dataset, while finding a significant effect in the longitudinal dataset, are superficially inconsistent with each other. If reproductive output is a permanent characteristic of an individual, a cumulative effect on telomere length could be expected. A similar hypothesis was proposed in common terns, in which individuals show consistent individual differences in reproductive success (Bauch et al., 2014). In this species, cross‐sectional and longitudinal analyses yielded consistent significant relations between reproduction and telomeres. However, great tits have low survival rates and hence their lifespan may be too short to detect such cumulative effects. Moreover, individual variation in reproductive output may be less consistent, because the production of recruits is probably highly stochastic, in which case the accumulation of effects would be more difficult to detect.
Individuals that were most likely to survive to the next year had longer telomeres and also lost fewer telomere base pairs, in line with findings in other species (Wilbourn et al., 2018), but none of these correlations reached significance in our study. The population of great tits on Vlieland is a relatively closed island system, and once males settle in the population as breeding birds they are not known to leave the island (Verhulst & van Eck, 1996). Therefore, selective emigration is unlikely to have biased our findings. Moreover, our sample size was relatively large, leading us to conclude that the correlation between survival and telomeres in great tits is apparently weak in our study population. In contrast, Salmón et al. (2017) did find a positive association between TL and survival in adult great tits, but in their telomere measurements they pooled all telomere classes (i.e. interstitial telomeric sequences and the ultralong class III telomeres that do not shorten with age in addition to class II telomeres, and hence their analysis is only superficially similar to ours that refers to class II telomeres up to 18kb only; Atema et al., 2019).
Contrary to what we observed in great tits, in some other species, including common terns, the individuals with higher reproductive output lost more telomere base pairs than less successful individuals (Bauch et al., 2013, 2014; Sudyka, 2019). This contrast raises an interesting question, which has received little attention, namely how the factors that determine who attains higher fitness varies between species. To some extent, these factors will be shared between species (e.g. ‘health’, although what constitutes ‘health’ may vary between species), but species differences in social and ecological factors are likely to result in different selection pressures in different species. For example, prey‐handling time affects the benefits of klepto‐parasitism, and thereby potential fitness benefits of social dominance (Ens et al., 1990). Indeed, differences in social and ecological factors are key to the causes of the evolution of species differences, but general patterns explaining variation between species in associations with individual variation in fitness remain to be identified. Since telomeres are evolutionary well conserved, and hence can in principle be studied in all vertebrate species, developing an explanation for interspecific variation in fitness/telomere dynamics association may be a fruitful starting point for this endeavour. The question can then be phrased as "What causes the variation between species in the association between reproductive success and telomere dynamics?".
One way to interpret individual variation in telomere dynamics is that it reflects variation in reproductive effort, with a larger effort accelerating telomere attrition. The small number of studies in which brood size was manipulated tend to support this interpretation (Sudyka, 2019). Interpreted in this way, our findings suggest that in great tits the more successful individuals made a smaller effort than the less successful individuals, while the opposite pattern emerged in common terns. For the contrast between great tits and common terns this interpretation is supported by information on the association between reproductive success and corticosterone, a hormone of which concentrations in plasma increase with increasing energy expenditure (e.g. Jimeno et al., 2017, 2018). In great tits, the individuals with high baseline corticosterone achieved the lowest reproductive success (Ouyang et al., 2013), while in common terns the individuals with high baseline corticosterone achieved the highest success (Bauch et al., 2016). An ecological explanation of this contrast may be that great tits are territorial, and hence each pair has their own resource pool, and individuals with a rich territory can provide more food with less effort compared to individuals on poor territories. Indeed, great tit energy expenditure during brood rearing is known to be lower on territories with higher food availability (Tinbergen & Dietz, 1994), while such territories yield a higher reproductive success (Verboven et al., 2001). In contrast, common terns are colonial, and thus have a shared resource pool, suggesting that reproductive success is likely to increase with increasing foraging effort, resulting in higher rates of provisioning. Thus, based on the comparison of these two well‐studied species, we propose that the social organisation as it determines resource access may contribute to determining whether successful individuals are either those that make the highest effort, and consequently lose more base pairs than less successful individuals, or, alternatively, are those individuals that achieve high success with low effort, and consequently lose relatively few base pairs. Obviously, more (longitudinal) studies of telomere dynamics and other traits that can be assumed to reflect reproductive effort are required to test this hypothesis and the underlying assumptions.
AUTHOR CONTRIBUTIONS
Project was designed by all three authors. Field data were collected by E.A. with assistance of A.J.v.N, and the telomere measurements were done by E.A. Data analysis and manuscript writing were done by E.A. and S.V.
5. CONFLICT OF INTEREST
The authors declare no conflict of interest.
OPEN RESEARCH BADGES
This article has earned an Open Data Badge for making publicly available the digitally‐shareable data necessary to reproduce the reported results. The data is available at https://doi.org/10.5061/dryad.qjq2bvqgv.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank the State Forestry Commission for permission to work on their property. We are grateful to J. den Ouden, E.A de Ruijter, N. Weites, A. Sibma, M. Rousselle, J. Zhang and C. Vinke for their great help in the field and to E. Mulder for her invaluable advice about the TRF analyses. Comments of Antoine Stier and an anonymous reviewer improved the manuscript. Sample collection and experimental procedures were approved by the Animal Experimental Committee of the Royal Netherlands Academy of Arts and Sciences (DEC‐KNAW, NIOO 11.03). E.A. was supported by NWO open competition grant 821.01.003 to AJvN and SV.
Atema, E. , van Noordwijk, A. J. , & Verhulst, S. (2022). Telomere dynamics in relation to experimentally increased locomotion costs and fitness in great tits. Molecular Ecology, 31, 6208–6215. 10.1111/mec.16162
DATA AVAILABILITY STATEMENT
The data used in these analyses have been deposited in the DRYAD repository with https://doi.org/10.5061/dryad.qjq2bvqgv.
REFERENCES
- Andrews, C. , Zuidersma, E. , Verhulst, S. , Nettle, D. , & Bateson, M. (2021). Exposure to food insecurity increases energy storage and reduces somatic maintenance in European starlings. Royal Society Open, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atema, E. , Mulder, E. , van Noordwijk, A. J. , & Verhulst, S. (2019). Ultra‐long telomeres shorten with age in nestling great tits but are static in adults and mask attrition of short telomeres. Molecular Ecology Resources, 19, 648–658. 10.1111/1755-0998.12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atema, E. , van Noordwijk, A. J. , Boonekamp, J. J. , & Verhulst, S. (2016). Costs of long‐term carrying of extra mass in a songbird. Behavioral Ecology, 27, 1087–1096. 10.1093/beheco/arw019 [DOI] [Google Scholar]
- Barha, C. K. , Hanna, C. W. , Salvante, K. G. , Wilson, S. L. , Robinson, W. P. , Altman, R. M. , & Nepomnaschy, P. A. (2016). Number of children and telomere length in women: a prospective, longitudinal evaluation. PLoS ONE, 11, 1–12. 10.1371/journal.pone.0146424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch, C. , Becker, P. H. , & Verhulst, S. (2013). Telomere length reflects phenotypic quality and costs of reproduction in a long‐lived seabird. Proceedings of the Royal Society B: Biological Sciences, 280, 20122540. 10.1098/rspb.2012.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch, C. , Becker, P. H. , & Verhulst, S. (2014). Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long‐lived seabird. Molecular Ecology, 23, 300–310. 10.1111/mec.12602 [DOI] [PubMed] [Google Scholar]
- Bauch, C. , Gatt, M. C. , Granadeiro, J. P. , Verhulst, S. , & Catry, P. (2020). Sex‐specific telomere length and dynamics in relation to age and reproductive success in Cory's shearwaters. Molecular Ecology, 29, 1344–1357. 10.1111/mec.15399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch, C. , Riechert, J. , Verhulst, S. , & Becker, P. (2016). Telomere length reflects reproductive effort indicated by corticosterone levels in a long‐lived seabird. Molecular Ecology, 25, 5785–5794. 10.1111/mec.13874 [DOI] [PubMed] [Google Scholar]
- Beaulieu, M. , Reichert, S. , Le Maho, Y. , Ancel, A. , & Criscuolo, F. (2011). Oxidative status and telomere length in a long‐lived bird facing a costly reproductive event. Functional Ecology, 25, 577–585. 10.1111/j.1365-2435.2010.01825.x [DOI] [Google Scholar]
- Blackburn, E. H. (1991). Telomeres. Trends in Biochemical Sciences, 16, 378–381. 10.1016/0968-0004(91)90155-O [DOI] [PubMed] [Google Scholar]
- Bouwhuis, S. , Sheldon, B. C. , Verhulst, S. , & Charmantier, A. (2009). Great tits growing old: Selective disappearance and the partitioning of senescence to stages within the breeding cycle. Proceedings of the Royal Society B: Biological Sciences, 276, 2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwhuis, S. , Van Noordwijk, A. J. , Sheldon, B. C. , Verhulst, S. , & Visser, M. E. (2010). Similar patterns of age‐specific reproduction in an island and mainland population of great tits Parus major. Journal of Avian Biology, 41, 615–620. 10.1111/j.1600-048X.2010.05111.x [DOI] [Google Scholar]
- Chatelain, M. , Drobniak, S. M. , & Szulkin, M. (2019). The association between stressors and telomeres in non‐human vertebrates: a meta‐analysis. Ecology Letters, 99, 21–18. [DOI] [PubMed] [Google Scholar]
- Eastwood, J. R. , Hall, M. L. , Teunissen, N. , Kingma, S. A. , Hidalgo Aranzamendi, H. , Fan, M. , Roast, M. , Verhulst, S. , & Peters, A. (2019). Early‐life telomere length predicts lifespan and lifetime reproductive success in a wild bird. Molecular Ecology, 28, 1127–1137. 10.1111/mec.15002 [DOI] [PubMed] [Google Scholar]
- Ens, B. J. , Esselink, P. , & Zwarts, L. (1990). Kleptoparasitism as a problem of prey choice: A study on mudflat‐feeding curlews, Numenius arquata . Animal Behaviour, 39, 219–230. 10.1016/S0003-3472(05)80866-8 [DOI] [Google Scholar]
- Houston, A. I. , McNamara, J. M. , & Hutchinson, J. M. C. (1993). General results concerning the trade‐off between gaining energy and avoiding predation. Philosophical Transactions of the Royal Society B: Biological Sciences, 341, 375–397. [Google Scholar]
- Jimeno, B. , Hau, M. , & Verhulst, S. (2017). Strong association between corticosterone and temperature dependent metabolic rate in individual zebra finches. Journal of Experimental Biology, 220, 3280–3289. 10.1242/jeb.166124 [DOI] [PubMed] [Google Scholar]
- Jimeno, B. , Hau, M. , & Verhulst, S. (2018). Corticosterone levels reflect variation in metabolic rate independent of ‘stress’. Scientific Reports, 8, 13020. 10.1038/s41598-018-31258-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, M. , Barbieri, M. , Gardner, J. P. , Skurnick, J. , Cao, X. , van Riel, N. , Rizzo, M. R. , Paoliso, G. , & Aviv, A. (2007). Leukocytes of exceptionally old persons display ultra‐short telomeres. American Journal of Physiology‐ Regulatory, Integrative and Comparative Physiology, 293, R2210–2217. 10.1152/ajpregu.00615.2007 [DOI] [PubMed] [Google Scholar]
- Lane, J. E. , Boutin, S. , Speakman, J. R. , & Humphries, M. M. (2010). Energetic costs of male reproduction in a scramble competition mating system. Journal of Animal Ecology, 79, 27–34. 10.1111/j.1365-2656.2009.01592.x [DOI] [PubMed] [Google Scholar]
- Le Vaillant, M. , Viblanc, V. A. , Saraux, C. , Le Bohec, C. , Le Maho, Y. , Kato, A. , Criscuolo, F. , & Ropert‐Coudert, Y. (2015). Telomere length reflects individual quality in free‐living adult king penguins. Polar Biology, 2059–2067. 10.1007/s00300-015-1766-0 [DOI] [Google Scholar]
- Olsson, M. , Shine, R. , & Wapstra, E. (2001). Costs of reproduction in a lizard species: A comparison of observational and experimental data. Oikos, 93, 121–125. 10.1034/j.1600-0706.2001.930113.x [DOI] [Google Scholar]
- Ouyang, J. Q. , Sharp, P. , Quetting, M. , & Hau, M. (2013). Endocrine phenotype, reproductive success and survival in the great tit, Parus major. Journal of Evolutionary Biology, 26, 1988–1998. [DOI] [PubMed] [Google Scholar]
- Pauliny, A. , Wagner, R. H. , Augustin, J. , Szép, T. , & Blomqvist, D. (2006). Age‐independent telomere length predicts fitness in two bird species. Molecular Ecology, 15, 1681–1687. 10.1111/j.1365-294X.2006.02862.x [DOI] [PubMed] [Google Scholar]
- Plot, V. , Criscuolo, F. , Zahn, S. , & Georges, J. Y. (2012). Telomeres, age and reproduction in a long‐lived reptile. PLoS ONE, 7, e40855. 10.1371/journal.pone.0040855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma, E. , & van Noordwijk, A. J. (2005). Gene flow maintains a large genetic difference in clutch size at a small spatial scale. Nature, 433, 65–68. 10.1038/nature03083 [DOI] [PubMed] [Google Scholar]
- R Core and Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Salmón, P. , Nilsson, J. F. , Watson, H. , Bensch, S. , & Isaksson, C. (2017). Selective disappearance of great tits with short telomeres in urban areas. Proceedings of the Royal Society B: Biological Sciences, 284, 20171349–20171358. 10.1098/rspb.2017.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons, H. M. , Mulder, G. A. , van de Zande, L. , Haussmann, M. F. , Linskens, M. H. K. , & Verhulst, S. (2009). Telomere shortening and survival in free‐living corvids. Proceedings of the Royal Society B: Biological Sciences, 276, 3157–3165. 10.1098/rspb.2009.0517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders, L. , van Rooij, E. P. , Burt, J. M. , Hinde, C. A. , van Oers, K. , & Naguib, M. (2014). Social networking in territorial great tits: slow explorers have the least central social network positions. Animal Behaviour, 98, 95–102. 10.1016/j.anbehav.2014.09.029 [DOI] [Google Scholar]
- Sudyka, J. (2019). Does Reproduction Shorten Telomeres? Towards Integrating Individual Quality with Life‐History Strategies in Telomere Biology. BioEssays, 32, 1900095–1900112. 10.1002/bies.201900095 [DOI] [PubMed] [Google Scholar]
- Tinbergen, J. M. , & Dietz, M. W. (1994). Parental energy expenditure during brood rearing in the great tit (Parus major) in relation to body mass, temperature, food availability and clutch size. Functional Ecology, 8, 563–572. 10.2307/2389916 [DOI] [Google Scholar]
- Tinbergen, J. M. , & Verhulst, S. (2000). A fixed energetic ceiling to parental effort in the great tit? Journal of Animal Ecology, 69, 323–334. 10.1046/j.1365-2656.2000.00395.x [DOI] [Google Scholar]
- Van de Pol, M. , & Verhulst, S. (2006). Age‐dependent traits: a new statistical model to separate within‐ and between‐individual effects. The American Naturalist, 167, 766–773. 10.1086/503331 [DOI] [PubMed] [Google Scholar]
- Van Noordwijk, A. J. , Van Balen, J. H. , & Scharloo, W. (1981). Genetic and environmental variation in clutch size of the Great tit Parus major. Netherlands Journal of Zoology, 31, 342–372. [Google Scholar]
- Verboven, N. , Tinbergen, J. M. , & Verhulst, S. (2001). Food, reproductive success and multiple breeding in the Great Tit Parus major”. Ardea, 89, 387–406. [Google Scholar]
- Verhulst, S. , & Van Eck, H. M. (1996). Gene flow and immigration rate in an island population of great tits. Journal of Evolutionary Biology, 9, 771–782. 10.1046/j.1420-9101.1996.9060771.x [DOI] [Google Scholar]
- Wilbourn, R. V. , Moatt, J. P. , Froy, H. , Walling, C. A. , Nussey, D. H. , & Boonekamp, J. J. (2018). The relationship between telomere length and mortality risk in non‐model vertebrate systems: a meta‐analysis. Philosophical Transactions of the Royal Society B: Biological Science, 373, 20160447–20160449. 10.1098/rstb.2016.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data used in these analyses have been deposited in the DRYAD repository with https://doi.org/10.5061/dryad.qjq2bvqgv.


