Summary
Sleep disorders have been observed among patients with heart failure. The aim of this study was to investigate whether acute sleep deprivation (SD) aggravates left heart function. Male C57B/L6 mice were assigned to four experimental groups. Ligation of the left anterior descending branch (LAD) caused myocardial infarction (MI) in mice in the LAD group and the LAD+SD group, while mice in the sham and sham+SD groups underwent the same surgery without ligation. Echocardiography was performed before and 8 weeks after ligation of the LAD to evaluate the left ventricular internal diameter at diastole (LVIDd), left ventricular internal diameter at systole (LVIDs), ejection fraction (EF), and fractional shortening (FS). Seven days of sleep deprivation induced using the modified single platform method resulted in a lower EF and FS and a higher LVIDd and LVIDs, as well as increased expression of the IL‐1β, IL‐18, and IL‐10 mRNAs in the left ventricular tissue of MI mice. ELISA also indicated higher levels of IL‐1β and IL‐10 in the LAD+SD group. It was concluded that acute sleep deprivation induced cardiovascular alterations in cardiac structure and function in HF mice, accompanied by increased levels of inflammatory cytokines.
Keywords: brain–heart interaction, cytokine, left ventricular function, sleep disorder
1. INTRODUCTION
Heart failure (HF) (Metra & Teerlink, 2017), which has high morbidity and mortality rates, contributes significantly to increasing health care costs. Much effort has been spent searching for valid therapies for heart failure, but no ideal drugs or regimens have been developed (Piek et al., 2018).
In the clinic, sleep disorders, such as insomnia and fragmentation, are common in patients with heart failure (Coniglio & Mentz, 2020). According to previous studies (Giampá et al., 2016; Wright et al., 2015), REM sleep deprivation increases the plasma levels of oxidative stress and inflammatory markers, accelerating myocardial necrosis and fibrosis. Some researchers have suggested that post infarct sleep disruption leads to an enlargement of the heart and increased oxidative stress and NO production, which may ultimately result in cardiac dysfunction (Aghajani et al., 2017). However, the effect of acute sleep deprivation on heart function after cardiac infarction has received little attention.
M‐type ultrasonic diagnostic instruments (also known as echocardiography instruments) can show the movement of organs, mainly used for the diagnosis of cardiovascular diseases, and has become a common functional test in many cardiac studies (Hauck et al., 2021; Zhang et al., 2021). By measuring the change in distance between the intima, the left ventricular internal diameter at diastole (LVIDd), left ventricular internal diameter at systole (LVIDs), heart rate (HR), ejection fraction (EF), and fractional shortening (FS) could be acquired. When mice had impaired cardiac systolic function, it was shown that the LVIDd and LVIDs increased, and FS and EF decreased.
A large body of evidence suggests that impaired cardiac function is associated with the induction of cytokines and chemokines. The pro‐inflammatory cytokines such as tumor necrosis factor (TNF)‐α, interleukin (IL)‐1, IL‐6, MCP‐1, and IL‐10 may contribute to the pathogenesis of adverse remodelling, and systolic and diastolic dysfunction (Hanna & Frangogiannis, 2020). IL‐1β could suppress systolic cardiomyocyte function through effects that may involve disruption of calcium handling (Kumar et al., 1996) or suppression of β‐adrenergic responses (Gulick et al., 1989). High IL‐18 levels correlate with an increased risk of developing cardiovascular disease (CVD) and with a worse prognosis in patients with established CVD (O'Brien et al., 2014). IL‐10 could activate fibroblasts, and stimulate collagen deposition, leading to impaired myocardial relaxation and increased myocardial stiffness (Hulsmans et al., 2018).
The aim of this work was to assess whether acute sleep deprivation affected the left ventricular function of a model of myocardial infarction and whether the underlying mechanism is related to the change of inflammatory factors in the heart tissue.
2. MATERIALS AND METHODS
2.1. Animal experimental protocols
All experiments were performed in accordance with the recommendations of national and international care and ethical guidelines and were approved by the Ethics Committee for Animal Research of Affiliated Suzhou Hospital, Nanjing Medical University (permit code: 2021111240). Adult male C57BL/6 mice (aged 6–8 weeks) were used in the present study. C57BL/6 mice (12 h light/dark cycle; water/food provided ad libitum) were distributed into the LAD group, LAD+SD group, sham+SD group, and sham group (n = 5).
2.2. Coronary artery ligation
Heart failure was conducted by ligating the left anterior descending artery. An elevation of ST indicated by ECG is considered to show successful ligation of the anterior descending branch. Heart failure was allowed to progress for 8 weeks during left ventricular remodelling. The mice in the LAD group received left anterior descending artery ligation, while mice in the LAD+SD group received 1 week of acute sleep deprivation 8 weeks after LAD ligation. Mice in the SHAM group underwent thoracotomy without ligation, while mice in the SHAM+SD group only received thoracotomy without ligation and the same process of sleep deprivation as the LAD+SD group.
2.3. Sleep deprivation
The acute sleep deprivation paradigm was conducted using the multiple small platform for 7 consecutive days. Five mice were placed in each cage (38 × 31 × 17 cm), and each cage contained 12 platforms (2.5 cm diameter). Each cage was spaced 7 cm apart and filled with water 1 cm below the upper surface of the platform. Thus, when animals entered the paradoxical phase of sleep and lost muscle tone, they were awoken by falling into the water. Food and water were available ad libitum.
2.4. Ultrasonography
Mice were anaesthetised and immobilised to assess cardiac function. Echocardiography was performed using a 16 MHz ultrasound probe with a GE Vivid 9 ultrasound platform (GE Health Care, Milwaukee, WI, USA). The left ventricular short axis was displayed by the probe, while the results of M‐mode measurements provided data on parameters such as the left ventricular internal diameter at diastole (LVIDd), left ventricular internal diameter at systole (LVIDs), and heart rate (HR). The EF and FS analyses were conducted using onboard ultrasound analysis software. Nine weeks after the operation and sleep deprivation, the haemodynamic parameters were measured using echocardiography.
2.5. Quantitative PCR
Immediately after the final echography session, the mice were anaesthetised, and noninfarcted heart tissue was dissected. Noninfarcted heart tissue was homogenised, the total RNA was isolated, and mRNA was transcribed into cDNAs using a TransScript All‐in‐one First‐strand cDNA Synthesis kit for qPCR (Code: AT341‐02). Quantitative polymerase chain reaction (PCR) amplification was performed using the GeneCopoeia platform (No: QP001). Gene expression was normalised to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) and analysed using the 2(−△△Ct) method. The primer sequences used in the present study are listed in Table 1.
TABLE 1.
Sequences of primers used for real‐time qPCR experiments
| Gene | Gene ID | Forward primer 5′‐3′ | Reverse primer 5′‐3′ |
|---|---|---|---|
| GAPDH | 287,362 | CACTGAGCAAGAGAGGCCCTAT | GCAGCGAACTTTATTGATGGTATT |
| IL‐10 | 25,166 | CCTCTGGATACAGCTGCGAC | GTAGATGCCGGGTGGTTCAA |
| IL‐1β | 24,494 | AGCTTCCTTGTGCAAGTGTCT | GACAGCCCAGGTCAAAGGTT |
| IL‐6 | 24,498 | ACGTAGCTAGCTAGTCGGTATG | TCGTAGCTTGGCTAGTCGATCG |
| IL‐18 | 24,599 | CAGGCCTGACATCTTCTGCAA | TCTGACATGGCAGCCATTGT |
| TNF‐α | 29,693 | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
2.6. ELISA
The IL‐1β (KE10003; Proteintech, Wuhan, China) and IL‐10 concentrations (CSB‐E04594m, CUSABIO, Wuhan, China) were measured using standard ELISA kits. Protein extracted from noninfarcted heart tissue was used for further tests. Data are presented for individual cytokines, as well as for the ratios between the levels of different cytokines.
2.7. Statistical analysis
All data were analysed using GraphPad Prism 9.0 statistical software. Data are presented as the mean ± standard deviation, and comparisons were performed with unpaired Welch's t‐test. A value of p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Acute sleep deprivation promotes a deterioration of cardiac function in mice with myocardial infarction
As shown in Figure 1a, at the beginning of the experiment, the mice in the LAD and LAD+SD groups underwent thoracotomy to ligate the left anterior descending branch, which was confirmed by electrocardiography (Figure 1b), while the mice in the sham and sham+SD groups underwent the same surgery without ligation (Figure 1c). Eight weeks after surgery, the mice in the LAD+SD and sham+SD groups were subjected to sleep deprivation for 1 week. The echocardiogram results showed that after 8 weeks of observation, with relatively equal heart rate levels (Figure 1d, n = 5), mice in the LAD group exhibited a significant decrease in EF and FS compared with the sham group (Figure 1e,f, n = 5, *p < 0.05), with increasing LVIDd and LVIDs (Figure 1g,h, n = 5, *p < 0.05). In addition, 1 week of sleep deprivation caused a significant deterioration of the cardiac function of mice in the LAD+SD group (Figure 2a,b). Mice in the LAD+SD group had an even lower EF and FS (Figure 2d,e, n = 5, *p < 0.05) and a higher LVIDd and LVIDs than mice in the LAD group (Figure 2f,g, n = 5, *p < 0.05). However, sleep deprivation had no effect on the cardiac systolic function mice in the sham group (Figure S1B–E, n = 5).
FIGURE 1.
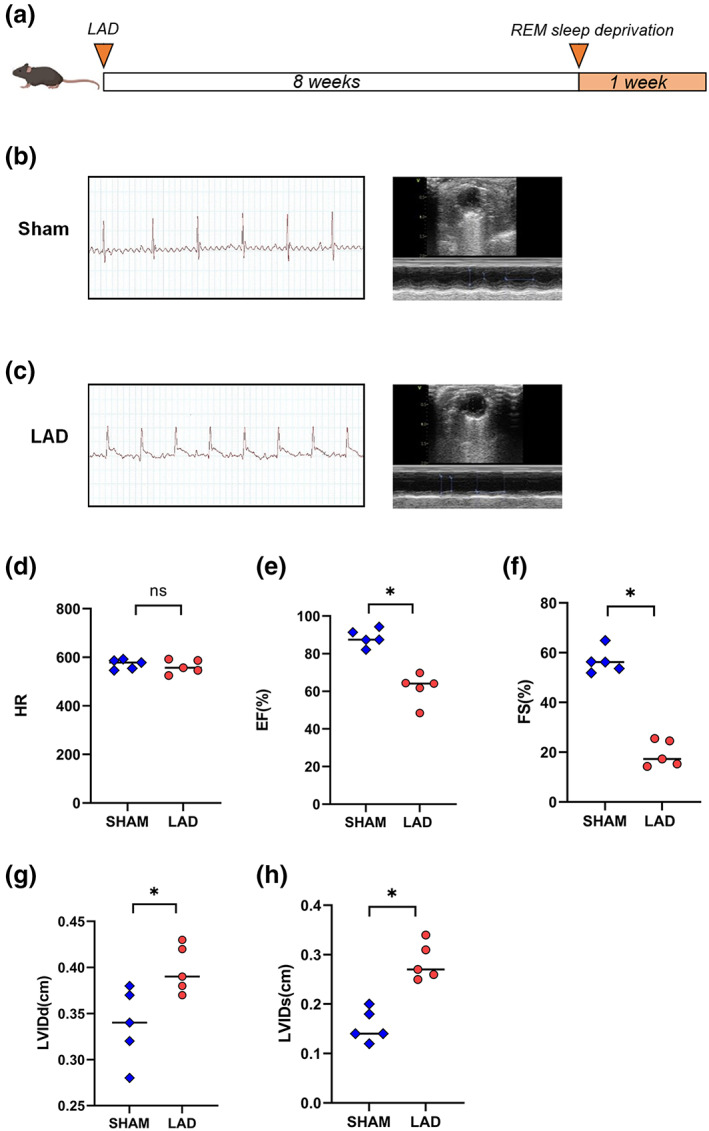
Confirmation of the LAD model and examination of left ventricular function in mice. (a) Schematic of the timeline for the animal experiment in which mice were subjected to sleep deprivation 8 weeks after LAD model establishment. (b) Electrocardiogram of control mice without ST segment elevation. (c) Electrocardiogram of LAD model mice with ST segment elevation. (d) The comparison of the HRs of mice in the LAD and sham groups is shown. (e) The comparison of the EF of mice in the LAD and sham groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (f) The comparison of the FS in mice from the LAD and sham groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (g) The comparison of the LVIDd of mice in the LAD and sham groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (h) The comparison of the LVIDs of mice in the LAD and sham groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05)
FIGURE 2.
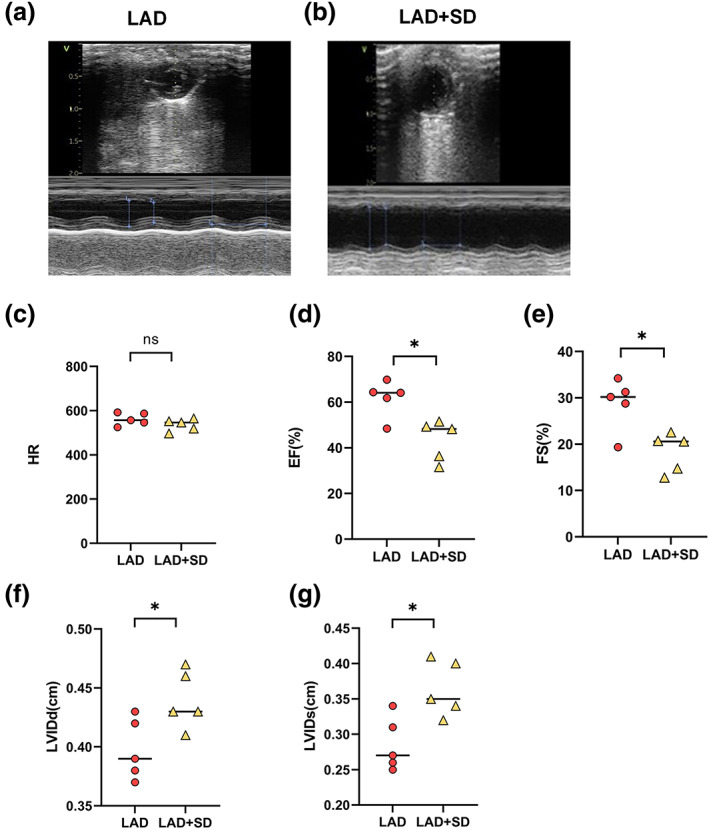
Sleep deprivation deteriorated the left ventricular function of LAD mice. (a,b) Echocardiography results from mice in the LAD and LAD+SD groups. (c) The comparison of the HRs of mice in the LAD and LAD+SD groups is shown. (d) The comparison of the EF of mice in the LAD and LAD+SD groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (e) The comparison of FS in mice from the LAD and LAD+SD groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (f) The comparison of the LVIDd of the LAD and LAD+SD groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (g) The comparison of the LVIDs in mice from the LAD and LAD+SD groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05)
3.2. Acute sleep deprivation increased inflammation in the post ischaemic heart tissue
After the acquisition of echocardiogram data, the left ventricular tissue was harvested for further detection. To explore the cause of the decline, we examined the expression of inflammatory factors in the mouse heart tissues to explore the cause of the decrease in the cardiac function of LAD mice induced by sleep deprivation. Using quantitative PCR, we found that acute sleep deprivation significantly increased IL‐1β, IL‐10, and IL‐18 levels in noninfarcted myocardium (Figure 3a,d,e, n = 5, *p < 0.05). The ELISA results further confirmed the trends of increased IL‐1β and IL‐10 expression in the noninfarcted myocardium of mice in the LAD+SD group compared with the LAD group (Figure 3h,i, n = 5, *p < 0.05). However, in comparison with the Sham group and the Sham+SD group, the expression of inflammatory factors in heart tissues was not significantly affected by sleep deprivation (Figure S1F–N, n = 5).
FIGURE 3.
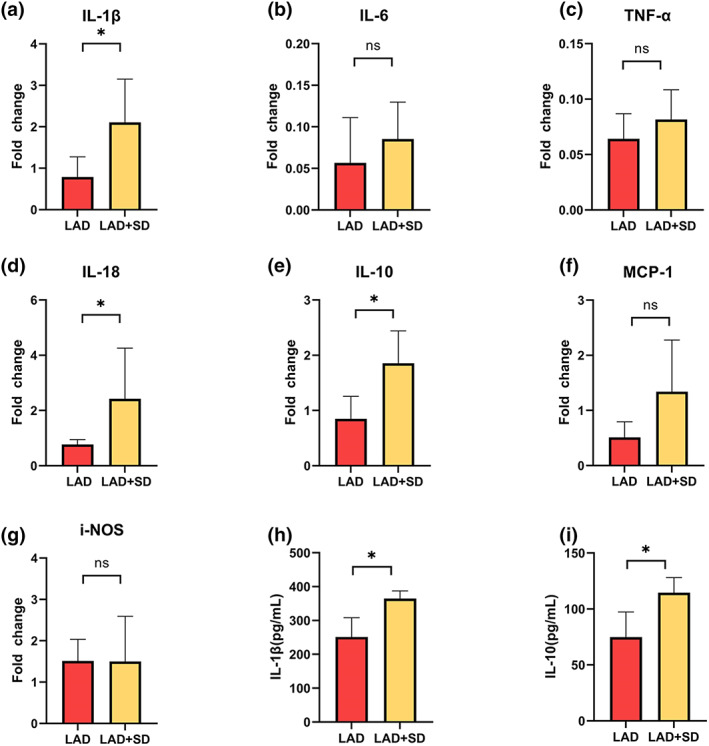
Changes in the levels of inflammatory factors in left ventricular tissue were analysed using qPCR and ELISA. (a) The comparison of IL‐1β mRNA levels in the left ventricles of mice in the LAD and LAD+SD groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (b) The comparison of IL‐6 mRNA levels in the left ventricles of mice in the LAD and LAD+SD groups is shown. (c) The comparison of TNF‐α mRNA levels in the left ventricles of mice in the LAD and LAD+SD groups is shown. (d) The comparison of IL‐18 mRNA levels in the left ventricles of mice in the LAD and LAD+SD groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (e) The comparison of IL‐10 mRNA levels in the left ventricles of mice in the LAD and LAD+SD groups is shown. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (f) The comparison of MCP‐1 mRNA levels in the left ventricles of mice in the LAD and LAD+SD groups is shown. (g) The comparison of iNOS mRNA levels in the left ventricles of mice in the LAD and LAD+SD groups is shown. (h) ELISA revealed increased levels of IL‐1β in the left ventricles of mice in the LAD+SD group. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05). (i) ELISA revealed increased levels of IL‐10 in the left ventricles of mice in the LAD+SD group. The significance of differences between groups was determined using Welch's t‐test (n = 5, *p < 0.05)
4. DISCUSSION
Although sleep disorders are considerably associated with arrhythmia and cardiovascular disease, the effect of acute sleep deprivation on myocardial infarction has received little attention. For the first time, our trial provides experimental data showing that acute sleep deprivation for 7 consecutive days aggravated the left ventricular dysfunction after myocardial infarction.
A study confirmed that the concentrations of corticosterone, cytokines (IL‐6, TNF, and IL‐10) and miRNAs (miR‐146a, miR‐155, miR‐223, miR‐16, miR‐126, and miR‐21) in serum were increased after 192 h of paradoxical sleep deprivation (Brianza‐Padilla et al., 2018). Another trial showed that sleep disruption participated in cardiac remodelling 24 h after myocardial infarction, accompanied by increased levels of NO, oxidative stress, and echocardiographic indices (p < 0.05) (Aghajani et al., 2017). Mice in the LAD+SD group exhibited a lower EF and FS and a higher expression of inflammatory cytokines (IL‐1β, IL‐18, and IL‐10). Increased levels of inflammatory cytokines correlate with a worse prognosis of heart failure (Adamo et al., 2020), and researchers have proposed that inflammation may promote disease development in patients with heart failure. Proinflammatory cytokines such as IL‐1β and IL‐18 exert adverse effects on cardiac remodeling, cytokine‐mediated systolic dysfunction, and heart failure (Gao et al., 2019; Xiao et al., 2018). IL‐10 and other inflammatory cytokines are also activated by cardiac injury, promoting the development of myocardial remodelling and fibrosis (Mahmoudi et al., 2019). In addition, the CANTOS trial showed that treatment with canakinumab (an IL‐1β antagonist) resulted in a dose‐dependent reduction in the rate of hospitalisations for heart failure, hospitalisation or heart failure‐related death (Everett et al., 2019). Therefore, we speculate that inflammatory inhibitors may improve the prognosis of patients with cardiac infarction after SD.
Sleep deprivation, an independent predictor of stroke, chronic heart failure, and cardiovascular and cerebrovascular diseases, increases the incidence of ventricular fibrillation and ventricular arrhythmias (Bhatwadekar et al., 2017). In the study by Wright, circadian misalignment significantly increased plasma tumor necrosis factor‐alpha (TNF‐α), interleukin 10 (IL‐10), and C‐reactive protein (CRP) levels (p < 0.05) (Wright et al., 2015). An increase in the levels of IL‐10 after long‐term sleep deprivation (18 h/day for 21 days) has also been reported previously (Venancio & Suchecki, 2015). Our trials indicated that IL‐1β, IL‐10, and IL‐18 levels in noninfarcted hearts were increased in the LAD+SD compared with the LAD groups. Markedly upregulated levels of the cytokines IL‐18, IL‐1β, and IL‐10, together with lower EF and FS, were observed in the LAD+SD group, indicating that REM sleep deprivation may affect the outcomes of myocardial infarction by upregulating IL‐1β and IL‐18.
The quality and length of sleep are vital for maintaining normal cardiovascular function (Venancio & Suchecki, 2015). Sleep deprivation alters the epigenetic and transcriptional characteristics of CLOCK genes, including Bmal‐1, CLOCK, cry‐1, and per‐171(Moreno‐Villanueva et al., 2018). Overexpression or restriction of sleep epigones may alter the progression of heart failure (Duong et al., 2019; Qin & Deng, 2015). Further studies exploring whether improving the sleep of patients with heart failure improves the prognosis would be worthwhile.
Our trials have some limitations. First, we did not explore the pathogenic mechanism by which sleep deprivation aggravates left ventricular dysfunction after myocardial infarction. Second, our study was performed exclusively in male animals. Some sex differences have been reported in rodent heart failure models (Regitz‐Zagrosek & Seeland, 2011), and in the early stages of myocardial infarction, sleep deprivation could increase the extent of ischaemia‐induced injury in a sex‐dependent manner (Zoladz et al., 2016). More notably, a cohort study also showed that there were gender differences in the association between insomnia and heart failure (Laugsand et al., 2014). Therefore, future studies are needed to explore the interaction between sleep deprivation and heart failure in female mice and to elucidate its mechanism.
In conclusion, sleep deprivation increased inflammation and exerted deleterious effects on the heart structure and function of mice with heart failure that were detectable using echocardiography.
AUTHOR CONTRIBUTION
Yumin Zhu was responsible for the study conception and execution.
Xian Chen, Lu Wang, Na Chen, and Yujie Xiao were responsible for animal experiment, data analysis, and interpretation.
Lizhe Guo was responsible for the study design and acquisition of data.
E. Wang was responsible for the study design and manuscript production.
FUNDING INFORMATION
This study was funded by the National Key Research and Development Programme of China (No: 2020YFC2005300).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Appendix S1 Supplementary Figure
ACKNOWLEDGMENTS
We are very grateful for all the anaesthetists who participated in the study.
Zhu, Y. , Chen, X. , Guo, L. , Wang, L. , Chen, N. , Xiao, Y. , & Wang, E. (2022). Acute sleep deprivation increases inflammation and aggravates heart failure after myocardial infarction. Journal of Sleep Research, 31(6), e13679. 10.1111/jsr.13679
Funding information National Key Research and Development Program of China, Grant/Award Number: 2020YFC2005300
DATA AVAILABILITY STATEMENT
Data available on request from the authors
REFERENCES
- Adamo, L. , Rocha‐Resende, C. , Prabhu, S. D. , & Mann, D. L. (2020). Reappraising the role of inflammation in heart failure. Nature Reviews. Cardiology, 17(5), 269–285. [DOI] [PubMed] [Google Scholar]
- Aghajani, M. , Faghihi, M. , Imani, A. , Vaez Mahdavi, M. R. , Shakoori, A. , Rastegar, T. , Parsa, H. , Mehrabi, S. , Moradi, F. , & Kazemi Moghaddam, E. (2017). Post‐infarct sleep disruption and its relation to cardiac remodeling in a rat model of myocardial infarction. Chronobiology International, 34(5), 587–600. [DOI] [PubMed] [Google Scholar]
- Bhatwadekar, A. D. , Beli, E. , Diao, Y. , Chen, J. , Luo, Q. , Alex, A. , Caballero, S. , Dominguez, J. M., II , Salazar, T. E. , Busik, J. V. , Segal, M. S. , & Grant, M. B. (2017). Conditional deletion of Bmal1 accentuates microvascular and macrovascular injury. The American Journal of Pathology, 187(6), 1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brianza‐Padilla, M. , Sánchez‐Muñoz, F. , Vázquez‐Palacios, G. , Huang, F. , Almanza‐Pérez, J. C. , Bojalil, R. , & Bonilla‐Jaime, H. (2018). Cytokine and microRNA levels during different periods of paradoxical sleep deprivation and sleep recovery in rats. PeerJ, 6, e5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coniglio, A. C. , & Mentz, R. J. (2020). Sleep breathing disorders in heart failure. Heart Failure Clinics, 16(1), 45–51. [DOI] [PubMed] [Google Scholar]
- Duong, A. T. H. , Reitz, C. J. , Louth, E. L. , Creighton, S. D. , Rasouli, M. , Zwaiman, A. , Kroetsch, J. T. , Bolz, S. S. , Winters, B. D. , Bailey, C. D. C. , & Martino, T. A. (2019). The clock mechanism influences neurobiology and adaptations to heart failure in clock (∆19/∆19) mice with implications for circadian medicine. Scientific Reports, 9(1), 4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett, B. M. , Cornel, J. H. , Lainscak, M. , Anker, S. D. , Abbate, A. , Thuren, T. , Libby, P. , Glynn, R. J. , & Ridker, P. M. (2019). Anti‐inflammatory therapy with Canakinumab for the prevention of hospitalization for heart failure. Circulation, 139(10), 1289–1299. [DOI] [PubMed] [Google Scholar]
- Gao, R. , Shi, H. , Chang, S. , Gao, Y. , Li, X. , Lv, C. , Yang, H. , Xiang, H. , Yang, J. , Xu, L. , & Tang, Y. (2019). The selective NLRP3‐inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. International Immunopharmacology, 74, 105575. [DOI] [PubMed] [Google Scholar]
- Giampá, S. Q. , Mônico‐Neto, M. , de Mello, M. T. , Souza, H. S. , Tufik, S. , Lee, K. S. , et al. (2016). Paradoxical sleep deprivation causes cardiac dysfunction and the impairment is attenuated by resistance training. PLoS One, 11(11), e0167029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick, T. , Chung, M. K. , Pieper, S. J. , Lange, L. G. , & Schreiner, G. F. (1989). Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta‐adrenergic responsiveness. Proceedings of the National Academy of Sciences of the United States of America, 86, 6753–6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna, A. , & Frangogiannis, N. G. (2020). Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovascular Drugs and Therapy, 34(6), 849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck, L. , Dadson, K. , Chauhan, S. , Grothe, D. , & Billia, F. (2021). Inhibiting the Pkm2/b‐catenin axis drives in vivo replication of adult cardiomyocytes following experimental MI. Cell Death and Differentiation, 28(4), 1398–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsmans, M. , Sager, H. B. , Roh, J. D. , Valero‐Muñoz, M. , Houstis, N. E. , Iwamoto, Y. , Sun, Y. , Wilson, R. M. , Wojtkiewicz, G. , Tricot, B. , Osborne, M. T. , Hung, J. , Vinegoni, C. , Naxerova, K. , Sosnovik, D. E. , Zile, M. R. , Bradshaw, A. D. , Liao, R. , Tawakol, A. , … Nahrendorf, M. (2018). Cardiac macrophages promote diastolic dysfunction. The Journal of Experimental Medicine, 215(2), 423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. , Thota, V. , Dee, L. , Olson, J. , Uretz, E. , & Parrillo, J. E. (1996). Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. The Journal of Experimental Medicine, 183, 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugsand, L. E. , Strand, L. B. , Platou, C. , Vatten, L. J. , & Janszky, I. (2014). Insomnia and the risk of incident heart failure: A population study. European Heart Journal, 35(21), 1382–1393. [DOI] [PubMed] [Google Scholar]
- Mahmoudi, M. J. , Hedayat, M. , Taghvaei, M. , Harsini, S. , Nematipour, E. , Rezaei, N. , et al. (2019). Interleukin‐10 and transforming growth factor Beta1 gene polymorphisms in chronic heart failure. Acta Bio‐Medica, 90(2), 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metra, M. , & Teerlink, J. R. (2017). Heart failure. Lancet, 390(10106), 1981–1995. [DOI] [PubMed] [Google Scholar]
- Moreno‐Villanueva, M. , von Scheven, G. , Feiveson, A. , Bürkle, A. , Wu, H. , & Goel, N. (2018). The degree of radiation‐induced DNA strand breaks is altered by acute sleep deprivation and psychological stress and is associated with cognitive performance in humans. Sleep, 41(7). [DOI] [PubMed] [Google Scholar]
- O'Brien, L. C. , Mezzaroma, E. , Van Tassell, B. W. , et al. (2014). Interleukin‐18 as a therapeutic target in acute myocardial infarction and heart failure. Molecular Medicine, 20(1), 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek, A. , Du, W. , de Boer, R. A. , & Silljé, H. H. W. (2018). Novel heart failure biomarkers: Why do we fail to exploit their potential? Critical Reviews in Clinical Laboratory Sciences, 55(4), 246–263. [DOI] [PubMed] [Google Scholar]
- Qin, B. , & Deng, Y. (2015). Overexpression of circadian clock protein cryptochrome (CRY) 1 alleviates sleep deprivation‐induced vascular inflammation in a mouse model. Immunology Letters, 163(1), 76–83. [DOI] [PubMed] [Google Scholar]
- Regitz‐Zagrosek, V. , & Seeland, U. (2011). Sex and gender differences in myocardial hypertrophy and heart failure. Wiener Medizinische Wochenschrift (1946), 161(5–6), 109–116. [DOI] [PubMed] [Google Scholar]
- Venancio, D. P. , & Suchecki, D. (2015). Prolonged REM sleep restriction induces metabolic syndrome‐related changes: Mediation by pro‐inflammatory cytokines. Brain, Behavior, and Immunity, 47, 109–117. [DOI] [PubMed] [Google Scholar]
- Wright, K. P., Jr. , Drake, A. L. , Frey, D. J. , Fleshner, M. , Desouza, C. A. , Gronfier, C. , & Czeisler, C. A. (2015). Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain, Behavior, and Immunity, 47, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, H. , Li, H. , Wang, J. J. , Zhang, J. S. , Shen, J. , An, X. B. , Zhang, C. C. , Wu, J. M. , Song, Y. , Wang, X. Y. , Yu, H. Y. , Deng, X. N. , Li, Z. J. , Xu, M. , Lu, Z. Z. , du, J. , Gao, W. , Zhang, A. H. , Feng, Y. , & Zhang, Y. Y. (2018). IL‐18 cleavage triggers cardiac inflammation and fibrosis upon β‐adrenergic insult. European Heart Journal, 39(1), 60–69. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Zheng, C. , Gao, Z. , Wang, L. , Chen, C. , Zheng, Y. , & Meng, Y. (2021). PKM2 promotes angiotensin‐II‐induced cardiac remodelling by activating TGF‐β/Smad2/3 and Jak2/Stat3 pathways through oxidative stress. Journal of Cellular and Molecular Medicine, 25(22), 10711–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz, P. R. , Krivenko, A. , Eisenmann, E. D. , Bui, A. D. , Seeley, S. L. , Fry, M. E. , Johnson, B. L. , & Rorabaugh, B. R. (2016). Sex‐dependent effects of sleep deprivation on myocardial sensitivity to ischemic injury. Stress, 19(2), 264–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Figure
Data Availability Statement
Data available on request from the authors


