Abstract
Immunity to Plasmodium falciparum develops slowly in areas of endemicity, and this is often ascribed to poorly immunogenic or highly variant parasite antigens. However, among populations newly exposed to malaria, adults acquire immunity more rapidly than children. We examined the relationship between pubertal development and resistance to P. falciparum. During two transmission seasons in western Kenya, we treated the same cohort of young males to eradicate P. falciparum and then obtained blood smears each week for 4 months. We determined pubertal development by Tanner staging and by levels of dehydroepiandrosterone sulfate (DHEAS) and testosterone in plasma. In multivariate and age-stratified analyses, we examined the effect of pubertal development on resistance to malaria. In both seasons (n = 248 and 144 volunteers, respectively), older males were less susceptible than younger males. Age-related decreases in the frequency and density of parasitemia were greatest during puberty (15- to 20-year-olds). DHEAS and testosterone were significant independent predictors of resistance to P. falciparum parasitemia, even after accounting for the effect of age. Fifteen- to 20-year-old males with high DHEAS levels had a 72% lower mean parasite density (P < 0.01) than individuals with low DHEAS levels. Similarly, 21- to 35-year-old males with high DHEAS levels had a 92% lower mean parasite density (P < 0.001) and 48% lower frequency of parasitemia (P < 0.05) than individuals with low DHEAS levels. These data suggest that the long period needed to attain full immunity could be explained as a consequence of host development rather than as the requirement to recognize variant or poorly immunogenic parasite antigens.
Plasmodium falciparum malaria is a leading cause of morbidity and mortality in developing countries, infecting hundreds of millions of individuals and killing up to 1 million children in sub-Saharan Africa each year (2). This death toll will rise as drug-resistant parasites spread (35), and meanwhile the promise of a broadly effective malaria vaccine remains unfulfilled despite important technological advances (27). Residents of areas of endemicity develop protective immunity that limits parasitemia and disease, providing a model for vaccine development, but the responses conferring naturally occurring protection have not been elucidated.
P. falciparum infection is more frequent and severe in children than in adults (23; reviewed in reference 4), and resistance is acquired over years of exposure. The long period required to develop resistance has supported the widely held views that the parasite is poorly immunogenic or that protective immunity is strain specific and requires exposure to the many parasite variants circulating in a community (10, 14). The recent observation that adult migrants to an area of endemicity acquire resistance more rapidly than their younger counterparts implicates host development in decreased susceptibility to P. falciparum (5, 7). In an area of holoendemicity in Kenya, where malaria is ubiquitous, we examined pubertal hormones as predictors of immunity to P. falciparum parasitemia. We show for the first time that pubertal hormone levels are directly related to increased resistance to falciparum malaria in humans, and we conclude that present paradigms explaining malarial immunity are inadequate to account for the epidemiology of infection.
MATERIALS AND METHODS
Study population.
The study site in western Kenya was 10 km north of Lake Victoria, in the adjoining villages of Wangarot, Riwa Ojelo, and Waringa, Rarieda Division, Nyanza Province. The entomological inoculation rate in this area can exceed 300 infectious bites per person per year (8). This study was conducted according to a protocol approved by ethical review boards of both the Walter Reed Army Institute of Research and the Kenya Medical Research Institute. All volunteers gave signed informed consent prior to entry into the study.
After the exclusion of individuals with abnormal hemograms or evidence of chronic disease on physical examination, 248 males aged 12 to 35 years entered the study at the beginning of the high-transmission season in April 1996. To initiate the study, volunteers were simultaneously treated for 3 days with quinine sulfate (10 mg/kg twice daily) and for 7 days with doxycycline (100 mg twice daily) to eradicate current malaria infections. Five volunteers were removed from first-season analysis because they did not complete the eradication treatment; the remaining 243 volunteers became aparasitemic during the week following treatment with quinine and doxycycline.
Thick and thin blood smears were obtained from each volunteer prior to initial treatment with quinine and doxycycline and then weekly for 16 weeks after initial treatment. Each smear was interpreted by two microscopists, and the mean of the two values was used to quantify parasitemia. Posttreatment blood smears were used to calculate malaria infection end points for the season. These end points included time to reappearance of parasitemia, mean parasitemia on all blood smears taken during the season, and frequency of parasitemia. Analyses using mean parasitemia of only positive blood smears produced results similar to those using mean parasitemia of all blood smears, and therefore only the latter results are reported.
To minimize unreported antimalarial use, trained field workers visited volunteers each day to assess their well-being. Sick volunteers were transported to the study clinic for a complete physical exam. Volunteers with positive blood films and clinical syndromes suggestive of acute malaria were treated with three tablets of Fansidar (Hoffman-LaRoche).
In April 1997, 144 of the 248 volunteers who entered the first-season study were reenrolled at the start of the subsequent high-transmission season, again treated with quinine and doxycycline, and then monitored for 18 weeks in the same manner as for the first season. One volunteer was removed from second-season analysis because his parasitemia persisted during the week following treatment with quinine and doxycycline. Immunologic studies with these volunteers have been reported elsewhere (25).
Entomology.
At the end of the first season, mosquitoes were collected by the daytime resting indoors method (19). This procedure was performed once at each volunteer's house between 22 July and 1 August 1996. The number of anopheline mosquitoes in each house was used to control for recent exposure in subsequent analyses.
Blood collection and processing.
In the second season, volunteers donated 10 ml of blood into heparinized tubes 2 weeks after treatment with quinine and doxycycline. Samples were centrifuged within 4 h of collection, and plasma was aliquoted and stored at −70°C for subsequent hormonal analyses.
Clinical laboratory tests.
Hemograms were obtained from heparinized blood by using a model T-890 cell counter (Coulter Corp., Hialeah, Fla.). ABO blood group and hemoglobin phenotype were determined for 154 volunteers with commercially available reagents (Sigma, St. Louis, Mo.).
Hormonal assays.
Quantitative assays for total plasma testosterone levels (Immuno-1; Bayer, Tarrytown, N.Y.) (normal range for 20- to 49-year-old males in the United States, 2.7 to 11.94 ng/ml) and dehydroepiandrosterone sulfate (DHEAS) levels (Immunolite; DPC, Los Angeles, Calif.) (normal range for 12- to 17-year-old males in the United States, 30 to 550 μg/dl; normal range for 18- to 29-year-old males in the United States, 280 to 640 μg/dl) were performed at a College of American Pathologists accredited clinical laboratory using automated enzyme immunoassay-based techniques.
Tanner staging.
Tanner staging was performed for 141 volunteers in the second season by a single physician (J.D.K.) according to standard techniques (32). Briefly, testicular size and scrotal-penile development were scored on an ordinal scale from 1 (prepubescent) to 5 (adult). Pubic hair quantity and distribution were also scored on an ordinal scale from 1 to 6. The results of these two scores were averaged to produce the Tanner stage.
Statistical analyses.
We examined relationships among age, puberty, and parasitemia. Measures of parasitemia included time to reappearance of parasitemia, frequency of parasitemia, and mean parasitemia. Data for time to reappearance of parasitemia were examined with Kaplan-Meier models for nominal covariates (group differences were evaluated with a log rank test) and Cox proportional hazards models for continuous covariates. The density and frequency of parasitemia were loge transformed [ln(value + 1)] to normalize the data; the logarithmic mean was obtained by taking the antiloge of the mean of transformed data. Mean parasitemia and frequency of parasitemia were evaluated with Pearson's correlation analysis. Potential confounding by ABO blood group, hemoglobin phenotype, and recent exposure was explored with analysis of covariance and multivariate linear regression where appropriate. Stratified analyses were performed to examine the association between age and parasitemia in several age strata. The relationship between pretreatment prevalence and age was examined with Student's two-tailed t test.
Multivariate linear regression was used to evaluate the association between measures of puberty and parasitemia after accounting for the effects of age. Stratified analyses were performed to further explore the associations among pubertal hormone levels, age, and parasitemia. Within each age strata, differences in parasitemia between hormone strata were evaluated by analysis of variance with Fisher's protected least-significant difference (PLSD) for post-hoc contrasts. DHEAS and testosterone levels were stratified into high, medium, and low strata based on the means and standard deviations of the values for adults in the cohort (ages 21 to 35).
End points of malaria infection were calculated using blood smears obtained before the first treatment with Fansidar. All analyses were performed with StatView version 5.0.1 on Macintosh computers.
RESULTS
Parasitemia reappeared in most volunteers after eradication treatment.
At the start of the rainy season in 1996 and again in 1997, the same cohort of young Kenyan males were treated to eradicate parasitemia and then monitored with weekly blood smears to quantify frequency and density of parasitemia. In the first season, 48% of the volunteers had P. falciparum present on the blood smear prior to eradication treatment. Among the 243 volunteers included for analysis, parasitemia reappeared in 50% within 5 weeks (Kaplan-Meier estimate) and reappeared in a total of 223 within 16 weeks.
In the second season, 53% of the volunteers had P. falciparum present on the blood smear prior to eradication treatment. Among the 143 volunteers included for analysis, parasitemia reappeared in 50% within 6 weeks (Kaplan-Meier estimate) and reappeared in a total of 135 within 18 weeks.
Increasing age predicts resistance to P. falciparum.
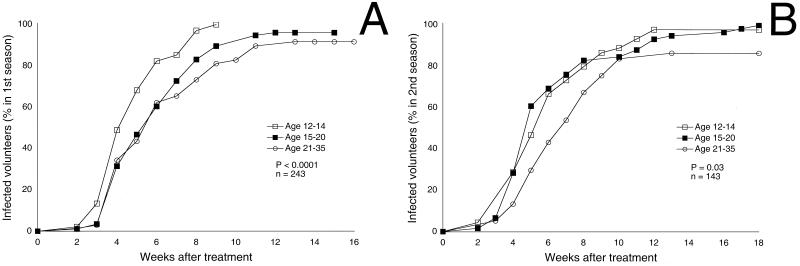
At the start of the first season, individuals with negative pretreatment blood smears were 3.64 years older than individuals with positive pretreatment blood smears (P < 0.0001). After malaria eradication, older individuals were more resistant to P. falciparum than younger individuals as assessed by Pearson's analysis of age versus mean parasitemia (r = −0.195; P < 0.005) and frequency of parasitemia (r = −0.298; P < 0.001) and Kaplan-Meier analysis of time to reappearance of parasitemia (P < 0.0001) (Fig. 1A). In multivariate analyses, older individuals were more resistant to parasitemia by all three measures than younger individuals after accounting for ABO blood type, hemoglobin phenotype, or recent exposure measured by the daytime resting indoors method (all P < 0.01).
FIG. 1.
Increasing age is associated with longer time to reappearance of parasitemia. Kaplan-Meier analysis of time to reappearance of parasitemia in the first season (A) and second season (B) is shown.
Similarly, at the start of the second season, individuals with negative pretreatment blood smears were 2.95 years older and 0.7 Tanner stage higher than individuals with positive pretreatment blood smears (P < 0.004 and P < 0.01, respectively). After malaria eradication, resistance to parasitemia increased with age. Older individuals had lower mean parasitemia (r = −0.383; P < 0.001), frequency of parasitemia (r = −0.404; P < 0.001), and longer time to reappearance of parasitemia (P = 0.03) (Fig. 1B) than younger individuals.
Resistance to P. falciparum increases most rapidly during puberty.
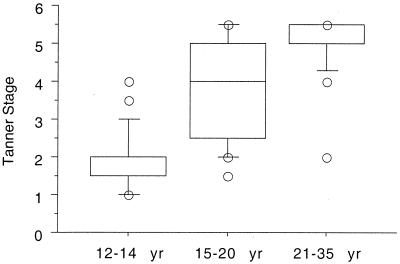
We examined the development of resistance before (12- to 14-year-olds), during (15- to 20-year-olds) and after (21- to 35-year-olds) puberty. These age strata were selected based on the results of Tanner staging in this population (Fig. 2). In both seasons, resistance to P. falciparum increased with age during puberty but not before puberty (Table 1). After puberty, the frequency of parasitemia decreased with age in the second season. Neither time to reappearance of parasitemia nor parasite density was associated with age in this group.
FIG. 2.
Associations between Tanner stage (a physical measure of pubertal development) and age. Puberty occurs at between 15 and 20 years of age in this study group. Box plots indicate medians (center lines), 75th percentiles (boxes), and 90th percentiles (bars). Remaining values are individually plotted as circles.
TABLE 1.
Associations between age and parasitemia split by age strataa
| Age stratum (yr) | Season
1
|
Season 2
|
||||||
|---|---|---|---|---|---|---|---|---|
| n | MPb | FPb | TRc | n | MP | FP | TR | |
| 12–14 | 90 | −0.06 | −0.113 | 0.013 | 45 | 0.06 | −0.07 | 0.05 |
| 15–20 | 86 | −0.231d | −0.361e | −0.233e | 60 | −0.265d | −0.252d | −0.09 |
| 21–35 | 67 | −0.138 | −0.141 | −0.008 | 38 | −0.189 | −0.351d | −0.04 |
n, sample sizes; MP, mean parasitemia; FP, frequency of parasitemia; TR, time to reappearance of parasitemia.
Pearson's correlation coefficient (r) for relationship between age and parasitemia, stratified by age group.
Coefficient for age in Cox proportional hazard model of time to reappearance of parasitemia.
P ≤ 0.05.
P < 0.005.
Physical and hormonal measures of puberty predict increased resistance to P. falciparum.
The mean Tanner stage and DHEAS and testosterone levels increased with age. The physical changes associated with puberty (Tanner stage) occurred principally in the 15- to 20-year-old age group (Fig. 2). All three pubertal measures were strongly correlated with age (r = 0.56 to 0.68; all P < 0.0001) and themselves (r = 0.56 to 0.82; all P < 0.0001).
Increased Tanner stage and DHEAS and testosterone levels, measured at the start of the second season, were each associated with lower mean parasitemia (P < 0.005), lower frequency of parasitemia (P < 0.005), and longer time to reappearance of parasitemia (P < 0.05) (Table 2).
TABLE 2.
Correlation between measures of pubertal development and parasitemiaa
| Pubertal measure (n) | MPb | FPb | TRc |
|---|---|---|---|
| Tanner stage (141) | −0.293e | −0.339e | −0.122d |
| DHEAS (139) | −0.376e | −0.325e | −0.003d |
| Testosterone (140) | −0.383e | −0.363e | −0.072d |
MP, mean parasitemia; FP, frequency of parasitemia; TR, time to reappearance of parasitemia.
Pearson's correlation coefficient (r) for relationship between age and parasitemia.
Coefficient for age in Cox proportional hazard model of time to reappearance of parasitemia.
P < 0.05.
P < 0.005.
Increased DHEAS and testosterone predict resistance to P. falciparum independent of age.
In a multivariate linear model, DHEAS was a significant predictor of mean parasitemia (P = 0.02) even after accounting for the effect of age, hemoglobin phenotype, ABO blood group, and recent exposure. Among volunteers 15 years or older (when the effects of puberty on resistance are apparent), DHEAS was a significant predictor of mean parasitemia (P = 0.02) after accounting for both age and testosterone. Individuals with higher DHEAS levels, independent of their age and testosterone level, had lower mean parasitemias than individuals with lower DHEAS levels. After accounting for age, increased DHEAS was associated with decreased frequency of parasitemia, and this association was significant in the older age groups (P = 0.1 for all volunteers; P = 0.04 for volunteers 15 years and older).
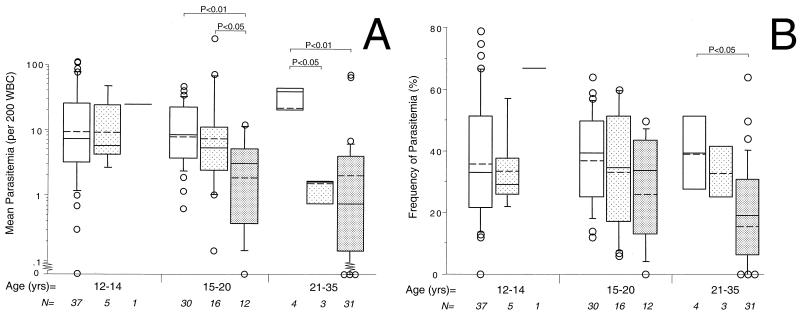
When stratified by age and DHEAS levels, 15- to 20-year-old males with high DHEAS levels had 66 to 72% lower mean parasitemias than individuals with lower levels of DHEAS (P < 0.05 to 0.01) (Fig. 3A). Twenty-one- to 35-year-old men with medium or high DHEAS levels had 92 to 94% lower mean parasitemias than individuals with low DHEAS levels (P < 0.01 to 0.001) (Fig. 3A). Twenty-one- to 35-year-olds with high DHEAS levels had a 52% lower frequency of parasitemia than individuals with low DHEAS levels (P < 0.05) (Fig. 3B). In the two older age groups, individuals with higher DHEAS levels were more resistant to P. falciparum than individuals with lower DHEAS levels.
FIG. 3.
Increasing DHEAS levels are associated with increased resistance to parasitemia in older age groups. Associations during the second season among age, DHEAS, and mean parasitemia (A) or frequency of parasitemia (B) are shown. Groups are stratified by age and DHEAS levels (low [□], intermediate [ ], and high [░⃞]). Box plots indicate logarithmic means (dashed lines), medians (solid lines), 75th percentiles (boxes), and 90th percentiles (bars). Remaining values are individually plotted as circles. Sample sizes are indicated at the bottom.
In a multivariate linear model, testosterone was also a significant predictor of mean parasitemia (P = 0.02) after accounting for age but lost significance after accounting for the effect of DHEAS (P = 0.07 for all volunteers; P = 0.1 for volunteers 15 years and older). Individuals with higher testosterone levels, independent of their age, had lower mean parasitemias than individuals with lower testosterone levels. After accounting for age, increased testosterone was associated with decreased frequency of parasitemia, but this did not achieve statistical significance (P = 0.07). Among 15- to 20-year-olds, individuals with high testosterone levels had a 48 to 55% lower frequency of parasitemia than individuals with medium or low testosterone levels (P < 0.05 and P < 0.005, respectively). No other significant differences were observed when measures of malaria were stratified by age and testosterone level.
DISCUSSION
In areas of endemicity, children are more susceptible to malaria than adults (23). Resistance to parasitemia develops over many years, and the lengthy nature of this process has been attributed to the poor immunogenicity or highly variant nature of parasite antigens (10, 14). Adults acquire resistance to malaria more rapidly than children when nonimmune families move into malarious areas (5, 6), an observation at odds with the notion that parasite immunoevasion alone accounts for the slow acquisition of immunity. Adults and children do not differ in susceptibility at the time of their first exposure to malaria, so innate resistance cannot account for age-related differences. Instead, acquired immunity develops more quickly in adults than in children with similar exposure histories.
In this prospective study of 12- to 35-year-old males, age significantly predicted resistance to malaria. Malaria is ubiquitous in this area and reappeared after eradication in nearly all volunteers during both seasons. However, older individuals were infected less frequently and at lower parasite density than younger individuals. This relationship between increasing age and increasing resistance was most apparent among 15- to 20-year-olds, the age when this population of males undergoes puberty (Fig. 2), and was not apparent among 12- to 14-year olds. In the years just before puberty, therefore, continued exposure to malaria does not result in increased immunity. Resistance increases during puberty, which could result from either additional exposure, developmentally regulated mechanisms of resistance, or the combined influence of both factors.
To evaluate these possibilities, we considered increasing age to be a correlate of increasing cumulative exposure. Resistance increased as the Tanner stage of pubertal development increased, but this effect could not be separated from the effect of age (i.e., increased cumulative exposure). However, increased DHEAS (a measure of adrenarche) and testosterone (a measure of gonadarche) were significant predictors of increasing resistance, even after accounting for age (and, for DHEAS, even after accounting for both age and testosterone). We inferred that human pubertal development contributes to resistance to malaria, separate from any effect of increasing cumulative exposure. In healthy populations, puberty begins at a younger age in children with African as opposed to European ancestry (11, 21, 31), and it is tempting to speculate that this may be due to the selective effect of malaria in human evolution (20). Although our cohort experienced a delayed onset of puberty compared to the standards of the developed world, this is likely a consequence of malnutrition and disease associated with their low socioeconomic status (11, 13).
The increase in resistance during puberty could be mediated by innate or acquired mechanisms. Nonimmune populations require several infections with P. falciparum before adults demonstrate resistance greater than children (5), suggesting an acquired response. Our results parallel these earlier findings: moderate elevations of DHEAS were associated with increased resistance in the oldest, but not the younger, age strata (Fig. 3), implying that exposure subsequent to the elevation of DHEAS augments resistance. Thus, puberty-related resistance in humans may be an acquired form of immunity, distinguishing it from the innate resistance observed in older chicks (17, 24) and rats (18), which are less susceptible than their younger counterparts at first exposure to malaria.
DHEAS predicted a lower frequency and density of parasitemia in multiple comparisons, effects that could be mediated by the potent immunoactivating properties of this hormone. In humans, DHEAS increases specific antibody responses (3, 15) and augments NK cell number and function (12). Both immune mechanisms contribute to resistance to malaria. Human NK cells lyse P. falciparum-infected erythrocytes (30), and increased NK cell cytokine production is associated with decreased parasitemia and mortality in murine Plasmodium chabaudi infection (28). In humans, specific P. falciparum antibodies are associated with resistance to both severe parasitemia and disease (1, 16, 33).
Testosterone levels were associated only with decreased frequency of parasitemia in a single age strata. In multivariate analyses, the effect of testosterone on resistance lost significance after accounting for DHEAS and age. Although testosterone also has immunomodulatory effects (9, 22, 34), earlier studies do not support a role for testosterone in resistance to malaria. Testosterone increases the susceptibility of female mice to fatal P. chabaudi infection (9). In humans, parasite densities are lower in nonpregnant adolescent females than in adolescent males (26, 29). We believe that testosterone is a marker for another puberty-associated change (such as DHEAS) that confers increased resistance to malaria. This possibility should be addressed in studies with sample sizes sufficient to simultaneously evaluate age, DHEAS, and testosterone, as well as parallel studies in females.
In summary, resistance to falciparum malaria increased with age in males during puberty but not in males just before puberty. Pubertal hormones were related to immunity independent of host age and cumulative exposure. Further investigation is needed to define the relative contributions of DHEAS and testosterone in resistance to parasitemia and to elucidate the effects of these hormones on protective antimalarial immune responses. We conclude that resistance to malaria does not result solely from the accumulated recognition of parasite variants or from additional exposure to poorly immunogenic antigens. Host development independently predicts resistance, and an understanding of protective immune responses that are developmentally regulated could lead to new vaccine strategies. Whether susceptibility to malaria is related to development during early life, when mortality is greatest, remains an important area for future study.
ACKNOWLEDGMENTS
This work was supported by the Military Infectious Disease Research Program of the U.S. Department of Defense and by the Department of Pathology and Laboratory Medicine, University of Pennsylvania. J.D.K. was a fellow of the National Research Council and the American Society of Tropical Medicine and Hygiene/Becton-Dickinson.
We gratefully thank Raphael Onyango and Samuel Oduor Wangowe for excellent supervision of the field studies, the volunteers for their participation, Jennifer Friedman and Philip Gruppuso for helpful discussions on pubertal development, Stephen McGarvey and Michal Fried for critical review of the manuscript, and David Goodman for supervising the hormonal assays. This work is published with the permission of the director of the Kenya Medical Research Institute.
REFERENCES
- 1.al-Yaman F, Genton B, Kramer K J, Chang S P, Hui G S, Baisor M, Alpers M P. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparummerozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg. 1996;54:443–448. doi: 10.4269/ajtmh.1996.54.443. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. World malaria situation in 1994. I. Population at risk. Wkly Epidemiol Rec. 1997;72:269–274. [PubMed] [Google Scholar]
- 3.Araneo B, Dowell T, Woods M L, Daynes R, Judd M, Evans T. DHEAS as an effective vaccine adjuvant in elderly humans. Proof-of-principle studies. Ann NY Acad Sci. 1995;774:232–248. doi: 10.1111/j.1749-6632.1995.tb17384.x-i1. [DOI] [PubMed] [Google Scholar]
- 4.Baird J K. Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol. 1998;92:367–390. doi: 10.1080/00034989859366. [DOI] [PubMed] [Google Scholar]
- 5.Baird J K. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 6.Baird J K, Jones T R, Danudirgo E W, Annis B A, Bangs M J, Basri H, Purnomo, Masbar S. Age-dependent acquired protection against Plasmodium falciparumin people having two years exposure to hyperendemic malaria. Am J Trop Med Hyg. 1991;45:65–76. doi: 10.4269/ajtmh.1991.45.65. [DOI] [PubMed] [Google Scholar]
- 7.Baird J K, Purnomo, Basri H, Bangs M J, Andersen E M, Jones T R, Masbar S, Harjosuwarno S, Subianto B, Arbani P R. Age-specific prevalence of Plasmodium falciparumamong six populations with limited histories of exposure to endemic malaria. Am J Trop Med Hyg. 1993;49:707–719. doi: 10.4269/ajtmh.1993.49.707. [DOI] [PubMed] [Google Scholar]
- 8.Beier J C, Oster C N, Onyango F K, Bales J D, Sherwood J A, Perkins P V, Chumo D K, Koech D V, Whitmire R E, Roberts C R, Diggs C L, Hoffman S L. Plasmodium falciparumincidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am J Trop Med Hyg. 1994;50:529–536. doi: 10.4269/ajtmh.1994.50.529. [DOI] [PubMed] [Google Scholar]
- 9.Benten W P, Ulrich P, Kuhn-Velten W N, Vohr H W, Wunderlich F. Testosterone-induced susceptibility to Plasmodium chabaudimalaria: persistence after withdrawal of testosterone. J Endocrinol. 1997;153:275–281. doi: 10.1677/joe.0.1530275. [DOI] [PubMed] [Google Scholar]
- 10.Brown K. Protective immunity to malaria provides a model for the survival of cells in an immunologically hostile environment. Nature. 1971;230:163–167. [Google Scholar]
- 11.Cameron N, Grieve C A, Kruger A, Leschner K F. Secondary sexual development in rural and urban South African black children. Ann Hum Biol. 1993;20:583–593. doi: 10.1080/03014469300002992. [DOI] [PubMed] [Google Scholar]
- 12.Casson P R, Andersen R N, Herrod H G, Stentz F B, Straughn A B, Abraham G E, Buster J E. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. Am J Obstet Gynecol. 1993;169:1536–1539. doi: 10.1016/0002-9378(93)90431-h. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury S, Shahabuddin A K, Seal A J, Talukder K K, Hassan Q, Begum R A, Rahman Q, Tomkins A, Costello A, Talukder M Q. Nutritional status and age at menarche in a rural area of Bangladesh. Ann Hum Biol. 2000;27:249–256. doi: 10.1080/030144600282136. [DOI] [PubMed] [Google Scholar]
- 14.Day K P, Marsh K. Naturally acquired immunity to Plasmodium falciparum. Immunol Today. 1991;12:A68–A71. doi: 10.1016/s0167-5699(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 15.Degelau J, Guay D, Hallgren H. The effect of DHEAS on influenza vaccination in aging adults. J Am Geriatr Soc. 1997;45:747–751. doi: 10.1111/j.1532-5415.1997.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 16.Egan A F, Morris J, Barnish G, Allen S, Greenwood B M, Kaslow D C, Holder A A, Riley E M. Clinical immunity to Plasmodium falciparummalaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 17.Garnham P C C. An introduction to protozoal immunity. In: Garnham P C C, Pierce A E, Roitt I, editors. Immunity to protozoa. F. A. Philadelphia, Pa: Davis Company; 1963. pp. 3–21. [Google Scholar]
- 18.Gravely S M, Hamburger J, Kreier J P. T and B cell population changes in young and in adult rats infected with Plasmodium berghei. Infect Immun. 1976;14:178–183. doi: 10.1128/iai.14.1.178-183.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunasekaran K, Jambulingam P, Sadanandane C, Sahu S S, Das P K. Reliability of light trap sampling for Anopheles fluviatilis, a vector of malaria. Acta Trop. 1994;58:1–11. doi: 10.1016/0001-706x(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 20.Haldane J B S. The rate of mutation of human genes. Proceedings of the 8th International Congress of Genetics. Hereditas. 1949;35(Suppl.):267–273. [Google Scholar]
- 21.Herman-Giddens M E, Slora E J, Wasserman R C, Bourdony C J, Bhapkar M V, Koch G G, Hasemeier C M. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 22.Kanda N, Tsuchida T, Tamaki K. Testosterone suppresses anti-DNA antibody production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:1703–1711. doi: 10.1002/art.1780400921. [DOI] [PubMed] [Google Scholar]
- 23.Koch R. Zweiter bericht über die thätigkeit der malaria-expedition. Dtsch Med Wochenschr. 1900;26:88–90. [Google Scholar]
- 24.Krettli A, Laurente K, Daher V, da Rocha E. Immunogenicity and infectivity of mature and immature Plasmodium gallinaceumsporozoites. Mem Inst Oswaldo Cruz. 1986;81(Suppl. II):115–122. [Google Scholar]
- 25.Kurtis J D, Lanar D E, Opollo M, Duffy P E. Interleukin-10 responses to liver-stage antigen 1 predict human resistance to Plasmodium falciparum. Infect Immun. 1999;67:3424–3429. doi: 10.1128/iai.67.7.3424-3429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landgraf B, Kollaritsch H, Wiedermann G, Wernsdorfer W H. Parasite density of Plasmodium falciparum malaria in Ghanaian schoolchildren: evidence for influence of sex hormones? Trans R Soc Trop Med Hyg. 1994;88:73–74. doi: 10.1016/0035-9203(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 27.Miller L H, Hoffman S L. Research toward vaccines against malaria. Nat Med. 1998;4:520–524. doi: 10.1038/nm0598supp-520. [DOI] [PubMed] [Google Scholar]
- 28.Mohan K, Moulin P, Stevenson M M. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudiAS infection. J Immunol. 1997;159:4990–4998. [PubMed] [Google Scholar]
- 29.Molineaux L, Gramiccia G. The Garki project: research on the epidemiology and control of malaria in the Sudan Savannah of West Africa. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 30.Orago A S, Facer C A. Cytotoxicity of human natural killer (NK) cell subsets for Plasmodium falciparumerythrocytic schizonts: stimulation by cytokines and inhibition by neomycin. Clin Exp Immunol. 1991;86:22–29. doi: 10.1111/j.1365-2249.1991.tb05768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards R J, Svec F, Bao W, Srinivasan S R, Berenson G S. Steroid hormones during puberty: racial (black-white) differences in androstenedione and estradiol—the Bogalusa Heart Study. J Clin Endocrinol Metab. 1992;75:624–631. doi: 10.1210/jcem.75.2.1639961. [DOI] [PubMed] [Google Scholar]
- 32.Rudolph A M, Kamei R K, Sagan P. Rudolph's fundamentals of pediatrics. Stamford, Conn: Appleton & Lange; 1998. [Google Scholar]
- 33.Sarthou J L, Angel G, Aribot G, Rogier C, Dieye A, Toure B A, Diatta B, Seignot P, Roussilhon C. Prognostic value of anti-Plasmodium falciparum-specific immunoglobulin G3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect Immun. 1997;65:3271–3276. doi: 10.1128/iai.65.8.3271-3276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Campbell H D, Young I G. Sex hormones and dexamethasone modulate interleukin-5 gene expression in T lymphocytes. J Steroid Biochem Mol Biol. 1993;44:203–210. doi: 10.1016/0960-0760(93)90080-g. [DOI] [PubMed] [Google Scholar]
- 35.White N J, Nosten F, Looareesuwan S, Watkins W M, Marsh K, Snow R W, Kokwaro G, Ouma J, Hien T T, Molyneux M E, Taylor T E, Newbold C I, Ruebush T K, 2nd, Danis M, Greenwood B M, Anderson R M, Olliaro P. Averting a malaria disaster. Lancet. 1999;353:1965–1967. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]