Abstract
Background
One main challenge in Helicobacter pylori (H. pylori) eradication is its increasing antibiotic resistance. Additionally, resistance rates vary between geographic areas and periods. However, data are limited since susceptibility testing is not routinely performed. Thus, it is valuable to gather data regarding H. pylori's resistance rates in Israel that would aid in better adjustment of treatment.
Materials and Methods
The study included 540 H. pylori isolates, recovered from gastric biopsy samples of patients who had undergone endoscopy, during 2015–2020, at the Padeh Poriya Medical Center. Antibiotic susceptibility testing to amoxicillin, clarithromycin, metronidazole, levofloxacin, rifampicin, and tetracycline was performed using the Etest technique. Data regarding participants' sex, age, and ethnic group were collected. For every antibiotic and for multi‐resistance, generalized linear models were used to estimate crude and adjusted estimated differences in mean MIC and odds ratios (ORs) for every year, compared with the reference year 2015.
Results
The highest resistance rates were for clarithromycin and metronidazole (46.3% and 16.3%, respectively). Patients above 18 had higher resistance rate to rifampicin and multi‐resistance (3.3% and 14.8%), compared with patients under 18 (0.5% and 8.4%, respectively). Resistance rates for levofloxacin, rifampicin, and multi‐resistance were significantly higher among Arab patients, compared with Jewish patients. During the 6‐year surveillance, a significant annual trend in MIC for metronidazole and in ORs for metronidazole, levofloxacin, and multi‐resistance were observed (after adjustment). During 2020 compared with 2015, significant increased ORs were observed for levofloxacin and metronidazole [5.72 (1.03–31.84); 4.28 (1.30–14.14), respectively].
Conclusions
In light of the remarkable changes in antibiotic resistance of H. pylori during the study's period and the increasing resistance rates to various antibiotics, it is very important to continuously monitor H. pylori antibiotic susceptibly. In order to increase eradication rates of this bacterium, therapy regimes must be based on an updated antibiotic resistance data.
Keywords: antibiotic resistance, H. Pylori, multi‐resistance
1. INTRODUCTION
One of the most common bacterial infections worldwide is caused by Helicobacter pylori (H. pylori), which affects approximately 50% of the global population, especially in developing areas. 1 Among H. pylori‐ associated diseases are peptic ulcers, chronic gastritis, gastric mucosa‐associated lymphoid tissue lymphoma, and gastric cancer. 1 , 2 Given to its contribution to the development of gastric cancer, the third cause of death among all cancers, the world health organization (WHO) have classified H. pylori as a group 1 carcinogen. 3 Therefore, early diagnosis and treatment of H. pylori infection are imperative.
First‐line treatment includes combination of proton pump inhibitors (PPI) with clarithromycin and amoxicillin or metronidazole for 14 days. In areas with clarithromycin resistance rate above 15%, metronidazole or bismuth‐quadruple therapy is used. 1 The bismuth‐quadruple therapy includes a PPI, bismuth, tetracycline, and a nitroimidazole for 10–14 days and is often given to patients with penicillin allergy or previous exposure to macrolides. Another antibiotic that is suggested as part of a first‐line treatment is levofloxacin, which is given with a PPI and amoxicillin for 10–14 days. 4
One of the main challenges in this bacterium eradication is its increasing antibiotic resistance. 5 For example, clarithromycin resistance was associated with approximately 70% decline in eradication rates. 6 , 7 , 8
Antibiotic resistance develops since most of the antibiotics prescribed to treat H. pylori infection are widely used for other infections. Correspondingly, clarithromycin‐resistant H. pylori has been recently added by the WHO to the list of pathogens with high priority for antibiotic development. 9 , 10 , 11
It should be noted that most clinical laboratories do not routinely perform antibiotic susceptibility testing for H. pylori due to difficulties in cultivating the bacterium, along the special conditions and long duration of growth. 12 Furthermore, antibiotic resistance rates vary between geographic areas and periods. 2 , 11 , 13 Thus, gathering an updated data regarding the local resistance rates of H. pylori are essential for better adjustment of treatment regimes.
The current study's objective was to assess trends in antibiotic resistance rates of H. pylori to various antibiotics, during the years 2015–2020, in north Israel and to investigate associations to gender, ethnicity, and age.
2. MATERIALS AND METHODS
2.1. Bacterial isolates and culture
The study included 540 H. pylori isolates from an isolates bank collected during the years 2015–2020 at the clinical microbiology laboratory of the Padeh Poriya Medical Center. Theses isolates were previously isolated from gastric biopsy samples obtained from patients who had undergone endoscopy at the medical center. The study was approved by the Baruch Padeh Medical Center Helsinki Committee, POR‐0007‐20, approved the study.
H. pylori identification was performed according to the routine identification tests of the clinical microbiology laboratory including a Gram stain, catalase, oxidase, and urease tests. Final identification was performed using the Bruker Biotyper system (Bruker Daltonics, Bremen, Germany), based on the matrix‐assisted laser desorption ionization‐time of flight (MALDI TOF) technique, with MALDI BIOTYPER 3.3 (Bruker Daltonics) software. The frozen isolates were seeded on selective growth agar media, BD Helicobacter Agar (BD Diagnostics), and plates were then incubated for up to 10 days at 35°C in a microaerobic atmosphere (5% O2 and 10% CO2) produced by a gas‐generating system adapted for Campylobacter (CampyGen™; Gamidor Diagnostics).
2.2. Antibiotic susceptibility testing
Antimicrobial susceptibility testing (AST) was performed using the Etest technique, which determines the minimum inhibitory concentration (MIC), that is, the minimal antibiotic concentration that inhibits the growth of 90% bacteria under specific conditions. H. pylori colonies were suspended in 0.85% NaCl solution to a 3.0 McFarland standard and this suspension was seeded on Mueller–Hinton agar with 10% horse blood (Hy‐labs). Then, gradient Etest strips (bioMérieux) were added to each agar plate for the determination of MIC to amoxicillin, clarithromycin, metronidazole, levofloxacin, rifampicin, and tetracycline. The agar plates were incubated for 72 h at 35°C in a microaerobic atmosphere (CampyGen™). MICs were defined as the lowest concentration of antimicrobial agent that inhibited visible growth of H. pylori bacteria. Interpretation of susceptibility test results was performed in accordance with British Society for Antimicrobial Chemotherapy (BSAC). H. pylori ATCC 43504 was used for MIC quality control.
2.3. Statistical analysis
We obtained information on the following characteristics and categorized them as follows: participant's sex (male/female), participant's ethnic group as declared at birth (Arab/Jewish), and age (≤18, years). Univariate tests were applied to analyze the differences in the resistance rates (for each antibiotic in addition to any resistance and multiple resistance) by year and by the other characteristics. Comparisons between groups were made using the chi‐squared test for independent samples for the categorical variables.
Generalized linear models with normal distribution and identity link function were used to estimate associations [estimated differences in mean MIC with 95% confidence intervals (CIs)] between every year, compared with the reference year (2015). Similarly, we fitted generalized linear models with binomial family and logit link function to estimate odds ratios (ORs) and 95% CIs for every antibiotic category resistance. Estimates for every antibiotic category were compared with the non‐resistance category and multiple resistance category were compared with others (non‐resistance or single resistance). In addition, annual linear trends were evaluated. The characteristics (sex, age, and ethnicity) included in the adjusted models.
Statistical significance was determined with the p‐value <.05. The data were analyzed using SPSS version 25.
3. RESULTS
3.1. Antibiotic resistance patterns
The study included 540 isolates that were isolated during 2015–2020 (2015: n = 52; 2016: n = 196; 2017: n = 143; 2018: n = 81; 2019: n = 36; 2020: n = 32). Overall resistance rates were 46.3% for clarithromycin, 16.3% for metronidazole, 6.8% for amoxicillin, 4.3% for tetracycline, 4.1% for levofloxacin, and 2.2% for rifampicin. First, we looked at the resistance rates of each antibiotic during six years (Figure 1).
FIGURE 1.
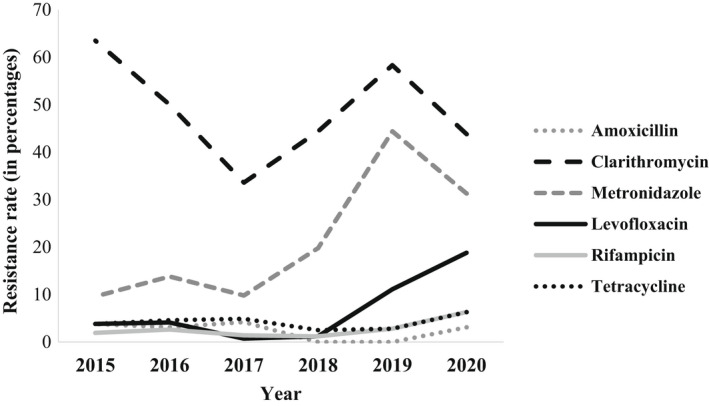
Resistance patterns of Helicobacter pylori during 2015–2020
During the 6‐year surveillance, resistance rates to clarithromycin, levofloxacin, and metronidazole significantly changed (p = .003, p = .001, p = .001, respectively) (Figure 1, Table 1). Resistance rates to clarithromycin were the highest (63.5%) in 2015, decreased during 2016–2017 (50% and 33.6%, respectively), and then increased during 2018–2019 (44.4% and 58.3%, respectively). In 2020, the resistance rate to clarithromycin decreased again (43.8%) and resembled the resistance rate in 2018 (44.4%). Similarly, resistance rates to metronidazole increased and decreased alternately during these years, with the highest rate (44.4%) in 2019. Resistance rate to levofloxacin decreased during 2015–2017 and then increased during 2018–2020.
TABLE 1.
Helicobacter pylori susceptibility patterns to Clarithromycin, Levofloxacin, and Metronidazole during 2015–2020
| Year (Total isolates) | Clarithromycin | Levofloxacin | Metronidazole | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S N (%) | R N (%) | p value a | S N (%) | R N (%) | p value | S N (%) | R N (%) | p value | |
| 2015 (52) | 19 (36.5) | 33 (63.5) | .003 | 50 (96.2) | 2 (3.8) | .001 | 47 (90.4) | 5 (9.6) | .001 |
| 2016 (196) | 98 (50) | 98 (50) | 188 (95.9) | 8 (4.1) | 169 (86.2) | 27 (13.8) | |||
| 2017 (143) | 95 (66.4) | 48 (33.6) | 142 (99.3) | 1 (0.7) | 129 (90.2) | 14 (9.8) | |||
| 2018 (81) | 45 (55.6) | 36 (44.4) | 80 (98.8) | 1 (1.2) | 65 (80.2) | 16 (19.8) | |||
| 2019 (36) | 15 (41.7) | 21 (58.3) | 32 (88.9) | 4 (11.1) | 20 (55.6) | 16 (44.4) | |||
| 2020 (32) | 18 (56.3) | 14 (43.8) | 26 (81.3) | 6 (18.8) | 22 (68.8) | 10 (31.3) | |||
| Total (540) | 290 (53.7) | 250 (46.3) | 518 (95.9) | 22 (4.1) | 452 (83.7) | 88 (16.3) | |||
p value refers to the differences in the resistance rates (for each antibiotic resistance) by year.
Resistance rates to tetracycline, amoxicillin and rifampicin were overall low during these years with no statistically significant changes between years (Figure 1, Table S1). Resistance rates to clarithromycin and metronidazole were the highest each year (Figure 1). Figures S1–S6 present the MIC distribution of all study's isolates to the 6 tested antibiotics.
Next, we assessed multi‐resistance among our isolates. As presented in Table S2, rates of multi‐resistance, defined as resistance to more than one antibiotic, significantly changed during the 6 years (p = .001) and were the highest in 2020 (31.3%). Overall, the rates of any resistance, defined as resistance to any antibiotic, decreased from 2015 (75%) to 2017 (50.3%) and then increased to 77.8% in 2019 (p = .003). In 2020, the rate of any resistance decreased a bit to 75% (Table S2).
3.2. Antibiotic resistance patterns according to age, gender, and ethnicity
We were interested whether antibiotic resistance was affected by the patients' demographic characteristics such as age, gender, and ethnicity. When comparing antibiotic resistance rates between patients under the age of 18 and above it, we did not find statistically significant differences (data not shown) except for a tendency to statistical significance for rifampicin (p = .030), for which the patients above 18 had higher resistance rate (3.3%), compared with patients under 18 (0.5%) (Table 2). Additionally, the rate of multi‐resistance was higher among patients above 18, compared with patients under 18 (14.8% and 8.4%, respectively, p = .027).
TABLE 2.
Helicobacter pylori susceptibility patterns according to age
| Age (Total isolates) | Rifampicin | Multi‐resistance a | ||||
|---|---|---|---|---|---|---|
| S n (%) | R n (%) | p value | No n (%) | Yes n (%) | p value | |
| ≤18 (203) | 202 (99.5) | 1 (0.5) | .030 | 186 (91.6) | 17 (8.4) | .027 |
| ˃18 (336) | 325 (96.7) | 11 (3.3) | 287 (85.2) | 50 (14.8) | ||
Multi‐resistance‐ No = no resistance at all or resistance to one antibiotic, Yes = resistance to more than one antibiotic.
No significant differences were found in resistance rates to antibiotics when comparing men and women (data not shown). Regarding ethnicity, resistance rates for levofloxacin and rifampicin were higher among Arab patients, compared with Jewish patients (p = .032, p = .035, respectively) (Table 3). The resistance rates for other antibiotics were not affected by ethnicity (data not shown). Multi‐resistance was more common among the Arab population (16.8%), compared with the Jewish population (9.8%) (p = .016).
TABLE 3.
Helicobacter pylori susceptibility patterns according to ethnicity
| Ethnicity (Total isolates) | Levofloxacin | Rifampicin | Multi‐resistance a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S n (%) | R n (%) | p value | S n (%) | R n (%) | p value | No n (%) | Yes n (%) | p value | |
| Jewish (338) | 329 (97.3) | 9 (2.7) | .032 | 333 (98.8) | 4 (1.2) | .035 | 305 (90.2) | 33 (9.8) | .016 |
| Arab (202) | 189 (93.6) | 13 (6.4) | 194 (96) | 8 (4) | 168 (83.2) | 34 (16.8) | |||
Multi‐resistance‐ No = no resistance at all or resistance to one antibiotic, Yes = resistance to more than one antibiotic.
3.3. Analysis of the crude and adjusted changes in mean MIC
Table S3 presents an analysis of the crude and adjusted changes in mean MIC for every antibiotic. After adjustment to ethnicity, age, and sex, a significant annual trend in MIC was observed for metronidazole [0.81 (0.51–1.11)]. During 2020 compared with 2015, significant increased mean MIC was observed for metronidazole [4.12 (2.15–6.09)] (Figure 2).
FIGURE 2.
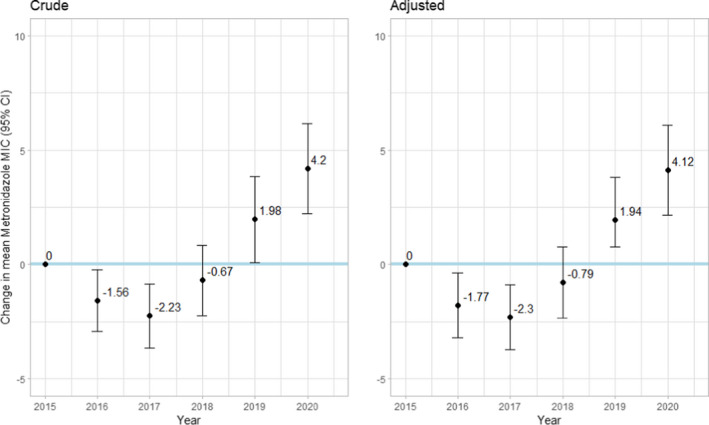
Crude and adjusted * change in mean minimum inhibitory concentration (MIC) and 95% confidence intervals (95% CI) by year, compared with the baseline of year 2015, for metronidazole. *Adjusted to sex, age, and ethnic group
Analysis of the crude and adjusted ORs for every antibiotic and for multi‐resistance is presented in Table S4. After adjustment to ethnicity, age, and sex, significant annual trend in ORs was observed for metronidazole [1.45 (1.23–1.73)], for levofloxacin [1.64 (1.19–2.25)] and for multi‐resistance [1.33(1.09–1.62)]. During 2020 compared with 2015, significant increased ORs were observed for metronidazole [4.28 (1.30–14.14)], for levofloxacin [5.72 (1.03–31.84)] (Figure 3).
FIGURE 3.
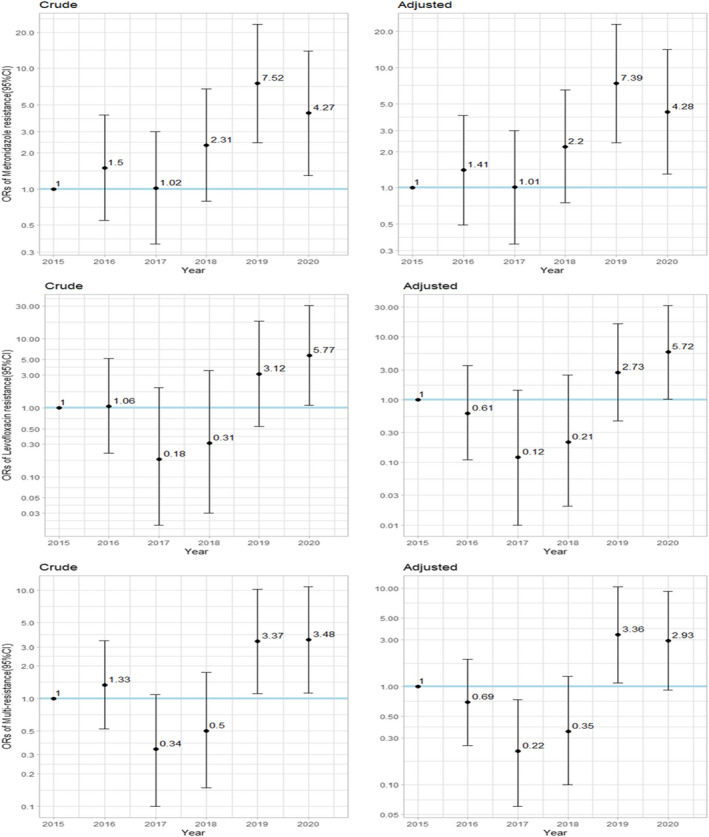
Crude and adjusted* ORs (Odds Ratio) and 95% Confidence intervals (95% CI) for metronidazole, levofloxacin, and multi‐resistance (resistance to more than one antibiotic), by year, compared with the baseline of year 2015. *Adjusted to sex, age, and ethnic group
4. DISCUSSION
The current study aimed to observe trends in antibiotic resistance rates of H. pylori in north Israel during 6 years. Among the tested antibiotics, the highest resistance rates were for clarithromycin and metronidazole. This finding correlates with previous studies that also reported on these two antibiotics associated with the most prevalent resistance rates, compared with other antibiotics. 2 , 14 , 15
Throughout the surveillance period, resistance rates to most antibiotics increased. Similar to our results, a previous study from Italy demonstrated an increased trend in resistance rates to clarithromycin and levofloxacin from 2009–2014 to 2015–2019. 16 Studies from other parts of the world also confirmed the increasing trend of antibiotic resistance in H. pylori, including, for example, the Asian Pacific region, 17 Southern Asian countries, 1 Taiwan, 18 and Australia. 2 , 19
In contrast to our results, a previous study from the European Registry reported on a decrease in metronidazole, clarithromycin, and levofloxacin resistance rates from 2013–2016 to 2017–2020. 14 However, it is well‐known that there is a notable variability in antibiotic resistance rates among different geographic areas, even within the same country. For example, the same study has found remarkable differences in H. pylori resistance rates between northern and southern European countries. 14 These differences may be attributed to variable prescribing patterns and antibiotic use that are prevalent in each area. Therefore, it would be interesting to monitor the antibiotic resistance rates of H. pylori in other areas in Israel and to compare with our data.
Along with the increasing resistance rates to each antibiotic, the rates of multi‐resistance also increased during the study period. This phenomenon is also common in other geographic areas and elucidates the decreasing rates of eradication success. 1 , 15 , 16 According to a recent report by the European Centre for Disease Prevention and Control regrading antimicrobial consumption in 27 European Union (EU) Member States and two European Economic Area (EEA) countries (Iceland and Norway), overall antibiotic consumption has been decreased during 2011–2020. However, an increasing trend was noticed in the consumption of broad‐spectrum antimicrobials, compared with narrow‐spectrum antibiotics during 2011–2020. 20 This can explain the rises in antibiotic resistance and multi‐resistance rates of H. pylori.
The rates of resistance to amoxicillin, tetracycline, and rifampicin were low during all study's years. This finding corresponds to several works that reported on low levels of resistance to amoxicillin, 1 , 5 , 14 , 15 , 18 , 19 tetracycline, 1 , 14 , 15 , 18 , 19 and rifampicin. 15 The low resistance rates to these antibiotics imply that their use for H. pylori treatment should be maintained; furthermore, their integration as a first‐line regime may be considered in areas with high resistance rates to clarithromycin and metronidazole.
Importantly, some of the changes in antibiotic resistance rates during the study's period were noticed also after adjustment to sex, ethnicity, and age; this implies that the observed trends are not confounded by these variables.
Another study's aim was to evaluate association of patients' demographic characteristics and antibiotic resistance of H. pylori. We found higher resistance rates to rifampicin and multi‐resistance rate among isolates from patients above the age of 18. This result can be related to increased use of antibiotics with increasing age. 21 , 22 Rifampicin is used to treat tuberculosis, and therefore is not usually given to H. pylori patients. 23 Thus, it would be interesting to investigate whether patients with rifampicin‐resistant isolates have been previously treated with rifampicin due to tuberculosis.
Surprisingly, we did not find significant differences in resistance rates to antibiotics between men and women. According to a previous work from Italy, H. pylori isolated from women were more likely to have double resistance to clarithromycin and metronidazole 16 and the authors concluded that this was related to the widely use of both antibiotics for treatment of urinary infections in women. 24 , 25 Additionally, several studies have associated the female gender with H. pylori eradication failure. 26 , 27
As for ethnicity, resistance rates for levofloxacin and rifampicin and multi‐resistance rate were higher among Arab patients, compared with Jewish patients. This result reaffirms the findings of our previous study, in which levofloxacin resistance rates and multi‐resistance rates were higher among isolates from Arab patients, compared with Jewish patients. We hypothesized that Arab population has different cultural behavior and less knowledge regarding antibiotics and their mechanism, leading to antibiotic overuse or misuse. 28 , 29 , 30 Also, as several doctors in northern Israel have noticed a high level of treatment failure in Arab patients, we assumed that it may be related to low compliance of the treatment. A recent study has identified rural residence as one of the factors associated with eradication failure. 31 Thus, the rural residence and high crowdedness in villages that are characteristic of the Arab society, may ease the transfer of resistant strains of H. pylori, thereby increasing the overall resistance rates.
5. CONCLUSIONS
In light of the changes in antibiotic resistance of H. pylori during recent years and the overall trend of increasing resistance rates to various antibiotics, including multi‐resistance rates, continuous monitoring of H. pylori antibiotic susceptibly is crucial/imperative. This way, each country can determine specific regimes for treatment that are based on updated data, and hopefully this strategy will diminish rates of treatment failure.
CONFLICTS OF INTEREST
All other authors declare no conflicts of interest.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We thank Mr. Wadie Abu Dahoud for the statistical analysis.
Azrad M, Vazana D, On A, et al. Antibiotic resistance patterns of Helicobacter pylori in North Israel – A six‐year study. Helicobacter. 2022;27:e12932. doi: 10.1111/hel.12932
Keren Agay‐Shay and Avi Peretz contributed equally to the research.
REFERENCES
- 1. Sukri A, Lopes BS, Hanafiah A. The emergence of multidrug‐resistant Helicobacter pylori in Southeast Asia: a systematic review on the trends and intervention strategies using antimicrobial peptides. Antibiotics (Basel). 2021;10(9):1061. doi: 10.3390/antibiotics10091061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan SF, Teng JC, Holmes JA, Perera C, Prendergast L, Waring L. Helicobacter pylori antimicrobial resistance in Melbourne, Australia. Time to review therapeutic guidelines? Intern Med J. 2021;51(11):1919‐1926. doi: 10.1111/imj.15355 [DOI] [PubMed] [Google Scholar]
- 3. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1‐241. [PMC free article] [PubMed] [Google Scholar]
- 4. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212‐239. doi: 10.1038/ajg.2016.563 [DOI] [PubMed] [Google Scholar]
- 5. Camorlinga‐Ponce M, Gomez‐Delgado A, Aguilar‐Zamora E, et al. Phenotypic and genotypic antibiotic resistance patterns in Helicobacter pylori strains from ethnically diverse population in Mexico. Front Cell Infect Microbiol. 2020;10:539115. doi: 10.3389/fcimb.2020.539115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Megraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374‐1384. doi: 10.1136/gut.2003.022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Connor A, Gisbert JP, McNamara D, O'Morain C. Treatment of Helicobacter pylori infection 2010. Helicobacter. 2010;15(suppl 1):46‐52. doi: 10.1111/j.1523-5378.2010.00774.x [DOI] [PubMed] [Google Scholar]
- 8. Nagaraja V, Eslick GD. Evidence‐based assessment of proton‐pump inhibitors in Helicobacter pylori eradication: a systematic review. World J Gastroenterol. 2014;20(40):14527‐14536. doi: 10.3748/wjg.v20.i40.14527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514‐533. doi: 10.1111/apt.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic‐resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318‐327. doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 11. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta‐analysis in World Health Organization regions. Gastroenterology. 2018;155(5):1372‐1382.e17. doi: 10.1053/j.gastro.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Megraud F, Hazell S, Glupczynski Y. Antibiotic susceptibility and resistance. In: HLT M, Mendz GL, Hazell SL, eds. Helicobacter Pylori: Physiology and Genetics. ASM Press; 2001. [PubMed] [Google Scholar]
- 13. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta‐analysis. Gastroenterology. 2017;153(2):420‐429. doi: 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 14. Bujanda L, Nyssen OP, Vaira D, et al. Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013–2020: results of the European registry on H. pylori management (Hp‐EuReg). Antibiotics (Basel). 2021;10(9):1058. doi: 10.3390/antibiotics10091058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Botija G, Garcia Rodriguez C, Recio Linares A, Campelo Gutierrez C, Perez‐Fernandez E, Barrio MA. Antibiotic resistances and eradication rates in Helicobacter pylori infection. An Pediatr (Engl ed). 2021;95(6):431‐437. doi: 10.1016/j.anpede.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 16. Saracino IM, Fiorini G, Zullo A, Pavoni M, Saccomanno L, Vaira D. Trends in primary antibiotic resistance in H. pylori strains isolated in Italy between 2009 and 2019. Antibiotics (Basel). 2020;9(1):26. doi: 10.3390/antibiotics9010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuo YT, Liou JM, El‐Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia‐Pacific region: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2017;2(10):707‐715. doi: 10.1016/S2468-1253(17)30219-4 [DOI] [PubMed] [Google Scholar]
- 18. Liang CM, Tai WC, Hsu PI, et al. Trend of changes in antibiotic resistance in Helicobacter pylori from 2013 to 2019: a multicentre report from Taiwan. Therap Adv Gastroenterol. 2020;13:1756284820976990. doi: 10.1177/1756284820976990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schubert JP, Warner MS, Rayner CK, et al. Increasing Helicobacter pylori clarithromycin resistance in Australia over 20 years. Intern Med J. 2021;52:1554‐1560. doi: 10.1111/imj.15640 [DOI] [PubMed] [Google Scholar]
- 20. European Centre for Disease Prevention and Control . Antimicrobial Consumption in the EU/EEA (ESAC‐Net) ‐ Annual Epidemiological Report 2020. ECDC; 2021. [Google Scholar]
- 21. McQuiston Haslund J, Rosborg Dinesen M, Sternhagen Nielsen AB, Llor C, Bjerrum L. Different recommendations for empiric first‐choice antibiotic treatment of uncomplicated urinary tract infections in Europe. Scand J Prim Health Care. 2013;31(4):235‐240. doi: 10.3109/02813432.2013.844410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ji Z, Han F, Meng F, Tu M, Yang N, Zhang J. The Association of Age and Antibiotic Resistance of Helicobacter pylori: a study in Jiaxing City, Zhejiang Province, China. Medicine (Baltimore). 2016;95(8):e2831. doi: 10.1097/MD.0000000000002831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiao L, Wang J, Ma H. Analysis of Helicobacter pylori's antibiotic resistance rate and research on its eradication treatment plan. Comput Math Methods Med. 2021;2021:6009602. doi: 10.1155/2021/6009602 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Ailloud F, Didelot X, Woltemate S, et al. Within‐host evolution of Helicobacter pylori shaped by niche‐specific adaptation, intragastric migrations and selective sweeps. Nat Commun. 2019;10(1):2273. doi: 10.1038/s41467-019-10050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lofmark S, Edlund C, Nord CE. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50(suppl 1):S16‐S23. doi: 10.1086/647939 [DOI] [PubMed] [Google Scholar]
- 26. Lim SG, Park RW, Shin SJ, et al. The relationship between the failure to eradicate Helicobacter pylori and previous antibiotics use. Dig Liver Dis. 2016;48:385‐390. doi: 10.1016/j.dld.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 27. Kumar S, Sangitha R, Nachamkin I, Metz DC. Resistance patterns of refractory H. pylori infection in a referral center in the Delaware Valley. GastroHep. 2020;2:6‐12. doi: 10.1002/ygh2.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abu Taha A, Abu‐Zaydeh AH, Ardah RA, et al. Public knowledge and attitudes regarding the use of antibiotics and resistance: findings from a cross‐sectional study among Palestinian adults. Zoonoses Public Health. 2016;63(6):449‐457. doi: 10.1111/zph.12249 [DOI] [PubMed] [Google Scholar]
- 29. Barah F, Goncalves V. Antibiotic use and knowledge in the community in Kalamoon, Syrian Arab Republic: a cross‐sectional study. East Mediterr Health J. 2010;16(5):516‐521. [PubMed] [Google Scholar]
- 30. Francavilla R, Lionetti E, Castellaneta S, et al. Clarithromycin‐resistant genotypes and eradication of Helicobacter pylori. J Pediatr. 2010;157(2):228‐232. doi: 10.1016/j.jpeds.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 31. Pena‐Galo E, Gotor J, Harb Y, Alonso M, Alcedo J. Socioeconomic and demographic factors associated with failure in Helicobacter pylori eradication using the standard triple therapy. Gastroenterol Hepatol Bed Bench. 2021;14(1):53‐58. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


