From 26 to 28 June 2021, an unprecedented atmospheric heatwave coincided with the lowest low tides of the year in the Pacific Northwest (i.e., the region consisting of the northwestern corner of the contiguous United States and southwestern Canada). This event broke numerous all‐time record high temperatures throughout the region and would have been virtually impossible without human‐caused climate change (Philip et al., 2021). During and immediately following the event, many scientists, resource managers, and members of the public reported dead and dying marine organisms, including barnacles, mussels, clams, and oysters, on intertidal beaches throughout the region (Figure 1). These observations raised alarms among many stakeholders because these species support important commercial, subsistence, and recreational fisheries and are major constituents of nearshore ecosystems.
FIGURE 1.

Scenes of invertebrate mortality post 26–28 June heatwave. (a) Dead Manila clams (Ruditapes philippinarum). (b) Dead and empty bay mussels (Mytilus spp.). (c) Dead Mytilus spp. (d) Empty and clean cockle (Clinocardium nuttallii). (e) Gaping cockle (C. nuttallii). (f) Mixed bed of dead barnacles (Balanus glandula and Chthamalus dalli). (g) Empty and clean Pacific oysters (Crassostrea gigas). (h) A healthy Olympia oyster (Ostrea lurida). (i) Turkey vultures foraging on tidelands in Sequim Bay, WA, following the heatwave event. (j) Sand dollars (Dendraster excentricus) turning yellow a week following the heatwave event. Detailed descriptions, locations, and credits can be found in Appendix S1.
In response, we developed and deployed a semi‐quantitative survey to a multiorganization network of collaborators to rapidly assess the postheatwave condition of nearshore invertebrates across the coastal Pacific Northwest and inland waters of Washington state and British Columbia known collectively as the Salish Sea (e.g., Strait of Juan de Fuca, Puget Sound, and the Strait of Georgia). Our goal was to inventory shellfish condition observations across a broad geographic scale to document the effects of the extreme heatwave and to serve as a starting point for future detailed quantitative research and monitoring. We asked local scientists (academic, tribal, and state and federal agencies) to rate shellfish condition in terms of the degree of postheatwave mortality (or, conversely, resilience) relative to what they would consider typical based on their prior experience with specific sites and species at the same time of year. We directed participants to only submit observations from locations where they possessed extensive local knowledge. In these situations, expert knowledge is a surrogate for empirical data collection because practitioners can develop quantitative information from the synthesis of their own observations, knowledge, and mental models of the system in question (Drescher et al., 2013). We used a five‐point postheatwave rating (PHWR) system to evaluate the condition of organisms: 1 = much worse than normal, 2 = worse than normal, 3 = normal, 4 = better than normal, 5 = much better than normal. Further information on our survey methods and rationale is given in Appendix S1.
We collected 203 observations from 108 sites spanning the outer and inner coasts (Figure 2), covering 24 species. Here we focus our discussion on acorn barnacles (Balanus glandula), California and so‐called bay mussels (Mytilus californianus, Mytilus spp.), butter clams (Saxidomus gigantea), cockles (Clinocardium nuttallii), native littleneck clams (Leukoma staminea), naturalized Manila clams (Ruditapes philippinarum), Olympia (Ostrea lurida) oysters, and naturalized Pacific oysters (Crassostrea gigas = Magallana gigas). We define bay mussels generally as Mytilus spp. due to difficulty differentiating M. trossulus, M. edulis, and M. galloprovincialis in the field and because of reports of hybridization between M. trossulus and M. galloprovincialis (E. Carrington, personal communication, July 26, 2021; C. A. Speck, unpublished). These species are conspicuous, well studied, and represent the majority of our observations (N = 171). They are also ecologically important, span a range of intertidal habitats, and support highly valued recreational, commercial, subsistence, and ceremonial harvest. All observations are reported in archived data (Raymond, 2022). We also note that we consider Manila clams and Pacific oysters as “naturalized” because they were introduced to the region ~100 years ago for aquaculture purposes but have established naturally reproducing populations.
FIGURE 2.
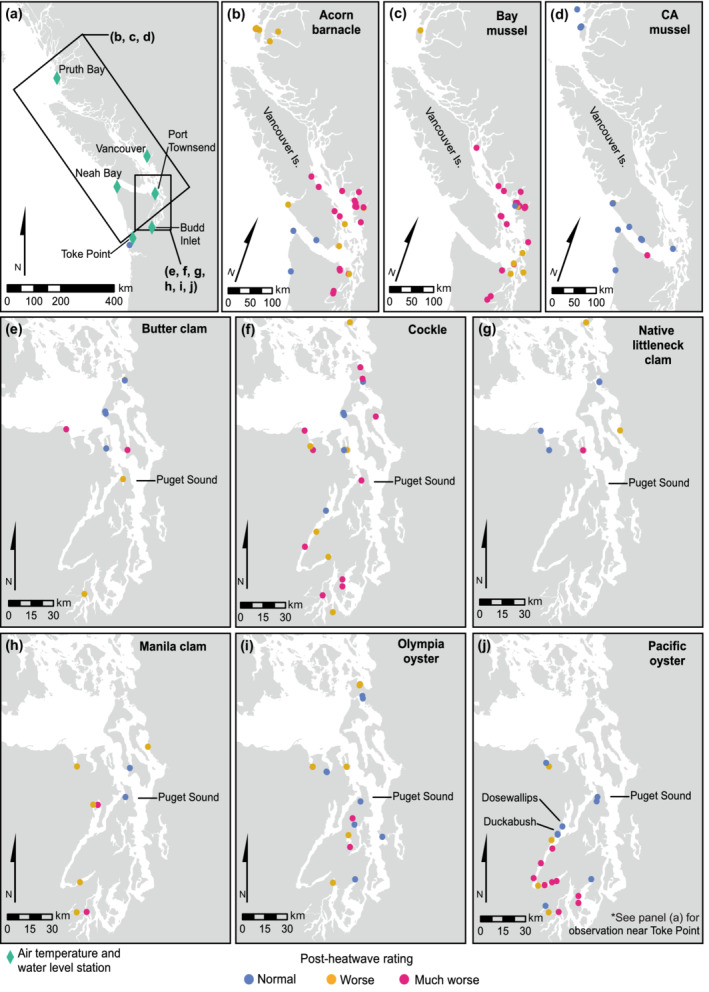
Study region (a) with locations of air temperature and water‐level data. Maps of postheatwave assessment for (b) acorn barnacles, (c) bay mussels, (d) California mussels, (e) butter clams, (f) cockles, (g) native littleneck clams, (h) Manila clams, (i) Olympia oysters, and (j) Pacific oysters
Region‐wide patterns of intertidal shellfish conditions reflect environmental gradients, the natural history of the species, and the intersection between them. A key factor that may contribute to these observed patterns is the difference in the timing of low tide over the days of the heatwave (Figure 3; Appendix S1: Figure S1). The outer coast of Washington state and British Columbia experienced low tide ~4 h before the inner coast sites of the Salish Sea, where low tide occurred very close to solar noon. Ambient air temperatures were also much higher at inner coast sites, potentially compounding the effects of a later low tide (Figure 3). Wave exposure, which is generally greater on the outer coast than the inner coast, may have moderated postheatwave ratings, along with the timing of low tide. The difference in the physical environment (tide timing and exposure) intersects the natural history of many of our focal species. For example, California mussels, which are almost exclusively found at outer coastal sites, largely avoided negative impacts (i.e., better condition) as opposed to congeneric bay mussels, which are found in more wave‐protected inner coast sites and were more likely to suffer negative impacts (Figure 2c,d). Furthermore, species found higher in the intertidal zone, such as acorn barnacles, were generally found in worse condition than species found lower in the intertidal zone, such as clams and oysters (Figure 2).Though we are not able to separate species‐specific effects here, this pattern highlights the range of thermal environments experienced by barnacles, mussels, clams, and oysters across the region. Thermal conditions at sites within the western Strait of Juan de Fuca (Figure 2) and along the outer coast are often buffered by winds, wave splash, or fog, and the only unusual barnacle mortality observed in this region was restricted to one less wave‐exposed, southeast‐facing shoreline (Figure 2b).
FIGURE 3.
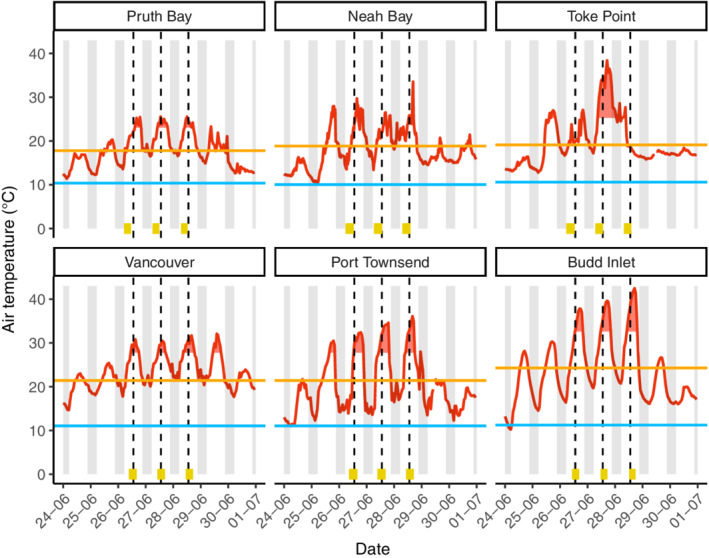
Air temperatures (red line) at outer (top row: Pruth Bay, Neah Bay, Toke Point) and inner coast sites (bottom row: Vancouver, Port Townsend, Budd Inlet) measured over the course of the heatwave (26–28 June 2021) and surrounding days plotted with the 1991–2020 mean summer (June–August) high (orange) and low (blue) temperatures. Red shaded regions are temperatures greater than two standard deviations above the 1991–2020 mean. Gray shaded regions are night. Vertical dashed lines are solar noon during the heatwave. Gold bars at bottom of plots represent duration of intertidal exposure to air below +1 m tide elevation relative to mean lower low water (MLLW). Temperature and tide data were obtained from multiple sources (Appendix S1).
Observed postheatwave condition of bivalves, including butter clams, cockles, native littleneck clams, Manila clams, and Olympia and Pacific oysters, varied among species in accordance with aspects of their natural history (Figure 2e–j). Butter clams, which often burrow >15 cm deep in sediment and live at lower tidal elevations than other clam species (Dethier, 2006), were less affected by the heatwave than surface‐dwelling cockles. Being buried deeper in the sediment, butter clams likely were buffered from high solar irradiance, high surface temperature, and high desiccation stress relative to animals such as cockles living on or near the surface. However, we did observe a range in butter clam condition among sites separated by ~30 km, indicating that local scale factors may also contribute to postheatwave clam condition. Manila and native littleneck clams varied in observed condition, but the low sample size for these species, owing to the opportunistic nature of our sampling or declining population size of native littleneck clams (J. Barber, unpublished), makes drawing broader conclusions difficult. Olympia oysters, which tend to be found lower in the intertidal zone (White et al., 2009), were less affected by the heatwave than Pacific oysters (Figure 2i,j). However, both oyster species experienced a range of observed conditions, again indicating the likely importance of local scale factors. Notably, more Pacific oysters were observed in poor condition in more southerly latitudes, which coincided with low tide and peak air temperatures. This may reflect the difference in air temperatures across the region, especially in southern Puget Sound, where air temperatures were greater than in northern Puget Sound (Figure 3). Pacific oyster observations near the Duckabush and Dosewallips estuaries were considered normal compared to other nearby locations in southern Hood Canal (Figure 2j). Observations by several contributing participants noted substantial increases to river flows associated with snow melt during the heatwave at these and other locations. Given these observations, it is possible that increased flow of surface water or groundwater could have provided thermal refuge for species at low tide; this requires further investigation.
Thermal stress is a common and well‐studied factor in ecology, a major structuring force in intertidal and nearshore ecosystems (Connell, 1961; Harley & Helmuth, 2003), and can affect the recruitment and energetics of organisms and the prevalence of biotoxins and infectious agents (references below). For example, clam populations in the region exhibit population synchrony at relatively large spatial scales, and adult clam biomass likely reflects larval recruitment success 4 years prior (Barber et al., 2019). Given that all of the bivalve species discussed here were likely reproductive during the heatwave (Anderson et al., 1982), it is possible that high mortality in certain species (e.g., cockles) may manifest itself in reduced adult populations in ~4 years. Because clam recruitment is naturally episodic (Hunt & Scheibling, 1997), attributing this event to a loss in a year class will require multiple years of population monitoring. Sublethal, but extreme, ambient temperatures also have a direct effect on the metabolism of marine invertebrates (Hand & Hardewig, 1996). Therefore, it is reasonable to expect that many organisms were under increased metabolic stress during the heatwave, potentially leading to delayed mortality or reduced overall condition, as documented in Manila and related clams (Macho et al., 2016). Unmeasured biological factors, such as biotoxins or infections, also could have increased susceptibility to thermal stress‐induced mortality in some species (Go et al., 2017; Green et al., 2019; King et al., 2021). For example, Pacific oysters with infections of the bacteria Vibrio sp. have reduced thermal tolerances compared to uninfected individuals (Wendling & Wegner, 2013).
Multiple factors and organismal traits can enhance or mitigate thermal stress in intertidal species, including morphology (body size and shell color), behavior (aspect), and environment (substrate, wind, and proximity to shade or freshwater runoff). Water quality factors, including pH, turbidity, and salinity, may also contribute to bivalve stress and mortality (Dethier et al., 2019). These unmeasured factors may have influenced observed postheatwave condition and may have delayed mortality for many species. Thus, further investigation into these factors and how they may have modulated thermal stress during this and future heatwaves will be critical for increasing our understanding of the effect of extreme heat events on intertidal organisms in the region. Moreover, the information could prove useful for identifying local climate refugia and incorporating climate adaptation into shellfish management. Long‐term population and environmental monitoring data will be particularly useful in separating heatwave effects from normal population fluctuations.
Our observations, though coarse, demonstrate the widespread negative impacts post heatwave to intertidal species across the waters of the Pacific Northwest and Salish Sea. Our broad survey suggests that the June 2021 heatwave may have far‐reaching and potentially multiyear effects on nearshore ecology, cultural connections, and fisheries. These observations represent just the beginning of our understanding of how the heatwave may have affected intertidal species and may serve as a bellwether for future extreme temperature events, which are predicted to become more frequent and more severe in a warmer climate (IPCC, 2021). The present work highlights heatwave responses by naturally occurring or enhanced sessile marine invertebrates; yet some of these species support a robust aquaculture industry in the Pacific Northwest. Identifying impacts on farmed shellfish was beyond the scope of this effort, and such impacts have yet to be examined. Continued population, recruitment, disease, and environmental monitoring and research will be needed to accurately assess species, community, and ecosystem responses, as well as impacts on human use. This project may also serve as a model for the power of research across a broad coalition of partners and as a method to rapidly assess unique or short‐lived weather events. The range of expertise, perspectives, and geographic location of project partners enabled a research product that will serve many user groups and has laid the groundwork for future collaborations.
AUTHOR CONTRIBUTIONS
Wendel W. Raymond conceived of the project and experimental design, participated in the execution of study, analyzed and interpreted data, wrote the manuscript, and helped secure funding. Julie S. Barber, Megan N. Dethier, Hilary A. Hayford, Christopher D. G. Harley, Teri L. King, Blair Paul, Camille A. Speck, Elizabeth D. Tobin, and Ann E.T. Raymond conceived of the project and experimental design, participated in the execution of study, and assisted in writing the manuscript. P. Sean McDonald conceived of the ideas and experimental design, participated in execution of study, assisted in writing the manuscript, and helped secure funding.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We would like to thank the more than 28 individuals representing 19 organizations who contributed observations for this project. Were it not for these observations, expertise gained over many years, and stewardship since time immemorial, this project would not have been possible. This work was conducted on traditional lands and waters of 42 Tribes and First Nations. A full list of contributors and associated lands and waters is available in Appendix S1. Additional thanks go to the following individuals and organization for providing temperature data; The Hakai Institute with financial support from the Tula Foundation, Evergreen State College, and C. Pfister and T. Wootton and the Makah Tribal Nation for allowing long‐term access to their lands. This work was funded in part by a grant from Washington Sea Grant, University of Washington, pursuant to National Oceanic and Atmospheric Administration (NOAA) Award No. NA14OAR4170095. The views expressed herein are those of the authors and do not necessarily reflect the views of NOAA or any of its subagencies.
Raymond, Wendel W. , Barber Julie S., Dethier Megan N., Hayford Hilary A., Harley Christopher D. G., King Teri L., Paul Blair, et al. 2022. “Assessment of the Impacts of an Unprecedented Heatwave on Intertidal Shellfish of the Salish Sea.” Ecology 103(10): e3798. 10.1002/ecy.3798
Handling Editor: John J. Pastor
Funding information National Oceanic and Atmospheric Administration, Grant/Award Number: NA14OAR4170095; Washington Sea Grant, University of Washington; Tula Foundation
DATA AVAILABILITY STATEMENT
Survey and air temperature data and statistical code are available at https://doi.org/10.5281/zenodo.6555076 (Raymond, 2022). Temperature and tide data were obtained from publically available sources and detailed in Appendix S1.
REFERENCES
- Anderson, G. J. , Miller M. B., and Chew K. K.. 1982. A Guide to Manila Clam Aquaculture in Puget Sound. Seattle, WA: Washington Sea Grant Program, University of Washington. [Google Scholar]
- Barber, J. S. , Ruff C. P., McArdle J. T., Hunter L. L., Speck C. A., Rogers D. W., and Greiner C. M.. 2019. “Intertidal Clams Exhibit Population Synchrony across Spatial and Temporal Scales.” Limnology and Oceanography 64: 284–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell, J. H. 1961. “The Influence of Interspecific Competition and Other Factors on the Distribution of the Barnacle Chthamalus stellatus .” Ecology 42: 710–23. [Google Scholar]
- Dethier, M. N. 2006. Native Shellfish in Nearshore Ecosystems of Puget Sound. Seattle, WA: Washington Sea Grant Program, University of Washington. [Google Scholar]
- Dethier, M. N. , Kobelt J., Yiu D., Wentzel L., and Ruesink J. L.. 2019. “Context‐Dependence of Abiotic and Biotic Factors Influencing Performance of Juvenile Clams.” Estuarine, Coastal and Shelf Science 219: 201–9. [Google Scholar]
- Drescher, M. , Perera A. H., Johnson C. J., Buse L. J., Drew C. A., and Burgman M. A.. 2013. “Toward Rigorous Use of Expert Knowledge in Ecological Research.” Ecosphere 4: 1–26. [Google Scholar]
- Go, J. , Deutscher A. T., Spiers Z. B., Dahle K., Kirkland P. D., and Jenkins C.. 2017. “Mass Mortalities of Unknown Aetiology in Pacific Oysters Crassostrea gigas in Port Stephens, New South Wales, Australia.” Diseases of Aquatic Organisms 125: 227–42. [DOI] [PubMed] [Google Scholar]
- Green, T. J. , Siboni N., King W. L., Labbate M., Seymour J. R., and Raftos D.. 2019. “Simulated Marine Heat Wave Alters Abundance and Structure of Vibrio Populations Associated with the Pacific Oyster Resulting in a Mass Mortality Event.” Microbial Ecology 77: 736–47. [DOI] [PubMed] [Google Scholar]
- Hand, S. C. , and Hardewig I.. 1996. “Downregulation of Cellular Metabolism during Environmental Stress: Mechanisms and Implications.” Annual Review of Physiology 58: 539–63. [DOI] [PubMed] [Google Scholar]
- Harley, C. D. G. , and Helmuth B. S. T.. 2003. “Local‐ and Regional‐Scale Effects of Wave Exposure, Thermal Stress, and Absolute Versus Effective Shore Level on Patterns of Intertidal Zonation.” Limnology and Oceanography 48: 1498–508. [Google Scholar]
- Hunt, H. L. , and Scheibling R. E.. 1997. “Role of Early Post‐Settlement Mortality in Recruitment of Benthic Marine Invertebrates.” Marine Ecology Progress Series 155: 269–301. [Google Scholar]
- IPCC . 2021. “Summary for policymakers.” In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, edited by V. Masson‐Delmotte, P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou. https://www.ipcc.ch/report/ar6/wg1/. [Google Scholar]
- King, T. L. , Nguyen N., Doucette G. J., Wang Z., Bill B. D., Peacock M. B., Madera S. L., Elston R. A., and Trainer V. L.. 2021. “Hiding in Plain Sight: Shellfish‐Killing Phytoplankton in Washington State.” Harmful Algae 105: 102032. [DOI] [PubMed] [Google Scholar]
- Macho, G. , Woodin S. A., Wethey D. S., and Vázquez E.. 2016. “Impacts of Sublethal and Lethal High Temperatures on Clams Exploited in European Fisheries.” Journal of Shellfish Research 35: 405–19. [Google Scholar]
- Philip, S. Y. , Kew S. F., Van Oldenborgh G. J., Yang W., Vecchi G. A., Anslow F. S., Li S., et al. 2021. “Rapid attribution analysis of the extraordinary heatwave on the Pacific Coast of the US and Canada June 2021.” Earth System Dynamics 2021: 1–34. [Google Scholar]
- Raymond, W. W. 2022. “wraymond/Ecology_heatwave‐salish‐sea‐shellfish: Accepted to Ecology (v1.0).” Zenodo. 10.5281/zenodo.6555076. [DOI]
- Wendling, C. C. , and Wegner K. M.. 2013. “Relative Contribution of Reproductive Investment, Thermal Stress and Vibrio Infection to Summer Mortality Phenomena in Pacific Oysters.” Aquaculture 412–413: 88–96. [Google Scholar]
- White, J. , Ruesink J. L., and Trimble A. C.. 2009. “The Nearly Forgotten Oyster: Ostrea lurida Carpenter 1864 (Olympia oyster) History and Management in Washington State.” Journal of Shellfish Research 28: 43–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Survey and air temperature data and statistical code are available at https://doi.org/10.5281/zenodo.6555076 (Raymond, 2022). Temperature and tide data were obtained from publically available sources and detailed in Appendix S1.


