Abstract
The contribution of granulocyte-macrophage colony-stimulating factor (GM-CSF), a hematopoietic and immunoregulatory cytokine, to resistance to blood-stage malaria was investigated by infecting GM-CSF-deficient (knockout [KO]) mice with Plasmodium chabaudi AS. KO mice were more susceptible to infection than wild-type (WT) mice, as evidenced by higher peak parasitemia, recurrent recrudescent parasitemia, and high mortality. P. chabaudi AS-infected KO mice had impaired splenomegaly and lower leukocytosis but equivalent levels of anemia compared to infected WT mice. Both bone marrow and splenic erythropoiesis were normal in infected KO mice. However, granulocyte-macrophage colony formation was significantly decreased in these tissues of uninfected and infected KO mice, and the numbers of macrophages in the spleen and peritoneal cavity were significantly lower than in infected WT mice. Serum levels of gamma interferon (IFN-γ) were found to be significantly higher in uninfected KO mice, and the level of this cytokine was not increased during infection. In contrast, IFN-γ levels were significantly above normal levels in infected WT mice. During infection, tumor necrosis factor alpha (TNF-α) levels were significantly increased in KO mice and were significantly higher than TNF-α levels in infected WT mice. Our results indicate that GM-CSF contributes to resistance to P. chabaudi AS infection and that it is involved in the development of splenomegaly, leukocytosis, and granulocyte-macrophage hematopoiesis. GM-CSF may also regulate IFN-γ and TNF-α production and activity in response to infection. The abnormal responses seen in infected KO mice may be due to the lack of GM-CSF during development, to the lack of GM-CSF in the infected mature mice, or to both.
Granulocyte-macrophage colony-stimulating factor (GM-CSF), a 23-kDa glycoprotein cytokine, is produced by a number of different cell types under a variety of circumstances. GM-CSF is produced by almost all tissues and organs and by various cell types such as activated T lymphocytes, macrophages, endothelial cells, and fibroblasts in response to cytokines, antigens, and inflammatory agents (28, 56). GM-CSF is thought to be involved in host response to microbial challenge because its expression is up-regulated during infection with various pathogens. In vivo treatment with anti-GM-CSF antibodies has detrimental effects, while treatment with recombinant GM-CSF has beneficial effects on the course of infection with organisms such as Listeria monocytogenes (6, 18, 44), Leishmania donovani (23, 48), Salmonella enterica serotype Typhimurium (22), Mycobacterium kansasii (4), and Trypanosoma cruzi (9, 29). In addition, observations of increased incidence of pulmonary and of soft tissue infections in GM-CSF gene-deficient (knockout [KO]) mice suggests impaired microbial killing in these animals (33). Following experimental infections with L. monocytogenes or Streptococcus group B, GM-CSF KO mice demonstrate increased susceptibility to infection (13, 55).
The possible mechanisms by which GM-CSF contributes to resistance to infection include regulation of hematopoiesis, regulation of cytokine production, and activation of effector functions of mature cells of the granulocytic and monocytic lineages. As a hematopoietic growth factor, GM-CSF acts on CFU of the granulocyte, macrophage, and granulocyte-macrophage lineages by stimulating proliferation and by maintaining viability of these precursor cells (14). As part of the cytokine network, GM-CSF induces monocyte and macrophage cytokine production of interleukin-6 (IL-6), IL-8, G-CSF, M-CSF, tumor necrosis factor alpha (TNF-α), and IL-1 (10, 14, 20, 21, 34, 42). GM-CSF affects various effector functions of granulocytes and macrophages. GM-CSF impairs neutrophil motility at sites of inflammation such that more phagocytes are available to combat infection and contributes to monocyte chemotaxis (4, 14, 42). In addition to affecting phagocyte migration, GM-CSF activates phagocytosis and microbial killing by neutrophils, monocytes, and macrophages (10, 20, 22). Antigen presentation by both monocytes and macrophages is enhanced by GM-CSF (1, 7, 14, 22). In addition, GM-CSF acts on humoral immunity by promoting differentiation of murine activated B cells to immunoglobulin secretion (35).
The biological effects of GM-CSF suggest that this molecule may be important in controlling Plasmodium infections. Serum levels of GM-CSF have been found to be elevated in severe human malaria caused by P. falciparum and in P. berghei-infected mice (26, 30). Up-regulation of GM-CSF mRNA expression has also been demonstrated in spleen cells from mice infected with P. yoelii (17) and in murine kidneys during a complication associated with P. berghei malaria (31). In vitro studies of neutrophils pretreated with human recombinant GM-CSF showed that this cytokine induces priming of human neutrophils for enhanced phagocytosis and killing of intraerythrocytic asexual stages of P. falciparum and up-regulates expression of complement and Fc receptors (12). Moreover, incorporation of a plasmid encoding murine GM-CSF in a DNA vaccine against the circumsporozoite protein of P. yoelii enhances the efficacy of the vaccine (52). The addition of GM-CSF to the vaccine increases antibody production, CD4+ T-cell proliferation, and IL-2 and gamma interferon (IFN-γ) responses.
To examine the role of GM-CSF in resistance to blood-stage malaria, GM-CSF KO mice were infected with P. chabaudi AS. Parasitemia and survival were measured to follow the course of infection in KO and wild-type (WT) mice. Since GM-CSF KO mice were found to be susceptible to this infection, we also characterized parameters indicative of a protective host response to infection in WT and KO mice: the development of splenomegaly, anemia, leukocytosis, and proinflammatory cytokine production, as well as bone marrow and splenic hematopoiesis.
MATERIALS AND METHODS
Experimental animals.
GM-CSF KO mice on the C57BL/6 × 129 background, generated as previously described (36), were bred at the New York Branch of The Ludwig Institute for Cancer Research from breeding stocks transferred from the Melbourne branch. The mice were maintained at the animal care facility of the Montreal General Hospital Research Institute for the duration of the experiments. Age- and sex-matched (B6 × 129)F2 mice purchased from the Jackson Laboratory (Bar Harbor, Maine) were used as WT controls in all experiments.
Parasite and infection protocol.
P. chabaudi AS was maintained in the laboratory by weekly passage as previously described (54). Mice were infected intraperitoneally with 106 P. chabaudi AS-parasitized red blood cells (PRBC), and the course and outcome of infection were monitored as previously described (38).
Preparation of cell suspensions.
Femurs and spleens were harvested aseptically after collecting heparinized blood from the ophthalmic venous plexus for hematologic studies. Femurs were flushed with 1 ml of cold Iscove's modified Dulbecco's medium (IMDM; Gibco-BRL, Burlington, Ontario, Canada) supplemented with 5% fetal calf serum (FCS; HyClone, Logan, Utah), 0.12% gentamicin (Schering Canada, Montreal, Quebec, Canada), and 2 mM glutamine (Gibco). Spleens were minced and passed through a sterile, fine wire mesh to obtain single-cell suspensions. Single-cell suspensions of spleen cells were resuspended in 15 ml of RPMI 1640 (Gibco) containing 10% FCS, 2% HEPES, and 0.12% gentamicin and centrifuged at 300 × g at 5°C for 10 min. Erythrocytes were lysed with cold 0.17 M NH4Cl, the cells were washed with Hanks' balanced salt solution (Gibco), and erythrocyte ghosts were removed by filtering cell suspensions through sterile gauze. Bone marrow and spleen cells were washed three times in IMDM. Total, viable cell counts were obtained using 0.1% trypan blue, and differential cell counts were determined on Diff-Quick (Baxter, McGaw Park, Ill.)-stained cytocentrifuge preparations. For hematopoietic assays, nucleated cells were counted using Turk's fluid and suspended at a concentration of 4 × 106 cells per ml for erythroid burst-forming unit (BFU-E) and granulocyte-macrophage CFU (CFU-GM) assays and at 2 × 106 cells per ml for erythroid CFU (CFU-E) assays.
Peritoneal cells from WT and KO mice were collected by peritoneal lavage using 10 ml of complete RPMI 1640 medium. The cells were washed and resuspended in 1.0 ml of culture medium. Total cell numbers and the percentages and numbers of macrophages were determined as described above.
Hematopoietic progenitor assays.
The numbers of BFU-E, CFU-E, and CFU-GM in single-cell suspensions from bone marrow and spleen were determined in colony-forming assays performed in semisolid media by previously described procedures (54). Briefly, CFU-E medium contained 0.8% methylcellulose from Iscove's 2.3% basic stock solution (Stem Cell Technologies, Vancouver, British Columbia, Canada), 30% FCS, 200 mU of recombinant human erythropoietin (tissue culture grade; R&D Systems, Minneapolis, Minn.) per ml, 2 mM glutamine, and 5 × 10−5 M 2-mercaptoethanol (Sigma, St. Louis, Mo.) in IMDM. Aliquots of 1.0 ml of bone marrow and spleen cells were plated in 35-mm-diameter dishes with grids (Sarstedt, Montreal, Quebec, Canada) at densities of 2 × 105 and 4 × 105 cells, respectively. For each mouse, triplicate cultures were established for both cell types. Dishes were incubated at 37°C in a humidified 5% CO2 incubator, and hemoglobin-synthesizing benzidine-positive colonies of eight or more cells were counted after 48 h. The BFU-E and CFU-GM medium consisted of 0.8% methylcellulose, 30% FCS, 10% pokeweed mitogen spleen cell-conditioned medium (SCM), 2,000 mU of erythropoietin per ml, 0.1 mM hemin (Eastman Kodak, Rochester, N.Y.), 2 mM glutamine, and 5 × 10−5 M 2-mercaptoethanol in IMDM. Cells were cultured at the same density as described for CFU-E cultures. Golden brown hemoglobinized colonies with at least 50 cells were scored for BFU-E counts, and CFU-GM counts were determined according to colony morphology after 7 days of incubation in a humidified 5% CO2 incubator at 37°C. Based on total spleen or bone marrow cell numbers, the final BFU-E, CFU-E, and CFU-GM counts are expressed per femur or per spleen.
Hematological analysis.
Hematocrit and total erythrocyte and leukocyte counts were determined on heparinized blood from individual mice using standard hematological procedures. The percentages of reticulocytes and of leukocyte populations were determined on Diff-Quick-stained blood smears prepared from tail vein blood of individual mice.
Cytokine ELISAs.
At the indicated times, blood samples were obtained from WT and KO mice by cardiac puncture and allowed to clot. Sera were separated by centrifugation at 13,800 × g for 30 s. Sera were stored at 4°C and analyzed for IFN-γ and TNF-α levels by two-site sandwich enzyme-linked immunosorbent assays (ELISAs) as previously described (11, 40).
Statistical analysis.
Data are presented as the mean ± standard error of the mean (SEM). Statistical significance of differences in means between two groups of mice was determined by Student's t test, with P < 0.05 considered significant. Data for mortality were analyzed using the nonparametric Kolmogorov-Smirnov two-sample test; an α value of <0.05 is significant. Data from male and female mice, except data for parasitemia and mortality, were pooled.
RESULTS AND DISCUSSION
Course of infection with P. chabaudi AS in WT and KO mice.
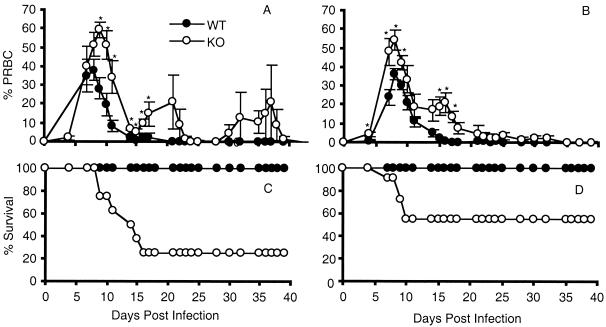
To determine whether GM-CSF deficiency affects resistance to blood-stage malaria, WT and KO mice were infected intraperitoneally with 106 P. chabaudi AS PRBC, and the course and outcome of infection were compared. WT mice had moderate levels of parasitemia, with peaks of 38 ± 6% PRBC in male mice (Fig. 1A) and 36 ± 3% PRBC in female mice (Fig. 1B); mice of both sexes cleared the infection by 4 weeks and exhibited 100% survival (Fig. 1C and D). In terms of parasitemia and survival in response to P. chabaudi AS infection, the WT mice used here are comparable to mice of the C57BL/6 strain from which they are derived. C57BL/6 mice are resistant to P. chabaudi AS, experience a peak parasitemia of approximately 35 to 40%, and have a Th1 response during the early, acute phase of infection (38, 39, 47). During the later phase, at about 2 weeks postinfection, the immune response switches to a Th2 response, which is characterized by clearance of the parasite and the production of cytokines important for B-cell activation and antibody production (39, 47). It is likely that WT mice experience the same sequence of protective Th cell-mediated immune responses as C57BL/6 mice, although we did not address this question in the present experiments.
FIG. 1.
Course of P. chabaudi AS infection in male (A and C) and female (B and D) WT and GM-CSF KO mice. (A and B) Course of parasitemia; (C and D) percent survival. Results are pooled from two replicate experiments and are expressed as mean ± SEM of 2 to 15 mice per genotype at each time point. Similar results were obtained in a third experiment. For panels A and B, statistically significant differences between WT and KO mice are indicated (∗, P < 0.05). Mortality (C and D) was analyzed using the Kolmogorov-Smirnov two-sample test (α < 0.01 for male WT and α = 0.02 for female WT versus KO mice).
In contrast, GM-CSF deficiency affected both the early and late phases of infection, resulting in significantly higher peak parasitemia in GM-CSF KO mice than in their WT counterparts (P < 0.01 in females; P < 0.05 in males) and in recurrent and significant recrudescence parasitemia later in infection (Fig. 1A and B). Peak parasitemia levels in P. chabaudi AS-susceptible A/J mice are >50% PRBC, while the peak levels attained by KO mice in the present experiments were 59% ± 4% and 54% ± 5% in male and female mice, respectively (38, 54). Compared to the WT control mice, mortality was significantly increased to 75% in male (α < 0.01) and 46% in female (α = 0.02) KO mice (Fig. 1C and D). Recovery was delayed until day 40 in some GM-CSF KO mice, and mortality occurred through day 16 postinfection. Among susceptible A/J mice, blood-stage P. chabaudi AS infection is usually 100% lethal within a few days of peak parasitemia, which occurs 9 to 12 days postinfection; the severe course and outcome of infection in this mouse strain correlates with an early Th2 response (38, 39).
These results suggest that GM-CSF is an important cytokine in the protective host response to P. chabaudi AS infection. GM-CSF may be required because of its effects on hematopoiesis and/or on the immune response to blood-stage malaria (28, 42). Alternatively, or in addition, the presence of GM-CSF during mouse development may be important in the development of a normal immune system and the subsequent ability of the mature immune response to mount a protective response to infection. While the course and outcome of infection in WT mice were similar to results for resistant C57BL/6 mice, KO mice had similar peak parasitemia levels but delayed and lower mortality than susceptible A/J mice. Although male KO mice displayed higher peak parasitemia (59 ± 4 versus 54 ± 5% PRBC) and lower survival (25% versus 54%) than female KO mice, the differences were not significant (P > 0.05), unlike what has been observed in P. chabaudi AS infections in KO mice deficient in other cytokines (32, 41). Consequently, data from males and females were pooled for the other parameters studied.
Development of splenomegaly during P. chabaudi AS infection in WT and KO mice.
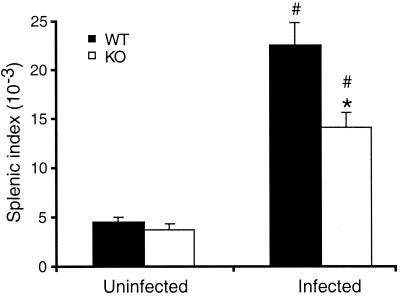
To investigate the underlying basis of the increased susceptibility of KO mice to malaria, we monitored parameters indicative of a protective host response to P. chabaudi AS infection in WT and KO mice. Since the degree of splenomegaly correlates with the level of resistance to P. chabaudi AS infection (37), we examined splenomegaly in WT and KO mice by determining the splenic index on day 7 postinfection. As shown in Fig. 2, there was no significant difference in the splenic indices of uninfected WT and uninfected KO mice (P = 0.27). Both strains of mice experienced significant splenomegaly on day 7 compared to their uninfected counterparts, but the splenic index was significantly higher in infected WT than in infected KO mice (P < 0.01), suggesting a role for GM-CSF in the development of malarial splenomegaly.
FIG. 2.
Increases in splenic index during P. chabaudi AS infection in WT and GM-CSF KO mice. Body and spleen weights of uninfected and infected mice were determined on day 7 postinfection. The splenic index was determined as the ratio of spleen weight to body weight. Data from male and female mice of each genotype were not significantly different and were pooled. Data are presented as mean ± SEM of five to seven mice per genotype analyzed individually. Statistically significant differences between infected WT and KO mice (∗, P < 0.01) and between infected and uninfected mice of each genotype (#, P < 0.001) are indicated.
The spleen is an important site of erythropoiesis as well as a site of PRBC clearance and immune system activation in response to blood-stage malaria (50, 54). There is expansion of the erythroid compartment as well as of the lymphoid, macrophage, and stromal compartments (51). As described below, our data suggest that it is unlikely that expansion of the splenic erythroid compartment was deficient in P. chabaudi AS-infected GM-CSF KO mice. Furthermore, total splenic leukocyte numbers in these mice were increased to levels comparable to those of WT mice on day 7 postinfection ([206 ± 27] × 106 for WT mice versus [223 ± 12] × 106 for KO mice; P > 0.05). However, the numbers of peripheral blood leukocytes were significantly lower in infected KO mice than in WT mice, and there were deficiencies in CFU-GM production in the spleen as well as the bone marrow of infected KO mice (see below).
Alternatively, inefficient splenomegaly in KO mice may be due to decreased sequestration of erythrocytes by the splenic reticuloendothelial system. Splenic clearance of parasitized as well as of uninfected erythrocytes is increased during malaria infections and is associated with splenomegaly as well as contributing to malarial anemia in humans and experimental animals (2, 15, 16, 27, 49). While splenic clearance is increased only after peak parasitemia in some models of malaria infection (2, 49), Yadava et al. (53) observed increased uptake of erythrocytes before peak parasitemia in P. chabaudi adami-infected mice. The decreased splenomegaly observed in KO mice on day 7 postinfection (before peak parasitemia), thus, may be associated with decreases in splenic uptake and clearance of infected erythrocytes which may contribute to impaired resolution of the infection and high parasitemia levels in these mice. Expansion of the stromal compartment may also be impaired in GM-CSF-deficient animals, leading to abnormal development of a blood-spleen barrier as observed in P. yoelii infections (49). Thus, the inadequate splenomegaly seen in P. chabaudi AS-infected GM-CSF KO mice may be due to deficiencies in expansion of one or many splenic compartments (erythroid, lymphoid, macrophage, or stromal), and splenic functions of hematopoiesis and clearance and sequestration of PRBC may be altered.
Leukocytosis and anemia during P. chabaudi AS infection in WT and KO mice.
Previous studies have shown that exogenous GM-CSF increases the numbers of peripheral blood leukocytes in mice infected with Leishmania donovani (23) or L. monocytogenes (6). Hence, we investigated the role of endogenous GM-CSF in regulating the numbers of peripheral blood leukocytes during P. chabaudi AS infection. Uninfected WT and KO mice had similar numbers and percentages of leukocytes in the blood (Table 1). On day 7 postinfection, there were significant and similar increases in the percentages of polymorphonuclear leukocytes and monocytes together with significant and similar decreases in the percentages of lymphocytes in both WT and KO mice compared to their uninfected counterparts. There were no significant differences between the two genotypes. However, total numbers of leukocytes as well as the numbers of lymphocytes and monocytes were significantly and markedly lower in KO mice than in WT mice on day 7 postinfection (P < 0.05). The number of polymorphonuclear leukocytes was lower in infected KO mice than in WT mice, but the difference was not significant. These results demonstrate that GM-CSF is required to induce increased numbers of peripheral blood leukocytes and absolute numbers of granulocytes and monocytes during blood-stage malaria but that the distribution of the cell types is not aberrant in mice lacking this cytokine.
TABLE 1.
Hematological parameters in WT and KO mice during P. chabaudi AS infectiona
| Mice | Leukocyte count (103/mm3) | Differential cell count (103/mm3)
|
Hematocrit (%) | Erythrocyte count (106/mm3) | ||
|---|---|---|---|---|---|---|
| Lymphocytes | Granulocytes | Monocytes | ||||
| Uninfected | ||||||
| WT | 11.7 ± 2.2 | 8.97 ± 2.2 (72 ± 3) | 2.31 ± 0.18 (24 ± 2) | 0.34 ± 0.06 (3.4 ± 0.1) | 53.7 ± 0.7 | 9.4 ± 0.3 |
| KO | 13.2 ± 1.9 | 9.56 ± 1.3 (74 ± 2) | 3.11 ± 0.6 (23 ± 2) | 0.4 ± 0.08 (2.9 ± 0.3) | 55.1 ± 0.3 | 9.3 ± 0.3 |
| Infected | ||||||
| WT | 22.3 ± 4.4 | 9.92 ± 2.1 (45 ± 2)b | 10.26 ± 1.95b (46 ± 2)b | 2.16 ± 0.51b (9.2 ± 1.3)b | 34.0 ± 3.8b | 6.0 ± 0.8b |
| KO | 9.84 ± 2.1d | 4.1 ± 0.8cd (44 ± 5)c | 5.0 ± 1.4c (48 ± 6)c | 0.74 ± 0.17d (7.9 ± 1.4)c | 43.4 ± 3.9c | 6.9 ± 0.8c |
Peripheral blood was collected from uninfected or day 7 infected WT and KO mice and was analyzed using standard hematological procedures. Data shown are mean ± SEM for five to seven individual mice per group. The percentage of each leukocyte population is shown in parentheses.
Statistically significant difference compared to uninfected WT mice (P < 0.01).
Statistically significant difference compared to uninfected KO mice (P < 0.05).
Statistically significant difference between infected KO and infected WT mice (P < 0.05).
We also examined the levels of anemia in WT and KO mice by determining hematocrits and the numbers of erythrocytes in peripheral blood. The severity of anemia has been observed to correlate with the severity of P. chabaudi AS infection in resistant C57BL/6 and susceptible A/J mice (38, 54). On day 7 postinfection, both WT and KO mice had significantly lower hematocrits and significantly fewer erythrocytes than uninfected mice, as expected (Table 1). There were no significant differences in these parameters between infected WT and KO mice at this time or on day 15 postinfection (data not shown). Interestingly, GM-CSF has been shown to be involved in murine autoimmune hemolytic anemia by enhancing erythrophagocytosis (5). Treatment with recombinant GM-CSF has also been associated with anemia due to accelerated hemolysis in a human subject (24). Our results, however, suggest that GM-CSF either does not contribute to malarial anemia or that compensatory mechanisms, such as increased erythropoiesis, may replace lost erythrocytes.
Erythropoiesis during P. chabaudi AS infection in WT and KO mice.
It has been shown that susceptible A/J mice experience higher parasitemia together with more severe anemia and defective splenic erythropoiesis during P. chabaudi AS infection compared to resistant C57BL/6 mice (54). Since high parasitemia was not associated with severe anemia during P. chabaudi AS infection in GM-CSF KO mice, we investigated if erythropoiesis in these mice is increased normally to compensate for erythrocyte destruction. The effects of GM-CSF on erythropoiesis are unclear. Studies have shown that GM-CSF inhibits BFU-E growth (45, 46), while others have reported stimulation of BFU-E and CFU-E proliferation by this growth factor (19). As shown in Table 2, there were no significant differences between uninfected WT and KO mice in the numbers of either BFU-E or CFU-E in bone marrow and spleen, consistent with a previous observation in these mice (36). The number of BFU-E in the bone marrow of WT but not KO mice was significantly higher in infected than in uninfected animals. Infection also resulted in significant increases in the numbers of CFU-E in the bone marrow of both WT and KO mice. Amplification of erythropoiesis was even more prominent in the spleen than the femora of infected animals, as previously described (51, 54). Approximately 3-fold increases in BFU-E and 30-fold increases in CFU-E numbers were apparent in the spleens of infected WT as well as KO mice. The increases were significant in both infected WT and KO mice compared to their uninfected counterparts, and there were no significant differences between the genotypes. As another measure of the erythropoietic response, we determined the percentage of reticulocytes in the peripheral blood (Table 2). While the increases in erythroid progenitors in response to P. chabaudi AS infection are usually seen on day 7 postinfection, reticulocytes begin to be released into the blood of C57BL/6 mice on day 12 (54). Consequently, we examined the frequency of reticulocytes on day 15 postinfection. Both WT and KO mice were observed to have significantly higher percentages of reticulocytes than their uninfected counterparts, and there was no significant difference between the genotypes. Thus, the erythropoietic response in GM-CSF-deficient mice during P. chabaudi AS infection was both appropriate to the degree of anemia and equivalent to that of infected WT mice, suggesting that GM-CSF deficiency does not alter the erythropoietic response to malarial anemia.
TABLE 2.
Erythropoiesis in WT and GM-CSF KO mice during P. chabaudi AS infection
| Mice | No. of progenitor cells (103/organ)a
|
% Reticulocytesb | |||
|---|---|---|---|---|---|
| Bone marrow
|
Spleen
|
||||
| BFU-E | CFU-E | BFU-E | CFU-E | ||
| Uninfected | |||||
| WT | 2.4 ± 0.2 | 4.8 ± 0.2 | 6.9 ± 0.5 | 7.4 ± 0.8 | 1.5 ± 0.3 |
| KO | 3.2 ± 0.4 | 3.9 ± 0.3 | 8.1 ± 1.6 | 5.9 ± 0.7 | 1.7 ± 0.3 |
| Infected | |||||
| WT | 3.3 ± 0.2c | 9.2 ± 5.0c | 25.3 ± 3.4d | 230 ± 45c | 22.5 ± 1.8d |
| KO | 3.4 ± 0.2 | 9.0 ± 3.3e | 23.5 ± 1.1e | 206 ± 22e | 27.2 ± 2.6e |
Mean ± SEM for five to seven individual mice per group, determined on day 7 postinfection.
Determined on day 15 postinfection.
Statistically significant difference compared to uninfected WT mice (P < 0.01).
Statistically significant difference compared to uninfected WT mice (P < 0.0001).
Statistically significant difference compared to uninfected KO mice (P < 0.001).
Granulocyte and macrophage hematopoiesis and macrophage numbers in tissues during P. chabaudi AS infection in WT and KO mice.
To further assess the role of GM-CSF in hematopoiesis during P. chabaudi AS infection, we also determined the numbers of early granulocyte-macrophage precursors or CFU-GM in the bone marrow and spleen. Although GM-CSF contributes to granulocyte and monocyte-macrophage hematopoiesis in normal mice, experiments in mice lacking GM-CSF suggest that GM-CSF is dispensable for normal steady-state hematopoiesis (36). Infection of GM-CSF KO mice with a high dose of L. monocytogenes, however, was observed to result in significantly decreased total bone marrow cellularity, due to deficiency in the number of granulocytes, and lower numbers of inflammatory macrophages in the peritoneal cavity (55). Together, these results suggest the importance of this molecule in emergency hematopoiesis.
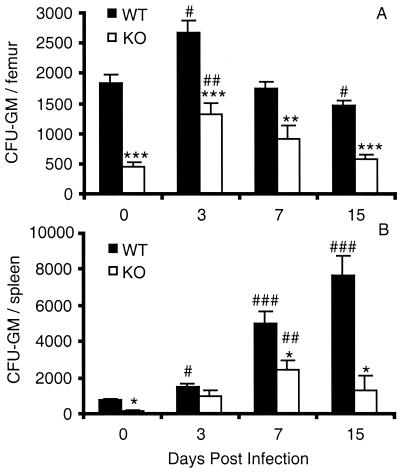
In this study, total femoral bone marrow cell numbers were not significantly different between uninfected or infected WT and KO mice (data not shown). Nevertheless, the numbers of bone marrow CFU-GM were significantly lower in KO than in WT mice (Fig. 3A). This was evident in both uninfected and infected KO mice through day 15 postinfection. There were, however, significant increases in both genotypes compared to uninfected mice on day 3. In contrast, Stanley et al. (36) observed that the frequencies of granulocyte and macrophage precursors were not significantly different between normal WT and KO mice. These investigators used various recombinant growth factors including GM-CSF, while the medium used here to stimulate the growth of CFU-GM contained SCM. Subsequent studies by Seymour et al. (33) showed that bone marrow cells from normal KO mice stimulated with SCM had lower frequencies of CFU-GM than WT mice but the difference was not significant, possibly because of low sample sizes.
FIG. 3.
Production of CFU-GM in bone marrow (A) and spleens (B) of WT and GM-CSF KO mice during P. chabaudi AS infection. Single-cell suspensions from each organ were prepared and cultured for CFU-GM as described in the text. Data from male and female mice of each genotype were not significantly different and were pooled. Data are expressed as the number of CFU-GM per organ and represent mean ± SEM of five to seven mice per genotype analyzed individually. Statistically significant differences between WT and KO mice (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001) and between infected and uninfected mice of each genotype (#, P < 0.05; ##, P < 0.01; ###, P < 0.001) are indicated.
The numbers of splenic CFU-GM were also significantly lower in KO than in WT mice on day 0 and on days 7 and 15 postinfection (Fig. 3B). While WT mice experienced progressive and significant increases in splenic CFU-GM numbers during infection, the numbers of splenic GM-CFU in KO mice were significantly higher than in uninfected KO mice only on day 7 postinfection (P < 0.01). Studies by Stanley et al. (36) have shown that splenic CFU-GM frequencies in response to SCM are significantly higher in uninfected KO than in WT mice, while a second study showed there is no significant difference between uninfected WT and KO mice (33).
Although the numbers of bone marrow and splenic CFU-GM were significantly lower in KO than in WT mice during infection, the numbers of macrophages in the spleen and peritoneal cavity were not significantly different between the two strains of mice during the first week of infection (data not shown). On day 15 postinfection, however, WT mice had significant increases in macrophage numbers in both tissues whereas KO mice had significantly lower numbers of macrophages than WT mice in the spleen ([60 ± 10] × 106 for WT mice versus [23 ± 6] × 106 for KO mice; P = 0.03) and peritoneal cavity ([2.3 ± 0.3] × 106 for WT mice versus KO [0.93 ± 0.08] × 106 for KO mice; P = 0.006). The differences in the numbers of tissue macrophages may reflect the observed deficiency in CFU-GM in the bone marrow and spleen of infected KO mice and likely contribute to the severity of malaria in these mice. In addition, these differences may reflect a defect in macrophage inflammation in malaria-infected GM-CSF KO animals.
Proinflammatory cytokine production during P. chabaudi AS infection in WT and KO mice.
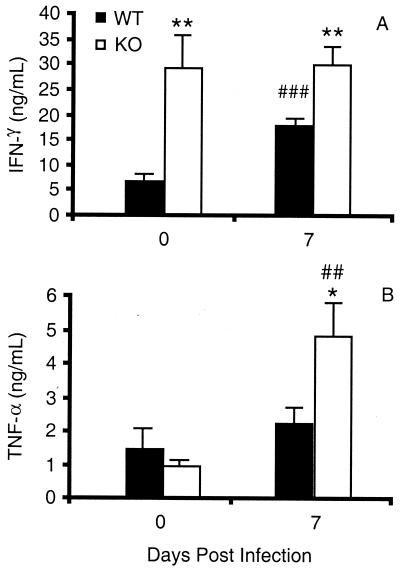
We also analyzed the levels of two important proinflammatory cytokines, IFN-γ and TNF-α, in the sera of WT and KO mice during malaria. The level of IFN-γ was significantly higher in uninfected KO mice than in their WT counterparts (P < 0.01) and on day 7 (P < 0.01) after infection with P. chabaudi AS (Fig. 4A). It is important to point out that while IFN-γ levels were significantly increased on day 7 in WT mice (P < 0.001), IFN-γ levels in infected and uninfected KO mice were similar. This suggests that regulation of IFN-γ production may be perturbed in GM-CSF KO mice. Previous studies suggest that the relationship between GM-CSF and IFN-γ is complex and dependent on the experimental system. T cells from KO mice immunized with keyhole limpet hemocyanin were found to be deficient in IFN-γ production in vitro, and KO mice challenged with lipopolysaccharide in vivo had lower levels of IFN-γ in the sera compared to WT mice (3, 25, 43). On the other hand, it has also been reported that treatment with exogenous IL-12 induces normal serum levels of IFN-γ in mice lacking GM-CSF (25) and that infection with Streptococcus group B leads to significantly higher levels of IFN-γ in lung homogenates of KO compared to WT mice (13). Moreover, GM-CSF has been shown to up-regulate IFN-γ receptor expression in vitro (8).
FIG. 4.
Serum levels of IFN-γ (A) and TNF-α (B) in WT and GM-CSF KO mice during P. chabaudi AS infection. Serum cytokine levels were determined by ELISA. Data from male and female mice of each genotype were not significantly different and were pooled. Data are presented as mean ± SEM of five or seven mice per time point analyzed individually. Statistically significant differences between WT and KO mice (∗, P < 0.05; ∗∗, P < 0.01) and between infected and uninfected mice of each genotype (##, P < 0.01; ###, P < 0.001) are indicated.
TNF-α production was also dysregulated in KO mice during P. chabaudi AS infection (Fig. 4B). Uninfected WT and KO mice had similar serum levels of this important proinflammatory cytokine. On day 7 postinfection, the level of TNF-α in KO mice was significantly increased compared to infected WT (P < 0.05) as well as uninfected KO (P < 0.01) mice. This observation is consistent with our previous observation of higher levels of TNF-α in the sera of susceptible A/J compared to resistant C57BL/6 mice around the time of peak parasitemia just before death occurs (11). A massive release of malaria antigens into the circulation of susceptible mice due to rupture of PRBC may result in higher levels of serum TNF-α in susceptible hosts. Alternatively, TNF-α may persist longer in the circulation of KO mice during malaria, as suggested by the results of a study of GM-CSF-deficient mice treated with lipopolysaccharide in vivo (3).
Because of the detrimental effects of high levels of TNF-α as well as of IFN-γ, the higher levels of these proinflammatory cytokines in KO mice may contribute to the high mortality of the animals in response to P. chabaudi AS infection. Alternatively, high levels of these two cytokines may also represent beneficial but unsuccessful attempts to control a more severe infection. Downstream effector mechanisms, possibly involving macrophages, may be deficient in these animals. This deficiency may be related to insufficient numbers of effector macrophages as we observed in infected GM-CSF KO mice as well as to qualitative differences in effector function per se. Other cytokines, such as IL-12, IL-4, and IL-10, and the effector molecule NO are also involved in the protective immune response to malaria. Further studies will be needed to characterize the cytokine response in P. chabaudi AS-infected KO mice.
In conclusion, we demonstrate that GM-CSF is an important cytokine in resistance to blood-stage malaria. Mice deficient in GM-CSF experienced higher levels of peak parasitemia, recrudescent parasitemia, and high mortality compared to WT mice. The underlying basis of the severity of blood-stage malaria in KO mice appears to be due to defects in important immune responses required for control of P. chabaudi AS infection. Each of these abnormalities may contribute to the high mortality of the KO mice: P. chabaudi AS-infected KO mice have impaired development of splenomegaly, impaired granulocyte-macrophage hematopoiesis in bone marrow and spleen, deficiencies in the numbers of peripheral blood leukocytes and tissue macrophages, and perturbed proinflammatory cytokine production. These responses may be defective in KO mice because of the lack of GM-CSF during the infection and/or may be a consequence of the lack of GM-CSF during mouse development. Normal immune system development may be impaired in the absence of GM-CSF, leading to a defective, mature immune response to infection. Taken together, our observations indicate that GM-CSF plays a critical role in protective immunity to blood-stage malaria due to its hematopoietic, immunoregulatory, and/or developmental properties.
ACKNOWLEDGMENT
This work was supported by a grant from the Medical Research Council of Canada (MT14663) to M.M.S.
REFERENCES
- 1.Alderson M R, Armitage R J, Tough T W, Strockbine L, Fanslow W C, Spriggs M K. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves H J, Weidanz W, Weiss L. The spleen in murine Plasmodium chabaudi adami malaria: stromal cells, T lymphocytes and hematopoiesis. Am J Trop Med Hyg. 1996;55:370–378. doi: 10.4269/ajtmh.1996.55.370. [DOI] [PubMed] [Google Scholar]
- 3.Basu S, Dunn A R, Marino M W, Savoia H, Hodgson G, Lieschke G J, Cebon J. Increased tolerance to endotoxin by granulocytemacrophage colony-stimulating factor-deficient mice. J Immunol. 1997;159:1412–1417. [PubMed] [Google Scholar]
- 4.Bermudez L E, Kemper C A, Deresinski S C. Dysfunctional monocytes from a patient with disseminated Mycobacterium kansasii infection are activated in vitro and in vivo by GM-CSF. Biotherapy. 1995;8:135–142. doi: 10.1007/BF01878497. [DOI] [PubMed] [Google Scholar]
- 5.Berney T, Shibata T, Merino R, Chicheportiche Y, Kindler V, Vassalli P, Izui S. Murine autoimmune hemolytic anemia resulting from Fcγ receptor-mediated erythrophagocytosis: protection by erythropoietin but not by interleukin-3, and aggravation by granulocyte-macrophage colony-stimulating factor. Blood. 1992;79:2960–2964. [PubMed] [Google Scholar]
- 6.Buisman A M, Langermans J A M, Van Furth R. Effect of granulocyte-macrophage colony-stimulating factor on the number of leucocytes and course of Listeria monocytogenes infection in naive and leucocytopenic mice. Immunology. 1998;93:73–79. doi: 10.1046/j.1365-2567.1998.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk L A, Wahl L M, Vogel S N. Analysis of Ia antigen expression in macrophages derived from bone marrow cells cultured in granulocyte-macrophage colony-stimulating factor or macrophage colony-stimulating factor. J Immunol. 1988;140:2652–2659. [PubMed] [Google Scholar]
- 8.Finbloom D S, Larner A C, Nakagawa Y, Hoovert D L. Culture of human monocyte with granulocyte-macrophage colony-stimulating factor results in enhancement of IFN-γ receptors but suppression of IFN-γ-induced expression of the gene IP-10. J Immunol. 1993;150:2383–2390. [PubMed] [Google Scholar]
- 9.Fontt E O, Heirman C, Thielemans K, Vray B. Granulocyte-macrophage colony-stimulating factor: involvement in control of Trypanosoma cruzi infection in mice. Infect Immun. 1996;64:3429–3434. doi: 10.1128/iai.64.8.3429-3434.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich S, Gong J H, Schmidt A, Nain M, Gemsa D. Macrophage activation by granulocyte-macrophage colony-stimulating factor. J Immunol. 1989;143:1198–1205. [PubMed] [Google Scholar]
- 11.Jacobs P, Radzioch D, Stevenson M M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumaratilake L M, Ferrante A, Jaeger T, Rzepczyk C. GM-CSF-induced priming of human neutrophils for enhanced phagocytosis and killing of asexual blood-stages of Plasmodium falciparum: synergistic effects of GM-CSF and TNF. Parasite Immunol. 1996;18:115–123. doi: 10.1046/j.1365-3024.1996.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 13.LeVine A M, Reed J A, Kurak K E, Cianciolo E, Whitsett J A. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Investig. 1999;103:563–569. doi: 10.1172/JCI5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liehl E, Hildebrandt J, Lam C, Mayer P. Prediction of the role of granulocyte-macrophage colony-stimulating factor in animals and man from in vitro results. Eur J Clin Microbiol Infect Dis. 1994;13:S9–S17. doi: 10.1007/BF01973596. [DOI] [PubMed] [Google Scholar]
- 15.Looareesuwan S, Ho M, Wattanagoon Y, White N J, Warrell D A, Bunnag D, Harinasuta T, Wyler D J. Dynamic alteration in splenic function during acute falciparum malaria. N Engl J Med. 1987;317:679–679. doi: 10.1056/NEJM198709103171105. [DOI] [PubMed] [Google Scholar]
- 16.Looareesuwan S, Mery A H, Phillips R E, Pleehachinda R, Wattanagoon Y, Ho M, Charoenlarp P, Warrell D A, Weatherall D J. Reduced erythrocyte survival following clearance of malarial parasitemia in Thai patients. Br J Haematol. 1987;67:473–478. doi: 10.1111/j.1365-2141.1987.tb06171.x. [DOI] [PubMed] [Google Scholar]
- 17.Lucas B, Smith K, Haque A. Plasmodium yoelii in mice: differential induction of cytokine gene expression during hyporesponsiveness induction and restimulation. Cell Immunol. 1995;160:79–90. doi: 10.1016/0008-8749(95)80012-8. [DOI] [PubMed] [Google Scholar]
- 18.Magee D M, Wing E J. Secretion of colony-stimulating factors by T cell clones. Role in adoptive protection against Listeria monocytogenes. J Immunol. 1989;143:2336–2341. [PubMed] [Google Scholar]
- 19.Metcalf D, Nicola N A. The regulatory factors controlling murine erythropoiesis in vitro. Prog Clin Biol Res. 1984;148:93–105. [PubMed] [Google Scholar]
- 20.Morrissey P J, Bressler L, Charrier K, Alpert A. Response of resident murine peritoneal macrophages to in vivo administration of granulocyte-macrophage colony-stimulating factor. J Immunol. 1988;140:1910–1915. [PubMed] [Google Scholar]
- 21.Morrissey P J, Bressler L, Park L S, Alpert A, Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presentation cells. J Immunol. 1987;139:1113–1119. [PubMed] [Google Scholar]
- 22.Morrissey P J, Charrier K. GM-CSF administration augments the survival of ITY-resistant A/J mice, but not ITY-susceptible C57BL/6 mice, to a lethal challenge with Salmonella typhimurium. J Immunol. 1990;144:557–561. [PubMed] [Google Scholar]
- 23.Murray H W, Cervia J S, Hariprashad J, Taylor A P, Stoeckle M Y, Hockman H. Effect of granulocyte-macrophage colony-stimulating factor in experimental visceral leishmaniasis. J Clin Investig. 1995;3:1183–1192. doi: 10.1172/JCI117767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan F E, Besa E C. GM-CSF and accelerated hemolysis. N Engl J Med. 1992;526:417. doi: 10.1056/NEJM199202063260617. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi Y, Wada H, Marino M W, Old L J. Regulation of IFN-γ production in granulocyte-macrophage colony-stimulating factor-deficient mice. Eur J Immunol. 1998;28:3980–3988. doi: 10.1002/(SICI)1521-4141(199812)28:12<3980::AID-IMMU3980>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Owhasi M, Kirai N, Asami M. Temporary appearance of a circulating granulocyte-macrophage colony-stimulating factor in lethal murine malaria. South Asian J Trop Med Public Health. 1996;27:530–534. [PubMed] [Google Scholar]
- 27.Quinn T C, Wyler D J. Intravascular clearance of parasitized erythrocytes in rodent malaria. J Clin Investig. 1979;63:1187–1194. doi: 10.1172/JCI109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasko J E J, Gough N M. Granulocyte-macrophage colony-stimulating factor. In: Thomson A, editor. The cytokine handbook. London, England: Academic Press Limited; 1994. pp. 343–369. [Google Scholar]
- 29.Reed S G, Nathan C F, Pihl D L, Rodricks P, Shanebeck K, Conlon P J, Grabstein K H. Recombinant granulocyte-macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. J Exp Med. 1987;12:1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ringwald P, Peyron F, Vuillez J P, Touze J E, Le Bras J, Deloron P. Levels of cytokines in plasma during Plasmodium falciparum malaria attacks. J Clin Microbiol. 1991;29:2076–2078. doi: 10.1128/jcm.29.9.2076-2078.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rui-Mei L, Kara A U, Sinniah R. Dysregulation of cytokine expression in tubulointerstitial nephritis associated with murine malaria. Kidney Int. 1998;53:845–852. doi: 10.1111/j.1523-1755.1998.00848.x. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 1999;67:4435–4442. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seymour J F, Lieschke G J, Grail D, Quilici C, Hodgson G, Dunn A R. Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survival. Blood. 1997;90:3037–3049. [PubMed] [Google Scholar]
- 34.Sisson S D, Dinarello C A. Production of interleukin-1α, interleukin-1β and tumor necrosis factor by human mononuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood. 1988;72:1368–1374. [PubMed] [Google Scholar]
- 35.Snapper C M, Moorman M A, Rosas F R, Kehry M R, Maliszewski C R, Mond J J. IL-3 and granulocyte-macrophage colony-stimulating factor strongly induce Ig secretion by sort-purified murine B cells activated through the membrane Ig, but not the CD40, signaling pathway. J Immunol. 1995;154:5842–5850. [PubMed] [Google Scholar]
- 36.Stanley E, Lieschke G J, Grail D, Metcalf D, Hodgson G, Gall J A M, Maher D W, Cebon J, Sinickas V, Dunn A R. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson M M, Skamene E. Murine malaria: resistance of AXB/BXA recombinant inbred mice to Plasmodium chabaudi. Infect Immun. 1985;47:452–456. doi: 10.1128/iai.47.2.452-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevenson M M, Lyanga J J, Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982;38:80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevenson M M, Tam M-F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993;92:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevenson M M, Tam M-F, Belosevic M, van der Meide P H, Podoba J E. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infect Immun. 1990;58:3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su Z, Stevenson M M. Central role of endogenous gamma interferon in protective immunity against acute blood-stage Plasmodium chabaudi AS infection. Infect Immun. 2000;68:4399–4406. doi: 10.1128/iai.68.8.4399-4406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarr P E. Granulocyte-macrophage colony-stimulating factor and the immune system. Med Oncol. 1996;13:133–140. doi: 10.1007/BF02990841. [DOI] [PubMed] [Google Scholar]
- 43.Wada H, Noguchi Y, Marino M W, Dunn A R, Old L J. T cell functions in granulocyte-macrophage colony-stimulating factor deficient mice. Proc Natl Acad Sci USA. 1997;94:12557–12561. doi: 10.1073/pnas.94.23.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner R D, Czuprynski C J. Cytokine mRNA expression in livers of mice infected with Listeria monocytogenes. J Leukoc Biol. 1993;53:525–531. doi: 10.1002/jlb.53.5.525. [DOI] [PubMed] [Google Scholar]
- 45.Wang C Q, Udupa K B, Lipschitz D A. Interferon-γ exerts its negative regulatory effect primarily on the earliest stages of murine erythroid progenitor cell development. J Cell Physiol. 1995;162:134–138. doi: 10.1002/jcp.1041620116. [DOI] [PubMed] [Google Scholar]
- 46.Wang C Q, Udupa K B, Lipschitz D A. The role of macrophages in the regulation of erythroid colony growth in vitro. Blood. 1992;80:1702–1709. [PubMed] [Google Scholar]
- 47.Weid T V D, Langhorne J. The roles of cytokines produced in the immune response to the erythrocytic stages of mouse malarias. Immunobiology. 1993;189:397–418. doi: 10.1016/s0171-2985(11)80367-0. [DOI] [PubMed] [Google Scholar]
- 48.Weiser W Y, Niel A V, Clark S C, David J R, Remold H G. Recombinant human granulocyte-macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987;166:1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss L. Mechanisms of splenic control of murine malaria: cellular reactions of the spleen in lethal (strain 17 XL) Plasmodium yoelii malaria in BALB/c mice, and the consequences of pre-infective splenectomy. Am J Trop Med Hyg. 1989;41:144–160. doi: 10.4269/ajtmh.1989.41.144. [DOI] [PubMed] [Google Scholar]
- 50.Weiss L. The spleen in malaria: the role of barrier cells. Immunol Lett. 1990;25:165–172. doi: 10.1016/0165-2478(90)90109-4. [DOI] [PubMed] [Google Scholar]
- 51.Weiss L, Johnson J, Weidanz W. Mechanisms of splenic control of murine malaria: tissue culture studies of the erythropoietic interplay of spleen, bone marrow, and blood in lethal (strain 17XL) Plasmodium yoelii malaria in BALB/c mice. Am J Trop Med Hyg. 1989;41:135–143. [PubMed] [Google Scholar]
- 52.Weiss W R, Ishii K J, Hedstrom R C, Sedegah M, Ichino M, Barnhart K, Klinman D M, Hoffman S L. A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine. J Immunol. 1998;161:2325–2332. [PubMed] [Google Scholar]
- 53.Yadava A, Kumar S, Dvorak J A, Milon G, Miller L H. Trafficking of Plasmodium chabaudi adami-infected erythrocytes within the mouse spleen. Proc Natl Acad Sci USA. 1996;93:4595–4599. doi: 10.1073/pnas.93.10.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yap G S, Stevenson M M. Plasmodium chabaudi AS: erythropoietic responses during infection in resistant and susceptible mice. Exp Parasitol. 1992;75:340–352. doi: 10.1016/0014-4894(92)90219-z. [DOI] [PubMed] [Google Scholar]
- 55.Zhan Y, Lieschke G J, Grail D, Dunn A R, Cheers C. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood. 1998;91:863–869. [PubMed] [Google Scholar]
- 56.Zhong W W, Burke P A, Hand A T, Walsh M J, Hughes L A, Forse R A. Regulation of cytokine mRNA expression in lipopolysaccharide-stimulated human macrophages. Arch Surg. 1992;128:158–164. doi: 10.1001/archsurg.1993.01420140035006. [DOI] [PubMed] [Google Scholar]