Abstract
Coronavirus is an ongoing global pandemic caused by severe acute respiratory syndrome coronavirus 2. Coronavirus disease 2019 known as COVID-19 is the worst pandemic since World War II. The outbreak of COVID-19 had a significant repercussion on the health, economy, politics, and environment, making coronavirus-related issues more complicated and becoming one of the most challenging pandemics of the last century with deadly outcomes and a high rate of the reproduction number. There are thousands of different types — or variants — of COVID circulating across the world. Viruses mutate all the time; it emphasizes the critical need for the designing of efficient vaccines to prevent virus infection, early and fast diagnosis, and effective antiviral and protective therapeutics. In this regard, the use of nanotechnology offers new opportunities for the development of novel strategies in terms of prevention, diagnosis, and treatment of COVID-19. This review presents an outline of the platforms developed using plasmonic nanoparticles in the detection, treatment, and prevention of SARS-CoV-2. We select the best strategies in each of these approaches. The properties of metallic plasmon NPs and their relevance in the development of novel point-of-care diagnosis approaches for COVID-19 are highlighted. Also, we discuss the current challenges and the future perspectives looking towards the clinical translation and the commercial aspects of nanotechnology and plasmonic NP-based diagnostic tools and therapy to fight COVID-19 pandemic. The article could be of significance for researchers dedicated to developing suitable plasmonic detection tools and therapy approaches for COVID-19 viruses and future pandemics.
Keywords: Coronavirus, COVID-19, Nanotechnology, Plasmonic nanoparticles, Diagnosis, Prevention, Therapy
Introduction
Coronavirus disease 2019 (COVID-19) is the worst pandemic since World War II. The disease was identified first in December 2019 in Wuhan city of China and has since rapidly spread across the world and remains uncontrolled. This newly emerging infectious disease is caused by the highly contagious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. As of 27 March 2022, the World Health Organization (WHO) declared more than 476,374,234 COVID-19 confirmed cases and around 6.10 million have died worldwide despite various emergency measures taken by many countries [2]. Based on full-length genomic phylogenetic analyses, SARS-CoV-2 has exhibit ~ 80% and 50% similarity with the genomes of the previous identified coronaviruses SARS-CoV in 2002 and MERS-CoV in 2012, respectively, in terms of causing sever acute respiratory distress (ARDS) [3, 4]. However, SARS-CoV-2 has a significantly higher transmissibility compared to the other two viruses [5]. The investigation reveals that SARS-CoV-2 is an enveloped virus from the coronavirus family with positive sense single-stranded ribonucleic acid RNA (( +)ssRNA) genomes (having the length of 30.000 nucleotides). It encodes 27 to 30 proteins in addition to an RNA-dependent RNA polymerase (RdRP), four major structural proteins and nine accessory proteins from a complement of 3´ORFs (ORF3a, 3b, 6, 7a,7b,8, 9b, 9c, and 10) [6, 7]. RdRP acts in conjuncture with nonstructural proteins to maintain genome fidelity. However, higher sequence diversity has been observed recently because of the changes in the viral genome [8]. The four structural proteins of SARS-CoV-2 include spike surface glycoprotein (S), small envelop protein (E), membrane (M) (maintains the membrane integrity of the viral particle), and nucleocapsid protein (N) (Fig. 1) [9, 10]. While the S gene of SARS-CoV-2 is divergent with less than 75% nucleotide sequence similarly compared to the previous SARS coronavirus, the three other structural proteins are conserved [11].
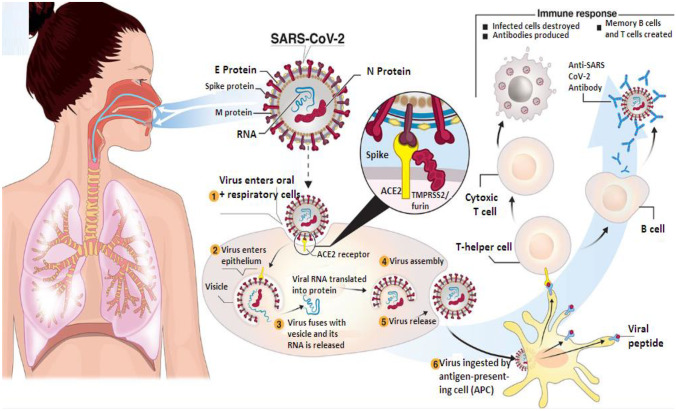
Fig. 1.
Transmission and life cycle of SARS-CoV-2 causing COVID-19. SARS-CoV-2 is transmitted via respiratory droplets of infected cases to oral and respiratory mucosal cells. The virus, possessing a single-stranded RNA genome wrapped in nucleocapsid (N) protein and three major surface proteins: membrane (M), envelope (E), and Spike, replicates and passes to the lower airways potentially leading to severe pneumonia. The gateway to host cell entry (magnified view) is via Spike-converting enzyme 2 (ACE2) interaction with cleavage of Spike in the prefusion state by proteases TMPRSS-2/furin. A simplified depiction of the life cycle of the virus is shown along with potential immune responses elicited [318].
Copyright© 2020 Funk
According to recent studies, the spike (S) proteins of SARS-CoV-2 are reported to be a class 1 fusion protein. These proteins are responsible for attachment to host human cell via the angiotensin converting enzyme 2 (ACE2) as a receptor in order to initiate the entrance procedure on the inside of the human host cell [12, 13]. As the respiratory tract has the highest expression of antigiotensio-convering enzyme 2 (ACE2), it becomes his main target. Indeed, ACE2 expresses strongly in the alveolar space of epithelial Type II cells (AT2) on the apical side of the lung [14, 15], the nasal, mucosa, bronchus, heart, esophagus, stomach, kidneys (PT cells), ileum, esophagus and in bladder urothetial cells, all of which will act as target and the virus will enter and kill them [16]. Scientists reported that the binding of SARS-CoV-2 and ACE2 glycoprotein is 10–20 folds higher than that of other coronaviruses, which could explain SARS-CoV-2’s transmission and infectious capability [17, 18]. The entry of virus into cell by binding of the S protein with ACE2 leads to a proteolytic cleavage into S1 and S2 subunits of S (Fig. 1).
The S1 protein consists of an N-terminal domain (NTD) and C-terminal domain (CTD). These two domains spanning from Arg319 to Phe541 called the receptor binding domain (RBD), which is the most variable part [19], and can bind to the peptidase domain of ACE2 precisely via the receptor-binding motif (RBM) region [20]. The S2 gene handles membrane fusion between viral envelope and host cell [21, 22]. It depicted that the variable part of coronavirus genome is the RBD region of the S protein. Researchers are working rapidly to decode the mechanism of operation of SARS-CoV-2 virus by focusing in the structure genome and the binding interface to ACE-2. The spike proteins of SARS-CoV-2 contain 1273 amino acids and four amino acid residues (PRRA) within the intersection of S1 along with S2. In general, the poly-basic cleavage sites in PRRA are generated due to these amino acid residues in SARS-CoV-2 [23]. According to structural study conducted, these cleavage sits decrease the stability of S protein and then facilitate its binding. The binding of S1 to ACE2 and S2 can be cleaved with a cellular proteins, including transmembrane protease serine-2 (TMPRSS2), -4) furin-like enzymes, and endosomal cathepsins B/L which are essential for viral spread and pathogenesis in the infected host, as shown in Fig. 1 [24–26]. Transmembrane protease serine 2 (TMPRSS2) exists in the surface cell and promotes printing and activation of the S protein. It permits the interaction of S2 fusion peptide (PT) with host membrane inducing membrane fusion and viral RNA release into the host [27].
Structural studies from SARS-CoV-2 Wuhan reference strain bound to human ACE2 have been conducted via X-ray crystallography [28] and molecular dynamic (MD) simulations [29] and elucidated that a total of 21 contact residues were specified on RBD which contact with 20 contact residues of ACE2 peptidase domain [30, 31]. The binding of the spike protein S of SARS-CoV-2 with ACE2 is critical for the first step of infection; therefore, the focus of interest in research is to disrupt this event. The life cycle of CoV is depicted in Fig. 1 that includes several steps: (i) endocytic entry into host cells (via ACE2 and TMPRSS2); (ii) RNA replication and transcription, and RNA-dependent RNA polymerase (RdRp) activation, translation, and proteolytic; (iii) virion assembly and release of new viruses through escocytic systems [32].
COVID-19 is a very contagious disease, the pandemic spread in the entire globe via human to human transmission. As reported, SARS-CoV-2 has 149 mutation sites which explain the high mutation and contagious rate [33]. Researches claim that people get infected with SARS-CoV-2 through direct, indirect, and close contact with an infected individual, mainly by inhaling virion particles in the droplets expelled into the air [34]. SARS-CoV-2 not only transmitted by these main routes, but also via aerosols where the droplet carrying the virus survives up to 3 h and via contaminated surfaces that individuals interact on a daily basis [35]. The primary symptoms of COVID-19-infected person include dry cough, fever, fatigue, loss of sense of taste and smell, body pain, severe respiratory illness, pneumonia, and dyspnea [36, 37]. SARS-CoV-2 viruses are generally spherical in shape and his particular has an approximate diameter ranging from 60 to 140 nm. The pandemic has affected globally the health, safety, and wellbeing of all the communities and economy and its fast rate of spread worldwide with lack of rapid diagnosis test have driven an unprecedented demand for emergency measures to response to the new viral threats. Researchers all over the globe have been devoted to look for novel ways to mitigate the contagion in terms of prevention, diagnostics, treatment strategies, and vaccines to tackle COVID-19 by harnessing all of the tools available technologies. Although the present vaccines available to treat COVID-19 as Pfizer vaccine (BNT-162), Moderna’s mRNA-vaccine (mRNA-1273), and other commercialized vaccines have been used for a year now as promising vaccines, there are high possibilities for the person to get infected even after the vaccination according to the cases reported by the World Health Organization (WHO). SARS-CoV-2 is an RNA virus with a high mutation rate up to day due to the high level of antigen drift that might enable the viral pathogen to look for more transmission ways and become more virulent. Due to the emergent of its different variants, SARS-CoV-2 resistances to vaccines challenge the efficacy and methods used in developing vaccines in the absence of full understanding about (i) its biological properties, epidemiology, etc. and (ii) the human immune responses naturally to SARS-CoV-2. Accordingly and with the lack of an effective diagnostics, therapy, or drug to eliminate the effect of this virus entirely, the key points are as follows: primarily, the early detect of COVID-19 cases and isolate the infected person to prevent further the infection and collect more sequence data with the additional mutations form occurred. Thus, scientists across the globe have been striving for the development of rapid diagnosis test. Currently, various methods have been developed for the detection of COVID-19 such as virus nucleic acid real-time PCR (RT-PCR) [38], CT imaging [39], and enzyme-linked immunosorbent assay (ELISA) [40]. Although those methods afford considerable reliability in detecting SARS-CoV-2 and diagnosing COVID-19 and its progression, most of them have a lot of disadvantages not only require multiple and lengthy process (including virus lysis, RNA extraction, reverse transcription, and amplification) but also have short coming effects [41]. Diagnosis is high cost, requires several hours, and despite their high sensitivity, false negative results were reported, which may delay the early treatment of patients and made the epidemic spread more. Second, further research should direct towards the design and development of effective vaccine to eradicate SARS-CoV-2. Given such limitations, it appears that a paradigm shift in our thinking is necessitated towards a new concept as an alternative. An urgent demand for platform technologies that can be easily adapted to this virus and to every new virus or even to virus mutations is a challenge. In this overall situation, the need for much simpler, rapid, repeatable, and more sensitive methods has been required for detecting SARS-CoV-2, providing timely treatment to infected individuals, and preventing the spread of the disease is mandatory with the new advancement available in nanotechnology.
Nanotechnology is a highly complex but possesses an enormous potential to understand the structure, growth, and life span of SARS-CoV-2 since this virus has to operate in a similar size scale which facilitate the interaction [42, 43]. The nanotechnology domain can become a bridge between diagnosis and therapy in the battle against viruses and especially COVID-19 and can offer a number of solutions in both outside and inside the host [44, 45]. So, the detection, neutralization, and even inhibition of this virus using nanomedicine/nanotechnology are imperative. With sufficient emphasis towards this virus, Fig. 2 illustrates the role of nanomaterials with their smaller sizes, highly active and tunable surfaces, low toxicity, and chemical modification capability providing an emerging tool for the advancement of point-of-care diagnostics, transporters for therapeutics, and antibody improvement in which nanodevices can be enhanced and adjusted to recognize, treat, and prevent SARS-CoV-2 spreading.
Fig. 2.
Nanomaterials for prevention and therapy of COVID-19. Integrating nanomaterials into personal protective equipment (PPE) can prevent the entrance of SARS-CoV-2 in the respiratory system. Nanomaterials could also be used to deliver drugs to the pulmonary system via inhalators. Cellular binding of viral particles at the alveoli can be inhibited using targeted nanoparticles (NPs) against angiotensin-converting enzyme 2 (ACE2) receptors or viral S protein. Various mechanisms can be used to inactivate viral particles systemically such as using neutralizing NPs or photocatalytic nanomaterials. Nanomaterial-based vaccines or immunomodulation can be used to prevent SARS-CoV-2 infection or even to boost the immune response during infection. PDT, photodynamic therapy. Reproduced with permission from [319].
Copyright© 2020 American Chemical Society
Along with the antiviral properties, nanoparticles and plasmonic NPs, to be specific, gold (AuNPs), silver (AgNPS), and copper (CuNPs), seem to be highly favorable. They can be specialized to fight the causal microbes and disable the viral pathogens before they invade the body. Plasmonic nanoparticles open new prospects in terms of the development of affordable and scalable detection and therapy strategies for the present epidemic and even for future epidemic [46]. These strategies consist of the development of plasmonic nanoparticles for (i) prevention measures and disinfectants, (ii) diagnostic tools for rapid, easy, sensitive and specific diagnostics, and (iii) therapeutic agents or vaccines to deliver antiviral agents into human body.
In this review, we provide an overview of current and new diagnosis, prevention, and therapy strategies that can be implemented with plasmonic NP-based platforms for SARS-CoV-2 early detection and treatment. We mainly focus on the role of plasmon nanoparticles and nanotechnology in photonic biosensors as a potential technology for rapid and efficient SARS-CoV-2 virus infection rapid, cost effective and early diagnosis tools. The electrochemical plasmon NP-based biosensors and lateral flow immunoassay technique are widely described together with examples of their recent applications for respiratory SARS-CoV-2 virus detection. Beyond conventional diagnosis techniques, colloidal gold (AuNP)-based lateral flow immunoassay (LFIA) developed during the COVID-19 is currently the most suitable point-of-care testing (POCT) method that has remarkable advantages of fast-acting, inexpensive, easy and rapid to use, portability, and therefore, facilitate the containment of the virus’s global spread. Not to mention the critical role of plasmon nanoparticles as a protective barriers against viruses in personal equipment (PPE) and surface disinfection. Given the limited time scientist spend for direct research on SARS-CoV-2 and its rapidly mutating variant, plasmonic NPs which considered nontoxic materials could be used in therapy strategy for inhibiting and inactivating SARS-CoV-2 pathogenesis.
Optical Properties of Plasmonic Nanoparticles Surface Plasmon Resonance (SPR)
Surface Plasmon Resonance
Metallic nanoparticles are aggregates whose dimensions in all three directions of space are less than a few tens of nanometers. They show an intermediate behavior between bulk and molecular system. Metallic nanoparticles have fascinated scientist and are heavily utilized in biomedical and engineering fields. These new materials with very small dimensions arouse a very large interest which resides in their great reactivity due mainly to the large number of atoms on the surface. At this scale, electronic confinement phenomena appear responsible for modifying the optical and photo-physical properties of matter [47]. This confinement results in an amplification of absorption as well as the appearance of resonance phenomena. Free electrons of the conduction band on the surface of metallic particle set in motion of collective oscillations if a metallic particle is subjected to an electromagnetic field having a very large wavelength compared to its size [48]. When the frequency of the electromagnetic wave and the natural frequency of the oscillation are equal, a resonance phenomenon is observed and called surface plasmon resonance (SPR) [49, 50]. Electronic oscillations create a separation of charges on the surface of the particle which is the origin of the creation of dipole moments. These induce a sharp increase in the electric field inside the particle which will tend to return the electron gas to its equilibrium position. The latter will perform oscillations around this position at the plasmon frequency. Resonance will take place when the wavelength of the incident radiation is equal to that of the oscillation of the electron gas. This effect is defined as surface plasmon resonance (SPR) [51]. It is a localized effect because of the diversity in the electron density in nanostructured plasmonic materials, which is strongly related to their morphology and size; it is therefore often referred to localized plasmon resonance (LSPR) [52]. The spectral position and the broadness of the band depend on the nature, size, shape, morphology, orientation, and dispersion in size and shape of the plasmonic nanoparticles [53]. Only a few metals exhibit surface plasmon resonance phenomena and are known as plasmonic nanoparticles such as lithium, sodium, and potassium, which belong to the alkali family. For the trivalents, only aluminum, gallium, indium, and finally copper, gold, and silver from noble metals are plasmon NPs. The position of the maximum of the plasmon band depends on the size of nanoparticles: the plasmon band is shifted towards blue as the size of the nanoparticles decreases [54]. For gold nanoparticles with a diameter of less than 2 nm, no band is observed [55]. Each mode of oscillation of the electrons corresponds to a resonant frequency which is specific to the shape of the nanoparticle. The most studied forms are as follows: nanospheres and nanorods. Nanospheres are the most ancient forms. In this case, there is only one resonance mode and only one plasmon band is observed in the absorption spectrum. Nanorods were synthesized by Martin et al. [56]. The two modes of electron resonance are due to the existence of two axes of symmetry. Two plasmon bands are therefore observed: an intense band located in the near IR domain due to the longitudinal oscillation of the electrons of the conduction band and a less intense band located in the visible domain due to the transverse oscillation. Another effect can be reported: dispersion in shape and size of NPs. This effect mainly affects the width of the band [57]. Indeed, the diversity of forms generates as many resonances as there are forms. Since the absorption spectrum is the average signal of the sample, the greater the dispersion, the wider the plasmon band. This widening is also observed in the case of a large dispersion in size. The plasmon resonance effect generates a strong electric field in the vicinity of the nanoparticles which significantly increase its surface sensitivity. In this plasmon region, the interaction of light with NPs is enhanced (Fig. 3). Therefore, the increase sensitivity of SPR can be applied in virus detection techniques and built various biosensors due to the strong optical response and signal enhancement at the macroscale/nanoscale [58, 59].
Fig. 3.
Schematic diagram of SPR-applied virus detection. The reflected light from the light irradiated on the Kretschmann configuration is captured by the detector or CCD camera and processed and analyzed as a signal. Reproduced with permission from [320].
Copyright © 2021, Takemura
Surface-Enhanced Raman Scattering (SERS)
Surface-enhanced Raman scattering (SERS) spectroscopy is a powerful sensing technique in which inelastic light scattering by molecules absorbed onto certain specially prepared metal surface such as silver (Ag), gold (Au), or copper (Cu) nanoparticles is greatly enhanced providing high detection limits of a wide variety of molecules at the single level. It used extensively for the determination of molecular structure and nature of binding in molecules. SERS phenomena occur due to the presence of a metal surface and contain two components: electromagnetic [60] and chemical [61]. SERS enhancement is determined primary by electromagnetic (EM) field which yields stronger enhancement of cross-section by several orders of magnitude (up to 106). The magnitude of enhancement in the cross-section depends not only on the chemical nature of the adsorbed molecules, but also the roughness of the surface and the optical properties of the adsorbent (generally Ag or Au) [62]. The enhancement is precisely due to the excitation of a surface plasmon which generates a strong, localized, secondary field that Raman scatters from the molecules together with the incident field in first place. The second reason is due to coupling between SERS photon and plasmons [63]. Experiment results investigating the origin of SERS have shown correlations between SERS enhancement and the excited surface plasmon which induced with the controlling surface plasmon resonance by different nanoparticles’ structure, size, and dielectric constant [64]. The experiments were confirmed with the models predict in considering the Raman enhancement on gold and silver spheres of 6 to 7 orders of magnitude [65]. Furthermore, the SERS enhancement has been specifically influenced by the size of gold and silver nanoparticles in colloids and core shell [66, 67]. The SERS enhancement is observed when the resonance plasmon surface wavelength is equal to λsp = (λexc + λRs)/2, where λsp, λexc, and λRs are the surface plasmon, excitation, and Raman wavelength respectively. It is a theoretical and experimental confirmed result [68]. However, the size of NPs influences not only the surface plasmon SP wavelength, but also the intensity of the electromagnetic field created in between the nanoparticles which lead to higher SERS enhancements [69].
Biomedical Properties of Plasmonic Nanoparticles
Plasmonic NPs are often studied because of their unique properties depending on the size such as good stability, high conductivity, improved solubility, large effective surface area adaptability, and multifunctionality. Because of their optoelectrical and chemical properties, primarily LSPR and photodynamic and photothermal capabilities for reactive oxygen species (ROS) generation, plasmon NPs such gold NPs, silver NPs, copper NPs, and their compositions are considered as multipurpose agents. These agents possess diverse applications in a variety of fields including biomedical, cosmetics, and disinfectant productions [70, 71]. These NPs have great potential as antiviral agents and broad-spectrum antimicrobial action against various bacteria, fungi, and viruses and participate to develop nanobiosensor-based platform, serve as antiviral agent or nanotherapy drug delivery, and play a pivotal role in the development of effective antimicrobial approaches [72, 73]. They were also considered being nontoxic materials in case of drug delivery and can offer diagnostic and therapeutic possibilities [74, 75]. Several studies have demonstrated the potent antiviral and antimicrobial action of plasmon NPs and their selectivity and sensitivity based-biosensor against human pathogenic viruses including respiratory syncytial virus (RSV), influenza virus, Norovirus, and human immunodeficiency virus (HIV) in human lives.
Gold nanoparticles (AuNPs) are inert, exhibit superior chemical stability, absorb light in visible and near IR region, and known for its tendency to form complexes with biological molecules. All of which are contributed to its antibacterial activity and low biological toxicity which is a significant advantage in in vitro and in vivo diagnosis [76]. Several studies highlight the biocompatibility of AuNPs with different sizes and shapes coupled with the SPR effect or functionalized with various agents (e.g., biomolecular polymer with antiviral properties). Specially gold NPs can attached with a number of macromolecules, which are capable of inducing immune response, including antibodies [77], antigenic proteins [78], T cell activating peptides derived from pathogens [79], and nucleic acid like siRNA [80] to develop new vaccine platforms in medicine. Thus, gold NPs can to be a suitable candidate not only for to treat virus or bacteria but also used as biosensing materials for detection of viruses [81]. The antibacterial and antifungal activity of AuNPs functionalized with 5-fluorouracil was demonstrated against Micrococcus luteus, S. aureus, P. eruginosa, E. coli, Aspergillus fumigates (A. fumigates), and Aspergillus niger (A. niger) [82]. The promising antimicrobial feature of AuNPs has been enhanced in recent years by functionalizing or coating gold NPs with plant extract. In this context, Su and Chang [83] demonstrated the discrete antimicrobial activity of AuNPs prepared by using gallic acid towards Echerichia coli and Staphylococcus aureus. Likewise, cinnamaldehyde-coated AuNPs (with an average diameter of 11 ± 3 nm) can be effective against the hyphae formation of Candida albicans and decrease pathogenicity of the organism [84]. In cytotoxicity assays, result showed that gold NPs not harmful to Vero cells up to concentration of 100 µg/mL. Many studies reported inactivation effects of AuNPs on many viruses that share structural similarities with SARS-CoV-2, AuNPs prepared with garlic extract (Allium sativa), as a reducing agent, displaying an effective inhibitory mechanism against measles (MeV) virus [85] and inhibition of hepatitis C virus by AuNP-based nanozymes [86]. Meanwhile, AuNPs used as surgical adjuvant to encapsulated antigens and help deliver them to specific targets for UV-inactivated SARS-coronavirus nanovaccines [87]. In addition, AuNPs were utilized to formulate synthetic VLPs (sVLPs) by incubating AuNPs (100 nm) with the spike S protein of Avian coronavirus infections bronchitis virus (IBV) [88]. The vaccination with these synthetic VLPs showed enhanced lymphatic antigen delivery (6-folds), stronger antibody titers, increased splenic T-cell response, and reduced infection-associated symptoms in an avian model of coronavirus infection. Aside from, AuNPs used as antigen carriers as well stimulated phagocytic activity of of lumphoid cell and induced release of inflammatory mediators [89]. Furthermore, surface-engineered Au nanorod nanocarriers were prepared to deliver immunodeficiency virus (HIV)-1 Env plasmid DNA for the immunization against HIV-1 [90]. Not only gold NPs serve as a carrier for antigens and adjuvant, but also can act as stimulators of immune response. In this regard, AuNPs combined with NALP3 inflammasome in order to prepare a vaccine adjuvant against SARS-CoV [78]. This drug was capable to activate dendritic cells (DCs). In another study, AuNPs conjugated with membrane matrix protein 2 (M2e) and TLR-9 to formulate a vaccine against influenza virus H1N1 and H5N1 [91]. Not only that, due to its capability to generate heat under suitable light near IR, gold NPs impregnated onto N95 respirator mask to obtain high filtration, high comfort level, and self-disinfection against virus [92]. In order to make use of the photothermal effect (PTT) appeared in the surface of NPs, the functionalized of AuNPs is one of the most approaches used to treat bacteria and inhibit viruses. In this regard, gold nanoparticles functionalized with DNA aptamer used specifically for targeting and inactivating methicillin-resistant Staphylococcus aureus (MRSA) via PTT [93]. To investigate the antiviral properties of plasmonic NPs against human norovirus, copper sulfide shell/gold core nanoparticles (Au@CuS NPs) of 2–5 nm were prepared to inhibit and inactivate up to 50% of virus (Broglie et al. 2014). The size of nanoparticles is critical to antiviral activity. Papp et al. [94] described the antiviral activity of sialic acid functionalized 2 nm and 14 nm gold AuNPs against enveloped influenza. This paper investigated the importance of NP size and proved that the larger AuNPs can inactivate respiratory influenza viruses through inhibition of viral fusion proteins needed for cell entry, while the smaller NPs did not had any effect. Considering the optimal size of AuNPs for antiviral effect, polysulfated gold NPs of diameter equal to and larger than the virus diameter (> 50 nm) were prepared to inhibit the vesicular stomatitis virus (VSV) than smaller particles [95]. The author explained his finding by the greater contact area and more interaction sites for AuNPs to attach to VSV. On the other hand, gold nanoparticle morphology played significant role to antiviral activity. Au nanosctructures of non-spherical morphologies have unique antiviral activity. Given this context, Bawage et al. [96] demonstrated in his research paper the antiviral activity of gold nanorods (45 nm × 10 nm) against respiratory syncytial virus (RSV), while Kim et al. [97] discussed the antiviral activity of porous gold nanoparticles against influenza (H1N1, N3N2, H9N2). A remarkable decrease of influenza viability was induced by binding to disulfide bonds in hemagglutinin HA, which consider one of the viral surface proteins involved in membrane fusion with the host cell, due to gold-thiol interactions. To further expand its bio-application expertise, hollow nanostructure multi-modified with AuNPs and DNA 3-way junction (HAuSN) was designed by an SPR method to detect label-free H5N1 avian influenza virus [98]. However, a glass carbon electrode-based immunosensor was fabricated by using AuNPs and zirconia NPs, in chitosan nanocomposites, to detect antigen (Ag) of hepatitis C virus (HCV) [99], while Justino et al. [100] used this transistors PET instrument to detect the nucleocapsid protein of SARS as a biomarker. Due to LRPS effect, gold NPs largely studied to develop a colorimetric assay to diagnosis viruses. The aggregation of AuNPs induced a redshift in the LRSP peak position which resulting a color change in the solution. This phenomenon can be observed with naked eye and can be caused by the plasmon coupling among the NPs when the colloidal NPs aggregate. Many studies have been reported the effective of gold NPs on a colorimetric assay to detect viruses. To detect avian influenza virus, a smartphone-based point of care platform formed with Au/Ag NPs that led to naked-eye detection of the virus was synthesized [101]. In addition, AuNPs were used to fabricate a naked-eye sensitive biosensing probe for the detection of Dengue virus by binding with the biotinylated target DNA-AuNPs [102]. Another example in colorimetric detection, a sandwich hybridization/nanoAu amplification/Ag-staining system for visual detection of hepatitis B virus (HBV) and hepatitis C virus (HCV) was developed [103]. Aiming to design a localized surface plasmon resonance (LSPR) efficient system to detect Zika virus, Adegoke et al. [104] designed Au/Ag core/shell and AuAg alloyed NPs functionalized with 3-mercaptopropionic acid which conjugated with CdSe QDs. However, Li and Rothberg [105] used AuNPs in a colorimetric assay detection of SARS-CoV RNAs to benefit from the difference in the electrostatic properties of single- and double-stranded DNA (ssDNA and dsDNA) which interact with citrate ions on the surface of AuNPs and confirmed the formation of dsDNA from viral ssRNA. Gold nanoislands (AuNIs) used to fabricate nanoplasmonic on-chip PCR to detect MERS-CoV via plasmonic photothermal heating [106]. However, colloidal AuNPs functionalized with streptavidin used to detect MARS-CoV nucleic acid by fabricating a vertical flow visualization strip (RT-LAMPVF) [107].
Silver nanoparticles (AgNPs) are well known as antimicrobial and antiviral nanomaterials very effective against many types of bacteria [108, 109], fungi [110], and virus. Silver’s mode of action is anticipated to be Ag+ ion released from AgNPs which inhibit bacterial growth binding with DNA function and through suppression of respiratory enzymes [111]. Previous studies proved the competent antibacterial efficacy of AgNPs (smaller than 10 nm) in defeating S. aureus solely [112] or beating MRSA in a composition with silica [113]. Ag2S NPs (3 and 4 nm of size) were used to inhibit the porcine epidemic diarrhea virus [114]. Silver NPs of size of 10 nm and 50 nm expand its antiviral against several others virus such as Hepatatis B virus (HBV) [115], Herpes simplex virus type 1 (HSV-1) and (HSV-2), and with human paraintfluenza virus type 3 (HPIV-3) [116]. In another study, colloidal AgNPs (10 nm) and silver nanowires (60 nm and 400 nm in diameter) diminished the infectivity of Transmissible gastroenteritis virus (TGEV) in ST cells by acting not only as an effective virucidal agent but also as an inhibitor of viral entry [117]. The antimirobial activity of AgNPs also has been investigated [118] against influenza virus H1N1. However, various silver NPs, such as AgNPs/chitosan [119], oseltamivir functionalized AgNPs [120], and zanmivir-functionalized AgNPs [121], have been investigated to inhibit the current seasonal influenza H1N1 virus that caused global epidemic. Add to influenza viruses, AgNPs modified with GO (GO-AgNPs) used to inhibit 53% of porcine reproductive and respiratory syndrome virus (PRRSV) [122]. AgNPs were used to target the viral outer envelope to block the infection and proliferation of H1V virus [123]. Furthermore, in another biomedical application, silver NPs are used as silver sulfadiazine in creams or wound dressing [124] and very recently are used to make potential antimicrobial materials such as surgery sutures [125, 126]. Silver NPs have also demonstrated promising antiviral capability to inactivate many infectious viruses such as monkeypox virus [127], bacteriophages UZ1 and MS2 [128], Tacaribe, and several respiratory pathogens, including adenovirus and influenza (H3N2) [129–132]. Smaller curcumin-functionalized AgNPs have been shown to inhibit cell entry of respiratory viruses due to the curcumin which act as a reducing agent [133]. In another coronavirus study, Alghrair et al. [134] demonstrated the effective antiviral activity of AgNPs conjugated to FluPep, a peptide, to inhibit influenza A viruses (IAVs). Specifically with regard to antiviral activities, some studied explained the excellent role of AgNPs to inhibit the entry of the virus into cells by interacting to envelope proteins through S binding of glycoprotein knobs gp120 of H1V1 virus [135]. Because of the multi-targeting and multi-directional mode of action, AgNPs combined with nutraceuticals could be a novel anti-viral agent. Tannic acid (TA) (a polyphenol with antiviral action) combined with AgNPs was used to treat the genital herpes infection and limit the viral spread [136]. Similarly, TA-functionalized silver and copper NPs (TA-AgNPs and TA-CuNPs) had directly block the entry of herpes simplex type 2 virus (HSV-2) into cells [137]. Far away, scientist tends to add nanomaterials in immunology as nanoadjuvant and delivery carriers to vaccine to minimize its side effects. Silver NPs used as mucosal vaccine adjuvant to deactivated influenza viruses [138]. In another example, Assis et al. [139] presented a nanocomposite of SiO2/Ag immobilized in a polymeric matrix with high antiviral activity that can kill bacteria Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), fungi (Candida albicans), and virus. Dung et al. [140] demonstrated the antiviral biocidal activity of AgNPs against a vast range of microbes including Salmonella enteric, E. coli, Vibrio cholera, and Coliform bacteria. The antiviral activity of silver nanoclusters NCs (5.3 nm) Ag2S capped with glutathione was examined by Du et al. [122] against coronaviruses with porcine epidemic diarrhea virus (PEDV) as a model. Similarly, the antiviral activity of AgNPs was discussed by Castro-Mayorga et al. [141] when produced composite polymer (a polyhydroxylkanoate bioplastic film) incorporating silver nanoparticles to inactivate norovirus surrogates, murine norovirus (MNV), and feline calicivirus (FCV). Nowadays, the biomedical products formed by AgNPs have been expanded to clothing, food container, ointments, and implant coating [142, 143]. Recently, Dung et al. [144] illustrated the antiseptic role of AgNPs (with an average size of 14 nm) against African swine fever virus (ASFV). Teengam et al. [145] have improved MERS-CoV RNA detection with the development of a paper sensor formed by AgNPs/pyrrolidinyl peptide nucleic acid (acpcPNA) where Kim et al. used AuNP-modified thiol in a colorimetric sensor. However, AgNPs deposited on antibody conjugated AuNPs forming plasmon platform employed to monitor hepatitis E virus [146].
Copper and its alloys are much less expensive compared to other noble metals. Its veridical and natural microbicide properties are known since the ancient time and became more widely recognized [147]. In general, copper considered to be safe for human contact, being non-irritating to the skin [148]. Copper nanoparticles (CuNPs) have excellent antibacterial ability for both gram-positive and gram-negative bacteria [149] and become an important metal with many applications such as sterile touch surfaces and medicines [150, 151]. Therefore, copper NPs have been incorporated into antimicrobial fabrics, polymers, and self-sterilizing PPE [152–154]. In general, copper NP antiviral activity issue from reactive oxygen species (ROS) generated by Cu(I) and Cu(II) ions released from CuNPs [140, 155, 156]. It is a common antiviral mechanism of copper nanoparticles that can be responsible of damaging DNA of biological molecules [157, 158]. Until now, a variety of studies has been exploited and depicted the effectiveness antiviral properties of copper NP surface to tackle many viruses caused human pathogen. In a coronavirus study, Warnes and Keevil [159] explained how the survival time of coronavirus 229E (HUCOV-2I29E) was reduced significantly on copper-containing. In another example, Hang et al. [160] illustrated the antiviral activity of cuprous oxide (Cu2O NPs) to inhibit hepatitis C virus (HCV) by explaining the high reactivity of ROS and its ability to cause oxidative damage to biological molecules. However, the effectiveness of antiviral activity of CuO NPs against herpes simplex virus type1 (HSV-1) was found to be 83.3% while considering the cytotoxicity of nanoparticles to the cells [161]. In addition, copper NPs can reduce the viability of cells which correlate essentially to the size, the shape, the chemical composition, and concentration of nanomaterials. Karlsson et al. [162] reported the role of CuO NPs to cause oxidative stress and DNA damage to lung epithelial cells and even reducing their viability to 50% after 18 h of exposure to 20 µg/mL. Comparative research conducted by Minoshima et al. [163] reported the high effective antiviral effect of Cu2O in comparison with CuO against both enveloped influenza A and non-enveloped bacteriophage. Moreover, a rapid decay within 4 h of SARS-CoV-1 was obtained when exposure to copper surface [164]. Copper oxide (Cu2O) was impregnated into respiratory protective masks N95 allowing them to have a potent biocidal properties in addition to inherent filtration properties of a human influenza A virus (H1N1) and avian influenza virus (H9N2) [153]. Recently, it was illustrated that polyurethane/CuO nanocomposites can effectively work as antimicrobial filters for air purification [165]. Due to its effectiveness to kill bacteria, prepared 800-nm Cu2O@ZrP hybrid nanosheets were reported to be effective (99% after 6 h) against the tow superbugs: methicillin-resistant Staphylococcus aureus (MARS) and vancomycin-resistant Enterococcus (VRE) [166]. However, CuS/GO nanocomposites with excellent capability to damage the bacterial membrane cell were used to kill multidrug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MARS) [167]. In the same context, Qiao et al. [168] explained the role of CuS nanorods (6 nm) to treat drug-resistant Gram-negative bacterial ESBL E. coli and MRAS-infected wounds. In an in vitro study, Au/CuS core/shell NPs can rapidly inactivate human norovirus G.I.1 virus-like particles. CuI generated OH− which responsible for the observed spike protein degradation of influenza [169]. Furthermore, CuO NPs were compared to the established drug Acyclovir against herpes simplex virus (HSV-1) [161]. At the highest non-toxic concentration of 100 µg/mL of CuO NPs, 83.3% inhibition rate of HSV-1 viral load was attained when exposure to CuO NPs explaining its effectiveness and antiviral potency. Investigating the difference of antiviral properties between cuprous oxide (Cu2O) and cupric oxide (CuO) is the main concern of many groups of research. Several studies have examined the two copper oxidation states. According to zeta potential measurement conducted by Mazurkow et al. [170] for the two materials, Cu2O possessed a higher isoelectric point (11) compared to CuO (7.4). This founding means that Cu2O have a higher positive surface charge to pH (7) allowing it to better absorb and inactivate bacteriophage MSe. Similarly, Sunada et al. [171] reported the reduction of bacteriophage Qβ with Cu2O loaded into glass substrate by 99.99% within 30 min which exceed that obtained with silver ions. In addition, Minoshima et al. [163] confirmed that the antiviral ability of Cu2O is more effective than CuO against both enveloped influenza A and non-enveloped bacteriophage. Furthermore, Warnes et al. [172] illustrated a rapid inactivation and distraction of murine norovirus MNV-1 plasmid and genomic nucleic acid on copper and copper alloy dry surface virus.
Since plasmonic NPs possess antiviral activities against the previous coronavirus and many other species of viruses and by examining the optoelectrical and chemical properties of plasmonic nanoparticles displaying the surface plasmon resonance effect, they may open a new perspective against COVID-19 as drug carriers, part of effective treatments and can be used for early detection. The functionalization of plasmonic NPs with versatile biomolecules that target SARS-CoV-2 would effectively develop new strategy for treatment and detection.
COVID-19 Prevention: Disinfection of Surfaces and Individual Protection Equipment
One of the major reasons for widespread COVID-19 infection is the contact between persons and the respiratory droplets of the infected person. Thus, especially the medical staff needs the use of appropriate PPE kits, masks, and gloves to protect themselves. As we know, the personal protection equipments (PPEs) available actually can be effective measures to limit the spread of SARS-CoV-2, yet without any intrinsic antimicrobial action can only protect the users temporary. In such difficult circumstances, current advances in nanotechnology have been providing an alternative by using metallic nanoparticles such a gold, silver, and copper, with antiviral properties to improve the effectiveness of PPEs, handle the pandemic, and further protect the people. Therefore, the development of antiviral surface coating and self-disinfection surfaces to inactivate SAS-CoV-2 is a highly demand matter. In these regards, many studies recently have presented to offer new perspective and highlight the use of plasmonic metallic nanoparticles associating with polymers and textiles to reduce the viability of viruses on surfaces, especially when expose to light [173, 174].
Face masks are the most preventive tools for fighting pandemic by prohibiting the widespread of virus, trapping them, and in advanced case inactivating. It is essential to cover the face for both infected and non-infected person to prevent virus transmission. However, most of the respiratory masks possess a large pore size than the SARS-CoV-2 size (around 120 nm). The performance of masks has been enhanced by using filter material like nanofiber and nanofiber webs [175] and by treating the surface with materials that have antimicrobial characteristics to mitigate viruses’ transmission. Various metal nanoparticles and its alloys have tested for making antibacterial face masks such as silver [176], copper, and oxide copper [177, 178]. Although masks are tools to prevent transmission, the increased demand also has raised concerns about the generation of waste. Same strategies using a sustainable polymer with plasmonic NPs on masks can reduce transmission and the impact of waste in the same time.
The N95 respirators are made of polymer fibers, such as polypropylene, even they possess hydrophobic surface; nevertheless, they lack self-disinfecting properties and their filtration efficiency remains around 80% because of its pore (≈ 300 nm). Thus, aqueous droplets can still stay on these fibers, which make the wearers specially the medical and the frontline staff face a huge need due to the global shortage of personnel protection equipments during the outbreak of COVID-19. The need of masks that can wear for long time is an urgent demand to inactivate the severe accurate respiratory syndrome coronavirus 2 viruses, on account of its instability of the spike and the RNA at temperature over 70 °C. The common decontamination methods for N95 respirators developed like chemical- and heat-based decontamination at 85 °C are the most used method to sterilize N95 for its reusing without damage, yet these methods need electrical and thermal sources to heat the samples. Based on this, the functionalization of the masks for improving their efficiency and reusability without external cleaning is crucial. It is worth mentioning the potential of photothermal properties of metallic NPs to disinfect surface.
Several studies and several disinfectant formulations and solutions based on AgNPs have been suggested to fight the COVID-19 pandemic. Zhong et al. [179] proposed the self-disinfecting challenges via generating a plasmon photothermal self-disinfecting and superhydrophobic coating on N95 mask respirators as an alternative low-cost method. The aim was to increase the antimicrobial properties of N95 by depositing silver nanoparticles, with wide size distribution from 5 to 50 nm, on the surface of the fibers of N95 respirators. This strategy provides better and long-term protection by improving the reusability, the antimicrobial activity, and self-decontamination of the masks. AgNPs have not only size-dependent plasmonic effect capability to convert photonic energy to heat and strong absorption of solar system but also exhibit an antibacterial properties depended on their size [180]. Accordingly, sunlight illumination can increase the surface temperature of the masks around 80 °C within 1 min and therefore can inactivate the SARS-CoV-2.In addition, this prossess is reproducible and it allows the reuse of the masks following a simple disinfection proceder under solar radiation. The author utilizes a 405-nm laser diode to excite AgNPs and then generate a strong heat. The Ag+ ion realize from AgNPs plays the disinfection role to block the droplets on the respirator surface and interact with the viral protein to inhibit their binding and penetration. However, a significant change can be detected at temperature over 120 °C, so it is necessary to control the laser temperature. This study may be a promising technique to provide more protection against SARS-CoV-2 and also the future pandemic.
Preliminary research study explained an antiviral nanohybrid coating made of silver Ag nanoclusters/silica composite (less than 200 nm) that could deposit on two disposable facial FFP3 masks [181]. The test towards SARS-CoV-2 showed a remarkable antiviral activity. Such coating can be deposited on practically every kind of filtering media and also on metallic, ceramic, polymeric, and glass surfaces and anywhere that is exposed to spread of virus. Another example reported that AgNPs can be incorporated and functionalized polyester/cotton fabrics using a simple pad-dry-cure method (a common textile fabric coating process in nature) to attain antibacterial, antifungal, and antiviral properties [182]. An antiviral test of AgNPs with different sizes ranging from 2 to 15 nm showed that the infectious amount of SARS-CoV-2 was reduced by 99.99% after an incubation period of 2 min. In addition, it caused 99.99% inhibition of the bacterial pathogens such as opportunity’s bacteria (E. coli, S. aureus, and C. albicans) and fungi, preventing cross-infections and did not cause allergies or photoirritation, photosensing, irritation nor sensitization process showing the safety of its use according to ANVISA’S Guide for Cosmetic Product Safety. The fabrication of these fabrics may provide new insight into the development of protection new textile material that can play an outstanding role as a new and important weapon against the current COVID-19 pandemic and other types of future viruses. Very recently, new surgical mask has been promoted that may be very promising to use in the current COVID-19 pandemic [183]. The author formulated a broad-spectrum disinfectant by impregnating AgNPs (with spherical shape and size of 5–13 xddfnm) in the textile fiber to modify surgical masks and its disinfection without altering its filtration effectiveness using the sputter coating technique. The well-embedded silver NPs in the silica glass substrate facilitated the conformal deposition of the hybrid composite onto the fibers. The AgNP-modified surgical masks illustrated an effective antibacterial effect towards the enveloped H5N1 virus and were inactivated within 15 min of disinfection. The nanodeveloped disinfectant was prepared to coat hydrophobic surgical masks with AgNPs, promoting antimicrobial activity, as well as viral inactivation efficiency for frontline clinical personnel to overcome the currently limited supply caused by SARS-CoV-2.
Silver AgNPs carry out their antiviral activity in several ways. For example, Nano Tech Surface, Italy, produced a durable and self-sterilizing disinfectant surface formula made of titanium dioxide and silver Ag+ ion (Stat Nano 0.2020). Similar to silver, copper is an important mineral that is necessary for a variety of biological processes. Generally, copper alloy surface is widely used in hospitals and can treat headaches, human fungal infection, and protect cops from bacterial pathogens [184]. The antiviral activity of copper and its alloys has been known because of its ability to generate reactive oxygen species (ROS) (one of the mechanism used by the immune system’s resisting microbial attack), which makes it potentially toxic to pathogenic bacteria and several viruses such as bronchitis virus, poliovirus, human immunodeficiency virus type 1 (H1V-1), other enveloped or nonenveloped, single- or double-stranded DNA and RNA viruses including SARS-CoV-2. It will result inactivation via viral protein oxidation and degradation of viral genomic DNA [185–187].
Recently, to reduce the activity of SARS-CoV-2 on solids, copper-based coating was used as a new designed [188]. The coating is made of composition on cuprous oxide (Cu2O) particles bound with polyurethane, which is already coating large items of everyday life. The study showed a reduction in the viral titer by about 99.9% within 1 h compared with the uncoated sample. The coating not only performs well in the glass and stainless steel as well as everyday item such as doorknob, a pen, and credit card, but also keeps its activity after 13 days of being immersed in water or after multiple cycles of expose to the virus.
Chilean/USA-based company, Copper 3D, has produced a face masks named Nano Hack, in which 5% copper oxide nanoparticles were impregnated in three of non-woven polypropylene filters layers allowing them to have an excellent antiviral properties against SARS-CoV-2 [189]. Promethean Particles Ltd, a UK-based Company, with collaboration with textile companies has developed fabrics and personnel protective equipment (PPE) for the healthcare sector by embedding copper nanoparticles into polymer fibers, such as nylon, via a met extrusion process. It is found that antimicrobial effect is higher than other similar antimicrobial fabrics in the market. This may be a lift in the production of PPE with efficient antiviral activity to defeat COVID-19. Very interested product has been produced by RESPILON Group, Czech Republic-based Company, it is a mask named ReSpimask® VK and it is available in the market as well [190]. This mask has nanofiber filter enrich with accelerated copper oxide nanoparticles resulting in 99.9% filtration efficiency for viruses and bacteria. The filter of the mask not only intercepts the viruses but also actively kills them. A filtration system from a nanofibirous respiratory facial mask containing multilayers of Cu nanoparticles/GO (graphene oxide) nanosheets dispersed in a nanofibirous matrix of biodegradable polyalactic acid (LA) or cellulose acetate (CA) was designed [191]. This designed system may be an innovative solution in the battle with SARS-CoV-2. Similarly, low-cost synthesis of novel Cu nanowires (20–35 nm)/ZIF-8 nanocomposites stabilized by an amphiphilic triblock copolymer (plunomic F-127) in a core shell structure was deposited onto a reusable face mask system (Kumar and Sharma 2020). The antiviral activity of his system is demonstrated using virus infected Vero E6 cells, and 55% inhibition of SAS-CoV-2 replication is obtained after 48 h at a concentration of 1 µg. A dual-channel spray-assisted nanocoating of shell ac/CuNP to a photoactivated antiviral facial mask with self-cleaning and reusability was reported with virucide effect originating from the release of copper and zinc ions, which interact with bacterial cell membrane (RNA). The ions released are responsible for the generation of reactive oxygen species (ROS), which cause damage of DNA.
Surface survival of COVID-19 was studied [164]. In this study, aerosol and surface stability of SARS-CoV-2 and its relation with SARS-CoV-1 were investigated in the same condition. They also explored how long the virus remained on these surfaces. They found that COVID-19 transmission in aerosol is plausible and remains for 3 h. On the other hand, SARS-CoV-2 is more stable on plastic and stainless than on copper. The experiment detected a viable SARRS-CoV-2 virus to 4 h on copper, up to 24 h on cardboard, on plastic and stainless steel surfaces from 2 to 3 days. Therefore, to prevent the transmission of COVID-19 from these surfaces, antimicrobial coating at the common place will be a preventive measure.
A dual-channel spray-assisted nanocoating hybrid of shell ac/copper nanoparticles (CuNPs) was added to nonwoven fibers used in surgical mask to increase its hydrophobicity of surface, as illustrated in Fig. 4 [192]. Due to the nanocomposite modification of the surface of nonwoven fibers and the unique depositing nanoparticles with spray technology, the facial mask (PMA) showed increasing temperature up 70 °C under solar illumination which increased the generation of ROS, as illustrated in Fig. 4c. An outstanding virucide effect has been resulted on the photoactivated facial mask (PMA). Considering the present challenges due to COVID-19, this recycling, self-sterilizing, and reusable mask could have a significant impact in our daily life.
Fig. 4.
Catalytic surface modification of nanocomposites on pristine surgical masks. a Diagram representing the individual components of the nanocomposite coating on the pristine surgical mask. b Schematic representation of the setup of the spray-based microfluidic device for the controlled deposition of the nanocomposite on nonwoven fibers of the pristine surgical mask. The spray device was designed to mix the solution of copper nanoparticles (CuNPs) and shellac at the junction, where the mixture meets the pressurized N2 air channel. c Schematic illustration of the inactivation of the virus in respiratory droplets through photothermal, photocatalytic, and hydrophobic self-cleaning processes after solar irradiation. d Optical image of the photoactive antiviral mask (PAM). e Representative scanning electron microscopy (SEM) images of commercial surgical masks to characterize the presence of propylene nonwoven fibers (left) and shellac-CuNP nanocomposite-coated nonwoven fibers (right). (Scale bar, 10 μm). f Photographs of a colored water droplet (30 μL) being held on the pristine mask (top) and PAM (bottom) after 1 h [321]. Copyright© 2021. American Chemical Society
The promising antiviral capabilities of plasmonic NPs are encouraging scientists to pursue such global crises with the spreading of COVID-19 through all surfaces. A rapid inhibition of SARS-CoV-2 only within 1 and 5 min was obtained on two different plated surfaces containing copper/silver nanohybrids with size of 26 nm for AgNPs and 212 nm for CuNPs respectively (Fig. 5) [193]. In this study, they explained that the antiviral activities are primarily attributed to Cu and its particulate forms. In the same time, the efficient inhibition of SARS-CoV-2 in the presence of Cu-Ag nanohybrids within 5 min is remarkable because such result has not been explored before; it was obtained with nanohybrids contained higher amounts of Cu (~ 65 and 78 wt%) and lower amounts of Ag (~ 7 and 9 wt%). Such surface coated with Cu-Ag was proposed in the aim to break the SARS-CoV-2 transmission chains within hospital and livestock settings and in public places.
Fig. 5.
Out-of-the-box surface administration of Cu-Ag nanohybrids rapidly inhibits SARS-CoV-2 (after 1 and 5 min), breaking the SARS-CoV-2 transmission chains and containing the pandemic within the hospital and livestock settings, and in public reservoirs. Nanohybrids A and B represent samples 2 and 3, containing ~ 65 and 78 wt% Cu and ~ 7 and 9 wt% Ag, respectively [193]
To further develop innovative eco-friendly materials capable to prevent transmission of SARS-CoV-2, Macelo et al. proposed a material constructed from SiO2-Ag composite immobilized in polymeric matrix (ethyl vinyl acetate, EVA) which exhibited high antibacterial activity not only towards SARS-CoV-2 but also Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) [139]. As shown in Fig. 6, the strategies are based on the plasmon effect and SiO2 semi-conductor capability to generate ROS in presence of H2O and O2 and lead to generate antiviral surfaces and then inhibit the replication activity of viruses.
Fig. 6.
A schematic representation of plasmon-induced hot electrons over SiO2-Ag composite: a in AgNP particles; b in metal semiconductor; and c proposed mechanism for biocidal activity. (CB and VB represent the conduction band and valence band, respectively [139])
The same group of research developed polyvinyl chloride (PVC)-SiO2-Ag composite with 0.83wt% on Ag safe and effective for preserving food with about 98% elimination of SARS-CoV-2 within only 3 h. The material obtained may be used for fabrication of packaging films to protect consumer products from infectious SARS-CoV-2 virus [194].
Plasmonic NPs Enabling Detection of SAR-CoV-2 and Diagnosis of COVID-19
PCR and Real time reverse-transcription polymerase chain reaction (RT-PCR) testing procedures are now the most acurrent, sensitive, and specific ways to identify SARS-CoV-2 which caused the novel coronavirus disease COVID-19 [195, 196]. However, these methods not only require multiple and lengthy process (including virus lysis, RNA extraction, reverse transcription, and amplification) but also have shortcoming effects. Diagnosis is high cost, require several hours, and despite their high sensitivity, false-negative results were reported, which may delay the early treatment of patients and made the epidemic spread more. At this point, it is a global consensus. There is an urgent demand for low-cost, rapid, and reliable SARS-CoV-2 antigen and virus detection. Several studies have been carried out over the past 2 years to highlight the use of nanotechnology and plasmonic nanoparticles as tools to develop new sensitive techniques for the rapid detection and identification of COVID-19. Thus, biosensors afford an alternative and reliable solution to clinical diagnosis by the developing of real-time biosensing platforms together with taking advantage of the properties of plasmonic NPs (such as gold AuNPs, silver AgNPs, and copper CuNPs) [197].
Plasmonic Biosensors
Owing to their chemical, physical, and electromagnetic properties, plasmonic nanoparticles have gained significant attention and help with the development of plasmonic biosensors. As explained in Fig. 7, the detection of specific viral molecules such as nucleic acid (NA) sequence, antigen, or protein that is present in the samples is the essential biorecognition functions of a biosensor. It used its combination of transducer and a biometric system, which show the concentration of analyte via an electronic signal, to react with the samples. On the other hand, the bio-functionalization of these nanotechnology-based sensors with antibodies, antigens, or nucleic acid can be used for a wide range of analytes and may enable the selective targeting detection of single virus particles with high sensitivity and specificity [198].
Fig. 7.
Principle of plasmonic biosensors.
Reproduced with permission from ref [322] Copyright© 2021 MPDI
Biosensor Platform-Based on LSPR and SERS Phenomena
By the developing of plasmonic biosensors based on photonics, the aim of the researchers is to overcome the difficulties to detect COVID-19 at early stage making use of various plasmonic phenomena such as surface plasmon resonance (SPR) (Fig. 8a), localized surface plasmon resonance (LSPR) (Fig. 8b), and surface-enhance Raman scattering (SERS) in combination with other detection formats, involving colorimetric, florescence, electrochemical, and other laser techniques. The plasmon biosensors are highly sensitive and take advantages of the local refractive index changes of the surface after the interaction between the target analyte and the immobilized biological receptor as well monitor the binding events occurring in the surface by SPR and LSPR, which both depend on the refraction index of the substrate or solution, and induce spectral shifts, as seen in Fig. 8 [199].
Fig. 8.
a Schematic illustration of the standard SPR-based biosensor configuration. b Schematic illustration of the resonance wavelength shift sensing based on LSPR sensor configuration [323]
From this perspective, the race to design a novel biosensor that would optimize COVID-19 tests in terms of cost, rate of testing, and sensitivity is underway. Various studies have been done to fabricate various plasmonic nanomaterial-based biosensors, especially with gold nanoparticles (AuNPs) due to its surface and biocompatibility affinity with protein and nucleic acid, to detect SARS-CoV-2 in clinical samples [200]. In this context, a proposed clinical approach to track and diagnose COVID-19 was developed by combining both the LSPR sensing transduction and the plasmonic photothermal (PPT) effects in an optical sensor for viral RNA samples [201]. The biosensor based on 2D gold nanoisland (AuNI) functionalized with thiol-cDNA, a complementary receptor for the RNA genome parts of COVID-19, can detect the selected sequence from SARS-CoV-2 including RdRp-COVID-19, ORF1ab-COVID-19, and E genes sequence grafted on gold nanoparticles very fast through nucleic acid hybridization. In this study, the author illustrated the value of the large optical cross-section of the plasmonic nanoparticles Au “nanoisland.” The localized (PPT) heat source generated near AuNPs at the plasmonic frequency capable to increase the in situ hybridization temperature, which increases the sensibility of the biosensor and discriminate with accuracy the sequence RdRp gene of nucleic acid from SARS-CoV and SARS-CoV-2. The sensing performance was improved via the thermoplasmonic heat generated in AuNIS chip, which used as a stable heat source. The sensor exhibited precise detection of the virus with a lower detection limit of 0.22 pM and could be a promising solution for the clinical COVID-19 diagnosis.
Based to LSPR, an opto-microfluidic sensing platform with gold nanospike was fabricated to detect SARS-CoV-2 spike protein in 30 min [202]. In this study, gold was electrodeposited on a 50 chromium layer on the glass substrates to produce the gold nanospike. To activate the surface of these gold nanospikes, Funari et al. functionalized them with a mixture of thiol:1:1 solution of 10 mM NHS (N-Hydroxysuccinimide) and 40 mM of EDC (1-Ethyl-3-(3-dimethyl aminopropyl carbodiimide)). The opto-microfluidic platform was produced by binding the gold nanospike to a polydimethylsiloxane (PDMS) slab using an 85-µm-thick adhesive polyester layer. This biosensor was used to quantify the anti-SARS-CoV-2 antibody concentration in diluted real human plasma which is correlated to the red shift of the LRSP peak of gold nanostructures in the microfluidic device. The limit of detection for the LSPR-based biosensor was around 0.08 ng/mL (~ 0.5 pM). With its high sensitivity, specificity, and without any labeling agents, this biosensor can be expanded as a good point-of-care antibody testing tool for SARS-CoV-2.
SERS offers higher sensitivities and chemical specificities than most modes of optical detection and is used to detect many viral molecules like influenza, Adeno, and West Nile. To diagnose COVID-19, a multiplex surface-enhanced Raman scattering (SERS) platform based on plasmonic paper was developed [203]. The paper was made of silver nanodots. The proposal method offers a highly sensitivity detection and identification of multiple fluorescent dyes targeting probes, reveals the multiplex detection capability of PCR-based SERS under exiting PCR condition without modifying the primer and the probe sequences (E and RdRp genes). This alternative optical technique leads to minimize contamination in diagnosis test. However, a novel protocol to detect SARS-CoV-2 in the body fluids such as saliva, nasopharyngeal secretion, and tears was determined by using microfluidic devices that involve connected microchannels coupled either with Au/Ag-coated carbon nanotubes or Ag-functionalized cellulose strips [204]. The result was obtained with surface enhanced Raman spectroscopy (SERS) directly using plasmonic properties of metallic nanostructures such as gold and silver. It is a highly rapid platform that could capture single coronavirus at real time. The carbon nanotubes, which are used in pharmacy and medicine, can absorb a wide range of molecules (drugs, proteins, antibodies, DNA, enzymes, etc.). The process could detect COVID-19 in polluted water at the single virus level (the detection limit was 80 copies mL−1, and the detection time was about 5 min) and the use of plasmonic material such as Au/Ag enhanced Raman signals and improved the range of detection of SARS-CoV-2 in nasal, throat, and saliva.
Colorimetric-Based Biosensors
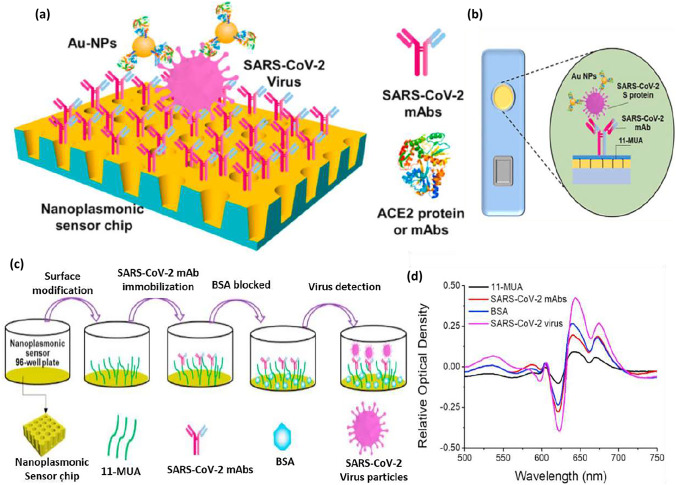
The colorimetric technique is the simplest procedure based on the detection of biomolecules such as proteins and nucleic acids by the color changes, which can be seen with naked eyes without complimented equipment [205, 206]. Several approved diagnostics are based on colorimetric lateral flow assay (LFA) when the targeted analyte is detected using antibodies immobilized on a membrane [207]. Huang et al. [208] investigated a low-cost nanoplasmonic biosensor for one step rapid detection and quantification of SARS-CoV-2 virus without sample preparation. They used Au-TiO2-Au nanocup array chip on a substrate made of silicon oxide wafer polymer (with 200-nm nanocup diameter) as a sensor without any external coupling optics (Fig. 9a). The plasmon resonance wavelength and intensity changing can be observed after the virus captured the sensor surface (Fig. 9d). In addition to being able to detect SARS-CoV-2 pseudovirus at low concentration (less than 370 Vp/mL), this nanoplasmonic sensor functionalized antibodies (SARS-CoV-2 mAbs or ACE2 proteins) and able to exhibit minimal responbses to SARS, MERS, and VSV pseudoviruses. (Fig. 9b, c). Therefore, using this nanoplasmonic sensor can be lead to an early diagnosis of COVID-19 disease. The biomarker in this study was the spike protein. The presence of gold nanoparticles (AuNPs) enhanced the sensitivity detection of the whole SARS-CoV-2 in one step. The limit of the biosensor was 30 SARS-CoV-2 virus particles in one step, within 15 min.
Fig. 9.
a Schematic diagram of the nanoplasmonic resonance sensor for determination of SARS-CoV-2 pseudovirus concentration. b The illustration shows the detection process of the sensor chip cartridge for specific SARS-CoV-2 detection. c Schematic of nanoplasmonic sensor chip surface functionalization as well as capturing and detecting SARS-CoV-2 pseudovirus. d The typical original spectra of adjacent modification steps and detection process with 2.5 × 108 vp/mL SARS-CoV-2 pseudovirus [207].
Copyright© 2020 Elsevier
Recently, a selective naked-eye approach was developed using the RNA sequence of SARS-CoV-2 as a target. Due to the lower sensitivity of the biosensors for the detection of N gene (nucleocapsid phosphoprotein gene) compared to the RdRP gene (RNA-dependent RNA polymerase gene) and E gene (envelope protein gene), a novel biosensor was developed to improve the detection of N gene of SARS-CoV-2 based on antisense oligonucleoide (ASO)-modified gold nanoparticles (AuNPs) [209].The colorimetric detection of these RNA sequences was performed without using any sophisticated instrument. Four antisense oligonucleoide (ASO) sequences were used for capping AuNPs.
All the ASO-capped AuNPs (Au-ASO1M, Au-ASO2L, Au-ASO3H, and Au-ASO4M) were mixed together, resulting in the formation of Au-ASOmix dispersed individually in the samples. However, Moitra described in this work that the thiol-modified ASO-capped AuNPs agglomerated selectively in the presence of its target N-gene sequence of SARS-CoV-2. The color change of the solution can be visualized by the naked eyes and the result confirmed by a redshirt in their absorbance spectra with 40 nm. For the naked-eye detection of the SARS-CoV-2 RNA, ribonuclease (HRNaseH) was added to the solution to cause a visually detectable precipitation of agglomerate ASO-capped AuNPs, as illustrated in Fig. 10c, d. The detection limit was 0.18 ng/μL for the SARS-CoV-2 RNA in the viral load. This biosensor showed a selectivity of the detection of COVID-19 even suitable with the muted N-gene forms of the virus.
Fig. 10.
a Comparison of response of the Au-ASOmix nanoparticles towards the RNA (1 ng/μL) isolated from noninfected Vero cells, Vero cells infected with MERS-CoV, and Vero cells infected with SARS-CoV-2 virus. Relative change in absorbance at 660-nm wavelength for the Au-ASOmix nanoparticle treated with SARS-CoV-2 RNA (1 ng/μL) followed by the addition of RNase H has been plotted in (b) when the mixture was incubated at different temperatures for 5 min. The schematic representation for the visual naked-eye detection of SARS-CoV-2 with the treatment of RNase H at 65 °C for 5 min is shown in (c). The error bar indicates the average results obtained from three such independent experiments performed in triplicate [209].
Copyright© 2020 American Chemical Society
A simple colorimetric technique making use of anti-spike antibody gold NPs for a rapid diagnosis of COVID-19 viral antigen within 5 min was developed [210]. In this work, 4-aminothiophenol was attached (4-ATP) to AuNPs via an Au–S bond and in the presence of COVID-19 antigen or virus particles (interaction antigen–antibody). AuNPs aggregated and the color of the solution changed from pink to blue. The result showed the detection of COVID-19 with the naked eyes even at a lower concentration of 1 nanogram (ng)/mL. SERS has been employed and a very strong signal obtained in the presence of (4-ATP), as a reporter molecular, banded to AuNPs due the “hot spot” formation in the presence of COVID-19 antigen or virus. The detection obtained even at a very low concentration of 4 picograms (pg)/mL and virus particles at a concentration of 18 virus/mL. By knowing that the spike protein binds to the human angiotensin-converting enzyme 2 (ACE2) receptor on the surface of cells, A. Pranami used pseudoSARS-CoV-2 as a model virus to demonstrate that anti-spike antibody and 4-ATP attached AuNP-based SERS may could have not only the ability to detect COVID-19 but also the ability to block viral replication and virus spread in the HEK293T cells, which express ACE2 human cells.
In the same context, Ventura et al. [211] used AuNPs in colorimetric detection of SARS-CoV-2. A new, fast, reliable, and cheap tools based on a colloidal solution of AuNPs (20 nm, DO = 1) functionalized against each of three surface proteins of anti-SARS-CoV-2 (anti-spike S, anti-envelope E, and anti-membrane M) without any link, due to the photochemical immobilization technique (PIT). Each of the antibodies was treated separately (Fig. 11a). The detection of COVID-19 is based on the interaction among the virus and the functionalized AuNP clinical samples. As a result, the presence of the antigen induced the formation of a nanoparticle layer on the surface of the biosensor causes a redshift of the optical density (D.O) in the extinction spectrum of the solution with color changes from red to purple in a few minutes even was visible by naked eyes, as shown in Fig. 11b, c. It was reported that one of the most significant advantages of this biosensor was its sensitivity to the virion instead of its content, which is RNA. This achievement can be used for COVID-19 testing and open a new perspective to detect other viruses.
Fig. 11.
a Sketch of the SARS-CoV-2 and functionalized AuNPs. SARS-CoV-2 proteins (spike, membrane, and envelope) and their corresponding antibody (S, E, and M) are highlighted in dark red, light violet, and gray, respectively. The inset shows the pink colloidal solution containing the anti-SARS-CoV-2 functionalized AuNPs (f-AuNPs). b The f-AuNPs surround the virion forming a nanoparticle layer on its surface. Their interaction leads to a shift of the resonance peak in the extinction spectrum and, hence, to a color change visible in the inset. c Extinction spectra reporting the OD of f-AuNP colloidal solution mixed with samples from patients with different viral load. At very low virion concentration (curve Ct32), the extinction spectrum is not distinguishable from the spectrum of f-AuNPs (black continuous line). At intermediate virion concentration (curve Ct15), the extinction spectrum is slightly red-shifted and its difference from the “control” (f-AuNPs) produces the curve Ct15-(f-AuNPs) that evidences the contribution entailed by the virion. At high virion concentration (curve Ct7), the extinction spectrum peaks at 560 nm as for Ct15-(f-AuNPs). The agreement between the curve C7 and the simulated spectrum (gold continuous line, scaled to the experimental one) from a dielectric sphere (100-nm diameter) surrounded by smaller AuNPs (20-nm diameter) confirms the interpretation of the extinction spectra as due to nanoparticle aggregation [211].
Copyright© 2020 American Chemical Society
Lateral Flow Immunoassay
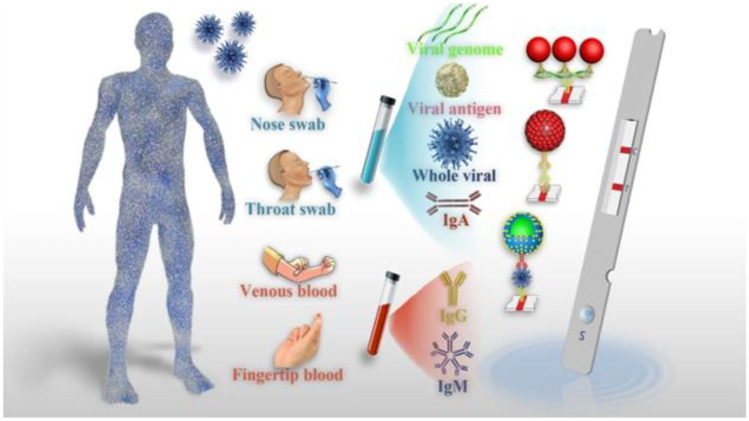
Ever since the discovery of COVID-19 in December 2019, the first step was to identify the viruses. Other than the usual detection technique for SARS-CoV-2 like rRT-qPCR or optic biosensors, a wide number of serological-based techniques have been employed for COVID-19 detection including enzyme-linked immunosorbent assays (ELISA), immunofluorescence, lateral flow assay (LFA), and isothermal amplification-assisted nucleic acid test. In general, these serological tests are used for asymptomatic patients. Although they are very specific to identify the virus by identifying the antibodies produced against the SARS-CoV-2 antigens and provide qualitative results with sensitivity, they require highly skilled personnel and expensive. Instead, LFIA has gained great success in COVID-19 diagnosis. As a complement to the current rRT-qPCR assay, LFA is highly sensitive, specific, and directly detect viral antigen or antibodies in clinical samples based on immunochromatography or lateral flow assays (LFIA) [212–214]. Lateral flow immunoassay (LFIA) has been considered one of the most popular point-of-care testing (POCT) techniques due to its simplicity, flexibility, speed, low cost, and wide adaptability which fulfill the requirement for the detection of SARS-CoV-2 [215]. It was a simple device designed to detect the analyte target in various clinical samples, such as whole blood, plasma, serum, and saliva (Fig. 12), without the need not only of specialists but also costly equipment. It was used in medical diagnosis even for home testing and POC testing to quickly recognize the presence of COVID-19 by analyzing specific biomarkers of SARS-CoV-2, such as nucleic acid, antibodies, and antigens. The test usually reveals results within 5 to 30 min. In the LFIA test strip, gold nanoparticles (AuNPs; labeling agent or reporter) and antibodies (recognition element) are the main constituents’ elements. Given this context, numerous rapid diagnosis technologies based on LFIA have been developed part of which have been developed as rapid test kits detecting SARS-CoV-2. The rapid tests involving LFIA techniques with the enhancement of specific optical plasmonic phenomena (such as SRS, LRSP, and SERS) improve COVID-19 diagnosis by targeting SARS-CoV-2-specific viral RNA, antibodies, antigens, and even the whole viruses. A major shift in the clinical diagnostic industry has begun with the LFIA point-of-care testing against COVID-19.
Fig. 12.
Schematic representation for the POC diagnostics of COVID-19 powered by LFA.
Reproduced with permission from ref [324]. Copyright© 2021 Elsevier B.V
Various serological are widely used as an efficient biomarker for the diagnosis of SARS-CoV-2 depending upon the characteristic of the virus and also on the collected samples from infected persons (Fig. 12). Various specific immunoglobulins are produced by the immune system in response to SARS-CoV-2, which appear and last different times [216]. In the limited resources in emergency division and in the need of antibody testing of SARS-CoV-2, LFIA raised as an accurate POCT that is capable of detecting specific proteins like antigens and antibodies IgM, IgG, and IgA in the various samples providing distinguished detection results.
Given established research into SARS-CoV-2 detection through nucleic acid testing, a feasible, low cost, and easy operation through immunochromatography assay was developed by using colloidal gold NPs (≈ 30 nm) to fabricate AuNP-based lateral flow (AuNP-LF) strips [217]. Anti-human IgM was conjugated with AuNP-LF as a detecting reporter that captured SARS-CoV-2 viral particles via antigen–antibody reaction. The best performance was obtained after optimization of pH value and concentration of anti-human IgM (pH = 8; 1.5 µg concentration of Anti-human IgM). The results were evaluated based on the notice test line which indicated the positivity of the samples. It showed an excellent sensitivity and specificity (100% and 93.3%), possessed satisfactory stability, and had a good consistency by the Kappa test (κ = 0.872). It can be confirmed that the applied method is rapid, fast, and can be used for potable detection of IgM antibody against SARS-CoV-2 virus.
However, the high concentration of the analyte limits the sensitivity and diminishes the reproducibility of the LFIA efficiency. To overcome these major drawbacks, LFIA has been integrated with other’s techniques such as SERS, RT-PCR, and ELISA to improve the detection of SARS-CoV-2 protein. SERS was combined with LFIA (lateral flow immunoassay) to form biosensing systems for detection of SARS-CoV-2 [218]. IgG/IgM biomolecular inspired by many previous kinds of research including heart diseases, tumor, infection biomarkers, and respiratory virus, using AgNPs for the sensitivity of the targets (Fig. 13). According to many studies in coronavirus, IgM raise after the first contact of virus with body to provide early defense and rapidly decrease after short time. However, IgG is responsible for long-term immunity and immunological memory and remains in blood after recovery even after 2 months [219, 220]. Liu et al. [218] used an advanced SERS tags in clinical samples to detect SARS-CoV-2, a dual-layers Raman dye molecule-loaded Ag shell-coated SiO2 (SiO2@Ag NPs with an average size of 200 nm), as shown in Fig. 13a. SARS-CoV-2 S protein immobilized on the SiO2@Ag SERS tags by amid reaction and then detected the anti-human SARS-CoV-2 IgG/IgM protein in human serum based on antibody-antigen reactions (Fig. 13b). Based on the SERS intensity analyses with anti-human IgG/IgM antibodies on the biosensor, it indicated an excellent intensity via SiO2@Ag NPs in the presence of SARS-CoV-2. The detection sensitivity of the proposed method for virus-specific IgM and IgG was 800 times more sensitive than that of the standard Au-based LFIA method. The clinical validation of the device was examined with 68 patient samples with 100% accuracy. Considering its high sensitivity, accuracy, and specificity, the proposed method may be used for rapid screening of COVID-19 during early infection, when the levels of target IgM and IgG antibodies are still low and difficult to detect by other detection methods, such as AuNP-based LFIA strip, indirect immunofluorescence assay, and ELISA.
Fig. 13.
a Schematic diagram of the preparation of the dual-layers DTNB-modified SiO2@Ag NPs. b Preparation of SARS-CoV-2 S protein modified SiO2@Ag SERS tags. c Operating principle of the high-sensitivity and simultaneous analysis of anti-SARS-CoV-2 IgM/IgG via the SERS-LFIA strip. Reproduced with permission from [218].
Copyright 2020 Elsevier
A similar study conducted and illustrated the same technique targeting anti-S Abs antibodies, the spike glycoprotein (IgM and IgG), of SARS-Co-2 [221]. Colloidal gold NP immuchromatographic assay (GICA) was used through serological test. The serum samples were collected from 306 COVID-19 recovery cases discharged from Wuhan Tongji Hospital during the next 6 months. Then, the obtained result of analyses was compared to the result detected by chemiluminescence microparticle immune assay of nAbs (neutralizing antibody) samples to verify its sensitivity. This approach could be suitable for primary screening periodic self-evaluation of the protective effects of nAbs in COVID-19 individual and post-vaccination populations. Furthermore, a serological test implementing flow through-dot-blot assay (FT-DBA) with anti-human IgG (and anti-mousse IgG)-biofunctionalized colloidal gold nanoparticles (AuNPs) (10 and 40 nm) was developed, as a signal reporter, to detect SARS-CoV-2 and specific IgG in human serum [222]. In order to verify its selectivity, the obtained analysis result with the kit was compared to the results obtained with RT-qPCR and it confirmed with good sensitivity. Compared with nucleocapsid (NCP), Commercial CLIA Elecsys SARS-CoV-2 Assay, or receptor-binding domain (RBD) ELISA(enzyme-linked immunosorbent assay) specific for SARS-CoV-2, used as capture antigen in the test platform, an additive sensitivity was observed duo to co-immobilization of both antigens to capture wide range of antibodies. The overall sensitivity and specificity of the FT-DBA kit were 98% and 98%, respectively.
To further satisfy the need of IgM and IgG detection in different circumstances such as port/entry exit, community, and in order to prevent transmission of SARS-CoV-2, lateral flow immunoassay (LFIA) must be more sensitive and easy to operate. Fluorescence lateral flow detection system has been known as an important POCT tool due to its advantages of high sensitivity and potable instrument. Recently QDs play a major role as fluorescence labels. Based on these, Wang et al. developed a colorimetric-fluorescence dual-mode lateral flow immunoassay biosensor tags SiO2@Au@QD using QD nanobeads for rapid, sensitive, and simultaneous detection of SARS-CoV-2-specific IgM and IgG in human serum [223]. The tag was fabricated via polyleneimine (PEI)-mediated electrostatic absorption method and it is consisted of monodisperse SiO2 core (200 m) as supporter, a layer of AuNPs (3 nm) to provide colorimetric signal, a layer of carboxylated CdSe@ZnS QDs shell outside the core as a reporter to provide fluorescence signal, and surface-modified vancomycin to bind to S. aureus. The fabricated strips contained two test lines T1 and T2 (human IgM and IgG) and one control line. The assay needs only 1µL of serum sample and could be completed within 15 min and is 100 more sensitive than the traditional colloidal AuNP-based LFIA. The SERS nanotags reported by the author can be used in place of colorimetric and other sensors as an ultrasensitive and highly specific POCT testing device.
The response to viral infection of SARS-CoV-2 begins with the production of specific immunoglobulins A (IgA), which plays an important role in protecting the respiratory mucosal epithelium [224]. While SAR-CoV-2 IgM and IgG can be detectable from 4 to 7 days, respectively [225], SARS-CoV-2 IgA can be detectable from 2 days of the initial symptoms [226]. Many studies showed that IgA serum is produced in large amounts than IgG. For the aforementioned reason, to detect IgA may be an alternative in serological test and more reliable for the detection of COVID-19-infected individuals than IgM [227]. IgA appeared first in the serum of the infected person (compared to IgM and IgG) and it has more advantages due to its existence in the saliva which makes it easy especially for point-of-care testing than blood samples. Furthermore, detection of immunoglobulin A (IgA) has been suggested by some researchers as a more accurate diagnostic tool. In this context, a dual optical/chemiluminescence format of a lateral flow immunoassay (LFIA) immunosensor to detect IgA in serum and saliva (Fig. 14) was fabricated [228]. It was a dual colorimetric chemiluminescence detection method for determination of SARS-CoV-2 IgA antibodies. A simple Smartphone-camera-based device was used to measure the color signal by gold nanoparticles (30 nm)-labeled anti-human IgA to reveal the IgA bound to the N antigen. For chemiluminescence transduction, a contact imaging portable device was applied and measured the light signal resulting from the reaction of the HPR-labeled anti-human IgA with a H2O2/luminal/enhancer substrate. It was the only immunosensor reported to detect immunoglobulin IgA and it may open the perspective to additional studies on the significance of IgA as biomarkers of immune response to COVID-19.
Fig. 14.

Schematic illustration of LFIA strip IgA antibody detection biosensor. Reproduced with permission from [227].
Copyright© 2020 Elsevier
In another study, a multi-target double line lateral flow immunoassay allowing the specific and sensitive detection of total immunoglobulins including IgG, IgM, and IgA directed towards the nucleocapsid protein (N) of SARS-CoV-2 was developed by [229]. The specific detection of anti-SARS-CoV-2 antibodies was assured by the probe, which composed by a recombination of SARS-CoV-2 nucleocapsid protein (N), gold nanoparticles AuNPs (30 nm), and biotin as colorimetric reporter. The LFIA device achieved a high specificity of 100% and 94.6% of sensitivity. Aside from the detection of IgA, the LFIA developed device seems to be a good early predictor of SARS-CoV-2 because IgA is intended to be produced at detectable levels earlier than IgG and IgM. By using colloidal gold nanoparticle (AuNP)-based lateral flow immunoassay, a point-of-care test (POCT) was successfully developed as a homemade portable reader, for rapid and specific detection of immunoglobulin G and M contain [230]. The colloidal gold nanoparticle (AuNP)-based lateral flow immunoassay test strip was able to detect virus within 15 min. The aim of this work was to fabricate (POCT) system with a good sensitivity that can detect anti-N IgM/IgG and anti-S-RBD IgG/IgM in human serum not only to prove the presence and exclude of SARS-CoV-2 but also to determine the levels and alterations of anti-SARS-CoV-2 IgG/IgM. A recent study illustrated a lateral flow immunoassay method for rapid detection of IgG antibodies against SARS-CoV-2 [231]. Gold nanoparticles (AuNPs) (30 nm) were used as a reporter which conjugated with anti-human IgG (mAbs). The performance of the assay was investigated using serum samples of the clinical diagnosed cases of COVID-19. The test had 69.1% sensitivity and 100% specificity.
To further speed up the detection of SARS-CoV-2 and monitor its spread, a very recent study conducted from the University of McMaster, Canada, to develop an enzyme-linked immunosorbent assay to rapid detection test of antibodies (Abs) using nanoprobes [232]. It is formed of a colloidal aqueous suspension of gold NPs (18 nm) functionalized with an aptamer thiol (SARS-CoV-2-RBD-4C) specific to detect the spike protein of SARS-CoV-2 membrane by binding with it. Aptamars are becoming interesting recently because of their good stability which offers better functionality for protein detection [233, 234]. The concept followed by the author was to add a coagulant MgCl2 salt solution and phosphate buffer saline (PBS) inducing nanoprobe agglomeration and forming complexes with the surface charges on the AuNPs (Fig. 15a, b).
Fig. 15.
Schematic illustrating the principle of the SARS-COV-2 test. a Nanoprobes are AuNPs functionalized with aptamers in an aqueous suspension. When the SARS-COV-2 spike protein is absent from the colloid, addition of the coagulant salt M neutralizes surface charges on the nanoprobes, inducing their agglomeration. b Nanoprobes with spike protein bind with aptamers and resist agglomeration, which depends on the extent of this binding. Protein binding provides additional charge to the nanoparticles enhancing steric stabilization. c Plasmon absorbance spectra for the nanoprobes show how agglomeration in a colloidal suspension broadens the absorbance spectrum and shifts peak absorbance to higher wavelengths [231].
Copyright© 2021 Elsevier
Therefore, the absorbance spectrum of the suspension is sensitive to the extent of nanoprobe agglomeration and the surface plasmon absorbance wavelength shifts to higher values (Fig. 15c). The spike protein was added to the suspension and binds through the aptamer on the surface of the nanoprobes which enhance steric stabilization and resist the agglomeration, as illustrated in Fig. 15a, b. The effect of this binding induces diminishment of the agglomeration and decreases the wavelength shift of the surface plasmon absorbance. The critical coagulant concentration, Cc, of the salt solution that induced agglomeration is a useful metric to determine the spike protein binding with the aptamer, which increase with increasing the spike protein concentration in the samples. The nanoprobes suggested can detect 3540 genome copies/µL and the high concentration of the inactivated SARS-CoV-2 virus obtained at an absorbance corresponding to 540 nm.
Electrochemical-Based Immunosensor
The electrochemical biosensor immunoassay is regarded as an effective sensing method in terms of sensitivity, quantitative, and accuracy for the detection. It can be an alternative detection approach to traditional assays which developed for spike protein or nucleocapsid protein detection. Accordingly, electrochemical immunosensor can encompass all the advantages of electrochemical procedures, specific immunorecognition reaction, and biosensor devices. For this reason, it becomes a potential detection strategy that can hold great attention from the scientist to make disposable devices for simple and rapid detection of viruses. In order to enhance the performance of the biosensors and immobilize the biological molecules on the electrode surface, different nanoparticles were used. In this context, several studies have been emerging to open a new perspective for the development and production of new electrochemical point-of-care devices that fulfill the need to fight COVID-19.
A paper-based electrochemical DNA biosensor developed to detect the presence of SAS-CoV-2 [235]. They constructed an electrode by using a thick layer of graphene on a paper surface. The biosensor uses highly thiol-specific antisense oligonucleotide (ssDNA)-capped gold nanoparticle (AuNPs) (10 nm) simultaneously targeting the four regions of the same viral nucleocapsid phosphoprotein (N-gene) of SARS-CoV-2, as illustrated in Fig. 16C–E. Accordingly, the author used gold nanoparticles to increase the sensitivity of the electrochemical platform because of their excellent properties. The graphene increases the conductivity of the sensor and also prevents the formation of a water tight composite on the sensor surface. The dynamic response of the sensor was obtained from various concentrations of viral RNA load extracted from Vero cells infected with SAS-CoV-2, SARS-CoV, and MERS-CoV RNA virus.
Fig. 16.
Schematic representation of the operation principle of the COVID-19 electrochemical sensing platform wherein step A: the infected samples will be collected from the nasal swab or saliva of the patients under observation; step B: the viral SARS-CoV-2 RNA will be extracted; step C: the viral RNA will be added on top of the graphene-ssDNA-AuNP platform; step D: incubation of 5 min; and step E: the digital electrochemical output will be recorded [235].
Copyright© 2020 American Chemical Society
The performance of the selectivity of the sensor ship to differentiate the positive COVID-19 from negatives ones was tested using 48 clinical samples and then compared and confirmed with using FDA-approved RT-PCR COVID-19 diagnostic kit. The biosensor provides a significant result about the presence of SARS-CoV-2 RNA only within 5 min; the sensitivity was 231 (copies µL−1)−1 and the LDO was 6.9 copies/µL with no amplification.
In another report, a label-free electrochemical nanobiodevice was fabricated as a biosensor for rapid detecting of spike protein to identify infected people to SARS-CoV-2 in order to facilitate the point-of-care diagnosis [236]. This sensor was fabricated by using Staphylococcal protein A (ProtA) coated with Cu2O nanocubes (Cu2O NCs), which has a large specific surface area and low toxicity effect, to increase the loading of biomolecules (human anti-SARS-CoV-2 spike protein antibody IgG) immobilized on screen printed electrode as a receptor element to detect the analyte target of SARS-CoV-2. The ultrasensitive electroanalytical nanobiodevice ProtA/Cu2O NC-modified SPCE was able to be used in clinical samples to detect SARS-CoV-2 virus within 20 min and it can be a useful tool to detect SARS-CoV-2 in early stage. Similarly, to facilitate the diagnosis and the detection of COVID-19, a sensitive, target specific, and cost-effective electrochemical biosensor approach based on gold nanoparticles (AuNPs) electro-deposited onto titanium surface as the sensing electrode was reported [237]. This sensing interface is prepared by physisorption of nanomaterials on a solid surface (titanium electrodes). AuNPs provide stability and protection to the electrode. A complementary single-stranded probe was designed with thiol to target the COVID-19-specific viral RNA or the corresponding c-DNA. This probe was attached to the NPs through Au-thiol self-assembly in the sensing domain. The coronavirus genome binds to the probe by DNA hybridization in the sensor and the diagnosis of COVID-19 is developed.
Recently, a sandwich assay electrochemical biosensor for the diagnosis of ORF1ab RNA of SARS-CoV-2 with a Smartphone was developed [238]. In this study, Au@Fe3O4 nanocomposite was synthesized and then functionalized with hexane-1-thiol in buffer solution. Graphene oxide (GO) was prepared and functionalized with p-sulfocalix[8]arene (SCX8) and then added the gold nanoparticles and TB fluorescence substance (toluidine blue) to form Au@SCX8-RGO-TB nanocomposites. The result displayed high specificity and selectivity to detect SARS-CoV-2 and it seems to be more significant when compared it with those obtained using RT-qPCR. The low limit of detection LOD of the device in clinical samples was 200copies/mL. The biosensor reported could be converted into portable biosensor by integration with Smartphone which allow it then to obtain result only in a few second. In another study, a rapid electrochemical diagnostic kit was fabricated that could detect pathogenic viruses such as SARS-CoV-2 through the differentiable fingerprint of their viral glycoproteins [239]. It composed of fixed/screen printed electrodes. The sensor was constituted of an electrode that was activated upon a layer of grapheme oxide (GO) and sensitive chemical compounds with gold nanostars (AuNS). This fabricated system can detect the trace of viruses in an aquatic biological media such as blood, saliva, and swab via interaction with active functional groups of their glycoproteins in 1 min. The limit of the detection LOD of the sensor towards SARS-CoV-2 was 1.6810−22µgmL−1, and the sensitivity was 0.0048μAμg mL−1 cm−2. The clinical evaluation of samples confirmed the high sensitivity and selectivity of the developed nanosensor to detect SARS-CoV-2.
Plasmonic NP-Based Therapy Against COVID-19
Nanotechnology has a great potential in contributing significantly to the fight against COVID-19 by developing effective therapies that can selectively eradicate the respiratory virus load. Because noble metal nanoparticles such as Ag, Au, and Cu reveal stability in the biological environment and remain stable in an intracellular environment and due to their small sizes, it makes them easily interact with bimolecular both on the surface and inside cells. The similarity of the genetic sequence of syndrome coronavirus SARS-CoV and SARS-CoV-2 with nearly the same structure might be inhibited by the same drug treatment as short-term solution. Hydroxychloroquine (HCQ) and chloroquine (CQ) have been used to treat SARS-CoV-2 and can be a potential therapeutic option against COVID-19. However, the use of this drug at a large scale for COVID-19 may lead to toxicity and even to cardiac death. As nanoparticles have the ability to transport drugs into the body to specific targets and act a drug delivery, scientists could have a better strategy to achieve a viable solution and alleviate these disadvantages. In this context, a calculation principle was determined to find out the affinity of HCQ/CQ molecules towards silver (Ag) and gold (Au) NPs for the purpose of using NPs as a delivery drugs [240]. Explained that the nitrogen N− and the oxygen O− of hydroxyl group in HCQ/CQ molecules have the highest affinity for interaction with noble metal cluster and it increases by changing the type of metal NP element AuNPs > AuAgNPs > AgNPs. The absorption spectra of plasmonic AgNPS, AuNPs, and their coating with HCQ/CQ drugs have a slight change for AgNPs, yet a remarkable change and shift to the blue with AuNPs. The obtained result from this combination of quantum mechanics and dynamic simulation may encourage and suggest these NPs as an efficient bridge to use HCQ/CQ and decrease its drug side effect for COVID-19.
As known before, silver nanoparticles with its first recorded medical have an effective antiviral activity. A research paper postulated a novel antiviral therapy for killing COVID-19 with minimal side effects [241]. It was a hypothesis of a potent drug against COVID-19 virus by using silver nanoparticles (AgNPs) (10 nm), dispersed in water, with bronchodilators in lungs through nebulization with simple nebulizer machine in corona patients. His idea has never been tried before, and it consists of speculating that Ag+ realize from AgNPs and bind with phosphorus or sulfur of glycoproteins of RNA viruses preventing the fusion of the virus to host cells. The Ag+ ion caused an alteration in the Ph of the respiratory epithelium to alkaline, which induce hostile environment for the viruses to survive. This mechanism led to a reduction of the viral load in the respiratory epithelium, therefore, decreasing the spread of the viruses from person to person.
Owing to its antimicrobial properties and its capability to absorb light in visible and NIR region, gold NPs are an appealing platform for the development of nanovaccines by antigen functionalization and may be valuable candidate in the treatment of COVID-19. Given this context, a proposed therapy method was developed as a paradigm that may be efficient to eradicate SARS-CoV-2 virus [242]. They used plasmonic photothermal therapy (PTT), an approach largely used in previous treatment to eliminate bacterial infection, murine leukemia virus, and cancer [243], as a localized treatment on targeting and eradicating the virus by using ACE-2-functionalized gold nanorods (AuNRs). Then, it followed with irradiation with near-infrared (NIR) light generating PTT effect to inactivate COVID-19 viral (Fig. 17). AuNRs have a maximum LSPR absorbance in the NIR region and a higher efficiency rate of converting photon into heat compared to the other gold NPs like nanospherics and nanoshells [244, 245]. In this study, the author suggested that an orientation in tip-to-tip AuNRs have a strong effect to LSPR coupling of AuNRs comes from a strong plasmonic coupling between the two facing tips in tip-to-tip AuNR dimer, which increase the intensity of absorbed light and then a higher localized photothermal heat generated. By taking this into account and by knowing that NIR light has a deeper tissue penetration than the visible light, it concluded that gold NRs may be the most suitable candidate for this approach to eliminate SARS-CoV-2 viral load in the respiratory tract of infected patients [243]. The functionalization of AuNRs with ACE-2, the natural host receptor in the human body to bind with a virus, enhances the selectivity of the NRs to SARS-CoV-2 in the airway. Both gold NR dispersion and laser energy were delivery into the distal airway using flexible bronchoscopy, which is the commonly performed procedure in pulmonary medicine for COVID-19 hospitalized patients [246]. Then, the light absorbed by protein ACE-2-functionalized AuNRs converted to heat energy and transformed to bound virus in the lung. This clinical approach could be an efficient method to save lives and may be have the potential to translate to clinical trials.
Fig. 17.
Novel approach used for management of hospitalized COVID-19 patients; a proposing plasmonic photothermal therapy as a safe and effective therapy using flexible bronchoscopy to introduce the AuNRs and apply NIR radiation locally in the distal airways. a Interaction of AuNRs with SARS-CoV-2 is dictated by surface-functionalization with ACE-2. b Virus-bound AuNRs absorb NIR light, c resulting in hyperthermia and loss of virus pathogenicity at elevated temperature [241] Copyright
© 2021. Springer Nature
Nanotechnology and COVID-19 Mutation Challenge
COVID-19 Spike Protein Mutations
The viral genome of SARS-CoV-2 has undergone a constant mutation throughout the emergent of COVID-19, which occurred spontaneously when there are replications. More than 9.711 million complete SARS-CoV-2 genome sequences have occurred, which are available at Global Initiative on Sharing Avian Inluence Data (GISID) since the emergence of the virus [247]. With this available data, a comprehensive epidemiology of SARS-CoV-2 related to variants (other terms used like strain or lineage) and its evolving in terms of adaptability to invade the immune system and enhanced infectivity can be achieved [248]. These large numbers of variants harbor mutations, not only in the gene encoding the S proteins with its two S1 and S2 subunit domains, but also can occur in any other region of SARS-CoV-2 genome, including N gene and NSP12 (RdRp) with high mutations rate [249]. The rate of mutation in SARS-CoV-2 is around 10−8 nucleotide/genome annually, which is very high for RNA viruses [250]. Although the huge numbers of mutations, most of them are silent and do not modify the primary amino acid sequences, which not have relevant effects in the viral transmissibility, yet they have been considered as putatively adaptive [251]. However, the accumulation of mutations can yield variants with selective and survival advantages [252]. These mutation variants can enhanced viral infectivity, replication, immune escape potential, and increased transmissibility [253–256].
Because the S gene is the most viable part of SARS-CoV-2, the mutation occurred in the S part is a major issue of concern for many research studies looking for an effective tool to alter the evolving of the virus and its pathogenicity. The S gene, with its major immunogenic regions NTD and RBD, is the most extensively studied viral infection protein and the main target to vaccine development. Currently, there are around 4000 mutations in the S protein gene [150].
The first and the most predominant mutation across the globe in S protein was a change from aspartic acid to glycine in the S1 subunit at the position 614 (S:D614G) [257, 258]. As described by Altmann et al. [253], this mutation was from B.1 lineage, and it did not resisted against vaccine but increased in vitro viral infection. It was first detected in viruses collected from Wuhan strain and Germany. Soon after its emergent in the initial of pandemic, this variant has becoming predominant in most country within 2 months. Many studies reported that the D614G have high fitness survival effect, infectivity, stability and transmissibility [259–261], and affinity with the ACE-2 receptor [262]. It has been noticed that D614G has become more predominant and appeared in all recently identified variants. According to the study conducted [253], the transition from D614 to G614 variant allowed this variant to be more predominant around the world and spread more rapidly than the original variant. They concluded that this new variant was more infectious, infected cells faster than D614, and had high viral RNA levels. New strain of the virus noted B.1.1.7 with multiple mutations was occurred in the RBD and NTD of spike in the UK [263]. By December 2020, this variant was responsible for one-quarter of the total COVID-19 cases worldwide and had 40–70% transmissibility compared to the original virus [258, 264]. This lineage has 23 mutations in the S, N, and ORF-8 and in its S protein, it contains several amino acid mutations including D614G and N501Y, and deletions DH69/DV70 [250]. The variant B.1.1.298 considered being the first to have the D614G mutation. It was appeared in Denmark mink farms [265], an affected 17 million Danish minks [266]. Many variants like Y453F in the RBD of the S protein contributed to the improvement of the viral fitness of the B.1.298 variant [267]. In addition, the N501Y is situated at the RBD-hACE-2 interface and strengthens the intermolecular hydrogen-binding between RBD-hACE-2 complexes. This mutant associated with immune escape against antibodies and enhanced transmissibility and affinity [265]. The lineages B.1429 and B.1.27 were assigned by the WHO as Epsilon variants; however, the lineage B.1.429 contains 5 distinct mutations in the S region such as S13I, W152C, and L452R [268]. Another interesting beta lineage B.1.351 emerged first in South Africa and then spread to other African countries and the world. By the beginning of 2021, it was responsible for more than 90% of all COVID-19 cases in South Africa [250, 269]. Several structural and non-structural mutations were induced from this lineage including L18F, D80A, D215G, Δ242, Δ243, Δ244, R246I, K417N, E484K, N501Y, D614G, N438K, and A701V in the S region [270, 271]. The critical three of them were in the RBD of the S protein (K417N, E484K, and N501Y). These three mutations enhanced viral fitness and adaptability. Particularly, the K417N variant escaped immune system and reduced vaccine effectively against COVID-19 [269]. However, the E484K variant enhanced the binding with ACE-2 [250] and decreased the response to Ab neutralization issued from previous infection or vaccination [260]. Li et al. [272] reported five mutations in the RBD of S which improved adaptation and increased spreading and reinfection cases previously infected with SARS-CoV-2 [273]. Oliveira et al. [274] reported the Gamma variant P.1 as an alias from the linage B.1.28.1. The variant P.1 contains 17 unique amino acid substitutions, 10 of which occurred in the S region including L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, H655Y, and T1027I [275, 276].
The B.1.1.7 lineage was reported first in the UK on 01 December 2020 by the WHO [276]. This variant has large numbers of genetic changes especially in the RBD and the furin cleavage. A total of 17 mutations were observed in the S protein and many of them were reported in other lineages [277]. The B.1.1.7 non-synonymous mutations and deletions that have been detected in the S proteins were deletion 69–70, deletion 144, N501Y, 570D, D614G, P681H, T716I, S982A, and D1118H [278, 279]. The three mutations N501Y, P681H, and the deletion at position 69–70 have reported to increase the binding affinity with ACE-2, increase infectivity, and affect replication. Dearlove et al. [258] reported that this lineage will not restrain immunity induced by vaccine.
The B.1.617 Pango lineage was first reported in India in December 2020 and it has many predominant mutations in RBD as E484Q, N439K, and L452R [280]. Interestingly, the substitution N439K was the most common RBD mutant reported in many other lineages such as B.1.1.7, B.1.351, and B.1.258 and it has been linked with an increased affinity with ACE-2 and immune escape [281]. B.1.617 lineage holds three sub-lineages B.1.617.1, B.1.617.2, and B.1.617.3. This variant contains 13 S protein mutations and the majority of them include the amino acid substitutions [282]. Many studies reported that this variant has resistant effect against neutralizing X593 and P2B-2F6 monoclonal antibodies [283, 284]. The B.1.617.2 is labeled as the Delta variant by WHO, and it was exhibited high transmissibility and risk of hospitalization than the original strain [285]. The most prevailing substitutions in Delta variant are T19R, Δ157-158, T478K/T478R, and D950N [281]. The other substitutions in the spike were G142D, L452R, R158G, DG14G, and P681R [286]. However, one of the sub-variant of Delta housing the substitution at K417N called Delta plus K417N (AY.1), also present in Beta variant, has appeared on June 2021 [287]. As this variant emerged, a concern has raised about the likelihood of immunity evasion and reinfection [288]. The three variants L452R, E484Q, and T478K have occurred in the S RBD while P681R occurred in the cleavage site between S1 and S2. As Kirola [289] reported, L452R mutation increased the binding affinity with ACE-2. Moreover, the T478K with L452R mutations assisted the stability of the RBD-ACE-2 complex and raised the virus infectivity rate [283]. The E484Q mutant enhanced the affinity to ACE-2 and also reduced antibody binding affinity, and it was much like what observed with E484K [290]. In his recent study, Liu et al. [290] elucidated that P681R modification leads to increase furin cleavage site (S1–S2 junction) which induce high infectivity rate compared to B.1.1.7 strain. The combination of mutations in B.1.617.2 (Delta variant) added advantages compared to the original virus and other variants by its high transmissibility, infectivity, and immune evasion [284, 291].
Very recently in November 2021, an emergent mutation of SARS-CoV-2 identified in South Africa and in many other regions in the globe. The WHO classified this variant as B.1.1.529 and named Omicron based in its severity and alarming situation in South Africa [255]. B.1.1.529 mutation holds many variants present in other variants such as N501Y (alpha), E484A–E484K (beta and gamma), and T478K; P681H-P681R (delta). As known, all the alpha, beta, gamma, and delta had high transmissibility and related to enhanced infectivity and affinity to ACE2, not to mention their potential to immune escape. Until now, omicron variant has more than 50 mutations; more than 30 of them are in the S protein [255]. In recent study [292], identified numerous mutations contain in omicron variants like Q498R and S477N. These two variants previously have been associated with elevated binding to ACE-2. However, there are a close connection between omicron and alpha variant [293]. But, it appeared from the various studies in the different variants that omicron variant does not increase virus severity.
Effect of Variants on Neutralization Activity and Vaccine Efficacy
While these mutations increasingly occurred, concern increase about the failure in the immune protection originates from a preceding infection or vaccination. The change in the genome sequence could lead to an increase in the severity of the infection which is the case for some variants like G614 and we did not avoid the fact that these variants emerged may be less infected than the original SARS-CoV-2, but with some of them, especially those whose mutated in the interface RBD, enhanced the interface binding RBD-ACE-2 concern may raise about the future cycle of the new variants and its effects in the host cells, its transmissibility, and its escape immune and vaccination. As we know, most COVID-19 vaccine target spike protein aiming to produce nAbs against the RBD region blocking binding sites to ACE-2 receptor in the host [294, 295]. However, all vaccines were developed according to the original SARS-CoV-2 without S protein amino acid mutations [259]. Several studies evaluated and demonstrated the effectiveness of current vaccines against SARS-CoV-2 variants [296–300]. According to these studies, many variants of SRAS-CoV-2 such as the B.1.1.7 [297], the B.1.351 [295, 298, 301], the P.1, and the B.1.617.2 [298, 302], associated with decreased of nAb activity, may lead to immune escape, while some variants contain the E484K (such as B.1.351 and B.1.1.248) were found to escape the immune response completely [295]. However, the reduction of nAbs is not a sign of vaccine failure [264]. For instance, Pfizer BioNTech vaccine extended 95% against the original SARS-CoV-2 [303]. Collier et al. [297] noticed that there is no decrease in the nAb activity against B.1.1.7 have been observed [298, 299]. But, there were a significant reduction of the nAb activity provided by BTN 162b2 vaccine against the B.1.351 variant [295, 302, 304]. However, a study conducted in Qatar to evaluate the effectiveness of BTN 162b2 vaccine revealed 89% effectiveness against B.1.1.7 and 75% against B.1.351 variant while the effectiveness of this vaccine against SARS-CoV-2 cases was 97.4% [296, 305]. Several studies conducted researches to demonstrate the impact of the mRNA-1273 (Moderna) [301] against SARS-CoV-2 strains (B.1.617.2, P.1). The vaccine still has efficacy against these variants. For example, against the B.1.1.7 mRNA vaccine reached 94% efficacy, there is no reduction in the nAbs [306]. Another study demonstrated that the mRNA-1273 vaccine reduced nAb activity against B.1.617.2 compared to the D614G strain [307]. Novavax vaccine offered 95% efficacy against original SARS-CoV-2 [270]; however, a low reduction of nAb activity was reported against B.1.1.7 pseudovirus [307]. The adenovirus vector-based ChAOx1-nCoV-19 (AstraZeneca) presented 66.7% against SARS-CoV-2 [308], yet against B.1.1.7 showed reduced nAb activity without affecting vaccine efficacy [309]. Likewise, this vaccine displayed a small reduction of nAb activity against P.1 and B.1.617.2 variants [303, 310]. Janssen also was another adenovirus vaccine with 72% efficacy against B.1.1.7 variant infection, while it was 89% against SARS-CoV-2. For Sputnik V vaccine, it was shown 91.6% against severe COVID-19 [311]. However, a reduction of the efficacy of this vaccine was reported against B.1.1.7, B.1.351, and P.1 to 81%, 59%, and 52%, respectively. Also, nAb serum activity was reduced against B.1.351 and P.1, but not against B.1.1.7 [312]. According to the studies conducted against various variants of SARS-CoV-2, more studies are critical to verify and conclude the effectiveness of the current vaccines against some current strains and the other future predicted variants.
So, it is also important to point out that most studies concern the effectiveness and efficacy of vaccine against variants were not yet clear. In addition, the more COVID-19 lasts, novel mutations that help the virus evade the immune response may emerge. Not only that, SARS-CoV-2 can acquire mutation with replication advantages and immunological resistance which will lead virus to antigenic drift and escape from immune recognition [313]. Specially, the existence of G614 variant may cause random drift because of the unknown of its effect on spike protein function in the entry and fusion. Thus, vaccine designed to inhibit the binding of spike protein to ACE-2 may reduce its efficacy in the presence of such variant. Furthermore, scientists and medical professionals face challenge to determine the impact of the new variants on the severity and infection of SARS-CoV-2 in first place. In the second, more studies about the efficacy of the vaccines and the variant’s potential immune response evasion in vaccinated person and also the reinfection cases should be conducted. Then, constant surveillance and new strategies towards harnessing nanotechnology and organic, inorganic, and plasmonic nanoparticles to develop selective and effective drugs against COVID-19 disease were an urgent demand to prevent more new variants and understand the impact of the current strains of SARS-CoV-2.
Nanoscience and Nanotherapy
Advanced in the field of nanoscience and the large literature studies of the biological effects of nanoparticles have been inspired researches to further pursuit the development of novel therapeutical strategies to face earlier SARS-CoV and the current COVID-19 pandemic. Since the end of 2019, SARS-CoV-2 has picked up mutations leading to patterns of genomic diversity affecting the detection assays and the vaccines developed. The large numbers of SARS-CoV-2 mutations pose obstacles and difficulties in the SARS-CoV-2 detection and targeted therapy, especially with some of them escape detection and develop resistance to existing drugs. In view of the absence of an effective drug or vaccine or treatment strategies against SARS-CoV-2, viral mutation studies can pave the way to develop an effective vaccines, an antiviral drugs targeting the mutation sites and diagnostic assays by harnessing the advanced of nanotechnology. Different low toxic nanomaterials have been successfully used and being used to develop therapeutic agents against SAR-CoV-2. Particularly, plasmonic nanoparticles have proven their efficiency as antiviral materials to treat several diseases with their specific physical properties allowing deliver drugs to selective targeted zone, not to miss their role to inhibit many viral targets [314]. These nanoparticles may play protective feature and prevent the encapsulated drugs from degradation due to their small size [315]. Gold nanoparticles have shown great interest in the production of vaccine. A research study conducted by Staroverov et al. [316] revealed that gold NPs conjugated to virus could be suggested as a possible vaccine. Additionally, silver nanoparticle is one of the most elements that had strong antimicrobial activity, while silver sulfadiazine was categorized by the WHO as an essential anti-infective topical medication [317]. The potential of plasmonic nanoparticles for the detection, the prevention, the diagnosis, and the treatment was explored in medicine.
Nanomedecine is one of the newly promising platforms for virus detection and neutralization. The increased mutation rates of SARS-CoV-2 inspired many research groups to open new direction towards the use of nanoparticles as new tool to unlock the nature of the molecule binding and the structure mechanism of SARS-CoV-2-ACE2 interface, to monitor the spread and the evolution of the virus, and also to deliver drugs to their direct target site using previous advances in cancer treatments as example. Many exiting therapists for COVID-19 use nanobased approach since its emergent in the early 2019. Several research studies have been underway to develop new vaccine as soon as possible, to develop new solution to face the actual universal public health threat and highlighted the new era of nanoscience.
Conclusion and Perspective
In this review, we exposed an overview concerning the use of nanotechnology in the development of new strategies in terms of prevention, diagnosis, and therapy of coronavirus disease 2019. The first part of the introduction was devoted to the identification of coronavirus while the second was dedicated to plasmonic nanoparticles in terms of their unique optical, chemical, and biological properties. Plasmonic nanoparticles especially AuNPs, AgNPs, and CuNPs have provided tremendous advantages in the infection recognition, the prevention of COVID-19, the disinfection, and individual protection equipment. The uses and the fascinated roles of plasmonic nanoparticles and nanocomposite in the detection and diagnosis of coronavirus were developed in the second part. We have discussed the plasmon-enhanced biosensors, colorimetric-plasmon sensor, electrochemical sensor, and lateral flow immunoassay detection of coronavirus disease 2019. LSPR and SPR were found to be the ideal candidate for the detection of nanoscale analytes because they exhibit great sensitivity to local variation. In addition, plasmonic nanoparticles have great potential as antiviral agents and broad spectrum antimicrobial action against various bacteria, fungi, and viruses and participate to develop nanobiosensor-based platform, serve as antiviral agent or nanotherapy drug delivery. Implementing noble metal nanoparticles such as Ag, Au, and Cu has a great potential in contributing significantly to the fight against COVID-19 by developing effective therapies. The nanotechnology domain can become a bridge between diagnosis and therapy in the battle against viruses specially COVID-19.
Advanced in nanomedicine has given a nudge to alter the clinical landscape for detection and antiviral therapies to tackle many unsolved issue related to viral infections in the modern era. Plasmonic nanoparticles can improve pharmacokinetic profiles and give reasonable understanding that can be leveraged based in their unique properties to improve antiviral therapy approaches in vivo and help formulate and deliver drug in current COVID-19 viruses and its future variants. These approaches are in their early stage of development for infection COVID-19 disease and strongly need improvements in its integral setup for broader applications in the pre-clinical and clinical trials to test the validity of these NPs against COVID-19. However, the safety profiles for each new nanotherapy approaches need to be considered and critically evaluated. So there is a vast scope for future development.
Author Contribution
All authors contributed to the study conception and design. Data collection was performed by Afef Yakoubi. The first draft of the manuscript was written by Afef Yakoubi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was self-funded.
Data Availability
The data sets generated during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Afef Yakoubi, Email: afefyakoubi88@gmail.com.
Cyrine El Baher Dhafer, Email: cedhafer@ju.edu.sa.
References
- 1.Wu F, Zhao S, Yu B, Chen YM, et al. A new corona virus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int
- 3.Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, et al. Diagnosing Covid-19:the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 4.Lu R, Zhao X, Li J, Niu P, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu T, Liu Y, Zhao M, Zhuang Q, et al. A comparison of COVID-19, SARS and MERS. Peer J. 2020;8:e9725. doi: 10.7717/peerj.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahanafrooz Z, Chen Z, Bao J, Li H, Lipworth L, Guo X. Gene. 2022;808:145963. doi: 10.1016/j.gene.2021.145963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkel Y, Mizrahi O, Nachshon A, Weingarten-Gabbay S, et al. The coding capacity of SARS-CoV-2. Nature. 2021;589(7840):125–130. doi: 10.1038/s41586-020-2739-1. [DOI] [PubMed] [Google Scholar]
- 8.Tang X, Wu C, Li X, Song Y, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. 2020;7:1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boopathi S, Poma AB, Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto V, Bolanos JF, Fiallos J, et al. COVID-19: review of a 21st century pandemic from etiology to neuro-psychiatric implications. J Alzheimers Dis. 2020;77(2):459–504. doi: 10.3233/JAD-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu A, Peng Y, Huang B, Ding X, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan G, Madou MJ, Kalra S, Chopra V, et al. Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano. 2020;14:7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- 13.Liu DX, Fung TS, Chong KK, Shukla A, et al. Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/jvi.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNAseq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019- nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Sen Tan K, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak: an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020 doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Moneim AS, Abdelwhab EM, Memish ZA (2021) Insights into SARS-CoV-2 evolution, potential antivirals, and vaccines. Virology 558:1–12. 10.1016/j.virol.2021.02.007 [DOI] [PMC free article] [PubMed]
- 21.Yan R, Zhang Y, Li Y, Xia L et al (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367:1444–1448. 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed]
- 22.Wong S K, Li W, Moore M J, Choe H, Farzan M (2004) A 193-amino-acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. 10.1074/jbc.C300520200 [DOI] [PMC free article] [PubMed]
- 23.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, et al. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, et al. ARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ou X, Liu, Lei X, Li P, Mi D, Ren L, Guo L et al (2020) Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11. 10.1038/s41467-020-15562-9 [DOI] [PMC free article] [PubMed]
- 26.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kielian M. Enhancing host cell infection by SARS-CoV-2. Science. 2020;370(6518):765–766. doi: 10.1126/science.abf0732. [DOI] [PubMed] [Google Scholar]
- 28.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Liu M, Gao J. Enhanced receptor binding of SARS-CoV-2 through networks of hydrogen-bonding and hydrophobic interactions. Proc Natl Acad Sci. 2020;117(25):13967–13974. doi: 10.1073/pnas.2008209117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181(2):281–92 e286 [DOI] [PMC free article] [PubMed]
- 32.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019- nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 33.Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27(12):2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 34.Prather KA, Wang CC, Schooley RT. Reducing transmission of SARS-CoV-2. Science. 2020;368:1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- 35.Lewis D. Is the coronavirus airborne? Experts can’t agree. Nature. 2020;580:175. doi: 10.1038/d41586-020-00974-w. [DOI] [PubMed] [Google Scholar]
- 36.Chan JFW, Yuan S, Kok KH, To KKW, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C, Wang Y, Li X, Ren L, et al. Clinical features of patients infected with 2019 novel coronavirus inWuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lan L, Xu D, Ye G, Xia C, et al. Positive RT-PCR test results in patients recovered from COVID-19. J Am Med Assoc. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang Y, Zhang H, Xie J, Lin M, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams ER, Ainsworth M, Anand R, Andersson MI, et al. Evaluation of antibody testing for SARS-CoV-2 using ELISA and lateral flow immunoassays. Wellcome Open Res. 2020 doi: 10.1101/2020.04.15.20066407v1. [DOI] [Google Scholar]
- 41.An J, Liao X, Xiao T, Qian S et al (2020) Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test. Ann Transl Med 8:1084. 10.1101/2020.03.26.20044222 [DOI] [PMC free article] [PubMed]
- 42.Laval JM, Mazeran PE, Thomas D. Nanobiotechnology and its role in the development of new analytical devices. Analyst. 2000;125:29–33. doi: 10.1039/a907827d. [DOI] [PubMed] [Google Scholar]
- 43.Hu TY, Frieman M, Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol. 2020;15:247–249. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asdaq SMB, Ikbal AMA, Sahu RK, Bhattacharjee B, et al. Nanotechnology integration for SARS-CoV-2 diagnosis and treatment: an approach to preventing pandemic. Nanomaterials. 2021;11:1841. doi: 10.3390/nano11071841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivasankarapillai VS, Lakshmi S, Dhanusurama MR (2021) Role of nanotechnology in facing SARS-CoV-2 pandemic: solving crux of the matter with a hopeful arrow in the quiver. Sens Int 2:100096. 10.1016/j.sintl.2021.100096 [DOI] [PMC free article] [PubMed]
- 46.Hassan MM, Sium FS, Islam F, Choudhury SM. A review on plasmonic and metamaterial based biosensing platforms for virus detection. Sens Bio-Sens Res. 2021;33:100429. doi: 10.1016/j.sbsr.2021.100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huakang Yu H, Peng Y, Yang Y, Li Z Y (2019) Plasmon-enhanced light–matter interactions and applications. NPJ Comput Mater 45
- 48.Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488.e11. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyazaki C M, Shimizu F M, Ferreira M (2017) 6 - Surface plasmon resonance (SPR) for sensors and biosensors. Micro Nano Technol 183–200. 10.1016/B978-0-323-49778-7.00006-0
- 50.Piliarik M, Homola J. Surface plasmon resonance (SPR) sensors: approaching their limits? Opt. Express. 2009;17:16505–16517. doi: 10.1364/OE.17.016505. [DOI] [PubMed] [Google Scholar]
- 51.Willets KA, VanDuyne RP. Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem. 2007;58:267–297. doi: 10.1146/annurev.physchem.58.032806.104607. [DOI] [PubMed] [Google Scholar]
- 52.Hutter E, Fendler JH. Exploitation of localized surface plasmon resonance. Adv Mater. 2004;16:1685–1706. doi: 10.1002/adma.200400271. [DOI] [Google Scholar]
- 53.Petryayeva E, Krull UJ (2011) Localized surface plasmon resonance: nanostructures, bioassays and biosensing. Anal Chim Acta 706:8–24. 10.1016/j.aca.2011.08.020 [DOI] [PubMed]
- 54.Krajczewski J, Kołątaj K, Kudelski A (2017) Plasmonic nanoparticles in chemical analysis. RSC Adv 7:17559–17576. 10.1039/C7RA01034F
- 55.Huang X, El-Sayed MA. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010;1:13–28. doi: 10.1016/j.jare.2010.02.002. [DOI] [Google Scholar]
- 56.Martin BR, Dermody DJ, Reiss BD, Mingming Fang M, et al. Orthogonal self-assembly on colloidal gold-platinum nanorods. Adv Mater. 1999;11(12):1021–1025. doi: 10.1002/(SICI)1521-4095(199908)11:12<1021::AID-ADMA1021>3.0.CO;2-S. [DOI] [Google Scholar]
- 57.Mansour Y, Battie Y, En-Naciri A, Chaoui N. Determination of the size distribution of metallic colloids from extinction spectroscopy. Nanomaterials. 2021;11:2872. doi: 10.3390/nano11112872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boltovets P, Snopok B, Boyko V, Shevchenko T et al (2004) Detection of plant viruses using a surface plasmon resonance via complexing with specific antibodies. J Virol Methods 121:101–106. 10.1016/j.jviromet.2004.06.019 [DOI] [PubMed]
- 59.Suenaga E, Mizuno H, Kumar PK (2012) Influenza virus surveillance using surface plasmon resonance. Virulence 3:464–470. 10.3390/bios11080250 [DOI] [PMC free article] [PubMed]
- 60.Moskovits M. Surface-enhanced spectroscopy. Rev Mod Phys. 1985;57:783–826. doi: 10.1103/RevModPhys.57.783. [DOI] [Google Scholar]
- 61.Persson BNJ. On the theory of surface-enhanced Raman scattering. Chem Phys Lett. 1981;82:561–565. doi: 10.1016/0009-2614(81)85441-3. [DOI] [Google Scholar]
- 62.Kneipp K, Kneipp H, Itzkan I, Dasari RR, et al. Surface-enhanced Raman scattering and biophysics. J Phys Condens Matter. 2002;14:R597–R624. doi: 10.1088/0953-8984/14/18/202. [DOI] [Google Scholar]
- 63.Kerker M, Wang DS, Chew H. Surface enhanced Raman scattering (SERS) by molecules adsorbed at spherical particles: errata. Appl Opt. 1980;19:4159–4174. doi: 10.1364/AO.19.004159. [DOI] [PubMed] [Google Scholar]
- 64.Haynes CL, Van Duyne RP. Plasmon-sampled surface enhanced Raman excitation spectroscopy. J Phys Chem B. 2003;107:7426–7433. doi: 10.1021/jp027749b. [DOI] [PubMed] [Google Scholar]
- 65.Barber PW, Chang RK, Massoudi H. Electrodynamic calculations of the surface-enhanced electric intensities on large Ag spheroids. Phys Rev B. 1983;27:7251–7261. doi: 10.1103/PhysRevB.27.7251. [DOI] [Google Scholar]
- 66.Rivas L, Sanchez-Cortes S, Garcia-Ramos JV, Morcillo G. Mixed silver/gold colloids: a study of their formation, morphology, and surface-enhanced Raman activity. Langmuir. 2000;16:972–9728. doi: 10.1021/la000557s. [DOI] [Google Scholar]
- 67.Mandal M, Jana NR, Kundu S, Ghosh SK, et al. Synthesis of Au core-Ag shell type bimetallic nanoparticles for single molecule detection in solution by SERS method. J Nanoparticle Res. 2004;6:53–61. doi: 10.1023/B:NANO.0000023227.17871.0f. [DOI] [Google Scholar]
- 68.Felidj N, Aubard J, Levi G, et al. Controlling the optical response of regular arrays of gold particles for surface enhanced Raman scattering. Phys Rev B. 2002;65(7):9. doi: 10.1103/PhysRevB.65.075419. [DOI] [Google Scholar]
- 69.Li K, Li X, Stockman MI, Bergman DJ. Surface plasmon amplification by stimulated emission in nanolenses. Phys Rev B. 2005;71:115409. doi: 10.1103/PhysRevB.71.115409. [DOI] [Google Scholar]
- 70.Shaikh WA, Chakraborty S, Owens G, Ul-IslamR R. A review of the phytochemical mediated synthesis of AgNP (silver nanoparticle): the wonder particle of the past decade. Appl Nanosci. 2021 doi: 10.1007/s13204-021-021355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferdous Z, Abderrahim Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci. 2020;21:2375. doi: 10.3390/ijms21072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fong KE, Yung L, –Y L, Localized surface plasmon resonance: a unique property of plasmonic nanoparticles for nucleic acid detection. Nanoscale. 2013;5:12043–12071. doi: 10.1039/C3NR02257A. [DOI] [PubMed] [Google Scholar]
- 73.Srinoi P, Chen YT, Vittur V, Marquez MD. Bimetallic nanoparticles:enhanced magnetic and optical properties for emerging biological applications. Appl Sci. 2018;8:1106. doi: 10.3390/app8071106. [DOI] [Google Scholar]
- 74.Speshock JL, Murdock RC, Braydich-Stolle LK, Schrand AM, et al. Interaction of silver nanoparticles with Tacaribe virus. J Nanobiotechnol. 2010;8:19. doi: 10.1186/1477-3155-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yen HJ, Hsu SH, Tsai CL. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. 2009;5:1553–1561. doi: 10.1002/smll.200900126. [DOI] [PubMed] [Google Scholar]
- 76.Jans H, Huo Q. Gold nanoparticle-enabled biological and chemical detection and analysis. Chem Soc Rev. 2012;41:2849–2866. doi: 10.1039/C1CS15280G. [DOI] [PubMed] [Google Scholar]
- 77.Cruz LJ, Tacken PJ, Rueda F, Domingo JC et al (2012) Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol 509:143-163.10.1016/B978-0-12-391858-1.00008-3 [DOI] [PubMed]
- 78.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 79.Lin AY, Lunsford J, Bear AS, Young JK, et al. High-density sub100-nm peptide-gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro. Nanoscale Res Lett. 2013;8:72. doi: 10.1186/1556-276X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paul AM, Shi Y, Acharya D, Douglas JR, et al. Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J Gen Virol. 2014;95:1712–1722. doi: 10.1099/vir.0.066084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doria G, Conde J, Veigas B, Giestas L, et al. Noble metal nanoparticles for biosensing applications. Sensors. 2012;12:1657–1687. doi: 10.3390/s120201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tiwari PM, Vig K, Dennis VA, Sing SR (2011) Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 1:31–63. [DOI] [PMC free article] [PubMed]
- 83.Su SS, Chang I (2018) Review of production routes of nanomaterials. Commercialization nanotechnol a case study approach 15–29. 10.1007/978-3-319-56979-6
- 84.Ramasamy M, Lee JH, Lee J. Direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles and their antibio film properties. Colloids Surf B Biointerfaces. 2017;160:639–648. doi: 10.1016/j.colsurfb.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 85.Meléndez-Villanueva MA, Morán-Santibañez K, Martínez-Sanmiguel JJ, et al. Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses. 2019;11:1111. doi: 10.3390/v11121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang K-P, Mehta AC, Turner JF (2012) Flexible bronchoscopy.West Sussex: Wiley-Blackwell. 10.1002/9781444346428
- 87.Sekimukai H, Iwata-Yoshikawa N, Fukushi S, Tani H, et al. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol Immunol. 2020;64:33–51. doi: 10.1111/13480421.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen HW, Huang CY, Lin SY, Fang ZS, et al. Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials. 2016;106:111–118. doi: 10.1016/j.biomaterials.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dykman LA, Khlebtsov NG. Immunological properties of gold nanoparticles. Chem Sci. 2017;8(3):1719–1735. doi: 10.1039/C6SC03631G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu L, Liu Y, Chen Z, Li W, et al. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12:2003–2012. doi: 10.1021/nl300027p. [DOI] [PubMed] [Google Scholar]
- 91.Tao W, Hurst BL, Shakya AK, Uddin ML, et al. Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza A viruses. Antivir Res. 2017;141:62–72. doi: 10.1016/j.antiviral.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pierini F, Guglielmelli A, Urbanek O, Nakielski P, et al. Thermoplasmonic-activated hydrogel based dynamic light attenuator. Adv Opt Mater. 2020;8:2000324. doi: 10.1002/adom.202000324. [DOI] [Google Scholar]
- 93.Ocsoy I, Yusufbeyoglu S, Yılmaz V, McLamore ES, et al. DNA aptamer functionalized gold nanostructures for molecular recognition and photothermal inactivation of methicillin-resistant Staphylococcus aureus. Colloids Surf B: Biointerfaces. 2017;159:16–22. doi: 10.1016/j.colsurfb.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 94.Papp I, Sieben C, Ludwig K, Roskamp M, et al. Inhibition of influenza virus infection by multivalent sialic-acidfunctionalized gold nanoparticles. Small. 2010;6:2900–2906. doi: 10.1002/smll.201001349. [DOI] [PubMed] [Google Scholar]
- 95.Vonnemann J, Sieben C, Wolff C, Ludwig K et al (2014) Virus inhibition induced by polyvalent nanoparticles of different sizes. Nanoscale 6:2353–2360. 10.1039/C3NR04449A [DOI] [PubMed]
- 96.Bawage SS, Tiwari PM, Singh A, Dixit S, et al. Gold nanorods inhibit respiratory syncytial virus by stimulating the innate immune response. Nanomed Nanotechnol Biol Med. 2016;12:2299–2310. doi: 10.1016/j.nano.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim J, Yeom M, Lee T, Kim HO, et al. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J Nanobiotechnol. 2020;18:54. doi: 10.1186/s12951-020-00611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee T, Kim GH, Kim SM, Hong K, et al. Label-free localized surface plasmon resonance biosensor composed of multi-functional DNA 3 way junction on hollow Au spike-like nanoparticles (HAuSN) for avian influenza virus detection. Colloids Surf B Biointerfaces. 2019;182:110341. doi: 10.1016/j.colsurfb.2019.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma C, Xie G, Zhang W, Liang M, et al. Label-free sandwich type of immunosensor for hepatitis C virus core antigen based on the use of gold nanoparticles on a nanostructured metal oxide surface. Microchim Acta. 2012;178:331–340. doi: 10.1007/s00604-012-0842-1. [DOI] [Google Scholar]
- 100.Justino CIL, Duarte AC, Rocha-Santos TAP. Critical overview on the application of sensors and biosensors for clinical analysis. Trends Analyt Chem. 2016;85:36–60. doi: 10.1016/j.trac.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia Y, Chen Y, Tang Y, Cheng G, et al. Smartphone-basedpoint-of-caremicrofluidic platform fabricated with a ZnO nanorod template for colorimetric virus detection. ACS Sens. 2019;4:3298–3307. doi: 10.1021/acssensors.9b01927. [DOI] [PubMed] [Google Scholar]
- 102.Choi JR, Yong KW, Tang R, Gong Y, et al. Lateral flow assay based on paper–hydrogelhybrid material for sensitive point-of-care detection of Dengue virus. Adv Healthc Mater. 2017;6:1600920. doi: 10.1002/adhm.201600920. [DOI] [PubMed] [Google Scholar]
- 103.Wang YF, Pang DW, Zhang ZL, Zheng HZ, et al. Visual gene diagnosis of HBV and HCV based on nanoparticle probe amplification and silver staining enhancement. J Med Virol. 2003;70:205–211. doi: 10.1002/jmv.10379. [DOI] [PubMed] [Google Scholar]
- 104.Adegoke O, Morita M, Kato T, Ito M, et al. Localized surface plasmon resonance-mediated fluorescence signals in plasmonic nanoparticle-quantum dot hybrids for ultrasensitive Zika virus RNA detection via hairpin hybridization assays. Biosens Bioelectron. 2017;94:513–522. doi: 10.1016/j.bios.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 105.Li H, Rothberg L. Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc Natl Acad Sci USA. 2004;101:14036–14039. doi: 10.1073/pnas.0406115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee Y, Kang B-H, Kang M, Chung DR, et al. Nanoplasmonic on-chip PCR for rapid precision molecular diagnostics. ACS Appl Mater Interfaces. 2020;12:12533–12540. doi: 10.1021/acsami.9b23591. [DOI] [PubMed] [Google Scholar]
- 107.Huang P, Wang H, Cao Z, Jin H, et al. A rapid and specific assay for the detection of MERS-CoV. Front Microbiol. 2018;9:1101. doi: 10.3389/fmicb.2018.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morones JR, Elechiguerra JL, Camacho A, Holt K, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 110.Gajbhiye M, Kesharwani J, Ingle A, Gade A, et al. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine. 2009;5:382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 111.Li Y, Leung P, Yao L, Song QW, et al. Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect. 2006;62:58–63. doi: 10.1016/j.jhin.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 112.Mosselhy DA, El-Aziz MA, Hanna M, Ahmed MA, et al. Comparative synthesis and antimicrobial action of silver nanoparticles and silver nitrate. J Nanopart Res. 2015;17:473. doi: 10.1007/s11051-015-3279-8. [DOI] [Google Scholar]
- 113.Mosselhy DA, Granbohm H, Hynönen U, Ge YA, et al. Nanosilver–silica composite. Nanomaterials. 2017;7:i261. doi: 10.3390/nano7090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Du T, Liang J, Dong N, Lu J, et al. Glutathione-capped Ag 2 S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl Mater Interfaces. 2018;10:4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- 115.Lu L, Sun RW, Chen R, Hui CK, et al. Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther. 2008;13:253–262. doi: 10.1177/135965350801300210. [DOI] [PubMed] [Google Scholar]
- 116.Gaikwad S, Ingle A, Gade A, Rai M, et al. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine. 2013;8:4303–4314. doi: 10.2147/IJN.S50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lv X, Wang P, Bai R, Cong Y et al (2014) Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 35:4195–4203 [DOI] [PMC free article] [PubMed]
- 118.Xiang DX, Chen Q, Pang L, Zheng CL. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J Virol Methods. 2011;178:137–142. doi: 10.1016/j.jviromet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 119.Mori Y, Tagawa T, Fujita M, Kuno T, et al. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res Lett. 2013;8:93. doi: 10.1186/1556-276X-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y, Lin Z, Zhao M, Guo M, et al. Silver nanoparticle based codelivery of Oseltamivir to inhibit the activity of the H1N1 influenza virus through ROS mediated signaling pathways. ACS Appl Mater Interfaces. 2016;8:24385–24393. doi: 10.1021/acsami.6b06613. [DOI] [PubMed] [Google Scholar]
- 121.Lin ZF, Li YH, Guo M, Xu Tet al (2017) The inhibition of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with zanamivir. RSC Adv 7:742–750. 10.1039/C6RA25010F
- 122.Du T, Lu J, Liu L, Dong N, et al. Antiviral activity of graphene oxide-silver nanocomposites by preventing viral entry and activation of the antiviral innate immune response. ACS Appl Bio Mater. 2018;1:1286–1293. doi: 10.1021/acsabm.8b00154. [DOI] [PubMed] [Google Scholar]
- 123.Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shao W, Liua H, Liub X, Wanga S, et al. Development of silver sulfadiazine loaded bacterial cellulose/sodium alginate composite films with enhanced antibacterial property. Carbo Hydr Polym. 2015;132:351–358. doi: 10.1016/j.carbpol.2015.06.057. [DOI] [PubMed] [Google Scholar]
- 125.Wu K, Yang Y, Zhang Y, Deng J, et al. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int J Nanomed. 2015;10:7241–7252. doi: 10.2147/IJN.S92307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dhas SP, Anbarasan S, Mukherjee A, Chandrasekaran N. Biobased silver nanocolloid coating on silk fibers for prevention of postsurgical wound infections. Int J Nanomed. 2015;10:159–170. doi: 10.2147/IJN.S82211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rogers JV, Parkinson CV, Choi YW, Speshock JL, et al. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res Lett. 2008;3:129–133. doi: 10.1007/s11671-008-9128-2. [DOI] [Google Scholar]
- 128.Park S, Ko YS, Jung H, Lee C, et al. Disinfection of waterborne viruses using silver nanoparticle-decorated silica hybrid composites in water environments. Sci Total Environ. 2018;625:477–485. doi: 10.1016/j.scitotenv.2017.12.318. [DOI] [PubMed] [Google Scholar]
- 129.Chen N, Zheng Y, Yin J, Li X, et al. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J Virol Methods. 2013;193:470–477. doi: 10.1016/j.jviromet.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 130.Galdiero S, Rai M, Gade A, Falanga A, Incoronato N, et al. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomed. 2013;8:4303–4314. doi: 10.2147/IJN.S50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bisht A, Mishra A, Bisht H, Tripathi RM. Nanomaterial based biosensors for detection of viruses including SARS-CoV-2: a review. J Anal Test. 2021;5:327–340. doi: 10.1007/s41664-021-00200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiang DX, Zheng Y, Duan W, et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int J Nanomed. 2013;8:4103–4114. doi: 10.2147/IJN.S53622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang XX, Li CM, Huang CZ. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 2016;8:3040–3048. doi: 10.1039/C5NR07918G. [DOI] [PubMed] [Google Scholar]
- 134.Alghrair ZK, Fernig DG, Ebrahimi B (2019) Enhanced inhibition of influenza virus infection by peptide-noble-metal nanoparticle conjugates. Beilstein J Nanotechnol 10:1038–104. 10.3762/bjnano.10.104 [DOI] [PMC free article] [PubMed]
- 135.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnology. 2010;8:1–10. doi: 10.1007/s10876-016-1100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Orłowski P, Kowalczyk A, Tomaszewska E et al (2018) Antiviral activity of tannic acid modified silver nanoparticles: potential to activate immune response in herpes genitalis. Viruses 10(10):524. 10.3390/v10100524 [DOI] [PMC free article] [PubMed]
- 137.Krzyzowska M, Tomaszewska E, Ranoszek-Soliwoda K et al (2017) Tannic acid modification of metal nanoparticles: possibility for new antiviral applications. Nanostructures for Oral Medicine Micro and Nano Technologies. Elsevier, Amsterdam 335–363. 10.1016/B978-0-323-47720-8.00013-4
- 138.Sanchez-Guzman D, Le Guen P, Villeret B, Sola N, et al. Silver nanoparticle-adjuvanted vaccine protects against lethal influenza infection through inducing BALT and IgA-mediated mucosalimmunity. Biomaterials. 2019;217:119308. doi: 10.1016/j.biomaterials.2019.119308. [DOI] [PubMed] [Google Scholar]
- 139.Assis M, Simoes LGP, Tremiliosi GC, Coelho D, et al. SiO2-Ag composite as a highly virucidal material: a roadmap that rapidly eliminates SARS-CoV-2. Nanomaterials. 2021;11:638. doi: 10.3390/nano11030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dung TTN, Buu NQ, Quang DV, Ha HT et al (2009) Synthesis of nanosilver particles by reverse micelle method and study of their bactericidal properties. J Phys Conf Ser 187:012054. 10.1088/1742-6596/187/1/012054
- 141.Castro-Mayorga JL, Randazzo W, Fabra MJ, Lagaron JM, et al. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT Food Sci Technol. 2017;79:503–510. doi: 10.1016/j.lwt.2017.01.065. [DOI] [Google Scholar]
- 142.Arora S, Jain J, Rajwade JM, Paknikar KM. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicol Lett. 2008;179:93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 143.Kumari A, Kumar P, Ajayan PM, John G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat Mater. 2008;7:236–241. doi: 10.1038/nmat2099. [DOI] [PubMed] [Google Scholar]
- 144.Dung TTN, Nam VN, Nhan TT, Ngoc TTB et al (2020) Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater Res Express 6:1250g9. 10.1088/2053-1591/ab6ad8
- 145.Teengam P, Siangproh W, Adisorn Tuantranont A, Vilaivan T. Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal Chem. 2017;89:5428–5435. doi: 10.1021/acs.analchem.7b0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khoris IM, Chowdhury AD, Li T-C, Suzuki T, et al. Advancement of capture immunoassay for real-time monitoring of hepatitis E virus-infected monkey. Anal Chim Acta. 2020;1110:64–71. doi: 10.1016/j.aca.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 147.Morrison J (2020) Copper’s virus-killing powers were known even to the ancients. Smithsonian Magazine. https://www.ncbi.nlm.nih.gov/search/research-news/9381/.14 . Accessed Apr 2020
- 148.Hostynek JJ, Maibach H I (2004) Copper hypersensitivity: dermatologic aspects. Dermatol Ther 17:328–333. 10.1111/j.1396-0296.2004.04035.x [DOI] [PubMed]
- 149.Usman MS, El Zowalaty ME, Shameli K, Zainuddin N, et al. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomed. 2013;8:4467. doi: 10.2147/IJN.S50837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grass G, Rensing C, Solioz M (2011) Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77:1541–1547. 10.1128/AEM.02766-10 [DOI] [PMC free article] [PubMed]
- 151.Ermini ML, Voliani V (2021) Antimicrobial nano-agents: the copper age. ACS Nano 15:6008–6029. 10.1021/acsnano.0c10756 [DOI] [PMC free article] [PubMed]
- 152.Mayorga JLC, Rovira MJF, Mas LC, Moragas GS, et al. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J Appl Polym Sci. 2018;135:45673. doi: 10.1002/app.45673. [DOI] [Google Scholar]
- 153.Borkow G, Zhou SS, Page T, Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE. 2010;5:e11295. doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Borkow G, Gabbay J. Putting copper into action: copper-impregnated products with potent biocidal activities. FASEB J. 2004;18:1728–1730. doi: 10.1096/fj.04-2029fje. [DOI] [PubMed] [Google Scholar]
- 155.Lee IC, Ko JW, Park SH, Lim JO, et al. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int J Nanomed. 2016;11:2883–2900. doi: 10.2147/IJN.S106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Applerot G, Lellouche J, Lipovsky A, Nitzan Y, et al. Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small. 2018;8:3326–3337. doi: 10.1002/smll.20120077. [DOI] [PubMed] [Google Scholar]
- 157.Qi Y, Ye J, Ren S, Lv J et al (2020) In-situ synthesis of metal nanoparticles@metal-organic frameworks: highly effective catalytic performance and synergistic antimicrobial activity. J Hazard Mater 387:121687. 10.1016/j.jhazmat.2019.121687 [DOI] [PubMed]
- 158.Hanagata N, Zhuang F, Connolly S, Li J, et al. Molecular responses of human lung epithelial cells to the toxicity of copper oxide nanoparticles inferred from whole genome expression analysis. ACS Nano. 2011;5:9326–9338. doi: 10.1021/nn202966t. [DOI] [PubMed] [Google Scholar]
- 159.Warnes SL, Keevil CW. Inactivation of Norovirus on dry copper alloy surfaces. PLoS ONE. 2013;8:e75017. doi: 10.1371/journal.pone.0075017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hang X, Peng H, Song H, Qi Z et al (2015) Antiviral activity of cuprous oxide nanoparticles against hepatitis C virus in vitro. J Virol Methods 222:150–157. 10.1016/j.jviromet.2015.06.010 [DOI] [PubMed]
- 161.Tavakoli A, Hashemzadeh MS. Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J Virol Methods. 2020;275:113688. doi: 10.1016/j.jviromet.2019.113688. [DOI] [PubMed] [Google Scholar]
- 162.Karlsson HL, Cronholm P, Gustafsson J, Möller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 163.Minoshima M, Lu Y, Kimura T, Nakano R, et al. Comparison of the antiviral effect of solid-state copper and silver compounds. J Hazard Mater. 2016;312:1–7. doi: 10.1016/j.jhazmat.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ungur G, Hruza J. Modified polyurethane nanofibers as antibacterial filters for air and water purification. RSC Adv. 2017;7:49177–49187. doi: 10.1039/C7RA06317B. [DOI] [Google Scholar]
- 166.Zhou J, Xiang H, Zabihi F, Yu S, et al. Intriguing anti-superbug Cu2O@ZrP hybrid nanosheet with enhanced antibacterial performance and weak cytotoxicity. Nano Res. 2019;12:1453–1460. doi: 10.1007/s12274-019-2406-8. [DOI] [Google Scholar]
- 167.Wang W S, Li B L, Yang H L, Lin Z F et al (2020) Efficient elimination of multidrug-resistant bacteria using copper sulfide nanozymes anchored to graphene oxide nanosheets. Nano Res 13:2156–2164. 10.1007/s12274-020-2824-7
- 168.Qiao Y, Ping Y, Zhang H, Zhou B et al (2019) Laser-activatable CuS nanodots to treat multidrug-resistant bacteria and release copper ion to accelerate healing of infected chronic nonhealing wounds. ACS Appl Mater Interfaces 11:3809–3822. 10.1021/acsami.8b21766 [DOI] [PMC free article] [PubMed]
- 169.Fujimori Y, Sato T, Hayata T, Nagao T et al (2012) Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol 78:951–955. 10.1128/AEM.06284-11 [DOI] [PMC free article] [PubMed]
- 170.Mazurkow JM, Yüzbasi NS, Domagala KW, Pfeiffer S, et al. Nano-sized copper (oxide) on alumina granules for water filtration: effect of copper oxidation state on virus removal performance. Environ Sci Technol. 2020;54:1214–1222. doi: 10.1021/acs.est.9b05211. [DOI] [PubMed] [Google Scholar]
- 171.Sunada K, Minoshima M, Hashimoto K (2012) Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J Hazard Mater 235–236:265–270. 10.1016/j.jhazmat.2012.07.052 [DOI] [PubMed]
- 172.Warnes S, Little Z, Keevil C (2015) Human coronavirus 229E remains infectious on common touch surface materials. mBio 6:e01697–15. 10.1128/mBio.01697-15 [DOI] [PMC free article] [PubMed]
- 173.DE Toledo G G, Toledo V H, Alexandre J C Lanfredi, Marcia Escote et al (2020) Promising nanostructured materials against enveloped virus. An Acad Bras Cienc 92:e20200718. 10.1590/0001-3765202020200718 [DOI] [PubMed]
- 174.Erkoc P, Ulucan-Karnak F. Nanotechnology-based antimicrobial and antiviral surface coating strategies. Prosthesis. 2021;3:25–52. doi: 10.3390/prosthesis3010005. [DOI] [Google Scholar]
- 175.Wang MW, Zhou MY, Ji GH et al (2020) Mask crisis during the COVID-19 outbreak. Eur Rev Med Pharm Sci 24:3397–3399. 10.26355/eurrev_202003_20707 [DOI] [PubMed]
- 176.Blevens MS, Pastrana HF, Mazzotta HC, Su-Jung Tsai C. Cloth face masks containing silver: evaluating the status. ACS Chem Health Saf. 2021;28:171–182. doi: 10.1021/acs.chas.1c00005. [DOI] [PubMed] [Google Scholar]
- 177.Jung S, Yang J-Y, Kim D-G, Lee D-G. Copper-coated polypropylene filter face mask with SARS-CoV-2 antiviral ability. Polymers. 2021;13:1367. doi: 10.3390/polym13091367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pollard ZA, Karod M, Goldfarb JL. Metal leaching from antimicrobial cloth face masks intended to slow the spread of COVID-19. Sci Rep. 2021;11:19216. doi: 10.1038/s41598-021-98577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhong H, Zhu Z, You P, Lin J, et al. Plasmonic and superhydrophobic self-decontaminating N95 respirators. ACS Nano. 2020;14:8846–8854. doi: 10.1021/acsnano.0c03504. [DOI] [PubMed] [Google Scholar]
- 180.Pilaquinga F, Morey J, Torres M, Seqqat R et al (2021) Silver nanoparticles as a potential treatment against SARS-CoV-2: a review Wiley Interdiscip. Rev Nanomed Nanobiotechnol 13:e1707. 10.1002/wnan.1707 [DOI] [PMC free article] [PubMed]
- 181.Balagna C, Perero S, Percivalle E, Nepita EV, et al. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceramics. 2020;1:100006. doi: 10.1016/j.oceram.2020.100006. [DOI] [Google Scholar]
- 182.Tremiliosi GC, Simoes LGP, Minozzi DT, Santos RI, Vilela DCB. Ag nanoparticles-based antimicrobial polycotton fabrics to prevent the transmission and spread of SARS-CoV-2. BIORXIV. 2020 doi: 10.1101/2020.06.26.152520Accessedjune26,2020. [DOI] [Google Scholar]
- 183.Valdez-Salas B, Beltran-Partida E, Cheng N, Salvador-Carlos J, et al. Promotion of surgical masks antimicrobial activity by disinfection and impregnation with disinfectant silver nanoparticles. Int J Nanomedicine. 2021;16:2689–2702. doi: 10.2147/IJN.S301212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tavakoli A, Hashemzadeh M-S. Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J Virol Methods. 2019;275:113688. doi: 10.1016/j.jviromet.2019.113688. [DOI] [PubMed] [Google Scholar]
- 185.Raha S, Mallick R, Basak S, Duttaroy AK. Is copper beneficial for COVID-19 patients? Med Hypotheses. 2020;142:109814. doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mostaghimi J, Pershin L, Salimijazi H, Nejad M, et al. Thermal spray copper alloy coatings as potent biocidal and virucidal surfaces. J Therm Spray Technol. 2021;30:25–39. doi: 10.1007/s11666-021-01161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Behzadinasab S, Chin A, Hosseini M, Poon L, et al. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl Mater Interfaces. 2020;12:34723–34727. doi: 10.1021/acsami.0c11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.StatNano. Technology against COVID-19: nano insights into prevention, diagnosis and treatment – respiratory masks. https://statnano.com/technology-against-covid-19-nano-insights#Respiratory-Masks
- 190.Nanotechnology Products Database. ReSpimaskR VK – Virus Killer (ADULT)
- 191.Ahmed MK, Afifi M, Uskokovic V. Protecting healthcare workers during COVID-19 pandemic with nanotechnology: a protocol for a new device from Egypt. J Infect Public Health. 2020;13:1243. doi: 10.1016/j.jiph.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kumar S, Karmacharya M, Joshi S R, Gulenko O et al (2020) Photoactive antiviral face mask with self-sterilization and reusability. Nano Letters acs 0c03725. 10.1021/acs.nanolett.0c03725 [DOI] [PubMed]
- 193.Mosselhy DA, Kareinen L, Kivistö I, Aaltonen K, et al. Copper-silver nanohybrids: SARS-CoV-2 inhibitory surfaces. Nanomaterials. 2021;11:1820. doi: 10.3390/nano11071820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Assis M, Simoes LGP, Tremiliosi GC, Ribeiro LK, et al. PVC-SiO2-Ag composite as a powerful biocide and anti-SARS-CoV-2 material. J Polym Res. 2021;28:361. doi: 10.1007/s10965-021-02729-1. [DOI] [Google Scholar]
- 195.Sethi S, Chakraborty T. Molecular (real-time reverse transcription polymerase chain reaction) diagnosis of SARS-CoV-2 infections: complexity and challenges. J Lab Med. 2021;45:135–142. doi: 10.1515/labmed-2020-0135. [DOI] [Google Scholar]
- 196.Layfield LJ, Camp S, Bowers K, Mille DC. SARS-CoV-2 detection by reverse transcriptase polymerase chain reaction testing: analysis of false positive results and recommendations for quality control measures. Pathol Res Pract. 2021;225:153579. doi: 10.1016/j.prp.2021.153579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Oliverio M, Perotto S, Messina GC, Lovato L, et al. Chemical functionalization of plasmonic surface biosensors: a tutorial review on issues, strategies, and costs. ACS Appl Mater Interfaces. 2017;9:29394–29411. doi: 10.1021/acsami.7b01583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tang L, Li J. Plasmon-based colorimetric nanosensors for ultrasensitive molecular diagnostics. ACS Sens. 2017;2:857–875. doi: 10.1021/acssensors.7b00282. [DOI] [PubMed] [Google Scholar]
- 199.Camarca A, Varriale A, Capo A, Pennacchio A, et al. Emergent biosensing technologies based on fluorescence spectroscopy and surface plasmon resonance. Sensors. 2021;21:906. doi: 10.3390/s21030906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mauriz E. Recent progress in plasmonic biosensing schemes for virus detection. Sensors (Basel) 2020;20:4745. doi: 10.3390/s20174745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qiu G, Gai Z, Tao Y, Schmitt J, et al. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14:5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 202.Funari R, Chu KY, Shen AQ. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an optomicrofluidic chip. Biosens Bioelectron. 2020;169:112578. doi: 10.1016/j.bios.2020.112578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim EJ, Kim H, Park E, Kim T, et al. Paper-based multiplex surface-enhanced Raman scattering detection using polymerase chain reaction probe codification. Anal Chem. 2021;93:3677–3685. doi: 10.1021/acs.analchem.0c05285. [DOI] [PubMed] [Google Scholar]
- 204.Jadhav SA, Biji P, Panthalingal MK, Krishna CM, et al. Development of integrated microfluidic platform coupled with surface-enhanced Raman spectroscopy for diagnosis of COVID-19. Medical Hypothesis. 2021;146:110356. doi: 10.1016/j.mehy.2020.110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Giancarla A, Camilla Z, Lisa Rita M, Raffaela B. Gold and silver nanoparticle-based colorimetric sensors: new trends and applications. Chemosensors. 2021;9:305. doi: 10.3390/chemosensors9110305. [DOI] [Google Scholar]
- 206.Ma X, He S, Qiu B, Luo F, et al. Noble metal nanoparticle-based multicolor immunoassays: an approach toward visual quantification of the analytes with the naked eye. ACS Sens. 2019;4:782–791. doi: 10.1021/acssensors.9b00438. [DOI] [PubMed] [Google Scholar]
- 207.Aldewachi H, Chalati T, Woodroofe MN, Bricklebank N, et al. Gold nanoparticle-based colorimetric biosensors Nanoscale. 2018;10:18–33. doi: 10.1039/C7NR06367A. [DOI] [PubMed] [Google Scholar]
- 208.Huang L, Ding L, Zhou J, Chen S, et al. One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens Bioelectron. 2021;171:112685. doi: 10.1016/j.bios.2020.112685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moitra P, Alafeef M, Dighe K, Frieman MB et al (2020) Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 14:7617–7627. 10.1021/acsnano.0c03822 [DOI] [PMC free article] [PubMed]
- 210.Pramanik A, Gao Y, Patibandla S, Mitra D, et al. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv. 2021;3:1588. doi: 10.1039/d0na01007c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ventura BD, Cennamo M, Minopoli A, Campanile R, et al. Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. ACS Sens. 2020;5:3043–3048. doi: 10.1021/acssensors.0c01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Long QX, Liu BZ, Deng HJ, Wu GC et al (2020) Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26:845–848. [DOI] [PubMed]
- 213.Zhao J, Yuan Q, Wang H, Liu W et al (2020) Antibody responses to SARSCoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis 71:2027–2034. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed]
- 214.Dortet L, Emeraud C, Vauloup-Fellous C, Khecharem M, et al. Rapid determination of SARS-CoV-2 antibodies using a bedside, point-of-care, serological test. Emerg Microbes Infect. 2020;9:2212–2221. doi: 10.1080/22221751.2020.1826892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Carter LJ, Garner LV, Smoot W, Li Y, et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci. 2020;6:591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2020;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Huang C, Wen T, Shi FJ, Zeng X Yet al (2020b) Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega 5:12550–12556. 10.1021/acsomega.0c01554 [DOI] [PMC free article] [PubMed]
- 218.Liu H, Dai E, Xiao R, Zhou Z et al (2021) Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of anti-SARS-CoV-2 IgM/IgG in clinical samples. Sens Actuators B 329:129196. 10.1016/j.snb.2020.129196 [DOI] [PMC free article] [PubMed]
- 219.Xu X, Sun J, Nie S, Li H, et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 220.Hou H, Wang T, Zhang B, Luo Yet al (2020) Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunol 9:e01136. 10.1002/cti2.1136 [DOI] [PMC free article] [PubMed]
- 221.Li X, Yin Y, Pang L, Xu S, et al. Colloidal gold immunochromatographic assay (GICA) is an effective screening method for identifying detectable anti-SARS-CoV-2 neutralizing antibodies. Int J Infect Dis. 2021;108:483–486. doi: 10.1016/j.ijid.2021.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bijon KS, Mohd RJ, Md AH, Mohib UK et al (2020) AuNP coupled rapid flow-through dot-blot immuno-assay for enhanced detection of SARS-CoV-2 specific nucleocapsid and receptor binding domain IgG. Int J Nanomed 16:4739–4753. 10.2147/IJN.S313140 [DOI] [PMC free article] [PubMed]
- 223.Wang C, Yang X, Gu B, Liu H, et al. Sensitive and simultaneous detection of SARS-CoV-2-specific IgM/IgG using lateral flow immunoassay based on dual-mode quantum dot nanobeads Anal. Chem. 2020;92:15542–15549. doi: 10.1021/acs.analchem.0c03484. [DOI] [PubMed] [Google Scholar]
- 224.Dahlke C, Heidepriem J, Kobbe R, Santer R et al (2020) Distinct early IgA profile may determine severity of COVID-19 symptoms: an immunological case series. ID-UKE COVID-19 Study Group. 10.1101/2020.04.14.20059733
- 225.Li Z, Yi Y, Luo X, Xiong N, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yu H, Sun B, Fang Z, Zhao J, et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020;56:2001526. doi: 10.1183/13993003.01526-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Padoan A, Sciacovelli L, Basso D, Negrini D et al 2(020) IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin Chim Acta 507:164–166. 10.1016/j.cca.2020.04.026 [DOI] [PMC free article] [PubMed]
- 228.Roda A, Cavalera S, Nardo F, Calabria D, et al. Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens Bioelectron. 2021;172:112765. doi: 10.1016/j.bios.2020.112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cavalera S, Colitti B, Rosati S, Ferrara G, et al. A multi-target lateral flow immunoassay enabling the specific and sensitive detection of total antibodies to SARS COV-2. Talanta. 2021;223:121737. doi: 10.1016/j.talanta.2020.121737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Peng T, Sui Z, Huang Z, Xie J, et al. Point-of-care test system for detection of immunoglobulin-G and -M against nucleocapsid protein and spike glycoprotein of SARS-CoV-2. Sens Actuators B Chem. 2021;331:129415. doi: 10.1016/j.snb.2020.129415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wen T, Huang C, Shi FJ, Zeng XY, et al. Development of a lateral flow immunoassay strip for rapid detection of IgG antibody against SARS-CoV-2 virus. Analyst. 2020;145:5345–5352. doi: 10.1039/D0AN00629G. [DOI] [PubMed] [Google Scholar]
- 232.Aithal S, Mishriki S, Gupta R, Sahu RP, et al. SARS-CoV-2 detection with aptamer-functionalized gold nanoparticles. Talanta. 2022;236:122841. doi: 10.1016/j.talanta.2021.122841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Iarossi M, Schiattarella C, Rea I, De Stefano L, et al. Colorimetric immunosensor by aggregation of photochemically functionalized gold nanoparticles. ACS Omega. 2018;3:3805–3812. doi: 10.1021/acsomega.8b00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Liu Y, Zhang L, Wei W, Zhao H, et al. Colorimetric detection of influenza A virus using antibody-functionalized gold nanoparticles. Analyst. 2015;140:3989–3995. doi: 10.1039/C5AN00407A. [DOI] [PubMed] [Google Scholar]
- 235.Alafeef M, Dighe K, Moitra P, Pan D. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano ACS Nano. 2020;14:17028–17045. doi: 10.1021/acsnano.0c06392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rahmati Z, Roushani M, Hosseini H, Choobin H (2021) Electrochemical immunosensor with Cu2O nanocube coating for detection of SARS-CoV-2 spike protein Microchim Acta 188:105–245. 10.1007/s00604-021-04762-9 [DOI] [PMC free article] [PubMed]
- 237.Tripathy S, Singh SG. Label-free electrochemical detection of DNA hybridization: a method for COVID-19 diagnosis. Trans Indian Natl Acad Eng. 2020;5:205–209. doi: 10.1007/s41403-020-00103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhao H, Liu F, Xie W et al (2021) Ultrasensitive supersandwich-type electrochemical sensor for SARSCoV-2 from the infected COVID-19 patients using a smartphone. Sens Actuators B Chem 327:128899. 10.1016/j.snb.2020.128899 [DOI] [PMC free article] [PubMed]
- 239.Hashemi SA, Golab Behbahan NG, Bahrani S, Mousavi SM, et al. Ultra-sensitive viral glycoprotein detection NanoSystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosens Bioelectron. 2021;171:112731. doi: 10.1016/j.bios.2020.112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Morad R, Akbari M, Rezaee P, et al. First principle simulation of coated hydroxychloroquine on Ag. Au and Pt nanoparticles Scientific Reports. 2021;11:2131. doi: 10.1038/s41598-021-81617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sarkar S (2020) Silver nanoparticles with bronchodilators through nebulisation to treat COVID 19 patients. Curr Med Res Opin 3:2589–8760. 10.15520/jcmro.v3i04.276
- 242.Labouta HI, Hooshmand N, Upreti T, El-Say MA. Localized plasmonic photothermal therapy as a life-saving treatment paradigm for hospitalized COVID-19 patients. Plasmonics. 2021;16:1029–1033. doi: 10.1007/s11468-020-01353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nazari M , Xi M, Lerch S, Alizadeh MH et al (2017) Plasmonic enhancement of selective photonic virus inactivation. Sci Rep 7:1195110. 10.1038/s41598-017-12377-5 [DOI] [PMC free article] [PubMed]
- 244.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 245.Huang X, Jain P K, El-Sayed IH, El-Sayed MA (2007) Plasmonic photothermal therapy (PPTT) using gold nanoparticles Lasers Med Sci 23:217–228. 10.1007/s10103-007-0470-x [DOI] [PubMed]
- 246.Wang Z, Liu H, Yang SH, Wang T, et al. Nanoparticle-based artificial RNA silencing machinery for antiviral therapy. Proc Natl Acad Sci U S A. 2012;109:12387–12392. doi: 10.1073/pnas.1207766109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.GISAID - Initiative. https://gisaid.org/resources/in-focus-archive/. Accessed 14 Feb 2022
- 248.Takeda M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol Immunol. 2022;66(1):15–23. doi: 10.1111/13480421.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Focosi D, Maggi F. Neutralising antibody escape of SARS-CoV-2 spike protein: risk assessment for antibody-based Covid-19 therapeutics and vaccines. Rev Med Virol. 2021;31(6):e2231. doi: 10.1002/rmv.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jia Q, Bielefeldt-Ohmann H, Maison R, Masleša-Galić S, Bowen R, Horwitz MA (2020) Analysis of the mutation dynamics of SARS-CoV-2 reveals the spread history and emergence of RBD mutant with lower ACE2 binding affinity. bioRxiv (Preprint)
- 251.Akkiz H. Implications of the novel mutations in the SARS-CoV-2 genome for transmission, disease severity, and the vaccine development. Front Med. 2021;8:636532. doi: 10.3389/fmed.2021.636532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Korber B, Fischer WM, Gnanakaran S, Yoon H, Thelier J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:1–16. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Altmann DM, Boyton RJ, Beale R. Immunity to SARS-CoV-2 variants of concern. Science. 2021;371(6534):1103–1104. doi: 10.1126/science.abg7404. [DOI] [PubMed] [Google Scholar]
- 254.Callaway E. Making sense of coronavirus mutations. Nature. 2020;585:174–177. doi: 10.1038/d41586-020-02544-6. [DOI] [PubMed] [Google Scholar]
- 255.Khateeb J, Li Y, Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit Care. 2021;25(1):244. doi: 10.1186/s13054-021-03662-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Nguyen TT, Pathirana PN, Nguyen T, Nguyen QVH, Bhatt A, Nguyen DC, et al. Genomic mutations and changes in protein secondary structure and solvent accessibility of SARS-CoV-2 (COVID-19 virus) Sci Rep. 2021;11(1):3487. doi: 10.1038/s41598-021-83105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Conti P, Caraffa CE, Kritas SK, Gallenga CE, Frydos I, Younes A et al (2021) The British variant of the new coronavirus-19 (SARS-CoV-2) should not create a vaccine problem. J Biol Regul 35:1–4. 10.23812/21-3-E [PubMed]
- 258.Dearlove B, Lewitus E, Bai H, Li Y, Reeves DB, Joyce MG et al (2020) A SARS-CoV-2 vaccine candidate would likely match all currently circulating variants. Proc Natl Acad Sci USA 117(38): 23652–23662. 10.1073/pnas.2008281117 [DOI] [PMC free article] [PubMed]
- 259.Hou YJ, Chiba S, Halfmann P, Ehre C, et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370:1464–1468. doi: 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Daniloski Z, Jordan TX, Ilmain JK, Guo X, Bhabha G, tenOever BR, Sanjana NE. The spike D614G mutation increases SARS-CoV-2 infection of multiple human cell types. Elife. 2021;10:e65365. doi: 10.7554/eLife.65365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhou B, Thi Nhu Thao T, Hoffmann D, et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021;592:122–127. doi: 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]
- 262.Ozono S, Zhang Y, Ode H, Sano K, Tan TS, Imai K, et al. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat Commun. 2021;12:848. doi: 10.1038/s41467-021-21118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hirabara SM, Serdan TDA, Gorjao R, Masi LN, Pithon-Curi TC, Covas DT, Curi R, Durigon EL. SARSCOV-2 variants: differences and potential of immune evasion. Front Cell Infect Microbiol. 2022;11:781429. doi: 10.3389/fcimb.2021.781429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Graham MS, Sudre CH, May A, Antonelli M, Murray B, Varsavsky T et al (2021) Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. Lancet Pub Health 6(5):e335–e345. 10.1016/S2468-2667(21)00055-4 [DOI] [PMC free article] [PubMed]
- 265.Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, et al. Transmission of SARS-CoV-2 on mink farms be- tween humans and mink and back to humans. Science. 2021;371:172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Garcia MA. Surface plasmons in metallic nanoparticles, fundamentals and applications. J Phys D: Appl Phys. 2011;44:283001. doi: 10.1088/0022-3727/44/28/283001. [DOI] [Google Scholar]
- 267.Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2020;592(7852):116–121. doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Janik E, Niemcewicz M, Podogrocki M, et al. The emerging concern and interest SARS-CoV-2 variants. Pathogens. 2021;10:633. doi: 10.3390/pathogens10060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Callaway E, Mallapaty S. Novavax offers first evidence that COVID vaccines protect people against variants. Nature. 2021;590(7844):17. doi: 10.1038/d41586-021-00268-9. [DOI] [PubMed] [Google Scholar]
- 270.Kim YJ, Jang US, Soh SM et al (2021) The impact on infectivity and neutralization efficiency of SARS-CoV-2 lineage b.1.351 pseudovirus. Viruses 13:633. 10.3390/v13040633 [DOI] [PMC free article] [PubMed]
- 271.Nelson-Sathi S, Umasankar PK, Sreekumar E, Radhakrishnan Nair R, et al. Mutational landscape and in silico structure models of SARS-CoV-2 spike receptor binding domain reveal key molecular determinants for virus-host interaction. BMC Molecular Cell Biol. 2022;23:2–12. doi: 10.1186/s12860-021-00403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Li Q, Nie J, Wu J, Zhang L, Ding R, Wang H et al (2021) SARS-CoV-2 501y.V2 variants lack higher infectivity but do have immune escape. Cell 184 (9):2362–2371.e9 10.1016/j.cell.2021.02.042 [DOI] [PMC free article] [PubMed]
- 273.Staub T, Arendt V, la Vega ECL, Braquet P, Michaux C, Kohnen M et al (2021) Case series of four re-infections with a SARS-CoV-2 B.1.351 variant. Euro Surveill 26(18):2100423. 10.2807/1560-7917.ES.2021.26.18.2100423 [DOI] [PMC free article] [PubMed]
- 274.Oliveira MDL, Oliveira KMT, Silva JN et al (2021) Theoretical causes of the Brazilian P.1 and P.2 lineages of the SARS-CoV-2 virus through molecular dynamics. bioRxiv 2021.04.09.439181. 10.1101/2021.04.09.439181
- 275.Hirotsu Y, Omata M. Discovery of a SARS-CoV-2 variant from the P.1 lineage harboring K417T/E484K/N501Y mutations in Kofu. Japan J Infect. 2021;82:276–316. doi: 10.1016/j.jinf.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rambaut A, Loman N, Pybus O, Barclay W, Barret J, Corabelli A et al (2021) Preliminary genomic characterization of an emergent SARS-CoV-2 lineage in the United Kingdom defined by a novel set of spike mutation. COVID-19 Genomic Consortium UK (CoG-UK)
- 277.Pachetti M, Matini B, Benedetti F, Giudici F, Mauro E, Storici P, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18:179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.World Health Organisation (2020) SARS-CoV-2 variant – United Kingdom of Great Britain and Northern Ireland. https://www.who.int/emergencies/disease-outbreaknews/item/2020DON304#:~:text=On%2014%20December%202020%2C%20authorities,month%2012%2C%20variant%2001)
- 279.European Centre for Disease Prevention and Control (2020) Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom. European Centre for Disease Prevention and Control
- 280.Kumar V, Singh J, Hasnain SE, Sundar D (2021) Possible link between higher transmissibility of B.1.617 and B.1.1.7 variants of SARS-CoV-2 and increased structural stability of its spike protein and hACE2 affinity. bioRxiv 1–12. 10.1101/2021.04.29.441933 [DOI] [PMC free article] [PubMed]
- 281.Thomson EC, Rosen LE, Shepherd JG, et al. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell. 2021;184(1171–1187):e20. doi: 10.1016/j.cell.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Cherian S, Potdar V, Jadhav S, et al. Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID-19 in Maharashtra. India bioRxiv. 2021 doi: 10.1101/2021.04.22.440932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Singh J, Rahman SA, Ehtesham NZ, et al. SARS-CoV-2 variants of concern are emerging in India. Nat Med. 2021;27:1131–1133. doi: 10.1038/s41591-021-01397-4. [DOI] [PubMed] [Google Scholar]
- 284.Yadav PD, Sapkal GN, Abraham P et al (2021) Neutralization potential of Covishield vaccinated individuals sera against B.1.617.1. Clin Infect Di ciab483. 10.1093/cid/ciab483 [DOI] [PubMed]
- 285.Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Toh MPHS et al (2021) Clinical and virological features of SARS CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta) and B.1.617.2 (Delta). SSRN J. 10.2139/ssrn.3861566 [DOI] [PMC free article] [PubMed]
- 286.Boehm E, Kronig I, Neher RA, et al. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect. 2021;27:1–24. doi: 10.1016/j.cmi.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Osina NA, Krasnov YM, Guseva NP et al (2021) Moleculargenetic monitoring of sARs-CoV-2 genovariants in the territory of the volga federal district of the Russian Federation. Communication. Probl Osobo Opasnykh Infektsii 122–127. 10.21055/0370-1069-2021-1-122-127
- 288.Shrestha UK. SARS-CoV-2 vaccines and their challenges against the variants. J Adv Intern Med. 2021;10:1–3. doi: 10.3126/jaim.v10i1.37080. [DOI] [Google Scholar]
- 289.Kirola L (2021) Genetic emergence of B.1.617.2 in COVID-19. New Microbes New Infect 43:100929. 10.1016/j.nmni.2021.100929 [DOI] [PMC free article] [PubMed]
- 290.Li X, Wang W, Zhao X, Zai J, Zhao Q, Li Y, Chaillon A. Transmission dynamics and evolutionary history of 2019-nCoV. J Med Virol. 2020;92:501–511. doi: 10.1002/jmv.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S et al (2021) Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 385(7):585–594. 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed]
- 292.Wang L, Cheng G. Sequence analysis of the emerging SARS-CoV-2 variant Omicron in South Africa. J Med Virol. 2021 doi: 10.1002/jmv.27516. [DOI] [PubMed] [Google Scholar]
- 293.Kandeel M, Mohamed MEM, Abd El-Lateef HM, Venugopala KN, El-Beltagi HS. Omicron variant genome evolution and phylogenetics. J Med Virol. 2021 doi: 10.1002/jmv.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chen RE, Zhang X, Case JB, Winkler ES, Liu Y, VanBlargan LA, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27(4):717–726. doi: 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Abu-Raddad L J, Chemaitelly H, Butt A A (2021) National study group for COVID-19 vaccination effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 385(2):187–189. 10.1056/NEJMc2104974 [DOI] [PMC free article] [PubMed]
- 296.Collier DA, De Marco A, Ferreira IATM, Meng B, Datir RP, Walls AC et al (2021) Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 593(7857):136–141. 10.1038/s41586-021-03412-7 [DOI] [PMC free article] [PubMed]
- 297.Lustig Y, Zuckerman N, Nemet I, Atari N, Kliker L, Regev-Yochay G et al (2021) Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Euro Surveill 26(26):2100557. 10.2807/1560-7917.ES.2021.26.26.2100557 [DOI] [PMC free article] [PubMed]
- 298.Muik A, Wallisch A K, Sänger B, Swanson K A, Mühl J, Chen W et al (2021) Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science 371(6534):1152–1153. 10.1126/science.abg6105 [DOI] [PMC free article] [PubMed]
- 299.Edara VV, Norwood C, Floyd K, Lai L, Davis-Gardner ME, Hudson WH (2021b) Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 29(4):516–521. 10.1016/j.chom.2021.03.009 [DOI] [PMC free article] [PubMed]
- 300.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kuzmina A, Khalaila Y, Voloshin O, Keren-Naus A, Boehm-Cohen L, Raviv Y, et al. SARS-CoV-2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post-vaccination sera. Cell Host Microbe. 2021;29(4):522–528.e2. doi: 10.1016/j.chom.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 303.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hoffmann M, Arora P, Groß R, Seidel A, Hörnich BF, Hahn AS et al (2021) SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell 184(9):2384–2393.e12. 10.1016/j.cell.2021.03.036 [DOI] [PMC free article] [PubMed]
- 305.Tang P, HasanM R, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27(12):2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 306.Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J et al (2021) SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe 29(4):529–539.e3. 10.1016/j.chom.2021.03.002 [DOI] [PMC free article] [PubMed]
- 307.Choi A, Koch M, Wu K, Dixon G, Oestreicher J, Legault H, et al. Serum neutralizing activity of mRNA-1273 against SARS-CoV-2 variants. J Virol. 2021;95(23):e0131321. doi: 10.1128/JVI.01313-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, et al. Safety and efficacy of the ChAdOx1 Ncov-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Supasa P, Zhou D, Dejnirattisai W, Liu C, Mentzer AJ, Ginn HM et al (2021) Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell 184(8):2201–2211.e7. 10.1016/j.cell.2021.02.033 [DOI] [PMC free article] [PubMed]
- 310.Dejnirattisai W, Zhou D, Supasa P, Liu C, Mentzer A J, Ginn H M et al (2021) Antibody evasion by the P.1 strain of SARS-CoV-2. Cell 184 (11):2939–2954.e9. 10.1016/j.cell.2021.03.055 [DOI] [PMC free article] [PubMed]
- 311.Moore JP, Offit PA. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325(9):821–822. doi: 10.1001/jama.2021.1114. [DOI] [PubMed] [Google Scholar]
- 312.Gushchin VA, Dolzhikova IV, Shchetinin AM, Odintsova AS, Siniavin AE, Nikiforova MA et al (2021) Neutralizing activity of sera from Sputnik V-vaccinated people against variants of concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow endemic SARS-CoV-2 variants. Vaccines 9:779. 10.3390/vaccines9070779 [DOI] [PMC free article] [PubMed]
- 313.Koyama T, Weerraratne D, Snowdon JL, Parida L. Emergence of drift variants that may affect COVID-19 vaccine development and antibody treatment. Pathogens. 2020;9:324. doi: 10.3390/pathogens9050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nasrollahzadeh M, Sajjadi M, Soufi GJ, Iravani S, Varma RS. Nanomaterials and nanotechnology-associated innovations against viral infections with a focus on coronaviruses. Nanomaterials. 2020 doi: 10.3390/nano10061072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wang H, Zhu W, Feng L, Chen Q, Chao Y, Dong Z, Liu Z. Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer. Nano Res. 2018;11:3244–3257. doi: 10.1007/s12274-017-1858-y. [DOI] [Google Scholar]
- 316.Staroverov SA, Vidyasheva IV, Gabalov KP, Vasilenko OA, Laskavyi VN, Dykman LA. Immunostimulatory effect of gold nanoparticles conjugated with transmissible gastroenteritis virus. Bull Exp Biol Med. 2011;151:436. doi: 10.1007/s10517-011-1350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.World Health Organization (2015) 19th WHO model list of essential medicines. 10.1016/S1473-3099(14)70780-7
- 318.Funk CD, Laferrière C, Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front Pharmaco l. 2020;1:937. doi: 10.3389/fphar.2020.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Weiss C, Carriere M, Fusco L, Capua I, et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020 doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 320.Takemura T (2021) Surface plasmon resonance (SPR)- and localized SPR (LSPR)-based virus sensing systems: optical vibration of nano- and micro-metallic materials for the development of next-generation virus detection technology. Biosensors 11:250. 10.3390/bios11080250 [DOI] [PMC free article] [PubMed]
- 321.Kumar A, Sharma A, Chen Y, Jones MM, et al. Copper@ZIF-8 core-shell nanowires for reusable antimicrobial face masks. Adv Funct Mater. 2020 doi: 10.1002/adfm.202008054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Pradhan A, Lahare P, Sinha P, Singh N, et al. Biosensors as nano-analytical tools for COVID-19 detection. Sensors. 2021;21:7823. doi: 10.3390/s21237823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Asghari A, Chao Wang C, Yoo KM, Ali Rostamian A, Xu X, et al. Fast, accurate, point-of-care COVID-19 pandemic diagnosis enabled through advanced lab-on-chip optical biosensors: opportunities and challenges. Appl Phys Rev. 2020;8:031313. doi: 10.1063/5.0022211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zhou Y, Wu Y, Ding L, Huang X, et al. Point-of-care COVID-19 diagnostics powered by lateral flow assay. Trends Analyt Chem. 2021;145:116452. doi: 10.1016/j.trac.2021.116452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during the current study are available from the corresponding author on reasonable request.