Abstract
Introduction
We aimed to systematically evaluate quality of shared decision‐making (SDM) in colorectal cancer (CRC) screening clinical practice guidelines (CPGs) and consensus statements (CSs).
Methods
Search for CRC screening guidances was from 2010 to November 2021 in EMBASE, Web of Science, MEDLINE, Scopus and CDSR, and the World Wide Web. Three independent reviewers and an arbitrator rated the quality of each guidance using a SDM quality assessment tool (maximum score: 31). Reviewer agreement was 0.88.
Results
SDM appeared in 41/83 (49.4%) CPGs and 9/19 (47.4%) CSs. None met all the quality criteria, and 51.0% (52/102) failed to meet any quality items. Overall compliance was low (mean 1.63, IQR 0–2). Quality was better in guidances published after 2015 (mean 1, IQR 0–3 vs. mean 0.5, IQR 0–1.5; p = 0.048) and when the term SDM was specifically reported (mean 4.5, IQR 2.5–4.5 vs. mean 0.5, IQR 0–1.5; p < 0.001). CPGs underpinned by systematic reviews showed better SDM quality than consensus (mean 1, IQR 0–3 vs. mean 0, IQR 0–2, p = 0.040).
Conclusion
SDM quality was suboptimal and mentioned in less than half of the guidances, and recommendations were scarce. Guideline developers should incorporate evidence‐based SDM recommendations in guidances to underpin the translation of evidence into practice.
Keywords: ‘clinical practice guidelines’, ‘colorectal cancer screening’, ‘consensus’, ‘quality of guidelines’, ‘shared decision‐making’
INTRODUCTION
Colorectal cancer (CRC) incidence and mortality have decreased due to screening programmes, removing precancerous polyps with colonoscopy, and advances in management (Cotton et al., 1996; Zauber et al., 2012). In screening programmes, the patient's overall health, prior screening history, and preferences and values must be considered in selecting an individualised approach adapted to the patient's risk of acquiring CRC (Hoffmann et al., 2020; US Preventive Services Task Force et al., 2021). Shared decision‐making (SDM) is essential for decision‐making, weighing up risks vs. benefits for each individual patient (Schrager & Burnside, 2017). In recent years, health‐care policymaking has emphasised SDM as a cornerstone of evidence‐based and patient‐centred care (Barry & Edgman‐Levitan, 2012; Elwyn et al., 2010). Institutional promotion is fundamental for improving SDM application (Senate and House of Representatives, 2010), and clinical practice guidelines (CPGs) and consensus statements (CSs) should promote it and advise about its execution (Maes‐Carballo, Munoz‐Nunez, et al., 2020). Although CPGs and CSs increasingly support it (Gärtner et al., 2019), they remain unclear on accomplishing SDM in routine practice (Elwyn et al., 2012). Therefore, the analysis of the quality of SDM in guidances is critically essential. To the best of our knowledge, no systematic review has investigated SDM in CRC screening guidances.
Considering this background, this systematic review aimed to analyze SDM in CRC screening CPGs and CSs, evaluating the quality of recommendations about SDM.
1. METHODS
Following prospective registration (Prospero no: CRD42021286156), this systematic review was conducted following advocated methods for search, assessment and reporting of guidelines using the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement (see Appendix S1) (Liberati et al., 2009; Moher et al., 2009).
1.1. Search strategy, data sources, inclusion and exclusion criteria
A search for relevant publications covering major electronic databases (EMBASE, Web of Science, MEDLINE, Scopus and CDSR) was developed without language restrictions to capture peer‐reviewed and grey literature from 2010 until November 2021. We also searched 59 websites of important professional societies and eight guidance‐specific databases. We explored the World Wide Web to include professional societies from countries with global CRC scientific production bigger than 0.5% (Maes‐Carballo et al., 2021, 2022; Maes‐Carballo, Mignini, et al., 2020). A total of 85,932 ‘Colorectal Cancer and Health’ records were scrutinised from Scopus on 10 March 2022 to calculate the scientific production of each country. This decision is adhered to rules followed by other previous peer‐reviewed published systematic reviews (Maes‐Carballo et al., 2021, 2022; Maes‐Carballo, Mignini, et al., 2020; Maes‐Carballo, Munoz‐Nunez, et al., 2020). We revised references from the guidances retrieved to search for conceivable additional CPGs or CSs. The final search integrated MeSH terms ‘practice guidelines’, ‘guidelines’, ‘consensus’, ‘colorectal neoplasms’, ‘colorectal cancer’, ‘screening’ and including word alternatives. Details of the search strategy were documented in Appendix S2. Endnote X9 software was employed to handle the searches downloaded.
Selection criteria captured eligible guidances about CRC screening produced by national or international professional organisations and societies or governmental agencies. Those guidances in which screening was the central issue and those in which there was a section dedicated to screening or prevention were included in our systematic review. We excluded protocols or screening programme documents, CPGs and CSs about diagnosis and treatment, out‐of‐date guidelines replaced by updates from the same organisation, and CPG and CSs for education and information purposes, randomised controlled trials, observational studies, narrative reviews, scientific reports, discussion papers, conference abstracts, and posters.
Studies were chosen through a multi‐step approach, including deleting duplicates, reading titles and abstracts, and assessing full texts. Initially, titles and abstracts were considered for eligibility by two reviewers (CR‐E and AI‐A). Then, full texts were appraised for eligibility also by these two reviewers. Potential disagreements or inconsistencies were decided by arbitration of another reviewer (MM‐C). Articles in duplication were recognised and excluded. When several versions of the same guidance were discovered, the most updated version was incorporated. Data were extracted independently and duplicated by three reviewers (YG‐F, CR‐E and AI‐A). Disagreements were solved by consensus or arbitration (MM‐C).
1.2. Quality appraisal of guidances and data extraction
The included guidance's characteristics and quality were extracted into a piloted electronic data extraction sheet. The methodological quality assessment was estimated using an already published appraisal tool consisting of a 31‐item checklist grouped into 11 domains (Maes‐Carballo, Munoz‐Nunez, et al., 2020). Three reviewers (YG‐F, CRE‐L and AI‐A) evaluated the quality of SDM in CRC screening guidances (Appendix S3). The 11 domains were basic information (items 1–4), background (items 5–7), selection criteria (items 8–9), strengths and limitations (items 10–14), recommendations about SDM (items 15–17), facilitators and barriers (items 18–19), implementation (items 20–21), resource implications (items 22–24), monitoring and auditing criteria (items 25–27), recommendations for further research and limitations about these recommendations described (items 28–29), and editorial independence and declaration of interest (items 30–31). In those general guidances on the management of CRC, SDM was only considered if it was covered in the screening section. The questions were designed for a binary ‘yes/no’ answer: ‘Yes’ or 1 if the item was met and ‘no’ or ‘0’ if the criterion was not accomplished. The high quality was related to a higher quantity of items completed in the CPG or CS evaluated. No formal score or cut‐off point was specified to determine the quality (Maes‐Carballo, Munoz‐Nunez, et al., 2020). All three reviewers (YG‐F, CRE‐L and AI‐A) had a prior meeting receiving a training seminar and workshop from the arbitrator and creator of the tool (MM‐C) that included education on SDM and the process and application of the SDM tool.
1.3. Evidence synthesis, investigation of heterogeneity and data analysis
A descriptive analysis of the characteristics and quality of the selected guidances was conducted. Statistical data analysis was completed using Stata 16. The Kruskal–Wallis test was utilised to compare scores and stratify for factors or characteristics that may influence the quality of SDM in CPGs and CSs. Values were assessed statistically significant when p < 0.05. Inter‐rater reliability between reviewers in data extraction was calculated using the intra‐class correlation coefficient (ICC). A result of more than 0.75 was considered good (Koo & Li, 2016).
2. RESULTS
2.1. Study selection
The study selection process is illustrated in the flow diagram in Figure 1. The initial search identified 8229 citations. We removed 439 duplicates and 7676 records for not meeting the selection criteria (inappropriate population, publication, development group or outdated guidance). A total of 114 records were filtered through reviews of titles and abstracts. Later, we obtained the full text of 114 citations for eligibility assessment, and finally, 102 guidances (83 CPGs) (Alberta, 2020; Alsanea et al., 2015; Aranda et al., 2015; Aranda‐Hernandez et al., 2016; Atkin et al., 2012; Austoker et al., 2012; Benton et al., 2016; Bo In Lee et al., 2012; Brenner et al., 2017; Brouwers et al., 2011; Canadian task Force on preventive health C, 2016; Clarke & Feuerstein, 2019; Cubiella et al., 2018; Day et al., 2011; Del Giudice et al., 2014; Duffy et al., 2014; European Colorectal Cancer Screening Guidelines Working Group et al., 2013; European Commission, 2010; Fabio Leonel Gil Parada et al., 2015; Gupta et al., 2019; Halloran et al., 2012; Hassan et al., 2013; Helsingen et al., 2019; Hospital provincial Neuquén, 2016; Instituto Mexicano del Seguro Social. Guía de Práctica Clínica, 2010; Instituto Nacional del Cáncer, 2015; Jenkins et al., 2018; Jenkinson & Steele, 2010; Jover et al., 2012; Kwaan & Jones‐Webb, 2018; Lam et al., 2018; Lansdorp‐Vogelaar et al., 2012; Leddin et al., 2013; Lee et al., 2012; Leong et al., 2017; Lieberman, 2012; Lopes et al., 2018; Malila et al., 2012; Ministry of Health, 2010, 2016; Minozzi et al., 2012; Monahan et al., 2019; Moreno et al., 2018; Moss et al., 2012; Network NCC, 2021; New Brunswick Colon Cancer Screening, 2013; NHS, 2021; Ong et al., 2014; Provenzale et al., 2016; Qaseem et al., 2019; Quirke et al., 2011, 2012; Recommended Cancer Screenings, 2013; Regula & Kaminski, 2010; Rex et al., 2017; Rubeca et al., 2017; Salzman et al., 2016; SemFYC AEdG, 2018; Seppälä et al., 2021; Shaukat et al., 2021; Society AC, 2018; Spada et al., 2014; Steele, Pox et al., 2012; Steele, Rey et al., 2012; Steinwachs et al., 2010; Stoffel et al., 2015; Tanaka et al., 2015; Telford, 2011; Tinmouth et al., 2014; Uruguay MdSd. Ministerio de Salud de Uruguay, 2018; US Preventive Services Task Force et al., 2021; Valori et al., 2012; Vasen et al., 2014; Vieth et al., 2012; von Karsa et al., 2012; Washington KFHPo, 2021; Wilkins et al., 2018; Wilkinson et al., 2019; Wolf et al., 2018; Wong et al., 2015; Yang et al., 2020; Zeimet et al., 2017; 손대경 김, 박윤희, 서민아, 신애선, 이희영, 임종필, 조현민, 홍성필, 김백희, 김용수, 김정욱, 김현수, 남정모, 박동일, 엄준원, 오순남, 임환섭, 장희진, 함상근, 정지혜, 김수영, 김열, 이원철, 정승용, 2015) and 19 CSs (Asociación Mexicana de Endoscopia Gastrointestinal y Colegio de Profesionistas, 2016; ANM. Programa Nacional de Consensos Inter‐Sociedades, 2010; Basu et al., 2021; García‐Carbonero et al., 2017; Giardiello et al., 2014; Hadjiliadis et al., 2018; Heresbach et al., 2016; Hyer et al., 2019; Johnson et al., 2014; Leddin et al., 2010, 2018; Lieberman et al., 2012; Rembacken et al., 2012; Robertson et al., 2017; Schmiegel et al., 2010; Schmoll et al., 2012; Sollano et al., 2017; Sung et al., 2015; 中国抗癌协会肿瘤内镜学专业委员会 国上国中医中中消中中中金国, 2019) were included in our study for the last appraisal.
FIGURE 1.

The flow diagram detailing the study selection
2.2. Characteristics of guidances and quality appraisal
The guidance's characteristics (type of document, entity, country, year, journal of publication, version and evidence analysis) were reported in Table 1. The guidances' mean number of items related to SDM was 1.63 (IQR 0–2). The quality assessment results using the SDM instrument are shown in Figure 2 and Appendix S4. No significant differences were obtained between CPGs and CSs concerning the SDM quality (p = 0.959). A total of 50 guidances (49.0%), 41/83 (49.4%) CPGs and 9/19 (47.4%) CSs, reported something about SDM. None of the guidelines met all the quality criteria, and 51.0% of the guidelines accomplished 0 items, and only 5.9% of them accomplished more than 25% items (8/31). When the SDM term was specifically cited in the guidance (n = 13), the quality of the CPG or CSs concerning SDM was better than when it did not appear (n = 89) (mean 4.5, IQR 2.5–4.5 vs. mean 0.5, IQR 0–1.5; p < 0.001).
TABLE 1.
Description of the screening clinical practice guidelines (CPGs) and consensus (CSs) (n=102) selected for the systematic review on the quality of reporting for SDM
| Name of the CPG or protocol | Abbreviated name | Type of document | Entity | Country | Year | Publication in a journal | Version | Evidence analysis | Quality tool referral | Type of cancer | Last updated date (months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Screening for colorectal cancer. US preventive services task Force recommendation statement | 2021 USPSTF CRC screening | CPG | USPSTF | USA | 2021 | JAMA | 5 | Systematic review | No | CRC | 4 |
| 2 | Bowel cancer screening: Pathology guidance on reporting lesions | 2021 UK government CRC screening | CPG | UK government | UK | 2021 | Not published | 3 | Review | No | CRC | 8 |
| 3 | NCCN clinical practice guidelines in oncology (NCCN Guidelines). Colorectal Cancer Screening | 2021 NCCN CRC screening | CPG | NCCN | USA | 2021 | Not published | 2 | Systematic review, consensus | No | CRC | 8 |
| 4 | Colorectal cancer screening guideline | 2021 KFHPW CRC screening | CPG | KFHPW | USA | 2021 | Not published | 2 | Not reported | No | CRC | 3 |
| 5 | European guidelines from the EHTG and ESCP for Lynch syndrome: An updated third edition of the Mallorca guidelines based on gene and gender | 2021 EHTG/ESCP Lynch syndrome | CPG | EHTG/ESCP | Europe | 2021 | BJS | 3 | Systematic review, Delphi consensus | Yes | CRC | 7 |
| 6 | Cancer screening in the coronavirus pandemic era: Adjusting to a new situation | 2021 COVID pandemic cancer screening | CS | ASCO | USA | 2021 | JCO global oncology | 1 | Scoping review | No | CRC/breast/cervical | 7 |
| 7 | ACG clinical guidelines: Colorectal cancer screening 2021 | 2021 ACG CRC Screening | CPG | ACG | USA | 2021 | Am J Gastroenteroly | 3 | Systematic review, GRADE | No | CRC | 9 |
| 8 | Colorectal cancer screening. Clinical practice guideline. Nov 2013 (Revised 2020) | 2020 CCAl CRC screening | CPG | CCAl | Canada | 2020 | Not published | 6 | Not reported | No | CRC | 23 |
| 9 | American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes | 2020 ASGE polyposis syndromes | CPG | ASGE | USA | 2020 | Gastrointest Endosc | 1 | Systematic review, consensus | No | CRC | 18 |
| 10 | Colorectal cancer screening | 2019 USMSTF CRC screening | CPG | USMSTF | USA | 2019 | JAMA | 1 | Systematic review, GRADE | No | CRC | 31 |
| 11 | Colorectal cancer screening for patients with a family history of colorectal cancer or adenomas | 2019 Ottawa CRC screening | CPG | University of Ottawa | Canada | 2019 | CFP | 1 | Systematic review, meta‐analysis, GRADE | No | CRC | 25 |
| 12 | Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a clinical practice guideline | 2019 MAGIC CRC screening | CPG | MAGIC | UK | 2019 | BMJ | 1 | Systematic review, GRADE | No | CRC | 34 |
| 13 | Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group | 2019 ESPGHAN polyposis syndromes | CS | ESPGHAN | Europe | 2019 | JPGN | 1 | Systematic literature, GRADE | No | CRC | 33 |
| 14 | Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments | 2019 CRC in IBD | CPG | HMS | USA | 2019 | WJG | 2 | Not reported | No | CRC | 28 |
| 15 | 中国早期结直肠癌筛查流程专家共识意见(2019, 上海) | 2019 Chinese CRC screening CS | CS | SMMU | China | 2019 | CGP | 1 | Not reported | No | CRC | 25 |
| 16 | Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG) | 2019 BSG/ACPGBI/UKCGG CRC screening | CPG | BSG/ACPGBI/UKCGG | UK | 2019 | BMJ | 1 | Systematic review, GRADE, Delphi consensus | No | CRC | 26 |
| 17 | Clinical practice guideline. Diagnosis and prevention of colorectal cancer. 2018 update | 2019 ACP CRC screening CPG | CPG | ACP | USA | 2019 | Ann Intern Med | 2 | Not reported | No | CRC | 29 |
| 18 | Screening for Colorectal Cancer in asymptomatic average‐risk adults: A guidance statement from the American College of Physicians | 2019 ACP average‐risk CRC screening | CPG | ACP | USA | 2019 | Ann Intern Med | 1 | Review | Yes | CRC | 25 |
| 19 | Guía de práctica clínica de tamizaje del cáncer colo‐rectal 2018 | 2018 Uruguay CRC screening | CPG | Ministerio de Salud. Uruguay | Uruguay | 2018 | Not published | 2 | Systematic review | Yes | CRC | 48 |
| 20 | Recommendations on prevention and screening for colorectal cancer in Hong Kong | 2018 Hong Kong CRC screening | CPG | CEWGCPS | China | 2018 | Hong Kong med J | 3 | Not reported | No | CRC | 36 |
| 21 | Early detection for colorectal cancer: ASCO resource‐stratified guideline | 2018 early detection for CRC | CPG | ASCO | USA | 2018 | JGO | 1 | Review, consensus | Yes | CRC | 36 |
| 22 | Cystic fibrosis colorectal cancer screening consensus recommendations | 2018 cystic fibrosis CRC screening | CS | AGA | USA | 2018 | Gastroenterology | 1 | Systematic review, consensus | No | CRC | 46 |
| 23 | Colorectal Cancer Screening in black men: Recommendations for best practices | 2018 CRC screening in black men | CPG | NIH | USA | 2018 | Am J Prev Med | 1 | Review | No | CRC | 38 |
| 24 | Colorectal Cancer Screening and prevention | 2018 CRC Screening and prevention | CPG | AAFP | USA | 2018 | Not published | 1 | Not reported | No | CRC | 42 |
| 25 | Clinical practice guideline on screening for colorectal cancer in individuals with a family history of non‐hereditary colorectal cancer or adenoma: The Canadian Association of Gastroenterology Banff Consensus | 2018 Banff consensus | CS | CAG | Canada | 2018 | Gastroenterology | 1 | Systematic review, GRADE, consensus | No | CRC | 37 |
| 26 | Revised Australian national guidelines for colorectal cancer screening: Family history | 2018 Australian CRC screening | CPG | CCA | Australia | 2018 | MJA | 2 | Systematic review, consensus | No | CRC | 36 |
| 27 | Diagnóstico y prevención del Cáncer Colorectal | 2018 AEC CRC screening | CPG | AEG | Spain | 2018 | Not published | 2 | Review, GRADE | No | CRC | 38 |
| 28 | Detección temprana, diagnóstico y clasificación por etapas | 2018 ACS CRC screening | CPG | ACS | USA | 2018 | Not published | 3 | Not reported | No | CRC | 46 |
| 29 | Colorectal cancer screening for average‐risk adults: 2018 guideline update from the American Cancer Society | 2018 ACS average‐risk CRC screening | CPG | ACS | USA | 2018 | CA Cancer J Clin | 2 | Systematic review, GRADE | No | CRC | 31 |
| 30 | ACR appropriateness criteria. Colorectal cancer screening | 2018 ACR CRC Screening | CPG | ACR | USA | 2018 | ACR | 2 | Systematic review, GRADE | No | CRC | 38 |
| 31 | Recommendations on faecal immunochemical testing to screen for colorectal neoplasia: A consensus statement by the US multi‐society task force on colorectal cancer | 2017 USPSTF FOBT | CS | USPSTF | USA | 2017 | Gastroenterology | 1 | Review, consensus | No | CRC | 58 |
| 32 | Colorectal cancer screening: Recommendations for physicians and patients from the U.S. multi‐society task force on colorectal cancer | 2017 USPSTF CRC screening | CPG | USPSTF | USA | 2017 | Am J Gastroenterol | 2 | Systematic review, GRADE | No | CRC | 52 |
| 33 | The Joint Philippine Society of Gastroenterology (PSG) and Philippine Society of Digestive Endoscopy (PSDE) Consensus Guidelines on the Management of Colorectal Carcinoma | 2017 Philippine CRC screening CS | CS | PSG, PSDE | Philippines | 2017 | PJIM | 1 | Review, consensus | No | CRC | 55 |
| 34 | Guidance for faecal occult blood testing: quantitative immunochemical method (FIT‐HB) in colorectal cancer screening programmes | 2017 Italian FOBT CRC screening | CPG | Grupo Italiano Screening Colorettale | Italy | 2017 | Not published | 1 | Systematic review | No | CRC | 48 |
| 35 | AGO Austria recommendation on screening and diagnosis of Lynch syndrome (LS) | 2017 AGO Lynch CRC screening | CS | AGO | Austria | 2017 | Arch Gynecol Obstet | 1 | Consensus | No | CRC | 54 |
| 36 | Association of coloproctology of Great Britain and Ireland (ACPGBI): Guidelines for the management of cancer of the colon, rectum and anus (2017) – Diagnosis, investigations and screening | 2017 ACPGBI CRC and anal cancer | CPG | ACPGBI | UK, Ireland | 2017 | Colorectal disease | 1 | Not reported | No | CRC, anal cancer | 58 |
| 37 | Colorectal cancer screening in average risk patients | 2017 CRC average risk patients | CPG | University of North Carolina | USA | 2017 | Med Clin North Am. | 1 | Review | No | CRC | 53 |
| 38 | Turkey Cancer Control Programme | 2016 Turkey CRC screening | CPG | Ministry of Health. Turkey | Turkey | 2016 | Not published | 1 | Not reported | No | CRC/breast/cervical | 70 |
| 39 | Prévention du cancer colorectal par coloscopie, en dehors du dépistage en population. Consensus et position de la SFED | 2016 SFED CRC screening | CS | SFED | France | 2016 | Acta Endosc | 2 | Consensus | No | CRC | 70 |
| 40 | NICE referral guidelines for suspected cancer: Colorectal cancer and faecal occult blood testing | 2016 NICE CRC screening | CPG | NICE | UK | 2016 | Annals of Clinical Biochemistry | 1 | Review | No | CRC | 70 |
| 41 | Guías de prevención y manejo endoscópico del cáncer colorrectal | 2016 Mexico CRC screening | CS | AMEG | Mexico | 2016 | Not published | 1 | Review, consensus, GRADE | No | CRC | 70 |
| 42 | Genetic/familial high‐risk assessment: Colorectal. Version 1.2016 | 2016 JNCCN CRC high risk | CPG | NCCN | USA | 2016 | JNCCN | 1 | Systematic review | No | CRC | 65 |
| 43 | Detección temprana de Cáncer Colorectal en población adulta | 2016 HPN CRC screening | CPG | HPN | Argentina | 2016 | Not published | 1 | Systematic review | AGREE II | CRC | 70 |
| 44 | 2016 gastrointestinal endoscopy: Global view. Seeing better – Evidence based recommendations on optimising colonoscopy adenoma detection rate | 2016 GI endoscopy | CPG | University of Toronto | Canada | 2016 | WJG | 1 | Review | No | CRC | 69 |
| 45 | Cancer screening in older patients | 2016 CRC screening old patients | CPG | Thomas Jefferson University | USA | 2016 | American Family Physician | 1 | Review | No | CRC | 67 |
| 46 | Guía de práctica clínica para la tamización de cáncer colorrectal | 2016 Colombia CRC screening | CPG | ACGEDCH | Colombia | 2016 | Rev Col Gastroenterol | 1 | Systematic review, GRADE | AGREE II | CRC | 62 |
| 47 | Recommendations on screening for colorectal cancer in primary care | 2016 Canadian task force screening | CPG | CTFPHC | Canada | 2016 | CMAJ | 1 | Systematic review, GRADE | No | CRC | 66 |
| 48 | SEOM/SERAM consensus statement on radiological diagnosis, response assessment and follow‐up in colorectal cancer | 2015 SEOM SERAM CRC screening | CS | SEOM, SERAM | Spain | 2015 | Clin Transl Oncol | 1 | Consensus | No | CRC | 78 |
| 49 | SEOM clinical guidelines for diagnosis and treatment of metastatic colorectal cancer 2015 | 2015 SEOM CRC screening | CPG | SEOM | Spain | 2015 | Clin Transl Oncol | 1 | Review, GRADE | No | CRC | 72 |
| 50 | National Guidelines for Colorectal Cancer Screening in Saudi Arabia with strength of recommendations and quality of evidence | 2015 Saudi Arabia CSC screening | CPG | SSCRS/ SGA/SOS/MH | Saudi Arabia | 2015 | Ann Saudi Med | 1 | Systematic review | No | CRC | 77 |
| 51 | 대장암 검진 권고안 | 2015 Korea CRC screening | CPG | NCCK | Korea | 2015 | J Korean Med Assoc | 1 | Review | No | CRC | 77 |
| 52 | Evidence‐based clinical practice guidelines for management of colorectal polyps | 2015 JSGE CR polyps | CPG | JSGE | Japan | 2015 | J Gastroenterol | 1 | Review, consensus | No | CRC | 81 |
| 53 | Targeted screening for colorectal cancer in high‐risk individuals | 2015 CRC screening high‐risk | CPG | CUHK | China | 2015 | Best Practice and Research Clinical Gastroenterology | 1 | Review | No | CRC | 82 |
| 54 | Colorectal cancer surveillance after index colonoscopy: Guidance from the Canadian Association of Gastroenterology | 2015 CAG CRC surveillance | CPG | CAG | Canada | 2015 | Can J Gastroenterol | 1 | Review, consensus | No | CRC | 82 |
| 55 | Hereditary colorectal cancer syndromes: American Society of Clinical Oncology clinical practice guideline endorsement of the familial risk–colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines | 2015 ASCO hereditary CRC | CPG | ASCO | USA | 2015 | JCO | 1 | Review | AGREE II | CRC | 82 |
| 56 | GUÍA PARA EQUIPOS DE ATENCIÓN PRIMARIA DE LA SALUD. Información para la prevención y detección temprana del cáncer colorrectal | 2015 Argentinian CRC screening | CPG | Ministerio de Salud. Argentina | Argentina | 2015 | Not published | 1 | Not reported | No | CRC | 78 |
| 57 | Guidelines on genetic evaluation and management of Lynch syndrome: A consensus statement by the US multi‐society task force on colorectal cancer | 2014 USPSTF Lynch syndrome | CPG | USPSTF | USA | 2014 | American journal of GASTROENTEROLOGY | 1 | Systematic review | No | CRC | 87 |
| 58 | Optimising adequacy of bowel cleansing for colonoscopy: Recommendations from the US multi‐society task force on colorectal cancer | 2014 USPSTF bowel cleansing | CS | USPSTF | USA | 2014 | Gastroenterology | 1 | Systematic review, GRADE | No | CRC | 94 |
| 59 | Colonoscopy quality assurance in Ontario: Systematic review and clinical practice guideline | 2014 Ontario CRC screening | CPG | CCO | Canada | 2014 | Can J Gastroenterol Hepatol | 1 | Systematic review | AGREE II | CRC | 89 |
| 60 | Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) guideline | 2014 ESGE ESGAR CTC | CPG | ESGE, ESGAR | Europe | 2014 | Endoscopy | 1 | Review, GRADE | No | CRC | 86 |
| 61 | Guideline for referral of patients with suspected colorectal cancer by family physicians and other primary care providers | 2014 CRC screening primary care | CPG | SAFHT | Canada | 2014 | Can Fam Physician | 1 | Systematic review | No | CRC | 84 |
| 62 | An updated Asia Pacific consensus recommendations on colorectal cancer screening | 2014 Asia Pacific CRC screening CS | CS | APWGCRCS | Asia | 2014 | Gut | 2 | Consensus | No | CRC | 91 |
| 63 | Regional and national guideline recommendations for digital ano‐rectal examination as a means for anal cancer screening in HIV positive men who have sex with men: A systematic review | 2014 ano‐rectal screening | CPG | University of Melbourne | Australia | 2014 | BMC Cancer | 1 | Systematic review | No | Anal and rectal cancer | 95 |
| 64 | Recommended Cancer screenings | 2013 Swedish CRC screening | CPG | Swedish Cancer institute | Sweden | 2013 | Not published | 1 | Not reported | No | CRC | 106 |
| 65 | New Brunswick colon cancer screening. Clinical practice guidelines | 2013 NBCN CRC screening | CPG | NBCN | Canada | 2013 | Not published | 1 | Review | No | CRC | 98 |
| 66 | Guidelines for surveillance of individuals with constitutional mismatch repair‐deficiency proposed by the European Consortium ‘Care for CMMR‐D’ (C4CMMR‐D) | 2013 Lynch CRC screening | CPG | LUMC | Netherlands | 2013 | J Med Genet | 1 | Not reported | No | CRC | 96 |
| 67 | Post‐polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline | 2013 ESGE endoscopy | CPG | ESGE | Europe | 2013 | Endoscopy | 1 | Systematic review | No | CRC | 96 |
| 68 | Tumour markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumour markers 2014 guidelines update | 2013 EGTM CRC tumour makers | CPG | University College Dublin | Ireland | 2013 | IJC | 1 | Not reported | No | CRC | 96 |
| 69 | Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US multi‐society task force on colorectal cancer | 2012 USPSTF screening surveillance | CS | USPSTF | USA | 2012 | Gastroenterology | 2 | Review, consensus | No | CRC | 118 |
| 70 | Clinical practice guidelines: Quality of colonoscopy in colorectal cancer screening | 2012 SEED AEG colonoscopy | CPG | SEED, AEG | Spain | 2012 | Endoscopy | 1 | Review | No | CRC | 118 |
| 71 | Colorectal cancer screening: Practice guidelines | 2012 Oregon CRC screening | CPG | OHSU | USA | 2012 | Dig Dis | 1 | Review | No | CRC | 119 |
| 72 | 대장암 선별과 대장폴립 진단검사 가이드라인 | 2012 Korean CRC screening | CPG | Universidad de Yonsei | Korea | 2012 | Korean J Gastroenterol Vol | 1 | Systematic review, meta‐analysis | No | CRC | 118 |
| 73 | Korean guidelines for colorectal cancer screening and polyp detection | 2012 Korea CRC screening | CPG | Yonsei University | Korea | 2012 | Clin Endosc | 1 | Review | No | CRC | 118 |
| 74 | ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalised approach to clinical decision‐making | 2012 ESMO CRC screening | CS | ESMO | Europe | 2012 | Annals of Oncology | 1 | Consensus, GRADE | No | CRC | 112 |
| 75 | Quality in screening colonoscopy: Position statement of the European Society of Gastrointestinal Endoscopy (ESGE) | 2012 ESGE quality in CRC screening | CS | ESGE | Europe | 2012 | Endoscopy | 1 | Review | No | CRC | 118 |
| 76 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition professional requirements and training | 2012 EC CRC screening. Training | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 77 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition colonoscopic surveillance following adenoma removal | 2012 EC CRC screening. Surveillance | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 78 | European guidelines for quality assurance in colorectal cancer screening and diagnosis: Overview and introduction to the full supplement publication | 2012 EC CRC screening. Supplement | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 109 |
| 79 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition executive summary | 2012 EC CRC screening. Summary | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 80 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition quality assurance in pathology in colorectal cancer screening and diagnosis | 2012 EC CRC screening. Pathology | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 81 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition organisation | 2012 EC CRC screening. Organisation | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 82 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition management of lesions detected in colorectal cancer screening | 2012 EC CRC screening. Lesions | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 83 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition introduction | 2012 EC CRC screening. Introduction | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 84 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition faecal occult blood testing | 2012 EC CRC screening. FOBT | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 85 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition evaluation and interpretation of screening outcomes | 2012 EC CRC screening. Evaluation | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 86 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition quality assurance in endoscopy in colorectal cancer screening and diagnosis | 2012 EC CRC screening. Endoscopy | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 87 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition annotations of colorectal lesions | 2012 EC CRC screening. CRC lesions | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 88 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition communication | 2012 EC CRC screening. Communication | CPG | European Commission | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 89 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition principles of evidence assessment and methods for reaching recommendations | 2012 CPO CRC screening principles | CPG | CPO Piemonte | Europe | 2012 | Endoscopy | 1 | Systematic review | No | CRC | 118 |
| 90 | Effective interventions to facilitate the uptake of breast, cervical and colorectal cancer screening: An implementation guideline | 2011 CCO effective interventions | CPG | CCO | Canada | 2011 | BMC | 1 | Systematic review | AGREE | CRC | 130 |
| 91 | Canadian guidelines for colorectal cancer screening | 2011 Canadian CRC screening | CPG | University of British Columbia | Canada | 2011 | Can J Gastroenterol | 1 | Not reported | No | CRC | 120 |
| 92 | Colorectal cancer screening and surveillance in the elderly patient | 2011 ACG CRC screening in old | CPG | ACG | USA | 2011 | Am J Gastroenterol | 1 | Review | No | CRC | 125 |
| 93 | Cancer screening | 2010 Singapore CRC screening | CPG | Ministry of Health. Singapore | Singapore | 2010 | Not published | 1 | Systematic review | No | CRC | 140 |
| 94 | Quality assurance in pathology in colorectal cancer screening and diagnosis—European recommendations | 2010 quality assurance CRC | CPG | University of Leeds | UK | 2010 | Virchows Arch | 1 | Review, consensus | No | CRC | 142 |
| 95 | Targeting risk groups for screening | 2010 Poland risk groups screening | CPG | Institute of Oncology, roentgen | Poland | 2010 | Best Practice and Research Clinical Gastroenterology | 1 | Not reported | No | CRC | 142 |
| 96 | NIH state‐of‐the‐science conference statement on enhancing use and quality of colorectal cancer screening | 2010 NIH CRC screening | CPG | NIH | UK | 2010 | Not published | 1 | Systematic review | No | CRC | 142 |
| 97 | Guía de Práctica Clínica. Detección Oportuna y Diagnóstico de Cáncer de Colon y Recto no Hereditario en Adultos en Primero, Segundo y Tercer Nivel de Atención | 2010 Mexico CRC screening | CPG | AMC | Mexico | 2010 | Not published | 1 | Systematic review | No | CRC | 138 |
| 98 | European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition | 2010 EC CRC screening | CPG | European Commission | Europe | 2010 | Not published | 1 | Systematic review | No | CRC | 142 |
| 99 | Colorectal cancer screening—Methodology | 2010 Dundee CRC screening | CPG | University of Dundee | UK | 2010 | The Surgeon | 1 | Review | No | CRC | 142 |
| 100 | S3 Guidelines for colorectal carcinoma results of an evidence‐based consensus conference on February 6/7, 2004 and June 8/9, 2007 (for the topics IV, VI and VII) | 2010 CRC screening | CS | Ruhr‐Universität Bochum | Germany | 2010 | Z Gastroenterol | 3 | Consensus, GRADE | No | CRC | 142 |
| 101 | Canadian Association of Gastroenterology position statement on screening individuals at average risk for developing colorectal cancer: 2010 | 2010 CAG risk CRC screening CS | CS | CAG | Canada | 2010 | Can J Gastroentero | 2 | Not reported | No | CRC | 130 |
| 102 | Programa Nacional de Consensos Inter‐Sociedades. Programa Argentino de Consensos de Enfermedades Oncológicas. GUÍA DE RECOMENDACIONES PARA LA PREVENCIÓN Y DETECCIÓN PRECOZ DEL CÁNCER COLORRECTAL. Septiembre 2010 | 2010 Argentinian CRC screening CS | CS | ANM | Argentina | 2010 | Not published | 1 | Consensus | AGREE II | CRC | 133 |
FIGURE 2.
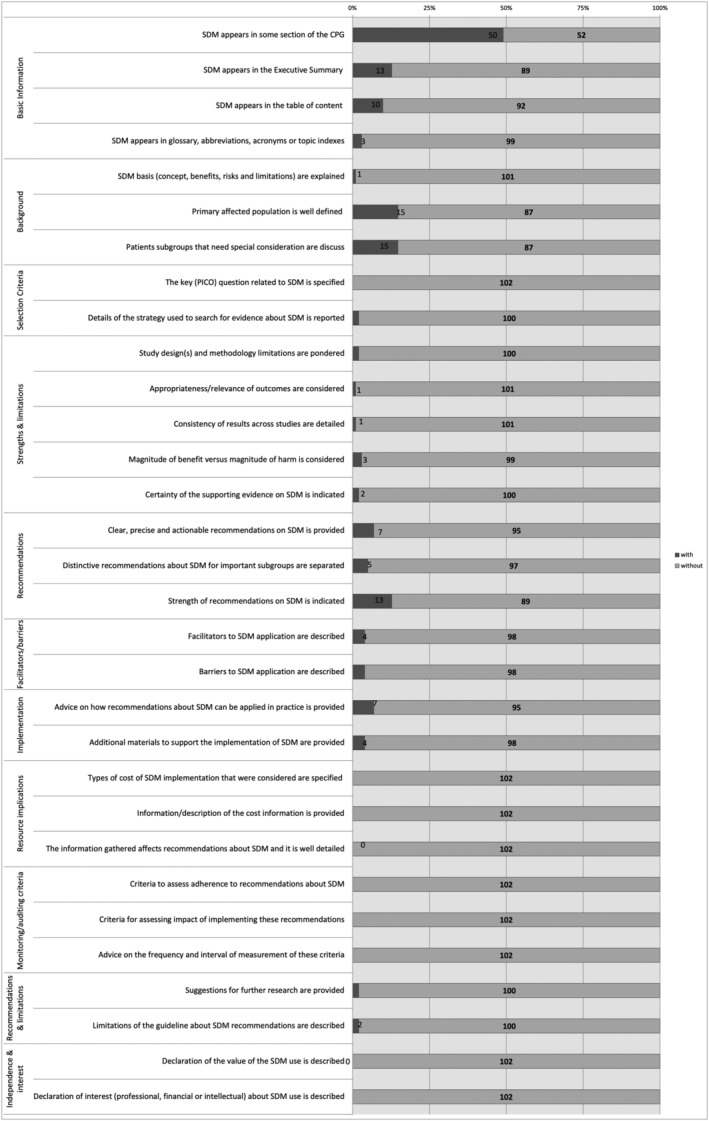
The compliance of the items with the SDM quality and reporting analysis tool
The best‐scored domains were basic information (domain 1) with a range of guidances accomplishing items from 3 to 50, background (domain 2) with a range from 1 to 15, and recommendations (domain 5) with a range from 5 to 13. Resource implications (domain 8), monitoring and auditing criteria (domain 9), and independence and conflict of interest (domain 11) did not appraise any of the items in any guidance. Only 13/102 (12.7%), 10/102 (9.8%) and 3/102 (2.9%) guidances informed SDM in their executive summary, table of content, glossary, abbreviations, acronyms or topic indexes. SDM concept was only explained in one (1.0%) guidance. Both the primary population and patient subgroups with special consideration were characterised in 15/102 (14.7%). The search strategy and the study design and methodology limitations were reported in 2/102 (2.0%), respectively. The importance of the outcomes and the consistency of the results were detailed in only one guidance (1.0%) each. No PICO question was identified in any guidance. The benefits vs. harms of SDM in CRC screening were considered only in 3/102 (2.9%). The evidence of using SDM was exhibited in 2/102 (2.0%). Recommendations about SDM use were clear, precise and reliable in only 7/102 (6.9%) guidance documents, and these recommendations were well‐reported regarding specific subgroups in 5/102 (4.9%) guidances. Facilitators and barriers for SDM application were well‐described in 4/102 (3.9%), advice on applying SDM in clinical routine in 7/102 (6.9%), and additional materials were provided in other 7/102 (6.9%). Suggestions for further research were located in 2/102 (2.0%), and limitations about SDM recommendations were also described in 2/102 (2.0%). No information about SDM implementation cost, any criteria to assess and measure SDM adherence, conflict of interest regarding SDM or a declaration of the value of SDM in clinical practice. The European Commission (Austoker et al., 2012; European Commission, 2010) guidances and the American Cancer Society (ACS) (Wolf et al., 2018) CPG achieved the highest number of quality SDM items completed (Appendix S4).
2.3. Analysis of guidances' characteristics
The countries' distribution concerning SDM was erratic. Most of the guidances were from Europe (41/102; %) or North America (40/102; %). Table 2 shows factors than may influence the SDM quality of the guidances. Two CPGs or CSs were from Africa or Oceania (2/102; %). Asia and South America had 12/102 (%) and 5/12 (%), respectively. The quality of SDM did not vary between continents (p = 0.233).
TABLE 2.
Factors that may influence the SDM quality and reporting of the CRC screening guidances
| Variable | Mean (items) | IQR range | p value |
|---|---|---|---|
| Type of document | |||
| CPGs | 1 | 0–2 | |
| CSs | 1 | 0–2 | p = 0.959 |
| Country | |||
| Europe | 0.5 | 0–1.5 | |
| North America | 1 | 0–3 | |
| Other countries | 1 | 0–1.5 | p = 0.233 |
| Publication year | |||
| Before or in 2015 | 0.5 | 0–1.5 | |
| After 2015 | 1 | 0–3 | p = 0.048 |
| Publication in a journal | |||
| Yes | 0.5 | 0–2 | |
| No | 1 | 0.3–3.5 | p = 0.131 |
| Version number | |||
| 1 | 0.8 | 0–2 | |
| 2 or more | 1 | 0–3 | p = 0.416 |
| Evidence analysis | |||
| Systematic review | 1 | 0–3 | p = 0.040 |
| Consensus or reviews | 0 | 0–2 | |
| Not reported | 0 | 0–1 | |
| Quality tool referral | |||
| Yes | 0.8 | 0–1.5 | |
| No | 1 | 0–2 | p = 0.902 |
| SDM specifically named | |||
| Yes | 4.5 | 2.5–4.5 | |
| No | 0.5 | 0–1.5 | p < 0.001 |
A greater tendency to introduce and recommend SDM was observed in the most recent guidances (Figure 3). The publication year after 2015 had an important influence on the quality than older publications (mean 1, IQR 0–3 vs. mean 0.5, IQR 0–1.5; p = 0.048). The publication in a journal (p = 0.131), and the version number (p = 0.416). The specific quality tool referral increased the quality and reporting of SDM on guidances (p < 0.001). CPGs following systematic reviews had better quality than consensus or literature reviews or when it was not reported (mean 1, IQR 0–3 vs. mean 0, IQR 0–2 vs. mean 0, IQR 0–1; p = 0.040).
FIGURE 3.

Appearance of SDM concerning the published year of the guidance document
3. DISCUSSION
3.1. Main findings
Our results showed that CPGs and CSs for SDM in CRC screening were of low quality, with variation between guidances and across domains. Recent guidances had better quality, but there is extensive room for improvement. CPGs based on systematic reviews scored better than CSs or guidances that did not report any of it for evidence analysis. Guidances that contained a description of the use of a specific quality tool such as AGREE II or RIGHT demonstrated higher quality.
3.2. Strengths and weaknesses
To the best of our knowledge, this systematic review is the first to investigate the quality of SDM in CRC screening guidances. One of the main strengths of our review was its comprehensive search based on a broad conceptual framework with no language barriers. SDM is a trendy term of relatively recent appearance. Most methodological recommendation manuals remark a 2‐ to 3‐year window for guidance renovation (Vernooij et al., 2014). However, for studying it, we included more than 10 years of published guidance documents to scrutinise the situation of SDM through time. Our study contained guidance documents of professional organisations from scientifically active nations with more than 0.5% of the global CRC research production.
A rigorous methodology in conducting systematic reviews is mandatory to guarantee reliable results. In this concern, the trustworthiness of the study selection and the data extraction process is critical. As in similar investigations that use this SDM instrument, there is a possibility of empirical limitations related to the subjectivity of quality data extraction. To minimise this inconvenience, four reviewers performed a preliminary meeting to explain and understand the tool where doubts were solved; and three reviewers worked independently and in duplicate, with double checks included throughout the work. Arbitration was accomplished by a fourth experienced reviewer and creator of the SDM instrument used. The reviewer agreement was good (ICC = 0.88), implying reliable results (Appendix S5).
Our systematic review aimed to study the quality of SDM. We are conscious that ‘not all the items can have the same relevance and weight’ (Maes‐Carballo, Munoz‐Nunez, et al., 2020). This procedure involving a quality assessment instrument specifies if SDM was cited and which aspects were often considered. Further studies should focus on rating quality.
3.3. Implications
CRC early diagnosis could decrease morbimortality by discovering less invasive lesions and permitting more efficient treatments. Furthermore, the debate about the effectiveness and overtreatment due to false‐positive results has appeared on the scene. CRC screening is costly and annoying and could increase the risk of false positives or negatives, which may incur unnecessary stress or procedures and a false sense of security. The mortality reduction is not statistically significant at all ages, and the benefit vs. harm balance is unknown. So, screening should be tailored to the characteristics (age, genetic factors, race, etc.), desires and values of women. Screening programmes are an excellent area for SDM practice as there are different options with similar benefits and harms, and option choice might depend on the patient's values and preferences (Wieringa et al., 2017). The practice of SDM by clinicians could support evidence‐based decisions (Heen et al., 2021) and increase patient satisfaction and treatment engagement (Baca‐Dietz et al., 2020).
Our systematic review showed that SDM had gained notoriety over the years, with an increasing tendency of SDM presence and recommendations related to them. However, it has also demonstrated that SDM advice merits improvement in all the areas but specifically urgently in the reporting of the SDM resource implications and conflict of interest and the explanation of monitoring and auditing SDM use. It is essential its presence in CPGs and CSs, which hold the potential to influence the care delivered by health‐care providers and the outcomes for patients. Guidances should provide clear and reasonable recommendations for SDM applicability (Rabi et al., 2020; Woolf et al., 1999). More efforts should be made in SDM (Keating & Pace, 2018), and future guidelines should play a more important role in SDM implementation (Gärtner et al., 2019).
SDM is a new trend, and recent guidances are starting to increase recommendations about basic concepts, evidence and applicability. However, it merits consideration to keep working in that direction. The evidence analysis showed that guidances underpinned by systematic reviews had better quality than consensus or not reported. The referral to SDM term in guidances has shown an improvement in SDM quality, which seems logical as normally improved precision and clearance of recommendations.
3.4. Conclusions
This systematic review demonstrated that SDM quality in guidance documents was suboptimal as it did not appear in half of the guidances analyzed. SDM recommendations were scarce and unclear. Recent guidances following systematic reviews and referring to quality tools (e.g. AGREE II or RIGHT) had better SDM quality. Therefore, guideline developers, professional institutions, medical journals and policymakers should consider including evidence‐based SDM recommendations in trustworthy and well‐developed guidances to ensure proper translation of evidence into practice.
CONFLICTS OF INTEREST
There are no conflicts of interest.
Supporting information
Appendix S0: PRISMA 2020 Checklist
Appendix S1: Data sources and search strategy.
Appendix S2: Quality assessment tool for Shared Decision Making (SDM) recommendations in Breast Cancer Management Clinical Practice Guidelines (CPG) and consensus (CS).
Appendix S3: Quality assessment tool for Shared Decision Making (SDM) recommendations in Breast Cancer Management Clinical Practice Guidelines (CPG) and consensus (CS).
Appendix S4: Data extraction analysis.
Appendix S5: The reviewer agreement calculated using the intra‐class correlation coefficient (corr).
ACKNOWLEDGEMENTS
KSK is a Distinguished Investigator funded by the Beatriz Galindo (senior modality) Programme grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Spanish Government. Funding for open access charge: Universidad de Granada / CBUA.
Maes‐Carballo, M. , García‐García, M. , Gómez‐Fandiño, Y. , Estrada‐López, C. R. , Iglesias‐Álvarez, A. , Bueno‐Cavanillas, A. , & Khan, K. S. (2022). Systematic review of shared decision‐making in guidelines about colorectal cancer screening. European Journal of Cancer Care, 31(6), e13738. 10.1111/ecc.13738
Funding information None declared.
DATA AVAILABILITY STATEMENT
All the supporting information can be accessed upon request via email to the corresponding authors of this review.
REFERENCES
- Alberta CC. Colorectal cancer screening. Clinical Practice Guideline|Nov 2013 (Revised 2020). 2020.
- Alsanea, N. , Almadi, M. A. , Abduljabbar, A. S. , Alhomoud, S. , Alshaban, T. A. , Alsuhaibani, A. , Alzahrani, A. , Batwa, F. , Hassan, A. H. , Hibbert, D. , Nooh, R. , Alothman, M. , Rochwerg, B. , Alhazzani, W. , & Morgan, R. L. (2015). National guidelines for colorectal cancer screening in Saudi Arabia with strength of recommendations and quality of evidence. Annals of Saudi Medicine, 35(3), 189–195. 10.5144/0256-4947.2015.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANM. Programa Nacional de Consensos Inter‐Sociedades . Programa Argentino de Consensos de Enfermedades Oncológicas. GUÍA DE RECOMENDACIONES PARA LA PREVENCIÓN Y DETECCIÓN PRECOZ DEL CÁNCER COLORRECTAL. Septiembre 2010. https://www.researchgate.net/publication/324877262_Programa_Nacional_de_Consensos_Inter-Sociedades_Programa_Argentino_de_Consensos_de_Enfermedades_Oncologicas_ALTO_RIESGO_PARA_CANCER_DE_MAMA_CONSENSO_NACIONAL_INTER-SOCIEDADES. 2010.
- Aranda, E. , Aparicio, J. , Alonso, V. , Garcia‐Albeniz, X. , Garcia‐Alfonso, P. , Salazar, R. , Valladares, M. , Vera, R. , Vieitez, J. M. , & Garcia‐Carbonero, R. (2015). SEOM clinical guidelines for diagnosis and treatment of metastatic colorectal cancer 2015. Clinical & Translational Oncology, 17(12), 972–981. 10.1007/s12094-015-1434-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda‐Hernandez, J. , Hwang, J. , & Kandel, G. (2016). Seeing better—Evidence based recommendations on optimizing colonoscopy adenoma detection rate. World Journal of Gastroenterology, 22(5), 1767–1778. 10.3748/wjg.v22.i5.1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asociación Mexicana de Endoscopia Gastrointestinal y Colegio de Profesionistas . Guías de prevención y manejo endoscópico del cáncer colorrectal. https://www.amegendoscopia.org.mx/index.php/component/phocadownload/category/1?Itemid=170&lang=es. 2016.
- Atkin, W. S. , Valori, R. , Kuipers, E. J. , Hoff, G. , Senore, C. , Segnan, N. , Jover, R. , Schmiegel, W. , Lambert, R. , Pox, C. , & International Agency for Research on Cancer . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Colonoscopic surveillance following adenoma removal. Endoscopy, 44, SE151–SE163. 10.1055/s-0032-1309821 [DOI] [PubMed] [Google Scholar]
- Austoker, J. , Giordano, L. , Hewitson, P. , Villain, P. , & International Agency for Research on C . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Communication. Endoscopy, 44, SE164–SE185. 10.1055/s-0032-1309809 [DOI] [PubMed] [Google Scholar]
- Baca‐Dietz, D. , Wojnar, D. M. , & Espina, C. R. (2020). The shared decision‐making model: Providers' and patients' knowledge and understanding in clinical practice. Journal of the American Association of Nurse Practitioners, 33, 529–536. 10.1097/JXX.0000000000000401 [DOI] [PubMed] [Google Scholar]
- Barry, M. J. , & Edgman‐Levitan, S. (2012). Shared decision making—The pinnacle of patient‐centered care. The New England Journal of Medicine, 366(9), 780–781. 10.1056/NEJMp1109283 [DOI] [PubMed] [Google Scholar]
- Basu, P. , Alhomoud, S. , Taghavi, K. , Carvalho, A. L. , Lucas, E. , & Baussano, I. (2021). Cancer screening in the coronavirus pandemic era: Adjusting to a new situation. JCO Global Oncology, 7, 416–424. 10.1200/GO.21.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton, S. , Steele, R. , Logan, R. , Djedovic, N. , Smith, S. , & Addison, C. (2016). NICE referral guidelines for suspected cancer: Colorectal cancer and faecal occult blood testing. Annals of Clinical Biochemistry, 53(Pt 1), 7–9. 10.1177/0004563215612507 [DOI] [PubMed] [Google Scholar]
- Bo In Lee, S. P. H. , Kim, S.‐E. , Kim, S. H. , Kim, H.‐S. , Hong, S. N. , Yang, D.‐H. , Shin, S. J. , Lee, S.‐H. , Kim, Y.‐H. , Park, D. I. , Kim, H. J. , Yang, S.‐K. , Kim, H. J. , & Jeon, H. J. (2012). 대장암 선별과 대장폴립 진단검사 가이드라인. (Korean guidelines for colorectal cancer screening and polyp detection) https://www.irjournal.org/upload/pdf/IR010-01-03.pdf. Intest Res, 10, 67–68. [Google Scholar]
- Brenner, A. T. , Dougherty, M. , & Reuland, D. S. (2017). Colorectal cancer screening in average risk patients. The Medical Clinics of North America, 101(4), 755–767. 10.1016/j.mcna.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers, M. C. , de Vito, C. , Bahirathan, L. , Carol, A. , Carroll, J. C. , Cotterchio, M. , Dobbins, M. , Lent, B. , Levitt, C. , Lewis, N. , McGregor, S. E. , Paszat, L. , Rand, C. , & Wathen, N. (2011). Effective interventions to facilitate the uptake of breast, cervical and colorectal cancer screening: An implementation guideline. Implementation Science, 6, 112. 10.1186/1748-5908-6-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian task Force on preventive health C . (2016). Recommendations on screening for colorectal cancer in primary care. CMAJ, 188(5), 340–348. 10.1503/cmaj.151125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, W. T. , & Feuerstein, J. D. (2019). Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World Journal of Gastroenterology, 25(30), 4148–4157. 10.3748/wjg.v25.i30.4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton, S. , Sharp, L. , & Little, J. (1996). The adenoma‐carcinoma sequence and prospects for the prevention of colorectal neoplasia. Critical Reviews in Oncogenesis, 7(5–6), 293–342. 10.1615/CritRevOncog.v7.i5-6.10 [DOI] [PubMed] [Google Scholar]
- Cubiella, J. , Marzo‐Castillejo, M. , Mascort‐Roca, J. J. , Amador‐Romero, F. J. , Bellas‐Beceiro, B. , Clofent‐Vilaplana, J. , Carballal, S. , Ferrándiz‐Santos, J. , Gimeno‐García, A. Z. , Jover, R. , Mangas‐Sanjuán, C. , Moreira, L. , Pellisè, M. , Quintero, E. , Rodríguez‐Camacho, E. , Vega‐Villaamil, P. , & Sociedad Española de Medicina de Familia y Comunitaria y Asociación Española de Gastroenterología . (2018). Clinical practice guideline. Diagnosis and prevention of colorectal cancer. 2018 update. Gastroenterología y Hepatología, 41(9), 585–596. 10.1016/j.gastrohep.2018.07.012 [DOI] [PubMed] [Google Scholar]
- Day, L. W. , Walter, L. C. , & Velayos, F. (2011). Colorectal cancer screening and surveillance in the elderly patient. The American Journal of Gastroenterology, 106(7), 1197–1206. 10.1038/ajg.2011.128 [DOI] [PubMed] [Google Scholar]
- Del Giudice, M. E. , Vella, E. T. , Hey, A. , Simunovic, M. , Harris, W. , & Levitt, C. (2014). Guideline for referral of patients with suspected colorectal cancer by family physicians and other primary care providers. Canadian Family Physician, 60(8), e405–e415. [PMC free article] [PubMed] [Google Scholar]
- Duffy, M. J. , Lamerz, R. , Haglund, C. , Nicolini, A. , Kalousová, M. , Holubec, L. , & Sturgeon, C. (2014). Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. International Journal of Cancer, 134(11), 2513–2522. 10.1002/ijc.28384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn, G. , Frosch, D. , Thomson, R. , Joseph‐Williams, N. , Lloyd, A. , Kinnersley, P. , Cording, E. , Tomson, D. , Dodd, C. , Rollnick, S. , Edwards, A. , & Barry, M. (2012). Shared decision making: A model for clinical practice. Journal of General Internal Medicine, 27(10), 1361–1367. 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn, G. , Laitner, S. , Coulter, A. , Walker, E. , Watson, P. , & Thomson, R. (2010). Implementing shared decision making in the NHS. BMJ, 341, c5146. 10.1136/bmj.c5146 [DOI] [PubMed] [Google Scholar]
- European Colorectal Cancer Screening Guidelines Working Group , von Karsa, L. , Patnick, J. , Segnan, N. , Atkin, W. , Halloran, S. , Lansdorp‐Vogelaar, I. , Malila, N. , Minozzi, S. , Moss, S. , Quirke, P. , Steele, R. J. , Vieth, M. , Aabakken, L. , Altenhofen, L. , Ancelle‐Park, R. , Antoljak, N. , Anttila, A. , Armaroli, P. , … Valori, R. (2013). European guidelines for quality assurance in colorectal cancer screening and diagnosis: Overview and introduction to the full supplement publication. Endoscopy, 45(1), 51–59. 10.1055/s-0032-1325997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission . European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition. https://op.europa.eu/es/publication-detail/-/publication/e1ef52d8-8786-4ac4-9f91-4da2261ee535. 2010.
- Fabio Leonel Gil Parada FLTAM, Riveros Santoya, S. V. , Castaño Llano, R. , Ibáñez, H. , Huertas Quintero, M. M. , Carmona, R. , Pardo, R. , Otero, W. , & Sabbagh, L. Guía de práctica clínica para la tamización de cáncer colorrectal. http://www.scielo.org.co/pdf/rcg/v30s1/v30s1a08.pdf. Asociaciones Colombianas de Gastroenterología, Endoscopia Digestiva, Coloproctología y Hepatología. 2015.
- García‐Carbonero, R. , Vera, R. , Rivera, F. , Parlorio, E. , Pagés, M. , González‐Flores, E. , Fernández‐Martos, C. , Corral, M. Á. , Bouzas, R. , & Matute, F. (2017). SEOM/SERAM consensus statement on radiological diagnosis, response assessment and follow‐up in colorectal cancer. Clinical & Translational Oncology, 19(2), 135–148. 10.1007/s12094-016-1518-9 [DOI] [PubMed] [Google Scholar]
- Gärtner, F. R. , Portielje, J. E. , Langendam, M. , Hairwassers, D. , Agoritsas, T. , Gijsen, B. , Liefers, G. J. , Pieterse, A. H. , & Stiggelbout, A. M. (2019). Role of patient preferences in clinical practice guidelines: A multiple methods study using guidelines from oncology as a case. BMJ Open, 9(12), e032483. 10.1136/bmjopen-2019-032483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello, F. M. , Allen, J. I. , Axilbund, J. E. , Boland, C. R. , Burke, C. A. , Burt, R. W. , Church, J. M. , Dominitz, J. A. , Johnson, D. A. , Kaltenbach, T. , Levin, T. R. , Lieberman, D. A. , Robertson, D. J. , Syngal, S. , & Rex, D. K. (2014). Guidelines on genetic evaluation and management of Lynch syndrome: A consensus statement by the US multi‐society task force on colorectal cancer. The American Journal of Gastroenterology, 109(8), 1159–1179. 10.1038/ajg.2014.186 [DOI] [PubMed] [Google Scholar]
- Gupta, N. , Kupfer, S. S. , & Davis, A. M. (2019). Colorectal cancer screening. Journal of the American Medical Association, 321(20), 2022–2023. 10.1001/jama.2019.4842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiliadis, D. , Khoruts, A. , Zauber, A. G. , Hempstead, S. E. , Maisonneuve, P. , Lowenfels, A. B. , Braid, A. L. , Cullina, J. , Daggett, A. , Fink, A. , Gini, A. , Hadjiliadis, D. , Harron, P. F. Jr. , Hempstead, S. , Khoruts, A. , Lansdorp‐Vogelaar, I. , Lieberman, D. , Liou, T. , Lomas, P. , … Sabadosa, K. (2018). Cystic fibrosis colorectal cancer screening consensus recommendations. Gastroenterology, 154(3), 736–45.e14. 10.1053/j.gastro.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran, S. P. , Launoy, G. , Zappa, M. , & International Agency for Research on C . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Faecal occult blood testing. Endoscopy, 44, SE65–SE87. 10.1055/s-0032-1309791 [DOI] [PubMed] [Google Scholar]
- Hassan, C. , Quintero, E. , Dumonceau, J. M. , Regula, J. , Brandão, C. , Chaussade, S. , Dekker, E. , Dinis‐Ribeiro, M. , Ferlitsch, M. , Gimeno‐García, A. , Hazewinkel, Y. , Jover, R. , Kalager, M. , Loberg, M. , Pox, C. , Rembacken, B. , Lieberman, D. , & European Society of Gastrointestinal Endoscopy . (2013). Post‐polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy, 45(10), 842–851. 10.1055/s-0033-1344548 [DOI] [PubMed] [Google Scholar]
- Heen, A. F. , Vandvik, P. O. , Brandt, L. , Montori, V. M. , Lytvyn, L. , Guyatt, G. , Quinlan, C. , & Agoritsas, T. (2021). A framework for practical issues was developed to inform shared decision‐making tools and clinical guidelines. Journal of Clinical Epidemiology, 129, 104–113. 10.1016/j.jclinepi.2020.10.002 [DOI] [PubMed] [Google Scholar]
- Helsingen, L. M. , Vandvik, P. O. , Jodal, H. C. , Agoritsas, T. , Lytvyn, L. , Anderson, J. C. , Auer, R. , Murphy, S. B. , Almadi, M. A. , Corley, D. A. , Quinlan, C. , Fuchs, J. M. , McKinnon, A. , Qaseem, A. , Heen, A. F. , Siemieniuk, R. A. C. , Kalager, M. , Usher‐Smith, J. A. , Lansdorp‐Vogelaar, I. , … Guyatt, G. (2019). Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A clinical practice guideline. BMJ, 367, l5515. 10.1136/bmj.l5515 [DOI] [PubMed] [Google Scholar]
- Heresbach, D. P. P. , Chaussade, S. , Barthet, M. , Bories, E. , Canard, J. M. , Cellier, C. , Dalbies, P. , Hochberger, J. , Joly, I. , Koch, S. , Lapuelle, J. , Lecomte, T. , Lefort, C. , Lesur, G. , Letard, J. C. , Palazzo, L. , Ponchon, T. , Syschenko, R. , Tarrerias, A. R. , … Bulois, P. (2016). Prévention du cancer colorectal par coloscopie, en dehors du dépistage en population. Consensus et position de la SFED. Acta Endosc, 46, 68–73. https://acen.revuesonline.com/articles/lvacen/abs/2016/01/101900068/101900068.html, 10.1007/s10190-016-0534-5 [DOI] [Google Scholar]
- Hoffmann, S. , Crispin, A. , Lindoerfer, D. , Sroczynski, G. , Siebert, U. , Mansmann, U. , & Consortium, F. A. R. K. O. R. (2020). Evaluating the effects of a risk‐adapted screening program for familial colorectal cancer in individuals between 25 and 50 years of age: Study protocol for the prospective population‐based intervention study FARKOR. BMC Gastroenterology, 20(1), 131. 10.1186/s12876-020-01247-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospital provincial Neuquén . Guía de Práctica Clínica. Detección temprana de Cáncer Colorectal en población adulta. https://www.hospitalneuquen.org.ar/wp-content/uploads/2016/11/Guia-Practica-Clinica-Cancer-colorrectal-2016.pdf. 2016. 2016.
- Hyer, W. , Cohen, S. , Attard, T. , Vila‐Miravet, V. , Pienar, C. , Auth, M. , Septer, S. , Hawkins, J. , Durno, C. , & Latchford, A. (2019). Management of familial adenomatous polyposis in children and adolescents: Position paper from the ESPGHAN polyposis working group. Journal of Pediatric Gastroenterology and Nutrition, 68(3), 428–441. 10.1097/MPG.0000000000002247 [DOI] [PubMed] [Google Scholar]
- Instituto Mexicano del Seguro Social. Guía de Práctica Clínica . Detección Oportuna y Diagnóstico de Cáncer de Colon y Recto no Hereditario en Adultos en Primero, Segundo y Tercer Nivel de Atención. imss.gob.mx/sites/all/statics/guiasclinicas/145GER.pdf. 2010.
- Instituto Nacional del Cáncer . Argentina. GUÍA PARA EQUIPOS DE ATENCIÓN PRIMARIA DE LA SALUD. Información para la prevención y detección temprana del cáncer colorrectal. https://sage.org.ar/wp-content/uploads/2019/05/PDF-guia-INC-CCR.pdf. 2015.
- Jenkins, M. A. , Ait Ouakrim, D. , Boussioutas, A. , Hopper, J. L. , Ee, H. C. , Emery, J. D. , Macrae, F. A. , Chetcuti, A. , Wuellner, L. , & St John, D. J. B. (2018). Revised Australian national guidelines for colorectal cancer screening: Family history. The Medical Journal of Australia, 209(10), 455–460. 10.5694/mja18.00142 [DOI] [PubMed] [Google Scholar]
- Jenkinson, F. , & Steele, R. J. (2010). Colorectal cancer screening—Methodology. The Surgeon, 8(3), 164–171. 10.1016/j.surge.2009.10.015 [DOI] [PubMed] [Google Scholar]
- Johnson, D. A. , Barkun, A. N. , Cohen, L. B. , Dominitz, J. A. , Kaltenbach, T. , Martel, M. , Robertson, D. J. , Boland, C. R. , Giardello, F. M. , Lieberman, D. A. , Levin, T. R. , Rex, D. K. , & US Multi‐Society Task Force on Colorectal Cancer . (2014). Optimizing adequacy of bowel cleansing for colonoscopy: Recommendations from the US multi‐society task force on colorectal cancer. Gastroenterology, 147(4), 903–924. 10.1053/j.gastro.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Jover, R. , Herráiz, M. , Alarcón, O. , Brullet, E. , Bujanda, L. , Bustamante, M. , Campo, R. , Carreño, R. , Castells, A. , Cubiella, J. , García‐Iglesias, P. , Hervás, A. , Menchén, P. , Ono, A. , Panadés, A. , Parra‐Blanco, A. , Pellisé, M. , Ponce, M. , Quintero, E. , … Vázquez Sequeiros, E. (2012). Clinical practice guidelines: Quality of colonoscopy in colorectal cancer screening. Endoscopy, 44(4), 444–451. 10.1055/s-0032-1306690 [DOI] [PubMed] [Google Scholar]
- Keating, N. L. , & Pace, L. E. (2018). Breast Cancer Screening in 2018. Time for shared decision making. JAMA Insights., 319(17), 1814–1815. [DOI] [PubMed] [Google Scholar]
- Koo, T. K. , & Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaan, M. R. , & Jones‐Webb, R. (2018). Colorectal cancer screening in black men: Recommendations for best practices. American Journal of Preventive Medicine, 55(5), S95–S102. 10.1016/j.amepre.2018.05.008 [DOI] [PubMed] [Google Scholar]
- Lam, T. H. , Wong, K. H. , Chan, K. K. , Chan, M. C. , Chao, D. V. , Cheung, A. N. , Fan, C. Y. , Ho, J. , Hui, E. P. , Lam, K. O. , Law, C. K. , Law, W. L. , Loong, H. H. , Ngan, R. K. , Tsang, T. H. , Wong, M. C. , Yeung, R. M. , Ying, A. C. , & Ching, R. (2018). Recommendations on prevention and screening for colorectal cancer in Hong Kong. Hong Kong Medical Journal, 24(5), 521–526. 10.12809/hkmj177095 [DOI] [PubMed] [Google Scholar]
- Lansdorp‐Vogelaar, I. , von Karsa, L. , & International Agency for Research on C . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Introduction. Endoscopy, 44, SE15–SE30. [DOI] [PubMed] [Google Scholar]
- Leddin, D. , Enns, R. , Hilsden, R. , Fallone, C. A. , Rabeneck, L. , Sadowski, D. C. , Singh, H. , & Canadian Association of Gastroenterology . (2013). Colorectal cancer surveillance after index colonoscopy: Guidance from the Canadian Association of Gastroenterology. Canadian Journal of Gastroenterology, 27(4), 224–228. 10.1155/2013/232769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddin, D. , Lieberman, D. A. , Tse, F. , Barkun, A. N. , Abou‐Setta, A. M. , Marshall, J. K. , Samadder, N. J. , Singh, H. , Telford, J. J. , Tinmouth, J. , Wilkinson, A. N. , & Leontiadis, G. I. (2018). Clinical practice guideline on screening for colorectal cancer in individuals with a family history of nonhereditary colorectal cancer or adenoma: The Canadian Association of Gastroenterology Banff consensus. Gastroenterology, 155(5), 1325–47.e3. 10.1053/j.gastro.2018.08.017 [DOI] [PubMed] [Google Scholar]
- Leddin, D. J. , Enns, R. , Hilsden, R. , Plourde, V. , Rabeneck, L. , Sadowski, D. C. , & Singh, H. (2010). Canadian Association of Gastroenterology position statement on screening individuals at average risk for developing colorectal cancer: 2010. Canadian Journal of Gastroenterology, 24(12), 705–714. 10.1155/2010/683171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B. I. , Hong, S. P. , Kim, S. E. , Kim, S. H. , Kim, H. S. , Hong, S. N. , Yang, D. H. , Shin, S. J. , Lee, S. H. , Park, D. I. , Kim, Y. H. , Kim, H. J. , Yang, S. K. , Kim, H. J. , Jeon, H. J. , & Multi‐Society Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management . (2012). Korean guidelines for colorectal cancer screening and polyp detection. Clinical Endoscopy, 45(1), 25–43. 10.5946/ce.2012.45.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong, K. , Hartley, J. , & Karandikar, S. (2017). Association of coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the management of cancer of the colon, rectum and anus (2017)—Follow up, Lifestyle and Survivorship. Colorectal Disease, 19(Suppl 1), 67–70. 10.1111/codi.13706 [DOI] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gøtzsche, P. C. , Ioannidis, J. P. , Clarke, M. , Devereaux, P. J. , Kleijnen, J. , & Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine, 151(4), W65–W94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- Lieberman, D. (2012). Colorectal cancer screening: Practice guidelines. Digestive Diseases, 30(Suppl 2), 34–38. 10.1159/000341891 [DOI] [PubMed] [Google Scholar]
- Lieberman, D. A. , Rex, D. K. , Winawer, S. J. , Giardiello, F. M. , Johnson, D. A. , & Levin, T. R. (2012). Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US multi‐society task force on colorectal cancer. Gastroenterology, 143(3), 844–857. 10.1053/j.gastro.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Lopes, G. , Stern, M. C. , Temin, S. , Sharara, A. I. , Cervantes, A. , Costas‐Chavarri, A. , Engineer, R. , Hamashima, C. , Ho, G. F. , Huitzil, F. D. , & Moghani, M. M. (2018). Early detection for colorectal cancer: ASCO resource‐stratified guideline. Journal of global oncology, 5, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes‐Carballo, M. , Mignini, L. , Martin‐Diaz, M. , Bueno‐Cavanillas, A. , & Khan, K. S. (2020). Quality and reporting of clinical guidelines for breast cancer treatment: A systematic review. Breast, 53, 201–211. 10.1016/j.breast.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes‐Carballo, M. , Mignini, L. , Martin‐Diaz, M. , Bueno‐Cavanillas, A. , & Khan, K. S. (2022). Clinical practice guidelines and consensus for the screening of breast cancer: A systematic appraisal of their quality and reporting. European Journal of Cancer Care, 31, e13540. 10.1111/ecc.13540 [DOI] [PubMed] [Google Scholar]
- Maes‐Carballo, M. , Moreno‐Asencio, T. , Martin‐Diaz, M. , Mignini, L. , Bueno‐Cavanillas, A. , & Khan, K. S. (2021). Shared decision making in breast cancer screening guidelines: A systematic review of their quality and reporting. European Journal of Public Health, 31, 873–883. 10.1093/eurpub/ckab084 [DOI] [PubMed] [Google Scholar]
- Maes‐Carballo, M. , Munoz‐Nunez, I. , Martin‐Diaz, M. , Mignini, L. , Bueno‐Cavanillas, A. , & Khan, K. S. (2020). Shared decision making in breast cancer treatment guidelines: Development of a quality assessment tool and a systematic review. Health Expectations, 23, 1045–1064. 10.1111/hex.13112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malila, N. , Senore, C. , Armaroli, P. , & International Agency for Research on C . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Organisation. Endoscopy, 44, SE31–SE48. 10.1055/s-0032-1309783 [DOI] [PubMed] [Google Scholar]
- Ministry of Health . Singapore. Cancer screening. https://www.moh.gov.sg/docs/librariesprovider4/guidelines/cpg_cancer-screening.pdf. 2010.
- Ministry of Health . Turkey. Turkey Cancer Control Programme. https://www.iccp-portal.org/system/files/plans/Turkiye_Kanser_Kontrol_Program_English.pdf. 2016.
- Minozzi, S. , Armaroli, P. , & Segnan, N. (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Principles of evidence assessment and methods for reaching recommendations. Endoscopy, 44, SE9–S14. 10.1055/s-0032-1309781 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & Group P . (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Open Med., 3(3), e123–e130. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan, K. J. , Bradshaw, N. , Dolwani, S. , Desouza, B. , Dunlop, M. G. , East, J. E. , Ilyas, M. , Kaur, A. , Lalloo, F. , Latchford, A. , Rutter, M. D. , Tomlinson, I. , Thomas, H. J. W. , Hill, J. , & Hereditary CRC guidelines eDelphi consensus group . (2019). Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom cancer genetics group (UKCGG). Gut, 69(3), 411–444. 10.1136/gutjnl-2019-319915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, C. , Kim, D. H. , Bartel, T. B. , Cash, B. D. , Chang, K. J. , Feig, B. W. , Fowler, K. J. , Garcia, E. M. , Kambadakone, A. R. , Lambert, D. L. , Levy, A. D. , Marin, D. , Peterson, C. M. , Scheirey, C. D. , Smith, M. P. , Weinstein, S. , & Carucci, L. R. (2018). ACR appropriateness criteria([R]) colorectal cancer screening. Journal of the American College of Radiology, 15(5S), S56–S68. 10.1016/j.jacr.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Moss, S. , Ancelle‐Park, R. , Brenner, H. , & International Agency for Research on C . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Evaluation and interpretation of screening outcomes. Endoscopy, 44, SE49–SE64. 10.1055/s-0032-1309788 [DOI] [PubMed] [Google Scholar]
- Network NCC . NCCN Guidelines: Colorectal Cancer Screening Version 2.2021. https://www.nccn.org/. 2021.
- New Brunswick Colon Cancer Screening . Clinical Practice Guidelines. https://www2.gnb.ca/content/gnb/en/departments/health/NewBrunswickCancerNetwork/content/ClinicalPracticeGuidelinesForColonCancerScreeningInNewBrunswick.html. 2013.
- NHS . NHS. Bowel cancer screening: Pathology guidance on reporting lesions. https://www.gov.uk/government/publications/bowel-cancer-screening-reporting-lesions. 2021.
- Ong, J. J. , Chen, M. , Grulich, A. E. , & Fairley, C. K. (2014). Regional and national guideline recommendations for digital ano‐rectal examination as a means for anal cancer screening in HIV positive men who have sex with men: A systematic review. BMC Cancer, 14, 557. 10.1186/1471-2407-14-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzale, D. , Gupta, S. , Ahnen, D. J. , Bray, T. , Cannon, J. A. , Cooper, G. , David, D. S. , Early, D. S. , Erwin, D. , Ford, J. M. , Giardiello, F. M. , Grady, W. , Halverson, A. L. , Hamilton, S. R. , Hampel, H. , Ismail, M. K. , Klapman, J. B. , Larson, D. W. , Lazenby, A. J. , … Darlow, S. (2016). Genetic/familial high‐risk assessment: Colorectal version 1.2016, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network, 14(8), 1010–1030. 10.6004/jnccn.2016.0108 [DOI] [PubMed] [Google Scholar]
- Qaseem, A. , Crandall, C. J. , Mustafa, R. A. , Hicks, L. A. , Clinical Guidelines Committee of the American College of Physicians , Forciea, M. A. , Fitterman, N. , Horwitch, C. A. , Kansagara, D. , Maroto, M. , McLean, R. , Roa, J. , & Tufte, J. (2019). Screening for colorectal cancer in asymptomatic average‐risk adults: A guidance statement from the American College of Physicians. Annals of Internal Medicine, 171(9), 643–654. 10.7326/M19-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirke, P. , Risio, M. , Lambert, R. , von Karsa, L. , & Vieth, M. (2011). Quality assurance in pathology in colorectal cancer screening and diagnosis‐European recommendations. Virchows Archiv, 458(1), 1–19. 10.1007/s00428-010-0977-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirke, P. , Risio, M. , Lambert, R. , von Karsa, L. , Vieth, M. , & International Agency for Research on C . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Quality assurance in pathology in colorectal cancer screening and diagnosis. Endoscopy, 44, SE116–SE130. 10.1055/s-0032-1309797 [DOI] [PubMed] [Google Scholar]
- Rabi, D. M. , Kunneman, M. , & Montori, V. M. (2020). When guidelines recommend shared decision‐making. Journal of the American Medical Association, 323(14), 1345–1346. 10.1001/jama.2020.1525 [DOI] [PubMed] [Google Scholar]
- Recommended Cancer Screenings . https://www.swedish.org/~/media/images/swedish/pdf/recommended%20cancer%20screenings%20pdf.pdf. 2013.
- Regula, J. , & Kaminski, M. F. (2010). Targeting risk groups for screening. Best Practice & Research. Clinical Gastroenterology, 24(4), 407–416. 10.1016/j.bpg.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Rembacken, B. , Hassan, C. , Riemann, J. F. , Chilton, A. , Rutter, M. , Dumonceau, J. M. , Omar, M. , & Ponchon, T. (2012). Quality in screening colonoscopy: Position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy, 44(10), 957–968. 10.1055/s-0032-1325686 [DOI] [PubMed] [Google Scholar]
- Rex, D. K. , Boland, C. R. , Dominitz, J. A. , Giardiello, F. M. , Johnson, D. A. , Kaltenbach, T. , Levin, T. R. , Lieberman, D. , & Robertson, D. J. (2017). Colorectal cancer screening: Recommendations for physicians and patients from the U.S. multi‐society task force on colorectal cancer. The American Journal of Gastroenterology, 112(7), 1016–1030. 10.1038/ajg.2017.174 [DOI] [PubMed] [Google Scholar]
- Robertson, D. J. , Lee, J. K. , Boland, C. R. , Dominitz, J. A. , Giardiello, F. M. , Johnson, D. A. , Kaltenbach, T. , Lieberman, D. , Levin, T. R. , & Rex, D. K. (2017). Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: A consensus statement by the US multi‐society task force on colorectal cancer. Gastroenterology, 152(5), 1217–37 e3. 10.1053/j.gastro.2016.08.053 [DOI] [PubMed] [Google Scholar]
- Rubeca, T. , Rapi, S. , Deandrea, S. , Malaspina, M. , Passamonti, B. U. , Grassi, E. , Cioccarelli, A. M. , Marchetti, E. , Boni, M. , Cellai, F. , & Pradella, M. (2017). Guidance for faecal occult blood testing: Quantitative immunochemical method (FIT‐HB) in colorectal cancer screening programmes. Epidemiologia e Prevenzione, 41(5–6), 1–31. [DOI] [PubMed] [Google Scholar]
- Salzman, B. , Beldowski, K. , & de la Paz, A. (2016). Cancer screening in older patients. American Family Physician, 93(8), 659–667. [PubMed] [Google Scholar]
- Schmiegel, W. , Pox, C. , Reinacher‐Schick, A. , Adler, G. , Arnold, D. , Fleig, W. , Fölsch, U. , Frühmorgen, P. , Graeven, U. , Heinemann, V. , Hohenberger, W. , Holstege, A. , Junginger, T. , Kopp, I. , Kühlbacher, T. , Porschen, R. , Propping, P. , Riemann, J. F. , Rödel, C. , … Selbmann, H. K. (2010). S3 guidelines for colorectal carcinoma: Results of an evidence‐based consensus conference on February 6/7, 2004 and June 8/9, 2007 (for the topics IV, VI and VII). Zeitschrift für Gastroenterologie, 48(1), 65–136. 10.1055/s-0028-1109936 [DOI] [PubMed] [Google Scholar]
- Schmoll, H. J. , van Cutsem, E. , Stein, A. , Valentini, V. , Glimelius, B. , Haustermans, K. , Nordlinger, B. , van de Velde, C. J. , Balmana, J. , Regula, J. , Nagtegaal, I. D. , Beets‐Tan, R. G. , Arnold, D. , Ciardiello, F. , Hoff, P. , Kerr, D. , Köhne, C. H. , Labianca, R. , Price, T. , … Cervantes, A. (2012). ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Annals of Oncology, 23(10), 2479–2516. 10.1093/annonc/mds236 [DOI] [PubMed] [Google Scholar]
- Schrager, S. P. G. , & Burnside, E. (2017). Shared decision making in cancer screening. Family Practice Management, 24(3), 5–10. [PMC free article] [PubMed] [Google Scholar]
- SemFYC AEdG . Diagnóstico y prevención del cáncer colorrectal. ACTUALIZACIÓN 2018. 2018.
- Senate and house of representatives . Patient protection and affordable care act. HR 3590. Washington: 2010. 2010.
- Seppälä, T. T. , Latchford, A. , Negoi, I. , Sampaio Soares, A. , Jimenez‐Rodriguez, R. , Sánchez‐Guillén, L. , Evans, D. G. , Ryan, N. , Crosbie, E. J. , Dominguez‐Valentin, M. , Burn, J. , Kloor, M. , Knebel Doeberitz, M. , Duijnhoven, F. J. B. , Quirke, P. , Sampson, J. R. , Møller, P. , Möslein, G. , & the European Hereditary Tumour Group (EHTG) and European Society of Coloproctology (ESCP) . (2021). European guidelines from the EHTG and ESCP for Lynch syndrome: An updated third edition of the Mallorca guidelines based on gene and gender. The British Journal of Surgery, 108(5), 484–498. 10.1002/bjs.11902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaukat, A. , Kahi, C. J. , Burke, C. A. , Rabeneck, L. , Sauer, B. G. , & Rex, D. K. (2021). ACG clinical guidelines: Colorectal cancer screening 2021. The American Journal of Gastroenterology, 116(3), 458–479. 10.14309/ajg.0000000000001122 [DOI] [PubMed] [Google Scholar]
- Society AC . America Cancer Society. Detección temprana, diagnóstico y clasificación por etapas. https://www.cancer.org/es/cancer/cancer-de-pulmon/deteccion-diagnostico-clasificacion-por-etapas.html. 2018.
- Sollano, J. D. , Lontok, M. A. , Lusong, M. A. , Romano, R. P. , Macatula, T. C. , Payawal, D. A. , Bocobo, J. C. , Dy, F. T. , Bondoc, E. M. , Olympia, E. G. , Ignacio, J. G. , Agas, F. C. , Naval, M. C. , Ong, E. G. , Co, A. L. , Moscoso, B. A. , Pangilinan, J. A. N. , Cloa, M. M. S. , Galang, J. A. G. , … Mina, Y. L. (2017). The Joint Philippine Society of Gastroenterology (PSG) and Philippine Society of Digestive Endoscopy (PSDE) consensus guidelines on the management of colorectal carcinoma. Philippine Journal of Internal Medicine, 55(1), 1–16. https://psde.org.ph/wp-content/uploads/2022/01/The_Joint_Philippine_Society_of_Gastroenterology_and_Philippine_Society_of_Digestive_Endoscopy_Consensus_Guidelines.pdf [Google Scholar]
- Spada, C. , Stoker, J. , Alarcon, O. , Barbaro, F. , Bellini, D. , Bretthauer, M. , de Haan, M. C. , Dumonceau, J. M. , Ferlitsch, M. , Halligan, S. , Helbren, E. , Hellstrom, M. , Kuipers, E. J. , Lefere, P. , Mang, T. , Neri, E. , Petruzziello, L. , Plumb, A. , Regge, D. , … European Society of Gastrointestinal and Abdominal Radiology . (2014). Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) guideline. Endoscopy, 46(10), 897–915. 10.1055/s-0034-1378092 [DOI] [PubMed] [Google Scholar]
- Steele, R. J. , Pox, C. , Kuipers, E. J. , Minoli, G. , Lambert, R. , & International Agency for Research on C . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Management of lesions detected in colorectal cancer screening. Endoscopy, 44, SE140–SE150. 10.1055/s-0032-1309802 [DOI] [PubMed] [Google Scholar]
- Steele, R. J. , Rey, J. F. , Lambert, R. , & International Agency for Research on C . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Management of lesions detected in colorectal cancer screening. Endoscopy, 44, SE140–SE150. 10.1055/s-0032-1309802 [DOI] [PubMed] [Google Scholar]
- Steinwachs, D. , Allen, J. D. , Barlow, W. E. , Duncan, R. P. , Egede, L. E. , Friedman, L. S. , Keating, N. L. , Kim, P. , Lave, J. R. , LaVeist, T. , Ness, R. B. , Optican, R. J. , & Virnig, B. A. (2010). NIH state‐of‐the‐science conference statement: Enhancing use and quality of colorectal cancer screening. NIH Consensus and State‐of‐the‐Science Statements, 27(1), 1–31. [PubMed] [Google Scholar]
- Stoffel, E. M. , Mangu, P. B. , Limburg, P. J. , American Society of Clinical Oncology , & European Society for Medical Oncology . (2015). Hereditary colorectal cancer syndromes: American Society of Clinical Oncology clinical practice guideline endorsement of the familial risk‐colorectal cancer: European Society for Medical Oncology clinical practice guidelines. Journal of Oncology Practice/ American Society of Clinical Oncology, 11(3), e437–e441. 10.1200/JOP.2015.003665 [DOI] [PubMed] [Google Scholar]
- Sung, J. J. , Ng, S. C. , Chan, F. K. , Chiu, H. M. , Kim, H. S. , Matsuda, T. , Ng, S. S. , Lau, J. Y. , Zheng, S. , Adler, S. , Reddy, N. , Yeoh, K. G. , Tsoi, K. K. , Ching, J. Y. , Kuipers, E. J. , Rabeneck, L. , Young, G. P. , Steele, R. J. , Lieberman, D. , … Asia Pacific Working Group . (2015). An updated Asia Pacific consensus recommendations on colorectal cancer screening. Gut, 64(1), 121–132. 10.1136/gutjnl-2013-306503 [DOI] [PubMed] [Google Scholar]
- Tanaka, S. , Saitoh, Y. , Matsuda, T. , Igarashi, M. , Matsumoto, T. , Iwao, Y. , Suzuki, Y. , Nishida, H. , Watanabe, T. , Sugai, T. , Sugihara, K. , Tsuruta, O. , Hirata, I. , Hiwatashi, N. , Saito, H. , Watanabe, M. , Sugano, K. , Shimosegawa, T. , & Japanese Society of Gastroenterology . (2015). Evidence‐based clinical practice guidelines for management of colorectal polyps. Journal of Gastroenterology, 50(3), 252–260. 10.1007/s00535-014-1021-4 [DOI] [PubMed] [Google Scholar]
- Telford, J. J. (2011). Canadian guidelines for colorectal cancer screening. Canadian Journal of Gastroenterology, 25(9), 479–481. 10.1155/2011/285926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinmouth, J. , Kennedy, E. B. , Baron, D. , Burke, M. , Feinberg, S. , Gould, M. , Baxter, N. , & Lewis, N. (2014). Colonoscopy quality assurance in Ontario: Systematic review and clinical practice guideline. Canadian Journal of Gastroenterology & Hepatology, 28(5), 251–274. 10.1155/2014/262816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruguay MdSd. Ministerio de Salud de Uruguay . Guía de práctica clínica de tamizaje del cáncer colo‐rectal 2018. https://www.paho.org/uru/dmdocuments/Guia%20de%20practica%20clinica%20de%20tamizaje%20del%20cancer%20colo‐rectal%202018.pdf. 2018.
- US Preventive Services Task Force , Davidson, K. W. , Barry, M. J. , Mangione, C. M. , Cabana, M. , Caughey, A. B. , Davis, E. M. , Donahue, K. E. , Doubeni, C. A. , Krist, A. H. , Kubik, M. , Li, L. , Ogedegbe, G. , Owens, D. K. , Pbert, L. , Silverstein, M. , Stevermer, J. , Tseng, C. W. , & Wong, J. B. (2021). Screening for colorectal cancer: US preventive services task force recommendation statement. Journal of the American Medical Association, 325(19), 1965–1977. 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- Valori, R. , Rey, J. F. , Atkin, W. S. , Bretthauer, M. , Senore, C. , Hoff, G. , Kuipers, E. , Altenhofen, L. , Lambert, R. , & Minoli, G. (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy, 44 Suppl 3, SE88–S105. 10.1055/s-0032-1309795 [DOI] [PubMed] [Google Scholar]
- Vasen, H. F. , Ghorbanoghli, Z. , Bourdeaut, F. , Cabaret, O. , Caron, O. , Duval, A. , Entz‐Werle, N. , Goldberg, Y. , Ilencikova, D. , Kratz, C. P. , Lavoine, N. , Loeffen, J. , Menko, F. H. , Muleris, M. , Sebille, G. , Colas, C. , Burkhardt, B. , Brugieres, L. , Wimmer, K. , & on behalf of the EU‐Consortium Care for CMMR‐D (C4CMMR‐D) . (2014). Guidelines for surveillance of individuals with constitutional mismatch repair‐deficiency proposed by the European Consortium “Care for CMMR‐D” (C4CMMR‐D). Journal of Medical Genetics, 51(5), 283–293. 10.1136/jmedgenet-2013-102238 [DOI] [PubMed] [Google Scholar]
- Vernooij, R. W. , Sanabria, A. J. , Sola, I. , Alonso‐Coello, P. , & Martinez, G. L. (2014). Guidance for updating clinical practice guidelines: A systematic review of methodological handbooks. Implementation Science, 9, 3. 10.1186/1748-5908-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth, M. , Quirke, P. , Lambert, R. , von Karsa, L. , Risio, M. , & International Agency for Research on C . (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Annotations of colorectal lesions. Endoscopy, 44 Suppl 3, SE131–SE139. 10.1055/s-0032-1309798 [DOI] [PubMed] [Google Scholar]
- von Karsa, L. , Patnick, J. , & Segnan, N. (2012). European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—Executive summary. Endoscopy, 44, SE1–SE8. 10.1055/s-0032-1309822 [DOI] [PubMed] [Google Scholar]
- Washington KFHPo . Kaiser Foundation Health Plan of Washington. Colorectal Cancer Screening Guideline. https://wa.kaiserpermanente.org/static/pdf/public/guidelines/colon.pdf. 2021.
- Wieringa, T. H. , Kunneman, M. , Rodriguez‐Gutierrez, R. , Montori, V. M. , de Wit, M. , Smets, E. M. A. , Schoonmade, L. J. , Spencer‐Bonilla, G. , & Snoek, F. J. (2017). A systematic review of decision aids that facilitate elements of shared decision‐making in chronic illnesses: A review protocol. Systematic Reviews, 6(1), 155. 10.1186/s13643-017-0557-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins, T. , McMechan, D. , & Talukder, A. (2018). Colorectal cancer screening and prevention. American Family Physician, 97(10), 658–665. [PubMed] [Google Scholar]
- Wilkinson, A. N. , Lieberman, D. , Leontiadis, G. I. , Tse, F. , Barkun, A. N. , Abou‐Setta, A. , Marshall, J. K. , Samadder, J. , Singh, H. , Telford, J. J. , Tinmouth, J. , & Leddin, D. (2019). Colorectal cancer screening for patients with a family history of colorectal cancer or adenomas. Canadian Family Physician, 65(11), 784–789. [PMC free article] [PubMed] [Google Scholar]
- Wolf, A. M. D. , Fontham, E. T. H. , Church, T. R. , Flowers, C. R. , Guerra, C. E. , LaMonte, S. J. , Etzioni, R. , McKenna, M. T. , Oeffinger, K. C. , Shih, Y. C. T. , Walter, L. C. , Andrews, K. S. , Brawley, O. W. , Brooks, D. , Fedewa, S. A. , Manassaram‐Baptiste, D. , Siegel, R. L. , Wender, R. C. , & Smith, R. A. (2018). Colorectal cancer screening for average‐risk adults: 2018 guideline update from the American Cancer Society. CA: a Cancer Journal for Clinicians, 68(4), 250–281. 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- Wong, M. C. , Wong, S. H. , Ng, S. C. , Wu, J. C. , Chan, F. K. , & Sung, J. J. (2015). Targeted screening for colorectal cancer in high‐risk individuals. Best Practice & Research. Clinical Gastroenterology, 29(6), 941–951. 10.1016/j.bpg.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Woolf, S. H. , Grol, R. , Hutchinson, A. , Eccles, M. , & Grimshaw, J. (1999). Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ, 318(7182), 527–530. 10.1136/bmj.318.7182.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Gurudu, S. R. , Koptiuch, C. , Agrawal, D. , Buxbaum, J. L. , Abbas Fehmi, S. M. , Fishman, D. S. , Khashab, M. A. , Jamil, L. H. , Jue, T. L. , Law, J. K. , Lee, J. K. , Naveed, M. , Qumseya, B. J. , Sawhney, M. S. , Thosani, N. , Wani, S. B. , & Samadder, N. J. (2020). American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointestinal Endoscopy, 91(5), 963–82.e2. 10.1016/j.gie.2020.01.028 [DOI] [PubMed] [Google Scholar]
- Zauber, A. G. , Winawer, S. J. , O'Brien, M. J. , Lansdorp‐Vogelaar, I. , van Ballegooijen, M. , Hankey, B. F. , Shi, W. , Bond, J. H. , Schapiro, M. , Panish, J. F. , Stewart, E. T. , & Waye, J. D. (2012). Colonoscopic polypectomy and long‐term prevention of colorectal‐cancer deaths. The New England Journal of Medicine, 366(8), 687–696. 10.1056/NEJMoa1100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeimet, A. G. , Mori, H. , Petru, E. , Polterauer, S. , Reinthaller, A. , Schauer, C. , Scholl‐Firon, T. , Singer, C. , Wimmer, K. , Zschocke, J. , & Marth, C. (2017). AGO Austria recommendation on screening and diagnosis of Lynch syndrome (LS). Archives of Gynecology and Obstetrics, 296(1), 123–127. 10.1007/s00404-017-4392-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 손대경 김, 박윤희, 서민아, 신애선, 이희영, 임종필, 조현민, 홍성필, 김백희, 김용수, 김정욱, 김현수, 남정모, 박동일, 엄준원, 오순남, 임환섭, 장희진, 함상근, 정지혜, 김수영, 김열, 이원철, 정승용 . 대장암 검진 권고안. 10.5124/jkma.2015.58.5.420. J Korean Med Assoc. 2015;58:420–32. [DOI] [Google Scholar]
- 中国抗癌协会肿瘤内镜学专业委员会 国上国中医中中消中中中金国 . 中国早期结直肠癌筛查流程专家共识意见 (2019, 上海). Natl Med J China. 2019;99.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S0: PRISMA 2020 Checklist
Appendix S1: Data sources and search strategy.
Appendix S2: Quality assessment tool for Shared Decision Making (SDM) recommendations in Breast Cancer Management Clinical Practice Guidelines (CPG) and consensus (CS).
Appendix S3: Quality assessment tool for Shared Decision Making (SDM) recommendations in Breast Cancer Management Clinical Practice Guidelines (CPG) and consensus (CS).
Appendix S4: Data extraction analysis.
Appendix S5: The reviewer agreement calculated using the intra‐class correlation coefficient (corr).
Data Availability Statement
All the supporting information can be accessed upon request via email to the corresponding authors of this review.


