Abstract
The ability of Toxoplasma gondii tachyzoites to differentiate into latent bradyzoite forms is essential for pathogenesis of clinical disease. We examined the effects of cyclic nucleotides on T. gondii bradyzoite differentiation in vitro. Differentiation of tachyzoites to bradyzoites was measured in an immunofluorescence assay using ME49 or its clonal derivative PLK, two well-characterized T. gondii strains. Treatment of human fibroblast cultures infected with T. gondii with 8-(4-chlorophenylthio)-cyclic GMP (CPT-cGMP), a membrane-permeable, nonhydrolyzable analogue of cGMP, resulted in an increased percentage of bradyzoite-positive vacuoles. Cyclic AMP (cAMP) also induced in vitro conversion of PLK, but the method of cAMP elevation was critical. Forskolin raises cAMP levels transiently and induced bradyzoites, whereas agents predicted to cause sustained elevation of cAMP were inhibitory to parasite conversion. Levels of cAMP were measured in host cells and extracellular tachyzoites. Forskolin, CPT-cGMP, and agents known to induce bradyzoite formation elevated cAMP in host cells and PLK parasites. These data suggest cyclic nucleotide signaling pathways are important in the stress-induced conversion of T. gondii tachyzoites to bradyzoites. Furthermore, because cAMP elevation was seen in PLK but not RH, a T. gondii strain that did not differentiate well in our assay, cAMP signaling within the parasite is likely to be critical.
Toxoplasma gondii is an obligate intracellular apicomplexan parasite responsible for encephalitis in immunocompromised individuals and birth defects in children infected in utero. Although some individuals present with toxoplasmosis during acute infection, most clinically apparent disease results from reactivation of dormant bradyzoites and their conversion to tachyzoites. Unchecked multiplication of the rapidly growing tachyzoite is thought to be responsible for disease, and control of tachyzoites by the immune system results in their conversion to latent bradyzoite forms. Thus, elucidation of the signaling pathways responsible for tachyzoite-bradyzoite interconversion is critical for understanding pathogenesis of toxoplasmosis.
Recent studies by several investigators have established that a variety of stress conditions including pH shock, heat shock, mitochondrial inhibitors, chemical stress, and nitric oxide induce bradyzoite formation (2, 3, 21, 23). Induction of a variety of heat shock proteins (HSPs) including HSP70 is associated with bradyzoite transition (19, 25), and knockout of a bradyzoite-specific small HSP gene, BAG1, results in reduced numbers of bradyzoites in mouse brains (29). These data collectively suggest that the transition from tachyzoite to bradyzoite is a stress-induced differentiation response. Because of the remarkable conservation of cyclic nucleotide signaling pathways in the stress response in a wide variety of organisms including other eukaryotic pathogens, we examined the role of cyclic nucleotide signaling in bradyzoite differentiation in T. gondii.
Our data suggest that both cyclic GMP (cGMP) and cyclic AMP (cAMP) can induce bradyzoite formation. These effects could be due to an increase in host or parasite cyclic nucleotides. PLK, a T. gondii strain able to differentiate in vitro, exhibited a rise in cAMP in response to bradyzoite-inducing conditions, but elevation of cAMP under the same conditions was not evident in RH, a strain that does not differentiate well. These data suggest that cAMP elevation within the parasite may be important for bradyzoite differentiation.
MATERIALS AND METHODS
Parasite and tissue culture.
ME49, PLK (a clonal derivative of ME49 [10]), and RH were the three T. gondii strains used. PLK parasites had been previously passaged though mice and were known to generate cysts efficiently in vivo (29). Parasites were maintained by serial passage in confluent monolayers of human foreskin fibroblasts (HFF) grown in Dulbecco's modified Eagle's medium (Gibco BRL, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (Gibco BRL) and 1% penicillin-streptomycin (Gibco BRL).
Materials.
Forskolin, 1-methyl-3-isobutylxanthine (IBMX), 8-(4-chlorophenylthio)-cAMP (CPT-cAMP), 8-(4-chlorophenylthio)-cGMP (CPT-cGMP), and sodium nitroprusside (SNP) were obtained from Sigma (St. Louis, Mo.). LY83583 (6-anilino-5,8-quinolinequinone) and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1- one (ODQ) were obtained from Calbiochem (La Jolla, Calif.). Forskolin, CPT-cAMP, CPT-cGMP, and IBMX were dissolved in dimethyl sulfoxide; SNP was dissolved in Dulbecco's modified Eagle's medium.
In vitro bradyzoite assay.
T. gondii in vitro differentiation assays were performed using an indirect immunofluorescence assay as described by Weiss et al. (23). Approximately 4,000 parasites were inoculated with the agent to be tested onto confluent HFF monolayers growing in four-chambered coverglass slides or Permanox slides (Lab-Tek; Fisher Scientific, Pittsburgh, Pa.). Each condition to be analyzed was set up in duplicate for each experiment. Cultures were grown for 2 or 3 days. (Cultures were monitored daily and fixed when tachyzoite vacuoles were on the verge of lysing [generally 2 days for RH and 3 days for PLK].) Fixation of cultures at 1 day was not routinely performed because staining was frequently weak or uneven and SNP-induced vacuoles were often too small to count reliably.
For each agent tested, control cultures were grown in medium with solvent. Bradyzoite-inducing conditions included pH shock (pH 8.1) and SNP, a nitric oxide donor. Medium for pH shock experiments contained 10 mM HEPES for buffering to the appropriate pH.
Cells were fixed in 3% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min and then permeabilized with 0.2% Triton X-100 in PBS for 20 min. Cells were then blocked with 3% bovine serum albumin and 0.2% Triton X-100 in PBS for at least 1 h. Slides were incubated for 1 h with rabbit antibody to T. gondii (diluted 1:500) and biotinylated Dolichos biflorus agglutinin (DBA; diluted 1:500; Vector Laboratories, Burlingame, Calif.) diluted with blocking solution. After three washes with PBS, cells were incubated for 1 h with goat anti-rabbit immunoglobulin G conjugated to fluorescein isothiocyanate (diluted 1:100; Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) and streptavidin-Texas red conjugate (diluted 1:500; Vector Laboratories). Similar results were obtained if cells were labeled with DBA, counted, and then stained with anti-Toxoplasma antibody. Controls with secondary reagents alone confirmed specificity of labeling by DBA and the T. gondii antisera. Cells were overlaid with 2.5% DABCO (Sigma) and 20% glycerol in PBS. Epifluorescence was detected with a Nikon Diaphot inverted U-V microscope. Parasite vacuoles and bradyzoite-specific vacuoles were counted manually or by analysis of images acquired with a Monospot Jr digital camera (Diagnostic Instrumentation Inc., Sterling Heights, Mich.) using Image Pro-plus (Media Cybernetics, Silver Spring, Md.) loaded onto an Apple Power Mac G3.
For comparison between experiments, percent induction for control and experimental conditions were calculated. Experimental conditions were normalized to the control values and expressed as fold induction. Data were analyzed by nonparametric statistics using the Wilcoxon signed rank test. Statistical analysis was performed with Sigma-Stat version 2.0 (SPSS Science, Chicago, Ill.).
Cyclic nucleotide measurements.
Freshly lysed RH, PLK, and ME49 strains of T. gondii were purified from host cells by serial passage through 20-, 23-, 25-, and 27-gauge needles and filtration through a 3.0-μm-pore-size Nuclepore filter. Parasites were washed and resuspended in complete medium supplemented with the agents to be tested. Incubations were performed at 37°C in a CO2 incubator to maintain normal medium pH. At 15, 30, and 60 min, cells were placed in 20 mM phosphate buffer (pH 7.0) containing 20 mM EDTA and 1 mM IBMX. The cells were then boiled for 7 min and quick cooled on ice. The extract was briefly centrifuged, and then the supernatant was stored at −20°C. Cyclic nucleotide levels were measured in duplicate using the cGMP and cAMP enzyme immunoassay (EIA) kits from Stratagene (La Jolla, Calif.) following the manufacturer's instructions. cGMP samples were acetylated prior to testing, and cAMP levels were measured with nonacetylated samples. Absolute levels of cyclic nucleotide were calculated by comparison to a standard curve generated during each experiment.
RESULTS
In vitro bradyzoite induction assay.
DBA lectin reactivity has been reported to be a sensitive early marker of bradyzoite differentiation similar to BAG1 and p36/BSR4 (4). The lectin recognizes carbohydrate modifications of a 116-kDa cyst wall glycoprotein previously identified with bradyzoite-specific monoclonal antibody 73.18 (24, 28). We adapted DBA to the in vitro differentiation assay. In our hands, DBA gave results similar to those obtained with BAG1 immunostaining (23).
Induction of bradyzoite formation by cyclic nucleotides.
Initially, the membrane-permeable nonhydrolyzable forms of cAMP and cGMP, CPT-cAMP and CPT-cGMP, were compared to control medium for the ability to induce bradyzoite conversion in culture. Parasites were infected onto HFF in medium supplemented with the agent to be tested and then grown for 3 days or until tachyzoites had begun to lyse. Each condition was tested in duplicate. Bradyzoite-positive vacuoles were counted for each experiment and compared to the control cultures. Cultures with SNP, which induces bradyzoite formation, were analyzed in parallel. Results from a representative experiment are shown in Table 1.
TABLE 1.
In vitro induction of bradyzoite formation by cyclic nucleotidesa
| Condition | Well | No. of bradyzoite vacuoles | No. of total vacuoles | % Bradyzoite vacuoles | Avg % bradyzoite vacuoles ± SEM | Fold induction |
|---|---|---|---|---|---|---|
| Control | 1 | 77 | 585 | 13 | 16.5 ± 3.5 | 1.0 |
| 2 | 137 | 670 | 20 | |||
| SNP | 1 | 384 | 655 | 59 | 73.5 ± 13.5 | 4.5 |
| 2 | 613 | 705 | 86 | |||
| CPT-cAMP | 1 | 32 | 820 | 4 | 3.5 ± 0.5 | 0.2 |
| 2 | 22 | 720 | 3 | |||
| CPT-cGMP | 1 | 190 | 660 | 29 | 37 ± 8.0 | 2.2 |
| 2 | 283 | 620 | 45 | |||
| IBMX | 1 | 18 | 410 | 4.4 | 4.5 ± 0.05 | 0.3 |
| 2 | 33 | 730 | 4.5 | |||
| Forskolin | 1 | 283 | 650 | 44 | 51.5 ± 7.5 | 3.1 |
| 2 | 414 | 695 | 59 | |||
| Forskolin-IBMX | 1 | 15 | 720 | 2 | 1.75 ± 0.25 | 0.1 |
| 2 | 13 | 860 | 1.5 |
PLK tachyzoites were inoculated in duplicate onto HFF with the indicated agents (100 μM SNP, 250 μM CPT-cAMP, 250 μM CPT-cGMP, 500 μM IBMX, and 10 μM forskolin) and incubated for 3 days. Slides were stained and counted as indicated in Materials and Methods.
Because of variation in endogenous switch rate, PLK and ME49 data were normalized to the control level (arbitrarily set at 1) to allow comparison between experiments (Table 2). Results for strains ME49 and PLK were comparable and therefore were pooled. The experimental conditions were compared to those for control media. CPT-cGMP treatment increased bradyzoite vacuole formation 2.3-fold over the control. Parasite vacuoles treated with CPT-cGMP were smaller than the control and in general contained fewer than five parasites, as did the parasites cultures treated with SNP. CPT-cAMP at 250 μM inhibited bradyzoite formation, with the average number of bradyzoite vacuoles 0.4-fold the control level (Table 2). Higher concentrations of CPT-cAMP sometimes led to bradyzoite induction, but only when accompanied by visible compromise of the integrity of the fibroblast monolayer over the 3 days that the experiment was conducted.
TABLE 2.
Average fold induction by agents affecting cyclic nucleotide signalinga
| Condition | No. of expts | Avg fold induction ± SEM | P |
|---|---|---|---|
| Control | 6 | 1.0 | NA |
| SNP | 6 | 6.4 ± 1.5 | 0.002 |
| CPT-cAMP | 5 | 0.4 ± 0.2 | 0.03 |
| CPT-cGMP | 6 | 2.3 ± 0.1 | 0.03 |
| Forskolin | 4 | 5.6 ± 2.9 | 0.01 |
| IBMX | 4 | 0.12 ± 0.02 | 0.01 |
PLK or ME49 tachyzoites were inoculated onto HFF in duplicate with the indicated agents (100 μM SNP, 250 μM CPT-cAMP, 250 μM CPT-cGMP, 500 μM IBMX, and 10 μM forskolin) and incubated for 3 days. (Results for PLK and ME49 were similar and therefore pooled.) Slides were stained and counted as indicated in Materials and Methods. Data for each experiment were analyzed as shown for a representative experiment in Table 1. P values were calculated by nonparametric statistics, using the Wilcoxon signed rank test. NA, not applicable.
Because the effects elicited by cAMP in other differentiation systems like Dictyostelium often depend on the duration and timing of cAMP elevation, we used different activators and inhibitors of the cAMP signaling pathway. Forskolin, a rapid and reversible adenylate cyclase activator, leads to a transient increase in cAMP. IBMX is a nonspecific inhibitor of most phosphodiesterases and would be expected to inhibit breakdown of both cGMP and cAMP.
Forskolin at 10 μM consistently induced bradyzoite formation, on average 5.6-fold (Table 2), comparable to SNP induction (6.4-fold). Cysts induced by forskolin were larger than those induced by CPT-cGMP or SNP and appeared to express bradyzoite antigens less abundantly when cultures were assayed after only 1 or 2 days in inducing conditions.
IBMX at 500 μM inhibited bradyzoite formation (0.12-fold the control level) more than any other treatment used (Table 2). IBMX also interfered with forskolin-induced bradyzoite differentiation, as treatment with both agents together inhibited bradyzoite formation (Table 1).
Effects of guanylate cyclase inhibitors.
Nitric oxide exerts some of its effects on cells by sequestering the heme group in soluble guanylate cyclase, leading to cyclase activation and increased levels of cGMP. The downstream effects of cGMP can be mediated by activation of cGMP-dependent kinase and either activation or inhibition of phosphodiesterase activity. Therefore, we tested the effects of the soluble guanylate cyclase inhibitors LY83583 (1) and ODQ (7, 18) on bradyzoite differentiation.
Induction of bradyzoite vacuoles by 100 μM SNP was inhibited by 1 μM LY83583 (1.1-fold induction compared to untreated controls; n = 4). Incubation of 1 mM cGMP (or a nonhydrolyzable cGMP analogue) with 1 μM LY83583 and 100 μM SNP restored bradyzoite differentiation to levels seen with 100 μM SNP alone. LY83583 at 1 μM had no effect on pH 8.1 induction of bradyzoite vacuoles. Because LY83583 has other effects (discussed in reference 7), we tested ODQ, which is reported to be an irreversible and more specific inhibitor of soluble guanylate cyclase (7, 18); 50 μM ODQ inhibited induction of bradyzoites by 100 μM SNP. The effects exerted by LY83583 and ODQ, however, are likely to be complex. Unlike LY83583, 50 μM ODQ alone induced bradyzoite formation to levels comparable to that induced by SNP.
Cyclic nucleotide measurements.
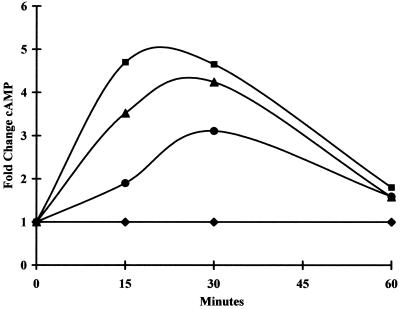
Cyclic nucleotide levels were measured in PLK and RH strain extracellular parasites as well as host cells by using cAMP and cGMP EIA assays. Samples were tested at 15, 30, and 60 min. For all conditions tested, absolute cGMP levels were lower than cAMP levels, and acetylated cGMP samples were used for detection. Acetylation increases the sensitivity of the assay at least 10-fold (Stratagene cAMP and cGMP EIA user manual). Host cells (HFF) exhibited cAMP elevation in response to 100 μM SNP, 100 μM forskolin, and pH 8.1 (Fig. 1). In HFF, SNP consistently led to an elevation of cGMP, but consistent results were not seen in response to other bradyzoite-inducing conditions (data not shown).
FIG. 1.
cAMP levels in HFF after exposure to various extracellular stimuli. Monolayers of host HFF were incubated in media with the indicated conditions. At the indicated times, samples were harvested and processed for cAMP measurements using a Stratagene EIA kit. Cyclic nucleotide concentration was determined by comparison to a standard curve. Numbers are plotted as fold change in concentration compared to untreated control cells. Shown are fold change in HFF cAMP levels in response to 100 μM SNP (circles), pH 8.1 (triangles), and 100 μM forskolin (squares) compared to untreated control cells (diamonds). Results of a representative experiment are shown.
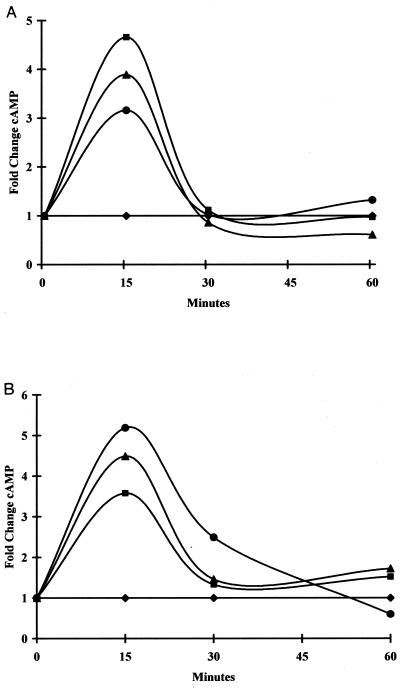
As demonstrated in Fig. 2A, T. gondii strain PLK responded to extracellular SNP, pH 8.1, and forskolin with an elevation in cAMP. CPT-cGMP, which we found induced bradyzoite formation, also increased cAMP in PLK (Fig. 2B). Thus, all four conditions that induced bradyzoite formation in PLK also induced elevations of cAMP levels in the parasites within 15 min. IBMX, which inhibits breakdown of phosphodiesterases, also elevated cAMP levels in PLK (Fig. 2B), suggesting that it was active on T. gondii phosphodiesterases. We could not accurately evaluate the duration of cAMP elevation, because incubation of control extracellular parasites in media exhibited gradual increase in absolute cAMP over time. Prolonged incubation of extracellular tachyzoites in media results in induction of expression of bradyzoite genes (27).
FIG. 2.
cAMP levels in T. gondii PLK tachyzoites in response to various stimuli. Extracellular PLK parasites were incubated in media with the indicated conditions. At the indicated times, samples were harvested and processed for cAMP measurements using a Stratagene EIA kit. cAMP concentration was determined by comparison to a standard curve generated for each experiment. Numbers are plotted as fold change in concentration compared to untreated control cells. (A) cAMP levels in PLK parasites exposed to 100 μM SNP (circles), pH 8.1 (triangles), and 100 μM forskolin (squares) compared to untreated control cells (diamonds). Results of a representative experiment are shown. (B) cAMP levels in PLK parasites treated with 100 μM SNP (circles), 250 μM CPT-cGMP (triangles), or 500 μM IBMX (squares) compared to untreated control cells (diamonds). Results of representative experiment are shown.
RH strain parasites, which did not form significant numbers of bradyzoite vacuoles in vitro, did not have a significant elevation in cAMP levels compared to control cultures with any of the stimuli tested (data not shown). cGMP levels were also measured in PLK and RH parasites. Consistent changes in cGMP were not observed in the PLK or RH strain (data not shown). cGMP levels are typically only 1 to 10% as high as cAMP levels, and it is possible that the levels of cGMP in parasites were too low for reliable detection of changes.
DISCUSSION
Studies from a number of laboratories suggest that bradyzoite formation is a response of parasites to environmental stress. Although changes in the parasite's environment frequently reflect changes in the host cell, there need not be changes in the host cell for bradyzoite induction to occur. Exposure of extracellular parasites to stress-inducing bradyzoite conditions is sufficient to induce bradyzoite differentiation whether pH induction or SNP is used (25). These data suggest that parasites sense and respond to changes in their extracellular environment by differentiating into bradyzoites and that continuous induction is not necessary. Similarly, Yahiaoui et al. (27) found that parasites incubated in media extracellularly for 12 h efficiently differentiated into bradyzoites once inoculated onto HFF grown in normal media.
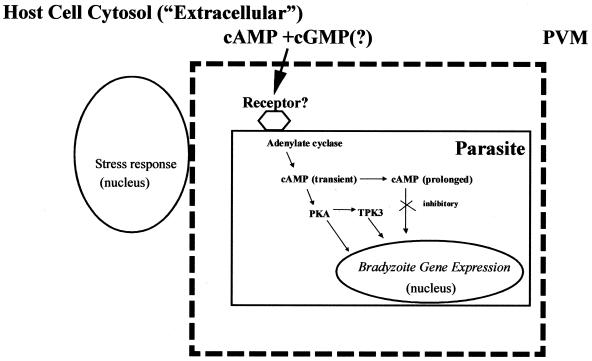
Our studies indicate that both cGMP and cAMP can mediate bradyzoite differentiation, and data from other systems suggest that these signaling pathways are likely to interact. It remains to be determined whether these effects are extracellular (i.e., host mediated), intracellular (i.e., within the parasite), or, more likely, due to stimulation of both host and parasite signaling pathways. It is likely that bradyzoite differentiation involves the interaction of multiple host and parasite signaling pathways including both cGMP and cAMP pathways (Fig. 3).
FIG. 3.
A working model for stress-induced bradyzoite differentiation. Stress conditions, including pH shock and exposure to nitric oxide, result in elevation of cAMP and cGMP (in response to nitric oxide) within host cells. Host cell stress and cAMP response genes are induced. Stress also induces formation of cAMP within the parasite by adenylate cyclase, which may be coupled to a receptor that senses an extracellular signaling molecule or nutrient. cAMP activates PKA and leads to transcription of bradyzoite-specific genes. Prolonged cAMP also activates pathways inhibitory to bradyzoite differentiation. TPK3, a GSK3 family member, may also interact with the PKA signaling pathway as seen in other stress-induced differentiation models such as Dictyostelium. PVM, parasitophorous vacuole membrane.
HFF generated a significant cAMP elevation after exposure to conditions associated with bradyzoite induction. It is likely that host cell environments including cAMP elevations contribute to the bradyzoite differentiation process. It is not known if T. gondii has a receptor or sensor for cyclic nucleotides in its extracellular environment. In Dictyostelium, cAMP secreted into the environment binds to cAMP receptors to regulate the differentiation program of cells within the fruiting body (20). A similar pathway may also be important in T. gondii, but elevation in host cell cAMP does not appear to be sufficient for differentiation given that T. gondii RH differentiated much less efficiently than T. gondii PLK or ME49.
The role of cGMP in T. gondii bradyzoite differentiation remains to be clarified. CPT-cGMP consistently induced PLK tachyzoites to differentiate into bradyzoites. CPT-cGMP also elevated cAMP levels in parasites, suggesting that cGMP effects may be due to regulation of cAMP levels. We addressed the role of cGMP in bradyzoite differentiation by using inhibitors of guanylate cyclase. While these experiments are consistent with a role for cGMP in nitric oxide-mediated bradyzoite induction, the effects of these inhibitors was complex and effects on other signaling molecules cannot be ruled out.
A cAMP response within the parasite may be associated with differentiation. We could not generate a significant cAMP response in RH parasites, but our RH isolate did not differentiate well under any conditions tested. In contrast, PLK parasites had a significant elevation in intracellular cAMP levels in conditions known to induce bradyzoite differentiation and were able to differentiate efficiently. Because the cyclic nucleotide measurements were performed on a population of cells, we were unable to determine if the small subpopulation of RH parasites differentiating to bradyzoites had changes in cyclic nucleotide levels.
It seems likely that cAMP has both stimulatory and antagonistic effects on bradyzoite differentiation. While both forskolin and IBMX elevated cAMP in parasites, only forskolin induced bradyzoite formation. Since IBMX, which inhibits cAMP degradation, blocked bradyzoite induction by forskolin, the degree of cAMP elevation or kinetics of cAMP elevation may be critical. It may be that sustained elevation of cAMP leads to activation of pathways that are inhibitory to bradyzoite differentiation. In support of this hypothesis, CPT-cAMP, which is not degraded by phosphodiesterases, was also inhibitory for bradyzoite formation. Signaling molecules such as the mitogen-activated protein kinase KSS1 (6, 13) and cAMP-dependent kinases (14, 17) have been described to have both positive and negative regulatory effects in stress-induced pseudohyphal differentiation in Saccharomyces cerevisiae.
Studies of many pathogens have correlated cAMP signaling with differentiation. cAMP signaling has been implicated in Plasmodium falciparum differentiation to gametocytes (11), but as with T. gondii, cAMP effects have not been observed in all strains (5, 9). cAMP signaling has also been found to be critical for virulence in a number of fungal pathogens, including Cryptococcus neoformans and Candida albicans (12).
Our data would predict that T. gondii protein kinase A (PKA) plays a significant role in the conversion of tachyzoites to bradyzoite forms. In P. falciparum, a correlation between PKA levels and ability to transform into gametocytes has been described (16). PKA could have stimulatory or inhibitory effects on bradyzoite gene transcription or effects on other kinases important in cell fate decisions and stress-induced differentiation (8) such as the T. gondii GSK3/shaggy homologue TPK3 (15, 26) (Fig. 3). Recent data for S. cerevisiae reveal that one of the three PKA catalytic subunits mediates stress-induced differentiation (14, 17), and studies of Dictyostelium have suggested that cAMP is not required for differentiation if sufficient levels of PKA activity are present (22).
Studies in other organisms suggest that the signaling pathways utilized by eukaryotic pathogens in response to stress are phylogenetically conserved and significantly affect virulence and pathogenicity. Further studies of T. gondii will be needed to clarify the complex interactions of cGMP and cAMP signaling molecules with upstream sensors and downstream effector molecules.
ACKNOWLEDGMENTS
L.A.K. is a Howard Hughes Medical Student Scholar. K.K. is a Burroughs Wellcome New Investigator in Molecular Parasitology. This study was supported by Public Health Service grants AI41058 (K.K.), AI01535 (K.K.), and AI39454 (L.M.W.) from the National Institute of Allergy and Infectious Diseases.
We thank Jianzhong Tang, Yan Fen Ma, and Denise LaPlace for expert technical assistance.
REFERENCES
- 1.Beasley D, Schwartz J H, Brenner B M. Interleukin 1 induces prolonged l-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Investig. 1991;87:602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohne W, Heesemann J, Gross U. Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect Immun. 1993;61:1141–1145. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boothroyd J C, Black M, Bonnefoy S, Hehl A, Knoll L J, Manger I D, Ortega-Barria E, Tomavo S. Genetic and biochemical analysis of development in Toxoplasma gondii. Philos Trans R Soc Lond B. 1997;352:1347–1354. doi: 10.1098/rstb.1997.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockelman C R. Conditions favoring gametocytogenesis in the continuous culture of Plasmodium falciparum. J Protozool. 1982;29:454–458. doi: 10.1111/j.1550-7408.1982.tb05432.x. [DOI] [PubMed] [Google Scholar]
- 6.Cook J G, Bardwell L, Thorner J. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signalling pathway. Nature. 1997;390:85–88. doi: 10.1038/36355. [DOI] [PubMed] [Google Scholar]
- 7.Garthwaite J, Southam E, Boulton C L, Nielsen E B, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 8.Harwood A, Plyte S, Woodgett J, Strutt H, Kay R. Glycogen synthase kinase-3 regulates cell fate in Dictyostelium. Cell. 1995;80:139–148. doi: 10.1016/0092-8674(95)90458-1. [DOI] [PubMed] [Google Scholar]
- 9.Inselburg J. Stage-specific inhibitory effect of cyclic AMP on asexual maturation and gametocyte formation of Plasmodium falciparum. J Parasitol. 1983;69:592–597. [PubMed] [Google Scholar]
- 10.Kasper L, Ware P. Recognition and characterization of stage-specific oocyst/sporozoite antigens of Toxoplasma gondii. J Clin Investig. 1985;75:1570. doi: 10.1172/JCI111862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushal D C, Carter R, Miller L H, Krishna G. Gametocytogenesis by malaria parasites in continuous culture. Nature. 1980;286:490–492. doi: 10.1038/286490a0. [DOI] [PubMed] [Google Scholar]
- 12.Madhani H D, Fink G R. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 1998;8:348–353. doi: 10.1016/s0962-8924(98)01298-7. [DOI] [PubMed] [Google Scholar]
- 13.Madhani H D, Styles C A, Fink G R. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell. 1997;91:673–684. doi: 10.1016/s0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 14.Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–4887. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin C-l, Tang J, Kim K. Cloning and in vitro expression of TPK3, a Toxoplasma gondii homologue of shaggy/glycogen synthase kinase-3 kinases. Mol Biochem Parasitol. 1998;93:273–282. doi: 10.1016/s0166-6851(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 16.Read L K, Mikkelsen R B. Comparison of adenylate cyclase and cAMP-dependent protein kinase in gametocytogenic and nongametocytogenic clones of Plasmodium falciparum. J Parasitol. 1991;77:346–352. [PubMed] [Google Scholar]
- 17.Robertson L S, Fink G R. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc Natl Acad Sci USA. 1998;95:13783–13787. doi: 10.1073/pnas.95.23.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- 19.Silva N M, Gazzinelli R T, Silva D A, Ferro E A, Kasper L H, Mineo J R. Expression of Toxoplasma gondii-specific heat shock protein 70 during in vivo conversion of bradyzoites to tachyzoites. Infect Immun. 1998;66:3959–3963. doi: 10.1128/iai.66.8.3959-3963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soderbom F, Loomis W F. Cell-cell signaling during Dictyostelium development. Trends Microbiol. 1998;6:402–406. doi: 10.1016/s0966-842x(98)01348-1. [DOI] [PubMed] [Google Scholar]
- 21.Soete M, Camus D, Dubremetz J F. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–370. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Kuspa A. Dictyostelium development in the absence of cAMP. Science. 1997;277:251–254. doi: 10.1126/science.277.5323.251. [DOI] [PubMed] [Google Scholar]
- 23.Weiss L M, Laplace D, Takvorian P M, Tanowitz H B, Cali A, Wittner M. A cell culture system for study of the development of Toxoplasma gondii bradyzoites. J Eukaryot Microbiol. 1995;42:150–157. doi: 10.1111/j.1550-7408.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 24.Weiss L M, LaPlace D, Tanowitz H B, Wittner M. Identification of Toxoplasma gondii bradyzoite-specific monoclonal antibodies. J Infect Dis. 1992;166:213–215. doi: 10.1093/infdis/166.1.213. [DOI] [PubMed] [Google Scholar]
- 25.Weiss L M, Ma Y F, Takvorian P M, Tanowitz H B, Wittner M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infect Immun. 1998;66:3295–3302. doi: 10.1128/iai.66.7.3295-3302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh G I, Wilson C, Proud C. GSK3: a SHAGGY frog story. Trends Cell Biol. 1996;6:274–279. doi: 10.1016/0962-8924(96)10023-4. [DOI] [PubMed] [Google Scholar]
- 27.Yahiaoui B, Dzierszinski F, Bernigaud A, Slomianny C, Camus D, Tomavo S. Isolation and characterization of a subtractive library enriched for developmentally regulated transcripts expressed during encystation of Toxoplasma gondii. Mol Biochem Parasitol. 1999;99:223–235. doi: 10.1016/s0166-6851(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y W, Halonen S K, Ma Y F, Wittner M, Weiss L M. Initial characterization of CST1: a Toxoplasma gondii cyst wall glycoprotein. Infect Immun. 2001;69:501–507. doi: 10.1128/IAI.69.1.501-507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y W, Kim K, Ma Y F, Wittner M, Tanowitz H B, Weiss L M. Disruption of the Toxoplasma gondii bradyzoite-specific gene BAG1 decreases in vivo cyst formation. Mol Microbiol. 1999;31:691–701. doi: 10.1046/j.1365-2958.1999.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]